Abstract
Background
Variation in blood pressure levels display circadian rhythms. The morning surge in blood pressure is known to increase the risk of myocardial events in the first several hours post awakening. A systematic review of the administration‐time‐related‐effects of evening versus morning dosing regimen of antihypertensive drugs in the management of patients with primary hypertension has not been conducted.
Objectives
To evaluate the administration‐time‐related‐effects of antihypertensive drugs administered as once daily monotherapy in the evening versus morning administration regimen on all cause mortality, cardiovascular morbidity and reduction of blood pressure in patients with primary hypertension.
Search methods
We searched Cochrane CENTRAL on Ovid (4th Quarter 2009), Ovid MEDLINE (1950 to October 2009), EMBASE (1974 to October 2009), the Chinese Biomedical literature database (1978 to 2009) and the reference lists of relevant articles. No language restrictions were applied.
Selection criteria
Randomized controlled trials comparing the administration‐time‐related effects of evening with morning dosing monotherapy regimens in patients with primary hypertension were included. Patients with known secondary hypertension, shift workers or white coat hypertension were excluded.
Data collection and analysis
Two authors independently extracted data and assessed trial quality. Disagreements were resolved by discussion or a third reviewer. Data synthesis and analysis were done using RevMan 5.1. Random effects meta‐analysis and sensitivity analysis were conducted.
Main results
21 randomized controlled trials (RCTs) in 1,993 patients with primary hypertension met the inclusion criteria for this review ‐ ACEIs (5 trials), CCBs (7 trials), ARBs (6 trials), diuretics (2 trials), alpha‐blockers (1 trial), and beta‐blockers (1 trial). Meta‐analysis showed significant heterogeneity across trials.
No RCT reported on all cause mortality, cardiovascular mortality, cardiovascular morbidity and serious adverse events.
There was no statistically significant difference for overall adverse events (RR=0.78, 95%CI: 0.37 to 1.65) and withdrawals due to adverse events (RR=0.53, 95%CI: 0.26 to 1.07).
No significant differences were noted for morning SBP (‐1.62 mm Hg, 95% CI: ‐4.19 to 0.95) and morning DBP (‐1.21 mm Hg, 95% CI: ‐3.28 to 0.86); but 24‐hour BP (SBP: ‐1.71 mm Hg, 95% CI: ‐2.78 to ‐0.65; DBP: ‐1.38 mm Hg, 95% CI: ‐2.13 to ‐0.62) showed a statistically significant difference.
Authors' conclusions
No RCT reported on clinically relevant outcome measures ‐ all cause mortality, cardiovascular morbidity and morbidity. There were no significant differences in overall adverse events and withdrawals due to adverse events among the evening versus morning dosing regimens. In terms of BP lowering efficacy, for 24‐hour SBP and DBP, the data suggests that better blood pressure control was achieved with bedtime dosing than morning administration of antihypertensive medication, the clinical significance of which is not known.
Plain language summary
Time effects of blood pressure lowering drugs for the treatment of high blood pressure
Elevated blood pressure is an important public health problem and once daily dosing regimen with blood pressure lowering drugs are recommended to reduce risk of strokes and heart attacks. This review examined the administration‐time‐related effects of once‐daily evening versus morning regimen on death, cardiovascular outcomes and blood pressure reduction. The interventions included chronotherapeutic delivery formulations and conventional antihypertensive agents. 21 trials, involving 1,993 patients with primary hypertension were identified. We concluded that evening dosing with antihypertensive drugs had a slightly better blood pressure control than the morning dosing regimen in 24‐hour BP, but its effect on death and adverse cardiovascular outcomes is not known.
Background
Description of the condition
Elevated blood pressure or hypertension (defined as resting blood pressure levels of 140/90 mm Hg or more) is estimated to affect 20% of the adult population in both developed and developing countries. It is associated with an increased risk of death, and cardiovascular disease (CVD).
Six main classes of antihypertensive drugs are used worldwide: diuretics, angiotensin converting enzyme inhibitors (ACEIs), calcium channel blockers (CCBs), beta‐adrenergic receptor blockers (BBs), angiotensin II receptor blockers (ARBs) and alpha‐adrenergic antagonists. It is a well known fact that variation in blood pressure levels display circadian rhythms. The morning surge in blood pressure is known to increase the risk of myocardial events in the first several hours post awakening. WHO recommends using once daily long acting antihypertensive drugs, since they provide a more consistent 24‐hour BP control, reduce BP variability, and improve adherence to therapy (Guidelines Subcommittee 1999). Antihypertensive drugs are traditionally administered either as monotherapy or in combinations in the morning upon arising from bed. This is mainly because this approach has been applied in the vast majority of outcome trials that showed benefits of treatment in reducing the risk of CVD. Another important reason is that a once‐daily morning regimen improves patients' adherence to the long‐term treatment (Chobanian 2003, Waeber 1999). However, the administration‐time‐effects of evening versus morning dosing regimen of antihypertensive drugs on clinically relevant outcomes such as death and cardiovascular outcomes in the management of patients with primary hypertension has not been studied in a systematic review.
Description of the intervention
In this review, the conventional or routine administration of antihypertensive drug therapy for essential hypertension means dosing in the morning upon arising from bed. The traditional antihypertensive agents include long acting medications or the conventional (so‐called homeostatically formulated) drugs administered without regard to BP circadian rhythm. They differ from chronotherapeutic formulations which are specially designed to provide peak plasma concentrations during the early morning hours when BP rises to peak and provide lower concentrations at night (Smolensky 1999).
Chronotherapeutics is defined as the purposeful timing of medications, whether or not they utilize special drug release technology, to proportion serum and tissue concentrations in synchrony with known circadian rhythms in disease processes and symptoms as a means of enhancing beneficial outcomes and/or attenuating or averting adverse effects (Smolensky 1996). The chronotherapy of hypertension specifically entails significant attenuation of the accelerated morning rise of SBP and DBP and this may be achieved through the use of special drug‐delivery technology (Smolensky 2005) or by changing the dosing timing of conventional BP‐lowering medications (Hermida 2004a, Hermida 2005e).
Ambulatory blood pressure monitoring (ABPM) is a valuable technique to determine antihypertensive efficacy both in clinical practice and in research settings (O'Brien 1991). The use of such monitoring makes it feasible to follow the time course of BP variation around the clock in large groups of subjects. Compared with traditional resting BP measurement, it allows the assessment of duration of action of antihypertensive agents and compensates for most of the limitations of office determinations (Hermida 1999). It also makes it possible to exclude pharmacotherapy in patients who have white coat hypertension, and allows the evaluation of the consistency of the antihypertensive effect of new drug‐chronotherapeutic agents (Canter 1994).
How the intervention might work
Blood pressure (BP) varies throughout the day, has a distinct and reproducible 24‐hour circadian rhythm in both normotensive and uncomplicated hypertensive patients (Hermida 2002, O'Brien 2003, White 1997a, White 1999). In patients who are awake during the daytime and asleep during the nighttime, their BP and HR have showed a typical circadian variation, with lower BP levels during nighttime sleep and an abrupt rise upon arising in the morning (Pickering 1993, White 1989, White 1997a, White 1999). This pattern is rapidly reversed when individuals work night shifts and sleep during the day (Sunderg 1988). It was previously reported that the morning BP surge upon arising from bed appeared to parallel the morning surge in the incidence of cardiovascular events and was significantly associated with a greater target organ damage and higher cardiovascular events risk (Kario 2003, Kuwajima 1995, Muller 1989, White 2001). Based on this rationale, it is hypothesized that antihypertensive medication targeted for early morning BP control in addition to providing 24‐hour BP control would result in a significant reduction of cardiovascular events in hypertensive patients. In other words, the medication is considered to lower BP consistently as well as reduce excessive peaks in pressure that may pose an additional cardiovascular risk.
Why it is important to do this review
Based on the above mentioned relationship, researchers began to apply the science of chronotherapeutics, or timing of drug effect to the treatment of essential hypertension to improve cardiovascular outcomes. A number of studies investigated the administration‐time‐dependent antihypertensive efficacy, e.g. ACEIs such as ramipril (Hermida 2009a, Myburgh 1995), trandolapril (Kuroda 2004), perindopril (Morgan 1997), and quinapril (Palatini 1992); CCBs such as diltiazem (Glasser 2003), nifedipine (Hermida 2007, Hermida 2008, Hermida 2009b), cilnidipine (Kitahara 2004), nisoldipine (White 1999a) and amlodipine (Nold 1998, Qiu 2003); diuretics such as torasemide (Calvo 2006a, Hermida 2008a); ARBs such as valsartan (Hermida 2003, Hermida 2005a, Hermida 2005b), telmisartan (Hermida 2007a), and olmesartan (Hermida 2009); α‐blockers such as doxazosin (Hermida 2004). A few clinical trials had found that nighttime dosing was more effective than morning administration to optimize morning BP control while maintaining 24‐hour efficacy (Glasser 2003, White 2004), but another trial found no difference in morning SBP between the two groups (Wright 2004). One large trial compared verapamil versus atenolol or HCTZ on reduction of BP and cardiovascular risk (Black 2003).
A study in hypertensive rats showed that dosing an ACE inhibitor, trandolapril, at night, had a better organ protective effect than dosing in the morning (Sugimoto 2001). Clinical trials have been performed in hypertensive patients by changing the time of dosing from morning to evening to enhance their effectiveness on cardiovascular outcomes (Fujimura 1999). There are also a few non‐systematic or traditional reviews focusing on this issue (Ezeugo 2009, Hermida 2007C, Hermida 2007d, Ohmori 2005, Stergiou 2007), some of which reported that nighttime administration of antihypertensive drugs had a larger blood pressure lowering effect during nighttime and the early morning hours. There is considerable evidence that the morning administration gives its full effect during daytime activities and a lesser effect during nighttime and the early morning hours, whereas bedtime administration has a larger effect during nighttime and the early morning hours. However, no systematic review and meta‐analysis has been conducted to confirm these findings. It might be argued that bedtime administration should be considered as an alternative strategy that has the potential benefits to provide more effective cardiovascular protection.
Objectives
To evaluate the effectiveness of administration‐time‐related effects of once‐daily evening versus conventional morning dosing antihypertensive drug therapy regimen on all cause mortality, cardiovascular mortality and morbidity, total adverse events, withdrawals due to adverse effects and reduction of systolic and diastolic blood pressure in patients with primary hypertension.
The secondary objective is to also compare once daily administration of antihypertensive chronotherapeutic delivery system (evening administration) versus a conventional monotherapy regimen (morning administration) in the management of patients with essential hypertension.
Methods
Criteria for considering studies for this review
Types of studies
Included studies must be randomised controlled trials of at least 3 weeks treatment duration. Randomized cross‐over trials which were restricted to designs with 2 interventions and 2 treatment periods were also included.
Types of participants
Adult patients with primary (essential) hypertension whose systolic and/or diastolic blood pressure levels were 140/90 mm Hg or greater were included. Patients with secondary causes of hypertension, white coat hypertension and alternating shift workers were excluded.
Types of interventions
Intervention: Monotherapy with an antihypertensive drug + administered once‐daily in the evening *
Control: Monotherapy with the same antihypertensive drug at the same dose administered once‐daily in the morning **.
+ Antihypertensive drug belonging to any one of the following six classes: angiotensin converting enzyme inhibitors (ACEIs), calcium channel blockers (CCBs), beta‐blockers (BBs), diuretics, angiotensin II receptor blockers (ARBs) and alpha‐blockers
*Evening administration was defined from 6:00 p.m. to 12:00 midnight
**Morning administration was defined from 6:00 a.m. to 12:00 noon
For the comparison between chronotherapeutic and conventional monotherapy drug regimen, the chronotherapeutic group should take the same drug and dose (evening administration) as the conventional regimen (morning administration only).
Types of outcome measures
Primary outcomes Death from all causesCardiovascular mortalityCardiovascular morbidity (stroke, myocardial infarction, congestive heart failure, aortic aneurysm)
Primary outcomes
Death from all causes
Cardiovascular mortality
Cardiovascular morbidity (stroke, myocardial infarction, congestive heart failure, aortic aneurysm)
Secondary outcomes
Serious adverse events
Overall adverse effects
Withdrawals from treatment due to adverse effects
Change from baseline in 24‐hour mean SBP and DBP by ambulatory BP monitoring
Change from baseline in morning SBP and DBP (assessed by ambulatory BP monitoring during the periods from 6 a.m. to 12 noon)
Search methods for identification of studies
Electronic searches
We searched the following electronic databases for randomised controlled trials (RCTs):
The Cochrane Central Register of Controlled Trials (CENTRAL) on Ovid (4th Quarter 2009)
Ovid MEDLINE from 1950 to October 2009
EMBASE.com from 1974 to October 2009
Chinese Biomedical Literature Database (CBLD) from 1978 to 2009
The Electronic databases were searched using a strategy combining a variation of the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision) with selected MeSH terms and free text terms relating to chronotherapy and hypertension. There were no language restrictions. The MEDLINE search strategy was translated into the other databases using the appropriate controlled vocabulary as applicable. The full electronic database search strategies are in Appendix 1, Appendix 2, Appendix 3, Appendix 4.
Searching other resources
Reference lists of meta‐analyses and relevant reviews were identified. Bibliographic citations from retrieved studies were also hand‐searched.
Authors of trials reporting incomplete information were contacted to provide the missing information.
Data collection and analysis
Selection of studies
All titles and the abstracts resulting from the search strategies were screened independently by two reviewers (Xu Ping and Zhao Ping). Articles were rejected on initial screening if they clearly did not meet the pre‐specified inclusion criteria. The full text of the remaining articles were then retrieved and translated to English where required. The bibliographies of pertinent articles, reviews and texts were searched for additional citations. Studies which met the inclusion criteria were examined in detail. Reasons for excluding any study were documented. Trials with more than one publication were counted only once. Discrepancies between reviewers were resolved by discussion, and when necessary by a third reviewer (Wan Chaomin or Wang Zhengrong). For the crossover trials, carryover effects were also assessed.
Data extraction and management
Data was extracted independently by two reviewers Xu Ping and Zhao Ping using a standard form and then cross‐checked. The differences in interpretation of data were resolved through further examination and consensus between the reviewers. If data were presented in tables, text or in figures, the numeric data were preferred because of possible measurement error when estimating from graphs. The data extracted from each study included patient characteristics, methods, interventions, outcomes and notes as mentioned in the table of included studies. All data, regardless of compliance or completion of follow up, was collected in order to allow for an intention to treat analysis.
In the case of missing information in the included studies, investigators were contacted by email to obtain the missing information. In the case of missing values for standard deviation of the change in blood pressure, the standard deviation was imputed based on the information in the same trial or from other trials using the same class of drug. The following hierarchy (listed from high to low preference) was used to impute standard deviation values:
Pooled standard deviation calculated either from the statistic corresponding to an exact p‐value reported or from the 95% confidence interval of the mean difference between two groups.
Standard deviation of blood pressure at the end of treatment.
Standard deviation of blood pressure at baseline (except if this measure was used for entry criteria). (Musini 2009)
Weighted mean standard deviation of change in blood pressure from other trials.
Assessment of risk of bias in included studies
Two independent reviewers (Xu Ping and Zhao Ping) assessed the risk of bias of all included trials and completed a Risk of Bias Table as described in chapter 8 of the Cochrane Handbook.
Measures of treatment effect
For evaluation of the primary outcomes (mortality, cardiovascular mortality, cardiovascular morbidity and adverse events), data on the total number of patients with at least one event within each trial was to be extracted and comparisons between groups would be presented as relative risk ratios with corresponding 95% confidence intervals. However, this was not done as none of the included trials reported any of these outcome measures.
Nine crossover RCTs that were included provided data on SBP and DBP. The data were obtained from texts, figures and tables. These data were entered using generic inverse variance. Subsequently for all other parallel group RCTs' blood pressure data were entered in a similar manner.
For parallel trials, we assessed the tolerability of the intervention by calculating the risk ratio (RR) of adverse events in the evening administration as compared to morning administration treatment arms. Random effects model was used to calculate a pooled risk ratio. Crossover trials are designed with the intention that all participants receive both the active and control interventions, and the treatment effect is estimated from the differences in response of the same participant to the different treatments. Hence participants who withdraw from either treatment cannot be included in the analysis and so the question of differential withdrawal between treatment arms does not arise.
Dealing with missing data
In general if there were missing data, the authors of the study were contacted using e‐mail for clarification. In cases where missing information was ultimately not available, the best estimate was included based on information in the same trial or information from other trials using the same class of drug. For instance, if standard error of the change for blood pressure was not provided, the value was imputed using the pooled standard error of change data from other similar trials and by calculating a weighted pooled standard error.
Assessment of heterogeneity
Heterogeneity between trial results was tested using the I2 statistic where percentages greater than 50% were taken to indicate significant heterogeneity. If heterogeneity was detected for outcomes, a random effects model was used.
