Abstract
The Omicron (B.1.1.529) variant was first reported in South Africa and rapidly spread worldwide in early November 2021. This caused panic in various countries, so it is necessary to understand Omicron Variant. This paper summarizes omicron variant-related research achievements. Studies have shown that Omicron Variant contains many mutations that make it more infectious and transmissible. At the same time, immune escape is also caused, resulting in reduced efficacy of existing vaccines, increased risk of reinfection, treatment failure or reduction of monoclonal antibody therapies, and detection failure. However, current data indicate that Omicron Variant causes mild clinical symptoms and few severe cases and deaths. Omicron Variant is valid for a range of nonpharmaceutical interventions against SARS-CoV-2. Improving diagnostic accuracy and enabling timely isolation and treatment of diagnosed cases is also critical to interrupting the spread of omicron variants. COVID-19 vaccine boosters could undoubtedly help control Omicron spread and infection. However, developing a vaccine specific to Omicron Variant is also imminent.
Keywords: SARS-CoV-2, COVID-19, Omicron variants, B.1.1.529, Immune escape
Introduction
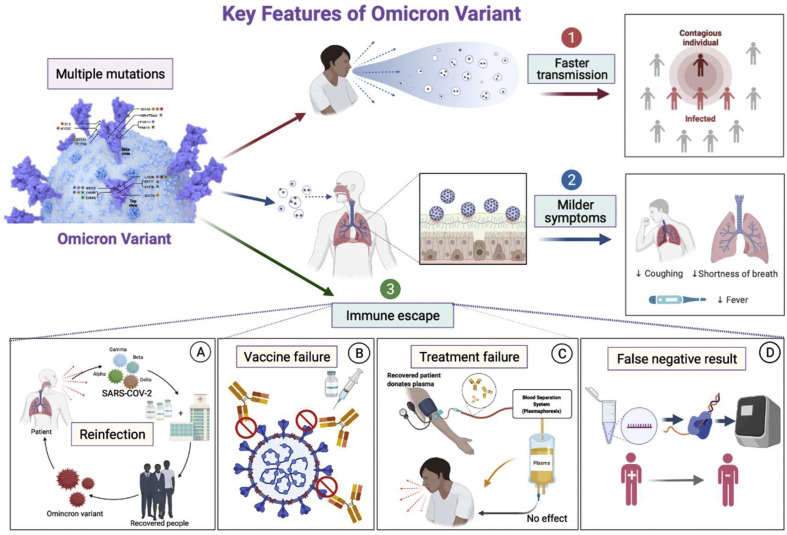
COVID-19, caused by SARS-CoV-2 and its various emerging variants, is a severe health threat to humans.1 , 2 However, a more worrying issue has recently emerged: a rapid surge of patients is being seen globally. A new SARS-CoV-2 variant makes its way to the world.3 The WHO named the omicron variant a variant of concern (VOCs) on November 26, 2021.4 In November 2021, the omicron variant was first found in South Africa. Subsequently, it quickly became the main variant in Gauteng. As of November 15, more than 75% of COVID-19–positive samples detected the omicron variant in South Africa.5 By November 29, 2021, cases with omicron variant infection had spread to many other countries. The alarming virus is sweeping the world again (see Fig. 1 ).
Figure 1.
The characteristics of Omicron Variant. Omicron variants contain many mutations that make them more infectious and transmissible. At the same time, immune escape is also caused, resulting in an increased risk of reinfection, reduced efficacy of existing vaccines, treatment failure of monoclonal antibody therapies, and detection failure.
Mutations of omicron variant
Overall, more than 60 mutations have been detected in Omicron variants.6 Among all SARS-CoV-2 variants, Omicron variants had the most mutation sites. In ORF1a, the Omicron variant has six substituents (A2710T, T3255I, K856R, L2084I, I3758 V, and P3395H) and two deletions of a total of four amino acids: amino acids 3674–3676 and amino acid 2083.7 Within ORF1b, the variant possesses P314 L and I1566 V substitutions. Additionally, a three-residue deletion and a P10S substitution are observed in ORF9b at positions. For the structural proteins, there are Q19E, D3G, and A63T substitutions in the membrane (M), one substitution (T9I) in the envelope (E), a three-residue deletion and three substitutions in the nucleocapsid (N) proteins.8
Although the mutations mentioned above occur throughout the whole viral genome, more than half of Omicron mutations have been detected in Spike. Several mutations in Spike have been confirmed to be involved in transmissibility, immune escape, and disease severity. These include 30 substitutions of S371 L, S373P, S375F, T95I, Y145D, A67 V, T478K, L212I, K417N, N440K, G446S, G339D, N679K, N501Y, S477N, Y505H, T547K, E484A, G496S, Q493R, Q498R, D614G, H655Y, P681H combination, D796Y, N764K, Q954H, N969K, N856K, and L981F, three deletions of N211, H69/V70 and G142/V143/Y144, and one insertion of three amino acids at position 214. Some reports have described these changes as V143/Y144/Y145 deletions with G142D and L212 deletions with N211I. The mutations in spikes found in Omicron were approximately 3-4 times greater than those observed in the other four VOC variants. It is worth noting that these five VOCs contain D614G mutations. Previous studies have established that D614G is associated with higher upper respiratory viral load and younger patients.9, 10, 11 In addition, the mutation of N501Y in the Omicron variant was also detected in alpha, beta, and gamma variants. It can enhance the binding force between spikes and angiotensin-converting enzyme 2, increasing transmission rates.12 The transmission rate of Omicron variants may be further enhanced in the presence of concurrent H69/V70 deletions.13 In addition, Omicron also had P681H and N679K mutations near furin cleavage sites. Binding to basic amino acids around the cleavage site of furin promotes the cleavage of the spikes into S1 and S2, thereby enhancing virus-cell fusion and viral infection.14
In summary, the Omicron variant possesses the most mutation sites in all SARS-CoV-2 variants identified to date. These mutations are involved in transmissibility, disease severity, and immune escape.
