Abstract
Background
Despite strong evidence of benefit, uptake of newer glucose-lowering medications that reduce cardiovascular risk has been low. We sought to examine global trends and predictors of use of SGLT2i and GLP-1 RA in patients with type 2 diabetes.
Methods
DISCOVER is a global, prospective, observational study of patients with diabetes enrolled from 2014–16 at initiation of second-line glucose-lowering therapy and followed for 3 years. We used hierarchical logistic regression to examine factors associated with use of either an SGLT2i or GLP-1 RA at last follow-up and to assess country-level variability.
Results
Among 14,576 patients from 37 countries, 1579 (10.8%) were started on an SGLT2i (1275; 8.7%) or GLP-1 RA (318; 2.2%) at enrollment, increasing to 16.1% at end of follow-up, with large variability across countries (range 0–62.7%). Use was highest in patients treated by cardiologists (26.1%) versus primary care physicians (10.4%), endocrinologists (16.9%), and other specialists (22.0%; p < 0.001). Coronary artery disease (OR 1.29, 95% CI 1.08–1.54) was associated with greater use of SGLT2i or GLP-1 RA while peripheral artery disease (OR 0.73, 95% CI 0.54–1.00) and chronic kidney disease (OR 0.73, 95% CI 0.58–0.94) were associated with lower use (OR 0.73, 95% CI 0.54–1.00). The country-level median odds ratio was 3.48, indicating a very large amount of variability in the use of SGLT2i or GLP-1 RA independent of patient demographic and clinical factors.
Conclusions
Global use of glucose-lowering medications with established cardiovascular benefits has increased over time but remains suboptimal, particularly in sub-groups most likely to benefit. Substantial country-level variability exists independent of patient factors, suggesting structural barriers may limit more widespread use of these medications.
Keywords: Diabetes, Cardiovascular disease, Quality of care
Background
Prior to 2015, neither a strategy of intensive glucose control nor individual glucose-lowering medications had been successful in reducing cardiovascular risk in patients with type 2 diabetes [1]. However, beginning with publication of the EMPA-REG OUTCOME [2] and Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trials [3], multiple trials and observational studies have shown cardiovascular risk reduction with the use of sodium-glucose co-transporter 2 inhibitors (SGLT2i) [4] and glucagonlike peptide-1 receptor agonists (GLP-1 RA) [5]. Despite this strong evidence of benefit, uptake has thus far been poor [6, 7], although notably these prior studies have been mostly cross-sectional studies from the US or Western European countries. We sought to more fully evaluate global trends in the use of these medications and to explore factors associated with differential use, including patient demographics, complications, physician specialty, and country.
Methods
The DISCOVER study is a prospective, observational study of individuals with type 2 diabetes enrolled from 38 countries at initiation of second-line glucose-lowering medication [8, 9]. Consecutive eligible adults were enrolled between December 2014 and June 2016 and followed at 6 months and 1, 2, and 3 years. Exclusions included first-line therapy with an injectable agent or herbal/natural medicine alone, pregnancy, dialysis, or kidney transplant. For this specific analysis, we also excluded patients who were on an SGTL2i as first-line therapy (n = 115). Data from China were excluded (n = 1292) due to regulations on data privacy released during the study. In line with the observational nature of the study, data were recorded according to routine clinical practice. Comorbidities and events were not adjudicated and relied on the judgement of the local investigators. The study protocol was approved by the appropriate clinical research ethics committees in each participating country and by the institutional review board at each site. All participants provided written informed consent.
The primary outcome for this analysis was being treated with an SGLT2i or GLP-1 RA at the last visit for each patient. Use was compared across key comorbidities, country (grouped by geographic region and by gross national income per capita [10]), and physician specialty. Given the large cohort size, unadjusted comparisons were made using standardized differences, where differences of > 10% are considered clinically relevant. We then constructed a hierarchical logistic regression model to examine the association of patient factors with use of SGLT2i or GLP-1 RA at last study visit. Baseline patient factors included were age, sex, diabetes duration, smoking, body mass index, and systolic blood pressure. As diagnoses both at baseline and throughout follow-up could impact the prescription of one of these glucose-lowering medications, coronary artery disease (CAD; including myocardial infarction, coronary revascularization, angina), heart failure, cerebrovascular disease (including stroke, transient ischemic attack, carotid endarterectomy or stenting), peripheral artery disease (PAD; including diabetic foot, amputation), and chronic kidney disease (CKD) were included in the model as time-dependent covariates. Country was included as a random effect to account for patient clustering within countries, and country-level variability independent of patient factors was assessed with a median odds ratio. The median odds ratio estimates the differences in the odds of being on SGLT2i or GLP-1 RA between two patients with identical risk factors from two randomly selected countries. Median odds ratios are always ≥ 1, with higher values indicating increased country-level variability in SGLT2i or GLP-1 RA use independent of patient factors. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, North Carolina), with statistical significance determined by p < 0.05.