Assessment of reporting biases
In the event that missing data was imputed, sensitivity analysis was performed to see if results were sensitive to the assumptions being made. The potential impact of missing data has been reviewed in the discussion section.
Data synthesis
Cochrane Review Manager software, RevMan 5.1, was used for all data syntheses and analyses.
Quantitative analyses of outcomes were based on intention‐to‐treat principles as much as possible. Relative risks were calculated for dichotomous clinical outcomes but was not done as none of the trials provided this data. Data for blood pressure reduction was combined using generic inverse variance, which entailed entering the end of study mean blood pressure difference and pooled standard error of the difference.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were performed, grouping the trials into those using drugs from different antihypertensive classes: α‐blockers, β‐blockers, ACEIs, ARBs, CCBs, and diuretics.
Sensitivity analysis
We intended to conduct a sensitivity analysis by methodological quality:
Exclusion of non double‐blind trials.
Exclusion of trials not reporting the method of generation of the allocation sequence.
Exclusion of trials not reporting the method of blinding.
Exclusion of trials with inadequate allocation concealment.
Exclusion of trials with imputed data.
The planned sensitivity analysis could not be conducted as few trials met the inclusion criteria and data within those trials was limited.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
The search strategy found 8416 references in CENTRAL, MEDLINE, EMBASE and CBLD, whose titles and abstracts were screened by Zhao P and Xu P. 8318 references were excluded and the remaining 98 articles were retrieved for detailed evaluation. On detailed examination, we excluded 68 articles (64 trials) for the following reasons: not a RCT (16 trials), treatment period less than three weeks (7 trials), placebo controlled with no comparator treatment arm (4 trials), triple‐way crossover RCT (2 trials), not monotherapy (13 trials), healthy people (2 trials), no relevant endpoints (11 trials), lack of data (1 trial), different drugs in comparator arms (6 trials), different dose in all patients (2 trials). See table Characteristics of excluded studies.
The 23 remaining articles described 21 RCTs that met the inclusion criteria and are described in the table Characteristics of included studies.
The 7 remaining references have not been retrieved yet (see Characteristics of studies awaiting classification).
Included studies
21 RCTs that provided data on 1,993 patients are included in the meta‐analysis. In thirteen trials parallel design was used (Calvo 2006a; Glasser 2003; Hermida 2003; Hermida 2004; Hermida 2005a; Hermida 2005b; Hermida 2007; Hermida 2007a; Hermida 2008; Hermida 2008a; Hermida 2009; Hermida 2009a; Hermida 2009b) and eight used crossover design (Morgan 1997, Myburgh 1995, Neutel 2005, Nold 1998, Palatini 1992, Pechere 1998, Qiu 2003, White 1999a).
The number of participants in each trial ranged from 10 to 259. The entry criteria of the 21 included RCTs were similar with respect to DBP, requiring participants with DBP 90‐115 mm Hg, but exclusion criteria varied between trials. The age ranged from 18 to 78 years. The gender mix of participants was different between trials (range 32% to 100% male). Seven trials reported ethnicity (Glasser 2003, Hermida 2003, Hermida 2004, Hermida 2005b, Neutel 2005, Qiu 2003, White 1999a), and most participants were white. Thirteen trials not reporting ethnicity were conducted in Europe or Australia (Calvo 2006a, Hermida 2005a, Hermida 2007, Hermida 2007a, Hermida 2008, Hermida 2008a, Hermida 2009, Hermida 2009a, Hermida 2009b, Morgan 1997, Nold 1998, Palatini 1992, Pechere 1998), therefore it was likely that most of the participants were white. One trial was conducted in South Africa (Myburgh 1995).
Each trial administered once‐daily dose antihypertensive drug at night (6 p.m. to midnight) or in the morning (6 a.m. to noon), but the antihypertensive drug and dose used between trials were different, thus there was substantial heterogeneity observed between trials.
No trial reported all cause mortality, cardiovascular outcomes and serious adverse events.
All trials reported the changes from baseline to endpoint in 24‐hour blood pressure. The data of SBP, DBP and SE were obtained from texts, figures and tables.
Three trials reported the changes in the morning blood pressure from 6 a.m. to noon with SD (Glasser 2003, Neutel 2005) and SE (White 1999a).
Ten trials reported adverse events (Calvo 2006a, Glasser 2003, Hermida 2007, Hermida 2007a, Hermida 2008, Hermida 2008a, Hermida 2009, Hermida 2009a, Hermida 2009b, Myburgh 1995).
Excluded studies
64 trials were excluded and the reasons for exclusion are reported in Characteristics of excluded studies.
Risk of bias in included studies
For the overall assessment of the risk of bias in included studies see Figure 1 and Figure 2.
1.
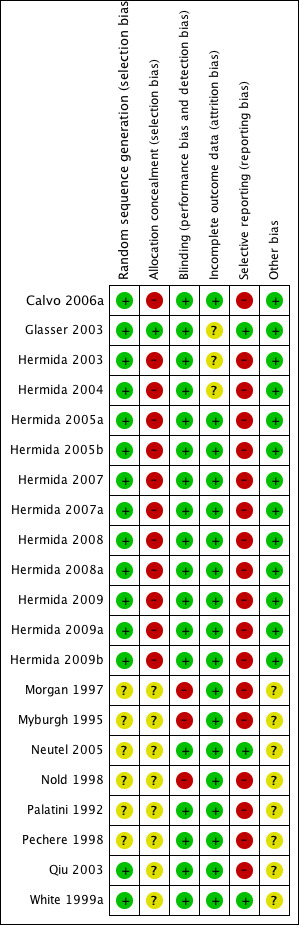
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
2.
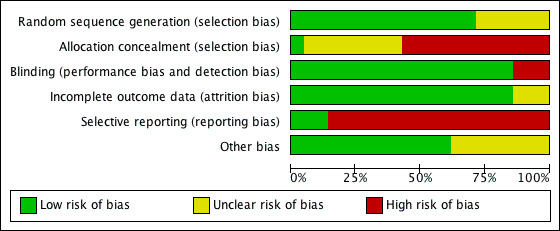
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Although all the included trials were randomised, the quality was downgraded due to lack of allocation concealment and selective reporting as major risks of bias.
Allocation
The method of randomization was confirmed to be adequate in 15 trials (Calvo 2006a, Glasser 2003, Hermida 2003, Hermida 2004, Hermida 2005a, Hermida 2005b, Hermida 2007, Hermida 2007a, Hermida 2008, Hermida 2008a, Hermida 2009, Hermida 2009a, Hermida 2009b, Qiu 2003, White 1999a) and was not reported in the other 6 trials (Morgan 1997, Myburgh 1995, Neutel 2005, Nold 1998, Palatini 1992, Pechere 1998). Concealment of allocation was confirmed as adequate in only one trial (Glasser 2003).
Blinding
Six trials blinded both treatment providers and participants (Glasser 2003, Neutel 2005, Palatini 1992, Pechere 1998, Qiu 2003, White 1999a), twelve trials blinded investigators obtaining the BP data and outcome assessors (Calvo 2006a, Hermida 2003, Hermida 2004, Hermida 2005a, Hermida 2005b, Hermida 2007, Hermida 2007a, Hermida 2008, Hermida 2008a, Hermida 2009, Hermida 2009a, Hermida 2009b), three trials did not implement blinding (Morgan 1997, Myburgh 1995, Nold 1998).
Incomplete outcome data
Loss to follow‐up was reported in all trials, but three trials did not report the distribution according to treatment group (Glasser 2003, Hermida 2003, Hermida 2004).
Selective reporting
We identified selective outcome reporting bias in nineteen trials (Calvo 2006a, Hermida 2003, Hermida 2004, Hermida 2005a, Hermida 2005b, Hermida 2007, Hermida 2007a, Hermida 2008, Hermida 2008a, Hermida 2009, Hermida 2009a, Hermida 2009b, Morgan 1997, Myburgh 1995, Nold 1998, Palatini 1992, Pechere 1998, Qiu 2003, White 1999a).
Other potential sources of bias
Eleven trials were supported by grants (Calvo 2006a, Hermida 2004, Hermida 2005a, Hermida 2007, Hermida 2007a, Hermida 2008, Hermida 2008a, Hermida 2009, Hermida 2009a. In 2 trials (Hermida 2009b, Nold 1998), conflict of interest was not declared. None of the eight crossover trials reported the carryover effects (Morgan 1997, Myburgh 1995, Neutel 2005, Nold 1998, Palatini 1992, Pechere 1998, Qiu 2003, White 1999a).
Effects of interventions
All trials reported data on BP, and 10 trials reported adverse events. Findings from these trials were aggregated in a meta‐analysis. Forest plots of these results are given in Analysis 1.1, Analysis 1.2, Analysis 1.3, Analysis 1.4, Analysis 1.5, Analysis 1.6.
1.1. Analysis.
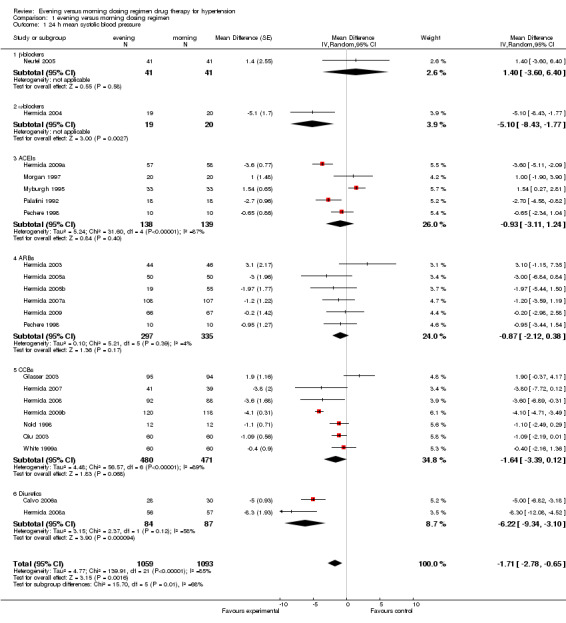
Comparison 1 evening versus morning dosing regimen, Outcome 1 24 h mean systolic blood pressure.
1.2. Analysis.
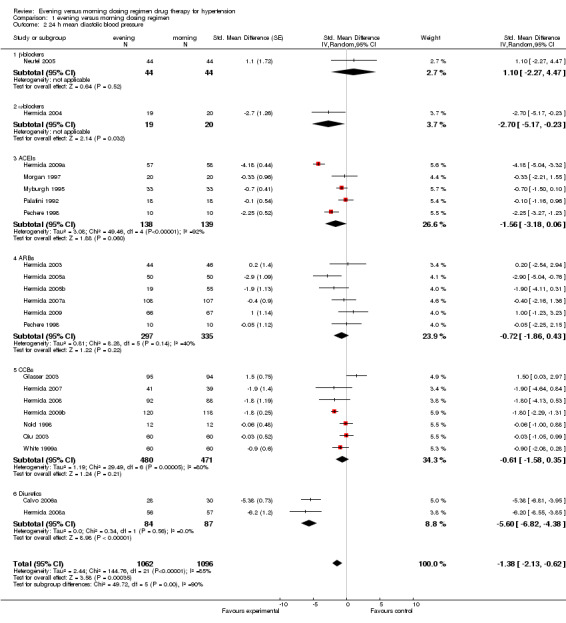
Comparison 1 evening versus morning dosing regimen, Outcome 2 24 h mean diastolic blood pressure.
1.3. Analysis.
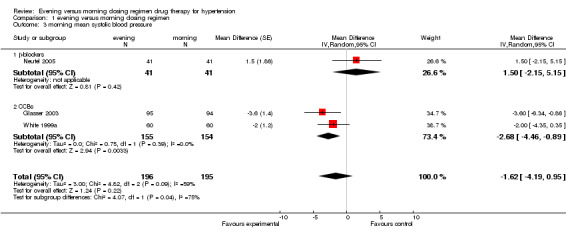
Comparison 1 evening versus morning dosing regimen, Outcome 3 morning mean systolic blood pressure.
1.4. Analysis.
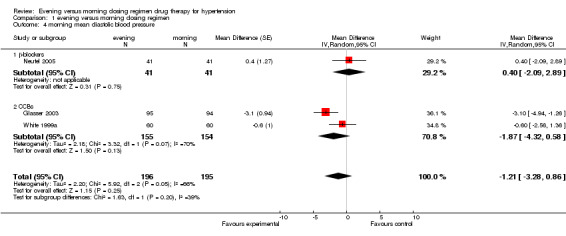
Comparison 1 evening versus morning dosing regimen, Outcome 4 morning mean diastolic blood pressure.
1.5. Analysis.
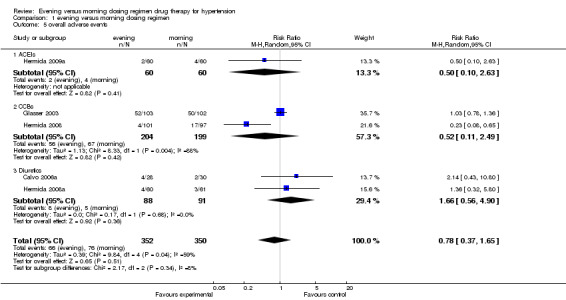
Comparison 1 evening versus morning dosing regimen, Outcome 5 overall adverse events.
1.6. Analysis.
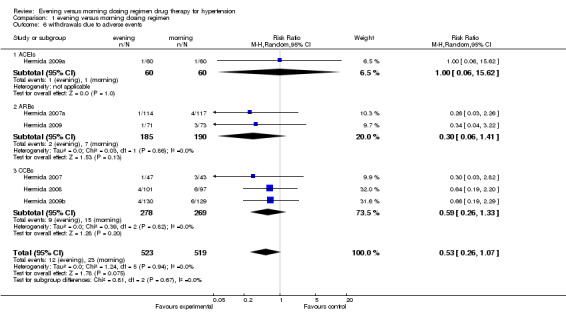
Comparison 1 evening versus morning dosing regimen, Outcome 6 withdrawals due to adverse events.
Mortality, cardiovascular mortality and morbidity outcomes
No RCTs that met the inclusion criteria reported data on mortality, cardiovascular mortality or morbidity.
None of the trials reported serious adverse events.
Blood pressure outcomes
Change in 24‐hour SBP
In general, the analysis of the overall mean difference in 24‐hour SBP (Analysis 1.1) found that the evening regimen reduced 24‐hour SBP by ‐1.71 mm Hg (95%CI ‐2.78 to ‐0.65), which was a statistically significant difference. Significant heterogeneity (I2=85%) was observed.
For the subgroup analysis of mean difference in 24‐hour SBP, evening versus morning dosing regimen, no differences were found with beta‐blocker chronotherapeutic agents, ACEIs, ARBs and CCBs. Evening dosing reduced 24‐hour SBP by 1.40 mm Hg (95%CI ‐3.60 to 6.40), ‐0.93 mm Hg (95%CI ‐3.11 to 1.24), ‐0.87 mm Hg (95%CI ‐2.12 to 0.38) and ‐1.64 mm Hg (95%CI ‐3.39 to 0.12) respectively compared with morning dosing. There were statistically significant differences found in alpha‐blockers and diuretics evening versus morning dosing regimen, evening dosing reduced 24‐hour SBP by ‐5.10 mm Hg (95%CI ‐8.43 to ‐1.77) and ‐6.22 mm Hg (95%CI ‐9.34 to ‐3.10) respectively.
Change in 24‐hour DBP
The analysis of mean difference in 24‐hour DBP (Analysis 1.2) found that the evening regimen significantly reduced 24‐hour DBP by ‐1.38 mm Hg (95%CI ‐2.13 to ‐0.62), but there was significant heterogeneity (I2=85%).
For the subgroup analysis of mean difference in 24‐hour DBP, statistical significant differences were observed in alpha‐blockers and diuretics, evening dosing reduced 24‐hour DBP by ‐2.70 mm Hg (95%CI ‐5.17 to ‐0.23) and ‐5.60 mm Hg (95%CI ‐6.82 to ‐4.38) respectively compared with morning regimen. No differences were found in evening versus morning dosing regimen with beta‐blocker chronotherapeutic agents, and conventional ACEIs, ARBs and CCBs. Evening dosing reduced 24‐hour DBP by 1.10 mm Hg (95%CI ‐2.27 to 4.47), ‐1.56 mm Hg (95%CI ‐3.18 to 0.06), ‐0.72 mm Hg ( 95%CI ‐1.86 to 0.43) and ‐0.61 mm Hg (95%CI ‐1.58 to 0.35) respectively compared with morning dosing.
Change in morning SBP
The analysis of mean difference in morning SBP (Analysis 1.3), based on very limited data, found no statistical difference in evening dosing versus morning dosing regimen, ‐1.62 mm Hg (95%CI ‐4.19 to 0.95, I2=59%). For the subgroup analysis of mean difference in morning SBP, there was statistical difference found in CCBs evening versus conventional morning dosing, ‐2.68 mm Hg (95%CI ‐4.46 to ‐0.89); no statistical difference was found with beta‐blocker chronotherapeutic formulation versus conventional medication, 1.50 mm Hg (95%CI ‐2.51 to 5.15).
