Abstract
Inferring from patterns observed in biomedical research, ecoimmunological theory predicts that oxidative stress is a ubiquitous physiological cost that contributes to generating variation in immune function between individuals or species. This prediction is, however, often challenged by empirical studies testing the relationship between immune response or infection and oxidative status markers. This points out the importance of combining ecological immunology and oxidative stress ecology to further our understanding of the proximate causes and fitness consequences of individual variation in health, and adaptability to natural and anthropogenic environmental changes. I reviewed evidence and performed phylogenetic meta-analyses of changes in oxidative status markers owing to either injection of an antigen or infection in captive and free-living vertebrates (141 studies, 1262 effect sizes, 97 species). The dataset was dominated by studies on fish, birds and mammals, which provided 95.8% of effect sizes. Both antigen injection and parasite exposure were associated with changes of oxidative status. There were significant effects of taxonomic class and experimental environment (captivity vs. wild). In contrast with my predictions, age category (young vs. adult), study design (correlational vs. experimental) and proxies of pace of life (clutch size, litter size, and body mass; for birds and mammals only) were negligible in this dataset. Several methodological aspects (type of immunostimulant, laboratory assay, tissue analysed) showed significant effects on both strength and direction of effect. My results suggest that alterations of oxidative status are a widespread consequence of immune function across vertebrates. However, this work also identified heterogeneity in strength and direction of effect sizes, which suggests that immune function does not necessarily result in oxidative stress. Finally, this work identifies methodological caveats that might be relevant for the interpretation and comparability of results and for the application in conservation programs.
Keywords: wildlife diseases, oxidative stress, life-history trade-offs, inflammation, ecoimmunology, antioxidant
Introduction
Immunological function plays a crucial role in the maintenance of physiological homeostasis of living organisms, recognizing and protecting against infectious agents. Parasites and pathogens can have detrimental effects on survival and Darwinian fitness in animals. Infections are often associated with reductions in host energetic stores, harm to tissues, changes in behaviour, or impaired reproduction (e.g. Moore, 2002; Graham et al., 2011; Watson, 2013; Asghar et al., 2015; Sánchez et al., 2018). Consequently, while individuals with well-functioning immune responses should be maintained by natural selection, poorly functioning ones should be counterselected. Thus, why is there large variation among individuals in immunological function? One answer to this question might lie with the factors that shape immune function, and the costs and benefits of an immune reaction, which in turn influence host–parasite relationships, disease outcomes and success of conservation actions (Mangalmurti and Hunter, 2020; Martin et al., 2011; Ohmer et al., 2021; Sheldon and Verhulst, 1996; Sorci et al., 2013; Sorci et al., 2017). For example, several authors proposed that physiological costs associated with an immune response might favour the maintenance of immunological variation because these costs are likely to vary with individual condition, environment, sex, age and high diversity of pathogens the hosts are exposed to (e.g. Klasing, 2004; Maizels and Nussey, 2013; Martin et al., 2008; Seppälä and Jokela, 2010; Sheldon and Verhulst, 1996).
Immune responses require various kinds of resources, which can lead to trade-offs with other physiological processes. Estimated energetic costs (percentage of increment in resting metabolic rate as compared to controls) of mounting an immune response during vaccination or sepsis may vary from 10% to 57% in humans or laboratory rodents (Lochmiller and Deerenberg, 2000). In birds, resting metabolic rates of several species increased from 5% to 15% following activation of humoral and cell-mediated immunities (Hasselquist and Nilsson, 2012). Apart from the dietary energy supply, nutritional modulation of the immune system is also reliant on the typology of micronutrients and macronutrients that are available. Growing evidence suggests that shortage of certain nutrients may impair immune functions, including reduced resistance to infections (e.g. Calder and Jackson, 2000; Gombart et al., 2020; Maggini et al., 2018; Wu et al., 2019).
Immune responses can also have other physiological effects. A hypothesis of immune-oxidative ecology states that perturbations of cellular oxidative status following an immune response might be costly for the organism (reviewed in Costantini, 2014). These perturbations, generally referred to as oxidative stress, may manifest as oxidative damage to biomolecules, oxidation of the key cellular antioxidant glutathione or changes in concentrations of antioxidant enzymes. It is increasingly recognized that oxidative stress might affect the risk of chronic diseases, cell senescence, fertility or longevity (reviewed in Costantini, 2014, 2019). Several lines of evidence provide strong support for a mechanistic link between immune response (including inflammation) and oxidative stress (Sorci and Faivre, 2009). For example, immune cells produce pro-oxidant chemicals (oxidative burst) to kill invading microorganisms (Sorci and Faivre, 2009). Pathogens with impaired antioxidant defences are more sensitive to phagocytic killing mediated by reactive oxygen species, supporting a role for pro-oxidant chemicals as microbicidal agents in living organisms (Halliwell and Gutteridge, 2015). Also, studies on knock-out mice (lacking a functional NADPH phagocyte oxidase or iNOS) suggested that individuals incapable of producing pro-oxidants are susceptible to severe bacterial or fungal infections (e.g. Mastroeni et al., 2000; Nathan and Shiloh, 2000). Beyond oxidative burst, immune response also raises oxygen consumption of cells (Hasselquist and Nilsson, 2012; Lochmiller and Deerenberg, 2000), which in turn might contribute to an increase in the generation of pro-oxidants.
Pro-oxidants produced by immune cells do not specifically target pathogens, so that they can also cause oxidative modifications to biomolecules (e.g. lipids, proteins, DNA), resulting in mutation, inflammation and tissue injury (Sorci and Faivre, 2009). Immune cells can themselves be damaged by their own pro-oxidants. For example, neutrophils with impaired glutathione reductase activity are more rapidly inactivated during phagocytosis than normal neutrophils (Halliwell and Gutteridge, 2015). Thus, hosts may experience trade-offs resulting from altered investment in immune function in relation to the need of regulating their oxidative status. However, empirical studies show that relationships between oxidative status markers, immunological reactions or infections can vary in strength and direction. For example, studies on bats and birds show large within- and among-species variation in the effects of immune response on oxidative status (e.g. Costantini and Møller, 2009; Cram et al., 2015; Fritze et al., 2019; Norte et al., 2018; Schneeberger et al., 2013; Sepp et al., 2012).
In this article, I reviewed evidence and used a phylogenetic meta-analytical approach to evaluate strength and direction of changes in oxidative status markers following immune response owing to antigen injection (any substance that is capable of stimulating an immune response; https://www.britannica.com/science/antigen) or exposure (natural or experimentally induced) to live parasites in vertebrates. I included free-living and captive populations for comparison to gain insight into what extent environmental variation in available resources can affect the physiological consequences of immune response. I expected that effect sizes would be larger for free-living animals than for captive animals because free-living animals might not readily recover from the costs of an immune response owing to stronger resource-based trade-offs. I also compared young (i.e. individuals sampled before sexual maturity) and adult individuals; I expected young individuals to suffer more oxidative stress than adults because they have immature antioxidant mechanisms (e.g. Fontagné et al., 2008; Surai, 2002). I also tested the effects of methodological aspects of study designs on effect sizes to assess the degree to which we need to refine our methods to improve interpretability and comparability of results. Finally, I tested the relationships between effect sizes and traits related to the pace of life in birds and in mammals, such as clutch size, litter size and body mass (e.g. Grainger and Levine, 2021; Sepp et al., 2018). I expected that species with faster paces of life would experience higher costs of immune response because of their higher metabolism and stronger investment in reproduction, which would come at a cost of antioxidant protection.
Materials and methods
Literature search
I performed a literature search (Supplementary Fig. S1) in the Web of Science All Databases for studies published between January 1950 and March 2021 about changes of oxidative status markers in relation to (i) injection of antigens, (ii) experimentally induced infections with live parasites (e.g. injection of a pathogen into the peritoneum) and (iii) comparisons between naturally infected and non-infected individuals in both captive and wild vertebrates, with the exclusion of laboratory mice or highly domesticated taxa because artificial selection might generate phenotypic responses that are not comparable to those observed in wild animals. Relying on the keywords used for the search on online databases, additional searches were carried out through the screening of cited literature in the selected articles from which data were taken. Finally, I have also consulted Google Scholar and PubMed, but I could not find any additional articles. As keywords, I used the following terms: ((immu* OR pathog* OR infect* OR parasit*) AND (antiox* OR oxida*) NOT (clinic* OR hospital OR mouse OR chicken OR human OR industry OR plant* OR agric* OR rat OR patient OR crab OR shrimp OR in vivo OR cell culture OR in vitro OR mutant)). The search was refined by selecting the following categories in WoS, which enabled me to collect only papers that fell into one of these categories: ecology or veterinary sciences or fisheries or marine freshwater biology or evolutionary biology or parasitology or behavioural sciences or environmental sciences or zoology or ornithology or biodiversity conservation. After the completion of this search, I screened all papers applying these following exclusion criteria: (i) studies that measured only the expression of antioxidant genes because I was interested in the biochemical outcomes, such as oxidation products or concentration of given antioxidants, which provide a more direct representation of cellular oxidative status; (ii) studies that only quantified the free radical generation because assays are not specific for a given free radical nor do they allow to infer about oxidative damage (e.g. Jaguezeski et al., 2020; Tobler et al., 2011); and (iii) studies that were not carried out on vertebrates.
I categorized oxidative status markers as follows: (i) oxidation [e.g. protein carbonyls, dROMs (reactive oxygen metabolites), MDA (malondialdehyde), TBARS (thiobarbituric acid reactive substances), GSSG (oxidized glutathione)]; (ii) non-enzymatic antioxidants [e.g. GSH (reduced glutathione), total thiols, assays of antioxidant capacity (OXY)]; and (iii) antioxidant enzymes [e.g. CAT (catalase), GST (glutathione-S-transferase), GPx (glutathione peroxidase), SOD (superoxide dismutase), GR (glutathione reductase)]. I also categorized the oxidative status markers by the typology of laboratory assay used (e.g. TBARS, MDA by HPLC, d-ROMs, KRL, GSH) and by tissue (e.g. blood, brain, liver, muscle; Supplementary Table S1). From each paper, I also extracted information about age category of experimental animals, which included young (before sexual maturity) and adult individuals, species, experimental environment (captivity vs. wild), type of parasite (e.g. worm, virus; Supplementary Table S2), type of antigen injection (e.g. LPS, PHA) and time elapsed from antigen injection or parasite exposure to final tissue sampling. Information on sex was available for a small number of studies, so that it could not be considered in subsequent analyses. I used all these metrics as moderators in the models.
Calculation of effect sizes
I used the compute.es package (del Re, 2013) in R version 4.0.5 to calculate the standardized effect size Hedges’ g and its sampling variance from descriptive statistics (means and standard deviations) and sample sizes or test statistics (e.g. t-values or F ratios). If data were not reported in the text or tables, I extracted them from graphs using the software GetData Graph Digitizer (Fedorov, 2014). When extraction of data was not possible, I contacted first and/or last authors. I made requests for 44 articles; only 7 did not respond, and their articles were excluded from the work. In total, I extracted 1262 effect sizes, of which 1209 were derived from descriptive statistics.
In the statistical analyses, as dependent variables, I used the unsigned and the signed effect sizes in separate models, respectively. Unsigned effect sizes indicate the strength of the difference in a given marker between groups (i.e. the magnitude of the effect). However, unsigned effect sizes do not provide information on the direction of the change, e.g. if oxidative damage increased or decreased in immune-challenged individuals. This is relevant information to make inferences about oxidative stress. Thus, I also used signed effect sizes. To do so, effect sizes were given a (i) positive value when either oxidative damage was higher or a given antioxidant was lower in immunostimulated or infected animals than in control animals and (ii) a negative value when either oxidative damage was lower or a given antioxidant was higher in immunostimulated or infected animals than in control animals. A positive effect size would imply higher biochemical oxidative stress (for a similar approach, see Costantini and Møller, 2009).
