Abstract
Background
Venous thromboembolism (VTE) is a major complication in patients with malignant tumors and orthopedic disorders. Although it is known that patients undergoing surgery for malignant musculoskeletal tumor are at an increased risk of thromboembolic events, only few studies have investigated this risk in detail. Therefore, the aim of this study was to determine the prevalence and risk factors for preoperative VTE in malignant musculoskeletal tumors patients.
Methods
We retrospectively reviewed the medical records of 270 patients who underwent surgical procedures, including biopsy for malignant musculoskeletal tumor, have undergone measurements of preoperative D-dimer levels, and were subsequently screened for VTE by lower extremity venous ultrasonography and/or contrast-enhanced computed tomography scans. Statistical analyses were performed to examine the prevalence and risk factors for VTE. Receiver operating characteristic (ROC) analysis was performed to verify the D-dimer cutoff value for the diagnosis of VTE.
Results
Overall, 199 patients (103 with primary soft tissue sarcomas, 38 with primary bone sarcomas, 46 with metastatic tumors, and 12 with hematologic malignancies) were included. D-dimer levels were high in 79 patients; VTE was detected in 19 patients (9.5%). Multivariate analysis indicated that age ≥ 60 years (P = 0.021) and tumor location in the lower limbs (P = 0.048) were independent risk factors for VTE. ROC analysis showed that the D-dimer cutoff value for the diagnosis of VTE was 1.53 µg/mL; the sensitivity and specificity were 89.5% and 79.4%, respectively.
Conclusions
Our study indicated that age and tumor location in the lower limbs were independent risk factors for preoperative VTE in malignant musculoskeletal tumors patients. D-dimer levels were not associated with VTE in the multivariate analysis, likely because they are affected by a wide variety of conditions, such as malignancy and aging. D-dimer is useful for exclusion diagnosis because of its high sensitivity, but patients with high age and tumor location in the lower limbs are a high-risk group and should be considered for imaging evaluation such as ultrasonography regardless of D-dimer levels.
Trial registration
Our study was approved by the institutional review board. The registration number is B200600056. The registration date was July 13, 2020.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12959-022-00382-2.
Keywords: Preoperative venous thromboembolism, VTE, Deep vein thrombosis, DVT, Malignant musculoskeletal tumor, Malignant musculoskeletal tumor, D-dimer
Background
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a major complication in orthopedic surgery. VTE is also known to be associated with malignancy, which increases the risk of VTE by 2- to sevenfold [1–3]. Several mechanisms may be involved in the promotion of thromboembolic events in cancer patients [4]. Because the risk of VTE is reported to be particularly high during the first few months after the diagnosis of malignancy [3], it is important to assess affected patients regarding this condition before initiating treatment.
D-dimer, the breakdown product of stabilized fibrin, is frequently used to screen for VTE as there is well-established evidence that levels below 0.5 µg/mL have an exceedingly high negative predictive value for the exclusion of PE [5–7]. Although lower extremity venography is the mainstay for the diagnosis of DVT, it has recently been replaced by lower extremity venous ultrasonography and contrast-enhanced computed tomography (CT) [8–10]. The combination of clinical symptoms, D-dimer concentrations, and contrast-enhanced CT has made it possible to diagnose 97.9% of VTE cases [11, 12].
The prevalence of perioperative VTE in malignant musculoskeletal tumors patients ranges from 2.7% to 22% [13–21]. A myriad of factors, such as age, size and location of the tumor, chemotherapy, Ewing sarcoma family of tumors, metastases, pathological fracture, and type of surgery, have been suggested to be related to the occurrence of VTE [13–17, 20–23]. However, most previous studies analyzed postoperative, rather than preoperative, patients who underwent orthopedic surgery [13, 15–18, 22]. In addition, routine screening for VTE was not considered; therefore, the reported rate of VTE represented only those that were clinically symptomatic [15, 17].
