Abstract
Granulomatous lobular mastitis (GLM) is a rare and chronic benign inflammatory disease of the breast. Difficulties exist in the management of GLM for many front-line surgeons and medical specialists who care for patients with inflammatory disorders of the breast. This consensus is summarized to establish evidence-based recommendations for the management of GLM. Literature was reviewed using PubMed from January 1, 1971 to July 31, 2020. Sixty-six international experienced multidisciplinary experts from 11 countries or regions were invited to review the evidence. Levels of evidence were determined using the American College of Physicians grading system, and recommendations were discussed until consensus. Experts discussed and concluded 30 recommendations on historical definitions, etiology and predisposing factors, diagnosis criteria, treatment, clinical stages, relapse and recurrence of GLM. GLM was recommended as a widely accepted definition. In addition, this consensus introduced a new clinical stages and management algorithm for GLM to provide individual treatment strategies. In conclusion, diagnosis of GLM depends on a combination of history, clinical manifestations, imaging examinations, laboratory examinations and pathology. The approach to treatment of GLM should be applied according to the different clinical stage of GLM. This evidence-based consensus would be valuable to assist front-line surgeons and medical specialists in the optimal management of GLM.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40779-022-00380-5.
Keywords: Granulomatous mastitis, Granulomatous lobular mastitis, Idiopathic granulomatous mastitis, Diagnosis, Treatment
Introduction
Granulomatous lobular mastitis (GLM) is a rare, chronic benign inflammatory disease of the breast. Pathologically, GLM typically manifests as non-caseating granulomatous lesions with leukomonocytes, lymphocytes, neutrophils and multinucleated giant cells, located in the center of breast lobules. With a rapidly increasing morbidity in the last two decades, GLM tends to occur in child-bearing women with a prolonged and recurrent course. Although clinical findings and histopathological features are necessary in the diagnosis of GLM, currently there are no international unified guidelines for GLM diagnosis and treatment. Difficulties may exist in the management of GLM for a significant number of front-line surgeons and medical specialists who care for patients with inflammatory disorders of the breast. To promote the standardization process of the diagnosis and treatment of GLM, 66 international experienced multidisciplinary experts from 11 countries or regions proposed the guideline about diagnostic strategy and management algorithm of GLM based on published literature. This guideline statement aims to standardize diagnostic approach, differential diagnoses and clinical management strategies which can be applied to all medical institutions managing GLM patients.
Methods
Literature evidence
Search parameters for the literature were set from January 1, 1971 to July 31, 2020. This time frame was extended back to allow for inclusion of the first published literature of GLM. For each topic, the primary coauthor conducted a search in PubMed using the Medical Subject Headings and Boolean operators for “granulomatous lobular mastitis”, “granulomatous mastitis”, “idiopathic granulomatous mastitis” search terms. References included language in English and several in Chinese. The literature search retrieved a total of 509 articles from PubMed.
Grading of practice recommendations
The 2010 American College of Physicians (ACP) grading system, which employs a validated scale to critically interpret and evaluate the strength and quality of the evidence and provide guidance on how to best apply the recommendations to individual patients, was utilized in manuscript preparation [1]. The ACP system applies the terms “strong” when benefits clearly outweigh risks and/or the recommendation should be applied to all or most patients without reservation, ‘‘weak’’ when benefits are finely balanced with risks or appreciable uncertainty exists. The quality of the evidence was graded “high” for well-done randomized controlled trials or overwhelming evidence, “moderate” for randomized controlled trials with important limitations, well-designed cohort or case–control studies, or large observational studies, and “low” for potentially biased, small observational, or case studies. When the evidence is insufficient to determine for or against routinely provided service, the authors grade the recommendation as “insufficient evidence to determine net benefits or risks”.
Historical perspective and definitions
Recommendation 1: The consensus experts group recommends GLM as a widely accepted definition. (Strong recommendation, high quality of evidence)
Comments: In 1971, Miller et al. [2] proposed the concept of granulomatous mastitis (GM) which presents as breast lobules infiltrated with acute and chronic inflammatory exudate with a mass of foreign body giant cell. In 1972, Kessler and Wolloch [3] described the characteristics of GM: child-bearing women, 1.5–5 years since the last delivery, multiple granulomas and abscess formation. In 1987, Going et al. [4] emphasized the histologic characteristics (the lesions located in the center of breast lobule) and recommended replacing GM with GLM. In 1994, Donn et al. [5] began to apply the definition of idiopathic granulomatous mastitis (IGM) to emphasize the unclear etiology. In 2010, Boarki and Labib [6] proposed idiopathic granulomatous lobular mastitis (IGLM) based on the etiological and histologic diagnosis. In 2011, Renshaw et al. [7] described a special histologic category, vacuole-like cavity formed by neutrophils, and Gram-positive bacilli could be detected in the cavity concomitant with diffuse granuloma, which was defined as cystic neutrophilic granulomatous mastitis (CNGM). Some researchers considered CNGM as a subtype of GLM [8, 9], and others classified CNGM as GM [10–12].
As understanding of this disease becomes more comprehensive, related terms and definitions continue to evolve, bringing confusion to clinicians. GLM is a histopathological diagnosis while IGM is an etiologic diagnosis. GLM has been found to be closely related with Corynebacterium infection [10, 13]. However, whether the detection of Corynebacterium is positive or negative, the same pathological characteristics are shown in specimens with GLM. Therefore, GLM avoids the problem about undetermined etiological relationship between Corynebacterium infection and disease development.
Even though GLM and GM are both pathological terms describing inflammatory changes in breast, GM may be further divided into primary (idiopathic) and secondary (infectious and non-infectious): (1) Primary (idiopathic) granulomatous mastitis: An underlying cause cannot be detected by routine pathogenic examination. Idiopathic granulomatous mastitis is a diagnosis that has excluded other forms of granulomatous mastitis with definite etiologies. (2) Secondary granulomatous mastitis: 1) infectious granulomatous mastitis: pathogenic microorganism can be detected by etiological examination, such as tuberculosis, actinomycetes, parasites, fungi and histoplasma, etc.; 2) non-infectious granulomatous mastitis: etiology is clear while no pathogen can be detected. Owing to the breast involvement by autoimmune disease or immune rejection response to exogenous material, non-infectious granulomatous mastitis tends to be a benign breast disease concomitant with granulomatous changes. This includes Wegener's granulomatous mastitis, giant cell arteritis mastitis, sclerosing lymphogranulomatous inflammation of the breast, foreign body granulomatosis, sarcoidosis and fat necrosis, etc..
Etiology and predisposing factors of GLM
Predisposing factors of GLM
Recommendation 2: Lactation disorders resulting in milk stasis, hyperprolactinemia, and blunt trauma of breast are the predisposing factors of GLM. (Strong recommendation, moderate quality of evidence)
Recommendation 3: The prevalence of GLM is associated with race and region. (Strong recommendation, moderate quality of evidence)
Comments: Possible predisposing factors of GLM include lactation disorders that result in milk stasis, hyperprolactinemia, and blunt trauma of breast [14–17]. Milk stasis plays a key role with breast tissue developing into a hypertrophic state subsequent to pregnancy, lactation, and hyperprolactinemia. Pituitary adenoma, antipsychotic drugs (such as potent D2 receptor antagonists, risperidone), and antidepressant drugs (such as selective serotonin reuptake inhibitors, fluoxetine) can lead to hyperprolactinemia [18, 19]. While the permeability of breast ducts increases, the immunogenic substance (retained milk) enters into lobular mesenchyme of the breast, causing T cell-mediated immune response and granuloma formation [20]. It is not clear in the literature how oral contraceptives are predisposing factors for GLM [21]. The nature of extramammary manifestations of GLM, including inflammatory arthritis, arthralgias, episcleritis, and erythema nodosum, is suggestive of an underlying immune process [22]. A favorable response to corticosteroids is supportive of this pathogenesis [22]. However, serological tests that are usually positive in patients with autoimmune diseases, such as rheumatoid factor and antinuclear antibodies have not shown consistent results with diagnostic or prognostic value [23].
Differences in race and region exist in the prevalence of GLM. GLM may be associated with dietary habits and genetic factors [14, 21]. As GLM usually occurs in the Mediterranean region and developing Asian countries. This prevalence might be the reflection of under-diagnosis of tuberculosis mastitis. Sometimes routine histology studies are not sufficient to rule out the diagnosis of tuberculosis mastitis. Presence of atypical mycobacteria may also be involved in the pathogenesis of GLM which are difficult to isolate under routine culture conditions. This may also be related to poor habits of lactation and weaning in Mediterranean region that may lead to milk stasis (galactostasis) which is the most important predisposing factor for GLM. [(1) Some GLM patients depend on lactation from one breast only and neglect lactation from the other one which develop GLM due to milk stasis. (2) Many patients neglect the routine breast massage and complete evacuation of both breasts after each time of lactation. (3) Many patients also neglect the routine breast massage and complete evacuation of both breasts during the first weeks of weaning.]
Pathogenesis of GLM
The most widely adopted theory considers GLM to be an immune reaction that involves both humoral and cell-mediated immunity stimulated by patients’ secretions such as retained milk [22, 24]. Deng et al. [25] retrospectively investigated steroids administration after the vacuum-assisted biopsy (VAB) of GLM, and immunohistochemical (IHC) staining for immune-related antigens (CD3, CD4, CD8, CD79a, IgG, and IgM) was performed. The results revealed that CD3, CD4 and CD8 lymphocytes were present diffusely in the lesion, indicating that cell-mediated immunity is involved in the development of GLM, and CD79a lymphocyte positivity indicates that humoral immunity is involved [25]. Kessler et al. [3] first reported the self-limited course of GLM, Cohen [26] also considered GLM as focal lesions of autoimmune disease. Available evidence on the effectiveness of glucocorticoids and immunosuppressive therapy for treatment of GLM supports this hypothesis [27].
