Abstract
Background
This is one of series of reviews of cervical ripening and labour induction using standardised methodology. Homoeopathy involves the use, in dilution, of substances which cause symptoms in their undiluted form. A type of herb, 'caulophyllum' is one type of homoeopathic therapy that has been used to induce labour.
Objectives
To determine the effects of homoeopathy for third trimester cervical ripening or induction of labour.
Search methods
The Cochrane Pregnancy and Childbirth Group's Trials Register (1 December 2009), and bibliographies of relevant papers.
Selection criteria
Randomised controlled trials comparing homeopathy used for third trimester cervical ripening or labour induction with placebo/no treatment or other methods listed above it on a predefined list of labour induction methods.
Data collection and analysis
A generic strategy was developed to deal with the large volume and complexity of trial data relating to labour induction. This involved a two‐stage method of data extraction. The initial data extraction was done centrally.
Main results
Two trials, involving 133 women, were included in the review. The trials were placebo controlled and double blind, but the quality was not high. Insufficient information was available on the method of randomisation and the study lacked clinically meaningful outcomes. This trials demonstrated no differences in any primary or secondary outcome between the treatment and control group.
Authors' conclusions
There is insufficient evidence to recommend the use of homoeopathy as a method of induction. It is likely that the demand for complementary medicine will continue and women will continue to consult a homoeopath during their pregnancy. Although caulophyllum is a commonly used homoeopathic therapy to induce labour, the treatment strategy used in the one trial in which it was evaluated may not reflect routine homoeopathy practice. Rigorous evaluations of individualised homeopathic therapies for induction of labour are needed.
Plain language summary
Homoeopathy for induction of labour
There is not enough evidence to show the effect of homoeopathy for inducing labour. Sometimes it is necessary to induce labour (getting labour started artificially) when a pregnant woman or her unborn child are at risk. Homoeopathy involves the use of diluted substances which in their undiluted form, cause certain symptoms. The principle is that a homoeopathic substance will stimulate the body and healing functions so that a state of balance is gained and symptoms are relieved. The review of two trials, involving 133 women, found there was not enough evidence to show the effect of a homoeopathy as a method of induction. More research is needed.
Background
Sometimes it is necessary to bring on labour artificially because of safety concerns for the mother or baby. This review is one of a series of reviews of methods of labour induction using a standardised protocol. For more detailed information on the rationale for this methodological approach, please refer to the currently published 'generic' protocol (Hofmeyr 2009). The generic protocol describes how a number of standardised reviews will be combined to compare various methods of preparing the cervix of the uterus and inducing labour. Homoeopathy is used around the world and is most widely used in Europe and India. Homoeopathy is a form of pharmacological therapy based on the concept that a substance which gives rise to specific symptoms, when given in pharmacological doses to healthy individuals, can be used to treat patients presenting with the same symptoms. This is described as the Law of Similars. Homoeopathy seeks to strengthen the body's immune system, the principle of the treatment being that the homoeopathic substance will stimulate the body and healing functions so that a state of balance is attained and the symptoms are relieved. Homoeopathic remedies are all natural medicines, with some remedies derived from herbs, minerals or other natural substances.
Homeopathic remedies are applied as potencies as a result of tiny and highly diluted amounts of the substances from which they are derived. They are prepared by a process of step by step repeated dilution and vigorous shaking, which is thought to make them capable of stimulating the body's own defence system. The resulting potency is labelled on the basis of the ratio of dilutent and diluted agent (D = decimal dilution = 1/10 diluted agent/dilutent; C = centesimal dilution = 1/100 diluted agent/dilutent) and the number of dilution steps (e.g. C5 indicates 5 dilution steps 1/100). The repetitive dilutions are thought to produce results more quickly, act on symptoms more effectively and are less likely to lead to side effects than the original substances.
The resulting homoeopathic medicine may contain very few or no single molecules of the original solute. For this reason many scientists have suggested the clinical effects resulting from homoeopathic remedies are due to the placebo effect (Vandenbrouke 1997). However, data from two meta‐analyses of placebo controlled clinical trials have found a greater therapeutic effect from homoeopathy compared with the placebo (Boissel 1996; Linde 1997). The precise biophysical mechanism underlying homoeopathy remains undefined.
There are different traditions in the prescribing of homoeopathic formulations. Classical homoeopathy refers to the practitioner prescribing a single therapy to treat the individual's illness based on the patient's general constitution. This involves consideration of the individual's current illness, medical history, personality and behaviour. Other practitioners prescribe a combination of homoeopathic therapies, 'complex homoeopathy', on the basis of a conventional diagnosis. Clinical homoeopathy uses the same remedy in patients presenting with a homogenous pathology or constellation of symptoms. There is no evidence that describes the benefits of one approach compared with another approach. Homoeopathy is practiced on different levels. Many homoeopathic therapies are available over the counter in pharmacies and health food shops. However, homoeopaths require several years of study to achieve their qualification. The trained homoeopath will treat an individual based on a detailed case history and the homoeopathic treatment will be tailored to the individual's constitution.
In recent years the use of alternative and complementary medicine has become popular in many Western countries (MacLennan 2002). Unconventional therapies are more common among women of reproductive age, with almost half of all women (49%) reporting that they have used them (Eisenberg 1998). It is possible that a significant proportion of women are using these therapies during pregnancy. The use of homoeopathy has been applied at the time of conception, pregnancy and labour to treat some of the discomforts and imbalances that can arise during pregnancy such as backache, constipation, morning sickness and heartburn. A recent survey described the prevalence and use of complementary therapies among 82 nurse‐midwives in North Carolina (Allaire 2000). Over 30% of nurse‐midwives reported recommending homoeopathy for use in pregnancy. Homoeopathy was recommended for use to ripen the cervix, induce labour and to augment labour.