Results
Among 14,576 patients with diabetes from 37 countries, mean age was 57.5 ± 12.0 years, 6718 (46.1%) were women, mean diabetes duration was 5.7 ± 5.3 years, and mean HbA1c was 67 ± 7 mmol/mol (8.3 ± 1.7%). At enrollment, 1579 patients (10.8%) were started on an SGLT2i or GLP-1 RA as second-line glucose-lowering treatment (1261 [8.7%] SGLT2i only, 304 [2.1%] GLP-1 RA only, 14 [0.1%] both), which increased over the 3 years of follow-up, such that at last study visit, 2348 patients (16.1%) were on an SGLT2i or GLP-1 RA (1870 [12.8%] SGLT2i only, 376 [2.6%] GLP-1 RA only, 102 [0.7%] both). Patient characteristics of those on versus not on an SGLT2i or GLP-1 RA are shown in Table 1.
Table 1.
Characteristics of patients treated versus not treated with SGLT2i or GLP-1 RA at last follow-up
| SGLT2i or GLP-1 RA | Standardized Difference (%)a | ||
|---|---|---|---|
|
Yes n = 2348 |
No n = 12,228 |
||
| Patient factors at enrollment | |||
| Age (years) | 56.1 ± 11.5 | 57.7 ± 12.1 | 13.8 |
| Female | 42.4% | 46.8% | 8.8 |
| Education level | 26.2 | ||
| Primary or none | 10.1% | 20.0% | |
| Secondary | 51.6% | 49.1% | |
| University | 37.3% | 30.9% | |
| Current smoker | 18.0% | 12.5% | 21.5 |
| Body mass index (kg/m2) | 31.8 ± 6.5 | 28.9 ± 5.8 | 47.0 |
| Duration of diabetes | 5.6 ± 5.0 | 5.8 ± 5.3 | 3.9 |
| HbA1c (%) | 8.3 ± 1.6 | 8.3 ± 1.7 | 2.6 |
| Creatinine (mg/dL) | 0.9 ± 1.0 | 1.0 ± 1.0 | 5.1 |
| Specialty of Main Investigator | 28.3 | ||
| Primary care | 20.2% | 31.4% | |
| Endocrinology | 74.0% | 65.5% | |
| Cardiology | 4.7% | 2.4% | |
| Other | 1.1% | 0.7% | |
| Region | 60.3 | ||
| Africa | 0.6% | 6.5% | |
| Americas | 18.1% | 12.8% | |
| Europe | 34.1% | 21.8% | |
| Middle East | 11.7% | 15.5% | |
| Southeast Asia | 10.3% | 25.4% | |
| Western Pacific | 25.2% | 17.9% | |
| Comorbidities at last follow-up | |||
| Coronary artery disease | 14.8% | 10.1% | 14.4 |
| Cerebrovascular disease | 2.9% | 2.9% | 0.2 |
| Heart failure | 7.5% | 4.3% | 13.2 |
| Peripheral artery disease | 3.4% | 3.3% | 0.6 |
| Chronic kidney disease | 6.1% | 5.1% | 4.6 |
a > 10% difference is considered clinically relevant
Use by patient comorbidity, country, and physician specialty
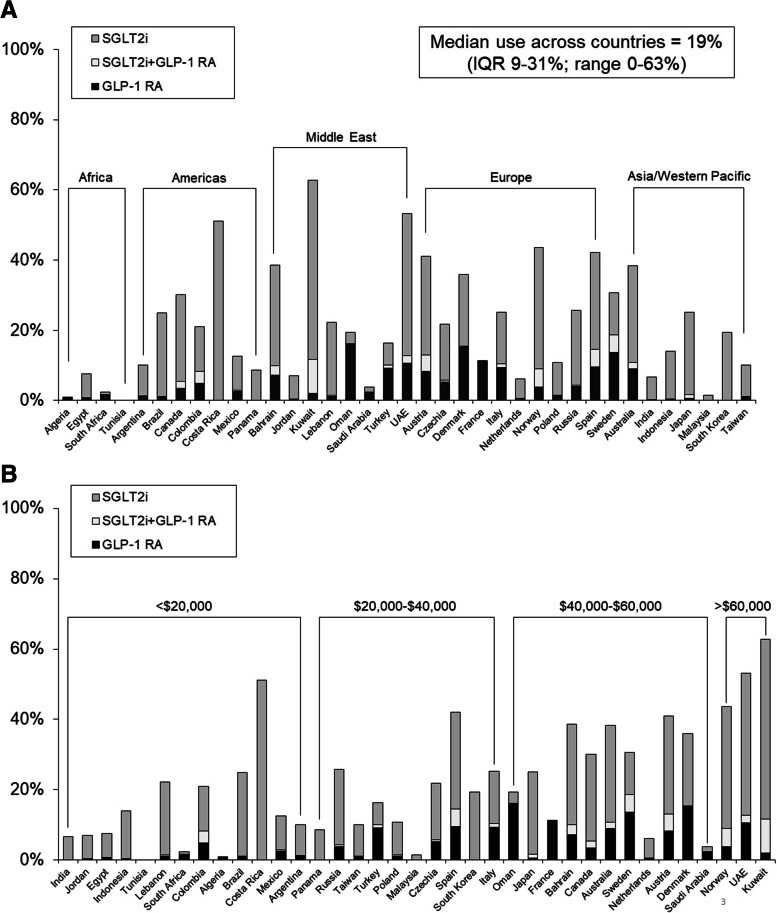
Patients with (vs. without) CAD, heart failure, and CKD were more likely to be on SGLT2i or GLP-1 RA (CAD: 20.0% vs. 13.8%; heart failure: 22.5% vs. 14.1%; CKD: 17.1% vs. 14.4%; all p < 0.001), whereas use was similar in those with vs. without cerebrovascular disease (14.7% vs. 14.5%, p = 0.18) and PAD (14.9% vs. 14.5%, p = 0.11). The median use of either SGLT2i or GLP-1 RA at end of follow-up across the 37 countries