Because such limitations may cause an underestimation of the prevalence of VTE, only limited evidence is available for understanding preoperative VTE and associated risks for malignant musculoskeletal tumors patients. Therefore, we investigated the prevalence and risk factors for VTE in these patients and hypothesized that a combination of measuring D-dimer levels and imaging-based examination may be the best screening strategy for VTE.
Methods
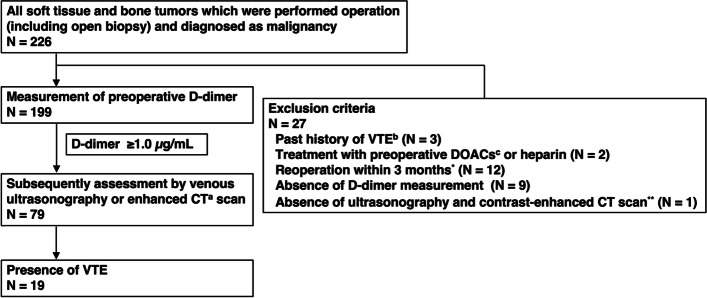
After obtaining approval from our institutional review board, we retrospectively reviewed the medical records of patients treated at our institution between January 2014 and June 2020. Out of 270 malignant musculoskeletal tumors patients who underwent orthopedic surgical procedures, including open biopsy, and were assessed for preoperative VTE according to a flowchart developed by our institution, a total of 199 patients were included (Fig. 1). VTE was screened by measuring preoperative D-dimer levels and subsequently performing lower extremity venous ultrasonography and/or contrast-enhanced CT. D-dimer levels were measured by LPIA D-dimer (Mitsubishi Chemical Medience, Tokyo, Japan); owing to the sensitivity of this assay, levels < 0.50 µg/mL were considered 0.50 µg/mL. Ultrasonography and CT scans were performed if the D-dimer level was ≥ 1.0 µg/mL. In this study, DVT in the lower extremities that involved the popliteal vein or those above was defined as the proximal type; DVT involving the area below the popliteal vein was defined as the distal type.
Fig. 1.
Flow chart depicting the venous thromboembolism assessment strategy. Patients were evaluated using D-dimer screening, lower extremity venous ultrasonography, and contrast-enhanced computed tomography. A total of 23 patients were not included based on the exclusion criteria. *Additional extensive resections for unplanned resections or positive margins and non-orthopedic surgery patients were included. **Only cases with D-dimer > 1.0 μg/ml and not yet evaluated imaging studies were indicated. a CT: computed tomography; b VTE: venous thromboembolism; c DOACs: direct oral anticoagulant
The clinical profiles of patients with VTE were retrospectively assessed. The anatomical location of the tumor was defined as the lower limb, including the pelvis, hip, thigh, knee, lower leg, ankle, and foot. Tumor location was also assessed based on the depth of the tumor. A soft tissue tumor located on the surface of the fascia was defined as superficial. Soft tissue tumors deeper than the fascia and bone tumors were defined as deep.
The Mann–Whitney test was used for continuous variables, and the chi-squared test or Fisher’s exact test were used for categorical variables. Logistic regression analysis was used for the multivariate analysis. Receiver operating characteristic (ROC) analysis was performed to verify the cutoff value of D-dimer for the diagnosis of VTE. Statistical significance was set at P < 0.05. All statistical analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria).