Phylogenetic meta-analyses
Analyses were carried out following Nakagawa and Cuthill (2007), Hadfield (2010), Nakagawa and Santos (2012), Garamszegi (2014) and Nakagawa et al. (2022). Note that, as the distribution of unsigned effect sizes is a folded normal distribution (Morryssey, 2016a, 2016b), I did not derive effect sizes because, while contrasts between estimates are reliable because they are placed on the same scale, magnitudes can be inflated. Moreover, for the goals of this work, signed effect sizes were granted as more relevant for ecological and conservation sciences.
First, I ran a phylogenetic meta-analysis in R using the package MCMCglmm to calculate the meta-analytical mean for signed effect sizes for the whole dataset. I also included the sampling variance associated with each effect size inserting the mev argument of the MCMCglmm package. To account for pseudo-replication, I included multiple random factors: species (non-independence of multiple effect sizes obtained from the same species), phylogenetic relatedness, article (non-independence of effect sizes from the same study), tissue where a given marker was analysed (variation in tissues analysed across studies), laboratory assay (variation in assays performed across studies) and type of immunostimulant (injection of an antigen or exposure to a given parasite). The phylogeny used to compute a phylogenetic covariance matrix was obtained from http://www.timetree.org. Eight species were replaced with closely related species in order to build the tree; the exclusion of these species did not affect outcomes (Supplementary Table S4), so that they were retained in the final models. The phylogenetic signal was calculated as lambda (λ) using the package phytools.
Then, I conducted two phylogenetic meta-analyses on the full dataset including the unsigned and the signed effect sizes as response variables, separately. In both models, I included the taxonomic class, the experimental environment (captivity or wild), the age category (young, adult or mix for studies that used a pool of young and adults), the marker category (oxidation, antioxidant enzyme or non-enzymatic antioxidant) and the study design (experimental or non-experimental) as fixed explanatory variables (termed moderators in meta-analysis). Note that studies carried out on captive and wild animals included both non-experimental (correlative) and experimental studies. I also included the sampling variance associated with each effect size using the mev argument. As random factors, I included the same factors as those used to calculate the meta-analytical means.
In a second set of analyses, I focussed on the effects of methodological approaches, which vary largely among studies. I ran meta-analyses on sub-sets of data including the following factors: hours elapsed from immunostimulation to final sampling (included as a covariate) and the random factors (species + phylogeny + article + tissue + assay + type of immunostimulant); hours elapsed from the start of infection to final sampling (included as a covariate) and the random factors (species + phylogeny + article + tissue + assay + type of immunostimulant); type of immunostimulant as fixed factor for the categories with sample sizes >40 and the random factors (species + phylogeny + article + tissue + assay); laboratory assay of oxidative status marker as fixed factor for the categories with sample sizes >40 and the random factors (species + phylogeny + article + tissue + type of immunostimulant); and tissue analysed as fixed factor for the categories with sample sizes >40 and the random factors (species + phylogeny + article + assay + type of immunostimulant). In each of these additional meta-analyses, I always included the sampling variance associated with each effect size using the mev argument.
Finally, I ran two separate meta-analyses for birds and mammals, respectively, to test the effects of proxies of pace of life (clutch size for birds and litter size for mammals; body mass for both birds and mammals; data obtained from https://worldspecies.org/). In each of these two models, I included the experimental environment, the age, the marker and the study design as additional fixed factors, and the sampling variance associated with each effect size using the mev argument. As random factors, I included species, article, laboratory assay, tissue, type of immunostimulant and phylogeny.
I ran all the Markov chain Monte Carlo models for 150 000 iterations, with sampling every 50 iterations with the first 50 000 removed as burn-in (e.g. Sanders, Frago et al., 2021). Then I tested the autocorrelation using the function autocorr.diag: lag values were always <0.1, indicating that autocorrelation between subsequent iterations was negligible. Convergence of the chains was also inspected visually to ensure that there were no trends in the chain and that posterior distributions were not skewed (for full datasets, see Supplementary Figs S2 and S3). Since I did not have any a priori knowledge on the distribution of data, I used flat priors: V = 1 and nu = 0.002. To assess the influence of the informativeness of the priors (nu) on the models, I repeated the analyses with different priors, with no detectable effect on the results. I reported significance as the pMCMC. Effect sizes were considered to be small when g = 0.2 (1% of the variance explained by a factor with a g value of 0.2), intermediate when g = 0.5 (9% of the variance explained by a factor with a g value of 0.5) or large when g = 0.8 (25% of the variance explained by a factor with a g value of 0.8) as described by Cohen (1988) and Møller and Jennions (2002).
Publication bias was assessed by examining funnel plots of effect sizes against the log10 of sample sizes for the full dataset (Møller and Jennions, 2001) and by performing full multi-level meta-regressions including log10 of sample sizes and the effective sample size as described by Nakagawa et al. (2022), respectively. In so doing, the publication bias test is conditioned on all the various sources affecting heterogeneity (i.e. fixed effects and random effects included in the model for the full dataset). Additionally, I tested for the presence of a time trend in signed effect sizes by fitting a continuous year variable in an MCMC model that included the taxonomic class, the experimental environment, the age category, the marker and the study design as fixed explanatory variables; species + phylogeny + article + tissue + assay + type of immunostimulant as random effects; and the sampling variance associated with each effect size using the mev argument.
Results
Dataset description
My final dataset included 141 studies that involved 1262 effect sizes from 97 species (Table 1): 4 amphibians (44 effect sizes), 34 birds (253 effect sizes), 33 fish (790 effect sizes), 22 mammals (166 effect sizes) and 4 reptiles (9 effect sizes). There were 803 and 459 effect sizes from captivity and wild studies, respectively; 766 and 496 from experimental and non-experimental studies, respectively; 631, 247 and 384 from enzymatic antioxidants, non-enzymatic antioxidants and oxidation markers, respectively; 490, 503 and 269 from studies using adults, young and both adults and young (mix), respectively. The time elapsed from the injection of an antigen to final sampling varied from 6 to 1440 hours and was available for 49 studies; the time elapsed from the start of infection to final sampling varied from 6 to 7392 hours and was available for 78 studies.
Table 1.
List of 97 species and 141 articles included in the meta-analysis
Results of full dataset
Meta-analytical mean of signed effect sizes was intermediate and the 95% CI included zero (0.567, −0.160/1.281, PMCMC = 0.099; Fig. 1). Unsigned effect sizes were significantly larger in studies on captive animals than in those on free-living animals and marginally smaller in birds than in fish or mammals (Supplementary Table S3). By contrast, age category, marker category or study design did not explain variation in effect sizes (Supplementary Table S3). The lambda phylogenetic signal was 0.0441 (posterior mode = 0.0015, HPD interval = 0.0001/0.2132). Signed effect sizes were significantly larger for oxidation markers than for enzymatic or non-enzymatic antioxidant markers (Fig. 1; Supplementary Table S3). Taxonomic class, age category, experimental environment and study design did not explain variation in effect sizes. The lambda phylogenetic signal was 0.1377 (posterior mode = 0.0019, HPD interval = 0.00005/0.4018). Results of models for both unsigned and signed effect sizes were unchanged if very large effect sizes (>10, n = 48) were removed from the models (Supplementary Table S5).
Figure 1.
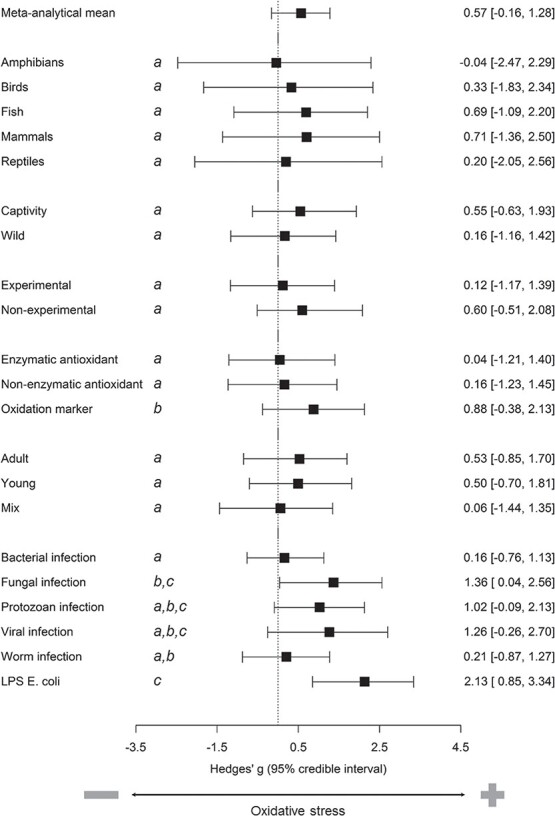
Summary of the evidence for changes in oxidative status markers in individuals challenged with an immunostimulant (antigen or live parasite). Forest plots show predictions of models for the overall meta-analytical mean with 95% credible intervals and for the effects of moderators on this mean. Outcomes of contrasts between levels are shown as letters: shared letters (e.g. both levels with a) indicate that the two levels of a given moderator do not differ significantly. The dataset included 141 studies that involved 1262 effect sizes from 97 species.
Effect of sampling time
Models included 158 and 518 effect sizes for antigen type and for infection with a parasite, respectively. Results show that both unsigned and signed effect sizes were not related to the time elapsed from antigen injection to final sampling nor to the time elapsed since the start of the infection until the final sampling, with or without centering of the covariate (Supplementary Table S6).
Effects of immunostimulant type, laboratory assay and tissue
The most common types of immunostimulant in the dataset (with a number of effect sizes >40) were bacterial infection (428), fungal infection (102), LPS Escherichia coli injection (94), protozoan infection (180), viral infection (59) and worm infection (170), which represented altogether 81.9% of effect sizes. The model testing the effects of immunostimulant type (1033 effect sizes) shows that unsigned effect sizes were similar among these six types of immunostimulant, with the exception of LPS E. coli injection whose effect size was significantly larger than that of worm infection (Supplementary Table S7). Signed effect sizes of LPS E. coli injection and of fungal infection were the only types of immunostimulant to differ significantly from zero (Fig. 1; Supplementary Table S6). Effect sizes of LPS E. coli injection were significantly larger than bacterial infection and worm infection; effect sizes of fungal infection were significantly larger than bacterial infection (Supplementary Table S7).
The model testing the laboratory assay effect (1093 effect sizes) included 10 assays with a number of effect sizes >40, which represented altogether 86.6% of effect sizes: three assays of oxidative damage [dROMs (52), TBARS (218) and protein carbonyls (41)], two non-enzymatic antioxidants [OXY (49), GSH (102)] and five enzymatic antioxidants [SOD (189), GPx (99), catalase (177), GST (87) and GR (79)]. Effect size of (i) protein carbonyls was significantly larger than that of all other assays with the exceptions of GPx and GST, (ii) GST was significantly larger than that of SOD and of OXY and (iii) GPx was significantly larger than that of OXY, SOD and GR (Supplementary Table S8). Signed effect sizes were significantly different from zero for protein carbonyls and TBARS (Fig. 2; Supplementary Table S8). Effect size of (i) protein carbonyls was significantly larger than that of all other assays; (ii) TBARS was significantly larger than that of CAT, GPX, GSH, GST, OXY, SOD and GR; and (iii) GST was significantly smaller than that of CAT, dROMs, GPx, GSH and SOD (Supplementary Table S8).
Figure 2.