Change in morning DBP
The analysis of mean difference in morning DBP (Analysis 1.4), based on very limited data, found that evening dosing did not significantly lower morning DBP compared with conventional dosing regimen, ‐1.21 mm Hg (95%CI ‐3.28 to 0.86, I2=66%). There was no statistical differences found in CCBs evening versus conventional morning dosing, ‐1.87 mm Hg (95%CI ‐4.32 to 0.58). There was no statistically significant difference with beta‐blocker chronotherapeutic formulation versus conventional medication, 0.40 mm Hg (95% CI ‐2.09 to 2.89).
Adverse events
Five parallel trials (Calvo 2006a, Glasser 2003, Hermida 2008, Hermida 2008a, Hermida 2009a) reported overall adverse effects and six trials (Hermida 2007, Hermida 2007a, Hermida 2008, Hermida 2009, Hermida 2009a, Hermida 2009b) reported withdrawals due to adverse events.
One crossover trial (Myburgh 1995) reported three patient withdrawals due to adverse events. No patient was reported to have any side effects during the entire study period in the crossover trial (Palatini 1992) and the remaining six crossover studies (Morgan 1997, Neutel 2005, Nold 1998, Pechere 1998, Qiu 2003, White 1999a) did not report whether participants suffered any adverse effects.
The meta‐analysis of 5 parallel trials showed that there was no statistically significant difference between evening and conventional dosing regimen in the incidence of overall adverse events (RR 0.78, 95%CI: 0.37 to 1.65, I2=59%, Analysis 1.5) and withdrawals due to adverse events (RR 0.53, 95%CI: 0.26 to 1.07, I2=0%, Analysis 1.6). For the subgroup analysis of overall adverse events, similar results were found for ACEIs, CCBs and diuretics evening compared with morning dosing regimen (RR 0.50, 95%CI: 0.10 to 2.63; RR = 0.52, 95%CI: 0.11 to 2.49; RR = 1.66, 95%CI: 0.56 to 4.90; respectively); for withdrawal due to adverse events, no differences were found in ACEIs, ARBs and CCBs evening versus morning dosing regimen (RR = 1.00, 95%CI: 0.06 to 15.62; RR = 0.30, 95%CI: 0.06 to 1.41; RR = 0.59, 95%CI: 0.26 to 1.33; respectively).
Funnel plot analysis
Funnel plots of 24‐hour SBP and DBP outcome data indicate evidence of publication bias (see Figure 3, Figure 4).
3.
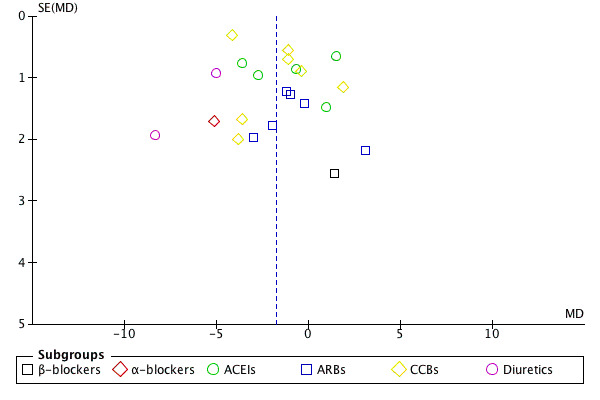
Funnel plot of comparison: 1 evening versus morning dosing regimen, outcome: 1.1 24 h mean systolic blood pressure.
4.
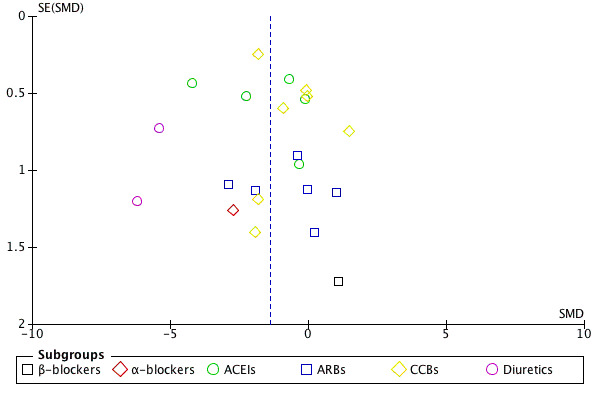
Funnel plot of comparison: 1 evening versus morning dosing regimen, outcome: 1.2 24 h mean diastolic blood pressure
Discussion
Summary of main results
No eligible studies evaluated mortality or morbidity for the six traditional antihypertensive drug classes morning versus evening once daily monotherapy regimens.
There were no significant differences in overall adverse effects and withdrawals due to adverse effects among the two dosing regimens. Subgroup analysis of overall adverse events found no differences with ACEIs, CCBs and diuretic drug class evening versus morning dosing regimen; for the withdrawal profile, similar results were found with ACEIs, ARBs and CCBs between the two dosing regimens.
This review provided very limited morning BP data for beta‐blockers and CCBs. Adverse events data (ACEIs, CCBs and diuretics) and withdrawals due to adverse effect (ACEIs, ARBs and CCBs) were reported, but no serious adverse events data for all the six conventional class antihypertensive agents were reported.
In a subgroups analysis of 24‐hour SBP, no differences were found for beta‐blockers, ACEIs, ARBs and CCBs evening versus morning dosing regimen. Statistically significant differences were found between evening versus morning dosing regimen for two drug classes, alpha‐blockers (limited to one trial data Hermida 2004) and diuretics (limited to 2 trials Calvo 2006a and Hermida 2008a).
In a subgroups analysis of 24‐hour DBP, no differences were found for beta‐blockers, ACEIs, ARBs and CCBs evening versus morning dosing regimen. Statistically significant differences were found between evening versus morning dosing regimen for alpha‐blockers (limited to one trial Hermida 2004) and diuretics (limited to 2 trials Calvo 2006a and Hermida 2008a).
Quality of the evidence
Most trials had risks of bias in at least two of several key criteria. One trial had risk of bias due to incomplete outcome data (Glasser 2003). See Figure 1
Nineteen of the 21 included studies were double‐blind, involving 97% (N=1,928) of the entire studied population. Fifteen trials reported adequate sequence generation. However, only one trial reported adequate concealment of allocation [N=205, 11% (205/1993) of total randomized participants], so the number of patients randomized with adequate concealment of allocation was very low.
Three of the 21 included studies had incomplete outcome data. However, risk of bias due to selective reporting was found in 19 of the 21 included studies had a bias.
Thirteen trials (N=1729, 87%) had no other bias.
See Figure 1 and Figure 2 for a graphic representation of the overall risks of bias detected in the 21 included studies.
Funnel plot analysis
We performed a funnel plot analysis and found evidence of publication bias since the trials in lower right hand and left hand area in the funnel plot are missing (see Figure 3, Figure 4).
Authors' conclusions
Implications for practice.
Based on data for 6 classes of antihypertensive drugs, evening administration lowered 24‐hour SBP by 1.61 mm Hg and 24‐hour DBP by 1.23 mm Hg. In particular the alpha‐blocker doxazosin GITS (4 mg/day) and the diuretic torasemide (5 mg/day) evening administration reduced 24‐hour SBP by 5.10 and 6.24 mm Hg respectively and 24‐hour DBP by 2.70 and 5.95 mm Hg respectively. The clinical relevance of this decrease is not known, since very limited data has been reported for morning SBP and DBP, and mortality and morbidity data have not been reported. There were no significant differences in overall adverse effects and withdrawals due to adverse effects among the two regimens.
This systematic review found that nighttime dosing of antihypertensive drugs is more effective than morning administration to lower 24‐hour BP, but did not find adequate data to determine which of the two regimens may have more beneficial effects on cardiovascular outcomes or adverse events.
Implications for research.
The short‐term trials conducted to date, which report the mean BP lowering efficacy as a surrogate outcome, are not adequate to establish which of the two dosing regimens may be better. Large double‐blind randomized controlled trials are needed to evaluate the administration time‐related ‐effects of different antihypertensive drug classes given as monotherapy or as first line drugs with stepped up therapy on mortality and cardiovascular morbidity, with long‐term follow up data of at least 3 to 5 years duration.
What's new
| Date | Event | Description |
|---|---|---|
| 23 September 2011 | Amended | amended contact details and corrected appendix 4 |
Notes
Medical Subject Headings (MeSH)
*Chronotherapy; Antihypertensive Agents [therapeutic use]; Blood Pressure [drug effects]; Hypertension [drug therapy]; Randomized Controlled Trials as Topic
MeSH check words
Humans
Acknowledgements
We would like to acknowledge the assistance provided by the Cochrane Hypertension Group, particularly Ciprian Jauca for his unconditional help and mentoring.
We are in deep gratitude to the editor Vijaya Musini who provided us with methodology guidance, analysing data using generic inverse variance, revising the draft, and help with writing/editing the final draft of the review.
We would also like to acknowledge the assistance provided by professor Liu Ming and Xu Liangzhi from the Chinese Cochrane Centre.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials search strategy
4th Quarter 2009
1 chronotherap$.af. 2 chronomodulat$.af. 3 chronopharm$.af. 4 1 or 2 or 3 5 hypertens$.mp. 6 exp Hypertension/ 7 blood pressure.mp. or exp Blood Pressure/ 8 5 or 6 or 7 9 (morning or day or am or diurnal$ or daytim$ or awak$).mp. 10 (evening or bedtim$ or night$ or nocturnal$ or pm).mp. 11 4 and 8 12 (coer or covera or codas or cardizem or innopran).mp. 13 8 and 9 and 10 14 11 or 12 or 13
Appendix 2. MEDLINE search strategy
Ovid MEDLINE(R) 1950 to Present with Daily Update
1 randomized controlled trial.pt. 2 controlled clinical trial.pt. 3 randomized.ab. 4 placebo.ab. 5 drug therapy.fs. 6 randomly.ab. 7 trial.ab. 8 groups.ab. 9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10 (animals not (humans and animals)).sh. 11 9 not 10 12 Hypertension/ 13 blood pressure$.mp. 14 hypertens$.mp. 15 exp blood pressure/ 16 13 or 14 or 12 or 15 17 exp Chronotherapy/ 18 (chronopharm$ or chronomodulat$ or chronotherap$).mp. 19 18 or 17 20 (morning or day or am or diurnal$ or daytim$ or awak$).mp. 21 (evening or bedtim$ or night$ or nocturnal$ or pm).mp. 22 21 and 20 23 16 and (19 or 22) 24 (coer or covera or codas or cardizem or innopran).mp. 25 11 and (23 or 24)
Appendix 3. EMBASE.COM search strategy
1974 to Oct 2009
#1 random* OR factorial* OR crossover* OR placebo* OR assign* OR allocat* OR volunteer* OR doubl* NEAR/5 blind* OR singl* NEAR/5 blind* #2 'crossover procedure'/exp #3 'double‐blind procedure'/exp #4 'randomized controlled trial'/exp #5 'single blind procedure'/exp #6 #1 OR #2 OR #3 OR #4 OR #5 #7 'hypertension'/exp #8 hypertens* #9 'blood pressure'/exp #10 #7 OR #8 OR #9 #11 'chronotherapy'/exp #12 chronopharm* OR chronomodulat* OR chronotherap* #13 morning OR day OR am OR diurnal* OR daytim* OR awak* #14 evening OR bedtim* OR night* OR nocturnal* OR pm #15 #13 AND #14 #16 #11 OR #12 OR #15 #17 #6 AND #10 AND #16
Appendix 4. Chinese Biomedical Literature Database (CBM) search strategy
1978 to 2009
1 分类号=R544.1/扩展/全部复分
2 主题词:高血压/全部树/全部副主题词
3 主题词:时间疗法/全部树/全部副主题词
4 主题词:时间治疗学/全部树/全部副主题词
5 缺省:时间 or 时辰 or 择时
6 缺省:(早上 or 白天 or 醒后 or 清晨) and (晚上 or 夜间 or 睡前)
7 缺省:(早晨 or 起床 or 凌晨 or 早间 or 上午) and (晚上 or 夜间 or 睡前)
8 缺省:早晚
9 #8 or #7 or #6 or #5 or #4 or #3
10 (#1 or #2) and #9
Data and analyses
Comparison 1. evening versus morning dosing regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 24 h mean systolic blood pressure | 21 | 2152 | Mean Difference (Random, 95% CI) | ‐1.71 [‐2.78, ‐0.65] |
| 1.1 β‐blockers | 1 | 82 | Mean Difference (Random, 95% CI) | 1.4 [‐3.60, 6.40] |
| 1.2 α‐blockers | 1 | 39 | Mean Difference (Random, 95% CI) | ‐5.1 [‐8.43, ‐1.77] |
| 1.3 ACEIs | 5 | 277 | Mean Difference (Random, 95% CI) | ‐0.93 [‐3.11, 1.24] |
| 1.4 ARBs | 6 | 632 | Mean Difference (Random, 95% CI) | ‐0.87 [‐2.12, 0.38] |
| 1.5 CCBs | 7 | 951 | Mean Difference (Random, 95% CI) | ‐1.64 [‐3.39, 0.12] |
| 1.6 Diuretics | 2 | 171 | Mean Difference (Random, 95% CI) | ‐6.22 [‐9.34, ‐3.10] |
| 2 24 h mean diastolic blood pressure | 21 | 2158 | Std. Mean Difference (Random, 95% CI) | ‐1.38 [‐2.13, ‐0.62] |
| 2.1 β‐blockers | 1 | 88 | Std. Mean Difference (Random, 95% CI) | 1.1 [‐2.27, 4.47] |
| 2.2 α‐blockers | 1 | 39 | Std. Mean Difference (Random, 95% CI) | ‐2.7 [‐5.17, ‐0.23] |
| 2.3 ACEIs | 5 | 277 | Std. Mean Difference (Random, 95% CI) | ‐1.56 [‐3.18, 0.06] |
| 2.4 ARBs | 6 | 632 | Std. Mean Difference (Random, 95% CI) | ‐0.72 [‐1.86, 0.43] |
| 2.5 CCBs | 7 | 951 | Std. Mean Difference (Random, 95% CI) | ‐0.61 [‐1.58, 0.35] |
| 2.6 Diuretics | 2 | 171 | Std. Mean Difference (Random, 95% CI) | ‐5.60 [‐6.82, ‐4.38] |
| 3 morning mean systolic blood pressure | 3 | 391 | Mean Difference (Random, 95% CI) | ‐1.62 [‐4.19, 0.95] |
| 3.1 β‐blockers | 1 | 82 | Mean Difference (Random, 95% CI) | 1.5 [‐2.15, 5.15] |
| 3.2 CCBs | 2 | 309 | Mean Difference (Random, 95% CI) | ‐2.68 [‐4.46, ‐0.89] |
| 4 morning mean diastolic blood pressure | 3 | 391 | Mean Difference (Random, 95% CI) | ‐1.21 [‐3.28, 0.86] |
| 4.1 β‐blockers | 1 | 82 | Mean Difference (Random, 95% CI) | 0.4 [‐2.09, 2.89] |
| 4.2 CCBs | 2 | 309 | Mean Difference (Random, 95% CI) | ‐1.87 [‐4.32, 0.58] |
| 5 overall adverse events | 5 | 702 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.37, 1.65] |
| 5.1 ACEIs | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.63] |
| 5.2 CCBs | 2 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.11, 2.49] |
| 5.3 Diuretics | 2 | 179 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [0.56, 4.90] |
| 6 withdrawals due to adverse events | 6 | 1042 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.26, 1.07] |
| 6.1 ACEIs | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.06, 15.62] |
| 6.2 ARBs | 2 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.06, 1.41] |
| 6.3 CCBs | 3 | 547 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.26, 1.33] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Calvo 2006a.
| Methods | prospective randomized open‐label, blinded endpoint, parallel‐group trial. Subjects ingested the single daily tablet of torasemide for 6 weeks. Baseline similarity: age, height, eight, BMI, waist and hip perimeters, BP, laboratory chemistry parameters sample size calculation:not reported |
|
| Participants | Country: Spain Number randomised: 58 Mean age: 48.7±11.9(SD) years gender: 25 men, 33 women. Ethnicity: not reported Inclusion Criteria: age>18 years, conventional SBP between 140 and 179 mm Hg or DBP between 90 and 109 mm Hg, and ABPM diurnal mean >135/85 mm Hg, or the nocturnal mean > 120/70 mm Hg. Exclusion criteria: grade 3 essential hypertension, shift workers, heavy drinkers, and cardiovascular disorders | |
| Interventions | torasemide (5 mg od) on awakening: N=30 torasemide (5 mg od) at bedtime:N=28 |
|
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data:24h BP change by 48h ABPM, data was obtained from graph and text (fig 5 on page 728) Adverse Events: overall adverse events |
|
| Notes | supported in part by grants from Xunta de Galicia (PGIDIT03‐PXIB‐32201PR), Hospital Clınico Universitario de Santiago and University of Vigo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computerized random‐number generator |
| Allocation concealment (selection bias) | High risk | one member of the research team used a list of random numbers |
| Blinding (performance bias and detection bias) All outcomes | Low risk | investigator obtaining the BP measurements, outcome assessors blinded. Benefits of the PROBE design and its validity compared with double‐blind, placebo‐controlled trials in assessing antihypertensive efficacy based on blinded ABPM measurements have been documented previously (Smith 2003) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were reported. 6 lost to follow‐up for no second ABPM available, 3 in awakening group, 3 in bedtime group. |
| Selective reporting (reporting bias) | High risk | Morning SBP, DBP, serious adverse events were not reported. |
| Other bias | Low risk | This trial was a part of MAPEC (http://www.clinicaltrials.gov/ct2/show/NCT00295542?term=NCT00295542). The funding body has no role in the study design, analysis and interpretation of data, writing of the reports, or the decision to submit articles to publication (Hermida 2007b) |
Glasser 2003.