Results
We excluded patients if they met any of the following criteria: benign condition; history of VTE; treatment with direct oral anticoagulants or heparin before the assessment of VTE; reoperation within 3 months; absence of preoperative D-dimer measurements; and absence of lower extremity venous ultrasonography or contrast-enhanced CT examinations in cases with D-dimer > 1.0 μg/ml. Baseline information was summarized in Table 1. The patients comprised 113 men and 86 women, with a mean age of 60.9 years (range, 14–91 years). The tumor location was the lower limb in 133 patients. The D-dimer concentration was ≥ 1.0 µg/mL in 79 (39.7%) patients. The overall prevalence of preoperative VTE detected by ultrasonography and contrast-enhanced CT scans was 9.5% (19 patients). VTE was detected in 8.7% and 7.9% of patients with soft tissue and bone sarcomas, respectively. The prevalence of VTE in patients with metastases was 10.9%. The tumors comprised 103 primary soft tissue sarcomas, 38 primary bone sarcomas, 46 metastatic tumors, and 12 hematologic malignancies. The pathological diagnoses of the patients are summarized in Table 2. Proximal VTE and PE were detected in 3 (2.5%) and 2 (1.7%) patients, respectively. Most patients had asymptomatic VTE, but 3/19 patients (15.8%) had local edema or pain. The clinical profiles of the patients with VTE are summarized in Table 3. The patients comprised 10 men and 9 women with a mean age of 59.7 years (range, 19–91 years). The tumor was located in the lower limbs in 17 patients, accounting for 89.5% of the patients with VTE. The mean D-dimer level was 2.64 µg/mL (range, 1.05–11.72 µg/mL). Among the patients with VTE, 9 had soft tissue sarcomas and 3 had bone sarcomas. The cases of soft tissue sarcoma included 4 cases of myxofibrosarcomas, 3 undifferentiated pleomorphic sarcomas (UPSs), and 2 leiomyosarcomas. The tumor was located on the surface of the fascia in 4 patients and deeper than the fascia in 5 patients. The cases of bone sarcoma included 2 UPSs of the bone and 1 osteosarcoma. Seven patients had bone metastases and hematologic malignancies. ROC analysis showed that the cutoff value of D-dimer for the diagnosis of VTE was 1.53 µg/mL using by Youden's index; the area under the curve was 0.857 (95% CI, 0.804—0.911), and the sensitivity and specificity were 90.0% and 79.6%, respectively.
Table 1.
Demographic and clinical variables for patients
| Variable | Number of patients | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| VTE (-) | VTE ( +) | Odds ratio | 95% confidence interval | p-value | Odds ratio | 95% confidence interval | p-value | ||
| Number of patients (n = 199) | 180 | 19 (9.5%) | |||||||
| Diagnosis | Soft tissue sarcoma | 94 | 9 (8.7%) | ||||||
| Bone sarcoma | 35 | 3 (7.9%) | |||||||
| Metastasis | 41 | 5 (10.9%) | |||||||
| Hematologic malignancy | 10 | 2 (16.7%) | |||||||
| Sex | Male | 103 | 10 | ||||||
| Female | 77 | 9 | 0.81 | 0.28–2.31 | 0.85a | ||||
| Age (years) | 59.7 ± 1.4 | 70.8 ± 3.7 | |||||||
| < 60 | 74 | 2 | |||||||
| ≧ 60 | 106 | 17 | 5.89 | 1.33–54.1 | 0.011a | 5.80 | 1.32–26.5 | 0.021c | |
| BMI (cm/kg) | 22.46 | 21.39 | 0.24b | ||||||
| Size (mm) | 80.4 | 65.8 | 0.79b | ||||||
| Location | Lower limb | 116 | 17 | ||||||
| Other | 64 | 2 | 4.66 | 1.05–42.9 | 0.038a | 4.56 | 1.01–20.4 | 0.048c | |
| Origin | Bone | 80 | 10 | ||||||
| Soft | 100 | 9 | 0.72 | 0.25–2.08 | 0.63a | ||||
| Subcutaneous ~ fascia | 36 | 4 | |||||||
| Fascia ~ bone | 144 | 15 | 1.14 | 0.26–3.90 | 0.77a | ||||
| D-dimer | 0.65 | 2.64 | > 0.001b | 1.01 | 0.96–1.08 | 0.51c | |||
| Performance Status | 0–2 | 154 | 16 | ||||||
| 3–4 | 26 | 3 | 1.11 | 0.19–4.29 | 0.74a | ||||
| Pathological fracture | 23 | 3 | 1.28 | 0.22–4.99 | 0.71a | ||||
| Hematologic status | Hemoglobin (g/dL) | 12.9 | 13 | 0.88b | |||||
| White blood cell (/μL) | 7010 | 6080 | 0.38b | ||||||
| Platelet (× 104/μL) | 28 | 26.2 | 0.99b | ||||||
| C related protein (mg/L) | 1.78 | 0.56 | 0.80b | ||||||
aFisher’s exact test
bMann-Whitney U test
cLogistic regression analysis
Table 2.
