Muskoxen are increasingly exposed to stressors in the rapidly changing Arctic. Hair cortisol, an indicator of stress, varied among years, sex, and geographical location in wild muskoxen and was associated with poorer body condition and lungworms. These results support use of hair cortisol as a tool for monitoring muskox health and informing management.
Keywords: Arctic, hair cortisol, hunter-based sampling, muskox, qiviut, wildlife
Abstract
Glucocorticoid (GC) levels are increasingly and widely used as biomarkers of hypothalamic–pituitary–adrenal (HPA) axis activity to study the effects of environmental changes and other perturbations on wildlife individuals and populations. However, identifying the intrinsic and extrinsic factors that influence GC levels is a key step in endocrinology studies to ensure accurate interpretation of GC responses. In muskoxen, qiviut (fine woolly undercoat hair) cortisol concentration is an integrative biomarker of HPA axis activity over the course of the hair’s growth. We gathered data from 219 wild muskoxen harvested in the Canadian Arctic between October 2015 and May 2019. We examined the relationship between qiviut cortisol and various intrinsic (sex, age, body condition and incisor breakage) and extrinsic biotic factors (lungworm and gastrointestinal parasite infections and exposure to bacteria), as well as broader non-specific landscape and temporal features (geographical location, season and year). A Bayesian approach, which allows for the joint estimation of missing values in the data and model parameters estimates, was applied for the statistical analyses. The main findings include the following: (i) higher qiviut cortisol levels in males than in females; (ii) inter-annual variations; (iii) higher qiviut cortisol levels in a declining population compared to a stable population; (iv) a negative association between qiviut cortisol and marrow fat percentage; (v) a relationship between qiviut cortisol and the infection intensity of the lungworm Umingmakstrongylus pallikuukensis, which varied depending on the geographical location; and (vi) no association between qiviut cortisol and other pathogen exposure/infection intensity metrics. This study confirmed and further identified important sources of variability in qiviut cortisol levels, while providing important insights on the relationship between GC levels and pathogen exposure/infection intensity. Results support the use of qiviut cortisol as a tool to monitor temporal changes in HPA axis activity at a population level and to inform management and conservation actions.
Introduction
Climate warming and other anthropogenic changes are occurring at an unprecedented pace worldwide. Understanding the effects that these changes and their associated stressors have on wildlife species is essential for biodiversity conservation (IPCC, 2014; Ribera d’Alcalà, 2019). This is particularly relevant in high-latitude environments such as the Arctic, where climate changes have been especially rapid and pronounced and have multiple impacts, including an increased frequency of extreme weather events, the loss of sea ice, higher surface temperatures and modifications in species associations and abundance (AMAP, 2017; Cuyler et al., 2020b).
Muskoxen (Ovibos moschatus), an iconic Arctic ungulate species, are essential for the nutrition, economy and culture of local Indigenous communities (Tomaselli et al., 2018a; Cuyler et al., 2020a). Over the past two decades, muskoxen have undergone substantial population declines on Banks and Victoria Islands in the western Canadian Arctic Archipelago (Tomaselli et al., 2018b; Cuyler et al., 2020b). These declines are linked to multiple factors, including icing events and large-scale disease-associated mortality and morbidity (Nagy and Gunn, 2009; Nagy et al., 2009; Kutz et al., 2017; Cuyler et al., 2020b). Recent emerging pathogens and disease syndromes of concern include Erysipelothrix rhusiopathiae, brucellosis, orf (Parapoxvirus) and expanding lungworm infections (Kutz et al., 2015; Tomaselli et al., 2016, 2018b, 2019; Kafle et al., 2020; Mavrot et al., 2020). The recent population declines, along with emerging diseases and the very low genetic diversity of muskoxen (Prewer et al., 2020), suggest that this species may be particularly threatened by the multiple environmental changes and associated stressors to which it is increasingly exposed (Kutz et al., 2017; Cuyler et al., 2020b).
The activation of the hypothalamic–pituitary–adrenal (HPA) axis, which leads to the release of glucocorticoids (GCs) (mainly cortisol in muskoxen; Koren et al., 2012), is an important part of the physiological stress response in mammals and plays a key role in energy regulation (Romero and Butler, 2007; Busch and Hayward, 2009). GC levels are increasingly and widely used as biomarkers of HPA axis activity to study the effects of various environmental changes and challenges on wildlife individuals and populations (Busch and Hayward, 2009; Dantzer et al., 2014). These hormones are incorporated and can be readily quantified in various biological matrices including blood, saliva, faeces and urine (in the form of metabolites), and hair (reviewed in Dantzer et al., 2014). Among these matrices, blood, saliva, urine and faeces provide short-term information on an animal’s GC levels at a single point in time or over hours to days, whereas hair is thought to provide a cumulative measurement of GC levels during the period of the hair’s growth—this may be weeks to months depending on turnover patterns and sampling regime (Sheriff et al., 2011; Russell et al., 2012). Hair GC concentrations may, therefore, represent a non-specific integrative measure of all the stressors experienced by the animal over the course of the hair’s growth (Cattet et al., 2014).
Hair GCs have been widely used to investigate the stress response of wildlife species to anthropogenic disturbances, extreme weather events and pathogens (e.g. Bryan et al., 2015; Ewacha et al., 2017; Fardi et al., 2018; Madslien et al., 2020). Less frequently, hair GCs have also been used to examine the possible consequences of elevated GCs on fitness indicators (e.g. survival probability, neonate birthweight and pregnancy success; Rakotoniaina et al., 2017; Downs et al., 2018). Even more rarely, studies have attempted to demonstrate the link between a stressor, elevated GCs and the effect of these hormones on fitness (but see Dulude-de Broin et al., 2020). Hair GCs are influenced by a wide variety of extrinsic (e.g. season, social status) and intrinsic (e.g. sex, reproductive status) factors that may affect both baseline GC levels and the magnitude of the response to stressors, as well as the incorporation of GCs into the hair (Dantzer et al., 2014; Heimbürge et al., 2019). These predictable influencing factors must be identified and controlled for in each species of interest in order to adequately interpret GC responses to environmental changes and disturbances. While many studies have assessed the relationship between GC levels and a single or few extrinsic and/or intrinsic factors (e.g. Salas et al., 2016; Fardi et al., 2018), the simultaneous inclusion of multiple factors in the analyses is rare (but see Santos et al., 2018). In particular, studies that investigated the association between GC levels and pathogens were generally focused on a single type of pathogen/parasite while only accounting for a few other extrinsic and/or intrinsic factors (e.g. Chapman et al., 2006; Madslien et al., 2020, but see Hoby et al., 2006; Cizauskas et al., 2015).
Cortisol concentration in the qiviut (fine woolly undercoat hair) of muskoxen can be used as a biomarker of HPA axis activity during the period of the hair’s growth (Di Francesco et al., 2021). Here, we use a large-scale cross-sectional study of wild muskoxen in the Canadian Arctic to examine the relationship between qiviut cortisol levels and various intrinsic (sex, age, body condition and incisor breakage) and extrinsic biotic factors [lungworm and gastrointestinal (GI) parasite infection intensities, parasite richness, exposure to bacteria], as well as broader landscape and temporal features (geographical location, season and year). Building on our previous work (Di Francesco et al., 2017), this study confirms and further identifies important sources of variability in qiviut cortisol levels.
Material and methods
Animals and sampling procedure
This study was approved by the Veterinary Sciences Animal Care Committee, University of Calgary (protocol #AC13-0121). Samples were obtained under the Wildlife Research Permit #2016-058 for Nunavut (NU) and the Wildlife Research Permits #WL500257, WL5004469 and WL500664 for the Northwest Territories (NWT).
Samples from 219 muskoxen, harvested as part of a community-based muskox health surveillance program in Ekaluktutiak and Kugluktuk, NU, and in Ulukhaktok, NWT, were obtained between October 2015 and May 2019 (Fig. 1). The surveillance program was developed through a partnership among these communities, Hunters and Trappers Organizations/Committees, guided hunting organizations, government biologists and academic researchers. Samples were obtained through individual subsistence, community and guided hunts.
Figure 1.
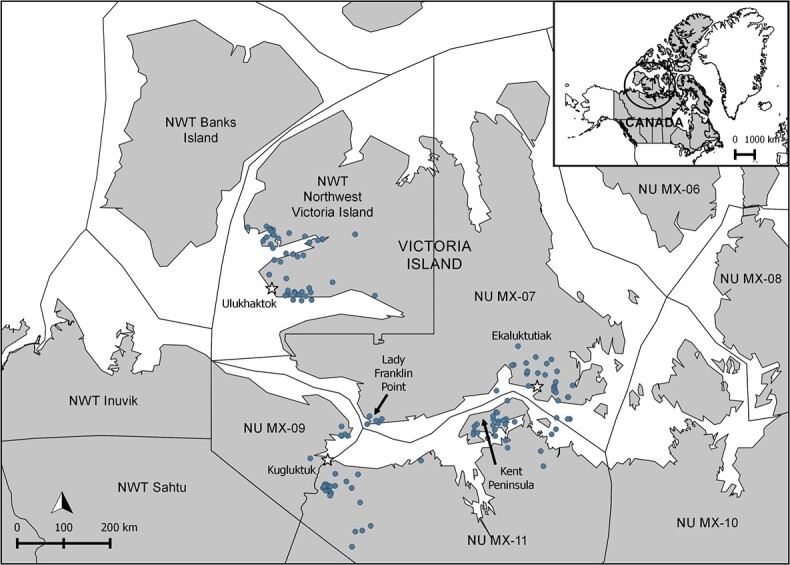
Map showing the muskox management units (MMUs) in the NWT and NU and the five specific geographical locations from which samples were obtained [communities of Ulukhaktok, Kugluktuk and Ekaluktutiak (black and white stars), and Lady Franklin Point and Kent Peninsula (black arrows)], with the geo-referenced harvesting locations of the muskoxen when available (blue points). Geographic coordinates were unavailable for 15 of the 211 muskoxen; these animals were assigned to a specific geographical location based on the MMUs in which they were harvested and on the community from which the kit was submitted (Ekaluktutiak, n = 8; Kugluktuk, n = 2; Ulukhaktok, n = 3; Kent Peninsula, n = 2). Map generated in QGIS version 2.8.9 using the shapefile from Cuyler et al., 2020b. For the purposes of the analyses, animals from Ekaluktutiak, Lady Franklin Point and Ulukhaktok were grouped within the broad geographical location of Victoria Island, which is only divided into two MMUs because of political boundaries. Animals harvested near Kugluktuk (NU MX-09 and west part of NU MX-11) and on the Kent Peninsula (east part of NU MX-11) were treated as two separate broad geographical locations (i.e. west and east mainland, respectively), because of ecological and muskox health status differences (Leclerc, 2014; Walker et al., 2005).
Hunters and guides collected samples using standardized kits available to them at the local wildlife office and/or Hunters and Trappers Organization/Committee. Participation in this program was voluntary and they received financial compensation for each completed kit. Kits were modified from those described by Kutz et al. (2013b) for caribou. A fully completed kit would include the following: blood collected on Nobuto filter paper strips (Toyo Roshi Kaisha, Ltd, Tokyo, Japan), faeces, the lower left hind leg, the lower jaw and a piece of skin with fur from the rump measuring ~10 × 10 cm (Fig. 2). The geographic coordinates of the kill location, the age class and sex of the muskox, a measure of its back fat thickness, a subjective assessment of its body condition (i.e. categories ‘skinny’, ‘not bad’, ‘fat’ or ‘really fat’) and observations of abnormalities seen during butchering were recorded by the hunter using the datasheet provided with the kit (Supplementary Fig. 1). All samples were stored at approximately −20°C until analysis. Not all returned kits and information forms were complete, and the amount of a sample collected was sometimes not sufficient for all the tests to be performed (e.g. faecal samples and parasitology testing), which led to missing values in the dataset (Table 1; Supplementary Fig. 2).