| Methods | multicenter (N=39), double‐blind, parallel‐group, dose‐response, placebo‐controlled, randomized study. The study consisted of an initial screening period followed by a 3‐ to 4‐week single‐blind, placebo run‐in period, and 7‐week double‐blind active treatment period. Baseline similarity: age, height, weight, gender, ethnicity, BP, HR Sample size calculation: reported | |
| Participants | Country: the United States Number randomised: 478 age range: 18‐70 years gender: 303 men, 175 women; relevant treatment group: 130 men, 75 women ethnicity: 302 White, 132 African‐American, 44 other; relevant treatment group: 133 White, 56 African‐American, 16 other inclusion criteria: seated SBP <200 mm Hg, 100 mm Hg≤mean seated DBP≤114 mm Hg, and 90 mm Hg ≤mean daytime (8AM‐4PM) DBP ≤114 mm Hg exclusion criteria: recent history of serious cardiovascular or cerebrovascular events, secondary hypertension, any serious chronic or uncontrolled medical conditions, nightshift workers and sensitivity to diltiazem | |
| Interventions | placebo group:N=69
GRD 120 mg PM group: N=67
GRD 240 mg PM group: N=68
GRD 360 mg AM group: N=102
GRD 360 mg PM group: N=103
GRD 540 mg PMgroup : N=69
The relevant treatment groups for this review are following two arms: GRD 360 mg AM group, GRD was taken each morning at 8 AM +/‐1 h (N=102) GRD 360 mg PM group, GRD was taken each evening at 10 PM +/‐ 1h (N=103) |
|
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data: morning BP (6AM‐noon) change by 24 h ABPM (table 2 on page 55); 24h BP change by 24h ABPM (table 2 on page 55) Adverse Events:Overall adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | central telephone |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | clinicians, patients blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants (N=478) were reported. 49 withdrawals are explained. 3.2% of GRD‐treated patients and 4.3% of placebo treated patients withdrawal due to adverse event, the other reasons for withdrawal included noncompliance, withdrawal of consent, and lack of efficacy, but distribution according to which treatment group was not reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes were not reported. |
| Other bias | Low risk | None identified |
Hermida 2003.
| Methods | prospective, randomized, open‐label, blinded end point, parallel‐group trial. 2‐ to 4‐week washout period and 3 months timed active treatment . Baseline similarity: age, height, weight, BMI, waist and hip perimeters, SBP, and DBP, laboratory chemistry variables Sample size calculation: not reported | |
| Participants | Country: Spain Number randomised:90 Mean age: 49.0±14.3(SD) years gender: 30 men, 60 women Ethnicity: White Inclusion Criteria: conventional SBP between 140 and 179 mm Hg, or DBP between 90 to 109 mm Hg, and ABPM 24 hour mean SBP/DBP > 130/80 mm Hg, diurnal mean >135/85 mm Hg, or the nocturnal mean > 120/70 mm Hg. Exclusion criteria: shift workers, heavy drinkers, smokers, and heavy exercisers, severe arterial hypertension, secondary arterial hypertension, cardiovascular disorders, including angina, heart failure, stroke, nephropathy, retinopathy, prior myocardial infarction or coronary revascularization. | |
| Interventions | valsartan 160 mg/d awakening: N=46 valsartan 160 mg/d bedtime: N=44 |
|
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data:24h BP change by 48h ABPM, data was obtained from graph and text (fig 3 on page 288) Adverse Events: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computerized random‐number generator |
| Allocation concealment (selection bias) | High risk | "Assignment of subjects to treatment groups was done by 1 member of the research team, according to the order of recruitment, following an allocation table constructed by a computerized random‐number generator." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | investigator obtaining the BP measurements and outcome assessors blinded. Benefits of the PROBE design and its validity compared with double‐blind, placebo‐controlled trials in assessing antihypertensive efficacy based on blinded ABPM measurements have been documented previously (Smith 2003). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants were reported. 6 subjects missing ABPM data were eliminated, 3 subjects discontinued timed treatment or they failed to return for the second ABPM at the end of treatment, but distribution according to group not reported |
| Selective reporting (reporting bias) | High risk | Morning SBP, DBP were not reported. |
| Other bias | Low risk | None identified |
Hermida 2004.
| Methods | prospective randomized open‐label, blinded endpoint, parallel‐group trial. 3 months timed active treatment Baseline similarity: age, height, weight, BMI, waist and hip perimeters, SBP, DBP,laboratory chemistry variables sample size calculation: not reported | |
| Participants | Country: Spain Number randomised:91 Mean age: 56.7±11.2(SD) years gender: 49 men, 42 women; relevant treatment groups: 27 men, 12 women Ethnicity: Caucasian Inclusion Criteria: conventional SBP between 140 and 179 mm Hg, or DBP between 90 to 109 mm Hg, and ABPM 24 hour mean SBP/DBP > 130/80 mm Hg, diurnal mean >135/85 mm Hg, or the nocturnal mean > 120/70 mm Hg. Exclusion criteria: shift workers, heavy drinkers, smokers, and heavy exercisers, severe arterial hypertension, secondary arterial hypertension, angina, heart failure, stroke, nephropathy, retinopathy, prior myocardial infarction or coronary revascularization. | |
| Interventions | morning monotherapy: a single daily tablet of doxazosin GITS(4 mg/day) was taken in the morning (N=20) bedtime monotherapy: a single daily tablet of doxazosin GITS(4 mg/day) was taken at bedtime (N=19) morning polytherapy: N=24 bedtime polytherapy: N=28 polytherapy group allowed combination of antihypertensive medications was restricted to angiotensin receptor blockers plus either a diuretic or calcium channel blocker, and ACE inhibitors plus either a diuretic or calcium channel blocker, each group received a single daily tablet of doxazosin GITS(4 mg/day), so the polytherapy arms were excluded for this review. |
|
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data:24h BP change by 48h ABPM, data was obtained from graph and text (fig 5 on page 290) Adverse Events: not reported |
|
| Notes | supported in part by grants from Xunta de Galicia (PGIDIT03‐PXIB‐32201PR), and Vicerrectorado de Investigacion, University of Vigo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computerized random‐number generator |
| Allocation concealment (selection bias) | High risk | one member of the research team used a list of random numbers |
| Blinding (performance bias and detection bias) All outcomes | Low risk | investigator obtaining the BP measurements, outcome assessors blinded. Benefits of the PROBE design and its validity compared with double‐blind, placebo‐controlled trials in assessing antihypertensive efficacy based on blinded ABPM measurements have been documented previously (Smith 2003) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants were reported. "BP profiles of seven subjects, originally randomized but not incorporated in this efficacy evaluation, were eliminated because of missing ABPM data", but distribution according to group was not reported |
| Selective reporting (reporting bias) | High risk | Morning SBP, DBP were not reported. |
| Other bias | Low risk | This trial was a part of MAPEC (http://www.clinicaltrials.gov/ct2/show/NCT00295542?term=NCT00295542). The funding body has no role in the study design, analysis and interpretation of data, writing of the reports, or the decision to submit articles to publication (Hermida 2007b). |
Hermida 2005a.
| Methods | prospective randomized open‐label, blinded endpoint, parallel‐group trial. 3 months of intervention Baseline similarity: age, height, weight, BMI, waist and hip perimeters, SBP, DBP,laboratory chemistry variables sample size calculation:not reported | |
| Participants | Country: Spain Number randomised: 105,100 completed Mean age: 68.2±4.9(SD) years gender: 34 men, 66 women. Ethnicity: not reported Inclusion Criteria: untreated, age≥60years, conventional SBP between 140 and 179 mm Hg or DBP between 90 and 109 mm Hg, and ABPM diurnal mean SBP/DBP>135/85 mm Hg, or the nocturnal mean > 120/70 mm Hg. Exclusion criteria: shift workers, heavy drinkers, smokers, heavy exercisers, severe arterial or secondary arterial hypertension, nephropathy and retinopathy and/or cardiovascular disorders. | |
| Interventions | valsartan (160mg/d) on awakening: N=53 valsartan monotherapy (160mg/d) at bedtime:N=52 |
|
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data:24h BP change by 48h ABPM, data was obtained from graph and text (fig 5 on page 770) Adverse Events: not reported |
|
| Notes | supported in part by grants from Xunta de Galicia (PGIDIT03‐PXIB‐32201PR), and Vicerrectorado de Investigacion, University of Vigo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computerized random‐number generator |
| Allocation concealment (selection bias) | High risk | one member of the research team use of a list of random numbers |
| Blinding (performance bias and detection bias) All outcomes | Low risk | investigator obtaining the BP measurements and outcome assessors blinded. Benefits of the PROBE design and its validity compared with double‐blind, placebo‐controlled trials in assessing antihypertensive efficacy based on blinded ABPM measurements have been documented previously (Smith 2003) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were reported. 5 subjects were eliminated for second ABPM, 3 in the morning treatment and 2 in bedtime treatment. |
| Selective reporting (reporting bias) | High risk | Morning SBP, DBP were not reported. |
| Other bias | Low risk | This trial was a part of MAPEC (http://www.clinicaltrials.gov/ct2/show/NCT00295542?term=NCT00295542). "The funding body has no role in the study design, analysis and interpretation of data, writing of the reports, or the decision to submit articles to publication (Hermida 2007b)". |
Hermida 2005b.
| Methods | prospective randomized open‐label, blinded endpoint, parallel‐group trial. After 2‐4 week washout period, subjects received timed active treatment for 3 months Baseline similarity: age, height, eight, BMI, waist and hip perimeters, BP, laboratory chemistry variables sample size calculation:not reported | |
| Participants | Country: Spain Number randomised: 152,148 completed Mean age: 53.0±12.6(SD) years gender: 50 men, 98 women. Ethnicity: white Inclusion Criteria: conventional SBP between 140 and 179 mm Hg or DBP between 90 and 109 mm Hg, and ABPM 24 hour mean SBP/DBP > 130/80 mm Hg, diurnal mean >135/85 mm Hg, or the nocturnal mean > 120/70 mm Hg. Exclusion criteria: shift workers, heavy drinkers, smokers, heavy exercisers, severe arterial or secondary arterial hypertension, nephropathy and retinopathy and/or cardiovascular disorders. | |
| Interventions | valsartan monotherapy (160mg od) on awakening: N=75 valsartan monotherapy (160mg od) at bedtime:N=77 |
|
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data:24h BP change by 48h ABPM, data was obtained from graph and text (fig 4 on page 1919) Adverse Events: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computerized random‐number generator |
| Allocation concealment (selection bias) | High risk | one member of the research team used a list of random numbers |
| Blinding (performance bias and detection bias) All outcomes | Low risk | investigator obtaining the BP measurements, outcome assessors blinded. Benefits of the PROBE design and its validity compared with double‐blind, placebo‐controlled trials in assessing antihypertensive efficacy based on blinded ABPM measurements have been documented previously (Smith 2003) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were reported. "Baseline blood pressure profiles of four additional subjects (three originally assigned to morning treatment and one to bedtime treatment) were eliminated because the patients failed to return for the second ABPM at the end of treatment." |
| Selective reporting (reporting bias) | High risk | Morning SBP, DBP were not reported. |
| Other bias | Low risk | None identified |
Hermida 2007.
| Methods | prospective randomized open‐label, blinded endpoint, parallel‐group trial. Subjects ingested the single daily tablet of nifedipine GITS (30 mg/day) for eight weeks. After this first stage of timed treatment, uncontrolled patients were asked to remain in the trial and be up‐titrated to 60 mg/day nifedipine GITS for another eight weeks at the same circadian time. Baseline similarity: age, height, eight, BMI, waist and hip perimeters, BP, laboratory chemistry variables sample size calculation:not reported |
|
| Participants | Country: Spain Number randomised: 90, 80 completed Mean age: 52.1±10.7(SD) years gender: 36 men, 44 women. Ethnicity: not reported Inclusion Criteria: conventional SBP between 140 and 179 mm Hg or DBP between 90 and 109 mm Hg, and ABPM 24 hour mean SBP/DBP > 130/80 mm Hg, diurnal mean >135/85 mm Hg, or the nocturnal mean > 120/70 mm Hg. Exclusion criteria: shift workers, heavy drinkers, smokers, heavy exercisers, severe arterial or secondary arterial hypertension, nephropathy and retinopathy and/or cardiovascular disorders | |
| Interventions | nifedipine GITS (30 mg od) on awakening: N=43 nifedipine GITS (30 mg od) at bedtime:N=47 nifedipine GITS (60 mg od) on awakening:N=21 nifedipine GITS (60 mg od) at bedtime:N=19 |
|
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data: 24h BP change by 48h ABPM, data was obtained from graph and text (fig 6 on page 485) Adverse Events: withdrawals due to adverse events |
|
| Notes | supported in part by grants from Ministerio de Educacio´n y Ciencia, Xunta de Galicia , Quımica Farmaceutica Bayer, Hospital Clınico Universitario de Santiago, and University of Vigo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computerized random‐number generator |
| Allocation concealment (selection bias) | High risk | one member of the research team use of a list of random numbers |
| Blinding (performance bias and detection bias) All outcomes | Low risk | investigator obtaining the BP measurements, outcome assessors blinded. Benefits of the PROBE design and its validity compared with double‐blind, placebo‐controlled trials in assessing antihypertensive efficacy based on blinded ABPM measurements have been documented previously (Smith 2003) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported. 90 randomised, 80 completed. At the first stage of timed treatment, 6 lost to follow‐up for no second ABPM available, 1 in daytime group, 5 in bedtime group, 4 withdrawn due to adverse effects, 3 in daytime group, 1 in bedtime group;At the second stage of timed treatment (uncontrolled BP, N=40), 5 discontinued because of adverse effects, 3 in daytime group, 2 in bedtime group |
| Selective reporting (reporting bias) | High risk | Morning SBP, DBP, overall adverse events, serious adverse events were not reported. |
| Other bias | Low risk | This trial was a part of MAPEC (http://www.clinicaltrials.gov/ct2/show/NCT00295542?term=NCT00295542). "The funding body has no role in the study design, analysis and interpretation of data, writing of the reports, or the decision to submit articles to publication (Hermida 2007b)". |
Hermida 2007a.
| Methods | prospective randomized open‐label, blinded endpoint, parallel‐group trial.Subjects ingested the single daily tablet of telmisartan (80 mg/day) for 12 weeks Baseline similarity: age, height, eight, BMI, waist and hip perimeters, BP, laboratory chemistry variables sample size calculation: reported |
|
| Participants | Country: Spain Number randomised: 231, 215 completed Mean age: 46.4±12.0(SD) years gender: 114 men, 101 women. Ethnicity: not reported Inclusion Criteria: age≥18 years, conventional SBP between 140 and 179 mm Hg or DBP between 90 and 109 mm Hg, and ABPM diurnal mean >135/85 mm Hg, or the nocturnal mean > 120/70 mm Hg. Exclusion criteria: shift workers, heavy drinkers, smokers, heavy exercisers, severe arterial or secondary arterial hypertension, type 1 diabetes, and cardiovascular disorders | |
| Interventions | telmisartan (80mg od) on awakening: N=117 telmisartan (80mg od) at bedtime: N=114 |
|
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data: 24h BP change by 48h ABPM, data was obtained from graph and text (fig 2 on page 720) Adverse Events: withdrawals due to adverse events |
|
| Notes | supported in part by grants from Ministerio de Educacion y Ciencia, Xunta de Galicia, Hospital Clınico Universitario de Santiago, and University of Vigo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computerized random‐number generator |
| Allocation concealment (selection bias) | High risk | one member of the research team use of a list of random numbers |
| Blinding (performance bias and detection bias) All outcomes | Low risk | investigator obtaining the BP measurements, outcome assessors blinded. Benefits of the PROBE design and its validity compared with double‐blind, placebo‐controlled trials in assessing antihypertensive efficacy based on blinded ABPM measurements have been documented previously (Smith 2003) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported. 231 randomised, 215 completed. 11 lost to follow‐up for no second ABPM available, 6 in daytime group, 5 in bedtime group; 5 withdrawn due to adverse effects, 4 in daytime group, 1 in bedtime group |
| Selective reporting (reporting bias) | High risk | Morning SBP, DBP were not reported. Compliance was measured but data was not provided |
| Other bias | Low risk | This was an investigator‐promoted independent research. |
Hermida 2008.