Summary of the evidence for effect of laboratory assay of oxidative status markers. Forest plot show predictions of models (mean with 95% credible intervals). Outcomes of contrasts between levels are shown as letters: shared letters (e.g. both levels with a) indicate that the two levels of a given moderator do not differ significantly.
Finally, the model testing the effect of tissue (1183 effect sizes) included blood (499), gills (120), gut (52), kidney (42), liver (294), muscle (132) and spleen (44), which were the most common tissues analysed, representing altogether 93.7% of effect sizes. Unsigned effect size of (i) gut was significantly larger than that of any other tissue, (ii) blood was significantly smaller than liver and muscle and (iii) kidney was significantly smaller than that of liver and muscle (Supplementary Table S9). Signed effect sizes were significantly different from zero for gut and muscle (Fig. 3; Supplementary Table S9). Effect size of (i) gut was significantly larger than that of gills and liver and (ii) muscle was significantly larger than that of blood, gills, liver and kidney (Fig. 3; Supplementary Table S9).
Figure 3.

Summary of the evidence for effect of tissue analysed. Forest plot show predictions of models (mean with 95% credible intervals). Outcomes of contrasts between levels are shown as letters: shared letters (e.g. both levels with a) indicate that the two levels of a given moderator do not differ significantly.
Pace of life
Models testing the effects of pace of life included 253 and 166 effect sizes for birds and mammals, respectively. Results showed that neither clutch size nor body mass were significantly related to both unsigned and signed effects sizes in birds (Supplementary Table S10). Similarly, in mammals, results showed that neither litter size nor body mass were significantly related to both unsigned and signed effect sizes (Supplementary Table S10).
Publication bias
The plot calculated on the full dataset appears in the shape of a ‘funnel’ (Supplementary Fig. S4). A meta-regression model showed that signed effect sizes were not related to the log10 of sample size (estimate/ci.lb/ci.ub: −0.169/−0.864/0.499, pMCMC = 0.623) nor to the effect sample size (estimate/ci.lb/ci.ub: 0.00002/−0.00483/0.00491). There was no time trend in effect sizes for both unsigned (publication year: postmean and 95% CI: −0.006, −0.047/0.036) and signed (publication year: postmean and 95% CI: −0.021, −0.080/0.031) effect sizes. These results are indicative of absence of publication bias.
Discussion
Inferring from the patterns observed in humans and laboratory rodents, ecoimmunology predicts that alterations of the cellular oxidative status are one relevant physiological cost of immune function that might impinge on fitness. The outcomes of these phylogenetic meta-analyses suggest that immune response owing to both antigen injection and exposure to a parasite cause change of oxidative status in vertebrates. These meta-analyses also show that there is substantial variation in the direction and strength of effects across studies. Thus, a change in oxidative status did not necessarily result in strong oxidative stress. These results were robust for variation among studies in laboratory assays used to assess the oxidative status, the tissue analysed or the type of immunostimulant, which were all controlled for in the models.
Immune response caused significant and strong changes of oxidative status markers across all five classes of vertebrates. The directionality of these changes was similar across vertebrates, but it was very variable within each class. These results suggest that immune response did not always necessarily result in increased oxidative damage or decreased antioxidant levels in any taxonomic class. My sample size was not, however, sufficiently high to draw robust conclusions about amphibians and reptiles. The dataset was actually dominated by studies on fish, birds and mammals, which provided 95.8% of effect sizes. This similarity in effect sizes among these three taxonomic classes suggests that endothermy or reproductive mode might not have a strong influence on general patterns of oxidative status variation in immune-challenged vertebrates. A recent meta-analysis found that relationships between infection and metrics of body condition (indicative of energetic status) were generally more strongly negative among endotherms than ectotherms, possibly because warm-blooded animals pay a metabolic cost of body temperature regulation and offer a constant endogenous environment that might favour parasite replication (Sánchez et al., 2018). By contrast, oxidative status might not be strongly linked to these metabolic costs because (i) immune function also affects oxidative status through pathways that are independent from energetic balance (e.g. oxidative burst in leukocytes; Sorci and Faivre, 2009) and (ii) increased metabolic demands caused by immune response do not induce proportional increases in generation of pro-oxidants (e.g. through the uncoupling between mitochondrial energy production and free radical generation; Herrero and Barja, 1997). Costs of immunity, however, can be highly variable depending on the type of infection and the tolerance/resistance of the infected species and show up in different ways (e.g. the emergence of immunopathologies). The results of my meta-analyses actually show heterogeneity in effect sizes, which might be due to some degree to this variation in costs among types of immunostimulant, masking any relationships between proxies of pace of life and effect sizes.
Contrary to my expectations, unsigned effect sizes were significantly larger in studies on captive animals than in those on free-living animals. This result does not confirm the preliminary observations from Costantini and Møller (2009) indicating that the oxidative status homeostasis of free-living birds might be less resistant or resilient to an immune challenge than that of captive birds. This greater sensitivity of captive animals might be attributable to a high investment of resources into the immune response. As compared to wild animals, laboratory animals are not subject to a full complement of natural and sexual selection pressures. For example, food is provided ad libitum and metabolic costs for foraging or mating are kept at minimum. Trade-offs would therefore be less stringent under the conditions of relaxed selection in captivity. As a consequence, captive animals might have invested more resources in the immune response, which came at a cost for the oxidative status homeostasis. On the other hand, captive animals might also experience different parasite loads or suboptimal conditions, such as space constraints that favour social stress, inadequate nutrient intakes and stressful abiotic conditions (e.g. temperature, humidity or light regimes), that might further impair physiological homeostasis. Also, captive and wild co-specific individuals might rely on different immune strategies that could generate heterogeneity in effect sizes. For example, Seeber et al. (2020) found that energetically cheap markers of constitutive innate immunity were lower in captive than in wild zebras, whereas energetically costly markers of the induced innate immunity were more highly expressed in captive zebras. Flies et al. (2015) show that wild hyenas have significantly higher serum antibody concentrations, natural antibodies and autoantibodies than do captive hyenas, but similar bacterial killing capacity of sera between captive and wild hyenas. Thus, captive animals might not be always adequate models to estimate the costs of immune function in wild animals.
This dataset included both correlational and experimental studies. A larger effect in correlational studies would indicate that co-variation between effect sizes and a given moderator might not be causal (e.g. Simons et al., 2012). This aspect of research design was negligible in this dataset. Effect sizes were indeed similar between experimental and correlational studies, indicating that differences in oxidative status between naturally infected and non-infected individuals in observational studies might reflect a cause-effect relationship. This result is not in line with a meta-analysis that found that relationships between infection and metrics of body condition were more strongly negative for experimental (e.g. parasite removal or manipulation of host nutritional state) than for observational studies (Sánchez et al., 2018). As discussed previously, alterations of oxidative status can also be induced by factors (e.g. oxidative burst) that operate independently from energetic status. Thus, relying on a single category of metrics to estimate costs of immune function might not be adequate for capturing the complexity of the multifaceted nature of these physiological effects.
The present meta-analyses also show that young and adult individuals have similar effect sizes. In vertebrates, individuals are born with the innate arm of the immune system, which matures and acquires memory with time, and then declines in old age (reviewed in Georgountzou and Papadopoulos, 2017). Similarly, levels of endogenous antioxidants are low in young and increase from birth to adulthood (e.g. Surai, 2002; Fontagné et al., 2008; reviewed in Costantini, 2014). This result suggests that maturations of immune function and of oxidative status might be synergistically co-programmed and canalized to some degree in order to balance costs and benefits. This comparison between young and adult individuals is, however, limited by the lack of control for individual age (e.g. middle-age adults vs. senescent adults) that might affect senescence of the immune function (Peters et al., 2019) or control of metabolic demands of growth in young individuals, which have a significant effect on oxidative stress (Smith et al., 2016).
This work also identifies methodological caveats that might be relevant for the interpretation and comparability of results. Both the time elapsed from (i) the injection of an antigen to final sampling and (ii) the start of infection with a living parasite to final sampling did not have a significant effect on effect sizes in the present dataset. By contrast, these meta-analyses detected significant effects of the type of immunostimulant, the laboratory assay of oxidative status and the tissue analysed. LPS E. coli injection and fungal infection generated strong effects as compared to other immunostimulants. It is becoming clear that parasites differ in their modes of interaction with the host immune defences, including oxidative burst and antioxidant levels (Paiva and Bozza, 2014). For example, different types of viruses sustain their replication cycle by promoting a pro-oxidant environment in the infected cell, actively interfering with its redox homeostasis and antioxidant defence systems (Camini et al., 2017). By contrast, parasites of the genus Leishmania may inhibit the production of pro-oxidants by phagocytes (Panaro et al., 1998), while helminths can release a number of metabolites with pro-oxidant properties that may result in mutagenic and carcinogenic effects (Brindley et al., 2015). Sampling design might also generate variation in effect sizes among types of immunostimulants. A total of 11 out of 16 LPS studies of which information on sampling was available collected samples for oxidative status analyses within 24 hours from antigen injection. This is because LPS studies aim to test acute inflammatory effects of innate immune response to a simulated bacterial infection. By contrast, sampling in studies that infected the animals experimentally with live parasites was carried out on average many days after parasite injection (e.g. 392 hours for bacterial infection; 1269 hours for worm infection). Thus, while LPS studies provide relevant information about acute effects, they might not be appropriate to infer adequately about long-term effects on oxidative status associated with prolonged infections. Hosts might also detoxify from parasites or start to tolerate them better and have a milder response because the parasite replication is dampened. Dose of antigen injected into the animal and parasite load might be two additional relevant factors that generate variation in effect sizes. I could not test for their effects because of the limited information available. Comparisons of groups of animals that were experimentally injected with different amounts of a given pathogen showed that oxidative damage is not always higher in those animals that were exposed to higher parasite concentrations, possibly because the amount injected weakly reflected the actual individual parasite load (e.g. Adeyemi, 2014; Armour et al., 2020; Sun et al., 2020). On the other hand, several studies suggested that the dose of antigen has an effect on the immune response (e.g. Fehr and Ochsenbein, 2004; Munang'andu et al., 2013; Rothoeft et al., 2003), and might also have a significant effect on the change of oxidative status (Armour et al., 2020).
Second, when investigating the effects of immune response or infection on oxidative status, I found that effect sizes for markers of oxidation were significantly larger than those of non-enzymatic antioxidant or enzymatic antioxidant markers. A closer investigation into the effect of each particular assay suggests that the choice of laboratory assay might affect results of an immunostimulation experiment. Two markers of oxidative damage (protein carbonyls and TBARS) showed a strong increase in immunostimulated individuals, indicating that they might be candidate tools to assess the physiological consequences of infections or the effects of pharmacological treatments on sick animals. By contrast, there was some variation in the direction of the response among non-enzymatic and enzymatic antioxidants. These results suggest that the antioxidant response to immunostimulation might not be easily predictable without consideration of factors that might affect it, such as the individual condition or quality, the phase of the immune response, life-history stage or the quality of diet (e.g. van de Crommenacker et al., 2012; Garcia et al., 2011; Zhao et al., 2019). For example, the up- or down-regulation of antioxidant enzymes might be regulated in such a manner in order to maintain an efficient immune response. The pro-oxidant hydrogen peroxide is produced by immune cells, and it can also be precursor of other compounds, such as the hypochlorous acid, which is a powerful antibacterial and antifungal pro-oxidant (Halliwell and Gutteridge, 2015; Winterbourn, 2002). Thus, a strict regulation of antioxidant enzymes, such as catalase or glutathione peroxidase that both remove hydrogen peroxide from tissues, might be important to not compromise the activity of immune cells in the initial stages of an immune response.