Figure 2.
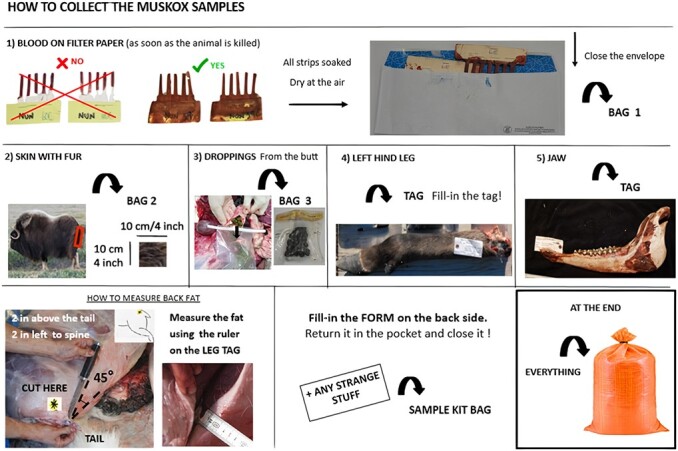
Instructions provided to the hunters with the kits to collect the samples.
Table 1.
Variables, and their respective abbreviations, descriptions and sample sizes, evaluated as potential predictors of qiviut cortisol levels in the 211 muskoxen. None of the continuous variables were normally distributed, so all are summarized as median (range). Missing values (i.e. insufficient sample for all laboratory analyses or information not recorded on data sheet), except those for back fat thickness and incisor breakage score, were estimated through the Bayesian analyses
| Information | Variable | Variable description | Number of muskoxen (%) | |
|---|---|---|---|---|
| General | Season (season) |
Categorical
Fall–early winter Mid-winter–late winter |
75 136 |
(35.5) (64.5) |
| Year (i.e. hair growth year) (year) |
Categorical
2015 2016 2017 2018 |
28 41 80 62 |
(13.3) (19.4) (37.9) (29.4) |
|
| Sex (sex) |
Categorical
Female Male |
91 120 |
(43.1) (56.9) |
|
| Age (age) |
Categorical
Adult Juvenile |
191 20 |
(90.5) (9.5) |
|
| Specific geographical location |
Categorical
Ekaluktutiak Kent Peninsula Kugluktuk Lady Franklin Point Ulukhaktok |
39 30 70 13 59 |
(18.5) (14.2) (33.2) (6.2) (27.9) |
|
| Broad geographical location (location) |
Categorical
East mainland (Kent Peninsula) Victoria Island (Ekaluktutiak, Lady Franklin Point and Ulukhaktok) West mainland (Kugluktuk) |
30 111 70 |
(14.2) (52.6) (33.2) |
|
| Lungworms | U. pallikuukensis larval counts (Up_lpg) |
Continuous
43.1 lpg (0–1669.8) Missing |
200 11 |
(94.8) (5.2) |
| V. eleguneniensis larval counts (Ve_lpg) |
Continuous
1.3 lpg (0–442.7) Missing |
200 11 |
(94.8) (5.2) |
|
| Lungworm richness (lung_richness) |
Categorical
0 (no lungworms detected) 1 (Up or Ve detected) 2 (both Up and Ve detected) Missing |
28 54 118 11 |
(13.3) (25.6) (55.9) (5.2) |
|
| GI parasites | Moniezia spp. infection (moniezia_YN) |
Categorical
No Yes Missing |
152 23 36 |
(72.0) (10.9) (17.1) |
| Eimeria spp. oocyst counts (eimeria_epg) |
Continuous
2.5 epg (0–822.6) Missing |
175 36 |
(82.9) (17.1) |
|
| Nematodirines egg counts (nematodirines_epg) |
Continuous
0 epg (0–16.1) Missing |
175 36 |
(82.9) (17.1) |
|
| M. marshalli egg counts (marshallagia_epg) |
Continuous
0 epg (0–10.4) Missing |
175 36 |
(82.9) (17.1) |
|
| GI parasite richness (GI_richness) |
Categorical
0 (no GI parasites detected) 1 (1 GI parasite species/group detected) 2 (2 GI parasite species/groups detected) 3 (3 GI parasite species/groups detected) 4 (4 GI parasite species/groups detected) Missing |
5 61 58 41 10 36 |
(2.4) (28.9) (27.5) (19.4) (4.7) (17.1) |
|
| Body condition | Metatarsus percentage marrow fat (marrow_fat) |
Continuous
91.1% (9.9–94.8)a Missing |
197 14 |
(93.4) (6.6) |
| Hunter condition assessment (condition_hunter) |
Categorical
Skinny Not bad Fat Really fat Missing |
21 53 93 20 24 |
(9.9) (25.1) (44.1) (9.5) (11.4) |
|
| Back fat thickness (back_fat) |
Continuous
2 cm (0–6.4) Missingb |
145 66 |
(68.7) (31.3) |
|
| Bacteria exposure | E. rhusiopathiae percentage positivity (erysipelothrix_PP) |
Continuous
22.4% (1–496.1)a Missing |
203 8 |
(96.2) (3.8) |
| Brucella serology (brucella_serology) |
Categorical
Negative Positive Missing |
192 11 8 |
(91.0) (5.2) (3.8) |
|
| Jaw health | Incisor breakage score (incisor_breakage) |
Continuous
0 (0–1) Missingb |
152 59 |
(72.0) (28.0) |
| Hormone levels (outcome) | Qiviut cortisol levels (qiviut_cortisol) |
Continuous
16.3 (3.8–84.5) |
||
aThese values were divided by 100 for the statistical analyses.
bNot estimated through Bayesian analyses.
Samples were classified to two different seasons based on their collection date: late fall–early winter (last week of September to end of December) and mid-winter–late winter (January to mid-May). There was a large overlap between the growth periods of some of the late fall-early winter and mid-winter–late winter qiviut samples, as qiviut grows approximately from early April to late November with no growth observed between mid-December and March (Flood et al., 1989; Robertson, 2000). However, this categorization was decided for consistency with a previous seasonal classification (Di Francesco et al., 2017) and due to the high seasonality of some of the other health indicators, such as body condition. The year was defined as the year of qiviut growth, not the year of sample collection (i.e. muskoxen harvested in January through mid-May were assigned to the year prior to harvest because the qiviut sampled at the time of harvest had grown during the previous summer and fall).
When left blank or indicated as ‘unknown’ on the information sheet, the sex of the muskox was determined using genetic analyses as previously detailed by Di Francesco et al. (2017). The age was categorized as ‘juvenile’ (calves and yearlings) or ‘adult’ (>2 years) for the analyses. This was estimated either based on the teeth eruption patterns when the lower jaw was collected (Henrichsen and Grue, 1980) or by the hunter if the jaw was not available. If neither of these sources of information were available, the age class was determined based on the metatarsus length for muskoxen harvested on Victoria Island. We plotted the distribution of metatarsus length for animals of known age class and developed a cut-off of 16 cm, above which muskoxen were classified as adults and below which the age remained ‘unknown’ (Supplementary Fig. 3). The eight muskoxen with an ‘unknown’ age were removed from the statistical analyses, as we chose not to impute the missing data for this variable, leaving a total of 211 animals to be included.
Sample analyses
Parasitology
Approximately 5 g from each faecal sample was weighed (as little as 2 g were used if the amount was limited) and analysed using the modified beaker Baermann technique for the presence of protostrongylid lungworm first stage larvae (L1) (Forrester and Lankester, 1997). Modifications were as follows: (i) tissue paper was replaced by a single layer of cheesecloth; (ii) samples sat for 5 min after removal of the faecal envelope before the supernatant was aspirated (using gentle vacuum suction) down to 15 ml and transferred in a test tube; (iii) the beaker was rinsed with 2 ml of tap water, which were added to the test tube; and (iv) samples were then centrifuged, supernatant aspirated down to 2 ml and, depending on the amount of L1 expected, either the entire sample or three 50 or 100 μl aliquots were analysed. The L1 recovered were identified microscopically under 40× magnification as Umingmakstrongylus pallikuukensis (Up) or Varestrongylus eleguneniensis (Ve) based on their caudal morphology following the keys developed by Kafle et al. (2015). Each species was quantified separately and L1 counts are expressed as larvae per gramme of faeces (lpg).
Approximately 2–4 g (depending on the amount available) from each faecal sample was weighed and evaluated for the presence of GI parasite eggs and oocysts using the modified Wisconsin double-centrifugation sugar flotation technique (Egwang and Slocombe, 1982). Modifications included straining the water-faecal mixture through a single layer of cheesecloth instead of a tea strainer and centrifuging the samples for 10 min at 1500 rpm both times. The eggs and oocysts recovered were counted and identified microscopically under 100× magnification to the group or species level (nematodirines (Nematodirus spp. and Nematodirella spp.), Marshallagia marshallagi, Eimeria spp. or Moniezia spp.) based on their morphological characteristics. The eggs of Teladorsagia boreoarcticus and Ostertagia gruehneri, two major GI parasites of muskoxen, were not counted as egg production occurs mainly throughout the summer (reviewed in Kutz et al., 2012) and freezing of faecal samples results in a rapid decrease in the number of detectable eggs of these species (De Bruyn, 2010). All egg and oocyst counts are expressed as eggs per gramme of faeces (epg), except for Moniezia spp. for which only the absence or presence of eggs was recorded as entire proglottids are typically shed in the faeces and quantification of eggs in faeces is not considered representative of actual parasite burden (Taylor et al., 2015).
We assumed that larval and egg/oocyst counts served as proxies for their corresponding parasite infection intensities (with the exception of Moniezia spp. as explained above). The correlations between the number of adult worms in the lungs or GI tract and the amount of L1, eggs or oocysts shed in the faeces have not been investigated in muskoxen. However, a study in Svalbard reindeer (Rangifer tarandus platyrhynchus) showed that faecal egg counts were positively associated with adult worm burden for M. marshalli (Irvine et al., 2001). An experimental study on a limited number of muskoxen suggested a similar relationship between faecal larval counts and adult worm burden for Up (Kutz et al., 1999).
Parasite richness was recorded for lungworms and GI parasites by counting the number of different species (lungworms) and species or groups (GI parasites) infecting each muskox.
Hormone analyses
For each animal, qiviut, intermediate hairs and guard hairs from an area free from obvious blood or other contamination were cut away from the skin using a scalpel blade. Qiviut was manually separated from guard and intermediate hairs using forceps and was divided into two subsamples of 0.05 g, which were independently analysed. Qiviut cortisol analyses were done at the Endocrinology Laboratory of the Toronto Zoo following the procedure detailed in Di Francesco et al. (2021). The mean concentration between the two subsamples, measured in nanograms of cortisol per gramme of qiviut (ng/g), was used as the final response variable. Duplicate subsamples had a median coefficient of variation [CV, calculated as (standard deviation/mean) × 100] of 12.70% (range = 0.14–83.26%).
Serology
Filter paper sets were removed from the freezer and air-dried at room temperature for at least 24 h. They were then eluted following the method described by Curry et al. (2011) and eluates were stored at −20°C until testing.
Eluates were tested for antibodies against E. rhusiopathiae with a modified enzyme-linked immunosorbent assay (ELISA) (Mavrot et al., 2020). Results were standardized across all ELISA plates and expressed as percentage positivity (PP) of a reference muskox positive control. The PP was calculated as (ODsample − ODblank)/(ODcont − ODblank) × 100, with ODsample corresponding to the optic density (OD) value measured for the sample, ODcont to the OD value of the positive control, and ODblank to the OD value of the ELISA plate’s blank well (Mavrot et al., 2020).