| Methods | prospective randomized open‐label, blinded endpoint, parallel‐group trial. Subjects ingested the single daily tablet of nifedipine GITS (30 mg/day) for 8 weeks. Baseline similarity: age, height, eight, BMI, waist perimeters, BP,HR, laboratory chemistry variables sample size calculation: reported |
|
| Participants | Country: Spain Number randomised: 198, 180 completed Mean age: 52.5±10.7(SD) years gender: 86 men, 94 women. Ethnicity: not reported Inclusion Criteria: untreated, age≥18 years, conventional SBP between 140 and 179 mm Hg or DBP between 90 and 109 mm Hg, and ABPM diurnal mean >135/85 mm Hg, or the nocturnal mean > 120/70 mm Hg. Exclusion criteria: shift workers, heavy drinkers, smokers, heavy exercisers, severe arterial or secondary arterial hypertension, type 1 diabetes, and cardiovascular disorders | |
| Interventions | nifedipine GITS (30 mg od) on awakening: N=97 nifedipine GITS (30 mg od) at bedtime:N=101 |
|
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data: 24h BP change by 48h ABPM, data was obtained from graph and text (fig 3 on page 952) Adverse Events: overall adverse events; withdrawals due to adverse events |
|
| Notes | supported in part by grants from Ministerio de Educación y Ciencia, Xunta de Galicia, Hospital Clınico Universitario de Santiago, and University of Vigo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computerized random‐number generator |
| Allocation concealment (selection bias) | High risk | one member of the research team use of a list of random numbers |
| Blinding (performance bias and detection bias) All outcomes | Low risk | investigator obtaining the BP measurements, outcome assessors blinded. Benefits of the PROBE design and its validity compared with double‐blind, placebo‐controlled trials in assessing antihypertensive efficacy based on blinded ABPM measurements have been documented previously (Smith 2003) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were reported. 8 lost to follow‐up for no second ABPM available, 3 in daytime group, 5 in bedtime group; 10 withdrawn due to adverse effects, 6 in daytime group, 4 in bedtime group |
| Selective reporting (reporting bias) | High risk | Morning SBP, DBP, serious adverse events were not reported. |
| Other bias | Low risk | The authors declared no conflict of interest |
Hermida 2008a.
| Methods | prospective randomized open‐label, blinded endpoint, parallel‐group trial. 6 weeks of intervention Baseline similarity: age, height, eight, BMI, waist perimeters, BP,HR, laboratory chemistry variables sample size calculation: reported |
|
| Participants | Country: Spain Number randomised: 121, 113 completed Mean age: 51.7±10.67(SD) years gender: 44 men, 69 women. Ethnicity: not reported Inclusion Criteria: untreated, age≥18 years, conventional SBP between 140 and 179 mm Hg or DBP between 90 and 109 mm Hg, and ABPM awake BP of mean ≥135/85 mm Hg, or asleep mean ≥120/70 mm Hg. Exclusion criteria: pregnant women, shift workers, heavy drinkers, smokers, heavy exercisers, severe arterial or secondary arterial hypertension, type 1 diabetes, and cardiovascular disorders | |
| Interventions | torasemide (5 mg od) on awakening: N=61 torasemide (5 mg od) at bedtime:N=60 |
|
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data: 24h BP change by 48h ABPM, data was obtained from graph and text (fig 3 on page 961) Adverse Events: overall adverse events |
|
| Notes | supported in part by grants from Ministerio de Educación y Ciencia, Xunta de Galicia, Hospital Clınico Universitario de Santiago, and University of Vigo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computerized random‐number generator |
| Allocation concealment (selection bias) | High risk | one member of the research team use of a list of random numbers |
| Blinding (performance bias and detection bias) All outcomes | Low risk | investigator obtaining the BP measurements, outcome assessors blinded. Benefits of the PROBE design and its validity compared with double‐blind, placebo‐controlled trials in assessing antihypertensive efficacy based on blinded ABPM measurements have been documented previously (Smith 2003) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were reported. 8 lost to follow‐up for no second ABPM available, 4 in awakening group, 4 in bedtime group |
| Selective reporting (reporting bias) | High risk | Morning SBP, DBP, serious adverse events were not reported. Compliance was measured but data was not provided. |
| Other bias | Low risk | "The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper". |
Hermida 2009.
| Methods | prospective randomized open‐label, blinded endpoint, parallel‐group trial. 3 months of intervention Baseline similarity: age, height, eight, BMI, waist perimeters, BP,HR, laboratory chemistry variables sample size calculation: reported |
|
| Participants | Country: Spain Number randomised: 144, 133 completed Mean age: 45.5±11.9(SD) years(awakening),47.6±12.7(SD) years (bedtime) gender: 43 men, 90 women. Ethnicity: not reported Inclusion Criteria: untreated, age≥18 years, conventional SBP between 140 and 179 mm Hg or DBP between 90 and 109 mm Hg, and ABPM awake BP of mean ≥135/85 mm Hg, or asleep mean ≥120/70 mm Hg. Exclusion criteria: pregnant women, shift workers, heavy drinkers, smokers, heavy exercisers, severe arterial or secondary arterial hypertension, type 1 diabetes, and cardiovascular disorders | |
| Interventions | olmesartan (20 mg od) on awakening: N=73 olmesartan (20 mg od) at bedtime:N=71 |
|
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data: 24h BP change by 48h ABPM, data was obtained from graph and text (fig 4 on page 72) Adverse Events: withdrawals due to adverse events |
|
| Notes | supported in part by grants from Ministerio de Educación y Ciencia, Xunta de Galicia, and University of Vigo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computerized random‐number generator |
| Allocation concealment (selection bias) | High risk | one member of the research team use of a list of random numbers |
| Blinding (performance bias and detection bias) All outcomes | Low risk | investigator obtaining the BP measurements, outcome assessors blinded. Benefits of the PROBE design and its validity compared with double‐blind, placebo‐controlled trials in assessing antihypertensive efficacy based on blinded ABPM measurements have been documented previously (Smith 2003) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported. 7 lost to follow‐up for no second ABPM available, 3 in daytime group, 4 in bedtime group; 4 withdrawn due to adverse effects, 3 in daytime group, 1 in bedtime group |
| Selective reporting (reporting bias) | High risk | Morning SBP, DBP were not reported. |
| Other bias | Low risk | "The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper". |
Hermida 2009a.
| Methods | prospective randomized open‐label, blinded endpoint, parallel‐group trial. 6 weeks of intervention Baseline similarity: age, BP,HR sample size calculation: reported |
|
| Participants | Country: Spain Number randomised: 120, 115 completed Mean age: 46.7±11.2(SD) years gender: 52 men, 63 women. Ethnicity: not reported Inclusion Criteria: untreated, age≥18 years, conventional SBP between 140 and 179 mm Hg or DBP between 90 and 109 mm Hg, and ABPM awake BP of mean ≥135/85 mm Hg, or asleep mean ≥120/70 mm Hg. Exclusion criteria: pregnant women, shift workers, heavy drinkers, smokers, heavy exercisers, severe arterial or secondary arterial hypertension, type 1 diabetes, and cardiovascular disorders | |
| Interventions | ramipril (5 mg od) on awakening: N=60 ramipril (5 mg od) at bedtime:N=60 |
|
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data: 24h BP change by 48h ABPM, data was obtained from graph and text (fig 3 on 44) Adverse Events: overall adverse events; withdrawals due to adverse events |
|
| Notes | supported in part by grants from Ministerio de Educación y Ciencia, King Pharmaceuticals, Xunta de Galicia, and University of Vigo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computerized random‐number generator |
| Allocation concealment (selection bias) | High risk | one member of the research team use of a list of random numbers (Hermida 2007b) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | investigator obtaining the BP measurements, outcome assessors blinded. Benefits of the PROBE design and its validity compared with double‐blind, placebo‐controlled trials in assessing antihypertensive efficacy based on blinded ABPM measurements have been documented previously (Smith 2003) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were reported. 3 lost to follow‐up for no second ABPM available, 1 in daytime group, 2 in bedtime group; 2 withdrawn due to adverse effects, 1 in daytime group, 1 in bedtime group |
| Selective reporting (reporting bias) | High risk | Morning SBP, DBP, serious adverse events were not reported. |
| Other bias | Low risk | none conflicts of interest |
Hermida 2009b.
| Methods | prospective randomized open‐label, blinded endpoint, parallel‐group trial. 8 weeks of intervention Baseline similarity: age, BP,HR sample size calculation: reported |
|
| Participants | Country: Spain Number randomised: 259, 238completed Mean age: 53.3±11.4(SD) years gender: 108 men, 130 women. Ethnicity: not reported Inclusion Criteria: untreated, age≧18 years, conventional SBP between 140 and 179 mm Hg or DBP between 90 and 109 mm Hg, and ABPM awake BP of mean ≧135/85 mm Hg, or asleep mean ≧120/70 mm Hg. Exclusion criteria: pregnant women, shift workers, heavy drinkers, smokers, heavy exercisers, severe arterial or secondary arterial hypertension, type 1 diabetes, and cardiovascular disorders | |
| Interventions | nifedipine GITS (30 mg od) on awakening: N=129 nifedipine GITS (30 mg od) at bedtime:N=130 |
|
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data:24h BP change by 48h ABPM, data was obtained from graph and text ( fig 2 on 157) Adverse Events: withdrawals due to adverse events |
|
| Notes | supported in part by grants from Ministerio de Educación y Ciencia, Xunta de Galicia, and University of Vigo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | computerized random‐number generator |
| Allocation concealment (selection bias) | High risk | one member of the research team use of a list of random numbers (Hermida 2007b) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | investigator obtaining the BP measurements, outcome assessors blinded. Benefits of the PROBE design and its validity compared with double‐blind, placebo‐controlled trials in assessing antihypertensive efficacy based on blinded ABPM measurements have been documented previously (Smith 2003) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were reported. 11 lost to follow‐up for no second ABPM available, 5 in daytime group, 6 in bedtime group; 10 withdrawn due to adverse effects, 6 in daytime group, 4 in bedtime group |
| Selective reporting (reporting bias) | High risk | Morning SBP, DBP were not reported. |
| Other bias | Low risk | none conflicts of interest |
Morgan 1997.
| Methods | randomised crossover trial. After 4 weeks placebo run‐in period, patients received perindopril in the morning and at bedtime each for 4 weeks
sample size calculation: not stated. carryover effects: not reported no washout period between treatment arms |
|
| Participants | Country: Australia Number randomised:20, 20 completed Age range: 33‐78 yeas mean age: 68±5 years gender: 20 male Ethnicity: not reported inclusion criteria: seated DBP 95‐110 mm Hg, less than 5 mm Hg difference between the two values, and mean 24 h DBP>85 mm Hg. exclusion criteria: clinic SBP >220 mm Hg, had a history of acute cerebrovascular or coronary events within the preceding 6 months, creatinine > 0.16 mmol/l, and liver function test results 50% greater than the normal range. | |
| Interventions | 4 mg od perindopril at 0900h or at 2100h | |
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data:24h BP change by 24h ABPM, data was obtained from graph and text ( fig 2 on 209) Adverse Events: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | not used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed, 2 patients ABPM data was eliminated |
| Selective reporting (reporting bias) | High risk | Morning SBP, DBP were not reported |
| Other bias | Unclear risk | carryover effects were not reported |
Myburgh 1995.
| Methods | open randomized crossover trial. After 4 weeks run‐in phase, patients received ramipril in the morning and at bedtime each for 4 weeks
Sample size calculation: not reported carryover effects: not reported no washout period between treatment arms |
|
| Participants | Country: South African Number randomised: 39 gender: 35 men, 4 women age range: 24‐73 years mean age: 49 years Ethnicity: not reported inclusion criteria: sitting DBP≥95 mm Hg and <114 mm Hg exclusion criteria: not stated | |
| Interventions | 2.5 mg od ramipril taken at 8 AM to 11AM or at 8 PM to 11PM | |
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data:24h BP change by 24h ABPM, data was obtained from graph and text (fig 1 on page 1302 and fig 2 on page 1303) Adverse Events: withdrawals due to adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | open label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were reported. Three patients was excluded due to increase dose of ramipril to 5 mg, three patients withdrawn because of adverse events |
| Selective reporting (reporting bias) | High risk | Morning SBP, DBP were not reported |
| Other bias | Unclear risk | carryover effects were not reported |
Neutel 2005.
| Methods | multicenter, double‐blind, double‐dummy, randomized, blinded end point, crossover study. After 4 weeks single‐blind placebo run‐in period, patients received chronotherapeutic propranolol and propranolol each for 4 weeks
Sample size calculation: not reported carryover effects: not reported no washout period between treatment arms |
|
| Participants | Country: not reported
Number randomised: 44
gender: 31 men, 13 women
mean age: 53.4±8.46 years Ethnicity: Caucasian 27, African American 5, Asian 3, Hispanic 5, Other 4 inclusion criteria: seated DBP of 95–114 mm Hg and a mean daytime ambulatory DBP (8 a.m.to 4 p.m.) of 90–114 mm Hg exclusion criteria: mean DBP ≥115 mm Hg and/or a mean SBP≥200 mm Hg |
|
| Interventions | 120 mg od chronotherapeutic delayed‐release propranolol (Innopran XL) dosed at bedtime: N=44 120 mg od traditional propranolol (Inderal LA) dosed in the morning : N=44 |
|
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data:24h BP change, morning (6 am to noon) BP change by 34h ABPM, data was obtained from text. Adverse Events: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | double‐dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were reported. Three patients were excluded, one was excluded by the investigator for being uncooperative and noncompliant with study medication, one for being off study medication for a significant period of time, and one patient was removed for alcohol abuse. |
| Selective reporting (reporting bias) | Low risk | all outcomes were reported. |
| Other bias | Unclear risk | carryover effects were not reported |
Nold 1998.
| Methods | open, randomized, crossover trial. After 1 week run‐in period, each patient received two treatment period( (each 3 weeks).
Sample size calculation: not reported
carryover effects: not reported no washout period between treatment arms |
|
| Participants | Country: Germany
Number randomised: 13, 12 completed
gender: 5 women, 7 men
mean age: 46.9±13.8 years
Ethnicity:not reported inclusion criteria: office DBP 95‐115 mm Hg, 18‐75 years, normal body weights exclusion criteria: malignant and secondary hypertension, history of angina pectoris, coronary heart disease, cerebrovascular event, myocardial infarction during the preceding 12 months, heart failure, arrhythmias, other severe concomitant pathological condition, child‐bearing women |
|
| Interventions | 5 mg od amlodipine was administered at 0800 h or at 2000h | |
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data:24h BP change by 24h ABPM, data was obtained from fig 1 on page 21 and fig 2 on page 22 Adverse Events: not reported |
|
| Notes | supported by grants from Pfizer GmbH, Karisruhe, Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | open |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported. one patient withdrawn for missing ABPM data. |
| Selective reporting (reporting bias) | High risk | Morning SBP, DBP were not reported |
| Other bias | Unclear risk | carryover effects were not reported; sponsorship or funding of this study and conflict of interest were not declared by the authors in the article |
Palatini 1992.
| Methods | randomized, doubled blind, crossover study. 2 weeks placebo run‐in period, two treatment period (each 4 weeks)
Sample size calculation: not reported carryover effects: not reported no washout period between treatment arms |
|
| Participants | Country: not reported
Number randomised: 18
gender: 12 men, 6 women
age: 48±7 years Ethnicity: not reported inclusion criteria: DBP 95‐114mm Hg exclusion criteria: secondary hypertension, renal or hepatic diseases, heart failure, postural hypotension, myocardial infarction or cerebrovascular accident within the past 6 months, unstable angina, valvular disease |
|
| Interventions | 20 mg od quinapril was administered at 8 AM and matching placebo was administered at 10pm for 4 weeks (N = 18) matching placebo was administered at 8 am and 20 mg od quinapril was administered at 10 PM for 4 weeks (N = 18) |
|
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data:24h BP change by 24h ABPM, data was obtained from fig 4 and fig 5 on page 1424 Adverse Events: No patient reported any side effects during the entire study period |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all patients completed the study |
| Selective reporting (reporting bias) | High risk | Morning SBP, DBP were not reported. |
| Other bias | Unclear risk | carryover effects were not reported |
Pechere 1998.