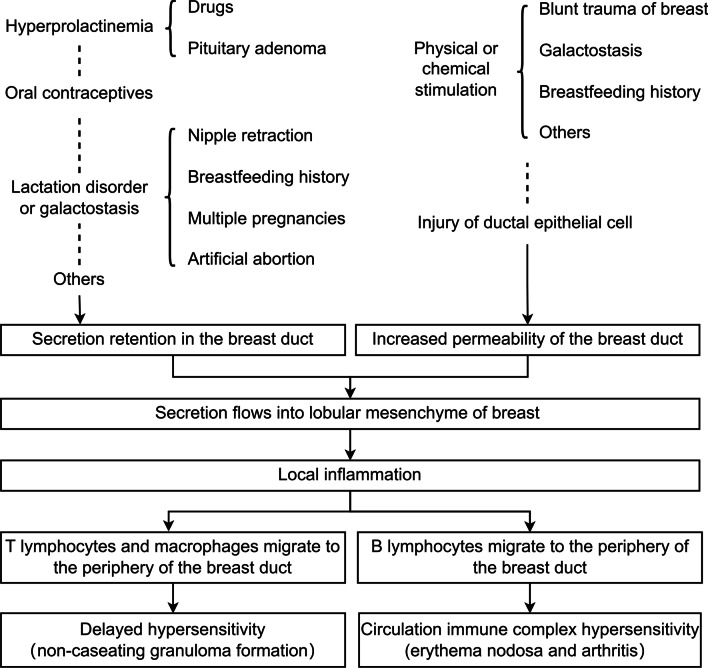
The pathogenesis of GLM may be due to increased permeability of breast duct caused by physical or chemical stimulation such as the infiltration of the lobular mesenchyme of breast with intraluminal secretions such as retained milk. This causes local inflammation in the mesenchyme which then induces the infiltration of immunocompetent cells to form delayed-type hypersensitivity. Finally, localized granulomas are formed (Fig. 1) [3, 28, 29].
Fig. 1.
Pathogenesis of granulomatous lobular mastitis (GLM)
Relationship between GLM and Corynebacterium infection
The relationship between GLM and Corynebacterium infection is not yet definitive. In 2003, Taylor et al. [30] first detected Corynebacterium in lesions of GM, of which Corynebacterium kroppenstedtii accounted for 14.1%. However, recent literature indicates a potential causal relation between CNGM and Corynebacterium. CNGM is a recently identified as an entity that raised the reconsideration of an underlying bacterial etiology for GLM [10, 12, 23, 31]. Typical biopsies of CNGM reveal a granulomatous inflammation with characteristic cystic spaces lined by neutrophils containing Gram-positive cocci [8]. Corynebacteria, especially Corynebacterium kroppenstedtii, and to a lesser extent Staphylococcal spp. were detected in some patients with CNGM by culture, or by 16S rRNA gene sequencing of specimens obtained at surgery or FNAC [8, 10]. Kivilcim et al. [32] evaluated multiple bacteriologic agents that might play a role in the etiology of GLM using a nucleic-acid-based assay with a universal primer on previously obtained GLM biopsies without evidence of CNGM [8]. They obtained no positive results [8]. The minority of cases diagnosed in the past as GLM with evidence of Corynebacterium may turn to be CNGM. In brief, when the characteristic histological findings of CNGM are encountered, the pathologist should make every effort to look for an associated primary bacterial infection. Culture of fresh specimens, Gram staining, or rRNA gene sequencing of specimens obtained at surgery might be considered in these cases [10, 31]. CNGM is now considered a secondary form of GM [10, 12, 23]. Early recognition of CNGM might call for a first line of treatment by lipophilic antimicrobials [12].
The normal flora in the breast is consistent with the skin commensal flora, mainly Propionibacteria, coagulase-negative Staphylococci and Corynebacteria, which can enter tissue through mammary ducts. Nipple retraction may be a predisposing factor leading to bacterial retrograde infection. Patients with Corynebacterium infection are more likely to present with fever and sinus formation [8]. If Corynebacteria are the causative factor in the development of GLM, the latter should be classified as infectious granulomatous mastitis. However, as a part of normal skin flora, the presence of Corynebacterium may be a colonization. Therefore, there are three hypotheses to explain the pathogenetic of GLM in relation with Corynebacterium. (1) Primary factor: As an independent immunogenic factor, Corynebacterium enters lobular mesenchyme of breast through increased permeability of breast ducts, inducing an autoimmune response. (2) Cofactor: Corynebacterium and immunogenic factors such as retained milk enter the lobular mesenchyme through increased permeability of breast ducts, and induce an autoimmune response. (3) Irrelevant factor: Corynebacteria does not participate in the process of autoimmune response. Other immunogenic factors acting as antigens enter lobular mesenchyme through increased permeability of breast ducts. Decreased local immune resistance and secondary Corynebacterium infection then exacerbate clinical symptoms.
Odds in the etiology
Despite a universal agreement on the relation of GLM to parity and lactation, possibly reflecting the impact of prolonged estrogen exposure, still it is rarely reported in nulliparous women, young girls, and males, expanding the dilemma about the etiology [22, 23, 33]. It is worth mentioning, however, that in a good percentage of cases reported in the male population, there was suggestion of increased endogenous estrogen to androgen ratio [33].
Diagnosis criteria
Recommendation 4: The diagnosis of GLM requires cooperation through the establishment of an effective multidisciplinary comprehensive treatment group, including clinicians, radiologists and pathologists. (Strong recommendation, moderate quality of evidence)
Recommendation 5: The diagnosis of GLM should combine history characteristics, clinical manifestations, physical examination, imaging examination and laboratory examination. (Strong recommendation, moderate quality of evidence)
Comments: There is evidence that the establishment of a multidisciplinary team for the management of GLM reduces rate of erroneous initial clinical impression of breast cancer [23]. Such a multidisciplinary approach achieved a higher rate of earlier preoperative diagnosis of GLM expanding the role of corticosteroid treatment in selected patients. False positive radiological diagnosis of breast carcinoma was also reduced [23].
Clinical history characteristics
Recommendation 6: GLM usually occurs in child-bearing women, seldom in pregnant and lactating women and very rarely in men. (Strong recommendation, high quality of evidence)
Recommendation 7: GLM in patients who have not been pregnant or delivered is associated with increased serum prolactin, or up-regulated prolactin receptors or hypersensitivity to the normally circulating prolactin. (Strong recommendation, high quality of evidence)
Comments: Clinical history information include age, body mass index (BMI), course of disease, history of child-bearing (number of pregnancies and deliveries, date of last delivery), history of lactation (episodes of acute mastitis during lactation, date of last breastfeeding, problems during breastfeeding, lactation from one breast only and negligence of lactation from the other one, negligence the routine breast massage and complete evacuation of both breasts after each time of lactation and during the first weeks of weaning), history of blunt trauma to the breast, history of taking oral contraceptives, history of taking psychiatric drugs, historical symptoms or history of autoimmune disease.
A descriptive study had found that median age for patients with GLM is 36 years (19–49 years) [34]. Azizi et al. [35] reported that patients with GLM usually had history of pregnancy (90.7%) and history of breastfeeding (82.7%). In the prospective study of Farouk et al. [21] that included 30 patients diagnosed with GLM, all patients were of reproductive age, and all had a history of breast feeding (100%). Male GLM had been documented in 13 cases, with a suggestion of higher endogenous estrogen to androgen ratio in a few of them [33]. Some case reports described GLM associated with hyperprolactinemia, which might result from specific drugs or pituitary adenoma, including antipsychotic drugs such as risperidone and antidepressant drugs such as fluoxetine [18, 36].
Clinical manifestations
Recommendation 8: In the early stage, breast mass can arise with or without pain, and without obvious local skin changes. The lesions usually spread to the areola from the periphery of the breast. (Strong recommendation, high quality of evidence)
Recommendation 9: Breast masses can enlarge rapidly with inflammatory manifestations including hyperaemia, swelling, warmth, and pain. Systemic inflammatory findings like fever usually does not occur. (Strong recommendation, high quality of evidence)
Recommendation 10: In a small number of patients with GLM, focal mastitis or tenderness may arise before the development of a mass by 2 to 3 months. (Strong recommendation, moderate quality of evidence)
Recommendation 11: During the progression of GLM, deep or subcutaneous abscesses can form. (Strong recommendation, high quality of evidence)
Recommendation 12: In the advanced stage, the main clinical manifestation is a fistula or sinus formation. The skin can be ulcerated and discharge purulent with prolonged healing time. (Strong recommendation, high quality of evidence)
Recommendation 13: Systemic involvement such as erythema nodosum and oligo- or poly-arthritis can appear at any stage and can be in favor of the diagnosis of GLM.
Comments: Clinical manifestations of GLM are diverse. A descriptive study reported 3060 patients with GLM, of which breast mass was the most frequent symptom (80%) with a mean size of 5 cm (3 – 9 cm), and 66% of them were painful [34]. Azizi et al. [35] described clinical symptoms of 474 GLM patients, 39.2% had skin lesions, 17.7% had concomitant nipple retraction, 15.6% had nipple discharge, and 4.6% had joint pain. Co et al. [37] reported 55.9% (57) of 102 GLM patients presenting with a painful mass, 28.4% presented with a painless mass and 15.7% had abscess formation. The median size of the inflammatory mass or abscess was 37 (6–92) mm.