Diagnosis of all patients in this study
| Tumor origin | Pathological diagnosis | Number of patients | |
|---|---|---|---|
| Primary | Bone | Chondrosarcoma | 15 |
| Osteosarcoma | 13 | ||
| UPS | 5 | ||
| Ewing sarcoma | 2 | ||
| Chordoma | 2 | ||
| Malignant giant cell tumor of bone | 1 | ||
| Subtotal | 38 | ||
| Soft tissue | Myxofibrosarcoma | 27 | |
| UPS | 24 | ||
| Myxoid liposarcoma | 10 | ||
| MPNST | 8 | ||
| Synovial sarcoma | 7 | ||
| Dedifferentiated liposarcoma | 6 | ||
| Leiomyosarcoma | 7 | ||
| Extraskeltal myxoid chondrosarcoma | 5 | ||
| Dermatofibrosarcoma protuberans | 3 | ||
| Pleomorphic liposarcoma | 2 | ||
| Rhabdomyosarcoma | 2 | ||
| Others | 2 | ||
| Subtotal | 103 | ||
| Metastasis | Renal cell cancer | 16 | |
| Lung cancer | 11 | ||
| Prostatic cancer | 5 | ||
| Breast cancer | 3 | ||
| Esophageal cancer | 2 | ||
| Colorectal cancer | 2 | ||
| Bladder cancer | 2 | ||
| Thyroid cancer | 1 | ||
| Gastric cancer | 1 | ||
| Liver cancer | 1 | ||
| Pancreatic cancer | 1 | ||
| Uterine cancer | 1 | ||
| Subtotal | 46 | ||
| Hematologic malignancy | Lymphoma | 9 | |
| Multiple myeloma | 3 | ||
| Subtotal | 12 | ||
| Total | 199 | ||
UPS Undifferentiated pleomorphic sarcoma, MPNST Malignant peripheral nerve sheath tumor
Table 3.
Characteristic of patients with venous thromboembolism
| Age | Sex | BMI (cm/kg) | Location | Depth | Diagnosis | Pathological fracture | PS | D-Dimer (μg/mL) | Location of VTE | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 76 | Male | 22.3 | Thigh | Superficial | Myxofibrosarcoma | - | 0 | 1.05 | Distal |
| 2 | 66 | Male | 18.7 | Femur | Bone | Lung cancer | - | 2 | 5.69 | Distal |
| 3 | 66 | Male | 29.1 | Thigh | Superficial | UPS | - | 0 | 1.58 | Distal |
| 4 | 86 | Female | 22.2 | Thigh | Deep | Leiomyosarcoma | - | 1 | 3.69 | Proximal |
| 5 | 79 | Male | 29.2 | Lower leg | Superficial | UPS | - | 1 | 2.16 | Distal |
| 6 | 77 | Female | 17.0 | Patella | Bone | Malignant lymphoma | - | 2 | 2.33 | PE, distal |
| 7 | 58 | Male | 19.8 | Femur | Bone | Lung cancer | + | 3 | 11.72 | PE, distal |
| 8 | 73 | Female | 16.4 | Femur | Bone | UPS | + | 4 | 2.43 | Distal |
| 9 | 64 | Female | 18.0 | Tibia | Bone | Uterine cancer | - | 1 | 4.65 | Distal |
| 10 | 91 | Male | 20.4 | Forearm | Superficial | Myxofibrosarcoma | - | 1 | 2.32 | Distal |
| 11 | 78 | Female | 20.2 | Pelvis | Bone | UPS | - | 1 | 4.23 | Proximal |
| 12 | 67 | Female | 17.9 | Buttocks | Deep | UPS | - | 1 | 2.71 | Distal |
| 13 | 76 | Female | 25.0 | Lower leg | Deep | Leiomyosarcoma | - | 1 | 1.21 | Distal |
| 14 | 19 | Male | 22.2 | Tibia | Bone | Osteosarcoma | - | 1 | 1.53 | Distal |
| 15 | 91 | Female | 20.4 | Elbow | Deep | Myxofibrosarcoma | - | 1 | 2.49 | Distal |
| 16 | 60 | Female | 23.2 | Femur | Bone | Breast cancer | + | 4 | 9.28 | Distal |
| 17 | 75 | Male | 24.5 | Pelvis | Bone | Malignant lymphoma | - | 1 | 1.76 | Distal |
| 18 | 85 | Male | 15.4 | Sacrum | Bone | Lung cancer | - | 1 | 2.85 | Distal |
| 19 | 79 | Male | 24.8 | Lower leg | Deep | Myxofibrosarcoma | - | 1 | 1.91 | Proximal |
BMI Body mass index, PS Performance status, VTE Venous thromboembolism, UPS Undifferentiated pleomorphic sarcoma
The risk factors for VTE were analyzed using univariate analysis; we found that age ≥ 60 years (P = 0.011), tumor location in the lower limbs (P = 0.038), and D-dimer levels (P < 0.001) were associated with the prevalence of VTE (Table 1). In contrast, sex, body mass index, size, origin, performance status, pathological fracture, and hematologic status, including hemoglobin, white blood cell, platelet, and C-reactive protein levels, were not identified as significant risk factors. Multivariate analysis indicated that age ≥ 60 years (P = 0.021), and tumor location in the lower limbs (P = 0.048) were independent risk factors for VTE. The D-dimer level was not significant in the multivariate analysis (P = 0.51).