| Methods | randomized, double‐blind, double‐dummy, crossover design. After 2 weeks single‐blind placebo period, each patient received two treatment periods (each 6 weeks)
Sample size calculation: not reported carryover effects: not reported no washout period between treatment arms |
|
| Participants | Country: Switzerland
Number randomised: 21, 20 completed
gender: 14 men, 7 women
age range: 35‐70 years Ethnicity: not reported inclusion criteria: uncomplicated, mild to moderate essential hypertension, normal serum creatinine levels, office DBP range 95‐115 mm Hg. exclusion criteria: not reported |
|
| Interventions | 100 mg od Irbesartan taken in the morning: N=10 100 mg od Irbesartan taken on the evening: N=10 20 mg od Enalapril taken in the morning: N=10 20 mg od Enalapril taken on the evening: N=10 |
|
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data:24h BP change by 24h ABPM, data was obtained from table 3 on page 390 Adverse Events: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | not reported |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | capsules of the same appearance, double dummy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were reported. One patient interrupted the study because his blood pressure increased markedly |
| Selective reporting (reporting bias) | High risk | Morning SBP, DBP were not reported |
| Other bias | Unclear risk | carryover effects were not reported |
Qiu 2003.
| Methods | perspective, double‐blind, randomized, crossover design.1‐ to 2‐week wash‐out period for patients who were currently receiving antihypertensive therapy; 2‐week single‐blind placebo run‐in period; 12‐week double‐blind crossover treatment period (each 6 weeks) sample size calculation: yes carryover effects: not reported no washout period between treatment arms | |
| Participants | Country: China
Number randomised: 62, 60 completed
mean age: 57.5±10.5 years
gender: 44 men, 16 women Ethnicity: Chinese inclusion criteria: aged 21‐77 years, 3 seated office DBP≥95 mm Hg and ≤114 mm Hg, and mean ambulatory daytime SBP≥135 mm Hg or DBP≥85 mm Hg. exclusion criteria: secondary hypertension, SBP>200 mm Hg or DBP≧ 115 mm Hg, bradycardia or tachycardial, stroke or myocardial infarction in the previous 6 months, congestive heart failure, clinically significant hepatic or renal disease, uncontrolled diabetes mellitus, life‐style factors such as night‐shift work, history of drug and alcohol abuse, neurologic and psychiatric illnesses, and women who were pregnant or breast‐feeding. |
|
| Interventions | 5 mg od amlodipine at 7AM, matching placebo at 9PM: N=62 5 mg od amlodipine at 9 PM, matching placebo at 7AM: N=62 | |
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data:24h BP by 24h ABPM, data was obtained from fig 2 on page338 Adverse Events: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | randomization schedule |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all patients were reported. Two patients withdrawn for adverse events |
| Selective reporting (reporting bias) | High risk | Morning SBP, DBP were not reported |
| Other bias | Unclear risk | carryover effects were not reported |
White 1999a.
| Methods | randomized, double blind , double‐dummy, crossover design. 3‐week single blind, placebo run‐in period, 8‐week double‐blind crossover treatment period (each 4 weeks) sample size calculation: yes carryover effects: not reported no washout period between treatment arms | |
| Participants | Country: the United States Number randomised: 85,75 completed Mean age: 57.8±9.1(SD) years gender: 43 men, 32 women, . Ethnicity: 46 White, 26 Black, 2 Hispanic, 1 Asian. Inclusion criteria: age≥21 years, seated office DBP≥90 mm Hg and ≥109 mm Hg exclusion criteria: secondary hypertension, SBP>200 mm Hg or DBP≥110 mm Hg, bradycardia , tachycardia , stroke or myocardial infarction in the previous 6 months, congestive heart failure, clinically significant hepatic or renal disease, uncontrolled diabetes mellitus, life‐style factors such as night‐shift work or regular naps during the daytime, or history of allergy or intolerance to study medications. | |
| Interventions | 20 mg nisoldipine ER in the morning , and matching placebo in the evening, N=85 20 mg nisoldipine ER in the evening, and matching placebo in the morning: N=85 | |
| Outcomes |
Mortality: not reported Morbidity: not reported Blood Pressure data: 24h BP change by 24h ABPM (table 3 on page 809) Adverse Events: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | randomization schedule |
| Allocation concealment (selection bias) | Unclear risk | not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | double‐dummy, matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data in all patients were reported. eight patient withdrawn for adverse events, two patients were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Unclear risk | carryover effects were not reported. |
BP: blood pressure
SBP: Systolic blood pressure
DBP: diastolic blood pressure
MI: Myocardial infarction
GITS: gastrointestinal therapeutic system
HR: heart rate
ABPM: ambulatory blood pressure monitoring
JNC: Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure
ER: extended‐release
SD: standard deviation
SE: standard error
h: hour
BMI: body mass index
WHO: World Health Organization
COER: controlled onset extended release
GRD: graded‐release diltiazem HCl extended‐release
PROBE: prospective, randomized, open‐label, blinded end point
MAPEC: Ambulatory Blood Pressure Monitoring in the Prediction of Cardiovascular Events and Effects of Chronotherapy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bakris 2002 | Different drugs were used in treatments arms (COER‐verapamil versus (Enalapril or Losartan)) |
| Beliaev 2002 | not RCT |
| Beliaev 2003 | not RCT |
| Black 2003 | Different drugs were used in treatments arms (COER‐verapamil versus (atenolol or HCTZ)) |
| Calvo 2006 | not monotherapy. patients were receiving 3 antihypertensive drugs in a single morning dose. Patients were randomly assigned to one of two groups according to the modification in their treatment strategy: a) Changing one of the drugs, but keeping all 3 in the morning. b) The same approach but prescribing one of the drugs to be taken at bedtime. |
| Carpentiere 1984 | RCT, but period of treatment was 1 week and the minimum for inclusion was 3 weeks. |
| Conte 1998 | not randomised, review article |
| Cooke 1994 | not randomised, review article |
| Fogari 1988 | not monotherapy |
| Fogari 1993 | triple‐way crossover design |
| Glasser 1999 | not randomised, review article |
| Glasser 2000 | not RCT |
| Greminger 1994 | randomized double‐blind crossover study, but period of treatment was 1 week and the minimum for inclusion is three weeks. |
| Gupta 1995 | healthy men, not RCT |
| Hermida 1997 | RCT, course was only one week , but the minimum for inclusion was three weeks. |
| Hermida 2003a | not monotherapy, HDR and ASA on awakening, or HDR and ASA at bedtime |
| Hermida 2005 | not monotherapy, HDR and ASA on awakening, or HDR and ASA at bedtime |
| Hermida 2005c | not monotherapy, HDR and ASA on awakening, or HDR and ASA at bedtime |
| Hermida 2005d | the scheme consisting of >=3 antihypertensive drugs |
| Hermida 2008b | not monotherapy |
| Huape‐Arreola 2006 | not monotherapy |
| Kitahara 2004 | the same drug, but not the same dose. cilnidipine (5 mg od) was administered at bedtime or in the morning. In one group, a morning dosing regimen of cilnidipine was started from an initial dose of 5 mg (once daily). The dose was increased until either the casual BP became optimal or a dose of 20 mg was reached; The dose at this time was continued for 8 weeks. Thereafter, a bedtime dosing regimen with the same dosage was followed for an additional 8 weeks. In the other group, bedtime dosing with cilnidipine was started from the same initial dose and increased in the same way; Thereafter the same dose was administered in the morning for an additional 8 weeks. So, for one patient in this trial, the dose was the same, but the dose wasn't the same in all patients. |
| Koga 2005 | Patients treated first‐line antihypertension drugs still had high blood pressure in the morning were given carvedilol. |
| Kuroda 2004 | the same drug, but not the same dose. Patients taken trandolapril (1mg od) at bedtime or just after breakfast. After 4 weeks of treatment the dosage was increased to 2 mg of trandolapril unless the patient's BP had already been reduced to below 150 mmHg in systole and 90 mmHg in diastole, or side effects had occurred. Mean dose in each groups was different (morning administration group: 1.4±0.5; Bedtime administration group: 1.2±0.5), so the dose in all patients was not the same. |
| Lauro 1984 | lack of the data. The trial showed there was no statistically difference in 24h blood pressure, but no data was reported. |
| Macchiarulo 1999 | triple‐way crossover design |
| Mallion 1992 | No relevant endpoints. Compliance was primary outcome |
| Mengden 1993 | outcomes of interest not reported |
| Neutel 1996 | compared with placebo |
| Niegowska 2000 | Not RCT |
| Panfilov 1988 | NOT RCT |
| Potter 1990 | 337 patients were studied, but 257patients completed the study (31 were not randomised). |
| Shiga 1993 | No relevant endpoints. Maximum plasma concentration (Cmax) and Time to maximum plasma concentration (Tmax) was primary outcome |
| Sica 2003 | Healthy male |
| Sica 2004 | placebo‐controlled |
| Smith 2001a | compared with placebo |
| Smolensky 2007 | not RCT |
| Sunaga 1995 | The treatment period for this trial was less than two weeks |
| Sundberg 1991 | not RCT |
| Tokbaeva 1996 | Not RCT |
| Tykarski 2003 | the trial has published in abstract form. |
| White 1995 | compared with placebo |
| White 1997 | placebo‐controlled |
| White 1998 | Different drugs in comparator arms (nifedipine GITS versus COER‐verapamil) |
| White 1999b | This was not an original study. It analysed the data from White 1998 (Comparison of effects of controlled onset extended release verapamil at bedtime and nifedipine gastrointestinal therapeutic system on arising on early morning blood pressure, heart rate, and the heart rate‐blood pressure product. Am J Cardiol 1998;81(4):424‐31) |
| White 1999c | This was not an original study. It compares pooled data from three independent studies. These three papers were not referenced. We had written to White WB seeking a clarification, but there has been no reply. |
| White 1999d | This were not an original study. It compares pooled data from three independent studies. These three papers were not referenced. We had written to White WB seeking a clarification, but there has been no reply. |
| White 2001a | This was not an original study. It compares pooled data from three independent studies. These three papers were not referenced. We had written to White WB seeking a clarification, but there has been no reply. |
| White 2001b | This was not an original study. It compares pooled data from three independent studies. These three papers were not referenced. We had written to White WB seeking a clarification, but there has been no reply. |
| White 2002a | Different drugs in comparator arms (COER‐verapamil versus (enalapril or losartan)) |
| White 2004 | Different drugs in comparator arms (diltiazem versus ramipril) |
| Witte 1993 | No relevant endpoints. Daytime,nighttime and rhythm of blood pressure were outcome. |
| Wright 1976 | not monotherapy, the drugs of control group administered by three times daily, and ambulatory 24 hour mean BP was not measured |
| Wright 1982 | duration of treatment only 2 weeks |
| Wright 2004 | Different drugs in comparator arms (diltiazem versus amlodipine) |
| Yan 2009 | not monotherapy, lifestyle modifications and ASA on awakening, or lifestyle modifications and ASA at bedtime |
| Zaslavskaia 1988 | not RCT |
| Zaslavskaia 1998a | RCT, but the treatment period for this trial was only 10 days |
| Zaslavskaia 1998b | RCT, but the treatment period for this trial was less than three weeks |
| Zaslavskaia 1999a | RCT, but the study evaluated the circadian rhythms of systolic, diastolic and mean arterial pressure, HR before and after ramipril intake throughout 24 h. |
| Zaslavskaia 1999b | not RCT |
| Zaslavskaia 2000b | RCT, but aimed at circadian study of blood pressure |
| Zaslavskaya 1995 | not RCT |
| Zhou 2004 | combination therapy |
RCT: randomized controlled trial
HCTZ: hydrochlorothiazide
ASA: aspirin
HDR: nonpharmacological hygienic‐dietary recommendations
Characteristics of studies awaiting assessment [ordered by study ID]
Bernard 1994.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting article retrieval |
Hermida 2003b.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting article retrieval |
Meilhac 1992.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting article retrieval |
Mori 2007.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting article retrieval |
White 2003.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting article retrieval (abstract) |
Zaslavskaia 1994.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting article retrieval |
Zaslavskaia 1996.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting article retrieval |
Differences between protocol and review
The title was changed to better reflect the objective of the review.
The protocol did not state that randomized cross‐over trials would be included. This type of trial was included in the systematic review as it was thought that properly done randomized cross‐over RCTs would add to the knowledge of the effects of evening versus conventional morning dosing regimen on blood pressure profile and cardiovascular outcomes.
Li Bingyan was a co‐author of the protocol but was unable to participate in the conduct of the full review, therefore his name does not appear in the list of authors.
Contributions of authors
All authors contributed work on this systematic review. Zhao Ping and Xu Ping formulated the idea for the review and developed the basis for the protocol.
Xu Ping and Zhao Ping both acted as independent reviewers and took the lead roles in searching, identifying, and assessing studies, in data abstraction and analyses, and in writing up the draft of this review. They were equivalent in this review.
Wan Chaomin and Wang Zhengrong helped with settling discrepancies in inclusion criteria or data abstraction, confirming accuracy of data, and making suggestions on writing the draft of this review.
Sources of support
Internal sources
Chinese Cochrane Centre, Chinese Centre of Evidence‐based Medicine, West China Hospital of Sichuan University, China.