Symptoms and virulence
SARS-CoV-2 invades the respiratory system and causes other organ injuries in severe cases, such as kidney injury,15 liver injury,16 myocardial injury,17 coagulation dysfunction18 , and gastrointestinal symptoms.2 , 19 Compared with the early original strains, many clinical studies have suggested that in cases with alpha, beta, and delta variants, the risks of hospitalization, ICU admission, and death were increased.13 , 20, 21, 22 However, the clinical characteristics of the Omicron variant are different.
In Canada, as of December 21, 2021, the KFL&A Region had the highest number of COVID-19 cases compared with any other region, with 1574 active cases.23 Of all confirmed or suspected cases of Omicron, 59% were aged between 18 and 24 years, and 27% were aged between 25 and 39 years, while the main outbreak settings were higher education and food and beverage settings. It is worth noting that no cases were hospitalized, and the first modeling estimates placed the Re for Omicron in this region at 1.5. Nasal congestion (73%) was the most common symptom, followed by cough (65%), headache (54%), sore throat (48%), chills (34%), and fever (32%). Asymptomatic cases accounted for 9.6 percent of the total. Remarkably, shortness of breath occurs in only 10% of cases. Reported confirmed cases with the Omicron variant from South Africa24 , 25 and the United States26 , 27 were also asymptomatic or had mild symptoms, and no deaths were reported. Subsequently, UKHSA announced that ten individuals infected with Omicron were hospitalized, and one died.28 As of December 20, 2021, seven deaths in those infected with Omicron have been reported.29
In conclusion, the virulence of the Omicron variant is weaker than that of other mutants, and patients are less severe after infection.
Infectivity and transmission
The Omicron variant rapidly spreads worldwide. Data from epidemiological surveys show that South Africa has recorded three large outbreaks of COVID-19 transmission since the beginning of 2020. Two of these are caused by beta and delta mutations. Epidemiological data found that the percentage of beta variant infections increased to 50% of the total daily infections in the approximately 100 days since the outbreak.30 During the same period, the percentage infected with the Delta variant increased to 80%. However, in South Africa, the percentage of Omicron infections reached 90% in approximately 25 days. Early doubling times for Beta, Delta and Omicron variants were 1.7, 1.5 and 1.2 days, respectively.30 These data suggest that omicron variants may be more infectious than beta and delta variants. It is also worth noting that a recent retrospective study based on population-wide epidemiological data from South Africa suggested a possible increased risk of reinfection with omicron-driven SARS-CoV-2.31 As a result, the likelihood of COVID-19 spreading in South Africa and even globally in the coming days is high.