Samples were tested for antibodies against Brucella using two different indirect enzyme-linked immunoassays (i-ELISA). All samples from hair growth year 2015 (n = 28) were analysed at the Arctic University of Norway, Research Group for Arctic Infection Biology (Tromsø, Norway; Nymo et al., 2013). The remaining samples (n = 183) were analysed at the Canadian Food Inspection Agency National Brucellosis Reference Laboratory (Ottawa, Canada; Nielsen et al., 1994, 2004). In both i-ELISAs, the antigen was smooth lipopolysaccharide from Brucella abortus and the enzyme conjugate was a peroxidase conjugated chimeric protein A/G (Nielsen et al., 2004; Nymo et al., 2013). Because of the clear cut-off between positive and negative samples, results were expressed as a positive/negative status.
Marrow fat measurement and other indices of body condition
The percentage of metatarsus marrow fat was measured following the protocol described by the CircumArctic Rangifer Monitoring and Assessment (CARMA) Network (2008), except marrow samples were dried at room temperature instead of in an oven (CARMA, 2008). The percentage of fat was calculated as (dry weight/fresh weight) × 100. We used the percentage of metatarsus marrow fat as a metric of body condition, along with the back fat thickness and the subjective body condition assessment by the hunter.
Lower jaw analysis
Lower jaws were assessed for incisor breakage (both permanent and deciduous). The incisor breakage score was calculated as number of incisors broken/number of incisors examined.
Statistical analyses
The effects of the different intrinsic (sex, age, body condition, jaw health), extrinsic abiotic (season, hair growth year, broad geographical location) and extrinsic biotic (lungworms, GI parasites, bacteria exposure) factors (Table 1) on qiviut cortisol were assessed using a Bayesian approach. This method allows for the joint estimation of missing data values and model parameters and consequently takes into account the uncertainty due to missing data when estimating parameters (see Erler et al., 2016). Back_fat and incisor_breakage were not included in the original modelling procedure as >25% of records for these variables were missing. The effects of these two variables and their corresponding interactions on qiviut cortisol were later assessed using the subset of animals for which they had been recorded by adding them into the final model.
Correlation analyses were performed to check for multicollinearity between the explanatory variables. This was done using Spearman’s rank correlation coefficient (rs) as these variables were not normally distributed (McCrum-Gardner, 2008). Biologically and ecologically plausible two-way interactions were originally identified through discussions among co-authors and based on the current knowledge in the available scientific literature (e.g. Baker et al., 2013). Only those for which the data were fairly balanced across the various levels of the variables were considered. Tested interactions are presented in Table 2. To determine which interactions would be important to keep for model building, we fitted a model with all the main effects, to which we added independently each identified interaction to check the impact on the deviance information criterion (DIC) (Spiegelhalter et al., 2002; Gelman et al., 2004) and whether it had an ‘important’ effect on qiviut cortisol [based on the 95% credible interval (CrI)]. For the purpose of parsimony, we only retained the three interactions in the model that produced the best improvement (i.e. largest reduction) of the DIC when compared with the model including only all the main effects (i.e. age and Ve_lpg, sex and year, year and erysipelothrix_PP), as well as some others that did not substantially improve the DIC, but for which there was suggestive evidence that they had an ‘important’ effect (i.e. location and Up_lpg, sex and season; see Supplementary Table 1). The resulting model, which we term the ‘stage 2’ model, included the five interaction terms and all the main effects, except for brucella_serology because of its absence of effect, low prevalence and unbalanced data (e.g. no positive muskoxen in the west mainland). From that ‘stage 2’ model, we proceeded through manual backward elimination to simplify the model and sequentially removed the variables or group of variables (i.e. GI parasite egg/oocyst counts) that did not appear to have an ‘important’ effect on qiviut cortisol when considering their 95% CrI. With each removal, the impact on the DIC (Supplementary Table 2) and on the parameter posterior estimates of the other variables was checked. As indicated in Spiegelhalter et al. (2002), a difference in DIC of <2 was considered ‘not important’, with variables being removed when their elimination resulted in such an increase. When checking the impact of each variable’s removal on the parameter estimates of the other variables, we observed that the parameter estimates remained relatively stable across all models. An interaction was never included without its main effects also being included in the model.
Table 2.
Biologically and ecologically plausible two-way interactions tested
We considered our missing data as missing at random and consequently the missing data mechanism as ‘ignorable’ (i.e. the value of the variable is independent of the probability of it being missing) (Ma and Chen, 2018). For all model parameters, we assumed a relatively flat normal prior distribution with mean zero and standard deviation of 1000, so that the parameter estimates were unlikely to be influenced by our choice of prior distribution. Additional prior information was included for the random imputation of certain variables: (i) specific geographical location for disease exposure; (ii) specific geographical location and age for lungworm larval counts; (iii) specific geographical location, age and season for GI parasite egg/oocyst counts; and (iv) sex, season, specific geographical location, Brucella serology and lungworm and GI parasite egg/oocyst counts for metatarsus marrow fat, and subsequently the same variables and the marrow fat for the hunter condition assessment. For GI parasite egg/oocyst and lungworm larval counts, all animals with an imputed value of ≤0.1 epg or lpg (the lowest amount of eggs/oocysts or L1 measured above zero) were considered not infected and assigned a count of zero. We assumed that the response variable, qiviut cortisol, followed a gamma distribution as its values were positive and right-skewed, and Up_lpg was rescaled using a square-root (sqrt) transformation for better model fit.
Parallel Markov chain Monte Carlo (MCMC) chains were run with 900 000 iterations per chain, a burn-in period of 10 000 iterations and thinning of 1000. Results reported here were based on two chains, whereas two additional chains were run (four chains in total) for model diagnostics. Marginal posterior distributions and the parameter trace plots were examined to check the convergence of the chains. Mixing of the chains was assessed visually using the MCMC trace plots for each parameter (Supplementary Fig. 4) and the Gelman–Rubin diagnostic, with the potential scale reduction factor and effective sample size adjusted for autocorrelation, can be found in Supplementary Table 3. Posterior median estimates and 95% CrIs are shown in the Results.
All statistical analyses were performed using R version 3.6.1, and rjags version 4-10 and coda version 0.19-4 packages were used for Bayesian data analysis and diagnostics (Plummer et al., 2006; Plummer, 2019; R Core Team, 2019).
Results
When assessing the two-by-two correlations between variables, we found a fairly high correlation only between Up_lpg and Ve_lpg (i.e. rs = 0.69, P < 0.001; all other rs were <0.40). However, we included both Up_lpg and Ve_lpg in the model selection process for research purposes as the two lungworms differ in their life history characteristics (e.g. fecundity, life span), morphology and pathology (Kutz et al., 1999; Verocai et al., 2014; Kafle et al., 2017). The final model obtained after manual backward elimination included sex, season, year, location, marrow_fat, sqrt(Up_lpg), as well as the interactions between location and sqrt(Up_lpg) and between sex and season.
Overall, males had higher qiviut cortisol levels than females and qiviut cortisol levels did not differ between late fall–early winter and mid-winter–late winter when both sexes were considered together (Table 3; Fig. 3). There appeared to be an interaction between sex and season, with males having lower qiviut cortisol levels in mid-winter–late winter than in late fall–early winter, whereas females had similar qiviut cortisol levels during the two seasons (Fig. 4). Metatarsus marrow fat was negatively associated with qiviut cortisol (Fig. 5). Qiviut cortisol levels were lower in 2016 and 2017 than in 2015, but similar in 2015 and 2018, and decreased from the east mainland (Kent Peninsula) to Victoria Island (Lady Franklin Point, Ekaluktutiak and Ulukhaktok) to the west mainland (Kugluktuk) (Table 3; Fig. 3).
Table 3.
Final model parameter posterior estimates (median and 95% CrI). The reference group for each categorical variable is listed first and all coefficients are on the natural-logarithmic scale.
| Variable | Levels | Median | 95%CrI |
|---|---|---|---|
| intercept | 4.337 | (3.534, 5.214) | |
| season | Late fall–early winter Mid-winter–late winter |
–0.034 | - (−0.264, 0.210) |
| year | 2015 | - | - |
| 2016 | −0.298 | (−0.557, −0.041) | |
| 2017 | −0.356 | (−0.580, −0.123) | |
| 2018 | 0.061 | (−0.193, 0.310) | |
| location | East mainland | - | - |
| Victoria Island | −0.404 | (−0.813, 0.003) | |
| West mainland | −0.890 | (−1.313, −0.450) | |
| marrow_fat | −1.098 | (−1.901, −0.404) | |
| sqrt(Up_lpg) | −0.038 | (−0.108, 0.025) | |
| sex | Female | - | - |
| Male | 0.460 | (0.225, 0.716) | |
| sqrt(Up_lpg) and location interaction | Victoria Island | 0.031 | (−0.034, 0.102) |
| West mainland | 0.062 | (−0.002, 0.137) | |
| sex and season interaction | Male, mid-winter–late winter | −0.297 | (−0.628, 0.015) |
Figure 3.
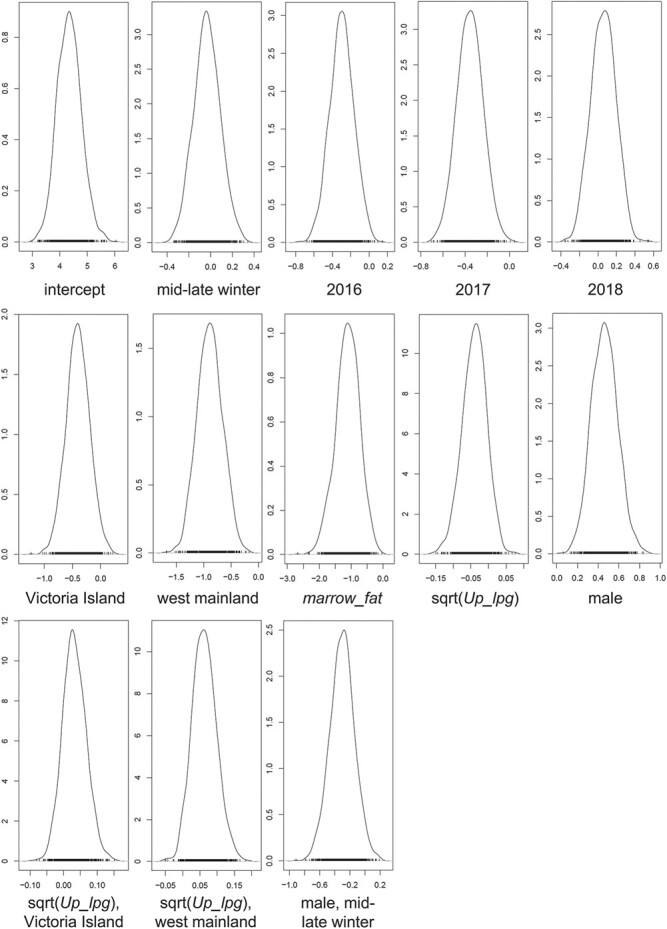
Marginal posterior distribution of the parameters. Y-axes correspond to the density, and X-axes correspond to the parameters. The peak of each distribution corresponds to the most likely parameter estimate; its spread corresponds to uncertainty about the parameter estimate. Positive values of a parameter imply that an increase in its variable is associated with higher qiviut cortisol levels, whereas negative values of a parameter imply that an increase in its variable is associated with lower qiviut cortisol levels. The intercept corresponds to the average qiviut cortisol values of females from the east mainland population in late fall–early winter 2015. All values shown are on the natural-logarithmic scale.
Figure 4.