A third caveat of this study is that effect sizes differed among tissues, and the stronger effects were detected for gut and muscle. This result is not surprising because it is widely accepted that tissues differ in sensitivity to oxidative stress or antioxidant expression (Halliwell and Gutteridge, 2015). The adaptive meaning of this tissue effect is not understood yet. It might be that animals strategically displace oxidative costs on those tissues that have a lower impact on fitness under particular circumstances. This hypothesis has been proposed several times to explain why the effects of reproductive investment or of migratory status on the oxidative status vary among tissues (e.g. Marasco et al., 2021; Yang et al., 2013). This hypothesis has not received, however, any formal investigation so far.
Another caveat of this study is that individuals facing with an experimental immunostimulation or an infection might suppress other functions (e.g. metabolic rate, foraging activity or reproduction) that in turn affect the oxidative status, generating heterogeneity in strength and direction of effect sizes. For example, immune-challenged Egyptian fruit bats (Rousettus aegyptiacus) reduce significantly their foraging activity compared to control bats (Moreno et al., 2021). Finally, the similarity in effect sizes between correlational and experimental studies suggests that the pro-oxidants produced by parasites might have a small effect on the host oxidative status. This topic has, however, been poorly investigated so far.
Finally, this work also identified several gaps and needs in current literature. Firstly, more work is needed for amphibians and reptiles, which were very underrepresented. This is particularly relevant given the high vulnerability of these two taxa to emerging infectious diseases and their deteriorating conservation status (e.g. Ferguson et al., 2018; Price et al., 2014). Secondly, I could not test any effects of sex because most studies did not assess whether oxidative costs of immune response differ between females and males. This is unfortunate because there is variation in immune function and oxidative status, as well as life-history trade-offs between sexes (Costantini, 2018; Klein and Flanagan, 2016). Thirdly, we need more long-term longitudinal studies to identify mechanisms that determine the costs of chronic infections and of co-infections, the roles of resistance-tolerance mechanisms, the relevance of selective disappearance, and the seasonality in responses. Fourthly, we also need more experimental manipulations of key resources (e.g. food quality and quantity, competition, pollutant exposure) that might influence the relationship between immune function and oxidative status. Finally, developing models that account for effects of physiological costs on immunological performance might be interesting, illuminating and important for (i) understanding the persistence and spatial spread of infectious agents, and the host–pathogen coevolution and (ii) implementing effective conservation actions.
Conclusions
The findings of these phylogenetic meta-analyses show congruent patterns in the link between either immune response or infection status and oxidative status across taxonomic classes. These results were consistent across experimental and correlational studies indicating that results of observational studies might suggest cause-effect relationships between infection and oxidative status. In contrast to my predictions, effect sizes were significantly larger in studies on captive animals than in those on free-living animals. Also, contrary to my predictions, oxidative costs of immune response were similar between young and adult individuals and were not associated with the species’ pace of life. These meta-analyses also show that it is very important to take into careful consideration some methodological aspects of study designs because they might affect interpretation and comparability of results and help understanding the mechanisms underlying the impacts of the type of immunostimulant and of tissue on oxidative status.
In conclusion, the outcomes of these meta-analyses show that perturbations of oxidative status are one widespread physiological effect of immune function across vertebrates that should be considered carefully when addressing the causes and consequences of variation in immunological performance in animal populations of conservation concern. Understanding physiological impacts of pathogens on organisms will be critical to assessing individual health and maintaining viable captive and wild populations.
Data availability statement
The full dataset is provided as supplementary material.
Supplementary Material
Acknowledgements
I thank Michael Beaulieu, Sophie Bourgeon, Michael Butler, Marie Charpentier, Philippe Christe, Janske van de Crommenacker, Greet De Coster, Jessica Delhaye, Tapio Eeva, Samia Elbahnaswy, Michel Fournier, Susannah S. French, Marcus Fritze, Andrée Gendron, Sveinn Are Hanssen, Ulalume Hernández-Arciga, Junyan Jin, Jimena López-Arrabé, Sylvain Losdat, Han Liu, David Marcogliese, Juan Moreno, Lorin A. Neuman-Lee, Claudia Norte, Bernd Pelster, Quentin Petitjean, Jeffrey D. Roth, Diego Rubolini, Karin Schneeberger, Lorenzo Serra, José Luis Soengas, Halyna Tkachenko and Eman Zaharan El-Naggar for kindly providing information needed for the calculation of effect sizes; Jarrod Hadfield, László Zsolt Garamszegi and Simone Messina for kindly providing statistical advice; Gabor Czirjak for valuable suggestions on the interpretation of results; and three anonymous reviewers for their thoughtful inputs that help me to revise and improve the article.
References
- Abasubong KP, Liu WB, Zhang DD, Yuan XY, Xia SL, Xu C, Li XF (2018) Fishmeal replacement by rice protein concentrate with xylooligosaccharides supplement benefits the growth performance, antioxidant capability and immune responses against Aeromonas hydrophila in blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol 78: 177–186. [DOI] [PubMed] [Google Scholar]
- Abdelkhalek NK, Risha E, El-Adl MA, Salama MF, Dawood MAO (2020) Antibacterial and antioxidant activity of clove oil against Streptococcus iniae infection in Nile tilapia (Oreochromis niloticus) and its effect on hepatic hepcidin expression. Fish Shellfish Immunol 104: 478–488. [DOI] [PubMed] [Google Scholar]
- Adeyemi JA (2014) Oxidative stress and antioxidant enzymes activities in the african catfish, Clarias gariepinus, experimentally challenged with Escherichia coli and vibrio fischeri. Fish Physiol Biochem 40: 347–354. [DOI] [PubMed] [Google Scholar]
- Akinsanya B, Isibor PO, Onadeko B, Tinuade AA (2020) Impacts of trace metals on african common toad, Amietophrynus regularis (Reuss, 1833) and depuration effects of the toad's enteric parasite, Amplicaecum africanum (Taylor, 1924) sampled within Lagos Metropolis, Nigeria. Heliyon 6: e03570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaasam VJ, Keehn JE, Durso AM, French SS, Feldman CR (2021) Ectoparasite load is reduced in side-blotched lizards (Uta stansburiana) at wind farms: implications for oxidative stress. Physiol Biochem Zool 94: 35–49. [DOI] [PubMed] [Google Scholar]
- Ali EH, Hashem M, Al-Salahy MB (2011) Pathogenicity and oxidative stress in Nile tilapia caused by Aphanomyces laevis and Phoma herbarum isolated from farmed fish. Diseas Aquat Organis 94: 17–28. [DOI] [PubMed] [Google Scholar]
- Allaam MA, Allam TS, Elkhatam AO (2014) Biochemical and circulating oxidative stress biomarkers in egyptian buffaloes (Bubalus bubalis) infested by sarcoptic mange. Glob Vet 13: 656–661. [Google Scholar]
- Alonso-Alvarez C, Bertrand S, Devevey G, Gaillard M, Prost J, Faivre B, Sorci G (2004) An experimental test of the dose-dependent effect of carotenoids and immune activation on sexual signals and antioxidant activity. Am Nat 164: 651–659. [DOI] [PubMed] [Google Scholar]
- Amin YA, Noseer EA, Fouad SS, Ali RA, Mahmoud H (2020) Changes of reproductive indices of the testis due to Trypanosoma evansi infection in dromedary bulls (Camelus dromedarius): semen picture, hormonal profile, histopathology, oxidative parameters, and hematobiochemical profile. J Adv Vet Anim Res 7: 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armour EM, Bruner TL, Hines JK, Butler MW (2020) Low-dose immune challenges result in detectable levels of oxidative damage. J Exp Biol 223: jeb220095. [DOI] [PubMed] [Google Scholar]
- Asghar M, Hasselquist D, Zehtindjiev P, Westerdahl H, Bensch S (2015) Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science 347: 436–438. [DOI] [PubMed] [Google Scholar]
- Azimzadeh K, Amniattalab A (2017) Total sialic acid, oxidative stress and histopathological changes in rainbow trout saprolegniasis (Oncorhynchus mykiss). Kafkas Universitesi Veteriner Fakultesi Dergisi 23: 55–62. [Google Scholar]
- Azma F, Razavi SM, Nazifi S, Rakhshandehroo E, Sanati AR (2015) A study on the status of inflammatory systems in camels naturally infected with Toxoplasma gondii. Trop Anim Health Prod 47: 909–914. [DOI] [PubMed] [Google Scholar]
- Balasch JC, Vargas R, Brandts I, Tvarijonaviciute A, Reyes-López F, Tort L, Teles M (2019) Divergent personalities influence the myogenic regulatory genes myostatin, myogenin and ghr2 transcript responses to vibrio anguillarum vaccination in fish fingerlings (Sparus aurata). Physiol Behav 212: 112697. [DOI] [PubMed] [Google Scholar]
- Baldissera MD, Souza CF, Moreira KLS, da Rocha M, da Veiga ML, Baldisserotto B (2017) Melaleuca alternifolia essential oil prevents oxidative stress and ameliorates the antioxidant system in the liver of silver catfish (Rhamdia quelen) naturally infected with Ichthyophthirius multifiliis. Aquaculture 480: 11–16. [Google Scholar]
- Baldissera MD, Souza CF, Descovi SN, Verdi CM, Zeppenfeld CC, da Silva AS, Santos RCV, Baldisserotto B (2019) Grape pomace flour ameliorates Pseudomonas aeruginosa-induced bioenergetic dysfunction in gills of grass carp. Aquaculture 506: 359–366. [Google Scholar]
- Baylor JL, Butler MW (2019) Immune challenge-induced oxidative damage may be mitigated by biliverdin. J Exp Biol 222: jeb200055. [DOI] [PubMed] [Google Scholar]
- Bello ARR, Fortes E, Bello-Klein A, Bello AA, Llesuy SF, Robaldo RB, Bianchini A (2000) Lipid peroxidation induced by Clinostomum detruncatum in muscle of the freshwater fish Rhamdia quelen. Dis Aquat Org 42: 233–236. [DOI] [PubMed] [Google Scholar]
- Bertrand S, Criscuolo F, Faivre B, Sorci G (2006) Immune activation increases susceptibility to oxidative tissue damage in zebra finches. Funct Ecol 20: 1022–1027. [Google Scholar]
- Brindley PJ, da Costa JMC, Sripa B (2015) Why does infection with some helminths cause cancer? Trends Cancer 1: 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broggi J, Soriguer RC, Figuerola J (2016) Transgenerational effects enhance specific immune response in a wild passerine. PeerJ 4: e1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder PC, Jackson AA (2000) Undernutrition, infection and immune function. Nutr Res Rev 13: 3–29. [DOI] [PubMed] [Google Scholar]