In vitro, computational studies applied affinity tests to detect the Delta and Omicron variants and showed that the Omicron variant had a higher affinity for human ACE2 than the Delta variant. The high affinity between the Omicron variant and ACE2 is attributed to its large number of mutations in the SARS-CoV-2 receptor-binding domain (RBD). Docking studies showed that mutations of Q493R, N501Y, S371 L, S373P, S375F, Q498R, and T478K were important reasons for high binding affinity with HUMAN ACE2. RBD is a true viral entity that mediates viral entry by recognizing ACE2 receptors.32 , 33 The Omicron variant has 15 mutations in the RBD, while the Delta variant had only the L452R and T478K mutations in the RBD. Among these substitutions, many residues are located near the bound ACE2 receptor. Therefore, these mutations may affect the infectivity and transmissibility of omicron variants. Omicron has a higher proportion of hydrophobic amino acids (such as leucine and phenylalanine) in the whole spike protein and RBD than the Delta type. These amino acids are located in the core of proteins and are necessary for structural stability. A disordered-ordered transformation was found in Omicron variants in the RBD region of the spike protein (468–473), which may have important implications in the effects of disordered residues/regions on spike protein stability and ACE2 binding.34
Another interesting finding can partly explain why Omicron Variant's virus spike has a stronger affinity for patients' ACE2. It was found that the positive electrostatic potential showed a clear upward trend from the original virus strain to the Delta virus strain and the recent Omicron variant. Since ACE2 plaques have a negative electrostatic surface potential, it is reasonable to speculate that the higher positive potential of the RBD may increase viral spike orientation and the overall interaction affinity of the ACE2 receptor. The relationship between the increase in positive electrostatic potential and affinity in the Delta VOCs (especially δ B.1.617.2) was discussed.35 If the electrostatic potential and receptor affinity have a direct relationship, then infectivity exists, then the hypothesis that the transmission of Omicron VOCs is higher than that of other VOCs will be supported. In addition, significant changes in the surface electrostatic potential of Omicron RBD can alter the interaction with macromolecules such as antibodies.36
In summary, the OM variant has stronger infectivity and transmission power due to more mutations and the surface electrostatic potential in the Omicron RBD.
Immune escape from omicron variant
Alterations in existing COVID-19 infection and vaccine-induced immune protection susceptibility may be associated with mutations in spike proteins.7 , 37 , 38
Risk of reinfection during the recent spread of the omicron variant
The sudden emergence of the Omicron variant in South Africa has again increased the risk of SARS-CoV-2 reinfection, suggesting that the Omicron variant may partially evade existing COVID-19 infection and immune responses induced by vaccines.31 Based on epidemiological data, we know of three outbreaks of COVID-19 in South Africa caused by the original virus, the beta variant, and the delta variant. Unfortunately, the whole country is again shrouded in the shadow of the Omicron variant.1 , 20 A preprint from NCID shows that the rate of new cases in the rapidly developing omicron wave was more than three times that of beta or Delta waves. However, the above data involved people who have already experienced COVID-19.39 However, it is unexpected that the estimated hazard ratio (HR) for reinfection versus primary infection was lower during the second (Beta) and third waves (Delta) than for the first wave (HR for the second wave versus the first wave: 0.75 (95% CI: 0.59–0.97); for the third wave versus the first wave: 0.71 (95% CI: 0.56–0.92)). In contrast, recent studies have reported that the increase in the reinfection hazard coefficient and the decrease in the primary infection hazard coefficient are related to the spread of the Omicron variant. More specifically, the HR for reinfection versus primary infection during the last 11 months (1 to November 27, 2021) versus the first wave was 2.39 (95% CI: 1.88–3.11).39 The same thing is happening in the UK. According to a recent report, a total of 5153 people were diagnosed with Omicron infection between November 1 and December 11, 2021, of which 305 were related to previously diagnosed infections. Meanwhile, we know that these 305 individuals had an interval from the previous positive test of at least 90 days.40 From the above data, we can roughly conclude that the Omicron variant may cause immune escape, resulting in a higher risk of reinfection than the Beta and Delta variants.
Many researchers are absorbedly studying the immune escape mechanism of Omicron variants. One of these studies successfully constructed a pseudomicron variant of SARS-CoV-2. They examined the sensitivity of serum samples from 28 COVID-19 convalescence patients infected with the original SARS-CoV-2 strain to pseudomicron and other variants of interest (VOCs, Alpha, Beta, Gamma, Delta), as well as several other variants of interest (VOIs, Lambda, Mu, etc.). The results showed that the average neutralizing ED50 of these sera to Omicron was approximately 8.4 times that of the D614G reference strain (ED50 = 556). However, the neutralization activity of other VOC and VOI pseudoviruses decreased only by approximately 1.2–4.5 times. This finding confirms that Omicron variants may evade immune protection from existing SARS-COV-2 infection.41
Protection of current COVID-19 vaccines against Omicron variants
In addition, along with the circulating variants escaping from the immune response to some degree, there is a heated discussion on whether the current COVID-19 vaccines still have a protective effect against the Omicron variant. Recently, a research team in the US used previous data to compare the efficacy of the COVID-19 vaccine against the earlier variant and the Omicron variant. Computer models they developed showed that Omicron was only approximately 30% effective against symptomatic infections after two doses of the mRNA vaccine from Pfizer/BioNTech or Moderna. However, the effectiveness against the Delta variant can be up to approximately 87%. The results suggested that the current COVID-19 vaccines are unlikely to provide enough immunity to the Omicron variant.42