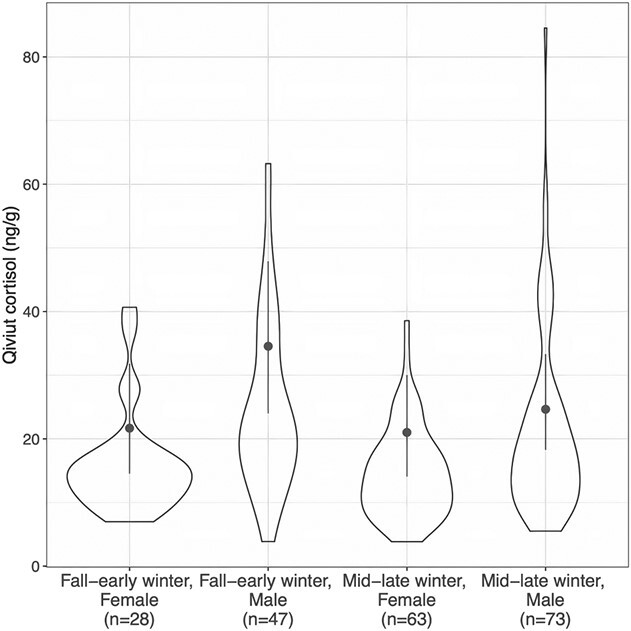
Relative qiviut cortisol levels of male and female muskoxen in fall–early winter and mid-winter–late winter. The violin plots represent the raw data. The dots show the posterior medians (the most likely estimates), and the lines show the 95% credible intervals (the uncertainty about the estimates) for qiviut cortisol by sex and season. All other categorical variables were fixed at the reference group and continuous variables at the median.
Figure 5.
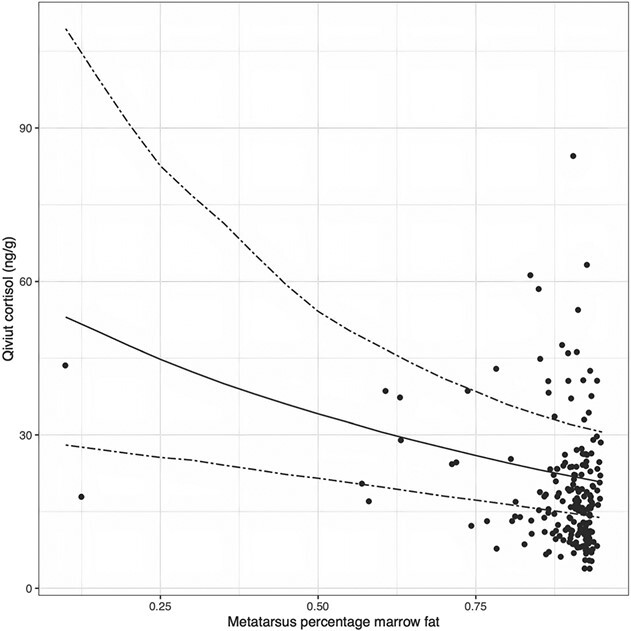
Effect of metatarsus percentage marrow fat on qiviut cortisol levels. The scatterplot shows the raw data. The solid line shows the posterior medians (the most likely estimates), and the dashed lines show the 95% credible intervals (the uncertainty about the estimates) for qiviut cortisol by metatarsus percentage marrow fat. All other categorical variables were fixed at the reference group and continuous variables at the median.
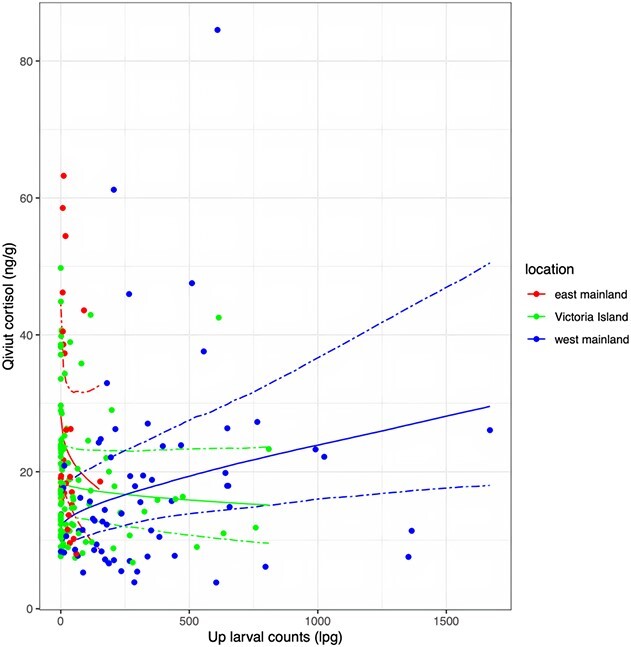
There appeared to be an interaction between Up_lpg and location. Qiviut cortisol levels tended to increase as Up larval counts increased on the west mainland, while they tended to decrease as Up larval counts increased on the east mainland, and no association was detected between qiviut cortisol and Up larval counts on Victoria Island (Table 3; Fig. 6). Umingmakstrongylus pallikuukensis abundance differed among these locations. Prevalence was 100% on the mainland (both east and west), whereas it was 72.4% on Victoria Island. Infection intensity also varied across sites: it was very low on Victoria Island [median (range) = 35.6 lpg (0.2–809.5); n = 76 (n = 29 were negative)] and the east mainland [median (range) = 16.6 lpg (0.2–153.2); n = 28], and much higher on the west mainland [median (range) = 236.5 lpg (0.4–1669.8); n = 67] (Fig. 7).
Figure 6.
Effect of U. pallikuukensis (Up) larval counts on qiviut cortisol levels by location. The scatterplots show the raw data. The solid line shows the posterior medians (the most likely estimates), and the dashed lines show the 95% credible intervals (the uncertainty about the estimates) for qiviut cortisol by location and Up larval counts. All other categorical variables were fixed at the reference group and continuous variables at the median. The x-axis shows the posterior values up to the maximum value of Up larval counts within each location.
Figure 7.
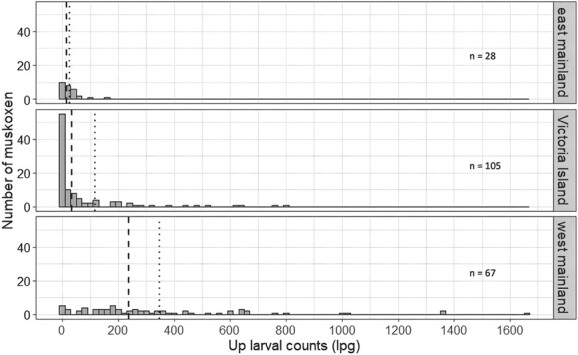
Histogram of U. pallikuukensis (Up) larval counts by location. Dashed lines and dotted lines indicate the median and mean infection intensities, respectively.
When added to the final model, back fat thickness (n = 145), incisor breakage score (n = 152) and their corresponding interactions had no effect on qiviut cortisol (results not shown).
Discussion
We evaluated the associations between hair GCs and multiple measurable intrinsic and extrinsic factors. We identified several important influential factors (i.e. sex, season, qiviut growth year, and geographical location) and relationships between hair GCs and metrics of health (i.e. body condition and pathogen exposure/infection intensity metrics), as well as interactions between geographical location and Up larval counts, and between sex and season.
Qiviut cortisol levels were higher in males than in females, consistent with previous findings in wild muskoxen (Di Francesco et al., 2017). Studies in a variety of wild mammalian species have differing results regarding variations in hair cortisol concentrations (HCCs) between sexes. Multiple studies report no differences in HCCs between sexes (Macbeth et al., 2010; Malcolm et al., 2013; Terwissen et al., 2013; Yamanashi et al., 2013; Carlitz et al., 2014; Caslini et al., 2016; Potratz et al., 2019), whereas others document higher HCCs in males (Lafferty et al., 2015; Schell et al., 2017; Azevedo et al., 2019; Santangeli et al., 2019; Madslien et al., 2020) or higher HCCs in females (Bechshøft et al., 2011; Laudenslager et al., 2012; Cattet et al., 2014; Dettmer et al., 2014; Dulude-de Broin et al., 2019). Variations in HCCs between sexes may reflect sex differences in basal HPA-axis activity or response to stressors (Rhodes and Rubin, 1999; Levine, 2002; Turner et al., 2002), as well as the experience of sex-specific stressors (e.g. rutting activity versus lactation) (Di Francesco et al., 2017; Di Francesco et al., in press). Sex differences in HCCs could also be an artefact of hair growth patterns and fibre morphological characteristics, which may influence cortisol incorporation into hair (Dulude-de Broin et al., 2019). Male muskoxen have coarser qiviut fibres and a higher variability in fibre diameter than females (Rowell et al., 2001) and timing of qiviut shedding as well as onset of qiviut growth differ between sexes (Wilkinson, 1974; Gray, 1987; Robertson, 2000). Increased faecal GC metabolite (FGM) levels in males during the rut are documented for several ungulate species (Mooring et al., 2006; McCoy and Ditchkoff, 2012; Corlatti et al., 2014). Additionally, according to Indigenous knowledge holders, male muskoxen experience high stress during this period (Di Francesco et al., in press). The rut is characterized by reduced nutritional intake and repeated and prolonged aggressive interactions with male counterparts, along with courtship behaviours, which are all highly energetically demanding (Di Francesco et al., in press; Gray, 1987). The lower qiviut cortisol levels of males measured in mid-winter–late winter may reflect that highly stressed individuals are less likely to survive post-rut (which occurs from August to October; see Di Francesco et al., in press), but may also be linked to the lower stress experienced after the rut, which may dilute the qiviut cortisol levels, although lower qiviut growth occurs after September. Female qiviut cortisol levels did not differ between the two seasons. Based on the qiviut growth cycle, approximately April–November, qiviut cortisol levels may have been affected by parturition, lactation and early pregnancy. These reproductive status data were not available, thus we were unable to assess their influence.
Although we did not detect any age differences, this may reflect the limitations of the unbalanced dataset with a low number of juveniles and the coarse scale age classification (i.e. only two categories; Table 1). In studies on white-tailed deer (Odocoileus virginianus) and red deer (Cervus elaphus), HCCs did not vary significantly among fawns, yearlings and adults (>2 years) (Caslini et al., 2016; Potratz et al., 2019), whereas age differences were detected in moose (Alces alces) and Rocky Mountain goats (Oreamnos americanus) (Dulude-de Broin et al., 2019; Madslien et al., 2020). Such differences among age classes have been attributed to variations in HPA axis activity and/or in hair growth and moulting patterns (Azevedo et al., 2019; Dulude-de Broin et al., 2019).
Qiviut cortisol levels varied among geographical locations. They were lowest in the stable muskox population from the west mainland (Kugluktuk, NU MX-09 and west section of NU MX-11), higher in the declining Victoria Island population and highest on the east mainland (Kent Peninsula, east section of NU MX-11, population status unknown) (Leclerc, 2014; Tomaselli et al., 2018b; Mavrot et al., unpubl. data). Differences in qiviut cortisol among geographical locations may reflect variations in biotic and abiotic environmental characteristics. Kugluktuk area is located in a different Bioclimate Subzone from Kent Peninsula and Victoria Island (Walker et al., 2005). Additionally, Kugluktuk IK holders described that Victoria Island has a dryer land, poorer vegetation (both in quantity and quality) and a greater relative increase in predators compared to around Kugluktuk (Di Francesco et al., in press).
Qiviut cortisol varied among years. Inter-annual variations in HCCs were previously detected in muskoxen (different set of data) (Di Francesco et al., 2017) and are described in other mammalian species, such as snowshoe hares (Lepus americanus) and rhesus monkeys (Macaca mulatta), in which higher HCCs were associated with increased predation risk and population density, respectively (Dettmer et al., 2014; Lavergne et al., 2020). Multiple biotic and abiotic factors may contribute to inter-annual differences in qiviut cortisol levels, such as variations in density, intraspecific competition, predator abundance, weather conditions, exposure to pathogens, food availability and abundance, human disturbance, etc. (Di Francesco et al., 2017; Cuyler et al., 2020b; Di Francesco et al., in press). The impact of some of these factors on GC levels has previously been highlighted in other wildlife species [e.g. anthropogenic activities and disturbances (Ewacha et al., 2017; Périquet et al., 2017; Santos et al., 2018), predation risk (Brunton et al., 2020; Lavergne et al., 2020), weather conditions and events (Cizauskas et al., 2015; Fardi et al., 2018)]. These factors could not be included in our analyses but are likely important drivers of physiological stress in muskoxen, which are crucial to understand in the context of accelerating climate change. However, our results indicate that yearly qiviut cortisol levels may serve as a useful tool to monitor the combined effects of these factors on HPA axis activity at a population level.