Discussion
We investigated the prevalence of preoperative VTE in malignant musculoskeletal tumors patients. The most important finding in this study was that more affected patients could potentially have VTE than that reported by previous studies. In this study, VTE was detected in 19 of the 199 included patients (9.5%); the prevalence was therefore higher than the rate of perioperative VTE reported in previous studies [13–18]. Several conditions in our study that differed from those in previous studies may have influenced the results. First, routine screening for VTE was performed. We performed DVT assessment in combination with routine D-dimer measurement, lower extremity ultrasonography, and contrast-enhanced CT scans. This screening strategy allowed for the diagnosis of patients with asymptomatic VTE. Symptomatic VTE in this study was 3/199 patients (1.5%). The necessity of anticoagulation therapy for these patients is controversial; however, Galanaud et al. reported that patients with cancer-related isolated distal DVT had a prognosis similar to that of patients with cancer-related isolated proximal DVT and a dramatically poorer prognosis than those with isolated distal DVT without cancer [24]. In addition, Gary et al. reported Asymptomatic deep vein thrombosis and superficial vein thrombosis in ambulatory cancer patients are associated with poor survival despite anticoagulation therapy [25]. In osteoarthritis, previous report suggested that performing MDCT before and after total knee arthroplasty may be useful to clarify the incidence of VTE and to develop appropriate strategies for treatment and prevention [26]. Therefore, for the management of preoperative malignant musculoskeletal tumors patients, it is crucial to recognize that more patients have asymptomatic VTE than previously thought. Second, our study included patients with metastases and hematologic malignancies. The rate of DVT in surgical oncology patients receiving no prophylaxis was reported to be 35.2% [27]. The cancer type affects the prevalence of VTE in adolescent and young adult patients; therefore, oncology patients, except for those with sarcomas, may have a different total prevalence of VTE.
Several risk factors associated with an increased susceptibility of preoperative VTE in malignant musculoskeletal tumors patients have been identified. Our study showed that increasing age and tumor location are independent risk factors. Age has been previously suggested to be associated with an increased risk of VTE [13, 20]. Kim et al. reported an odds ratio of 5.84 in patients older than 60 years [13]. Our results showed a similar trend, and only two patients with VTE were younger than 60 years of age. Patients with bone or soft tissue sarcomas located in the hip or thigh have been suggested to have an increased risk of VTE [17]. Yamaguchi et al. demonstrated a high prevalence of VTE (22%) in patients after resection of musculoskeletal tumors of the lower limb [20]. Since surgery involving the pelvis has been associated with the development of proximal DVT [22], patients with tumors in the lower limbs are suggested to be at an increased risk of VTE occurrence. In this study, 17 of 19 VTE patients had tumors in the lower limbs (89.5%), and the two VTE patients with tumors in the upper extremities were both older than 90 years of age.