External sources
China Medical Board of New York (Grant number:98‐680), USA.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Calvo 2006a {published data only}
- Calvo C, Hermida RC, Ayala DE, Lopez JE, Rodriguez M, Chayan L, et al. [Chronotherapy with torasemide in hypertensive patients: increased efficacy and therapeutic coverage with bedtime administration]. Medicina clinica 2006;127(19):721‐9. [DOI] [PubMed] [Google Scholar]
Glasser 2003 {published data only}
- Glasser SP, Neutel JM, Gana TJ, Albert KS. Efficacy and safety of a once daily graded‐release diltiazem formulation in essential hypertension. Am J Hypertens 2003;16(1):51‐8. [DOI] [PubMed] [Google Scholar]
Hermida 2003 {published data only}
- Hermida RC, Calvo C, Ayala DE, Dominguez MJ, Covelo M, Fernandez JR, et al. Administration time‐dependent effects of valsartan on ambulatory blood pressure in hypertensive subjects. Hypertension 2003;42(3):283‐290. [DOI] [PubMed] [Google Scholar]
Hermida 2004 {published data only}
- Hermida RC, Calvo C, Ayala DE, Dominguez MJ, Covelo M, Fernandez JR, et al. Administration‐time‐dependent effects of doxazosin gits on ambulatory blood pressure of hypertensive subjects. Chronobiol Int 2004;21(2):277‐296. [DOI] [PubMed] [Google Scholar]
Hermida 2005a {published data only}
- Hermida RC, Calvo C, Ayala DE, Mojon A, Rodriguez M, Chayan L, et al. Administration time‐dependent effects of valsartan on ambulatory blood pressure in elderly hypertensive subjects. Chronobiology international 2005;22(4):755‐76. [DOI] [PubMed] [Google Scholar]
Hermida 2005b {published data only}
- Hermida RC, Calvo C, Ayala DE, Fernandez JR, Covelo M, Mojon A, et al. Treatment of non‐dipper hypertension with bedtime administration of valsartan. Journal of hypertension 2005;23(10):1913‐22. [DOI] [PubMed] [Google Scholar]
Hermida 2007 {published data only}
- Hermida RC, Calvo C, Ayala DE, Lopez JE, Rodriguez M, Chayan L, et al. Dose‐ and administration time‐dependent effects of nifedipine gits on ambulatory blood pressure in hypertensive subjects. Chronobiology international 2007;24(3):471‐93. [DOI] [PubMed] [Google Scholar]
Hermida 2007a {published data only}
- Hermida RC, Ayala DE, Fernandez JR, Calvo C. Comparison of the efficacy of morning versus evening administration of telmisartan in essential hypertension. Hypertension 2007;50(4):715‐22. [DOI] [PubMed] [Google Scholar]
Hermida 2008 {published data only}
- Hermida RC, Ayala DE, Mojon A, Fernandez JR. Chronotherapy with nifedipine GITS in hypertensive patients: improved efficacy and safety with bedtime dosing. American journal of hypertension 2008;21(8):948‐54. [DOI] [PubMed] [Google Scholar]
Hermida 2008a {published data only}
- Hermida RC, Ayala DE, Mojon A, Chayan L, Dominguez MJ, Fontao MJ, et al. Comparison of the effects on ambulatory blood pressure of awakening versus bedtime administration of torasemide in essential hypertension. Chronobiology international 2008;25(6):950‐70. [DOI] [PubMed] [Google Scholar]
Hermida 2009 {published data only}
- Hermida RC, Ayala DE, Chayan L, Mojon A, Fernandez JR. Administration‐time‐dependent effects of olmesartan on the ambulatory blood pressure of essential hypertension patients. Chronobiology international 2009;26(1):61‐79. [DOI] [PubMed] [Google Scholar]
Hermida 2009a {published data only}
- Hermida RC, Ayala DE. Chronotherapy with the angiotensin‐converting enzyme inhibitor ramipril in essential hypertension: improved blood pressure control with bedtime dosing. Hypertension 2009;54(1):40‐6. [DOI] [PubMed] [Google Scholar]
Hermida 2009b {published data only}
- Hermida RC, Ayala DE, Mojon A, Alonso I, Fernandez JR. Reduction of morning blood pressure surge after treatment with nifedipine. GITS at bedtime, but not upon awakening, in essential hypertension. Blood Pressure Monitoring 2009;14(4):152‐9. [DOI] [PubMed] [Google Scholar]
Morgan 1997 {published data only}
- Morgan T, Anderson A, Jones E. The effect on 24 h blood pressure control of an angiotensin converting enzyme inhibitor (perindopril) administered in the morning or at night. J Hypertens 1997;15(2):205‐211. [DOI] [PubMed] [Google Scholar]
Myburgh 1995 {published data only}
- Myburgh DP, Verho M, Botes JH, Erasmus TP, Luus HG. 24‐hour blood pressure control with ramipril: comparison of once‐ daily morning and evening administration. Curr Ther Res Clin Exp 1995;56(12):1298‐306. [Google Scholar]
Neutel 2005 {published data only}
- Neutel JM, Rotenberg K. Comparison of a chronotherapeutically administered beta blocker vs. a traditionally administered beta blocker in patients with hypertension. Journal of clinical hypertension (Greenwich, Conn.) 2005;7(7):395‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nold 1998 {published data only}
- Nold G, Strobel G, Lemmer B. Morning versus evening amlodipine treatment: effect on circadian blood pressure profile in essential hypertensive patients. Blood Press Monit 1998;3(1):17‐25. [PubMed] [Google Scholar]
Palatini 1992 {published data only}
- Palatini P. Can an angiotensin‐converting enzyme inhibitor with a short half‐life effectively lower blood pressure for 24 hours?. Am Heart J 1992;123(5):1421‐5. [DOI] [PubMed] [Google Scholar]
- Palatini P, Racioppa A, Raule G, Zaninotto M, Penzo M, Pessina AC. Effect of timing of administration on the plasma ace inhibitory activity and the antihypertensive effect of quinapril. Clin Pharmacol Ther 1992;52(4):378‐83. [DOI] [PubMed] [Google Scholar]
Pechere 1998 {published data only}
- Pechere‐Bertschi A, Nussberger J, Decosterd L, Armagnac C, Sissmann J, Bouroudian M, et al. Renal response to the angiotensin ii receptor subtype 1 antagonist irbesartan versus enalapril in hypertensive patients. J Hypertens 1998;16(3):385‐393. [DOI] [PubMed] [Google Scholar]
Qiu 2003 {published data only}
- Qiu YG, Chen JZ, Zhu JH, Yao XY. Differential effects of morning or evening dosing of amlodipine on circadian blood pressure and heart rate. Cardiovasc Drugs Ther 2003;17(4):335‐341. [DOI] [PubMed] [Google Scholar]
- Qiu YG, Zheng Ping, Yao XY, He JL, Hu XS, Chen JZ, et al. Effect of 24 h Blood Pressure by Amlodipine Administered in Different Time [¶‾̬ѪѹÆÀ¼Û²»Í¬Ê±¼ä·þÓð±ÂȵØƽµÄ½µÑ¹Ð§Ó¦]. ¸ßѪѹÔÓÖ¾ 2000;8(3):207‐209. [Google Scholar]
White 1999a {published data only}
- White WB, Mansoor GA, Pickering TG, Vidt DG, Hutchinson HG, Johnson RB, et al. Differential effects of morning and evening dosing of nisoldipine ER on circadian blood pressure and heart rate. Am J Hypertens 1999;12(8 Pt 1):806‐14. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bakris 2002 {published data only}
- Bakris G, Sica D, Ram V, Fagan T, Vaitkus PT, Anders RJ. A comparative trial of controlled‐onset, extended‐release verapamil, enalapril, and losartan on blood pressure and heart rate changes. Am J Hypertens 2002;15(1 Pt 1):53‐57. [DOI] [PubMed] [Google Scholar]
Beliaev 2002 {published data only}
- Beliaev SD, Zaslavskaia RM. [Advantages of capoten chronotherapy of patients with hypertension in an outpatient setting]. Ter Arkh 2002;74(1):18‐21. [PubMed] [Google Scholar]
Beliaev 2003 {published data only}
- Beliaev SD, Zaslavskaia RM, Khetagurova LG. [Optimization of sanatorium treatment of patients with essential hypertension stage ii by chronotherapy]. Klin Med (Mosk) 2003;81(11):46‐50. [PubMed] [Google Scholar]
Black 2003 {published data only}
- Black HR, Elliott WJ, Grandits G, Grambsch P, Lucente T, Neaton JD, et al. Results of the Controlled Onset Verapamil INvestigation of Cardiovascular Endpoints (CONVINCE) trial by geographical region. Journal of hypertension 2005;23(5):1099‐106. [DOI] [PubMed] [Google Scholar]
- Black HR, Elliott WJ, Grandits G, Grambsch P, Lucente T, White WB, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA 2003;289(16):2073‐82. [DOI] [PubMed] [Google Scholar]
- Black HR, Elliott WJ, Neaton JD, Grandits G, Grambsch P, Grimm RH Jr, et al. Baseline Characteristics and Early Blood Pressure Control in the CONVINCE Trial. Hypertension 2001;37(1):12‐18. [DOI] [PubMed] [Google Scholar]
- Black HR, Elliott WJ, Neaton JD, Grandits G, Grambsch P, Grimm RH Jr, et al. Rationale and design for the controlled onset verapamil investigation of cardiovascular endpoints (CONVINCE) trial. Control Clin Trials 1998;19(4):370‐90. [DOI] [PubMed] [Google Scholar]
Calvo 2006 {published data only}
- Calvo C, Hermida RC, Ayala DE, Lopez JE, Fernandez JR, Mojon A, et al. [Effects of time‐dependent administration of antihypertensive treatment in patients with resistant hypertension]. Medicina clinica 2006;126(10):364‐72. [DOI] [PubMed] [Google Scholar]
Carpentiere 1984 {published data only}
- Carpentiere G, Martelli M, Castello F, Marino S. Time‐dependent effects of once‐daily methyldopa treatment. Curr Ther Res Clin Exp 1984;35(3):476‐82. [Google Scholar]
Conte 1998 {published data only}
- Conte G, Rigon N, Perrone A. [Application of chronotherapy to cardiovascular diseases]. Recenti Prog Med 1998;89(9):465‐9. [PubMed] [Google Scholar]
Cooke 1994 {published data only}
- Cooke HM, Lynch A. Biorhythms and chronotherapy in cardiovascular disease. Am J Hosp Pharm 1994;51(20):2569‐80. [PubMed] [Google Scholar]
Fogari 1988 {published data only}
- Fogari R, Tettamanti F, Zoppi A, Poletti L, Botta GF. Evaluation of the efficacy of once‐daily administration of captopril plus hydrochlorothiazide by 24‐hour ambulatory blood pressure monitoring. Curr Ther Res Clin Exp 1988;44(6):1050‐7. [Google Scholar]
Fogari 1993 {published data only}
- Fogari R, Malacco E, Tettamanti F, Gnemmi AE, Milani M. Evening vs morning isradipine sustained release in essential hypertension: a double‐blind study with 24 h ambulatory monitoring. Br J Clin Pharmacol 1993;35(1):51‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glasser 1999 {published data only}
- Glasser SP. Circadian variations and chronotherapeutic implications for cardiovascular management: a focus on coer verapamil. Heart Dis 1999;1(4):226‐32. [PubMed] [Google Scholar]
Glasser 2000 {published data only}
- Glasser SP, Frishman W, White WB, Stone P, Johnson MF. Circadian heart rate response to chronotherapy versus conventional therapy in patients with hypertension and myocardial ischemia. Clin Cardiol 2000;23(7):524‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greminger 1994 {published data only}
- Greminger P, Suter PM, Holm D, Kobelt R, Vetter W. Morning versus evening administration of nifedipine gastrointestinal therapeutic system in the management of essential hypertension. Clin Investig 1994;72(11):864‐9. [DOI] [PubMed] [Google Scholar]
Gupta 1995 {published data only}
- Gupta SK, Yih BM, Atkinson L, Longstreth J. The effect of food, time of dosing, and body position on the pharmacokinetics and pharmacodynamics of verapamil and norverapamil. Journal of Clinical Pharmacology 1995;35(11):1083‐93. [DOI] [PubMed] [Google Scholar]
Hermida 1997 {published data only}
- Hermida RC, Fernandez JR, Ayala DE, Mojon A, Iglesias M. Influence of aspirin usage on blood pressure: dose and administration‐time dependencies. Chronobiol Int 1997;14(6):619‐37. [DOI] [PubMed] [Google Scholar]
Hermida 2003a {published data only}
- Hermida RC, Ayala DE, Calvo C, Lopez JE, Fernandez JR, Mojon A, et al. Administration time‐dependent effects of aspirin on blood pressure in untreated hypertensive patients. Hypertension 2003;41(6):1259‐67. [DOI] [PubMed] [Google Scholar]
Hermida 2005 {published data only}
- Hermida RC, Ayala DE, Calvo C, Lopez JE, Mojon A, Rodriguez M, et al. Differing administration time‐dependent effects of aspirin on blood pressure in dipper and non‐dipper hypertensives. Hypertension 2005;46(4):1060‐8. [DOI] [PubMed] [Google Scholar]
Hermida 2005c {published data only}
- Hermida RC, Ayala DE, Calvo C, Lopez JE. Aspirin administered at bedtime, but not on awakening, has an effect on ambulatory blood pressure in hypertensive patients. Journal of the American College of Cardiology 2005;46(6):975‐83. [DOI] [PubMed] [Google Scholar]
Hermida 2005d {published data only}
- Hermida RC, Ayala DE, Calvo C, Lopez JE, Mojon A, Fontao MJ, et al. Effects of time of day of treatment on ambulatory blood pressure pattern of patients with resistant hypertension. Hypertension 2005;46(2):1053‐9. [DOI] [PubMed] [Google Scholar]
Hermida 2008b {published data only}
- Hermida RC, Ayala DE, Fernandez JR, Calvo C. Chronotherapy improves blood pressure control and reverts the nondipper pattern in patients with resistant hypertension. Hypertension 2008;51(1):69‐76. [DOI] [PubMed] [Google Scholar]
Huape‐Arreola 2006 {published data only}
- Huape‐Arreola MS, Urbina‐Arreola CG, Vargas‐Espinosa JM, Cervantes M, Ruiz‐Vega H. Circadian rhythm of blood pressure is more frequently attained under nocturnal rather than diurnal schedule of irbesartan administration in hypertensive patients. Proceedings of the Western Pharmacology Society 2006;49:165‐6. [Google Scholar]
Kitahara 2004 {published data only}
- Kitahara Y, Saito F, Akao M, Fujita H, Takahashi A, Taguchi H, et al. Effect of morning and bedtime dosing with cilnidipine on blood pressure, heart rate, and sympathetic nervous activity in essential hypertensive patients. J Cardiovasc Pharmacol 2004;43(1):68‐73. [DOI] [PubMed] [Google Scholar]
Koga 2005 {published data only}
- Koga H, Hayashi J, Yamamoto M, Kitamoto K. Prevention of morning surge of hypertension by the evening administration of carvedilol. JMAJ 2005;48(8):398‐403. [Google Scholar]
Kuroda 2004 {published data only}
- Kuroda T, Kario K, Hoshide S, Hashimoto T, Nomura Y, Saito Y, et al. Effects of bedtime vs. Morning administration of the long‐acting lipophilic angiotensin‐coverting enzyme inhibitor trandolapril on morning blood pressure in hypertensive patients. Hypertens Res 2004;27(1):15‐20. [DOI] [PubMed] [Google Scholar]
Lauro 1984 {published data only}
- Lauro R, Del MS, Ottolenghi L, et al. Effect of mepindolol on blood pressure variability in hypertensive patients. Curr Ther Res Clin Exp 1984;36(2):374‐8. [Google Scholar]
- Lauro R, Reda G, Del MS, et al. Chronobiological approach to the treatment of essential hypertension: preliminary data. Int J Clin Pharmacol Ther Toxicol 1984;22(11):630‐6. [PubMed] [Google Scholar]
Macchiarulo 1999 {published data only}
- Macchiarulo C, Pieri R, Mitolo DC, Pirrelli A. Management of antihypertensive treatment with lisinopril: a chronotherapeutic approach. Riv Eur Sci Med Farmacol 1999;3(6):269‐75. [PubMed] [Google Scholar]
Mallion 1992 {published data only}
- Mallion JM, Meilhac B, Tremel F, Calvez R, Bertholom N. Use of a microprocessor‐equipped tablet box in monitoring compliance with antihypertensive treatment. J Cardiovasc Pharmacol 1992;19(S2):S41‐8. [DOI] [PubMed] [Google Scholar]
Mengden 1993 {published data only}
- Mengden T, Binswanger B, Spuhler T, Weisser B, Vetter W. The use of self‐measured blood pressure determinations in assessing dynamics of drug compliance in a study with amlodipine once a day, morning versus evening. J Hypertens 1993;11(12):1403‐11. [DOI] [PubMed] [Google Scholar]
Neutel 1996 {published data only}
- Neutel JM, Alderman M, Anders RJ, Weber MA. Novel delivery system for verapamil designed to achieve maximal blood pressure control during the early morning. Am Heart J 1996;132(6):1202‐6. [DOI] [PubMed] [Google Scholar]
Niegowska 2000 {published data only}
- Niegowska J, Mastej M, Piotrowski W. [Controlled release diltiazem in monotherapy of hypertension‐‐time of drug administration and circadian blood pressure pattern]. Pol Arch Med Wewn 2000;104(6):853‐7. [PubMed] [Google Scholar]
Panfilov 1988 {published data only}
- Panfilov IuA, Kriukov NN, Lapina ML. [Chronotherapy of hypertensive patients]. Klin Med (Mosk) 1988;66(5):47‐51. [PubMed] [Google Scholar]
Potter 1990 {published data only}
- Potter C, Dews I, Pullan T, Wight L, Vandenburg M. Morning vs evening dosing of sustained‐release verapamil in mild to moderate hypertension. Br J Clin Pharmacol 1990;30(2):338P‐9P. [Google Scholar]
Shiga 1993 {published data only}
- Shiga T, Fujimura A, Tateishi T, Ohashi K, Ebihara A. Differences of chronopharmacokinetic profiles between propranolol and atenolol in hypertensive subjects. J Clin Pharmacol 1993;33(8):756‐61. [DOI] [PubMed] [Google Scholar]
Sica 2003 {published data only}
- Sica D, Frishman WH, Manowitz N. Pharmacokinetics of propranolol after single and multiple dosing with sustained release propranolol or propranolol CR (Innopran XL) , a new chronotherapeutic formulation. Heart Disease 2003;5(3):176‐81. [DOI] [PubMed] [Google Scholar]
Sica 2004 {published data only}
- Sica DA, Neutel JM, Weber MA, Manowitz N. The antihypertensive efficacy and safety of a chronotherapeutic formulation of propranolol in patients with hypertension. J Clin Hypertens (Greenwich) 2004;6(5):231‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2001a {published data only}