Qiviut cortisol was inversely associated with marrow fat percentage but not with the other two body condition metrics—hunter assessment and back fat thickness. In ungulates, as an animal’s nutritional status declines, fat stores are generally used sequentially, starting with subcutaneous deposits (back fat), followed by visceral deposits and ultimately by bone marrow fat (Harris, 1945; Bear, 1971). The presence of an association between qiviut cortisol and marrow_fat, but not with back_fat or condition_hunter may attest to some resilience that muskoxen have up to a critical threshold, at which point the marrow fat starts declining and we begin observing an association with qiviut cortisol levels. The course scale for condition_hunter may not have allowed differentiation of animal condition once visible body fat stores were depleted. Alternatively, the sample sizes for these two alternate measures may have been too small to detect an effect in the final model. While the cause and effect relationship remains unclear, multiple studies in wild mammalian species have reported negative associations between body condition metrics and HCCs (Macbeth et al., 2012; Cattet et al., 2014; Mislan et al., 2016; Potratz et al., 2019; Shave et al., 2019) or FGM levels (Cabezas et al., 2007; Pokharel et al., 2017; Wolf et al., 2018). Elevated levels of circulating GCs may cause a reduction in body condition through the mobilization of energy and the inhibition of protein synthesis (Landys et al., 2006; Cabezas et al., 2007; Sapolsky et al., 2000). Alternatively, nutritional stress may lead to reduced body condition and increased levels of circulating GCs as animals must mobilize energy stores (e.g. Jeanniard du Dot et al., 2009; Bryan et al., 2013). The combination of high qiviut cortisol and poor body condition in a muskox may, therefore, reflect not only nutritional stress, but also other long-term stressors (Cattet et al., 2014; Mislan et al., 2016).
The relationship between GC secretion and pathogen infections is highly complex and likely bidirectional, influenced by a multitude of factors related to the host and pathogen (e.g. pathogen type, host sex and age) (reviewed in Defolie et al., 2020). In this study, we only detected associations between Up larval counts and qiviut cortisol. The cross-sectional nature of our study (i.e. observations of individuals occurred at a single time point; Carlsson et al., 2019), the unique life histories of different pathogens and time lags between infection, cortisol incorporation into qiviut and sampling complicate interpretation of results.
The associations between Up larval counts and qiviut cortisol levels varied among locations. Consideration for the dynamic recent history of geographic colonization of this parasite provides some insights into these enigmatic findings. Umingmakstrongylus pallikuukensis is well established and abundant near Kugluktuk and has recently expanded its geographic range northward and eastward in response to rapid Arctic climate warming (Kafle et al., 2020). It was first detected on Victoria Island in 2008 near Lady Franklin Point and near Ekaluktutiak in 2012 (Kutz et al., 2013a); recent range expansion east of Kugluktuk towards Kent Peninsula has also recently occurred, but the parasite remains at lower prevalence and intensity in this area. The positive relationship with qiviut cortisol in Kugluktuk suggests a measurable impact associated with the high intensities of infection with Up in muskoxen in this region. In contrast, absence of a relationship on Victoria Island suggests that the parasite cost may be minimal at low intensities. The apparent inverse association on Kent Peninsula remains enigmatic but, given the small sample size (n = 30) and very low intensities of infection, it is perhaps a spurious relationship. We did not observe a relationship between qiviut cortisol levels and Ve larval counts or lungworm richness. Varestrongylus eleguneniensis was present at a lower prevalence and infection intensity in all three locations and is substantially smaller than Up with presumably less of an impact on muskoxen (Verocai et al., 2014). Two studies investigating the relationship between GC levels and protostrongylid lungworm infections in ungulates found conflicting results. A lungworm removal experiment in bighorn sheep (Ovis canadensis canadensis) showed no difference in FGM levels between the treated and control herds (Goldstein et al., 2005), whereas another study found a positive correlation between lungworm larval output and FGM levels in male Alpine chamois (Rupicapra rupicapra) (Hoby et al., 2006).
We did not find a relationship between GI parasite burdens and qiviut cortisol levels. This is consistent with other studies on naturally infected Artiodactyls measuring HCCs (e.g. Trevisan et al., 2017) or FGM levels (e.g. Cizauskas et al., 2015). The absence of association between qiviut cortisol and GI parasites in our study may be linked to the fairly low infection intensities, but it may also suggest that muskoxen use a tolerance strategy to deal with GI parasites. This has previously been suggested by Carlsson et al. (2016a) for reindeer (Rangifer tarandus tarandus), and Cizauskas et al. (2015) for plains zebras (Equus quagga) and springboks (Antidorcas marsupialis). Tolerance mechanisms generally evolve when virulence is low and rate of transmission is high (Restif and Koella, 2004).
Our opportunistic sampling design was a limiting factor for assessing the relationship between qiviut cortisol and parasite burden. Many Arctic parasites are highly seasonal (Kutz et al., 2012) and this can result in a temporal mismatch between the parasite burden measured through faecal larval/egg/oocyst counts at time of sampling (late fall/winter) and the parasite burden present during the summer qiviut growth period. This mismatch requires further exploration of the specific life cycle and thermal tolerances of each parasite species. For example, T. boreoarcticus, the dominant GI parasite of muskoxen, is most abundant and pathogenic from late spring to late summer, during peak qiviut growth. It produces eggs mainly throughout the summer and the few eggs produced during winter do not survive freezing, thus the association between this parasite and qiviut cortisol could not be evaluated based on our late fall/winter sampling design (Kutz et al., 2012). Marshallagia marshalli, another common abomasal nematode parasite of muskoxen, is transmitted throughout the year with peak faecal egg counts in winter. Mid-winter–late winter burdens may reflect recent acquisition during a time when the qiviut was no longer growing, and thus effects of the parasite on the HPA axis would not likely be reflected in the qiviut. In contrast, Up and Ve both have long pre-patent (time from exposure to presence of reproductively active adult parasites) and patent periods (lifespan of the adult parasites) and winter infection intensities may be associated with summer stressors that resulted in increased exposure or increased susceptibility to infection (Kutz et al., 1999; Kafle et al., 2017). However, effects of the winter burdens of adult parasites would not be reflected in the qiviut cortisol. For the intestinal protozoan Eimeria spp., high oocyst shedding generally occurs in immediate response to recent stress (Pyziel et al., 2011; Chartier and Paraud, 2012); this also would not be detectable in the qiviut in the absence of growth (Di Francesco et al., 2021). A shorter-term measure of GC levels, such as faecal GC metabolites, would provide complementary information to better unravel the complex relationships between parasite burden and GC levels.
We did not detect an association between qiviut cortisol levels and E. rhusiopathiae or Brucella exposure status. Seropositivity indicates exposure to these two bacteria, but does not provide information regarding the timing of exposure, occurrence of clinical disease or the subsequent possible recovery of the animal. Erysipelothrix rhusiopathiae was associated with acute mortality in muskoxen of the western Canadian Arctic archipelago (Kutz et al., 2015; Forde et al., 2016). While there is evidence of very few carrier animals, no chronic forms have been described in this species (see Forde et al., 2016). Assuming that positive cases of E. rhusiopathiae among our harvested animals are recovered, it is not surprising that we did not detect an association between exposure to this bacterium and qiviut cortisol levels. In contrast, Brucella suis biovar 4, reported increasingly in muskoxen from Victoria Island, is associated with chronic disease manifestations (Tomaselli et al., 2018b, 2019). We would have predicted higher qiviut cortisol levels in Brucella-positive muskoxen as chronic illnesses have previously been associated with increased HCCs in domestic cattle (Bos taurus) (Braun et al., 2017). The absence of association, however, may be due to the animals being exposed to the bacterium after the qiviut had stopped growing and/or to the unbalanced dataset and low prevalence.
The simultaneous infection of muskoxen by multiple pathogen species was common in this study; however, we only investigated associations between qiviut cortisol and single pathogens. Co-infecting pathogens may have synergistic or antagonistic interactions, which modify their impact on the infected host, when compared to single-pathogen infections (Joly and Messier, 2005; Jolles et al., 2008; Thumbi et al., 2013). Identifying and further exploring such interactions among pathogens and their association with qiviut cortisol may elucidate new insights.
We did not detect an association between qiviut cortisol and incisor breakage score. Severe wearing or breakage of incisors may affect browsing and food processing efficiency, leading to nutritional deficiencies (Kojola et al., 1998; Ericsson and Wallin, 2001). However, this effect may have been lower during the summer and fall, when the qiviut is growing, as the vegetation is more abundant and easier to access (Di Francesco et al., in press).
It is important to note that we encountered substantial computational limitations in the number of models we could fit due to the large amount of time necessary to run each MCMC analysis. We consequently chose to use two different stepwise variable selection procedures to decide which variables should be included in the model (i.e. addition of each interaction independently to the model with all the main effects to select the interactions and then backward elimination to simplify the model). However, there are important limitations associated with stepwise methods (e.g. bias in parameter estimation, problem of multiple hypothesis testing and increased type I error rates, etc.), which may have led to the exclusion of variables that were actually associated with qiviut cortisol, while including other variables that did not truly have an effect (Whittingham et al., 2006; Mundry and Nunn, 2009).
This study has highlighted some important limitations and challenges, including issues associated with temporal mismatch between qiviut cortisol and various health determinants (e.g. parasites), which can inform the experimental design of future studies in wildlife endocrinology. Although we found multiple associations between qiviut cortisol levels and extrinsic factors and metrics of health, due to the cross-sectional nature of this study, we were unable to infer causal relationships. Future research should focus on more specifically elucidating the relationship between a particular stressor, the increased cortisol levels it causes and the subsequent effect this has on health and fitness. This could be done through longitudinal studies, by monitoring and resampling marked individuals over several years (Carlsson et al., 2019). Such a long-term study of Rocky Mountain goats has recently demonstrated a link between increased predation risk and breeding suppression through chronically elevated cortisol levels (Dulude-de Broin et al., 2020).
While we mostly focused on evaluating associations between qiviut cortisol levels and measures of health at an individual level, our results also highlighted inter-annual variations and suggested differences in qiviut cortisol among geographical locations that are consistent with the local muskox population trends. Gathering long-term data from additional muskox populations for which population trends, and preferably annual productivity, are known would provide further insights as to whether these associations between population trajectory and qiviut cortisol levels hold true. If increased GC levels are shown to precede population declines, poor productivity or mortality events, these could be used as predictive biomarkers of population health and fitness and inform management and conservation actions pro-actively.
This study was made possible by the fruitful collaborations developed with the communities of Ekaluktutiak, Kugluktuk and Ulukhaktok and the strong participation of local hunters in sample collection. Hunter-based sampling programs can contribute substantially to ecological and wildlife health studies (Carlsson et al., 2016b; Kutz and Tomaselli, 2019; Tomaselli, 2019; Peacock et al., 2020). In particular, for this study, hunter-based sampling allowed for collection of a standardized set of samples, from a large number (n = 219) of animals, across a broad geographic area and over several years. The collection of these samples would not have been logistically, financially nor ethically feasible using conventional scientific approaches.