Whilst the level of D-dimer showed an association with preoperative VTE in the univariate analysis, this was not confirmed in the multivariate analysis. This was likely due to the influence of increased age on D-dimer levels, as they need to be corrected by age, and the value of this fibrin degradation product itself should be evaluated carefully [5, 7]. In addition, the D-dimer cutoff value in cancer patients was reported to be higher than that commonly used in clinical practice [28]. The cutoff value of D-dimer for the diagnosis of VTE was found to be 1.53 µg/mL in the present study, which was almost the same as that used for other cancer types [29, 30]. The appropriate consideration of D-dimer limits the overuse and added cost of ultrasonography without a negative impact [31]. If D-dimer exceeds a level of 1.53 µg/mL, additional assessment of VTE by lower extremity ultrasonography and contrast-enhanced CT should be performed. In particular, patients older than 60 years and with tumor location in the lower limbs are a high-risk group and should be considered for imaging evaluation such as ultrasonography regardless of D-dimer levels.
Our study has several limitations. First, it was retrospective in nature and had a relatively small sample size. Some predictive variables may not have had sufficient statistical power; therefore, some important variables may have been ignored. Second, patients with metastases and hematologic malignancies were included. Regarding the evaluation of VTE risk in bone and soft tissue sarcoma patients, these malignancies should be evaluated in separate categories with sufficient sample sizes. Finally, lower extremity ultrasonography and contrast-enhanced CT for VTE evaluation were not unified.
Conclusions
Our study indicated that age and tumor location in the lower limbs were independent risk factors for preoperative VTE in malignant musculoskeletal tumors patients. D-dimer levels were not identified as independent risk factors in the multivariate analysis because it is affected by a wide variety of conditions, including malignancy and aging. D-dimer is useful for exclusion diagnosis because of its high sensitivity, but patients older than 60 years and with tumor location in the lower limbs are a high-risk group and should be considered for imaging evaluation such as ultrasonography regardless of D-dimer levels.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- CT
Computed tomography
- DOAC
Direct oral anticoagulant
- DVT
Deep vein thrombosis
- PE
Pulmonary embolism
- ROC
Receiver operating characteristic
- VTE
Venous thromboembolism
Authors’ contributions
K.H. was a major contributor in writing the manuscript. Y.K. have made design of the work. K.S. analyzed the patient data. S.F. interpreted the patient data and prepared Table 1 and 2. I.K. was a major contributor in pathological assessment. H.C. and M.T. have made substantial contributions to the conception. Y.I. have approved the submitted version. All authors read and approved the final manuscript.
Funding
The authors declare that they have no funding.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This research has been approved by the IRB of the Yokohama City University Hospital. The approval number is B200600056. All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from all participants or, if participants are under 16, from a parent and/or legal guardian.