There is such a concern that the existing neutralizing activity might be insufficient for adequate immune protection when faced with the Omicron variant. Recently, an in vitro study compared the neutralization of omicron-infected cells in serum samples from participants who received two and three vaccine doses. There were significant differences in geometric mean titers between wild-type, Omicron, and other viruses. For example, after the second dose of vaccine, the geometric mean titers of wild-type and beta, Delta, and Omicron variants were 16.56, 1.27, 8.00, and 1.11, respectively. After the third dose, the data were 891.4, 152.2, 430.5, and 107.6, respectively.43 Taking the BNT162b2 vaccine as an example, the geometric mean titer against the Omicron variant rose from 1.11 after the second dose to 107.6 after the third dose, suggesting that the third dose of the BNT162b2 vaccine may efficiently neutralize infection of the omicron variant.43
Omicron variant hazards are continuing and expanding. By December 9, 2021, Denmark had reported 785 SARS-CoV-2 Omicron variant cases, most of whom were fully vaccinated (76%), or booster vaccinated (7.1%). Among them, 34 cases (4.3%) had a previous SARS-CoV-2 infection.44 These data may indicate that current COVID-19 vaccines are as ineffective against Omicron variants as other SARS-COV-2 variants. Many other studies have come to a similar conclusion. A recent study showed that serum obtained from vaccinated individuals was approximately 40 times less neutralizing against the Omicron variant than wild-type SARS-CoV-2.45
A managed care organization in South Africa used data from Discovery Health to estimate the vaccine effectiveness against hospitalization for COVID-19 caused by the Omicron variant after full vaccination (i.e., two doses of the BNT162b2 vaccine).5 They found that the BNT162b2 vaccine was 70% effective (95% confidence interval [CI], 62–76) during the proxy Omicron period, which was significantly different from the comparison period (93% (95% CI, 90–94)).5
In late November 2021, the omicron SARS-CoV-2 virus was found in Oslo, Norway, which could be traced to a Christmas party with 117 participants. The attack rate in this outbreak was approximately 74%, and most cases developed symptoms. However, none have been hospitalized as of December 13. Most participants were middle-aged (30–50 years old), among whom 96% were fully vaccinated. Most cases (n = 79; 98%) and noncases (n = 27; 93%) were fully vaccinated, with a median time of 79 days since receiving the last vaccine dose in the case group and 87 days in the noncase group (no significant difference, Wilcoxon rank-sum, P = 0.48). Fifty-five percent (41/75) of patients who received two vaccine doses received combination therapy (BNT162b2 mRNA, Biontech-Pfizer, Mainz, Germany/New York, USA (USA)). However, 23% (17/75) received spike wax (mRNA-1273, Moderna, Cambridge, US). Of the 25 noncases who received two vaccine doses, seven received community treatment, and 12 were treated with Spikewax.46 These findings, to some degree, can illustrate the hypothesis that the Omicron variant may be more transmissible, so the current vaccination may not be able to provide enough protective effect in preventing infection.46
In a recent study, geometric mean neutralizing antibody titers (GMTs) were significantly lower in those who received the BNT162b2 vaccine (5.43 and 6.42) of the Omicron variant than in the Beta and Delta variants. GMTs of the two Omicron variants were 35.7–39.9 times lower than that of the original strain (229.4). There was no significant difference in the GMT value between HKU691 and HKU344-R346K.47 The additional R346K mutation did not affect neutralization susceptibility, but the Omicron variant can dexterously escape neutralizing antibodies elicited by BNT162b2 or Coronavac, suggesting that the Omicron variant may result in lower COVID-19 vaccine effectiveness.43 However, several vaccine companies and research teams (including BioNTech and Pfizer companies and the Israeli Scientific Team) confirmed that the primary vaccine still has some protective effect against Omicron Variant and actual virus pseudovirus.48 On December 8, BioNTech and Pfizer claimed that receiving three doses of the vaccine provided better neutralization against wild-type and three variants of the virus than receiving two. A laboratory test found that their COVID-19 vaccine neutralized the new Omicron variant with a three-dose course. They also found that although the two doses produced much lower neutralizing antibodies, they still protected against severe illness.49 Similar findings to BioNTech and Pfizer, they compared to blood from 20 people who had received two doses of the vaccine with the same number who had received a booster. The results showed that people who received the second dose five or six months earlier still had some neutralization against the original virus, beta, and delta variants, but none against Omicron. Their research also indicated that booster vaccination might be indispensable in preventing infection from the newly identified variant, such as the Omicron variant.
At present, there are still no long-term monitoring data on the effectiveness of currently available COVID-19 vaccines to Omicron spike mutations. Still, we can show that existing vaccines based on wild-type SARS-CoV-2 are ineffective enough to prevent mutated infections.50 Therefore, it is necessary to develop mutation-specific vaccines based on the mutated Spike, especially for the Omicron variant, because vaccines based on the mutated Spike have higher levels of neutralizing antibodies against the mutated virus. In addition, although some vaccines have been developed based on other variants, these vaccine candidates can also be used to prevent Omicron infection and its transmission as long as they contain one or more Omicron mutations.