was 19.4% (IQR 8.7–30.6%; range 0–62.7%). Countries in Africa and Asia had notably low rates of use (Fig. 1A), and there was a trend toward higher use in countries with greater economic resources (Fig. 1B). Finally, use of SGLT2i and GLP-1 RA in patients treated by primary care physicians (n = 4105) was 10.4% [SGLT2i 7.7%, GLP-1 RA 3.2%]; endocrinologists (n = 9234): 16.9% [SGLT2i 14.7%, GLP-1 RA 2.8%]; cardiologists (n = 380): 26.1% [SGLT2i 25.0%, GLP-1 RA 1.1%]; and other specialists (n = 109): 22.0% [SGLT2i 13.8%, GLP-1 RA 9.2%] (p < 0.001).
Fig.1.
Use of SGLT2 inhibitors and GLP-1 receptor agonists by country. A Grouped by global region; B Ordered by gross national income per capita
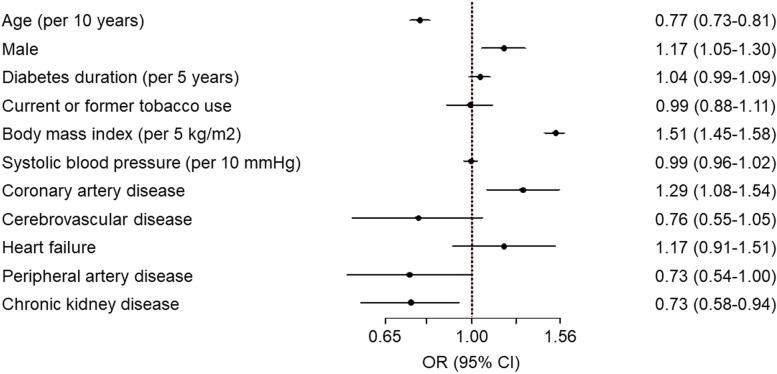
In the hierarchical logistic regression model, younger age (OR 0.77 per 10 year increase, 95% CI 0.73–0.81), male sex (OR 1.17, 95% CI 1.05–1.30), and higher body mass index (OR 1.51 per 5 kg/m2, 95% CI 1.45–1.58) were associated with a greater use of SGLT2i or GLP-1 RA (Fig. 2). In terms of comorbidities/cardiovascular events (both prior to enrollment and during follow-up), CAD (OR 1.29, 95% CI 1.08–1.54) was associated with greater use of SGLT2i or GLP-1 RA while PAD and CKD were associated with lower use (PAD: OR 0.73, 95% CI 0.54–1.00; CKD: OR 0.73, 95% CI 0.58–0.94). The country-level median odds ratio was 3.48, indicating a very large amount of variability in the use of SGLT2i or GLP-1 RA independent of patient demographic and clinical factors.
Fig. 2.
Use of SGLT2 inhibitors or GLP-1 receptor agonists by patient factors according to the hierarchical logistic regression model
Discussion
In a large, multinational, prospective cohort study of patients with type 2 diabetes enrolled at the time of initiation of second-line glucose-lowering medication, we found that use of glucose-lowering medications shown to have cardiovascular risk reduction has increased over time but remains suboptimal. The majority of increase was observed in the use of SGLT2i, with particularly high use among cardiologists. Although patients with CAD, heart failure, and CKD were more likely to be on these medications compared with patients without these conditions, after accounting for patient factors and concomitant comorbidities, only CAD was associated with a greater use of either SGLT2i or GLP-1 RA while PAD and CKD were associated with lower use. Finally, we saw a substantial degree of variability in the use of these medications across countries—both unadjusted and after accounting for patient factors and comorbidities—suggesting that structural barriers likely continue to limit broader use of these medications.