- Camini FC, da Silva Caetano CC, Almeida LT, de Brito Magalhães CL (2017) Implications of oxidative stress on viral pathogenesis. Arch Virol 162: 907–917. [DOI] [PubMed] [Google Scholar]
- Casagrande S, Costantini D, Groothuis TGG (2012) Interaction between sexual steroids and immune response in affecting oxidative status of birds. Comp Biochem Physiol Part A 163: 296–301. [DOI] [PubMed] [Google Scholar]
- Casasole G, Costantini D, Cichon M, Rutkowska J (2016) The effect of maternal immunization on female oxidative status, yolk antioxidants and offspring survival in a songbird. Comp Biochem Physiol Part A 194: 56–61. [DOI] [PubMed] [Google Scholar]
- Charoensuk L, Pinlaor P, Prakobwong S, Hiraku Y, Laothong U, Ruangjirachuporn W, Yongvanit P, Pinlaor S (2011) Curcumin induces a nuclear factor-erythroid 2-related factor 2-driven response against oxidative and nitrative stress after praziquantel treatment in liver fluke-infected hamsters. Int J Parasitol 41: 615–626. [DOI] [PubMed] [Google Scholar]
- Charpentier MJE, Boundenga L, Beaulieu M, Dibakou SE, Arnathau C, Sidobre C, Willaume E, Mercier-Delarue S, Simon F, Rougeron V et al. (2019) A longitudinal molecular study of the ecology of malaria infections in free-ranging mandrills. Int J Parasitol 10: 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Liu NN, Zhang HJ, Zhao YD, Cao XL (2020) The effects of Aeromonas hydrophila infection on oxidative stress, nonspecific immunity, autophagy, and apoptosis in the common carp. Dev Comp Immunol 105: 103587. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988) Statistical Power Analysis for the Behavioral Sciences. Lawrence Earlbaum Associates, Hillsdale, NJ, USA [Google Scholar]
- Costantini D (2014) Oxidative Stress and Hormesis in Evolutionary Ecology and Physiology: A Marriage Between Mechanistic and Evolutionary Approaches. Springer, Berlin Heidelberg [Google Scholar]
- Costantini D (2018) Meta-analysis reveals that reproductive strategies are associated with sexual differences in oxidative balance across vertebrates. Curr Zool 64: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini D (2019) Understanding diversity in oxidative status and oxidative stress: the opportunities and challenges ahead. J Exp Biol 222: jeb194688. [DOI] [PubMed] [Google Scholar]
- Costantini D, Dell’Omo G (2006) Effects of t-cell-mediated immune response on avian oxidative stress. Comp Biochem Physiol Part A 145: 137–142. [DOI] [PubMed] [Google Scholar]
- Costantini D, Møller AP (2009) Does immune response cause oxidative stress in birds? A meta-analysis. Comp Biochem Physiol Part A 153: 339–344. [DOI] [PubMed] [Google Scholar]
- Costantini D, Casagrande S, Casasole G, AbdElgawad H, Asard H, Pinxten R, Eens M (2015a) Immunization reduces vocal communication but does not increase oxidative stress in a songbird species. Behav Ecol Sociobiol 69: 829–839. [Google Scholar]
- Costantini D, Goutte A, Barbraud C, Faivre B, Sorci G, Weimerskirch H, Delord K, Chastel O (2015b) Demographic responses to oxidative stress and inflammation in the wandering albatross (Diomedea exulans). PLoS One 10: e0133967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini D, Seeber PA, Soilemetzidou SE, Azab W, Bohner J, Buuveibaatar B, Czirjak GA, East ML, Greunz EM, Kaczensky P et al. (2018) Physiological costs of infection: herpesvirus replication is linked to blood oxidative stress in equids. Sci Rep 8: 10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cram DL, Blount JD, York JE, Young AJ (2015) Immune response in a wild bird is predicted by oxidative status, but does not cause oxidative stress. PLoS One 10: e0122421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Crommenacker J, Richardson DS, Koltz AM, Hutchings K, Komdeur J (2012) Parasitic infection and oxidative status are associated and vary with breeding activity in the Seychelles warbler. Proc R Soc Lond B 279: 1466–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautremepuits C, Betoulle S, Vernet G (2003) Stimulation of antioxidant enzymes levels in carp (Cyprinus carpio L.) infected by Ptychobothrium sp. (Cestoda). Fish Shellfish Immunol 15: 467–471. [DOI] [PubMed] [Google Scholar]
- De Angeli DD, Silveira P, Ramos JA, Sousa JP, Braga ÉM, Norte AC (2017) Haemosporidian infections affect antioxidant defences in great tits Parus major but are not related to exposure to aerial pollutants. Parasitol Open 3: e4. [Google Scholar]
- De Coster G, De Neve L, Verhulst S, Lens L (2012) Maternal effects reduce oxidative stress in female nestlings under high parasite load. J Avian Biol 43: 177–185. [Google Scholar]
- Del Re AC (2013) Compute.es: compute effect sizes. http://cran.r–project.org/web/packages/compute.es.
- Delhaye J, Jenkins T, Christe P (2016) Plasmodium infection and oxidative status in breeding great tits, Parus major. Malar J 15: 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaye J, Glaizot O, Christe P (2018) The effect of dietary antioxidant supplementation in a vertebrate host on the infection dynamics and transmission of avian malaria to the vector. Parasitol Res 117: 2043–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devevey G, Bize P, Fournier S, Person E, Christe P (2010) Testing the predictive adaptive response in a host-parasite system. Funct Ecol 24: 178–185. [Google Scholar]
- Dkhil MA, Abdel-Baki AS, Al-Quraishy S, Abdel-Moneim AE (2013) Hepatic oxidative stress in mongolian gerbils experimentally infected with babesia divergens. Ticks Tick Borne Dis 4: 346–351. [DOI] [PubMed] [Google Scholar]
- Du YS, Yi MM, Xiao P, Meng LJ, Li X, Sun GX, Liu Y (2015) The impact of Aeromonas salmonicida infection on innate immune parameters of Atlantic salmon (Salmo salar). Fish Shellfish Immunol 44: 307–315. [DOI] [PubMed] [Google Scholar]
- El-Son MAM, Elbahnaswy S, Ibrahim I (2020) Molecular and histopathological characterization of Photobacterium damselae in naturally and experimentally infected Nile tilapia (Oreochromis niloticus). J Fish Dis 43: 1505–1517. [DOI] [PubMed] [Google Scholar]
- Espinal-Carrion T, Lopez-Lopez E (2010) Helminths and lipid peroxidation in Astyanax aeneus (pisces: Characidae) from a river in the humid subtropics of southeastern Mexico. Dis Aquat Organ 88: 215–224. [DOI] [PubMed] [Google Scholar]
- Fedorov S (2014). GetData Graph Digitizer, v.2.26.0.20. www.graph-digitizer.com.
- Fehr T, Ochsenbein AF (2004) Outcome of the antibody response: a question of antigen dose and distribution. Trends Immunol 25: 165–166. [DOI] [PubMed] [Google Scholar]
- Ferguson LV, Kortet R, Sinclair BJ (2018) Eco-immunology in the cold: the role of immunity in shaping the overwintering survival of ectotherms. J Exp Biol 221: jeb163873. [DOI] [PubMed] [Google Scholar]
- Flies AS, Mansfield LS, Grant CK, Weldele ML, Holekamp KE (2015) Markedly elevated antibody responses in wild versus captive spotted hyenas show that environmental and ecological factors are important modulators of immunity. PLoS One 10: e0137679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontagné S, Lataillade E, Brèque J, Kaushik S (2008) Lipid peroxidative stress and antioxidant defence status during ontogeny of rainbow trout (Oncorhynchus mykiss). Br J Nutr 100: 102–111. [DOI] [PubMed] [Google Scholar]
- Fritze M, Costantini D, Fickel J, Wehner D, Czirják GÁ, Voigt CC (2019) Immune response of hibernating european bats to a fungal challenge. Biol Open 8: bio046078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritze M, Puechmaille SJ, Costantini D, Fickel J, Voigt CC, Czirják GÁ (2021) Determinants of defence strategies of a hibernating european bat species towards the fungal pathogen Pseudogymnoascus destructans. Develop Comp Immunol 119: 104017. [DOI] [PubMed] [Google Scholar]
- Garamszegi LZ (2014) Modern Phylogenetic Comparative Methods and Their Application in Evolutionary Biology: Concepts and Practice. Springer, Heidelberg [Google Scholar]
- Garcia LO, Becker AG, Bertuzzi T, Cunha MA, Kochhann D, Finamor IA, Riffel APK, Llesuy S, Pavanato MA, Baldisserotto B (2011) Oxidative stress parameters in silver catfish (Rhamdia quelen) juveniles infected with Ichthyophthirius multifiliis and maintained at different levels of water pH. Vet Parasitol 178: 15–21. [DOI] [PubMed] [Google Scholar]
- Gasso D, Vicente J, Mentaberre G, Soriguer R, Rodriguez RJ, Navarro-Gonzalez N, Tvarijonaviciute A, Lavin S, Fernandez-Llario P, Segales J et al. (2016) Oxidative stress in wild boars naturally and experimentally infected with Mycobacterium bovis. PLoS One 11: e0163971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng RJ, Jia YY, Chi ML, Wang ZG, Liu H, Wang WM (2019) RNase1 alleviates the Aeromonas hydrophila-induced oxidative stress in blunt snout bream. Dev Comp Immunol 91: 8–16. [DOI] [PubMed] [Google Scholar]
- Georgountzou A, Papadopoulos NG (2017) Postnatal innate immune development: from birth to adulthood. Front Immunol 8: 957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghirardi R, Cazenave J, Lopez JA, Antoniazzi CE, Perotti MG (2018) Evaluation of stress responses to water molds in embryos of Physalaemus albonotatus (Anura: Leptodactylidae). Herpetol Conserv Biol 13: 216–223. [Google Scholar]
- Ghirardi R, Cazenave J, Lopez JA, Antoniazzi CE, Perotti MG (2020) Water mould exposure induces enzymatic antioxidant defences in embryos of the two-colored oval frog (Elachistocleis bicolor) (Anura: Microhylidae). Can J Zool 98: 411–416. [Google Scholar]
- Giraudeau M, Mousel M, Earl S, McGraw K (2014) Parasites in the city: degree of urbanization predicts poxvirus and coccidian infections in house finches (Haemorhous mexicanus). PLoS One 9: e86747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri SS, Kim HJ, Kim SG, Kim SW, Kwon J, Lee SB, Sukumaran V, Park SC (2020) Effectiveness of the guava leaf extracts against lipopolysaccharide-induced oxidative stress and immune responses in Cyprinus carpio. Fish Shellfish Immunol 105: 164–176. [DOI] [PubMed] [Google Scholar]
- Gombart AF, Pierre A, Maggini S (2020) A review of micronutrients and the immune system-working in harmony to reduce the risk of infection. Nutrients 12: 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Shuker DM, Pollitt LC, Auld S, Wilson AJ, Little TJ (2011) Fitness consequences of immune responses: strengthening the empirical framework for ecoimmunology. Funct Ecol 25: 5–17. [Google Scholar]
- Grainger TN, Levine JM (2021) Rapid evolution of life-history traits in response to warming, predation and competition: a meta-analysis. Ecol Lett. 25: 541–554. [DOI] [PubMed] [Google Scholar]