Omicron Variant's impact on existing monoclonal antibody therapies
Notably, Omicron has 15 substitutes in the RBD region, a major target for neutralizing antibodies. In addition, all of these mutations can be located at one or more antigenic sites of the RBD; including RBS-A, RBS-B, RBS-C, CR302, and S309.51 Thus, Omicron may be resistant to one or more monoclonal antibodies that target these sites. In clinical terms, the FDA has approved emergency use of a cocktail of LY-CoV555 (bamlanivimab) and LY-CoV016 (etevimab). According to previous studies, mutations at positions 484 and 417 were associated with immune evasion,52 and both beta and gamma variants are reported to be resistant to the neutralization of LY-CoV 016 (due to K417 N/T) and LY-CoV 555 (due to E484K).53 Thus, Omicron is likely to resist both antibodies as well, as E484A and K417N mutations have been found in the Omicron variant. To date, only two monoclonal antibodies have been found to be effective neutralizing agents against Omicron (IC50: 0.181 ng/L for Vir-7831; 0.287 ng/μl for DXP-604).54 In addition, an in vitro study of authentic Omicron virus showed that imdevimab and casirivimab could effectively prevent Delta infection, while Omicron was resistant to casirivimab and imdevimab.55
Influence of Omicron variant on detection of SARS-CoV-2
In addition to the influence on antibody efficacy, there is also the risk of detection failure. There is growing evidence that these mutations may reduce the reliability of commercial immunoassays to detect antibodies against SARS-CoV-2. The antigens (spikes or RBD) and epitopes of the prototype SARS-CoV-2 can no longer reflect the sequence of circulating virus variations. As a result, commercially available immunoassays may not be able to identify antibodies triggered by highly mutated SARS-CoV-2 variants.56
In contrast, mutations in the Omicron strain did not appear to affect the accuracy of the commonly used antigen-based rapid diagnostic test (AG-RDT) test. According to a recent study evaluating ten commercially available COVID-19 antigen tests, there was no significant difference between delta and omicron variants in the analytical sensitivity of the ten antigen kits.57 All ten kits detected Delta at 6.50 log10 copies/mL (Ct 25.4) and Omicron at 6.39 log10 copies/mL (Ct 25.8), which is consistent with previous studies of assay sensitivity of antigen kits.57 Neither Delta nor Omicron was detected in any ten kits at the lowest dilution concentrations (5.23 log10 copies/mL, Ct28.8 and 5.33 log10 copies/mL, Ct 28.8, respectively).57 This result is consistent with other work demonstrating the validity of antigen tests for SARS-CoV-2 variants. Most Omicron strains also affect the accuracy of conventional PCR detection. However, in RT–PCR, the BA.1 lineage showed S target gene failure (SGTF) due to multiple deletions of the S glycoprotein NTD, while the BA.2 lineage may skip SGTF due to NTD deletion.
In summary, immune escape caused by Omicron Variant causes reduced efficiency of existing vaccines, increased risk of reinfection, treatment failure of monoclonal antibody therapies, and detection failure.
Strategies for prevention of Omicron variant
To date, the detailed characteristics of the Omicron variant remain unclear. These mutations can also be observed in other VOCs. Of particular concern is that Omicron may have evolved to spread more easily among humans and resist current antibody therapies. Therefore, there is a definite need to maintain existing public health precautions, including frequent hand washing, physical distancing, and wearing protective equipment. These measures proved effective in preventing the spread of other variants and should also reduce the spread of the Omicron variant. In addition, early diagnosis and timely isolation are essential to reduce the transmission of the virus. Previous studies have shown that an increase in Omicron infections leads to a higher percentage of PCR tests for spike gene failure. Therefore, it is essential to improve diagnostic accuracy, ensure timely isolation and treatment of infected patients and cut off the cycle of Omicron mutations.
Several studies have reported a decrease in serum neutralizing antibodies six months after vaccination. At the same time, adding a booster can restore the vaccine's effectiveness to its original level, or even higher57, 58, 59, 60. We believe that additional doses of the COVID-19 vaccine will undoubtedly help prevent the spread of Omicron.
Conclusion
In summary, Omicron Variant contains many mutations that make it more infectious and transmissible. At the same time, immune escape is also caused, resulting in reduced efficacy of existing vaccines, increased risk of reinfection, treatment failure or reduction of monoclonal antibody therapies, and detection failure. However, current data indicate that Omicron Variant causes mild clinical symptoms and few severe cases and deaths. Omicron Variant is valid for a range of nonpharmaceutical interventions against SARS-CoV-2. Improving diagnostic accuracy and enabling timely isolation and treatment of diagnosed cases is also critical to interrupting the spread of omicron variants. Adding additional doses of the COVID-19 vaccine to vaccination schedules will undoubtedly help control the spread and infection of Omicron. However, developing a vaccine against the Omicron variant is also urgent.