The first ten reported cases of GLM by Kessler and Wolloch [3], and Cohen [26] shared the classical presentation of a hard cancer-mimicking breast mass occurring in young parous women. However, the spectrum of possible local presentations GLM has expanded to include acute breast-abscess-like presentations, and the subacute presentations with skin fungation, fistulization, and/or sinus formation [21]. Extramammary presentations in the form of erythema nodosum, arthritis, oligo-arthritis, and episcleritis are recognized as occasional findings [21, 22, 33, 38].
Imaging examinations
Recommendation 14: Ultrasound (US) is the first line modality for GLM. US helps to detect the inflammatory changes, abscess, and tunneling and sinus formation; US is also helpful to perform biopsy and do follow-up of remission or progression of GLM. (Strong recommendation, high quality of evidence)
Recommendation 15: Magnetic resonance imaging (MRI) is a useful imaging modality for the differential diagnosis with breast cancer and it also could be useful to indicate active lesions, locate the extent of the lesions, evaluate possible residual disease after treatment or monitor the disease in patients who underwent conservative treatment. (Strong recommendation, moderate quality of evidence)
Recommendation 16: Hyperprolactinaemia or suspected TB should be appropriately investigated. (Strong recommendation, moderate quality of evidence)
Comments: Irregular hypoechoic masses, tubular echoes, and multiple abscesses can be observed by US [39]. When the lesion is fibrous or blood flow of the lesion is rich, obvious acoustic shadow can be observed behind the lesion during US examination. In the absence of a mass lesion, US evidence of areas of mixed echo pattern with parenchymal deformity, multiple collection pockets and tracks, focal mastitis with interstitial edema, may indicate the presence of an inflammatory granulomatous process [40]. Alikhassi et al. [41] analyzed imaging features in 36 patients with GLM, 72.2% of them presented irregular, less uniform, hypoechoic masses with ill-defined margin, 50% of them formed tubular dilation and subcutaneous sinus formation, 28% of them showed floating debris, and 25% of them existed ductal ectasia.
In mammography, focal or global asymmetric density, blurred edges, with or without skin thickening and parenchymal distortion can be detected in GLM patients [42]. Aghajanzadeh et al. [43] reported that the major mammographic finding was an irregular mass in 118 (63.5%) of 186 GLM patients, asymmetric density and heterogeneously were found in 8.5% patients, 5% of them presented skin thickening or edema, and 3.5% of them showed an irregular or lobulated mass. Young child-bearing women who tend to develop GLM, have dense breast parenchyma is dense, making the detection of lesion difficult. Thus GLM could be misdiagnosed as breast cancer [44].
The characteristics of GLM detected by MRI commonly present as heterogeneous enhancing masses, segmental non-mass enhancement, or focal non-massive lesions [45]. MRI shows edema, inflammation, tumor-like lesions, as well as abscess fistula formation in the parenchyma [46]. Micro-lesions with fusion, T2 high signal intensity lesions and rim enhancing micro-abscesses are among other MRI presentations [47]. Most lesions in GLM show persistent dynamic curves, but the enhancement curves of different parts of a lesion in a patient may not be the same [48]. Decreased ADC sequence signal is observed in GLM on diffusion-weighted imaging (DWI), which is of little value in distinguishing from inflammatory breast cancer [49]. Zhang et al. [50] revealed that the accuracy of MRI-enhanced imaging in assessing the extent of GLM lesions was 88.9% (24/27), much higher than the accuracy of US alone or combined with mammography. Furthermore, MRI can provide more accurate information regarding the assessment of therapy success especially after the local therapy [51, 52]. MRI is a follow-up tool for aggressive, diffuse, and non-responsive diseases [53].
Laboratory examination
Recommendation 17: Laboratory examinations include routine blood test, erythrocyte sedimentation rate, purified protein derivative (PPD) test and inflammatory markers such as C-reactive protein, serum prolactin, and immunological examination such as antinuclear antibody (ANA) profile and rheumatism factor. (Strong recommendation, high quality of evidence)
Recommendation 18: Testing tissue for the presence or growth of Mycobacterium tuberculosis should be performed for patients with suspected tuberculosis. (Strong recommendation, moderate quality of evidence)
Comments: PPD of tuberculin test can be useful in weighing the differential diagnosis. There is a clinical anergic response to PPD among GLM patients that could be useful in ruling out TB and for diagnosis of latent TB while deciding to prescribe immunosuppressant medication to GLM patients [54]. Inflammatory cells increase in GLM patients. For GLM patients with erythema nodosa and arthritis, C-reactive protein abnormally increases, and ANA and rheumatoid are normal [55]. DNA test of Mycobacterium tuberculosis helps to distinguish tuberculous granulomatous mastitis [56]. Patients who have not been pregnant or delivered often suffer from hyperprolactinemia or up-regulated prolactin receptors or hypersensitivity to the normally circulating prolactin [57].
Histopathology
Recommendation 19: Core needle biopsy (CNB) can be performed as diagnostic histopathological examination (strong recommendation, high quality of evidence)
Comments: GLM is characterized histologically as non-caseating granulomatous lesions with epithelioid histiocytes and multinucleated giant cells, located in the center of the lobules (Additional file 1: Fig. S1). The surrounding tissue is mainly infiltrated by neutrophils, lymphocytes, plasma cells and a small number of eosinophils. The lesions can be multifocal and form micro-abscesses, and vary in size. Inflammation of GLM is usually confined to the breast lobules, seldomly involving the main ducts [58, 59]. The gross pathological appearance of GLM is nonspecific, and depends on the size of the lesion, degree of inflammation, fibrosis, and micro-abscess formation.
Routine biopsy methods include CNB, VAB, and fine needle aspiration (FNA); the former two methods have a high accuracy for diagnosis. Excisional biopsy can be performed if necessary. Frozen section evaluation can occasionally be useful in confirming the diagnosis and judging the extent of surgical resection [23]. A study by Hovanessian Larsen et al. [44] indicated that 21% (4/19) of GLM cases can be diagnosed by FNA, and 96% of GLM patients can be diagnosed by CNB. In addition, it is necessary to pay attention to the site selection of CNB, inserting the needle as close as possible to the margin of the areola. If a subsequent operation is planned, the puncture tunnel can be removed at the same time to reduce scars.
Differential diagnosis
GLM needs to be differentiated from ductal dilatation/periductal inflammation of breast, Zuska disease/subareolar abscess, and breast cancer (Table 1).
Table 1.
Differential diagnosis of GLM
| Etiology | Age | Clinical manifestations | Auxiliary examination | Histopathology | |
|---|---|---|---|---|---|
| GLM | Unknown etiology. Motivations are blunt breast trauma, lactation disorder, galactostasis, hyperprolactinemia, etc. [60] | Women who have delivered with breastfeeding history tend to develop GLM. Women who have not delivered seldom develop GLM | It frequently occurs on the periphery of the breast and concentrically involves the areola area. The subcutaneous abscess can spread to the whole breast, and can form recurrent ulcers or sinuses with a prolonged healing time | Ultrasonography often presents hypoechoic or uneven masses, with or without duct dilation | Non-caseating granulomas centered on the breast lobular, distributed multifocally, varying in size, with or without micro-abscesses |
| Ductal dilatation/periductal inflammation of the breast | Unknown etiology. Ductal dilatation may be associated with nipple deformities, blocked milk ducts, smoking, and bacterial infections [61] | Women in all age groups can develop ductal dilatation, more often in perimenopausal women. Women who have not been delivered can develop ductal dilatation as well | Manifestations include nipple discharge with nipple retraction. The lesion is centered on the areola [62], showing eccentric development. The large ducts behind the areola dilate, and an areola abscess may appear | Obvious duct dilation, fine light spots inside, and flow signs when pressurized can be observed by ultrasound. Dilated, tortuous, blocked and deformed duct can be observed by galactography | Breast duct is highly dilated, the wall of the duct is thickened or ruptured, and the cyst cavity is filled with pink granular thick material. Infiltration of lymphocytes, plasma cells and neutrophils can be seen around the dilated duct [63] |
| Zuska disease/subareolar abscess | Zuska may be associated with the breast duct obstruction, congenital malformation of breast duct, and nipple retraction | Zuska mainly occurs in non-lactating period, more common in women aged 14–66 years old, especially unmarried women | Swelling under the areola, swelling formation or abscess, lactiferous fistula and repeated attacks, prolonged non-healing are important characteristics of this disease | One or more hypoechoic or anechoic areas with blood flow signals can be detected around the areola by ultrasound | Squamous metaplasia of lactiferous tube columnar epithelium at the base of the nipple |
| Breast cancer | Breast cancer may be related with a family history of breast cancer, BRCA1/2 mutation [64], exposure of radiation, first menstruation before 12 years old, first pregnancy after 35 years old, no pregnancy, tobacco and alcohol, and psychological stress. Oncogenic and latent viruses such as HPV, CMV, EBV, MMTV and BLV are recently accused to be etiologic factors in the pathogenesis of breast cancer. Moreover, stress has a role in activation of these viral mechanisms | The incidence rate gradually rises after the age of 20, and more frequently in perimenopausal and post-menopausal women | Breast cancer usually presents a single mass with unclear borders, hard texture and poor mobility, and may be accompanied by enlarged ipsilateral axillary lymph nodes. As a rare subtype of breast cancer, inflammatory breast cancer develops rapidly, and local skin may show inflammation-like manifestations, including redness, edema, thickening, roughness, and increased surface temperature [65] | A hypoechoic mass with unclear borders and blood flow signals can be detected by Doppler ultrasound[66]. Ultrasound manifestations of inflammatory breast cancer include thickened skin and extensive parenchymal echo enhancement of the breast. Mammography shows an increased density of masses with irregularity margin or with Burr sign, small and dense calcification | – |
BLV bovine leukemia virus, CMV cytomegalovirus, EBV Epstein–Barr virus, GLM granulomatous lobular mastitis, HPV human papillomavirus, MMTV mice mammary tumor virus
Treatment
The treatment strategy should be the responsibility of a multidisciplinary team. Individual patient needs should be taken into consideration.