Prior studies
Prior studies have shown SGLT2i or GLP-1 RA uptake to be suboptimal, despite a number of trials and observational studies providing evidence of cardiovascular benefit and several position papers and guideline statements encouraging broader use [1, 11]. A recent study of ~ 10,000 patients from 13 countries with diabetes found 22% of patients were on an SGLT2i or GLP-1 RA (15% SGLT2i, 9% GLP-1 RA), with no differences according to the presence or absence of CAD or of cardiovascular disease [7]. We found use to be lower in our study, which likely represents differences in the enrolling countries, as countries in DISCOVER had a broader spectrum of socioeconomic status (e.g., Africa and Central America). The large country-level variability in the use of SGLT2i or GLP-1 RA in DISCOVER illustrates the importance of healthcare policy and access in the use of these medications and exposes the need to target not only individual physicians but structural issues within the healthcare system that could allow physicians to treat patients with the optimal medications. Notably, we found that patients in DISCOVER who had CAD were more likely to be treated with SGLT2i or GLP-1 RA. While more education continues to be needed about the potential benefit of these medications across the spectrum of cardiovascular and kidney disease, it is encouraging to see at least some targeted use of these medication in patients who are most likely to benefit.
Limitations
Although DISCOVER included many lower-income countries that have rarely been studied, the cohort remains under-representative of very poor countries in addition to patients within these countries who did not have access to medical care, both of which would lead to lower use of SGLT2i or GLP-1 RA. In addition, we did not have data on individual access to medications (e.g., medication coverage, socioeconomic status) and could only observe country-level effects.
Conclusion
In a large, multinational, prospective cohort study of patients with type 2 diabetes, use of glucose-lowering medications with cardiovascular risk reduction has increased over time (particularly SGLT2i) but remains suboptimal. While we observed some targeted use of SGLT2i or GLP-1 RAs in patients with CAD, other comorbidities (e.g., heart failure, chronic kidney disease) were not associated with increased use despite the known benefits in these clinical settings. Substantial country-level variability exists—both unadjusted and after accounting for patient factors and comorbidities—suggesting that structural barriers likely continue to limit broader use of these medications.
Acknowledgements
Not applicable.
Local ethics committees
Algeria (Central ethics committee of CHU Beni Messous), Argentina (Comite de Bioetica del Instituto de Investigaciones Clinicas Rosario, Comite de Bioetica en Investigacion de Ciencias de la Salud, Comite de Docencia e Investigacion, Hospital San Martin de Parana, Comite de Etica Dr Carlos Barclay, Comité de Ética y Revisión Institucional Centro Médico Famyl, Comité Independiente de Ética de Investigación en Salud Prof. Dr. Marcelino Rusculleda, Comite Provincial de Bioetica—Ministerio de Salud Provincia de Santa Fe, IEC—Comité Independiente de Ética para Ensayos en Farmacología Clínica (FEFyM), SIPROSA Sistema Provincial de Salud), Australia (Bellberry Human Research Ethics Committees), Austria (BASG—Bundesamt für Sicherheit im Gesundheitswesen, Ethics Committee Royal Medical Services, Bahrain Defence Force), Brazil (CEP da Faculdade de Jaguariúna (CEP-FAJ), CEP da Faculdade de Medicina da Universidade de São Paulo—FMUSP/SP, CEP da Faculdade de Medicina de São José do Rio Preto – FAMERP, CEP da Faculdade de Medicina do ABC/SP, CEP da Irmandade da Santa Casa de Misericórdia de Porto Alegre / RS, CEP da Irmandade da Santa Casa de Misericórdia de São Paulo/ISCMSP, CEP da Pontificia Universidade Catolica de Campinas / PUC Campinas, CEP da Pontifícia Universidade Católica do Paraná—PUC/PR, CEP da Real Benemérita Associaçao Portuguesa de Beneficência—Hospital São Joaquim, CEP da Universidade Caxias do Sul / RS, CEP da Universidade Positivo, CEP do Centro Universitário de Brasília – UNICEUB, CEP do Centro Universitario Franciscano, CEP do Hospital das Clínicas da Universidade Federal de Goiás, CEP do Hospital de Clinicas da Universidade Federal do Parana—HCUFPR / PR, CEP do