Ethical
Not relevant. We declare that reporting of the study was in line with the Declaration of Helsinki, as revised in 2013.
Funding
None declare.
Author contributions
Wenxia Shao wrote the manuscript and prepared figures. Wenxia Shao, Weiying Zhang, Xiang Fang, and Daojun Yu contributed to the data collection and designed the figure. Daojun Yu and Xianjun Wang conceived and contributed to the modification and revision of the manuscript. All authors contributed to this article and approved the submitted versions.
Acknowledgments
None declare.
References
- 1.Tian D., Sun Y., Zhou J., Ye Q. The global epidemic of the SARS-CoV-2 delta variant, key spike mutations and immune escape. Front Immunol. 2021;12:751778. doi: 10.3389/fimmu.2021.751778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou X., Ye Q. Cellular immune response to COVID-19 and potential immune modulators. Front Immunol. 2021;12:646333. doi: 10.3389/fimmu.2021.646333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahmani S., Rezaei N. Omicron (B.1.1.529) variant: development, dissemination, and dominance. J Med Virol. 2021;94:1787–1788. doi: 10.1002/jmv.27563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . 2021. Classification of omicron (B.1.1.529): SARS-CoV-2 variant of concern.https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b .1.1.529)-sars-cov-2-variant-of-concern [Google Scholar]
- 5.Collie S., Champion J., Moultrie H., Bekker L.G., Gray G. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N Engl J Med. 2021;386:494–496. doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GISAID . 2021. Covariants.https://covariants.org/variants/21K.Omicron [Google Scholar]
- 7.Tian D., Sun Y., Zhou J., Ye Q. The global epidemic of SARS-CoV-2 variants and their mutational immune escape. J Med Virol. 2022;94:847–857. doi: 10.1002/jmv.27376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.University. S. CORONAVIRUS ANTIVIRAL & RESISTANCE DATABASE. https://covdb.stanford.edu/page/susceptibility-data/. Last updated on 12/23/2021, 4:09:41 PM.
- 9.Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., et al. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;182:812–827. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plante J.A., Liu Y., Liu J., Xia H., Johnson B.A., Lokugamage K.G., et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature. 2021;592:116–121. doi: 10.1038/s41586-020-2895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volz E., Hill V., McCrone J.T., Price A., Jorgensen D., O’Toole Á, et al. Evaluating the effects of SARS-CoV-2 spike mutation D614G on transmissibility and pathogenicity. Cell. 2021;184:64–75. doi: 10.1016/j.cell.2020.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang T.-J., Yu P.-Y., Chang Y.-C., Liang K.-H., Tso H.-C., Ho M.-R., et al. Impacts on the structure-function relationship of SARS-CoV-2 spike by B.1.1.7 mutations. bioRxiv. 2021;5:443686. doi: 10.1101/2021.05.11.443686. [DOI] [Google Scholar]
- 13.Leung K., Shum M.H., Leung G.M., Lam T.T., Wu J.T. Early transmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom, October to November 2020. Euro Surveill. 2021;26:2002106. doi: 10.2807/1560-7917.ES.2020.26.1.2002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuckerman N.S., Fleishon S., Bucris E., Bar-Ilan D., Linial M., Bar-Or I., et al. A unique SARS-CoV-2 spike protein P681H variant detected in Israel. Vaccines (Basel) 2021;9:616. doi: 10.3390/vaccines9060616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han X., Ye Q. Kidney involvement in COVID-19 and its treatments. J Med Virol. 2021;93:1387–1395. doi: 10.1002/jmv.26653. [DOI] [PubMed] [Google Scholar]
- 16.Tian D., Ye Q. Hepatic complications of COVID-19 and its treatment. J Med Virol. 2020;92:1818–1824. doi: 10.1002/jmv.26036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye Q., Shang S., Fu J., Gong F., Shu Q., Mao J. Crosstalk between coronavirus disease 2019 and cardiovascular disease and its treatment. ESC Heart Fail. 2020;7:3464–3472. doi: 10.1002/ehf2.12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo H.C., You C.Y., Lu S.W., Fu Y.Q. Characteristics of coagulation alteration in patients with COVID-19. Ann Hematol. 2021;100:45–52. doi: 10.1007/s00277-020-04305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye Q., Wang B., Zhang T., Xu J., Shang S. The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19. Am J Physiol Gastrointest Liver Physiol. 2020;319:G245–G252. doi: 10.1152/ajpgi.00148.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makoni M. South Africa responds to new SARS-CoV-2 variant. Lancet. 2021;397:267. doi: 10.1016/S0140-6736(21)00144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh J., Rahman S.A., Ehtesham N.Z., Hira S., Hasnain S.E. SARS-CoV-2 variants of concern are emerging in India. Nat Med. 2021;27:1131–1133. doi: 10.1038/s41591-021-01397-4. [DOI] [PubMed] [Google Scholar]
- 22.Tian D., Sun Y., Zhou J., Ye Q. The global epidemic of SARS-CoV-2 variants and their mutational immune escape. J Med Virol. 2022;94:847–857. doi: 10.1002/jmv.27376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li A., Maier A., Carter M., Hugh Guan T. Omicron and S-gene target failure cases in the highest COVID-19 case rate region in Canada - december 2021. J Med Virol. 2022;94:1784–1786. doi: 10.1002/jmv.27562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frequently asked questions for the B.1.1.529 mutated SARS-COV-2 lineage in South Africa. https://www.nicd.ac.za/frequentlyasked-questions-for-the-b-1-1-529-mutated-sars-cov-2-lineage-in-south-africa/. 2021.