Etiologic treatment
Recommendation 20: GLM patients with hyperprolactinemia should be treated with bromocriptine and the etiology should be cured. For patients with hyperprolactinemia caused by antipsychotic drugs such as risperidone, drug substitution should be evaluated by the physician and psychiatrist. (Strong recommendation, high quality of evidence)
Comments: Hyperprolactinemia or up-regulated prolactin receptors or hypersensitivity to the normally circulating prolactin may be involved in the pathogenesis of GLM [67]. GLM patients with hyperprolactinemia have a higher risk of recurrence [68]. Nikolaev et al. [36] proposed that the usage of low-dose corticosteroids is effective in patients with hyperprolactinemia who have had pituitary adenoma resected. Aghajanzadeh et al. [43] revealed that the combination of glucocorticosteroid and bromocriptine (5–10 mg/day) was effective in 31% (5/16) GLM patients.
Clinical and sonographic follow-up
Recommendation 21: As a self-limiting disease, the symptoms of GLM can be relieved without any treatment. If small breast masses are the only symptoms, without other systemic symptoms, patients can be supervised after identifying the etiology of the mass. The progression of GLM needs to be closely monitored. (Weak recommendation, low quality of evidence)
Comments: 50% of the GLM patients would achieve complete remission at 2–24 months since disease onset, the other 50% have no progression in the disease [69]. Davis et al. [70] surveilled 120 GLM patients from 2006 to 2019, 112 of them achieved complete remission on average 5 months (0–20 months). Hur et al. [71] managed 50 GLM patients on grade according to the severity of symptoms. Eight patients with milder illness were supervised, 5 of them had single/multiple small lesions (1–2 cm). Seven patients achieved remission, and one patient with large mass (5 cm) developed into an abscess.
Medical treatment
Usually, drug acts as the first-line primary treatment or secondary treatment pre- and post-operation.
Antibiotics
Recommendation 22: Antibiotics can be applied based on the results of bacterial testing and drug susceptibility tests. (Strong recommendation, moderate quality of evidence)
Comments: GLM patients with Corynebacterium infection require antibiotics [72]. Dobinson et al. [13] conducted a drug susceptibility analysis for 27 Corynebacteria infected samples from breast, showing that Corynebacterium kluyveri was resistant to β-lactam antibiotics. Non-lipophilic Corynebacterium such as Corynebacterium glucoside, and Corynebacterium frenii are sensitive to multiple antibacterial drugs. For patients suspected of GLM, non-penicillin drugs such as clindamycin [73], levofloxacin and azithromycin can be applied empirically before the outcome of antibiotic susceptibility test [72, 73].
Mixed infective micro-organisms may be involved in the pathogenesis of GLM and some of these are atypical microorganisms, which are difficult to isolate under ordinary culture conditions. Rifampicin inhibits the growth of most Gram-positive and many Gram-negative bacteria, including atypical Mycobacteria that may be involved in the pathogenesis of GLM. Therefore, the use of Rifampicin can be used for GLM patients with Corynebacterium infection in all stages and may be applied for GLM patients without Corynebacterium infection as well [21]. In the prospective study that was included 30 patients diagnosed with GLM, Farouk et al. [21] have successfully proven the efficacy of a Rifampicin therapy regimen of 300 mg twice daily for a period of 6–9 months in the treatment of GLM for all patients at all stages with complete clinical and ultrasonographic response without any recurrent episodes after a median follow-up of 15.5 months (average 3–35 months) without any surgical excision or corticosteroid therapy.
Corticosteroids
Recommendation 23: Administration of corticosteroids for large lesions prior to surgery may help in obtaining better cosmesis. Treatment time needs to be adjusted according to the progression of disease. (Strong recommendation, high quality of evidence)
Recommendation 24: GLM patients with mainly skin changes or who suffered from side effects of oral corticosteroids can be treated with intralesional corticosteroid injection and topical steroid. (Weak recommendation, moderate quality of evidence)
Comments: A growing number of publications over the last two decades have shown the effectiveness of oral corticosteroid treatment in reducing the extent of surgery, or even alleviating the need for surgery in selected cases. On the other hand, the use of corticosteroids might be limited in pregnant, diabetic, or lactating women. The usage of glucocorticosteroid for a prolonged time may lead to weight gain, osteoporosis, and worsening infections [22, 33]. Although there were many studies considering the dosage, time, and methods to apply corticosteroids, there were no studies regarding the end-point to discontinue the treatment up to date. Complete clinical response (CCR) was the criteria to discontinue the treatment in the search of the effectiveness of steroid treatment, in accordance with the studies to date [24]. The usage of glucocorticosteroid should comply with the principle of minimum effective dose. However, Montazer et al. [74] had reported that high dose prednisolone has a high success rate with lower recurrence and could reduce the need for surgery. Çetin et al. [75] found the systemic side effects could not reduce significantly with the decreased dose of systemic steroids in combination with topical treatment.
Intralesional injection and topical corticosteroids can effectively reduce the side effects, especially in patients suffering from concomitant skin lesions (e.g., fistula, skin erosions, ulcers) [46, 75, 76]. For breast masses with single or multiple abscesses or even sinus, Xiao et al. [77] recommended aspiring pus repeatedly, guided by US, wash the abscess cavity using 0.9% NaCl solution, followed by injecting 40 mg triamcinolone acetonide into the abscess cavity through the aspiration needle or drainage tube. The therapeutic efficiency was 78.26% (18/23), and the effective time is (6.00 ± 2.09) d [77]. Tae Ik Eom recommended 6–7 times of triamcinolone acetonide injection (maximial dose 20 mg with interval every 3 weeks). Furthermore, intra-mammary corticosteroids injection can also be administered to perilesional fibroglandular area in patients without abscess formation. The major advantage of this method is that it can be applied in multiple sessions until satisfactory results were obtained [78]. Toktas et al. [79] divided 78 female patients diagnosed with GLM into the local steroid treatment group (intralesional steroid injection with topical steroid administration, group 1) and the peroral systemic steroid treatment group (group 2). The recurrence rates were significantly lower in group 1 (8.7%) compared to group 2 (46.9%, P = 0.001), and the need for surgical treatment was significantly less in group 1 (2.2%) than in group 2 (9.4%, P = 0.001), while the complication rates were similar between groups.
Non-corticosteroid immunosuppressive agents
Recommendation 25: For patients who are resistant to corticosteroids or intolerant of long-term corticosteroids therapy, non-corticosteroid immunosuppressive agents such as methotrexate (MTX) may be considered. (Weak recommendation, low quality of evidence)
Comments: The combination of MTX and corticosteroids has a synergistic effect on the control of disease progression, meanwhile the dosage of corticosteroids can be reduced [27, 80, 81]. The dosage of MTX is 5–15 mg/week for 6–24 months, and patients receiving MTX should be given two doses of folic acid per week before MTX use [82]. For women of reproductive age receiving MTX, contraception should be provided. Side effects should be closely observed during treatment, such as impairment of liver and renal function, bone marrow suppression, interstitial pneumonia, folic acid deficiency, and gastrointestinal reactions [54, 83]. Azidothymidine (AZT) could be an option for pregnant women [84].
Surgery
Recommendation 26: Surgical treatment (wide local excision) is most effective in complex lesions with limited focus, sinus tract, and without abscess, including abscess excision and drainage, segmental dissection, enlarged dissection and mastectomy of breast. (Strong recommendation, high quality of evidence)
Comments: Indications for surgical operations: (1) When patients are not sensitive to the corticosteroids and antibiotic therapy, or cannot tolerate the side effects of corticosteroids. (2) Patients who recur after corticosteroid or surgical treatment. (3) The lesions are extensively distributed in three quadrants of the breast. (4) Complicated lesions with acute and chronic manifestations such as abscesses, sinus, fistula formation, and persistent wound infection (skin ulcers and pus). (5) Patients with long course of disease combined with systemic manifestations such as erythema nodosa and polyarthritis of extremities.
Relative contraindications for surgery: (1) Patients with symptoms of acute infection or are in the advanced stage of disease. (2) Extensive lesions with involving more 2/3 of the breast, wide area of skin lesions, difficulties in guaranteeing a satisfactory recovery after surgery. (3) Pregnant patients.
Surgical approaches: There is no unified surgical methods for GLM, which mainly depend on the surgical extent and lesion location. Wide local excision has stood the test of time as being a corner stone in the treatment of GLM. First, locate lesions preoperatively by US or MRI to identify the surgical extent to fully remove the necrotic tissue and pus. Then, completely wash the wound with 3% hydrogen peroxide, iodine and 0.9% saline. Finally, breast plastic surgery can be performed with the glandular fascia flap and fascia tissue. Immediate breast reconstruction can be conducted if necessary.
Notifications: (1) The selection of operative incision should consider both areola and ulcer. (2) Changing instruments and gloves to avoid re-contamination during the reconstruction surgery. (3) Double-layer purse-string suture at the base of the nipple makes the nipple protrude to avoid postoperative indentation. (4) For patients with extensive local resection, autologous tissue displacement and shaping can be performed.
Summary of evidence: In a recent systematic review and meta-analysis surgical treatment (with or without corticosteroids) was associated with a high cure rate and a relatively low recurrence rate, the cure rates of oral corticosteroids and surgery were 90.6%, 94.5%, respectively, and the recurrence rates were 6.8%, 4.0%, respectively [24]. For patients with diffuse disease, recurrence, or ineffective conservative treatment, wide local excision can be applied.