- Guzmán-Villanueva LT, Ascencio-Valle F, Macías-Rodríguez ME, Tovar-Ramírez D (2014) Effects of dietary β-1,3/1,6-glucan on the antioxidant and digestive enzyme activities of pacific red snapper (Lutjanus peru) after exposure to lipopolysaccharides. Fish Physiol Biochem 40: 827–837. [DOI] [PubMed] [Google Scholar]
- Hadfield JD (2010) MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J Stat Softw 33: 1–22.20808728 [Google Scholar]
- Halliwell BH, Gutteridge JMC (2015) Free Radicals in Biology and Medicine. Oxford University Press, Oxford, UK [Google Scholar]
- Hanssen SA, Bustnes JO, Schnug L, Bourgeon S, Johnsen TV, Ballesteros M, Sonne C, Herzke D, Eulaers I, Jaspers VL et al. (2013) Antiparasite treatments reduce humoral immunity and impact oxidative status in raptor nestlings. Ecol Evol 3: 5157–5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harikrishnan R, Thamizharasan S, Devi G, Van Doan H, Kumar TTA, Hoseinifar SH, Balasundaram C (2020) Dried lemon peel enriched diet improves antioxidant activity, immune response and modulates immuno-antioxidant genes in Labeo rohita against Aeromonas sorbia. Fish Shellfish Immunol 106: 675–684. [DOI] [PubMed] [Google Scholar]
- Hasselquist D, Nilsson JA (2012) Physiological mechanisms mediating costs of immune responses: what can we learn from studies of birds? Anim Behav 83: 1303–1312. [Google Scholar]
- Hau M, Haussmann MF, Greives TJ, Matlack C, Costantini D, Quetting M, Adelman JS, Miranda AC, Partecke J (2015) Repeated stressors in adulthood increase the rate of biological ageing. Front Zool 12: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidarpour M, Mohri M, Borji H, Moghdass E (2012) Oxidative stress and trace elements in camel (Camelus dromedarius) with liver cystic echinococcosis. Vet Parasitol 187: 459–463. [DOI] [PubMed] [Google Scholar]
- Hernandez-Arciga U, Herrera LG, Konigsberg M, Valdez RA, Flores-Martinez JJ, Romano MC (2020) Synergetic effects of immune challenge and stress depress cortisol, inflammatory response and antioxidant activity in fish-eating Myotis. J Exp Biol 223: jeb234914. [DOI] [PubMed] [Google Scholar]
- Herrero A, Barja G (1997) ADP-regulation of mitochondrial free radical production is different with complex I- and complex II-linked substrates: implications for the exercise paradox and brain hypermetabolism. J Bioenerg Biomembr 29: 241–249. [DOI] [PubMed] [Google Scholar]
- Hoogenboom MO, Metcalfe NB, Groothuis TGG, de Vries B, Costantini D (2012) Relationship between oxidative stress and circulating testosterone and cortisol in pre-spawning female brown trout. Comp Biochem Physiol Part A 163: 379–387. [DOI] [PubMed] [Google Scholar]
- Horak P, Zilmer M, Saks L, Ots I, Karu U, Zilmer K (2006) Antioxidant protection, carotenoids and the costs of immune challenge in greenfinches. J Exp Biol 209: 4329–4338. [DOI] [PubMed] [Google Scholar]
- Horak P, Saks L, Zilmer M, Karu U, Zilmer K (2007) Notes and comments—do dietary antioxidants alleviate the cost of immune activation? An experiment with greenfinches. Am Nat 170: 625–635. [DOI] [PubMed] [Google Scholar]
- Isaksson C, Sepil I, Baramidze V, Sheldon B (2013) Explaining variance of avian malaria infection in the wild: the importance of host density, habitat, individual life-history and oxidative stress. BMC Ecol 13: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Kera A, Murata H, Yoshida T, Sakai T, Yamauchi K, Yamasaki Y, Yamaguchi T, Ukawa M (2000) Experimentally induced bacterial hemolytic jaundice of yellowtail and oxidative stress. Nippon Suisan Gakkaishi 66: 50–54. [Google Scholar]
- Jaguezeski AM, da Silva AS, Gomes TMA, Bottari NB, Lopes TF, Cechin RA, Morsch VM, Schetinger MRC, Giongo JL, de A Vaucher R (2020) Experimental listeriosis: a study of purinergic and cholinergic inflammatory pathway. Vet Microbiol 241: 108528. [DOI] [PubMed] [Google Scholar]
- Jiang B, Wang J, Luo HL, Lu GL, Li YW, Li AX (2017) L-amino acid oxidase expression profile and biochemical responses of rabbitfish (Siganus oramin) after exposure to a high dose of Cryptocaryon irritans. Fish Shellfish Immunol 69: 85–89. [DOI] [PubMed] [Google Scholar]
- Jolly S, Jaffal A, Delahaut L, Palluel O, Porcher JM, Geffard A, Sanchez W, Betoulle S (2014) Effects of aluminium and bacterial lipopolysaccharide on oxidative stress and immune parameters in roach, Rutilus rutilus L. Environ Sci Pollut Res 21: 13103–13117. [DOI] [PubMed] [Google Scholar]
- Kim YS, Ke F, Zhang QY (2009) Effect of beta-glucan on activity of antioxidant enzymes and Mx gene expression in virus infected grass carp. Fish Shellfish Immunol 27: 336–340. [DOI] [PubMed] [Google Scholar]
- Kim JH, Sohn S, Kim SK, Hur YB (2020) Effects on hematological parameters, antioxidant and immune responses, ache, and stress indicators of olive flounders, Paralichthys olivaceus, raised in bio-floc and seawater challenged by Edwardsiella tarda. Fish Shellfish Immunol 97: 194–203. [DOI] [PubMed] [Google Scholar]
- Klasing KC (2004) The costs of immunity. Acta Zool Sin 50: 961–969. [Google Scholar]
- Klein S, Flanagan K (2016) Sex differences in immune responses. Nat Rev Immunol 16: 626–638. [DOI] [PubMed] [Google Scholar]
- Kumar S, Raman RP, Prasad KP, Srivastava PP, Kumar S, Rajendran KV (2017) Modulation of innate immune responses and induction of oxidative stress biomarkers in Pangasianodon hypophthalmus following an experimental infection with Dactylogyrid monogeneans. Fish Shellfish Immunol 63: 334–343. [DOI] [PubMed] [Google Scholar]
- Kurhalyuk N, Tkachenko H (2011) Induction of oxidative stress and antioxidant defenses in the livers of sea trout, Salmo trutta L., with ulcerative dermal necrosis. Fish Aquat Life 19: 229–240. [Google Scholar]
- Kurhalyuk N, Tkachenko H, Palczynska K (2009) Antioxidant enzymes profile in the brown trout (Salmo trutta trutta) with ulcerative dermal necrosis. Bull Vet Inst Pulawy 53: 813–818. [Google Scholar]
- Kurhalyuk N, Tkachenko H, Palczynska K (2011) Resistance of erythrocytes from brown trout (Salmo trutta m. trutta L.) affected by ulcerative dermal necrosis syndrome. Polish J Vet Sci 14: 443–448. [DOI] [PubMed] [Google Scholar]
- Le Guernic A, Sanchez W, Palluel O, Bado-Nilles A, Floriani M, Turies C, Chadili E, Vedova CD, Cavalié I, Adam-Guillermin C et al. (2016) Acclimation capacity of the three-spined stickleback (Gasterosteus aculeatus, L.) to a sudden biological stress following a polymetallic exposure. Ecotoxicology 25: 1478–1499. [DOI] [PubMed] [Google Scholar]
- Li MY, Guo WQ, Guo GL, Zhu XM, Niu XT, Shan XF, Tian JX, Wang GQ, Zhang DM (2020) Effects of dietary astaxanthin on lipopolysaccharide-induced oxidative stress, immune responses and glucocorticoid receptor (gr)-related gene expression in Channa argus. Aquaculure 517: 734816. [Google Scholar]
- Lilley TM, Stauffer J, Kanerva M, Eeva T (2014) Interspecific variation in redox status regulation and immune defence in five bat species: the role of ectoparasites. Oecologia 175: 811–823. [DOI] [PubMed] [Google Scholar]
- Liu C, Liu HK, Han D, Xie SQ, Jin JY, Yang YX, Zhu XM (2020) Effects of dietary Arthrospira platensis supplementation on the growth performance, antioxidation and immune related-gene expression in yellow catfish (Pelteobagrus fulvidraco). Aquac Rep 17. [Google Scholar]
- Lochmiller RL, Deerenberg C (2000) Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88: 87–98. [Google Scholar]
- Lopez-Arrabe J, Cantarero A, Perez-Rodriguez L, Palma A, Alonso-Alvarez C, Gonzalez-Braojos S, Moreno J (2015) Nest-dwelling ectoparasites reduce antioxidant defences in females and nestlings of a passerine: a field experiment. Oecologia 179: 29–41. [DOI] [PubMed] [Google Scholar]
- Losdat S, Richner H, Blount JD, Helfenstein F (2011) Immune activation reduces sperm quality in the great tit. PLoS One 6: e22221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggini S, Pierre A, Calder PC (2018) Immune function and micronutrient requirements change over the life course. Nutrients 10: 1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels RM, Nussey DH (2013) Into the wild: digging at immunology's evolutionary roots. Nat Immunol 14: 879–883. [DOI] [PubMed] [Google Scholar]
- Mangalmurti N, Hunter CA (2020) Cytokine storms: understanding COVID-19. Immunity 53: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasco V, Sebastiano M, Costantini D, Pola G, Fusani L (2021) Controlled expression of the migratory phenotype affects oxidative status in birds. J Exp Biol 224: jeb233486. [DOI] [PubMed] [Google Scholar]
- Marcogliese DJ, Brambilla LG, Gagne F, Gendron AD (2005) Joint effects of parasitism and pollution on oxidative stress biomarkers in yellow perch Perca flavescens. Dis Aquat Org 63: 77–84. [DOI] [PubMed] [Google Scholar]
- Marcogliese DJ, Dautremepuits C, Gendron AD, Fournier M (2010) Interactions between parasites and pollutants in yellow perch (Perca flavescens) in the st. Lawrence river, Canada: implications for resistance and tolerance to parasites. Can J Zool 88: 247–258. [Google Scholar]
- Marcogliese DJ, King KC, Bates KA (2021) Effects of multiple stressors on northern leopard frogs in agricultural wetlands. Parasitology 148: 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maronde L, Losdat S, Richner H (2018) Do parasites and antioxidant availability affect begging behaviour, growth rate and resistance to oxidative stress? J Evol Biol 31: 904–913. [DOI] [PubMed] [Google Scholar]
- Marri V, Richner H (2015) Immune response, oxidative stress and dietary antioxidants in great tit nestlings. Comp Biochem Physiol Part A 179: 192–196. [DOI] [PubMed] [Google Scholar]
- Martin LB, Weil ZM, Nelson RJ (2008) Seasonal changes in vertebrate immune activity: mediation by physiological trade-offs. Phil Trans R Soc B 363: 321–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB, Hawley DM, Ardia DR (2011) An introduction to ecological immunology. Funct Ecol 25: 1–4. [Google Scholar]
- Mastroeni P, Vazquez-Torres A, Fang FC, Xu YS, Khan S, Hormaeche CE, Dougan G (2000) Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J Exp Med 192: 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina S, Edwards DP, Van Houtte N, Tomassi S, Benedick S, Eens M, Costantini D (2022) Impacts of selective logging on haemosporidian infection and physiological correlates in tropical birds. Int J Parasitol 52: 87–96. [DOI] [PubMed] [Google Scholar]
- Molbert N, Alliot F, Leroux-Coyau M, Medoc V, Biard C, Meylan S, Jacquin L, Santos R, Goutte A (2020) Potential benefits of acanthocephalan parasites for chub hosts in polluted environments. Environ Sci Technol 54: 5540–5549. [DOI] [PubMed] [Google Scholar]
- Møller AP, Jennions MD (2001) Testing and adjusting for publication bias. Trends Ecol Evol 16: 580–586. [Google Scholar]
- Møller A, Jennions MD (2002) How much variance can be explained by ecologists and evolutionary biologists? Oecologia 132: 492–500. [DOI] [PubMed] [Google Scholar]