- 25.Dyer O. Covid-19: South Africa’s surge in cases deepens alarm over omicron variant. BMJ. 2021;375:n3013. doi: 10.1136/bmj.n3013. [DOI] [PubMed] [Google Scholar]
- 26.Enhancing readiness for omicron (B.1.1.529): technical brief and priority actions for member States. 10 Dec 2021. https://www.who.int/publications/m/item/enhancing-readiness-for-omicron-(b.1.1.529)-technical-brief-and-priority-actions-for-member-states. 2021 [Google Scholar]
- 27.Omicron Variant: What You Need to Know. Updated Dec. 10, 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/omicron-variant.html. 2021.
- 28.COVID-19 variants identified in the UK. https://www.gov.uk/government/news/covid-19-variants-identified-in-the-uk. 2021.
- 29.Epidemiological update: omicron variant of concern (VOC) – data as of 19 December 2021 (12:00) 2021. https://www.ecdc.europa.eu/en/news-events/epidemiological-update-omicron-variant-concern-voc-data-19-december-2021 [Google Scholar]
- 30.Karim S.S.A., Karim Q.A. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398:2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pulliam J.R.C., van Schalkwyk C., Govender N., von Gottberg A., Cohen C., Groome M.J., et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. medRxiv. 2021;11:21266068. doi: 10.1101/2021.11.11.21266068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lan J., Ge J., Yu J., Shan S., Zhou H., Fan S., et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 33.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar S., Thambiraja T.S., Karuppanan K., Subramaniam G. Omicron and Delta variant of SARS-CoV-2: a comparative computational study of spike protein. J Med Virol. 2022;94:1641–1649. doi: 10.1002/jmv.27526. [DOI] [PubMed] [Google Scholar]
- 35.Pascarella S., Ciccozzi M., Zella D., Bianchi M., Benedetti F., Benvenuto D., et al. SARS-CoV-2 B.1.617 Indian variants: are electrostatic potential changes responsible for a higher transmission rate? J Med Virol. 2021;93:6551–6556. doi: 10.1002/jmv.27210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pascarella S, Ciccozzi M, Bianchi M, Benvenuto D, Cauda R, Cassone A. The electrostatic potential of the Omicron variant spike is higher than in Delta and Delta-plus variants: a hint to higher transmissibility? J Med Virol. 2022;94:1277–1280. doi: 10.1002/jmv.27528. [DOI] [PubMed] [Google Scholar]
- 37.Han X., Xu P., Ye Q. Analysis of COVID-19 vaccines: types, thoughts, and application. J Clin Lab Anal. 2021;35:e23937. doi: 10.1002/jcla.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han X., Ye Q. The variants of SARS-CoV-2 and the challenges of vaccines. J Med Virol. 2022;94:1366–1372. doi: 10.1002/jmv.27513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pulliam J.R.C., van Schalkwyk C., Govender N., von Gottberg A., Cohen C., Groome M.J., et al. 2021. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Latest updates 12 Dec 2021. on SARS-CoV-2 variants detected in the UK. https://www.gov.uk/government/news/covid-19-variants-identified-in-the-uk. 2021.