Hospital e Maternidade Angelina Caron/PR, CEP do Hospital Lifecenter, CEP do Hospital Moinhos de Vento/ RS, CEP do Hospital Pró-Cardíaco/RJ, CEP do Hospital Universitario—CAS/UFJF/MG, CEP do Hospital Universitário Pedro Ernesto, CEP do Hospital Universitario Walter Cantidio—HUWC / CE, CEP do Instituto de Ciência e Tecnologia—UNESP Campus de São José dos Campos, CEP Investiga—Instituto de Pesquisas, CONEP—Comissão Nacional de Ética em Pesquisa, Canada (Advarra’s CIRBI), Czechia (Státní ústav pro kontrolu léčiv (SUKL)), Colombia (Comité de Bioética en Investigación Clínica Farallones, Comité de Estudios Medicos S.A.S CREIMED, Comité de Ética e Investigación Biomédica de la Fundación Valle del Lili, Comité de Ética en Investigación Clínica de la Costa, Comite de Etica en Investigacion del Centro Medico Imbanaco, Comité de Ética en Investigación. Medplus Medicina Prepagada S.A., Comite de Etica en la Investigacion CAIMED, Comite de Investigaciones y Etica en Investigaciones Hospital Pablo Tobon Uribe, Comite Institucional de Etica e Investigacion Clinica, Comité Institucional de Ética en Investigación C.I.E.I CAFAM), Costa Rica (Comité Ético Científico del Instituto Costarricense de Investigaciones Clínicas, Comité Ético Cientifico Universidad Ciencias Médicas), Denmark (No ethics committee approval was needed for the DISCOVER study), Egypt (Ministry of Health & Population Central Directorate for Research and Health Development), France (CPP Sud Ouest et Outre Mer II), India (Clinical Trial Ethics Committee, CLINICOM, Ethics Committee—Apollo Hospitals, Ethics Committee of Diabetes Thyroid Hormone Research Institute, Ethics Committee, Diacon Hospital (Diabetes Care and Research Center), Ethics Committee, Inamdar Multispecialty Hospital, Independent Ethics Committee, BYL Nair Hospital & TN Medical College, Institutional Ethics Committee, BGS Global Hospital, Institutional Ethics Committee Clinical Studies, Institutional Ethics Committee Fortis Hospital, Institutional Ethics Committee of Jothydev's Diabetics and Research centre, Institutional Ethics Committee of Kovai Diabetes Speciality Centre and Hospital, Institutional Ethics Committee of Rama Krishna Mission Seva Prathisthan, Institutional Ethics Committee, Apollo Gleneagles Hospitals, Institutional Ethics Committee, Oyster and Pearl Hospital, Institutional Ethics Committee, Poona Medical Research Foundation pune, Institutional Ethics Committee-The Calcutta Medical Research Institute, Integrity Ethics Committee, League Health Ethics Committee, Manipal University Ethics Committee, Medanta Institutional Ethics Committee, Medisys clinisearch ethical review board, St John’s Medical College & Hospital Institutional Ethics Committee, The Ethics Committee, Dr. V. Seshiah Diabetes Research Institute, Dr. Balaji Diabetes Care Centre, Virtuous Institutional Medical Research Ethics Committee), Indonesia (Ethical Committee of Research Faculty of Medicine Brawijaya, Komisi Etik Penelitian Fakultas Kedokteran Universitas Udayana/RSUP Sanglah Denpas, The Committee of Medical Research Ethics of The Faculty of Medicine University of Indonesia), Italy (AIFA—Agenzia Italiana del Farmaco, CE MI Area 3, Comiitato Etico Unico (CEUR) per la Basilicata, Comitato Etico Area Vasta Nord Ovest, Comitato Etico ASST Sette Laghi, Comitato Etico dell’Area Vasta Emilia Nord (AVEN), Comitato Etico della Azienda Ospedaliera Ospedale Treviglio-Caravaggio di Treviglio, Comitato Etico della Romagna, Comitato Etico dell'Azienda Ospedaliero-Universitaria Ospedali Riuniti di Foggia, Comitato Etico dell'IRCCS Multimedica di Sesto San Giovanni, Comitato Etico Lazio 2, Comitato Etico Milano Area 3, Comitato Etico Referente per l'area di Pavia), Japan (Medical Corporation, Keiaikai, Saga Memorial Hospital, Ethics Review Committee, Local Incorporated Administrative Agency, Saga-ken Medical Centre, Koseikan, Medical Corporation, Shinwakai, Adachi Kyosai Hospital, Institutional Review Committee, "Local Independent Administrative Agency, Osaka Prefectural Hospital Organization,, Osaka General Medical Center, Clinical Trial / Clinical Research Review Committee", Medical Corporation, Sanseikai, Shin-yurigaoka General Hospital, Ethics Committee, Social Medical Corporation, Keigakukai, Ethics Committee, Medical Corporation, Midtown Clinic, Tokyo Midtown Clinic, Institutional Review Committee, Saga University