Finally, in this study, we only included broad extrinsic factors (i.e. year and geographic location) in the analyses, which did not allow us to identify the specific drivers of physiological stress in muskoxen. Muskoxen are likely impacted by multiple factors, many of which are predicted to increase with accelerating climate change (e.g. insect harassment, rain on snow, heat extremes, changing vegetation, air quality, etc.) (see Cuyler et al., 2020b; Di Francesco et al., in press). The use of both scientific knowledge and Indigenous knowledge to develop indicators/metrics for the various potential stressors of muskoxen (e.g. Di Francesco et al., in press; Peacock et al., 2020), such as indexes of insect harassment (some have already been developed for reindeer and caribou; Weladji et al., 2003; Witter et al., 2012) and quantitative and/or qualitative measures of plant abundance and quality, will help to inform and guide future conservation endocrinology studies.
Funding
J.D.F. was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC)-Collaborative Research and Training Experience (CREATE) Host–Parasite Interactions Training Program; the NSERC-CREATE Integrated Training Program in Infectious Diseases, Food Safety and Public Policy; the University of Calgary Faculty of Graduate Studies; and the Morris Animal Foundation [Fellowship Training Grant D18ZO-407]. S.K. was supported by grants from Polar Knowledge Canada [Grant NST-1718-0015], NSERC [Discovery Grant RGPIN/04171-2014 and Northern Supplement RGPNS/316244-2014], ArcticNet, Canada North Outfitting and the Shikar Foundation.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
Acknowledgements
We are highly grateful to all the hunters and biologists who participated in sample collection, as well as the Ekaluktutiak, Kugluktuk and Ulukhaktok Hunters and Trappers Organizations/Committees for their support and guidance. We also thank all the students and staff who helped coordinate the sampling program in the communities or contributed to sample processing and analyses, in particular Russell Akeeagok, Allen Niptanatiak, Terry Milton, Bessie Inuktalik, Denise Okheena, Angie Schneider, Matilde Tomaselli, Christine Gilman, Patricia Medd, Mélanie Meyer, Sanchit Chopra, Tessa Bailey, Akaysha Envik, Marriane Trudeau, Leslie Bottari, Kamala Sapkota, Consuelo Grassi and James Wang. Finally, we thank Stephanie Peacock for the stimulating discussions on the statistical analyses and Shane Black (Canada North Outfitting) and the Governments of NWT and Nunavut for their support.
Supplementary material
Supplementary material is available at Conservation Physiology online.
References
- AMAP (2017) Snow, water, ice and permafrost in the Arctic (SWIPA) 2017.
- Azevedo A, Bailey L, Bandeira V, Dehnhard M, Fonseca C, Sousa L, Jewgenow K (2019) Age, sex and storage time influence hair cortisol levels in a wild mammal population. PLoS One 14: e0221124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MR, Gobush KS, Vynne CH (2013) Review of factors influencing stress hormones in fish and wildlife. J Nat Conserv 21: 309–318. [Google Scholar]
- Bear GD (1971) Seasonal trends in fat levels of pronghorns, Antilocapra americana, in Colorado. J Mammal 52: 583–589. [Google Scholar]
- Bechshøft T, Sonne C, Dietz R, Born EW, Novak MA, Henchey E, Meyer JS (2011) Cortisol levels in hair of East Greenland polar bears. Sci Total Environ 409: 831–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun U, Clavadetscher G, Baumgartner M, Riond B, Binz T (2017) Hair cortisol concentration and adrenal gland weight in healthy and ill cows. Schweiz Arch Tierheilkd 159: 493–495. [DOI] [PubMed] [Google Scholar]
- Brunton EA, Clemente CJ, Burnett SE (2020) Not all urban landscapes are the same: interactions between urban land use and stress in a large herbivorous mammal. Ecol Appl 30: e02055. [DOI] [PubMed] [Google Scholar]
- Bryan HM, Darimont CT, Paquet PC, Wynne-Edwards KE, Smits JEG (2013) Stress and reproductive hormones in grizzly bears reflect nutritional benefits and social consequences of a salmon foraging niche. PLoS One 8: e80537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan HM, Smits JEG, Koren L, Paquet PC, Wynne-Edwards KE, Musiani M (2015) Heavily hunted wolves have higher stress and reproductive steroids than wolves with lower hunting pressure. Funct Ecol 29: 347–356. [Google Scholar]
- Busch DS, Hayward LS (2009) Stress in a conservation context: a discussion of glucocorticoid actions and how levels change with conservation-relevant variables. Biol Conserv 142: 2844–2853. [Google Scholar]
- Cabezas S, Blas J, Marchant TA, Moreno S (2007) Physiological stress levels predict survival probabilities in wild rabbits. Horm Behav 51: 313–320. [DOI] [PubMed] [Google Scholar]
- Carlitz EHD, Kirschbaum C, Stalder T, Schaik CP (2014) Hair as a long-term retrospective cortisol calendar in orang-utans (Pongo spp.): new perspectives for stress monitoring in captive management and conservation. Gen Comp Endocrinol 195: 151–156. [DOI] [PubMed] [Google Scholar]
- Carlsson AM, Dobson A, Kutz SJ (2019) The impact of infectious agents on Rangifer populations. In Tryland M, Kutz SJ, eds, Reindeer and Caribou: Health and Disease, Ed1st. CRC Press, Taylor & Francis Group, Boca Raton, USA, pp. 315–352. [Google Scholar]
- Carlsson AM, Mastromonaco G, Vandervalk E, Kutz S (2016a) Parasites, stress and reindeer: infection with abomasal nematodes is not associated with elevated glucocorticoid levels in hair or faeces. Conserv Physiol 4: cow058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson AM, Veitch AM, Popko RA, Behrens S, Kutz SJ (2016b) Monitoring wildlife health for conservation and food security in the Canadian North: a case study from the Sahtu settlement area in the Northwest Territories. In Cork S, Hall D, Karen L, eds, One Health Case Studies: Addressing Complex Problems in a Changing World, Ed1st. 5M Publishing, Essex, UK, pp. 132–151. [Google Scholar]
- CARMA (2008) CircumArctic Rangifer Monitoring and Assessment (CARMA) Network: Rangifer health & body condition monitoring, monitoring protocols level II, p. 54.
- Caslini C, Comin A, Peric T, Prandi A, Pedrotti L, Mattiello S (2016) Use of hair cortisol analysis for comparing population status in wild red deer (Cervus elaphus) living in areas with different characteristics. Eur J Wildl Res 62: 713–723. [Google Scholar]
- Cattet M, Macbeth BJ, Janz DM, Zedrosser A, Swenson JE, Dumond M, Stenhouse GB (2014) Quantifying long-term stress in brown bears with the hair cortisol concentration: a biomarker that may be confounded by rapid changes in response to capture and handling. Conserv Physiol 2: cou026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman CA, Wasserman MD, Gillespie TR, Speirs ML, Lawes MJ, Saj TL, Ziegler TE (2006) Do food availability, parasitism, and stress have synergistic effects on red colobus populations living in forest fragments? Am J Phys Anthropol 131: 525–534. [DOI] [PubMed] [Google Scholar]
- Chartier C, Paraud C (2012) Coccidiosis due to Eimeria in sheep and goats, a review. Small Rumin Res 103: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizauskas CA, Turner WC, Pitts N, Getz WM (2015) Seasonal patterns of hormones, macroparasites, and microparasites in wild African ungulates: the interplay among stress, reproduction, and disease. PLoS One 10: e0120800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corlatti L, Palme R, Lovari S (2014) Physiological response to etho-ecological stressors in male Alpine chamois: timescale matters! Naturwissenschaften 101: 577–586. [DOI] [PubMed] [Google Scholar]
- Curry PS, Elkin BT, Campbell M, Nielsen K, Hutchins W, Ribble C, Kutz SJ (2011) Filter-paper blood samples for ELISA detection of Brucella antibodies in caribou. J Wildl Dis 47: 12–20. [DOI] [PubMed] [Google Scholar]
- Cuyler C, Daniel CJ, Enghoff M, Levermann N, Møller-Lund N, Hansen PN, Damhus D, Danielsen F (2020a) Using local ecological knowledge as evidence to guide management: a community-led harvest calculator for muskoxen in Greenland. Conserv Sci Pract 2: e159. [Google Scholar]
- Cuyler C, Rowell J, Adamczewski J, Anderson M, Blake J, Bretten T, Brodeur V, Campbell M, Checkley SL, Cluff HDet al. (2020b) Muskox status, recent variation, and uncertain future. Ambio 49: 805–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer B, Fletcher QE, Boonstra R, Sheriff MJ (2014) Measures of physiological stress: a transparent or opaque window into the status, management and conservation of species? Conserv Physiol 2: cou023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruyn NP (2010) Gastrointestinal nematodes of western Canadian cervids: molecular diagnostics, faunal baselines and management considerations. Master’s thesis. Department of Biological Sciences, University of Calgary, Calgary, Alberta, Canada.