- 41.Wang Y., Zhang L., Li Q., Liang Z., Li T., Liu S., et al. The significant immune escape of pseudotyped SARS-CoV-2 variant Omicron. Emerg Microb Infect. 2022;11:1–5. doi: 10.1080/22221751.2021.2017757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardner BJ, Kilpatrick AM. Estimates of reduced vaccine effectiveness against hospitalization, infection, transmission and symptomatic disease of a new SARS-CoV-2 variant, Omicron (B.1.1.529), using neutralizing antibody titers. medRxiv. 2021;12:21267594. doi: 10.1101/2021.12.10.21267594. [DOI] [Google Scholar]
- 43.Nemet I., Kliker L., Lustig Y., Zuckerman N., Erster O., Cohen C., et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 omicron infection. N Engl J Med. 2022;386:492–494. doi: 10.1056/NEJMc2119358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Espenhain L., Funk T., Overvad M., Edslev S.M., Fonager J., Ingham A.C., et al. Epidemiological characterisation of the first 785 SARS-CoV-2 Omicron variant cases in Denmark, December 2021. Euro Surveill. 2021;26:2101146. doi: 10.2807/1560-7917.ES.2021.26.50.2101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cele S., Jackson L., Khoury D.S., Khan K., Moyo-Gwete T., Tegally H., et al. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv. 2021;12:21267417. doi: 10.1101/2021.12.08.21267417. [DOI] [Google Scholar]
- 46.Brandal L.T., MacDonald E., Veneti L., Ravlo T., Lange H., Naseer U., et al. Outbreak caused by the SARS-CoV-2 omicron variant in Norway, november to december 2021. Euro Surveill. 2021;26:2101147. doi: 10.2807/1560-7917.ES.2021.26.50.2101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu L., Mok B.W., Chen L.L., Chan J.M., Tsang O.T., Lam B.H., et al. Neutralization of SARS-CoV-2 Omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. Clin Infect Dis. 2021:ciab1041. doi: 10.1093/cid/ciab1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reuters . December 12, 2021. Israeli study finds Pfizer COVID-19 booster protects against Omicron.https://www.reuters.com/world/middle-east/israeli-study-finds-pfizer-covid-19-booster-protects-against-omicron-2021-12-11/.
- 49.Pfizer. Pfizer and BioNTech Provide Update on Omicron Variant. Press release. Wednesday, December 08, 2021 - 06:54am. https://www.pfizer.com/news/press-release/press-releasedetail/pfizer-and-biontech-provide-update-omicron-variant. (Last accessed December 12, 2021.
- 50.He X., Hong W., Pan X., Lu G., Wei X. SARS-CoV-2 Omicron variant: characteristics and prevention. MedComm. 2021;16:838–845. doi: 10.1002/mco2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuan M., Liu H., Wu N.C., Wilson I.A. Recognition of the SARS-CoV-2 receptor binding domain by neutralizing antibodies. Biochem Biophys Res Commun. 2021;538:192–203. doi: 10.1016/j.bbrc.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zimerman R.A., Gröhs Ferrareze P.A., Cadegiani F.A., Wambier C.G., Fonseca DdN., de Souza A.R., et al. Comparative genomics and characterization of SARS-CoV-2 P.1 (gamma) variant of concern (VOC) from Amazonas, Brazil. medRxiv. 2021;10:21265694. doi: 10.1101/2021.10.30.21265694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Starr T.N., Greaney A.J., Dingens A.S., Bloom J.D. Complete map of SARS-CoV-2 RBD mutations that escape the monoclonal antibody LY-CoV555 and its cocktail with LY-CoV016. Cell Rep Med. 2021;2:100255. doi: 10.1016/j.xcrm.2021.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao Y., Wang J., Jian F., Xiao T., Song W., Yisimayi A., et al. 2021. B.1.1.529 escape the majority of SARS-CoV-2 neutralizing antibodies of diverse epitopes. [Google Scholar]
- 55.Wilhelm A., Widera M., Grikscheit K., Toptan T., Schenk B., Pallas C., et al. 2021. Reduced neutralization of SARS-CoV-2 omicron variant by vaccine sera and monoclonal antibodies. [Google Scholar]
- 56.Lippi G., Adeli K., Plebani M. Commercial immunoassays for detection of anti-SARS-CoV-2 spike and RBD antibodies: urgent call for validation against new and highly mutated variants. Clin Chem Lab Med. 2022;60:338–342. doi: 10.1515/cclm-2021-1287. [DOI] [PubMed] [Google Scholar]
- 57.Bekliz M., Adea K., Essaidi-Laziosi M., Sacks J.A., Escadafal C., Kaiser L., et al. SARS-CoV-2 antigen-detecting rapid tests for the delta variant. Lancet Microbe. 2021;3:e90. doi: 10.1016/S2666-5247(21)00302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffmann M, Krüger N, Schulz S, Cossmann A, Rocha C, Kempf A, et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell. 2022;185:447–456. doi: 10.1016/j.cell.2021.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Planas D, Saunders N, Maes P, Guivel-Benhassine F, Planchais C, Buchrieser J, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602:671–675. doi: 10.1038/s41586-021-04389-z. [DOI] [PubMed] [Google Scholar]
- 60.Fabricius D, Ludwig C, Scholz J, Rode I, Tsamadou C, Jacobsen EM, et al. mRNA vaccines enhance neutralizing immunity against sars-cov-2 variants in convalescent and chadox1-primed subjects. Vaccines (Basel) 2021;9:918. doi: 10.3390/vaccines9080918. [DOI] [PMC free article] [PubMed] [Google Scholar]