Clinical management pathways
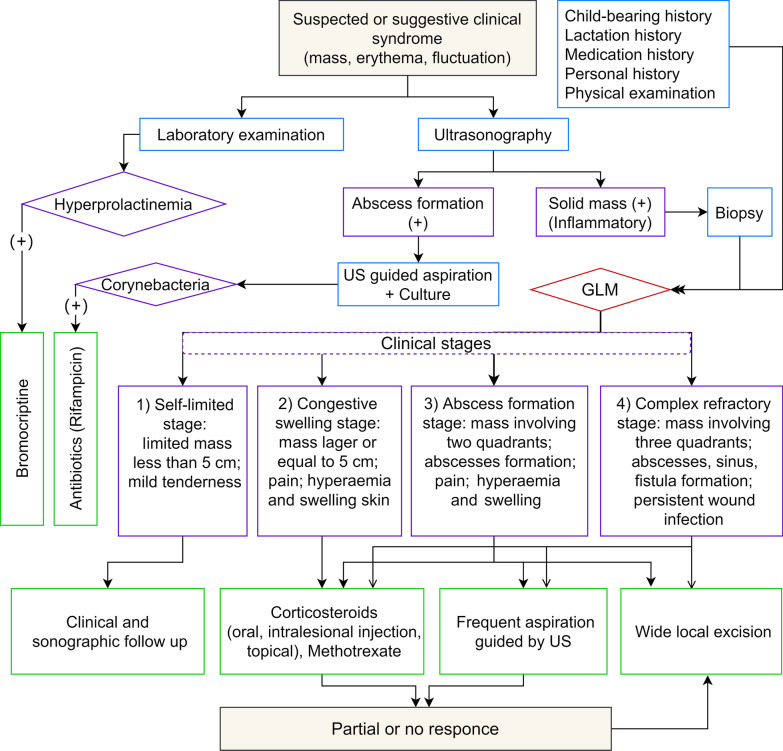
GLM is classified into four stages according to the progression of the GLM and the clinical manifestations (mass size, skin change, abscess, sinus and fistula formation): (1) self-limited stage, (2) congestive swelling stage, (3) abscess formation stage and (4) complex refractory stage. Treatment response was defined as partial response (improvement in all clinically significant symptoms, including pain, swelling, erythema, and induration) or complete response (complete resolution of the aforementioned symptoms) (Fig. 2) [85]. Notably, women do not have these discrete stages, they can be blurred and if they have multifocal disease then the different lesions can be at different stages and some skip stages.
Fig. 2.
Clinical management algorithm of GLM. US ultrasound, GLM granulomatous lobular mastitis
Self-limited stage
Recommendation 27: GLM patients in the self-limited stage can be managed by watchful waiting using clinical and imaging surveillance. (Weak recommendation, low quality of evidence)
Comments: One or more self-limited breast mass(es) usually present in the early stage of the GLM. The classic presentation is a breast mass less than 5 cm with mild pain and tenderness. The overlying skin can be normal and axillary lymphadenopathy are not common. No abscess is detected in physical examination or using an US. During this stage, GLM can be regarded as “white skin mass stage”. Watchful waiting by clinical examination and sonographic evaluation can be a reasonable approach at this stage. As a self-limited stage, symptoms may disappear or remain nonprogress for months or years. Only patients who show signs of disease progression need further treatment (Additional file 1: Fig. S2).
Congestive swelling stage
Recommendation 28: Patients with GLM in the hyperaemia and swelling progressive stage can be treated with oral corticosteroids, intralesional injection and topical corticosteroids, and non-corticosteroid immunosuppressive agents. (Strong recommendation, high quality of evidence)
Comments: Hyperaemia and swelling progressive stage progresses from mass alone stage. The lesions are larger than or equal to 5 cm, with pain and without abscesses. The skin is hyperaemic and swollen, which can be regarded as “red skin mass stage”. Corticosteroids are considered as the front-line treatment option at this stage. Oral glucocorticoid can shrink the mass, reduce the pain and tenderness, and decrease inflammatory changes of the overlying skin. Subsequently, the dosage can be tapered off gradually over a period of 6–8 weeks. However, a small number of patients may experience relapse or recurrence of symptoms following tapering or discounting of the glucocorticoids is reduced or stopped. Then, GLM will rapidly enter into a refractory stage. For patients who cannot tolerate the adverse effects of corticosteroids therapy, wide local excision can be considered. Attention should be paid to the adverse effects of corticosteroids, such as Cushing’s syndrome and hirsutism, hypertension and increased glucose tolerance. Local treatment could be an option for those with mild inflammatory changes who are at higher risk for corticosteroid complications (Additional file 1: Fig. S3).
Abscess formation stage
Recommendation 29: Patients with GLM in the abscess formation stage can be performed with corticosteroids and wide local excision. (Strong recommendation, moderate quality of evidence)
Comments: Abscess formation stage develops from mass alone stage or hyperaemia and swelling progressive stage. The formation of abscesses can be detected by physical examination or US. These lesions are large, often involving more than two quadrants, and are manifested in the acute stage, usually accompanied by axillary lymphadenopathy. Complicated sinuses and fistulas have not yet formed. Corticosteroid (oral, intralesional injection, topical), MTX and aspiration guided by US can be performed wide local excision can be performed if the aforementioned methods are not effective. For patients who refuse surgery, oral or topical corticosteroids can be applied if the single mass is limited, and intracavitary injection of triamcinolone acetonide guided by US is effective (Additional file 1: Fig. S4).
Complex refractory stage
Recommendation 30: As same as patients in the abscess formation stage, corticosteroids and wide local excision can also be applied for patients in the complex refractory stage. (Strong recommendation, moderate quality of evidence)
Comments: In this advanced stage, lesions are extensively distributed to more than three quadrants or with abscesses, sinus, fistula and persistent wound infection (skin ulceration and pus). Patients in this stage need comprehensive treatment based on wide local excision [17]. Corticosteroid (oral, intralesional injection, topical), MTX and aspiration guided by US can be applied, and wide local excision should be performed if the aforementioned methods are not effective (Additional file 1: Fig. S5).
Relapse and recurrence of GLM
Recurrence was considered as occurrence of inflammatory mass clinically or radiologically and was confirmed by Tru-cut biopsy [85]. In literature, recurrence rates of GLM were reported 15.4–24.8% [35, 86–89]. The patient who has a palpable mass at the time of discontinuation of medical therapy are at higher risk of relapse or recurrence than those without such finding. Younger age, corynebacterial infection, and pregnancy were associated with longer treatment durations [86]. Corynebacterial infection was associated with a 2.16–2.64 times higher risk of recurrence [73, 86]. Breast skin lesions were associated with a significantly higher odds of recurrence [35]. The difference of prolactin (PRL) level before and after treatment was an independent risk factor for recurrence and patients presenting with higher PRL after treatment than before treatment had a higher risk of recurrence [87].
Future recommendations
GLM is a rare disease, and requires multi-center studies and meta-analysis for further understanding. We call for a comprehensive disease classification depending on clinical, radiological, and pathological criteria.
Supplementary Information
Additional file 1: Fig. S1 Histological staining of granulomatous lobular mastitis. Fig. S2 Self-limited stage. Fig. S3 Congestive swelling stage. Fig. S4 Abscess formation stage. Fig. S5 Complex refractory stage.
Acknowledgements
Not applicable.
Abbreviations
- GLM
Granulomatous lobular mastitis
- IGM
Idiopathic granulomatous mastitis
- GM
Granulomatous mastitis
- IGLM
Idiopathic granulomatous lobular mastitis
- CNGM
Cystic neutrophilic granulomatous mastitis
- CNB
Core needle biopsy
- VAB
Vacuum-assisted biopsy
- FNA
Fine needle aspiration
- CCR
Complete clinical response
- MTX
Methotrexate
- PRL
Prolactin
Author contributions
GSW and XTZ designed and supported this study. QQY, LYL, QYF, RZ and YTD collected and assembled the references and data. All authors performed the data analysis and manuscript writing. GSW and SXX reviewed and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Improving the Ability of Diagnosis and Treatment of Difficult Diseases (ZLYNXM202009).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Qian-Qian Yuan, Email: Yuanqq11@whu.edu.cn.
Shu-Xuan Xiao, Email: shu-yuan.xiao@uchospitals.edu.
Omar Farouk, Email: dr_omarfarouk@yahoo.com.
Yu-Tang Du, Email: duyutang666@126.com.
Fereshte Sheybani, Email: Fereshtesheybani@gmail.com.
Qing Ting Tan, Email: tan.qing.ting@singhealth.com.sg.
Sami Akbulut, Email: akbulutsami@gmail.com.
Kenan Cetin, Email: drkenancetin@hotmail.com.
Afsaneh Alikhassi, Email: alikhassiafsaneh@gmail.com.
Rami Jalal Yaghan, Email: ramijy@agu.edu.bh.
Irmak Durur-Subasi, Email: irmakdurur@yahoo.com.
Fatih Altintoprak, Email: fatihaltintoprak@yahoo.com.
Tae Ik Eom, Email: redtake@naver.com.
Fatih Alper, Email: drfatihalper@gmail.com.
Mustafa Hasbahceci, Email: hasbahceci@yahoo.com.
David Martínez-Ramos, Email: doctormartinezramos@gmail.com.
Pelin Seher Oztekin, Email: pelinoztekintr@yahoo.com.
Ava Kwong, Email: avakwong@hku.hk.