- Defolie C, Merkling T, Fichtel C (2020) Patterns and variation in the mammal parasite–glucocorticoid relationship. Biol Rev 95: 74–93. [DOI] [PubMed] [Google Scholar]
- Dettmer AM, Novak MA, Meyer JS, Suomi SJ (2014) Population density-dependent hair cortisol concentrations in rhesus monkeys (Macaca mulatta). Psychoneuroendocrinology 42: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Francesco J, Mastromonaco GF, Checkley SL, Blake J, Rowell JE, Kutz S (2021) Qiviut cortisol reflects hypothalamic–pituitary–adrenal axis activity in muskoxen (Ovibos moschatus). Gen Comp Endocrinol 306: 113737. [DOI] [PubMed] [Google Scholar]
- Di Francesco J, Navarro-Gonzalez N, Wynne-Edwards K, Peacock S, Leclerc L-M, Tomaselli M, Davison T, Carlsson A, Kutz S (2017) Qiviut cortisol in muskoxen as a potential tool for informing conservation strategies. Conserv Physiol 5: cox052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs CJ, Boan BV, Lohuis TD, Stewart KM (2018) Investigating relationships between reproduction, immune defenses, and cortisol in Dall sheep. Front Immunol 9: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulude-de Broin F, Côté SD, Whiteside DP, Mastromonaco GF (2019) Faecal metabolites and hair cortisol as biological markers of HPA-axis activity in the Rocky Mountain goat. Gen Comp Endocrinol 280: 147–157. [DOI] [PubMed] [Google Scholar]
- Dulude-de Broin F, Hamel S, Mastromonaco GF, Côté SD (2020) Predation risk and mountain goat reproduction: evidence for stress-induced breeding suppression in a wild ungulate. Funct Ecol 34: 1003–1014. [Google Scholar]
- Egwang TG, Slocombe JO (1982) Evaluation of the Cornell-Wisconsin centrifugal flotation technique for recovering trichostrongylid eggs from bovine feces. Can J Comp Med 46: 133–137. [PMC free article] [PubMed] [Google Scholar]
- Ericsson G, Wallin K (2001) Age-specific moose (Alces alces) mortality in a predator-free environment: evidence for senescence in females. Écoscience 8: 157–163. [Google Scholar]
- Erler NS, Rizopoulos D, Rosmalen J, Jaddoe VWV, Franco OH, Lesaffre EMEH (2016) Dealing with missing covariates in epidemiologic studies: a comparison between multiple imputation and a full Bayesian approach. Stat Med 35: 2955–2974. [DOI] [PubMed] [Google Scholar]
- Ewacha MVA, Roth JD, Anderson WG, Brannen DC, Dupont DLJ (2017) Disturbance and chronic levels of cortisol in boreal woodland caribou. J Wildl Manag 81: 1266–1275. [Google Scholar]
- Fardi S, Sauther Michelle L, Cuozzo FP, Jacky IAY, Bernstein RM (2018) The effect of extreme weather events on hair cortisol and body weight in a wild ring-tailed lemur population (Lemur catta) in southwestern Madagascar. Am J Primatol 80: e22731. [DOI] [PubMed] [Google Scholar]
- Flood PF, Stalker MJ, Rowell JE (1989) The hair follicle density and seasonal shedding cycle of the muskox (Ovibos moschatus). Can J Zool 67: 1143–1147. [Google Scholar]
- Forde TL, Orsel K, Zadoks RN, Biek R, Adams LG, Checkley SL, Davison T, De Buck J, Dumond M, Elkin BTet al. (2016) Bacterial genomics reveal the complex epidemiology of an emerging pathogen in Arctic and boreal ungulates. Front Microbiol 7: 1759. 10.3389/fmicb.2016.01759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester SG, Lankester MW (1997) Extracting protostrongylid nematode larvae from ungulate feces. J Wildl Dis 33: 511–516. [DOI] [PubMed] [Google Scholar]
- Gelman A, Carlin JB, Stern HS, Rubin DB (2004) Chapter 6: Model checking and improvement. In Chatfield C, Tanner M, Zidek J, eds, Bayesian Data Analysis, Ed2nd. Chapman & Hall/CRC, Boca Raton, London, New York, Washington D.C., pp. 157–196. [Google Scholar]
- Goldstein EJ, Millspaugh JJ, Washburn BE, Brundige GC, Raedeke KJ (2005) Relationships among fecal lungworm loads, fecal glucocorticoid metabolites, and lamb recruitment in free-ranging Rocky Mountain bighorn sheep. J Wildl Dis 41: 416–425. [DOI] [PubMed] [Google Scholar]
- Gray DR (1987) The Muskoxen of Polar Bear Pass. Fitzhenry & Whiteside in trust for the National Museum of Natural Sciences, National Museums of Canada, Markham, Canada. [Google Scholar]
- Harris D (1945) Symptoms of malnutrition in deer. J Wildl Manag 9: 319. [Google Scholar]
- Heimbürge S, Kanitz E, Otten W (2019) The use of hair cortisol for the assessment of stress in animals. Gen Comp Endocrinol 270: 10–17. [DOI] [PubMed] [Google Scholar]
- Henrichsen P, Grue H (1980) Age criteria in the muskox (Ovibos moschatus) from Greenland. Dan Rev Game Biol 11. [Google Scholar]
- Hoby S, Schwarzenberger F, Doherr MG, Robert N, Walzer C (2006) Steroid hormone related male biased parasitism in chamois, Rupicapra rupicapra rupicapra. Vet Parasitol 138: 337–348. [DOI] [PubMed] [Google Scholar]
- IPCC (2014) Climate Change 2014: Impacts, Adaptation, and Vulnerability. Contribution of working group II to the fifth assessment report of the Intergovernmental Panel on Climate Change (IPCC). Cambridge University Press, Cambridge, UK. [Google Scholar]
- Irvine RJ, Stien A, Dallas JF, Halvorsen O, Langvatn R, Albon SD (2001) Contrasting regulation of fecundity in two abomasal nematodes of Svalbard reindeer (Rangifer tarandus platyrhynchus). Parasitology 122: 673–681. [DOI] [PubMed] [Google Scholar]
- Jeanniard du Dot T, Rosen DAS, Richmond JP, Kitaysky AS, Zinn SA, Trites AW (2009) Changes in glucocorticoids, IGF-I and thyroid hormones as indicators of nutritional stress and subsequent refeeding in Steller Sea lions (Eumetopias jubatus). Comp Biochem Physiol A Mol Integr Physiol 152: 524–534. [DOI] [PubMed] [Google Scholar]
- Jolles AE, Ezenwa VO, Etienne RS, Turner WC, Olff H (2008) Interactions between macroparasites and microparasites drive infection patterns in free-ranging African buffalo. Ecology 89: 2239–2250. [DOI] [PubMed] [Google Scholar]
- Joly DO, Messier F (2005) The effect of bovine tuberculosis and brucellosis on reproduction and survival of wood bison in Wood Buffalo National Park: effect of disease on bison survival. J Anim Ecol 74: 543–551. [Google Scholar]
- Kafle P, Lejeune M, Verocai GG, Hoberg EP, Kutz SJ (2015) Morphological and morphometric differentiation of dorsal-spined first stage larvae of lungworms (Nematoda: Protostrongylidae) infecting muskoxen (Ovibos moschatus) in the Central Canadian Arctic. Int J Parasitol Parasites Wildl 4: 283–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafle P, Peller P, Massolo A, Hoberg E, Leclerc L-M, Tomaselli M, Kutz S (2020) Range expansion of muskox lungworms track rapid arctic warming: implications for geographic colonization under climate forcing. Sci Rep 10: 17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafle P, Sullivan J, Verocai GG, Kutz SJ (2017) Experimental life-cycle of Varestrongylus eleguneniensis (Nematoda: Protostrongylidae) in a captive reindeer (Rangifer tarandus tarandus) and a muskox (Ovibos moschatus moschatus). J Parasitol 103: 584–587. [DOI] [PubMed] [Google Scholar]
- Kojola I, Helle T, Huhta E, Niva A (1998) Foraging conditions, tooth wear and herbivore body reserves: a study of female reindeer. Oecologia 117: 26–30. [DOI] [PubMed] [Google Scholar]
- Koren L, Whiteside D, Fahlman Å, Ruckstuhl K, Kutz S, Checkley S, Dumond M, Wynne-Edwards K (2012) Cortisol and corticosterone independence in cortisol-dominant wildlife. Gen Comp Endocrinol 177: 113–119. [DOI] [PubMed] [Google Scholar]
- Kutz S, Bollinger T, Branigan M, Checkley S, Davison T, Dumond M, Elkin B, Forde T, Hutchins W, Niptanatiak Aet al. (2015) Erysipelothrix rhusiopathiae associated with recent widespread muskox mortalities in the Canadian Arctic. Can Vet J 56: 560–563. [PMC free article] [PubMed] [Google Scholar]
- Kutz S, Checkley S, Verocai GG, Dumond M, Hoberg EP, Peacock R, Wu JP, Orsel K, Seegers K, Warren ALet al. (2013a) Invasion, establishment, and range expansion of two parasitic nematodes in the Canadian Arctic. Glob Change Biol 19: 3254–3262. [DOI] [PubMed] [Google Scholar]
- Kutz S, Ducrocq J, Cuyler C, Elkin B, Gunn A, Kolpashikov L, Russell D, White RG (2013b) Standardized monitoring of Rangifer health during international polar year. Rangifer 91–114. [Google Scholar]
- Kutz S, Rowell J, Adamczewski J, Gunn A, Cuyler C, Aleuy OA, Austin M, Berger J, Blake J, Bondo Ket al. (2017) Muskox health ecology symposium 2016: gathering to share knowledge on Umingmak in a time of rapid change. Arctic 70: 225. [Google Scholar]
- Kutz S, Tomaselli M (2019) “Two-eyed seeing” supports wildlife health. Science 364: 1135–1137. [DOI] [PubMed] [Google Scholar]
- Kutz SJ, Ducrocq J, Verocai GG, Hoar BM, Colwell DD, Beckmen KB, Polley L, Elkin BT, Hoberg EP (2012) Chapter 2: Parasites in ungulates of Arctic North America and Greenland: a view of contemporary diversity, ecology, and impact in a world under change. Adv Parasitol 79: 99–252. [DOI] [PubMed] [Google Scholar]
- Kutz SJ, Hoberg EP, Polley L (1999) Experimental infections of muskoxen (Ovibos moschatus) and domestic sheep with Umingmakstrongylus pallikuukensis (Nematoda: Protostrongylidae): parasite development, population structure, and pathology. Can J Zool 77: 1562–1572. [Google Scholar]
- Lafferty DJR, Laudenslager ML, Mowat G, Heard D, Belant JL (2015) Sex, diet, and the social environment: factors influencing hair cortisol concentration in free-ranging black bears (Ursus americanus). PLoS One 10: e0141489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landys MM, Ramenofsky M, Wingfield JC (2006) Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen Comp Endocrinol 148: 132–149. [DOI] [PubMed] [Google Scholar]
- Laudenslager ML, Jorgensen MJ, Fairbanks LA (2012) Developmental patterns of hair cortisol in male and female nonhuman primates: lower hair cortisol levels in vervet males emerge at puberty. Psychoneuroendocrinology 37: 1736–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavergne SG, Peers MJL, Mastromonaco G, Majchrzak YN, Nair A, Boutin S, Boonstra R (2020) Hair cortisol as a reliable indicator of stress physiology in the snowshoe hare: influence of body region, sex, season, and predator–prey population dynamics. Gen Comp Endocrinol 294: 113471. [DOI] [PubMed] [Google Scholar]
- Leclerc L-M (2014) Muskox aerial survey (Ovibos moschatus) of the Kitikmeot Region, Nunavut (final report). Department of Environment, Government of Nunavut, Canada. [Google Scholar]
- Levine JE (2002) Editorial: stressing the importance of sex. Endocrinology 143: 4502–4504. [DOI] [PubMed] [Google Scholar]
- Ma Z, Chen G (2018) Bayesian methods for dealing with missing data problems. J Korean Stat Soc 47: 297–313. [Google Scholar]
- Macbeth BJ, Cattet MRL, Obbard ME, Middel K, Janz DM (2012) Evaluation of hair cortisol concentration as a biomarker of long-term stress in free-ranging polar bears. Wildl Soc Bull 36: 747–758. [Google Scholar]
- Macbeth BJ, Cattet MRL, Stenhouse GB, Gibeau ML, Janz DM (2010) Hair cortisol concentration as a noninvasive measure of long-term stress in free-ranging grizzly bears (Ursus arctos): considerations with implications for other wildlife. Can J Zool 88: 935–949. [Google Scholar]
- Madslien K, Stubsjøen SM, Viljugrein H, Ytrehus B, Solberg EJ, Kapronczai L, Mysterud A, Godfroid J, Janz DM, Cattet M (2020) Hair cortisol concentration and body mass in moose (Alces alces) infested with deer keds (Lipoptena cervi). J Wildl Dis. 56: 687–692. [DOI] [PubMed] [Google Scholar]
- Malcolm KD, McShea WJ, Van Deelen TR, Bacon HJ, Liu F, Putman S, Zhu X, Brown JL (2013) Analyses of fecal and hair glucocorticoids to evaluate short- and long-term stress and recovery of Asiatic black bears (Ursus thibetanus) removed from bile farms in China. Gen Comp Endocrinol 185: 97–106. [DOI] [PubMed] [Google Scholar]
- Mavrot F, Orsel K, Hutchins W, Adams LG, Beckmen K, Blake JE, Checkley SL, Davison T, Di Francesco J, Elkin Bet al. (2020) Novel insights into serodiagnosis and epidemiology of Erysipelothrix rhusiopathiae, a newly recognized pathogen in muskoxen (Ovibos moschatus). PLoS One 15: e0231724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy JC, Ditchkoff SS (2012) Patterns of fecal hormones in a fenced population of white-tailed deer. Wildl Soc Bull 36: 641–646. [Google Scholar]
- McCrum-Gardner E (2008) Which is the correct statistical test to use? Br J Oral Maxillofac Surg 46: 38–41. [DOI] [PubMed] [Google Scholar]
- Mislan P, Derocher AE, St. Louis VL, Richardson E, Lunn NJ, Janz DM (2016) Assessing stress in Western Hudson Bay polar bears using hair cortisol concentration as a biomarker. Ecol Indic 71: 47–54. [Google Scholar]
- Mooring MS, Patton ML, Lance VA, Hall BM, Schaad EW, Fetter GA, Fortin SS, McPeak KM (2006) Glucocorticoids of bison bulls in relation to social status. Horm Behav 49: 369–375. [DOI] [PubMed] [Google Scholar]
- Mundry R, Nunn CL (2009) Stepwise model fitting and statistical inference: turning noise into signal pollution. Am Nat 173: 119–123. [DOI] [PubMed] [Google Scholar]
- Nagy JA, Gunn A (2009) Productivity of Peary caribou and muskoxen on Banks and Melville Islands, NT, July 2004 (manuscript report no. 204). Department of Environment and Natural Resources, Government of the Northwest Territories, Canada, p 25.