Hospital, Clinical Research Ethics Review Committee, Medical Corporation, Ishiikai, Ishii Hospital, Institutional Review Committee, Imari Arita Kyoritsu Hospital, Ethics Committee, Medical Corporation, Lifestyle Tomonaga Clinic, Institutional Review Committee, Independent administrative agency, Japan Organization of Occupational Health and Safety, Yokohama Rosai Hospital, Institutional Review Committee, Medical Corporation, Shintokai Yokohama Minoru Clinic, Review Committee, Medical Corporation, Medview, Tokyo Chidori Hospital, Institutional Review Committee, Social Medical Corporation, Yuuaikai, Institutional Review Committee, Local Incorporated Administrative Agency, Yamanashi Prefectural Hospital Organization, Yamanashi Prefectural Central Hospital, Clinical Trial / Clinical Research Review Committee, Medical Corporation, Shuuwakai, Shuuwa General Hospital, Ethics Committee, Medical Corporation, Jisenkai, Himorogi Phychiatric Institute, Ethics Committee), Jordan (Institutional Review Board Jordan University of Science and Technology, King Abdulla University Hospital, International Pharmaceutical Research Center, Istishari Hospital institutional review board, Jordan University Hospital institutional review board, Royal medical services institutional review board), Kuwait (Research Affairs/Ethical Review Committee, Dasman Diabetes Institute), Lebanon (American University of Beirut institutional review board, Ethics committee of Mount Lebanon Hospital, Hammoud hospital institutional review board, Institutional review board Rafik Hariri University hospital), Malaysia (Human Research Ethics Committee USM (HREC), Medical Research & Ethics Committee, National Medical Ethics Committee), Mexico (COFEPRIS, Comision de Bioetica del Instituto Nacional de Cardiologia Ignacio Chavez, Comite de Etica de la Fac de Med de la UANL y Hospital Universitario "Dr. Jose Eleuterio Gonzalez", Comite de Etica del Instituto Jalisciense de Investigacion Clinica, Comite de etica en Investigacion de Investigacion Biomedica para el Desarrollo de Farmacos, Comite de Etica en Investigacion de la Unidad de Investigacion en Salud de Chihuahua, S.C., Comité de Ética en Investigación de México Centre for Clinical Research S.A. de C.V., Comite de Etica en Investigacion de Unidad Clinica de Bioequivalencia S. de R.L. de C.V., Comité de Ética en Investigación del Centro de Estudios de Investigación Metabólicos y Cardio. S.C., Comite de Etica en Investigacion del Centro Hospitalario Vicor, SA de C.V. CHG Hospitales, Comite de Etica en Investigacion del Hospital General de Mexico, Comite de Etica en Investigacion del INCMNSZ (Nutricion), Comite de Etica en Investigacion del Instituto del Corazon de Queretaro SA de CV, Comite de Etica en Investigacion del Instituto Jalisciense de Investigacion Clinica, S.A. de C.V.), Netherlands (METC St Elisabeth), Norway (Regional Committees for Medical and Health Research Ethics), Oman (Ethics & Scientific Research Committee and Royal Hospital), Panama (Comite de Bioetica de la Investigacion del Instituto Conmemorativo Gorgas de Estudios de la Salud), Poland (Komisja Bioetyczna przy OIL w Katowicach), Russia (EC at First Clinical Emergency Hospital n.a. E.E.Volosevish, EC at Saint-Petersburg SBHI “City hospital №40 of Resort District”, EC under SEIHPE Novosibirsk State Medical University, Independent Interdisciplinary Committee for Ethical Examination of Clinical Studies, Independent Interdisciplinary Committee for Ethical Examination of Clinical Studies, LEC at FGBU Endocrinology Research Center of Minzdrav of Russia, LEC at FSAEI HE " First Moscow State Medical University n.a. I.M. Sechenov" of the MoH of the RF, LEC at FSBSI “Scientific Research Institute of Therapy and Preventive Medicine”, LEC at LLC "Best Clinical Practice", LEC at NIH "Central Clinical Hospital # 2 n.a.N.A. Semashko of OJSC "Russian Railways", LEC at SBEI HPE "Moscow State Medical and Dentistry University n.a. A. I. Evdokimov" of the MoH, LEC at SBHI «City Clinical Hospital #10 of Kanavinsky District», LEC at SHI of Yaroslavl region “Clinical Hospital №2”, LEC to City Cinical Hospital named after F.I. Inozemtseva, Reg. Endocrinol. Dispensary, SBIH of Moscow "Endocrinilogy Dispensary" of Department of Healthcare of Moscow, SEIHPE "Rostov SMU of MoH of RF", SPB SHI "Pokrovskaya City Hospital", The Independent Multidisciplinary Committee on