Cedric W. Pluguez-Turull, Email: cedricpluguez@gmail.com
Kirstyn E. Brownson, Email: Kirstyn.brownson@hci.utah.edu
Shirish Chandanwale, Email: shirishchandanwale@gmail.com.
Mehran Habibi, Email: mhabibi2@jhmi.edu.
Liu-Yi Lan, Email: 13797928460@163.com.
Rui Zhou, Email: 42193469@qq.com.
Xian-Tao Zeng, Email: zengxiantao1128@163.com.
Jiao Bai, Email: baijiao2013@163.com.
Jun-Wen Bai, Email: baijunwen2001@163.com.
Qiong-Rong Chen, Email: qiongrongchen@hotmail.com.
Xing Chen, Email: 763044127@qq.com.
Xiao-Ming Zha, Email: njzhaxm@qq.com.
Wen-Jie Dai, Email: hmudavid@163.com.
Zhi-Jun Dai, Email: dzj0911@126.com.
Qin-Yu Feng, Email: 844614239@qq.com.
Qing-Jun Gao, Email: gaoqingjun@163.com.
Run-Fang Gao, Email: xugaoran@163.com.
Bao-San Han, Email: shhanbs311@126.com.
Wei Hou, Email: 842630809@qq.com.
Hai-Ying Liao, Email: liaoliao000000@sina.com.
Hong Luo, Email: 23016605@qq.com.
Zheng-Ren Liu, Email: Caolei148@163.com.
Jing-Hua Lu, Email: 869674874@qq.com.
Bin Luo, Email: luobin@mail.tsinghua.edu.cn.
Xiao-Peng Ma, Email: doccmxp@126.com.
Jun Qian, Email: qianjun0404@foxmail.com.
Jian-Yong Qin, Email: 252365170@qq.com.
Wei Wei, Email: rxwei1123@163.com.
Gang Wei, Email: wuhanuniversitywei@qq.com.
Li-Ying Xu, Email: 2312777367@qq.com.
Hui-Chao Xue, Email: rofessorhcxue@outlook.com.
Hua-Wei Yang, Email: yanghuawei@stu.gxmu.edu.cn.
Wei-Ge Yang, Email: yangweige12308@126.com.
Chao-Jie Zhang, Email: zhangchaojie@hunnu.edu.cn.
Fan Zhang, Email: zhangfancgh@163.com.
Guan-Xin Zhang, Email: 443979975@qq.com.
Shao-Kun Zhang, Email: qingtonycc@163.com.
Shu-Qun Zhang, Email: zhangshuqun19711@aliyun.com.
Ye-Qiang Zhang, Email: 264510968@qq.com.
Yue-Peng Zhang, Email: z-ypeng@163.com.
Sheng-Chu Zhang, Email: xgzsc@126.com.
Dai-Wei Zhao, Email: zhaodw@hotmail.com.
Xiang-Min Zheng, Email: zhengxiangmin@yahoo.com.
Le-Wei Zheng, Email: 530030984@qq.com.
Gao-Ran Xu, Email: xugaoran@163.com.
Wen-Bo Zhou, Email: zwbmail@163.com.
Gao-Song Wu, Email: wugaosongtj@163.com.
References
- 1.Qaseem A, Snow V, Owens DK, Shekelle P. Clinical Guidelines Committee of the American College of Physicians. The development of clinical practice guidelines and guidance statements of the American College of Physicians: Summary of Methods. Ann Intern Med. 2010;153(3):194–9. doi: 10.7326/0003-4819-153-3-201008030-00010. [DOI] [PubMed] [Google Scholar]
- 2.Miller FSI, Smith C. Granulomatous mastitis. N Y State J Med. 1971;71(8):2194–5. [PubMed] [Google Scholar]
- 3.Kessler E, Wolloch Y. Granulomatous mastitis: a lesion clinically simulating carcinoma. Am J Clin Pathol. 1972;58(6):642–646. doi: 10.1093/ajcp/58.6.642. [DOI] [PubMed] [Google Scholar]
- 4.Going JJ, Anderson TJ, Wilkinson S, Chetty U. Granulomatous lobular mastitis. J Clin Pathol. 1987;40(5):535–540. doi: 10.1136/jcp.40.5.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donn W, Rebbeck P, Wilson C, Gilks CB. Idiopathic granulomatous mastitis: a report of three cases and review of the literature. Arch Pathol Lab Med. 1994;118(8):822–5. [PubMed] [Google Scholar]
- 6.Boarki K, Labib M. Imaging findings in Idiopathic lobular granulomattous mastitis, case report and review of literature. Gulf J Oncolog. 2010;7:46–52. [PubMed] [Google Scholar]
- 7.Renshaw AA, Derhagopian RP, Gould EW. Cystic neutrophilic granulomatous mastitis: an underappreciated pattern strongly associated with gram-positive bacilli. Am J Clin Pathol. 2011;136(3):424–427. doi: 10.1309/AJCP1W9JBRYOQSNZ. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Legolvan M, Chapin K, Mainiero M. Cystic neutrophilic granulomatous mastitis with Corynebacterium and Staphylococcus mimicking breast carcinoma. Clin Case Rep. 2018;6(11):2208–2210. doi: 10.1002/ccr3.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naik MA, Korlimarla A, Shetty ST, Fernandes AM, Pai SA. Cystic neutrophilic granulomatous mastitis: a clinicopathological study with 16s rRNA sequencing for the detection of Corynebacteria in formalin-fixed paraffin-embedded tissue. Int J Surg Pathol. 2020;28(4):371–381. doi: 10.1177/1066896919896021. [DOI] [PubMed] [Google Scholar]
- 10.Johnstone KJ, Robson J, Cherian SG, Wan Sai Cheong J, Kerr K, Bligh JF. Cystic neutrophilic granulomatous mastitis associated with Corynebacterium including Corynebacterium kroppenstedtii. Pathology. 2017;49(4):405–12. doi: 10.1016/j.pathol.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Sangoi AR. "Thick Section" gram stain yields improved detection of organisms in tissue sections of cystic neutrophilic granulomatous mastitis. Am J Clin Pathol. 2020;153(5):593–597. doi: 10.1093/ajcp/aqz200. [DOI] [PubMed] [Google Scholar]
- 12.Gautham I, Radford DM, Kovacs CS, Calhoun BC, Procop GW, Shepardson LB, et al. Cystic neutrophilic granulomatous mastitis: The Cleveland Clinic experience with diagnosis and management. Breast J. 2019;25(1):80–85. doi: 10.1111/tbj.13160. [DOI] [PubMed] [Google Scholar]
- 13.Dobinson HC, Anderson TP, Chambers ST, Doogue MP, Seaward L, Werno AM. Antimicrobial treatment options for granulomatous mastitis caused by Corynebacterium species. J Clin Microbiol. 2015;53(9):2895–2899. doi: 10.1128/JCM.00760-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altintoprak F, Kivilcim T, Ozkan OV. Aetiology of idiopathic granulomatous mastitis. World J Clin Cases. 2014;2(12):852–858. doi: 10.12998/wjcc.v2.i12.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benson DD., Jr Idiopathic granulomatous mastitis: presentation, investigation and management. Future Oncol (Lond, Engl) 2016;12(11):1381–1394. doi: 10.2217/fon-2015-0038. [DOI] [PubMed] [Google Scholar]
- 16.Sheybani F, Naderi HR, Gharib M, Sarvghad M, Mirfeizi Z. Idiopathic granulomatous mastitis: long-discussed but yet-to-be-known. Autoimmunity. 2016;49(4):236–239. doi: 10.3109/08916934.2016.1138221. [DOI] [PubMed] [Google Scholar]
- 17.Zhou R, Lan L, Zheng L, Feng Q, Liao Y, Yuan Q, et al. Diagnosis and treatment of patients with granulomatous lobular mastitis (in Chinese) Chin J General Surg. 2021;36(2):118–121. [Google Scholar]
- 18.Lin CH, Hsu CW, Tsao TY, Chou J. Idiopathic granulomatous mastitis associated with risperidone-induced hyperprolactinemia. Diagn Pathol. 2012;7:2. doi: 10.1186/1746-1596-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellavia M, Damiano G, Palumbo VD, Spinelli G, Tomasello G, Marrazzo A, et al. Granulomatous mastitis during chronic antidepressant therapy: is it possible a conservative therapeutic approach? J Breast Cancer. 2012;15(3):371–372. doi: 10.4048/jbc.2012.15.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Omranipour R, Mohammadi SF, Samimi P. Idiopathic granulomatous lobular mastitis—report of 43 cases from Iran; introducing a preliminary clinical practice guideline. Breast Care (Basel) 2013;8(6):439–443. doi: 10.1159/000357320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farouk O, Abdelkhalek M, Abdallah A, Shata A, Senbel A, Attia E, et al. Rifampicin for idiopathic granulomatous lobular mastitis: a promising alternative for treatment. World J Surg. 2017;41(5):1313–1321. doi: 10.1007/s00268-016-3857-7. [DOI] [PubMed] [Google Scholar]
- 22.Yaghan R, Hamouri S, Ayoub NM, Yaghan L, Mazahreh T. A proposal of a clinically based classification for idiopathic granulomatous mastitis. Asian Pac J Cancer Prev. 2019;20(3):929–934. doi: 10.31557/APJCP.2019.20.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yaghan RJ, Ayoub NM, Hamouri S, Al-Mohtaseb A, Gharaibeh M, Yaghan L, et al. The role of establishing a multidisciplinary team for idiopathic granulomatous mastitis in improving patient outcomes and spreading awareness about recent disease trends. Int J Breast Cancer. 2020;2020:5243958. doi: 10.1155/2020/5243958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lei X, Chen K, Zhu L, Song E, Su F, Li S. Treatments for idiopathic granulomatous mastitis: systematic review and meta-analysis. Breastfeed Med. 2017;12(7):415–421. doi: 10.1089/bfm.2017.0030. [DOI] [PubMed] [Google Scholar]
- 25.Deng JQ, Yu L, Yang Y, Feng XJ, Sun J, Liu J, et al. Steroids administered after vacuum-assisted biopsy in the management of idiopathic granulomatous mastitis. J Clin Pathol. 2017;70(10):827–831. doi: 10.1136/jclinpath-2016-204287. [DOI] [PubMed] [Google Scholar]