- Moore J (2002) Parasites and the Behavior of Animals. Oxford University Press, Oxford, UK [Google Scholar]
- Moore MS, Reichard JD, Murtha TD, Nabhan ML, Pian RE, Ferreira JS, Kunz TH (2013) Hibernating little brown myotis (Myotis lucifugus) show variable immunological responses to white-nose syndrome. PLoS One 8: e58976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno KR, Weinberg M, Harten L, Ramos VSB, Herrera MLG, Czirjak GA, Yovel Y (2021) Sick bats stay home alone: fruit bats practice social distancing when faced with an immunological challenge. Ann N Y Acad Sci 1505: 178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morryssey MB (2016a) Meta-analysis of magnitudes, differences and variation in evolutionary parameters. J Evol Biol 29: 1882–1904. [DOI] [PubMed] [Google Scholar]
- Morryssey MB (2016b) Rejoinder: further considerations for meta-analysis of transformed quantities such as absolute values. J Evol Biol 29: 1922–1931. [DOI] [PubMed] [Google Scholar]
- Mougeot F, Martinez-Padilla J, Webster LMI, Blount JD, Perez-Rodriguez L, Piertney SB (2009) Honest sexual signalling mediated by parasite and testosterone effects on oxidative balance. Proc R Soc Lond B 276: 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougeot F, Martinez-Padilla J, Blount JD, Perez-Rodriguez L, Webster LMI, Piertney SB (2010) Oxidative stress and the effect of parasites on a carotenoid-based ornament. J Exp Biol 213: 400–407. [DOI] [PubMed] [Google Scholar]
- Munang'andu HM, Fredriksen BN, Mutoloki S, Dalmo RA, Evensen Ø (2013) Antigen dose and humoral immune response correspond with protection for inactivated infectious pancreatic necrosis virus vaccines in Atlantic salmon (Salmo salar L). Vet Res 44: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82: 591–605. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Santos ESA (2012) Methodological issues and advances in biological meta-analysis. Evol Ecol 26: 1253–1274. [Google Scholar]
- Nakagawa S, Lagisz M, Jennions MD, Koricheva J, Noble DW, Parker TH, Sánchez-Tójar A, Yang Y, O'Dea RE (2022) Methods for testing publication bias in ecological and evolutionary meta-analyses. Meth Ecol Evol 13: 4–21. [Google Scholar]
- Nathan C, Shiloh MU (2000) Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci U S A 97: 8841–8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman-Lee LA, Van Wettere AJ, French SS (2019) Interrelations among multiple metrics of immune and physiological function in a squamate, the common gartersnake (Thamnophis sirtalis). Physiol Biochem Zool 92: 12–23. [DOI] [PubMed] [Google Scholar]
- Norte AC, Costantini D, Araujo PM, Eens M, Ramos JA, Heylen D (2018) Experimental infection by microparasites affects the oxidative balance in their avian reservoir host the blackbird turdus merula. Ticks Tick Borne Dis 9: 720–729. [DOI] [PubMed] [Google Scholar]
- Ohmer MEB, Costantini D, Czirják G, Downs CJ, Ferguson LV, Flies A, Franklin CE, Kayigwe AN, Knutie S, Richards-Zawacki CL et al. (2021) Applied ecoimmunology: using immunological tools to improve conservation efforts in a changing world. Conserv Physiol 9: coab074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orledge JM, Blount JD, Hoodless AN, Royle NJ (2012) Antioxidant supplementation during early development reduces parasite load but does not affect sexual ornament expression in adult ring-necked pheasants. Funct Ecol 26: 688–700. [Google Scholar]
- Pacios-Palma I, Moreno S, Selman C, Rouco C (2018) Oxidative stress in wild European rabbits naturally infected with Myxoma virus and rabbit haemorrhagic disease virus. Eur J Wildlife Res 64: 47. [Google Scholar]
- Paiva CN, Bozza MT (2014) Are reactive oxygen species always detrimental to pathogens? Antioxid Redox Signal 20: 1000–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Roy D, Ray S, Homechaudhuri S (2020) Aeromonas hydrophila induced mitochondrial dysfunction and apoptosis in liver and spleen of Labeo rohita mediated by calcium and reactive oxygen species. Turk J Fish Aquat Sci 20: 255–266. [Google Scholar]
- Panaro MA, Lisi S, Mitolo V, Acquafredda A, Fasanella A, Carelli MG, Brandonisio O (1998) Evaluation of killing, superoxide anion and nitric oxide production by Leishmania infantum-infected dog monocytes. Cytobios 95: 151–160. [PubMed] [Google Scholar]
- Pap PL, Vagasi CI, Czirjak GA, Titilincu A, Pintea A, Osvath G, Fulop A, Barta Z (2011) The effect of coccidians on the condition and immune profile of molting house sparrows (Passer domesticus). Auk 128: 330–339. [Google Scholar]
- Pap PL, Sesarman A, Vagasi CI, Buehler DM, Patras L, Versteegh MA, Banciu M (2014) No evidence for parasitism-linked changes in immune function or oxidative physiology over the annual cycle of an avian species. Physiol Biochem Zool 87: 729–739. [DOI] [PubMed] [Google Scholar]
- Peixoto MJ, Salas-Leiton E, Brito F, Pereira L, Svendsen J, Baptista T, Pereira R, Abreu H, Reis P, Gonçalves JFM et al. (2017) Effects of dietary Gracilaria sp. and Alaria sp. supplementation on growth performance, metabolic rates and health in meagre (Argyrosomus regius) subjected to pathogen infection. J Appl Phycol 29: 433–447. [Google Scholar]
- Perez-Rodriguez L, Fo M, Alonso-Alvarez C, Blas J, Viñuela J, Bortolotti GR (2008) Cell-mediated immune activation rapidly decreases plasma carotenoids but does not affect oxidative stress in red-legged partridges (Alectoris rufa). J Exp Biol 211: 2155–2161. [DOI] [PubMed] [Google Scholar]
- Peters A, Delhey K, Nakagawa S, Aulsebrook A, Verhulst S (2019) Immunosenescence in wild animals: meta-analysis and outlook. Ecol Lett 22: 1709–1722. [DOI] [PubMed] [Google Scholar]
- Petitjean Q, Jacquin L, LeHenaff M, Perrault A, Cousseau M, Laffaille P, Jean S (2021a) Dose- and time-dependent effects of an immune challenge on fish across biological levels. J Exp Zool Part A 335: 250–264. [DOI] [PubMed] [Google Scholar]
- Petitjean Q, Jacquin L, Riem L, Pitout M, Perrault A, Cousseau M, Laffaille P, Jean S (2021b) Intraspecific variability of responses to combined metal contamination and immune challenge among wild fish populations. Environ Pollut 272: 116042. [DOI] [PubMed] [Google Scholar]
- Price SJ, Garner TWJ, Nichols RA, Balloux F, Ayres C, Mora-Cabello de Alba A, Bosch J (2014) Collapse of amphibian communities due to an introduced Ranavirus. Curr Biol 24: 2586–2591. [DOI] [PubMed] [Google Scholar]
- Ramirez-Otarola N, Sarria M, Rivera DS, Sabat P, Bozinovic F (2019) Ecoimmunology in degus: interplay among diet, immune response, and oxidative stress. J Comp Physiol B 189: 143–152. [DOI] [PubMed] [Google Scholar]
- Ramirez-Otarola N, Maldonado K, Cavieres G, Bozinovic F, Sabat P (2021) Nutritional ecology and ecological immunology in degus: does early nutrition affect the postnatal development of the immune function? J Exp Zool Part A 335: 239–249. [DOI] [PubMed] [Google Scholar]
- Razavi SM, Nazifi S, Afsar M, Yazdanpanah Z, Rakhshandehroo E (2016) Evaluation of the blood oxidant-antioxidant interactions in pigeons naturally infected with Haemoproteus columbae. Vet Archiv 86: 395–405. [Google Scholar]
- Ren ZL, Wang SF, Cai Y, Wu Y, Tian LJ, Liao JQ, Wang SQ, Jiang L, Guo WL, Zhou YC (2020) Antioxidant capacity, non-specific immunity, histopathological analysis and immune-related genes expression in Nile tilapia Oreochromis niloticus infected with Aeromonas schubertii. Aquaculture 529: 735642. [Google Scholar]
- Rodriguez A, Broggi J, Alcaide M, Negro JJ, Figuerola J (2014) Determinants and short-term physiological consequences of pha immune response in lesser kestrel nestlings. J Exp Zool Part A 321: 376–386. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Quiroga JJ, Otero-Rodino C, Suarez P, Nieto TP, Estevez JMG, San Juan F, Soengas JL (2017) Differential effects of exposure to parasites and bacteria on stress response in turbot Scophthalmus maximus simultaneously stressed by low water depth. J Fish Biol 91: 242–259. [DOI] [PubMed] [Google Scholar]
- da Rosa VM, Ariotti K, Bressan CA, da Silva EG, Dallaporta M, Junior GB, da Costa ST, de Vargas AC, Baldisserotto B, Finamor IA et al. (2019) Dietary addition of rutin impairs inflammatory response and protects muscle of silver catfish (Rhamdia quelen) from apoptosis and oxidative stress in Aeromonas hydrophila-induced infection. Comp Biochem Physiol Part C 226: 108611. [DOI] [PubMed] [Google Scholar]
- Roth JD, Dobson FS, Criscuolo F, Uhlrich P, Zahariev A, Bergouignan A, Viblanc VA (2019) Subtle short-term physiological costs of an experimental augmentation of fleas in wild columbian ground squirrels. J Exp Biol 222: jeb203588. [DOI] [PubMed] [Google Scholar]
- Rothoeft T, Gonschorek A, Bartz H, Anhenn O, Schauer U (2003) Antigen dose, type of antigen-presenting cell and time of differentiation contribute to the T helper 1/T helper 2 polarization of naive T cells. Immunology 110: 430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy D, Pal S, Datta Ray S, Homechaudhuri S (2019) Evaluating oxidative stress in labeo rohita, infected asypmtomatically with native and invasive aeromonads using biochemical indices. Proc Nat Acad Sci India Section B 89: 973–978. [Google Scholar]
- Sahan A, Duman S, Colak SO, Cinar E, Bilgin R (2017) Determination of some hematological and non-specific immune defences, oxidative stress and histopathological status in rainbow trout (Oncorhynchus mykiss) fed rosehip (Rosa canina) to Yersinia ruckeri. Turk J Fish Aquat Sci 17: 91–100. [Google Scholar]
- Saleh MA, Al-Salahy MB, Sanousi SA (2009) Oxidative stress in blood of camels (Camelus dromedaries) naturally infected with Trypanosoma evansi. Vet Parasitol 162: 192–199. [DOI] [PubMed] [Google Scholar]
- Şanchez CA, Becker DJ, Teitelbaum CS, Barriga P, Brown LM, Majewska AA, Hall RJ, Altizer S (2018) On the relationship between body condition and parasite infection in wildlife: a review and meta-analysis. Ecol Lett 21: 1869–1884. [DOI] [PubMed] [Google Scholar]
- Sanders D, Frago E, Kehoe R, Patterson C, Gaston KJ (2021) A meta-analysis of biological impacts of artificial light at night. Nat Ecol Evol 5: 74–81. [DOI] [PubMed] [Google Scholar]
- Schneebauer G, Hanel R, Pelster B (2016) Anguillicola crassus impairs the silvering-related enhancements of the ROS defense capacity in swimbladder tissue of the european eel (Anguilla anguilla). J Comp Physiol B 186: 867–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger K, Czirjak GA, Voigt CC (2013) Inflammatory challenge increases measures of oxidative stress in a free-ranging, long-lived mammal. J Exp Biol 216: 4514–4519. [DOI] [PubMed] [Google Scholar]
- Schoenle LA, Kernbach M, Haussmann MF, Bonier F, Moore IT (2017) An experimental test of the physiological consequences of avian malaria infection. J Anim Ecol 86: 1483–1496. [DOI] [PubMed] [Google Scholar]