- Smith DH, Neutel JM, Weber MA. A new chronotherapeutic oral drug absorption system for verapamil optimizes blood pressure control in the morning. Am J Hyperten 2001;14(1):14‐9. [DOI] [PubMed] [Google Scholar]
Smolensky 2007 {published data only}
- Smolensky MH, Hermida RC, Portaluppi F. Comparison of the efficacy of morning versus evening administration of olmesartan in uncomplicated essential hypertension. Chronobiology International 2007;24(1):171‐81. [DOI] [PubMed] [Google Scholar]
Sunaga 1995 {published data only}
- Sunaga K, Fujimura A, Shiga T, Ebihara A. Chronopharmacology of enalapril in hypertensive patients. Eur J Clin Pharmacol 1995;48(6):441‐5. [DOI] [PubMed] [Google Scholar]
Sundberg 1991 {published data only}
- Sundberg S, Luurila OJ, Kohvakka A, Gordin A. The circadian heart rate but not blood pressure profile is influenced by the timing of beta‐block administration in hypertension. Eur J Clin Pharmacol 1991;40:435‐436. [DOI] [PubMed] [Google Scholar]
Tokbaeva 1996 {published data only}
- Tokbaeva KK, Zaslavskaia RM, Khalberg F, Teiblium MM. [A chronobiological approach to correcting disorders of time organization of clofelin hemodynamics in patients with stage ii essential hypertension]. Klin Med (Mosk) 1996;74(8):52‐4. [PubMed] [Google Scholar]
Tykarski 2003 {published data only}
- Tykarski A, Mastej M, Niklas A, Lopatka P, Krasinska B, Kosicka T. Blood pressure response during exercise test in hypertensive patients depending on time of drugs administration. Nephrol Dial Transplant 2003;18(S4):364. [Google Scholar]
White 1995 {published data only}
- White WB, Anders RJ, MacIntyre JM, Black HR, Sica DA, Bynny RL, et al. Nocturnal dosing of a novel delivery system of verapamil for systemic hypertension. Am J Cardiol 1995;76(5):375‐80. [DOI] [PubMed] [Google Scholar]
White 1997 {published data only}
- White WB, Mehrotra DV, Black HR, Fakouhi TD. Effects of controlled‐onset extended‐release verapamil on nocturnal blood pressure (dippers versus nondippers). Am J Cardiol 1997;80(4):469‐74. [DOI] [PubMed] [Google Scholar]
White 1998 {published data only}
- White WB, Black HR, Weber MA, Elliott WJ, Bryzinski B, Fakouhi TD. Comparison of effects of controlled onset extended release verapamil at bedtime and nifedipine gastrointestinal therapeutic system on arising on early morning blood pressure, heart rate, and the heart rate‐blood pressure product. Am J Cardiol 1998;81(4):424‐31. [DOI] [PubMed] [Google Scholar]
White 1999b {published data only}
- White WB. Heart rate and the rate‐pressure product as determinants of cardiovascular risk in patients with hypertension. Am J Hypertens 1999;12(2 II SUPPL):50S‐55S. [DOI] [PubMed] [Google Scholar]
White 1999c {published data only}
- White WB, Johnson MF, Black HR, Fakouhi TD. Effects of the chronotherapeutic delivery of verapamil on circadian blood pressure in African‐American patients with hypertension. Ethn Dis 1999;9(3):341‐9. [PubMed] [Google Scholar]
White 1999d {published data only}
- White WB, Johnson M. Heart rate and the rate‐pressure product in patients with hypertension: the usefulness of chronotherapy. Eur Heart J Suppl 1999;1(B):B18‐B23. [Google Scholar]
White 2001a {published data only}
- White WB, Elliott WJ, Johnson MF, Black HR. Chronotherapeutic delivery of verapamil in obese versus non‐obese patients with essential hypertension. J Hum Hypertens 2001;15(2):135‐141. [DOI] [PubMed] [Google Scholar]
White 2001b {published data only}
- White WB, Johnson MF, Black HR, Elliott WJ, Sica DA. Gender and age effects on the ambulatory blood pressure and heart rate responses to antihypertensive therapy. Am J Hypertens 2001;14(12):1239‐47. [DOI] [PubMed] [Google Scholar]
White 2002a {published data only}
- White WB, Sica DA, Calhoun D, Mansoor GA, Anders RJ. Preventing increases in early‐morning blood pressure, heart rate, and the rate‐pressure product with controlled onset extended release verapamil at bedtime versus enalapril, losartan, and placebo on arising. Am Heart J 2002;144(4):657‐65. [DOI] [PubMed] [Google Scholar]
White 2004 {published data only}
- White WB, Lacourciere Y, Gana T, Pascual MG, Smith DH, Albert KS. Effects of graded‐release diltiazem versus ramipril, dosed at bedtime, on early morning blood pressure, heart rate, and the rate‐pressure product. American heart journal 2004;148(4):628‐34. [DOI] [PubMed] [Google Scholar]
Witte 1993 {published data only}
- Witte K, Weisser K, Neubeck M, Mutschler E, Lehmann K, Hopf R, et al. Cardiovascular effects, pharmacokinetics, and converting enzyme inhibition of enalapril after morning versus evening administration. Clin Pharmacol Ther 1993;54(2):177‐86. [DOI] [PubMed] [Google Scholar]
Wright 1976 {published data only}
- Wright JM, McLeod PJ, McCullough W. Antihypertensive efficacy of a single bedtime dose of methyldopa. Clinical pharmacology and therapeutics 1976;20(6):733‐7. [DOI] [PubMed] [Google Scholar]
Wright 1982 {published data only}
- Wright JM, Orozco‐Gonzalez M, Polak G, Dollery CT. Duration of effect of single daily dose methyldopa therapy. British Journal of Clinical Pharmacology 1982;13(6):847‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 2004 {published data only}
- Wright JT, Sica DA, Gana TJ, Bohannon K, Pascual LG, Albert KS. Antihypertensive efficacy of night‐time graded‐release diltiazem versus morning amlodipine in African Americans. American journal of hypertension 2004;17(9):734‐42. [DOI] [PubMed] [Google Scholar]
Yan 2009 {published data only}
- Yan R, Lv JY, Duan LQ. [Differing administration time‐dependent effects of aspirin on blood pressure in mild hypertension]. Chinese Journal of Integrative Medicine on Cardio‐/Cerebrovascular Disease 2009;7(6):646‐647. [Google Scholar]
Zaslavskaia 1988 {published data only}
- Zaslavskaia RM, Varshitskii MG, Teiblium MM. [Chronotherapy of hypertension with dopegit]. Sov Med 1988;6:79‐81. [PubMed] [Google Scholar]
Zaslavskaia 1998a {published data only}
- Zaslavskaia RM, Zhamankulov KA, Zhumabaeva TN, Teiblium MM. [Chronopharmacodynamics of betacap (long‐acting propranolol) in patients with hypertension stage ii]. Klin Med (Mosk). 1998;76(4):38‐40. [PubMed] [Google Scholar]
Zaslavskaia 1998b {published data only}
- Zaslavskaia RM Zhumabaeva TN, Zhamankulov KA, Teiblium MM. [Hemodynamic chronosensitivity to metopress‐retard in patients with stage ii hypertension]. Klin Med (Mosk). 1998;76(10):42‐4. [PubMed] [Google Scholar]
Zaslavskaia 1999a {published data only}
- Zaslavskaia RM, Narmanova OZ, Teiblium MM, Kalimurzina BS. [Time‐dependent effects of ramipril in patients with hypertension of 2 stage]. Klin Med (Mosk) 1999;77(10):41‐4. [PubMed] [Google Scholar]
Zaslavskaia 1999b {published data only}
- Zaslavskaia RM, Iskakova MT, Teiblium MM. [Preventive chronotherapy with capoten in hypertensive outpatients]. Klin Med (Mosk) 1999;77(3):39‐41. [PubMed] [Google Scholar]
Zaslavskaia 2000b {published data only}
- Zaslavskaia RM, Biiasilov NS, Akhmetov KZh, Teiblium MM. [Capozide‐50 alone and in combination with melatonin in therapy of hypertension]. Klin Med (Mosk) 2000;78(11):39‐41. [PubMed] [Google Scholar]
Zaslavskaya 1995 {published data only}
- Zaslavskaya RM, Akhmetov Zh K, Teiblyum MM. Chronosensitivity to adelphan‐esidrex and sinepres and efficacy of hypotensive treatment. Klin Med 1995;73(4):57‐60. [PubMed] [Google Scholar]
Zhou 2004 {published data only}
- Zhou YJ, Hou JY, Zhang YZ. Effect of timing of administration on antihypertensive effect of losartan and hydrochlorothiazide. Clinical Focus 2004;19(5):F2. [Google Scholar]
References to studies awaiting assessment
Bernard 1994 {published data only}
- Bernard FMF, Renucci JF, Siche JP, Vaisse B, Poggi L, Mallion JM. Ambulatory blood pressure measurement and efficacy of diltiazem. Influence of the timing of drug doses and of initial blood pressure level. Ann Cardiol Angeiol (Paris) 1994;43(6):357‐64. [PubMed] [Google Scholar]
Hermida 2003b {published data only}
- Hermida RC, Ayala DE, Calvo C, Lopez JE, Fernandez JR, Mojon A, et al. [Administration‐time dependent effects of acetyl‐salicylic acid on blood pressure in patients with mild essential hypertension]. Med Clin (Barc) 2003;120(18):686‐92. [DOI] [PubMed] [Google Scholar]
Meilhac 1992 {published data only}
- Meilhac B, Mallion JM, Carre A, Chanudet X, Poggi L, Gosse P, et al. [Study of the influence of the time of administration on the antihypertensive effect and nitrendipine tolerance in mild to moderate essential hypertensive patients. Value of ambulatory recording of blood pressure on 24 hours]. Therapie 1992;47(3):205‐10. [PubMed] [Google Scholar]
Mori 2007 {published data only}
- Mori H. Comparison of effect on morning home blood pressure between administered telmisartan based antihypertensive drugs in morning and in evening. Therapeutic Research 2007;28(2):269‐74. [Google Scholar]
White 2003 {published data only}
- White WB, Smith DHG, Gana TJ, Pascual LG, Albert KS. Comparison of nocturnal dosing of diltiazem er and ramipril on early morning blood pressure, heart rate and the rate‐pressure product. American Journal Hypertension 2003; Vol. 16:133A.
Zaslavskaia 1994 {published data only}
- Zaslavskaia RM, Akhmetov KZh, Hulberg F, Teiblum MM. [The optimization of capoten treatment by taking into account its chronosensitivity in hypertension patients]. Ter Arkh 1994;66(8):7‐9. [PubMed] [Google Scholar]
Zaslavskaia 1996 {published data only}
- Zaslavskaia RM, Lilitsa GV, Akhmetov KZ, Teiblium MM. [Time‐related effect of betapressin+ in patients with essential hypertension stage ii]. Klin Med (Mosk). 1996;74(9):43‐5. [PubMed] [Google Scholar]
Additional references
Canter 1994
- Canter DA, Texter MJ, McLain RW. Short report: Ambulatory blood pressure monitoring can play an integral role in patient selection dosage adjustment and efficacy assessment in clinical trials of antihypertensive agents. J Hypertens 1994;12:491‐494. [PubMed] [Google Scholar]
Chobanian 2003
- Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. Hypertension 2003;42:1206‐1252. [DOI] [PubMed] [Google Scholar]
Ezeugo 2009
- Ezeugo U, Glasser SP. Clinical benefits versus shortcomings of diltiazem once‐daily in the chronotherapy of cardiovascular diseases. Expert Opinion on Pharmacotherapy 2009;10(3):485‐91. [DOI] [PubMed] [Google Scholar]
Fujimura 1999
- Fujimura A, Ebihara A, Shiigai T, Shimada K, Tagawa H, Gomi T, Nakamura Y, Suzuki M, Yokozuka H. Amelioration of enalapril‐induced dry cough by changing dosing time from morning to evening; a preliminary trial. Jpn J Clin Pharmacol Ther 1999;30(5):741‐744. [Google Scholar]
Guidelines Subcommittee 1999
- Guidelines Subcommittee. 1999 World Health Organization‐International Society of Hypertension guidelines for the management of hypertension. J Hypertens 1999;17:151‐183. [PubMed] [Google Scholar]
Hermida 1999
- Hermida RC. Time‐qualified reference values for 24 h ambulatory blood pressure monitoring. Blood Press Monit 1999;4:137‐147. [PubMed] [Google Scholar]
Hermida 2002
- Hermida RC, Ayala DE, Fernandez JR, et al. Modeling the circadian variability of ambulatorily monitored blood pressure by multiple component analysis. Chronobiol Int 2002;19:461‐81. [DOI] [PubMed] [Google Scholar]
Hermida 2004a
- Hermida RC, Smolensky MH. Chronotherapy of hypertension. Curr Opin Nephrol Hypertens 2004;13:501‐505. [DOI] [PubMed] [Google Scholar]
Hermida 2005e
- Hermida RC, Ayala DE, Calvo C. Administration time‐dependent effects of antihypertensive treatment on the circadian pattern of blood pressure. Curr Opin Nephrol Hypertens 2005;14:453‐459. [DOI] [PubMed] [Google Scholar]
Hermida 2007b
- Hermida RC. Ambulatory blood pressure monitoring in the prediction of cardiovascular events and effects of chronotherapy: rationale and design of the MAPEC study. Chronobiology International 2007;24(4):749‐75. [DOI] [PubMed] [Google Scholar]
Hermida 2007C
- Hermida RC, Ayala DE, Smolensky MH, Portaluppi F. Chronotherapy in hypertensive patients: administration‐time dependent effects of treatment on blood pressure regulation. Expert Review of Cardiovascular Therapy 2007;5(3):463‐75. [DOI] [PubMed] [Google Scholar]
Hermida 2007d
- Hermida RC, Ayala DE, Calvo C. Optimal timing for antihypertensive dosing: focus on valsartan. Therapeutics and Clinical Risk Management 2007;3(1):119‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kario 2003
- Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, MorinariM, Murata M, Kuroda T, Schwartz JE, Shimada K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation 2003;107:1401‐1406. [DOI] [PubMed] [Google Scholar]
Kuwajima 1995
- Kuwajima I, Mitani K, Miyao M, Suzuki Y, Kuramoto K, Ozawa T. Cardiac implications of the morning surge in blood pressure in elderly hypertensive patients: relation to arising time. Am J Hypertens 1995;8:29‐31. [DOI] [PubMed] [Google Scholar]
Muller 1989
- Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation 1989;79:733‐743. [DOI] [PubMed] [Google Scholar]
Musini 2009
- Musini VM, Wright JM. Factors Affecting Blood Pressure Variability: Lessons Learned from Two Systematic Reviews of Randomized Controlled Trials. PLoS ONE 22 May 2009;4(5):e5673. [DOI: 10.1371/journal.pone.0005673] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brien 1991
- O'Brien E, Cox J, O'Malley K. The role of twenty‐four‐hour ambulatory blood pressure measurement in clinical practice. J Hypertens 1991;9(Suppl 8):S63‐S65. [PubMed] [Google Scholar]
O'Brien 2003
- O'Brien E, Asmar R, Beilin L, et al. Society of Hypertension Working Group on Blood Pressure Monitoring: European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens 2003;21:821‐848. [DOI] [PubMed] [Google Scholar]
Ohmori 2005
- Ohmori M, Fujimura A. ACE inhibitors and Chronotherapy. Clinical and experimental hypertension 2005;27(2‐3):179‐185. [PubMed] [Google Scholar]
Pickering 1993
- Pickering TG, James GD. Determinants and consequences of the diurnal rhythm of blood pressure. Am J Hypertens 1993;6(6 Pt 2):166s‐169s. [DOI] [PubMed] [Google Scholar]
Smith 2003
- Smith DH, Neutel JM, Lacourciere Y, Kempthorne‐Rawson J. Prospective, randomized, open‐label, blinded‐endpoint (PROBE) designed trials yield the same results as double‐blind, placebo‐controlled trials with respect to ABPM measurements. Journal of hypertension 2003;21(7):1291‐98. [DOI] [PubMed] [Google Scholar]
Smolensky 1996
- Smolensky MH. Chronobiology and chronotherapeutics. Applications to cardiovascular medicine. American Journal of Hypertension 1996;9(4 Pt 3):11S‐21S. [DOI] [PubMed] [Google Scholar]
Smolensky 1999
- Smolensky MH, Portaluppi F. Chronopharmacology and chronotherapy of cardiovascular medications: relevance to prevention and treatment of coronary heart disease. American Heart Journal 1999;137(4 Pt 2):S14‐S24. [DOI] [PubMed] [Google Scholar]
Smolensky 2005
- Smolensky MH, Hermida RC, Portaluppi F, Haus E, Reinberg A. Chronotherapeutics in the treatment of hypertension . In: Oparil S, Weber MA editor(s). Hypertension: A Companion to Brenner and Rector's the Kidney. Second Edition. PA, USA: Elsevier Saunders, 2005:530‐540. [Google Scholar]
Stergiou 2007
- Stergioyu GS, Nasothimiou EG. Does dosing antihypertensive drugs at night alter renal or cardiovascular outcome: do we have the evidence?. Current Opinion Nephrology and Hypertenssion 2008;17:464‐469. [DOI] [PubMed] [Google Scholar]
Sugimoto 2001
- Sugimoto K, Ohmori M. Kawaguchi A, Tsuruoka S. Fujimura A. Dosing time‐dependent effect of trandolapril on the prevention of cardiac hypertrophy in rats with aortic banding. Jpn J Pharmacol 2001;87:86‐89. [DOI] [PubMed] [Google Scholar]
Sunderg 1988
- Sunderg S, Kohvakka A, Gordin A. Rapid reversal of circadian blood pressure rhythm in shift workers. J hypertens 1988;6:393‐396. [PubMed] [Google Scholar]
Waeber 1999
- Waeber B, Burnier M, Brunner HR. Compliance with antihypertensive therapy. Clin Exp Hypertens 1999;21:973‐985. [DOI] [PubMed] [Google Scholar]
White 1989
- White WB, Morganroth J. Usefulness of ambulatory monitoring of the blood pressure in assessing antihypertensive therapy. Am J Cardiol 1989;63:93‐98. [DOI] [PubMed] [Google Scholar]
White 1997a
- White WB. Circadian variation of blood pressure: Clinical relevance and implications for cardiovascular chronotherapeutics. Blood Press Monit 1997;2(1):47‐51. [PubMed] [Google Scholar]
White 1999
- White WB, Johnson M. Heart rate and the rate‐pressure product in patients with hypertension: The usefulness of chronotherapy. Euro Heart J Suppl 1999;1(B):B18‐B23. [Google Scholar]
White 2001
- White WB. Cardiovascular risk and therapeutic intervention for the early morning surge in blood pressure and heart rate. Blood Press Monit 2001;6:63‐72. [DOI] [PubMed] [Google Scholar]