- 26.Cohen C. Granulomatous mastitis: a review of 5 cases. S Afr Med J. 1977;52(1):14–6. [PubMed] [Google Scholar]
- 27.Akbulut S, Yilmaz D, Bakir S. Methotrexate in the management of idiopathic granulomatous mastitis: review of 108 published cases and report of four cases. Breast J. 2011;17(6):661–668. doi: 10.1111/j.1524-4741.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 28.Imoto S, Kitaya T, Kodama T, Hasebe T, Mukai K. Idiopathic granulomatous mastitis: case report and review of the literature. Jpn J Clin Oncol. 1997;27(4):274–277. doi: 10.1093/jjco/27.4.274. [DOI] [PubMed] [Google Scholar]
- 29.Altintoprak F, Karakece E, Kivilcim T, Dikicier E, Cakmak G, Celebi F, et al. Idiopathic granulomatous mastitis: an autoimmune disease? Sci World J. 2013;2013:148727. doi: 10.1155/2013/148727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor G, Paviour SD, Musaad S, Jones WO, Holland DJ. A clinicopathological review of 34 cases of inflammatory breast disease showing an association between Corynebacteria infection and granulomatous mastitis. Pathology. 2003;35(2):109–19. [PubMed] [Google Scholar]
- 31.Troxell ML, Gordon NT, Doggett JS, Ballard M, Vetto JT, Pommier RF, et al. Cystic neutrophilic granulomatous mastitis: association with gram-positive bacilli and Corynebacterium. Am J Clin Pathol. 2016;145(5):635–645. doi: 10.1093/ajcp/aqw046. [DOI] [PubMed] [Google Scholar]
- 32.Kivilcim T, Altintoprak F, Memis B, Ferhatoglu MF, Kartal A, Dikicier E, et al. Role of bacteriological agents in idiopathic granulomatous mastitis: real or not? Eur J Breast Health. 2019;15(1):32–36. doi: 10.5152/ejbh.2018.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaghan RJ, Ayoub NM, Shenawi HM, Yaghan LR. Idiopathic granulomatous mastitis in the male population: a clinical analysis of 13 reported cases. Breast J. 2020;26(7):1481–1482. doi: 10.1111/tbj.13778. [DOI] [PubMed] [Google Scholar]
- 34.Martinez-Ramos D, Simon-Monterde L, Suelves-Piqueres C, Queralt-Martin R, Granel-Villach L, Laguna-Sastre JM, et al. Idiopathic granulomatous mastitis: a systematic review of 3060 patients. Breast J. 2019;25(6):1245–1250. doi: 10.1111/tbj.13446. [DOI] [PubMed] [Google Scholar]
- 35.Azizi A, Prasath V, Canner J, Gharib M, Sadat Fattahi A, Naser Forghani M, et al. Idiopathic granulomatous mastitis: management and predictors of recurrence in 474 patients. Breast J. 2020;26(7):1358–1362. doi: 10.1111/tbj.13822. [DOI] [PubMed] [Google Scholar]
- 36.Nikolaev A, Blake CN, Carlson DL. Association between hyperprolactinemia and granulomatous mastitis. Breast J. 2016;22(2):224–231. doi: 10.1111/tbj.12552. [DOI] [PubMed] [Google Scholar]
- 37.Co M, Cheng VCC, Wei J, Wong SCY, Chan SMS, Shek T, et al. Idiopathic granulomatous mastitis: a 10-year study from a multicentre clinical database. Pathology. 2018;50(7):742–747. doi: 10.1016/j.pathol.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Sheybani F, Sarvghad M, Naderi H, Gharib M. Treatment for and clinical characteristics of granulomatous mastitis. Obstet Gynecol. 2015;125(4):801–807. doi: 10.1097/AOG.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 39.Pluguez-Turull CW, Nanyes JE, Quintero CJ, Alizai H, Mais DD, Kist KA, et al. idiopathic granulomatous mastitis: manifestations at multimodality imaging and pitfalls. Radiographics. 2018;38(2):330–356. doi: 10.1148/rg.2018170095. [DOI] [PubMed] [Google Scholar]
- 40.Kok KY, Telisinghe PU. Granulomatous mastitis: presentation, treatment and outcome in 43 patients. Surgeon. 2010;8(4):197–201. doi: 10.1016/j.surge.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Alikhassi A, Azizi F, Ensani F. Imaging features of granulomatous mastitis in 36 patients with new sonographic signs. J Ultrasound. 2020;23(1):61–68. doi: 10.1007/s40477-019-00392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasbahceci M, Kadioglu H. Use of imaging for the diagnosis of idiopathic granulomatous mastitis: a clinician's perspective. J Coll Physicians Surg Pak. 2018;28(11):862–867. doi: 10.29271/jcpsp.2018.11.862. [DOI] [PubMed] [Google Scholar]
- 43.Aghajanzadeh M, Hassanzadeh R, Alizadeh Sefat S, Alavi A, Hemmati H, Esmaeili Delshad MS, et al. Granulomatous mastitis: Presentations, diagnosis, treatment and outcome in 206 patients from the north of Iran. Breast. 2015;24(4):456–460. doi: 10.1016/j.breast.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Hovanessian Larsen LJ, Peyvandi B, Klipfel N, Grant E, Iyengar G. Granulomatous lobular mastitis: imaging, diagnosis, and treatment. AJR Am J Roentgenol. 2009;193(2):574–581. doi: 10.2214/AJR.08.1528. [DOI] [PubMed] [Google Scholar]
- 45.Al-Khawari HA, Al-Manfouhi HA, Madda JP, Kovacs A, Sheikh M, Roberts O. Radiologic features of granulomatous mastitis. Breast J. 2011;17(6):645–650. doi: 10.1111/j.1524-4741.2011.01154.x. [DOI] [PubMed] [Google Scholar]
- 46.Alper F, Karadeniz E, Guven F, Yilmaz Cankaya B, Ozden K, Akcay MN. The evaluation of the efficacy of local steroid administration in idiopathic granulomatous mastitis: the preliminary results. Breast J. 2020;26(2):309–311. doi: 10.1111/tbj.13588. [DOI] [PubMed] [Google Scholar]
- 47.Oztekin PS, Durhan G, Nercis Kosar P, Erel S, Hucumenoglu S. Imaging findings in patients with granulomatous mastitis. Iran J Radiol. 2016;13(3):e33900. doi: 10.5812/iranjradiol.33900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tse GM, Chaiwun B, Wong KT, Yeung DK, Pang AL, Tang AP, et al. Magnetic resonance imaging of breast lesions—a pathologic correlation. Breast Cancer Res Treat. 2007;103(1):1–10. doi: 10.1007/s10549-006-9352-3. [DOI] [PubMed] [Google Scholar]
- 49.Yilmaz R, Demir AA, Kaplan A, Sahin D, Ozkurt E, Dursun M, et al. Magnetic resonance imaging features of idiopathic granulomatous mastitis: is there any contribution of diffusion-weighted imaging in the differential diagnosis? Radiol Med. 2016;121(11):857–866. doi: 10.1007/s11547-016-0666-x. [DOI] [PubMed] [Google Scholar]
- 50.Zhang C, Fan P, Liu P, Zhang Z. Applicable value of dynamic magnetic resonance imaging in the evaluation of granulomatous mastitis surgery (in Chinese) China J Modem Med. 2012;22(21):86–9. [Google Scholar]
- 51.Fazzio RT, Shah SS, Sandhu NP, Glazebrook KN. Idiopathic granulomatous mastitis: imaging update and review. Insights Imaging. 2016;7(4):531–539. doi: 10.1007/s13244-016-0499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gunduz Y, Altintoprak F, Tatli Ayhan L, Kivilcim T, Celebi F. Effect of topical steroid treatment on idiopathic granulomatous mastitis: clinical and radiologic evaluation. Breast J. 2014;20(6):586–591. doi: 10.1111/tbj.12335. [DOI] [PubMed] [Google Scholar]
- 53.Durur-Subasi I. Diagnostic and interventional radiology in idiopathic granulomatous mastitis. Eurasian J Med. 2019;51(3):293–297. doi: 10.5152/eurasianjmed.2019.19211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haddad M, Sheybani F, Arian M, Gharib M. Methotrexate-based regimen as initial treatment of patients with idiopathic granulomatous mastitis. Breast J. 2020;26(2):325–327. doi: 10.1111/tbj.13590. [DOI] [PubMed] [Google Scholar]
- 55.Salesi M, Karimifar M, Salimi F, Mahzouni P. A case of granulomatous mastitis with erythema nodosum and arthritis. Rheumatol Int. 2011;31(8):1093–1095. doi: 10.1007/s00296-009-1273-0. [DOI] [PubMed] [Google Scholar]
- 56.Ben Abid F, Abdel Rahman SSH. A case report of TB versus idiopathic granulomatous mastitis with erythema nodosum, reactive arthritis, cough, and headache. Aging Male. 2020;23(5):411–4. doi: 10.1080/13685538.2018.1504915. [DOI] [PubMed] [Google Scholar]
- 57.Agrawal A, Pabolu S. A rare case of idiopathic granulomatous mastitis in a nulliparous woman with hyperprolactinemia. Cureus. 2019;11(5):e4680. doi: 10.7759/cureus.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou F, Yu LX, Ma ZB, Yu ZG. Granulomatous lobular mastitis. Chronic Dis Transl Med. 2016;2(1):17–21. doi: 10.1016/j.cdtm.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng J, Ding H, Du Y, Wu X, Dai X. Granlllomatous loblllar mastitis, mammary duct ectasia and erythema nodosmn: a cliIlicopathologic study of 4 cases with review of literature (in Chinese) Chin J Diagn Pathol. 2014;21(08):488–90. [Google Scholar]