Consent for publication
Consent for publication was obtained from all participants or, if participants are under 16, from a parent and/or legal guardian.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stein PD, Beemath A, Meyers FA, Skaf E, Sanchez J, Olson RE. Incidence of venous thromboembolism in patients hospitalized with cancer. Am J Med. 2006;119(1):60–68. doi: 10.1016/j.amjmed.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 2.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160(6):809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 3.Blom JW, Doggen CJM, Osanto S, Rosendaal FR. Malignancies, Prothrombotic Mutations, and the Risk of Venous Thrombosis. JAMA. 2005;293(6):715–722. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 4.Donnellan E, Khorana AA. Cancer and venous thromboembolic disease: a review. Oncologist. 2017;22(2):199–207. doi: 10.1634/theoncologist.2016-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Righini M, Perrier A, Moerloose PD, Bounameaux H. D-Dimer for venous thromboembolism diagnosis: 20 years later. J Thromb Haemost. 2008;6(7):1059–1071. doi: 10.1111/j.1538-7836.2008.02981.x. [DOI] [PubMed] [Google Scholar]
- 6.Nisio MD, Squizzato A, Rutjes AWS, Büller HR, Zwinderman PMM Bossuyt. Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: a systematic review. J Thromb Haemost. 2007;5(2):296–304. doi: 10.1111/j.1538-7836.2007.02328.x. [DOI] [PubMed] [Google Scholar]
- 7.Nybo M, Hvas AM. Age-adjusted D-dimer cut-off in the diagnostic strategy for deep vein thrombosis: a systematic review. Scand J Clin Lab Invest. 2017;77(8):568–573. doi: 10.1080/00365513.2017.1390783. [DOI] [PubMed] [Google Scholar]
- 8.Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e419S–e496S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernardi E, Camporese G, Büller HR, Siragusa S, Imberti D, Berchio A, et al. Serial 2-point ultrasonography plus D-dimer vs whole-leg color-coded Doppler ultrasonography for diagnosing suspected symptomatic deep vein thrombosis: a randomized controlled trial. JAMA. 2008;300(14):1653–1659. doi: 10.1001/jama.300.14.1653. [DOI] [PubMed] [Google Scholar]
- 10.Duwe KM, Shiau M, Budorick NE, Austin JH, Berkmen YM. Evaluation of the lower extremity veins in patients with suspected pulmonary embolism: a retrospective comparison of helical CT venography and sonography. 2000 ARRS Executive Council Award I. American Roentgen Ray Society. AJR Am J Roentgenol. 2000;175(6):1525–31. doi: 10.2214/ajr.175.6.1751525. [DOI] [PubMed] [Google Scholar]
- 11.Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, et al. Derivation of a simple clinical model to categorize patients’ probability of pulmonary embolism: increasing the model’s utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416–420. doi: 10.1055/s-0037-1613830. [DOI] [PubMed] [Google Scholar]
- 12.van Belle A, Büller HR, Huisman MV, Huisman PM, Kaasjager K, Kamphuisen PW, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA. 2006;295(2):172–179. doi: 10.1001/jama.295.2.172. [DOI] [PubMed] [Google Scholar]
- 13.Kim SM, Park JM, Shin SH, Seo SW. Risk factors for post-operative venous thromboembolism in patients with a malignancy of the lower limb. Bone Joint J. 2013;95-B(4):558–62. doi: 10.1302/0301-620X.95B4.30416. [DOI] [PubMed] [Google Scholar]
- 14.Morii T, Mochizuki K, Tajima T, Aoyagi T, Satomi K. Venous thromboembolism in the management of patients with musculoskeletal tumor. J Orthop Sci. 2010;15(6):810–815. doi: 10.1007/s00776-010-1539-0. [DOI] [PubMed] [Google Scholar]