Ethical Review of Clinical Trials), Saudi Arabia (KFMC IRB (King Fahad Medical City), Khamis Mushayt Armed Forces Hospital Research Ethics Committee, KSUMC-IRB (King Saud University Medical city), IMC-IRB (International Medical Center), SGH-IRB (Saudi German Hospital)), Spain (Agencia Española del Medicamento y Productos Sanitarios (Spain), CEIC Autonomico de Andalucia, CEIC del Area de Segovia, CEIC del Hospital Universitario de Fuenlabrada, CEIC Fundació Gol i Gurina, CEIC Hospital Clínico de Salamanca, CEIC Hospital Uiversitario Virgen del Rocio, CEIC Hospital Universitario Central de Asturias, CEIC Hospital Universitario de Elche, CEIC Hospital Universitario Puerta del Mar, CEIC Hospital Universitario Ramon y Cajal, CEIC Hospital Universitario Virgen Macarena, CEIC Hospital Virgen de la Arrixaca, Fundacion Jimenez Diaz, Hospital Universitari i Politecnic La Fe, Hospital Universitario de La Princesa, RA Asturias, RA Castilla y Leon, RA Cataluna, RA Comunidad Valenciana, RA Madrid, RA Murcia), South Africa (Pharma Ethics Research Ethics Committee, Stellenbosch University Health Research Ethics Committee, University of Cape Town Research Ethics Committee), South Korea (IRB of Bundang Jesaeng hospital, IRB of Chung-Ang University Hospital, IRB of Eulji University Hospital, IRB of Gangnam Severance Hospital, Yonsei University Health System, IRB of Gwangmyung Seongae hospital, IRB of Hyewon Medical Foundation SeJong General Hospital, IRB of Jeju National University Hospital, IRB of Kangwon National University Hospital, IRB of Myongji Hospital, IRB of Seoul National University Bundang Hospital, IRB of Severance Hospital, Yonsei University Health System, IRB of SoonChunHyang University Hospital Cheonan, IRB of Wonkwang University Hospital), Sweden (Regionala etikprövningsnämnden i Stockholm), Taiwan (Chang Gung Medical Foundation, Institutional Review Board, Changhua Christian Hospital Institutional Review Board, Cheng Hsin General Hospital, Institutional Review Board, Chi Mei Medical Center, Institutional Review Board, Chia-Yi Christian Hospital Institutional Review Board, China Medical University Hospital, Institutional Review Board, Chung Shan Medical University Hospital, Institutional Review Board, Institutional Review Board of the Cathay General Hospital, Mackay Memorial Hospital, Institutional Review Board, National Cheng Kung University Hospital, Institutional Review Board, National Taiwan University Hospital, Research Ethics Committee, Research Ethics Review Committee Far Estern Memorial Hospital, Taichung Veterans General Hospital, Institutional Review Board, Taipei Veterans General Hospital, Institutional Review Board, Tri-Service General Hospital, Institutional Review Board), Tunisia (Comité d’éthique de l’Institut National de Nutrition de Tunis (LEC), Comité de Protection des Personnes Nord de l'institut Pasteur de Tunis (CPP-N) (CEC)), Turkey (Erciyes University Medical Faculty Ethics committee), United Arab Emirates (Dubai Health Authority, IRB Sheikh Khalifa Medical City).
Abbreviations
- SGLT2i
Sodium-glucose co-transporter 2 inhibitors
- GLP-1 RA
Glucagonlike peptide-1 receptor agonists
- CAD
Coronary artery disease
- PAD
Peripheral artery disease
- CKD
Chronic kidney disease
Authors’ contributions
Conception and design of the work: SVA, FT, HC, MK; Acquisition of data: AC, HC, MBG, WR, IS, JV, HW, KK, MK; Data analysis: SVA, FT; Interpretation of data: SVA, FT, HC, MK; Drafted the work: SVA; Made critical revisions to the work: FT, AC, HC, MBG, WR, IS, JV, HW, KK, MK. The author(s) read and approved the final manuscript.
Funding
The DISCOVER study program is funded by AstraZeneca. Coauthors from AstraZeneca reviewed and edited the manuscript for intellectual content; however, the authors retained full control over decision to submit the manuscript for publication.
Availability of data and materials
The data that support the findings of this study are available from AstraZeneca. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of AstraZeneca.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the appropriate clinical research ethics committees in each participating country (listed below) and by the institutional review board at each site. All participants provided written informed consent. All methods were performed in accordance with STROBE guidelines for reporting of observational studies.