- 60.Zhao Q, Xie T, Fu C, Chen L, Bai Q, Grimm R, et al. Differentiation between idiopathic granulomatous mastitis and invasive breast carcinoma, both presenting with non-mass enhancement without rim-enhanced masses: the value of whole-lesion histogram and texture analysis using apparent diffusion coefficient. Eur J Radiol. 2020;123:108782. doi: 10.1016/j.ejrad.2019.108782. [DOI] [PubMed] [Google Scholar]
- 61.Yu H, Wang Q, Yang J, Lian Z, Zhang A, Li W, et al. Anti-mycobacteria drugs therapy for perductal mastitis with fistula (in Chinese) Chin J Surg. 2012;50(11):971–4. [PubMed] [Google Scholar]
- 62.Liu L, Zhou F, Wang P, Yu L, Ma Z, Li Y, et al. Periductal mastitis: an inflammatory disease related to bacterial infection and consequent immune responses? Mediators Inflamm. 2017;2017:5309081. doi: 10.1155/2017/5309081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang J, Wang Q, Zhang A, Xu J, Gao H, Li W, et al. Identification and treatment of periductal mastitis and granulomatous mastitis (in Chinese) Chin J Breast Dis (Electron Ed). 2011;5(3):306–12. [Google Scholar]
- 64.Zhou DD, Bai WQ, Zhai XT, Sun LP, Zhen YS, Li ZR, et al. Excellent efects and possible mechanisms of action of a new antibody–drug conjugate against EGFR-positive triple-negative breast cancer. Mil Med Res. 2021;8(1):63. doi: 10.1186/s40779-021-00358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Menta A, Fouad TM, Lucci A, Le-Petross H, Stauder MC, Woodward WA, et al. Inflammatory breast cancer: what to know about this unique, aggressive breast cancer. Surg Clin North Am. 2018;98(4):787–800. doi: 10.1016/j.suc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 66.Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389(10074):1134–1150. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 67.Lee JOK, Kim E, Kwack K, Jung W, Lee H. Radiologic and clinical features of idiopathic granulomatous lobular mastitis mimicking advanced breast cancer. Yonsei Med J. 2006;47(1):78–84. doi: 10.3349/ymj.2006.47.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rowe PH. Granulomatous mastitis associated with a pituitary prolactinoma. Br J Clin Pract. 1984;38(1):32–34. [PubMed] [Google Scholar]
- 69.Lai EC, Chan WC, Ma TK, Tang AP, Poon CS, Leong HT. The role of conservative treatment in idiopathic granulomatous mastitis. Breast J. 2005;11(6):454–456. doi: 10.1111/j.1075-122X.2005.00127.x. [DOI] [PubMed] [Google Scholar]
- 70.Davis J, Cocco D, Matz S, Hsu CH, Brown MJ, Lee J, et al. Re-evaluating if observation continues to be the best management of idiopathic granulomatous mastitis. Surgery. 2019;166(6):1176–1180. doi: 10.1016/j.surg.2019.06.030. [DOI] [PubMed] [Google Scholar]
- 71.Hur SM, Cho DH, Lee SK, Choi MY, Bae SY, Koo MY, et al. Experience of treatment of patients with granulomatous lobular mastitis. J Korean Surg Soc. 2013;85(1):1–6. doi: 10.4174/jkss.2013.85.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joseph KA, Luu X, Mor A. Granulomatous mastitis: a New York public hospital experience. Ann Surg Oncol. 2014;21(13):4159–4163. doi: 10.1245/s10434-014-3895-z. [DOI] [PubMed] [Google Scholar]
- 73.Tan QT, Tay SP, Gudi MA, Nadkarni NV, Lim SH, Chuwa EWL. Granulomatous mastitis and factors associated with recurrence: an 11-year single-centre study of 113 patients in Singapore. World J Surg. 2019;43(7):1737–1745. doi: 10.1007/s00268-019-05014-x. [DOI] [PubMed] [Google Scholar]
- 74.Montazer M, Dadashzadeh M, Moosavi Toomatari SE. Comparison of the outcome of low dose and high-dose corticosteroid in the treatment of idiopathic granulomatous mastitis. Asian Pac J Cancer Prev. 2020;21(4):993–996. doi: 10.31557/APJCP.2020.21.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Çetin K, Sıkar HE, Göret NE, Rona G, Barışık NO, Küçük HF, et al. Comparison of topical, systemic, and combined therapy with steroids on idiopathic granulomatous mastitis: a prospective randomized study. World J Surg. 2019;43(11):2865–2873. doi: 10.1007/s00268-019-05084-x. [DOI] [PubMed] [Google Scholar]
- 76.Altintoprak F, Kivilcim T, Yalkin O, Uzunoglu Y, Kahyaoglu Z, Dilek ON. Topical steroids are effective in the treatment of idiopathic granulomatous mastitis. World J Surg. 2015;39(11):2718–2723. doi: 10.1007/s00268-015-3147-9. [DOI] [PubMed] [Google Scholar]
- 77.Xiao M, Li S, Tong S. Clinical analysis of treatment with granulomatous lobular mastitis by ultrasonography-guided abscess puncture aspiration or drainage combined with local application of triamcinolone acetonide for 46 cases (in Chinese) Chin Arch Gen Surg (Electron Ed). 2019;13(1):30–3. [Google Scholar]
- 78.Korkut E, Akcay MN, Karadeniz E, Subasi ID, Gursan N. Granulomatous mastitis: a ten-year experience at a university hospital. Eurasian J Med. 2015;47(3):165–173. doi: 10.5152/eurasianjmed.2015.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Toktas O, Konca C, Trabulus DC, Soyder A, Koksal H, Karanlik H, et al. A novel first-line treatment alternative for noncomplicated idiopathic granulomatous mastitis: combined intralesional steroid injection with topical steroid administration. Breast Care (Basel) 2021;16(2):181–187. doi: 10.1159/000507951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Raj N, Macmillan RD, Ellis IO, Deighton CM. Rheumatologists and breasts: immunosuppressive therapy for granulomatous mastitis. Rheumatology (Oxford) 2004;43(8):1055–1056. doi: 10.1093/rheumatology/keh246. [DOI] [PubMed] [Google Scholar]
- 81.Ringsted S, Friedman M. A rheumatologic approach to granulomatous mastitis: a case series and review of the literature. Int J Rheum Dis. 2021;24(4):526–532. doi: 10.1111/1756-185X.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmajuk G, Genovese MC. First report of idiopathic granulomatous mastitis treated with methotrexate monotherapy. J Rheumatol. 2009;36(7):1559–1560. doi: 10.3899/jrheum.090091. [DOI] [PubMed] [Google Scholar]
- 83.Akbulut S, Arikanoglu Z, Senol A, Sogutcu N, Basbug M, Yeniaras E, et al. Is methotrexate an acceptable treatment in the management of idiopathic granulomatous mastitis? Arch Gynecol Obstet. 2011;284(5):1189–1195. doi: 10.1007/s00404-010-1825-2. [DOI] [PubMed] [Google Scholar]
- 84.Tekgoz E, Colak S, Cinar M, Yilmaz S. Treatment of idiopathic granulomatous mastitis and factors related with disease recurrence. Turk J Med Sci. 2020;50(5):1380–1386. doi: 10.3906/sag-2003-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Steuer AB, Stern MJ, Cobos G, Castilla C, Joseph KA, Pomeranz MK, et al. Clinical characteristics and medical management of idiopathic granulomatous mastitis. JAMA Dermatol. 2020;156(4):460–464. doi: 10.1001/jamadermatol.2019.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tsai MJ, Huang WC, Wang JT, Wang MY, Lee YH, Lin SW, et al. Factors associated with treatment duration and recurrence rate of complicated mastitis. J Microbiol Immunol Infect. 2020;53(6):875–881. doi: 10.1016/j.jmii.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 87.Huang Y, Wu H. A retrospective analysis of recurrence risk factors for granulomatous lobular mastitis in 130 patients: more attention should be paied to prolactin level. Ann Palliat Med. 2021;10(3):2824–2831. doi: 10.21037/apm-20-1972. [DOI] [PubMed] [Google Scholar]
- 88.Yılmaz TU, Gürel B, Güler SA, Baran MA, Erşan B, Duman S, et al. Scoring idiopathic granulomatous mastitis: an effective system for predicting recurrence? Eur J Breast Health. 2018;14(2):112–116. doi: 10.5152/ejbh.2018.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ahmed YS, Abd El Maksoud W. Evaluation of therapeutic mammoplasty techniques in the surgical management of female patients with idiopathic granulomatous mastitis with mild to moderate inflammatory symptoms in terms of recurrence and patients' satisfaction. Breast Dis. 2016;36(1):37–45. doi: 10.3233/BD-150198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1 Histological staining of granulomatous lobular mastitis. Fig. S2 Self-limited stage. Fig. S3 Congestive swelling stage. Fig. S4 Abscess formation stage. Fig. S5 Complex refractory stage.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.