- Sebastiano M, Eens M, Abd Elgawad H, de Thoisy B, Lacoste V, Pineau K, Asard H, Chastel O, Costantini D (2017) Oxidative stress biomarkers are associated with visible clinical signs of a disease in frigatebird nestlings. Sci Rep 7: 1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastiano M, Eens M, Messina S, AbdElgawad H, Pineau K, Beemster GTS, Chastel O, Costantini D (2018) Resveratrol supplementation reduces oxidative stress and modulates the immune response in free-living animals during a viral infection. Funct Ecol 32: 2509–2519. [Google Scholar]
- Seeber PA, Morrison T, Ortega A, East ML, Greenwood AD, Czirják GA (2020) Immune differences in captive and free-ranging zebras (Equus zebra and E. quagga). Mammal Biol 100: 155–164. [Google Scholar]
- Sepp T, Karu U, Blount JD, Sild E, Manniste M, Horak P (2012) Coccidian infection causes oxidative damage in greenfinches. PLoS One 7: e36495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepp T, McGraw KJ, Kaasik A, Giraudeau M (2018) A review of urban impacts on avian life-history evolution: does city living lead to slower pace of life? Glob Change Biol 24: 1452–1469. [DOI] [PubMed] [Google Scholar]
- Seppälä O, Jokela J (2010) Maintenance of genetic variation in immune defense of a freshwater snail: role of environmental heterogeneity. Evolution 64: 2397–2407. [DOI] [PubMed] [Google Scholar]
- Serra L, Pirrello S, Caprioli M, Griggio M, Andreotti A, Romano A, Pilastro A, Saino N, Sacchi R, Galeotti P et al. (2012) Seasonal decline of offspring quality in the European starling Sturnus vulgaris: an immune challenge experiment. Behav Ecol Sociobiol 66: 697–709. [Google Scholar]
- Sheldon BC, Verhulst S (1996) Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol 11: 317–321. [DOI] [PubMed] [Google Scholar]
- da Silva CC, Klein RD, Barcarolli IF, Bianchini A (2016) Metal contamination as a possible etiology of fibropapillomatosis in juvenile female green sea turtles Chelonia mydas from the southern Atlantic Ocean. Aquat Toxicol 170: 42–51. [DOI] [PubMed] [Google Scholar]
- Simons MJP, Cohen AA, Verhulst S (2012) What does carotenoid-dependent coloration tell? Plasma carotenoid level signals immunocompetence and oxidative stress state in birds–a meta-analysis. PLoS One 7: e43088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuratovskaya EN, Zav’yalov AV, Rudneva II (2015) Black Sea whiting Merlangus merlangus euxinus (Nordmann, 1840) to parasitic nematode Hysterothylacium aduncum (Rudolphi, 1802) infection. Bull Eur Assoc Fish Pathol 35: 170–176. [Google Scholar]
- Skuratovskaya EN, Yurakhno VM, Chesnokova II (2020) Biochemical response of two fish species of Gobiidae (Gobiiformes) to Cryptocotyle (Opisthorchiida, Heterophyidae) metacercariae infection from the mouth of the river Chernaya (Black Sea). Ecol Montenegrina 38: 158–165. [Google Scholar]
- Smith SM, Nager RG, Costantini D (2016) Meta-analysis indicates that oxidative stress is both a constraint on and a cost of growth. Ecol Evol 6: 2833–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobroza AO, Tonin AA, Da Silva AS, Dornelles GL, Wolkmer P, Duarte M, Hausen BS, Sangoi MB, Moresco RN, Stefani LM et al. (2014) Iron metabolism in hamsters experimentally infected with Leptospira interrogans serovar Pomona: influence on disease pathogenesis. Comp Immunol Microbiol Infect Dis 37: 299–304. [DOI] [PubMed] [Google Scholar]
- Sorci G, Faivre B (2009) Inflammation and oxidative stress in vertebrate host-parasite systems. Phil Trans R Soc Lond B 364: 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorci G, Cornet S, Faivre B (2013) Immune evasion, immunopathology and the regulation of the immune system. Pathogens 2: 71–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorci G, Lippens C, Lechenault C, Faivre B (2017) Benefits of immune protection versus immunopathology costs: a synthesis from cytokine KO models. Infect Genet Evol 54: 491–495. [DOI] [PubMed] [Google Scholar]
- Srivastava P, Puri SK, Dutta GP, Pandey VC (1992) Status of oxidative stress and antioxidant defenses during plasmodium knowlesi infection and chloroquine treatment in Macaca mulatta. Inter J Parasitol 22: 243–245. [DOI] [PubMed] [Google Scholar]
- Stauffer J, Bruneaux M, Panda B, Visse M, Vasemagi A, Ilmonen P (2017) Telomere length and antioxidant defense associate with parasite-induced retarded growth in wild brown trout. Oecologia 185: 365–374. [DOI] [PubMed] [Google Scholar]
- Stumbo AD, Goater CP, Hontela A (2012) Parasite-induced oxidative stress in liver tissue of fathead minnows exposed to trematode cercariae. Parasitology 139: 1666–1671. [DOI] [PubMed] [Google Scholar]
- Sun LL, Sun Y, Jiang M, Luo LJ, Yu XB, Yao WZ, Wu ZL (2020) Isolation, identification and pathogenicity of Proteus vulgaris from moribund common carp (Cyprinus carpio) farmed in China. Aquaculture 525: 735294. [Google Scholar]
- Surai P (2002) Natural Antioxidants in Avian Nutrition and Reproduction. Nottingham University Press, Nottingham [Google Scholar]
- Sweazea KL, Simperova A, Juan T, Gadau A, Brant SV, Deviche P, Jarrett C (2015) Pathophysiological responses to a schistosome infection in a wild population of mourning doves (Zenaida macroura). Fortschr Zool 118: 386–393. [DOI] [PubMed] [Google Scholar]
- Tancredo KR, Goncalves ELT, Brum A, Acchile M, Hashimoto GSO, Pereira SA, Martins ML (2015) Hemato-immunological and biochemical parameters of silver catfish Rhamdia quelen immunized with live theronts of Ichthyophthirius multifiliis. Fish Shellfish Immunol 45: 689–694. [DOI] [PubMed] [Google Scholar]
- Tkachenko H, Kurhalyuk N, Palczynska K (2011) Responses of antioxidant status in the gills of brown trout (Salmo trutta m. trutta L.) with ulcerative dermal necrosis. Baltic Coastal Zone. J Ecol Prot Coastl 15: 145–158. [Google Scholar]
- Tkachenko H, Kurhaluk N, Andriichuk A, Gasiuk E, Beschasniu S (2014) Oxidative stress biomarkers in liver of sea trout (Salmo trutta m. trutta L.) affected by ulcerative dermal necrosis syndrome. Turk J Fish Aquat Sci 14: 391–402. [Google Scholar]
- Tkachenko H, Grudniewska J, Pekala A, Terech-Majewska E (2016) Oxidative stress and antioxidant defence markers in muscle tissue of rainbow trout (Oncorhynchus mykiss) after vaccination against Yersinia ruckeri. J Vet Res 60: 25–33. [Google Scholar]
- Tobler M, Healey M, Wilson M, Olsson M (2011) Basal superoxide as a sex-specific immune constraint. Biol Lett 7: 906–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler M, Ballen C, Healey M, Wilson M, Olsson M (2015) Oxidant trade-offs in immunity: an experimental test in a lizard. PLoS One 10: e0126155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonin AA, da Silva AS, Thome GR, Bochi GV, Schetinger MRC, Moresco RN, Camillo G, Toscan G, Vogel FF, Lopes STA (2014) Oxidative stress in brain tissue of gerbils experimentally infected with Neospora caninum. J Parasitol 100: 154–156. [DOI] [PubMed] [Google Scholar]
- Torres R, Velando A (2007) Male reproductive senescence: the price of immune-induced oxidative damage on sexual attractiveness in the blue-footed booby. J Anim Ecol 76: 1161–1168. [DOI] [PubMed] [Google Scholar]
- Vitula F, Peckova L, Bandouchova H, Pohanka M, Novotny L, Jira D, Kral J, Ondracek K, Osickova J, Zendulkova D et al. (2011) Mycoplasma gallisepticum infection in the grey partridge Perdix perdix: outbreak description, histopathology, biochemistry and antioxidant parameters. BMC Vet Res 7: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt CC, Fritze M, Lindecke O, Costantini D, Petersons G, Czirjak GA (2020) The immune response of bats differs between pre-migration and migration seasons. Sci Rep 10: 17384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MJ (2013) What drives population-level effects of parasites? Meta-analysis meets life-history. Int J Parasitol 2: 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann M, Voegeli B, Richner H (2015) Physiological responses to increased brood size and ectoparasite infestation: adult great tits favour self-maintenance. Physiol Behav 141: 127–134. [DOI] [PubMed] [Google Scholar]
- Winterbourn CC (2002) Biological reactivity and biomarkers of the neutrophil oxidant, hypochlorous acid. Toxicology 181: 223–227. [DOI] [PubMed] [Google Scholar]
- Wu DY, Lewis ED, Pae MY, Meydani SN (2019) Nutritional modulation of immune function: analysis of evidence, mechanisms, and clinical relevance. Front Immunol 9: 3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WJ, Li HY, Wu LY, Jin JY, Zhu XM, Han D, Liu HK, Yang YX, Xu XD, Xie SQ (2020) Dietary Scenedesmus ovalternus improves disease resistance of overwintering gibel carp (Carassius gibelio) by alleviating toll-like receptor signaling activation. Fish Shellfish Immunol 97: 351–358. [DOI] [PubMed] [Google Scholar]
- Yang D-B, Xu Y-C, Wang D-H, Speakman JR (2013) Effects of reproduction on immunosuppression and oxidative damage, and hence support or otherwise for their roles as mechanisms underpinning life history trade-offs, are tissue and assay dependent. J Exp Biol 216: 4242–4250. [DOI] [PubMed] [Google Scholar]
- Yun S, Giri SS, Kim HJ, Kim SG, Kim SW, Kang JW, Han SJ, Kwon J, Oh WT, Chi C et al. (2019) Enhanced bath immersion vaccination through microbubble treatment in the cyprinid loach. Fish Shellfish Immunol 91: 12–18. [DOI] [PubMed] [Google Scholar]
- Zahran E, Risha E (2013) Protective role of adjuvant and potassium permanganate on oxidative stress response of Nile tilapia (Oreochromis niloticus) challenged with Saprolegnia ferax. Springerplus 2: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahran E, El-Gawad EAA, Risha E (2018) Dietary Withania sominefera root confers protective and immunotherapeutic effects against Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 80: 641–650. [DOI] [PubMed] [Google Scholar]
- Zahran E, Mahgoub HA, Abdelhamid F, Sadeyen JR, Risha E (2019) Experimental pathogenesis and host immune responses of enterococcus faecalis infection in Nile tilapia (Oreochromis niloticus). Aquaculture 512: 734319. [Google Scholar]
- Zhang YF, Hill GE, Ge ZY, Park NR, Taylor HA, Andreasen V, Tardy L, Kavazis AN, Bonneaud C, Hood WR (2021) Effects of a bacterial infection on mitochondrial function and oxidative stress in a songbird. Physiol Biochem Zool 94: 71–82. [DOI] [PubMed] [Google Scholar]
- Zhao H, Luo YE, Wu ZX, Zhou Y, Guo DY, Wang H, Chen XX (2019) Hepatic lipid metabolism and oxidative stress responses of grass carp (Ctenopharyngodon idella) fed diets of two different lipid levels against aeromonas hydrophila infection. Aquaculture 509: 149–158. [Google Scholar]
- Zhu XM, Li MY, Liu XY, Xia CG, Niu XT, Wang GQ, Zhang DM (2020) Effects of dietary astaxanthin on growth, blood biochemistry, antioxidant, immune and inflammatory response in lipopolysaccharide-challenged Channa argus. Aquac Res 51: 1980–1991. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The full dataset is provided as supplementary material.