Consent for publication
NA
Competing interests
AC, HC: Employees of AstraZeneca. MBG: Support from AstraZeneca to attend DISCOVER planning meetings; honoraria from Merck-Serono. WR: Research support from Novo Nordisk. IS: Honoraria from Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Kowa, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Novo Nordisk, Ono Pharmaceutical, Sanwa Kagaku Kenkyusho and Takeda Pharmaceutical, and research support from Astellas Pharma, AstraZeneca, Daiichi Sankyo, Eli Lilly, Japan Foundation for Applied Enzymology, Japan Science and Technology Agency, Kowa, Kyowa Hakko Kirin, Midori Health Management Center, Mitsubishi Tanabe Pharma, Novo Nordisk, Ono Pharmaceutical, Sanofi, Suzuken Memorial Foundation and Takeda Pharmaceutical. JV: Former employee of AstraZeneca. HW: Support from AstraZeneca to attend DISCOVER planning meetings; honoraria from Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Sumitomo Dainippon Pharma, Eli Lilly, Kissei Pharmaceutical, Kowa Pharmaceuticals America Inc., Kyowa Hakko Kirin, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Novartis, Novo Nordisk, Ono Pharmaceutical, Sanofi, Sanwa Kagaku Kenkyusho and Takeda; research support from Abbott, Astellas Pharma, AstraZeneca, Bayer, Benefit One Health Care, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Dainippon Sumitomo Pharma, Eli Lilly, Johnson & Johnson, Kissei Pharmaceutical, Kowa Pharmaceuticals America Inc., Kyowa Hakko Kirin, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Nitto Boseki, Novartis, Novo Nordisk, Ono Pharmaceutical, Pfizer, Sanofi, Sanwa Kagaku Kenkyusho, Taisho Toyama Pharmaceutical, Takeda and Terumo Corp. KK Investigator-initiated studies for Astra Zeneca, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly and Merck Sharp & Dohme, Boehringer Ingelheim, Bayer, Berlin-Chemie AG / Menarini Group, Janssen, and Napp. MK: Support from AstraZeneca to attend DISCOVER planning meetings; honoraria from Amgen, Applied Therapeutics AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Merck (Diabetes), Novo Nordisk, Sanofi and Vifor Pharma.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arnold SV, Bhatt DL, Barsness GW, Beatty AL, Deedwania PC, Inzucchi SE, Kosiborod M, Leiter LA, Lipska KJ, Newman JD, et al. Clinical management of stable coronary artery disease in patients with Type 2 Diabetes Mellitus: a scientific statement from the American heart association. Circulation. 2020;141(19):e779–e806. doi: 10.1161/CIR.0000000000000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 3.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, et al. Liraglutide and cardiovascular outcomes in Type 2 Diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, Pratley R, Greenberg M, Wang S, Huyck S, et al. Association of sglt2 inhibitors with cardiovascular and Kidney outcomes in patients with Type 2 Diabetes: a meta-analysis. JAMA Cardiol. 2021;6(2):148–158. doi: 10.1001/jamacardio.2020.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kristensen SL, Rorth R, Jhund PS, Docherty KF, Sattar N, Preiss D, Kober L, Petrie MC, McMurray JJV. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–785. doi: 10.1016/S2213-8587(19)30249-9. [DOI] [PubMed] [Google Scholar]
- 6.Arnold SV, Inzucchi SE, Tang F, McGuire DK, Mehta SN, Maddox TM, Goyal A, Sperling LS, Einhorn D, Wong ND, et al. Real-world use and modeled impact of glucose-lowering therapies evaluated in recent cardiovascular outcomes trials: an NCDR(R) Research to Practice project. Eur J Prev Cardiol. 2017;24(15):1637–1645. doi: 10.1177/2047487317729252. [DOI] [PubMed] [Google Scholar]
- 7.Vencio S, Alguwaihes A, Arenas JL, Bayram F, Darmon P, Dieuzeide G, Hettiarachchige N, Hong T, Kaltoft MS, Lengyel C, et al. Contemporary use of diabetes medications with a cardiovascular indication in adults with type 2 diabetes: a secondary analysis of the multinational CAPTURE study. 56th EASD Annual Meeting of the European Association for the Study of Diabetes. Diabetologia. 2020;63(1–485):A945. [Google Scholar]
- 8.Khunti K, Ji L, Medina J, Surmont F, Kosiborod M. Type 2 diabetes treatment and outcomes worldwide: A short review of the DISCOVER study programme. Diabetes Obes Metab. 2019;21(11):2349–2353. doi: 10.1111/dom.13817. [DOI] [PubMed] [Google Scholar]
- 9.Ji L, Bonnet F, Charbonnel B, Gomes MB, Kosiborod M, Khunti K, Nicolucci A, Pocock S, Rathmann W, Shestakova MV, et al. Towards an improved global understanding of treatment and outcomes in people with type 2 diabetes: Rationale and methods of the DISCOVER observational study program. J Diabetes Complications. 2017;31(7):1188–1196. doi: 10.1016/j.jdiacomp.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 10.World Bank Country Categorization by GNI/capita. http://databank.worldbank.org/data/download/site-content/OGHIST.xls. Accessed 1 Oct 2019.
- 11.Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, D'Alessio DA, Davies MJ. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. a consensus report by the American Diabetes Association (ADA) and the European association for the study of Diabetes (EASD) Diabetes Care. 2020;43(2):487–493. doi: 10.2337/dci19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from AstraZeneca. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of AstraZeneca.