- 15.Damron TA, Wardak Z, Glodny B, Grant W. Risk of venous thromboembolism in bone and soft-tissue sarcoma patients undergoing surgical intervention: a report from prior to the initiation of SCIP measures. J Surg Oncol. 2011;103(7):643–647. doi: 10.1002/jso.21884. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser CL, Freehan MK, Driscoll DA, Schwab JH, Bernstein KDA, Lozano-Calderon SA. Predictors of venous thromboembolism in patients with primary sarcoma of bone. Surg Oncol. 2017;26(4):506–510. doi: 10.1016/j.suronc.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell SY, Lingard EA, Kesteven P, McCaskie AW, Gerrand CH. Venous thromboembolism in patients with primary bone or soft-tissue sarcomas. J Bone Joint Surg Am. 2007;89(11):2433–2439. doi: 10.2106/00004623-200711000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Singh VA, Yong LM, Vijayananthan A. Is DVT prophylaxis necessary after oncology lower limb surgery? A pilot study. Springerplus. 2016;5(1):943. doi: 10.1186/s40064-016-2441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuy B, Bhate C, Beebe K, Patterson F, Benevenia J. IVC filters may prevent fatal pulmonary embolism in musculoskeletal tumor surgery. Clin Orthop Relat Res. 2009;467(1):239–245. doi: 10.1007/s11999-008-0607-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaguchi T, Matsumine A, Niimi R, Nakamura T, Asanuma K, Hasegawa M, et al. Deep-vein thrombosis after resection of musculoskeletal tumours of the lower limb. Bone Joint J. 2013;95-B(9):1280–4. doi: 10.1302/0301-620X.95B9.30905. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Guo Q, Hu W. Incidence, risk factors, and outcomes of venous thromboembolism after oncologic surgery: a systematic review and meta-analysis. Thromb Res. 2019;173:48–56. doi: 10.1016/j.thromres.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Nathan SS, Simmons KA, Lin PP, Hann LE, Morris CD, Athanasian EA, et al. Proximal deep vein thrombosis after hip replacement for oncologic indications. J Bone Joint Surg Am. 2006;88(5):1066–1070. doi: 10.2106/JBJS.D.02926. [DOI] [PubMed] [Google Scholar]
- 23.Ogura K, Yasunaga H, Horiguchi H, Ohe K, Kawano H. Incidence and risk factors for pulmonary embolism after primary musculoskeletal tumor surgery. Clin Orthop Relat Res. 2013;471(10):3310–3316. doi: 10.1007/s11999-013-3073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galanaud JP, Sevestre MA, Pernod G, Genty C, Richelet S, Kahn SR, et al. Long-term outcomes of cancer-related isolated distal deep vein thrombosis: the OPTIMEV study. J Thromb Haemost. 2017;15(5):907–916. doi: 10.1111/jth.13664. [DOI] [PubMed] [Google Scholar]
- 25.Gary T, Belaj K, Steidl K, Pichler M, Eisner F, Stöger H, et al. Asymptomatic deep vein thrombosis and superficial vein thrombosis in ambulatory cancer patients: impact on short-term survival. Br J Cancer. 2012;107(8):1244–1248. doi: 10.1038/bjc.2012.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe H, Sekiya H, Kariya Y, Hoshino Y, Sugimoto H, Hayasaka S. The incidence of venous thromboembolism before and after total knee arthroplasty using 16-row multidetector computed tomography. J Arthroplasty. 2011;26(8):1488–1493. doi: 10.1016/j.arth.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Leonardi MJ, McGory ML, Ko CY. A systematic review of deep venous thrombosis prophylaxis in cancer patients: implications for improving quality. Ann Surg Oncol. 2007;14(2):929–936. doi: 10.1245/s10434-006-9183-9. [DOI] [PubMed] [Google Scholar]
- 28.Chen C, Li G, Liu YD, Gu YJ. A new D-dimer cutoff value to improve the exclusion of deep vein thrombosis in cancer patients. Asian Pac J Cancer Prev. 2014;15(4):1655–1658. doi: 10.7314/APJCP.2014.15.4.1655. [DOI] [PubMed] [Google Scholar]
- 29.Kawaguchi R, Furukawa N, Kobayashi H. Cut-off value of D-dimer for prediction of deep venous thrombosis before treatment in ovarian cancer. J Gynecol Oncol. 2012;23(2):98–102. doi: 10.3802/jgo.2012.23.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osaki T, Saito H, Fukumoto Y, Kono Y, Murakami Y, Shishido Y, et al. Risk and incidence of perioperative deep vein thrombosis in patients undergoing gastric cancer surgery. Surg Today. 2018;48(5):525–533. doi: 10.1007/s00595-017-1617-4. [DOI] [PubMed] [Google Scholar]
- 31.Mousa AY, Broce M, Gill G, Kali M, Yacoub M, AbuRahma AF. Appropriate use of D-dimer testing can minimize over-utilization of venous duplex ultrasound in a contemporary high-volume hospital. Ann Vasc Surg. 2015;29(2):311–317. doi: 10.1016/j.avsg.2014.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.