- Nagy JA, Wright WH, Larter NC (2009) Population estimates for Peary caribou (Minto Inlet herd), Dolphin and Union caribou, and muskox on northwest Victoria Island, NT, July 2001 (manuscript report no. 202). Department of Environment and Natural Resources, Government of the Northwest Territories, Canada, p 36.
- Nielsen K, Kelly L, Gall D, Smith P, Bosse J, Nicoletti P, Kelly W (1994) The use of divalent cation chelating agents (EDTA/EGTA) to reduce non-specific serum protein interaction in enzyme immunoassay. Vet Res Commun 18: 433–437. [DOI] [PubMed] [Google Scholar]
- Nielsen K, Smith P, Yu W, Nicoletti P, Elzer P, Vigliocco A, Silva P, Bermudez R, Renteria T, Moreno Fet al. (2004) Enzyme immunoassay for the diagnosis of brucellosis: chimeric protein A–protein G as a common enzyme labeled detection reagent for sera for different animal species. Vet Microbiol 101: 123–129. [DOI] [PubMed] [Google Scholar]
- Nymo IH, Godfroid J, Åsbakk K, Larsen AK, Neves CG, Rødven R, Tryland M (2013) A protein A/G indirect enzyme-linked immunosorbent assay for the detection of anti-Brucella antibodies in Arctic wildlife. J Vet Diagn Invest 25: 369–375. [DOI] [PubMed] [Google Scholar]
- Peacock SJ, Mavrot F, Tomaselli M, Hanke A, Fenton H, Nathoo R, Aleuy OA, Di Francesco J, Aguilar XF, Jutha Net al. (2020) Linking co-monitoring to co-management: bringing together local, traditional, and scientific knowledge in a wildlife status assessment framework. Arct Sci 6: 247–266. [Google Scholar]
- Périquet S, Richardson P, Cameron EZ, Ganswindt A, Belton L, Loubser E, Dalerum F (2017) Effects of lions on behaviour and endocrine stress in plains zebras. Ethology 123: 667–674. [Google Scholar]
- Plummer M (2019) rjags: Bayesian graphical models using MCMC.
- Plummer M, Best N, Cowles K, Vines K (2006) CODA: convergence diagnosis and output analysis for MCMC. R News 6: 7–11. [Google Scholar]
- Pokharel SS, Seshagiri PB, Sukumar R (2017) Assessment of season-dependent body condition scores in relation to faecal glucocorticoid metabolites in free-ranging Asian elephants. Conserv Physiol 5: cox039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potratz EJ, Brown JS, Gallo T, Anchor C, Santymire RM (2019) Effects of demography and urbanization on stress and body condition in urban white-tailed deer. Urban Ecosyst 22: 807–816. [Google Scholar]
- Prewer E, Kutz S, Leclerc LM, Kyle CJ (2020) Already at the bottom? Demographic declines are unlikely further to undermine genetic diversity of a large Arctic ungulate: muskox, Ovibos moschatus (Artiodactyla: Bovidae). Biol J Linn Soc 129: 459–469. [Google Scholar]
- Pyziel AM, Kowalczyk R, Demiaszkiewicz AW (2011) The annual cycle of shedding Eimeria oocysts by European bison (Bison bonasus) in the Bialowieza Primeval Forest, Poland. J Parasitol 97: 737–739. [DOI] [PubMed] [Google Scholar]
- R Core Team (2019) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Rakotoniaina JH, Kappeler PM, Kaesler E, Hämäläinen AM, Kirschbaum C, Kraus C (2017) Hair cortisol concentrations correlate negatively with survival in a wild primate population. BMC Ecol 17: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restif O, Koella JC (2004) Concurrent evolution of resistance and tolerance to pathogens. Am Nat 164: E90–E102. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Rubin RT (1999) Functional sex differences (‘sexual diergism’) of central nervous system cholinergic systems, vasopressin, and hypothalamic–pituitary–adrenal axis activity in mammals: a selective review. Brain Res Rev 30: 135–152. [DOI] [PubMed] [Google Scholar]
- Ribera d’Alcalà M (2019) Similarities, differences and mechanisms of climate impact on terrestrial vs. marine ecosystems. Nat Conserv 34: 505–523. [Google Scholar]
- Robertson MA (2000) Maximizing qiviut growth in muskoxen. Master’s thesis. University of Alaska Fairbanks, Fairbanks, AK, USA.
- Romero LM, Butler LK (2007) Endocrinology of stress. Int J Comp Psychol 20: 89–95. [Google Scholar]
- Rowell JE, Lupton CJ, Robertson MA, Pfeiffer FA, Nagy JA, White RG (2001) Fiber characteristics of qiviut and guard hair from wild muskoxen (Ovibos moschatus). J Anim Sci 79: 1670. [DOI] [PubMed] [Google Scholar]
- Russell E, Koren G, Rieder M, Van Uum S (2012) Hair cortisol as a biological marker of chronic stress: current status, future directions and unanswered questions. Psychoneuroendocrinology 37: 589–601. [DOI] [PubMed] [Google Scholar]
- Salas M, Temple D, Abáigar T, Cuadrado M, Delclaux M, Enseñat C, Almagro V, Martínez-Nevado E, Quevedo MÁ, Carbajal Aet al. (2016) Aggressive behavior and hair cortisol levels in captive Dorcas gazelles (Gazella dorcas) as animal-based welfare indicators. Zoo Biol 35: 467–473. [DOI] [PubMed] [Google Scholar]
- Santangeli A, Wistbacka R, Morosinotto C, Raulo A (2019) Hair cortisol concentration in Siberian flying squirrels is unrelated to landscape and social factors. Sci Nat 106: 29. [DOI] [PubMed] [Google Scholar]
- Santos JPV, Acevedo P, Carvalho J, Queirós J, Villamuelas M, Fonseca C, Gortázar C, López-Olvera JR, Vicente J (2018) The importance of intrinsic traits, environment and human activities in modulating stress levels in a wild ungulate. Ecol Indic 89: 706–715. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21: 55–89. [DOI] [PubMed] [Google Scholar]
- Schell CJ, Young JK, Lonsdorf EV, Mateo JM, Santymire RM (2017) Investigation of techniques to measure cortisol and testosterone concentrations in coyote hair. Zoo Biol 36: 220–225. [DOI] [PubMed] [Google Scholar]
- Shave JR, Derocher AE, Cherry SG, Thiemann GW (2019) Chronic stress and body condition of wolf-killed prey in Prince Albert National Park, Saskatchewan. Conserv Physiol 7: coz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheriff MJ, Dantzer B, Delehanty B, Palme R, Boonstra R (2011) Measuring stress in wildlife: techniques for quantifying glucocorticoids. Oecologia 166: 869–887. [DOI] [PubMed] [Google Scholar]
- Spiegelhalter DJ, Best NG, Carlin BP, Linde A (2002) Bayesian measures of model complexity and fit. J R Stat Soc Series B Stat Methodol 64: 583–639. [Google Scholar]
- Taylor M, Coop R, Wall R (eds) (2015) Veterinary Parasitology: Taylor/Veterinary, Ed4th. John Wiley & Sons, Inc., Hoboken, NJ, USA. [Google Scholar]
- Terwissen CV, Mastromonaco GF, Murray DL (2013) Influence of adrenocorticotrophin hormone challenge and external factors (age, sex, and body region) on hair cortisol concentration in Canada lynx (Lynx canadensis). Gen Comp Endocrinol 194: 162–167. [DOI] [PubMed] [Google Scholar]
- Thumbi SM, C Bronsvoort BM, Poole EJ, Kiara H, Toye P, Ndila M, Conradie I, Jennings A, Handel IG, JAW Cet al. (2013) Parasite co-infections show synergistic and antagonistic interactions on growth performance of East African zebu cattle under one year. Parasitology 140: 1789–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tø B, Sonne C, Dietz R, Born EW, Novak MA, Henchey E, Meyer JS (2011) Cortisol levels in hair of East Greenland polar bears. Sci Total Environ 409: 831–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli M (2019) Improved wildlife health and disease surveillance through the combined use of local knowledge and scientific knowledge. PhD dissertation. Faculty of Veterinary Medicine, University of Calgary, Calgary, Alberta, Canada.
- Tomaselli M, Dalton C, Duignan PJ, Kutz S, Meer F, Kafle P, Surujballi O, Turcotte C, Checkley S (2016) Contagious ecthyma, rangiferine brucellosis, and lungworm infection in a muskox (Ovibos moschatus) from the Canadian Arctic, 2014. J Wildl Dis 52: 719–724. [DOI] [PubMed] [Google Scholar]
- Tomaselli M, Elkin B, Kutz S, Harms NJ, Nymo HI, Davison T, Leclerc L-M, Branigan M, Dumond M, Tryland Met al. (2019) A transdisciplinary approach to Brucella in muskoxen of the western Canadian Arctic 1989–2016. Ecohealth 16: 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli M, Gerlach SC, Kutz SJ, Checkley SL, The Community of Iqaluktutiaq (2018a) Iqaluktutiaq voices: local perspectives about the importance of muskoxen, contemporary and traditional use and practices. Arctic 71: 1–14. [Google Scholar]
- Tomaselli M, Kutz S, Gerlach C, Checkley S (2018b) Local knowledge to enhance wildlife population health surveillance: conserving muskoxen and caribou in the Canadian Arctic. Biol Conserv 217: 337–348. [Google Scholar]
- Trevisan C, Montillo M, Prandi A, Mkupasi EM, Ngowi HA, Johansen MV (2017) Hair cortisol and dehydroepiandrosterone concentrations in naturally Taenia solium infected pigs in Tanzania. Gen Comp Endocrinol 246: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A, Canny B, Hobbs R, Bond J, Clarke I, Tilbrook A (2002) Influence of sex and gonadal status of sheep on cortisol secretion in response to ACTH and on cortisol and LH secretion in response to stress: importance of different stressors. J Endocrinol 173: 113–122. [DOI] [PubMed] [Google Scholar]
- Verocai GG, Kutz SJ, Simard M, Hoberg EP (2014) Varestrongylus eleguneniensis sp. n. (Nematoda: Protostrongylidae): a widespread, multi-host lungworm of wild North American ungulates, with an emended diagnosis for the genus and explorations of biogeography. Parasit Vectors 7: 556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DA, Raynolds MK, Daniëls FJA, Einarsson E, Elvebakk A, Gould WA, Katenin AE, Kholod SS, Markon CJ, Melnikov ESet al. (2005) The circumpolar Arctic vegetation map. J Veg Sci 16: 267–282. [Google Scholar]
- Weladji RB, Holand Ø, Almøy T (2003) Use of climatic data to assess the effect of insect harassment on the autumn weight of reindeer (Rangifer tarandus) calves. J Zool 260: 79–85. [Google Scholar]
- Whittingham MJ, Stephens PA, Bradbury RB, Freckleton RP (2006) Why do we still use stepwise modelling in ecology and behaviour? Stepwise modelling in ecology and behaviour. J Anim Ecol 75: 1182–1189. [DOI] [PubMed] [Google Scholar]
- Wilkinson PF (1974) Wool shedding in musk oxen. Biol J Linn Soc 6: 127–141. [Google Scholar]
- Witter LA, Johnson CJ, Croft B, Gunn A, Poirier LM (2012) Gauging climate change effects at local scales: weather-based indices to monitor insect harassment in caribou. Ecol Appl 22: 1838–1851. [DOI] [PubMed] [Google Scholar]
- Wolf TE, Valades GB, Simelane P, Bennett NC, Ganswindt A (2018) The relationship between physical injury, body condition and stress-related hormone concentrations in free-ranging giraffes. Wildl Biol. 2018. [Google Scholar]
- Yamanashi Y, Morimura N, Mori Y, Hayashi M, Suzuki J (2013) Cortisol analysis of hair of captive chimpanzees (Pan troglodytes). Gen Comp Endocrinol 194: 55–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.