For some women with a prolonged pregnancy, an induction of labour may be perceived to intervene in the natural process of pregnancy and may drastically change their expected plan of care during pregnancy. The reasons why pregnant women are interested in using complementary therapies to ripen the cervix or induce labour, or both, is an important question and needs to be answered when evaluating new options of care. Serious adverse effects from homoeopathy are rare, and remedies recommended for use in pregnancy are not thought to cause any problems in pregnancy.
Caulophyllum thalictroides is proposed to be extremely useful with establishing labour, or when uterine contractions are short and irregular or when uterine contractions stop (Priestman 1988). Some homoeopaths suggest taking one tablet daily for the last few days before labour starts, or alternatively to dissolve a tablet in a glass of water and sip from the glass from time to time, or whenever a contraction is imminent. A non‐randomised clinical trial was carried out to examine the efficacy of caulophyllum before birth for the treatment of uterine inertia and reducing the risk of postpartum haemorrhage (Ventoskovskiy 1990). The authors concluded this remedy has a role in preventing poor contraction patterns.
Objectives
To determine, from the best available evidence, the effectiveness and safety of homoeopathy for third trimester cervical ripening and induction of labour.
Methods
Criteria for considering studies for this review
Types of studies
Clinical trials comparing homoeopathy for cervical ripening or labour induction, with placebo/no treatment or other methods listed on a predefined list of methods of labour induction (seeMethods); the trials included random allocation to either group; and they reported one or more of the prestated outcomes.
Types of participants
Pregnant women due for third trimester induction of labour, carrying a viable fetus.
Types of interventions
Homoeopathy compared with placebo/no treatment or any other method on a predefined list of methods of labour induction.
Types of outcome measures
Clinically relevant outcomes for trials of methods of cervical ripening/labour induction have been prespecified by two authors of labour induction reviews (Justus Hofmeyr and Zarko Alfirevic).
Primary outcomes
Five primary outcomes were chosen as being most representative of the clinically important measures of effectiveness and complications: (1) vaginal delivery not achieved within 24 hours; (2) uterine hyperstimulation with fetal heart rate (FHR) changes; (3) caesarean section; (4) serious neonatal morbidity or perinatal death (e.g. seizures, birth asphyxia defined by trialists, neonatal encephalopathy, disability in childhood); (5) serious maternal morbidity or death (e.g. uterine rupture, admission to intensive care unit, septicemia).
Perinatal and maternal morbidity and mortality are composite outcomes. This is not an ideal solution because some components are clearly less severe than others. It is possible for one intervention to cause more deaths but less severe morbidity. However, in the context of labour induction at term this is unlikely. All these events will be rare, and a modest change in their incidence will be easier to detect if composite outcomes are presented. Where possible, the incidence of individual components were explored as secondary outcomes (see below).
Secondary outcomes
Secondary outcomes relate to measures of effectiveness, complications and satisfaction.
Measures of effectiveness
(6) Cervix unfavourable/unchanged after 12 to 24 hours; (7) oxytocin augmentation.
Complications
(8) Uterine hyperstimulation without FHR changes; (9) uterine rupture; (10) epidural analgesia; (11) instrumental vaginal delivery; (12) meconium‐stained liquor; (13) Apgar score less than seven at five minutes; (14) neonatal intensive care unit admission; (15) neonatal encephalopathy; (16) perinatal death; (17) disability in childhood; (18) maternal side effects (all); (19) maternal nausea; (20) maternal vomiting; (21) maternal diarrhoea; (22) other maternal side‐effects; (23) postpartum haemorrhage (as defined by the trial authors); (24) serious maternal complications (e.g. intensive care unit admission, septicaemia but excluding uterine rupture); (25) maternal death.
Measures of satisfaction
(26) Woman not satisfied; (27) caregiver not satisfied. While all the above outcomes were sought, only those with data appear in the analysis tables.
The terminology of uterine hyperstimulation is problematic (Curtis 1987). In the reviews we use the term 'uterine hyperstimulation without FHR changes' to include uterine tachysystole (more than five contractions per 10 minutes for at least 20 minutes) and uterine hypersystole/hypertonus (a contraction lasting at least two minutes) and 'uterine hyperstimulation with FHR changes' to denote uterine hyperstimulation syndrome (tachysystole or hypersystole with fetal heart rate changes such as persistent decelerations, tachycardia or decreased short‐term variability).
Outcomes were included in the analysis if reasonable measures were taken to minimise observer bias; and data were available for analysis according to original allocation.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (1 December 2009).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists of identified papers.
We did not apply any language restrictions.
Data collection and analysis
A strategy was developed to deal with the large volume and complexity of trial data relating to labour induction. Many methods have been studied, in many different categories of women undergoing labour induction. Most trials are intervention‐driven, comparing two or more methods in various categories of women. Clinicians and parents need the data arranged by category of woman, to be able to choose which method is best for a particular clinical scenario. To extract these data from several hundred trial reports in a single step would be very difficult. We, therefore, developed a two‐stage method of data extraction. The initial data extraction is done in a series of reviews arranged by methods of induction of labour, following a standardised methodology.
To avoid duplication of data in the primary reviews, the labour induction methods have been listed in a specific order, from one to 25. Each review includes comparisons between one of the methods (from two to 25) with only those methods above it on the list. Thus, the review of intravenous oxytocin (4) includes only comparisons with intra cervical prostaglandins (3), vaginal prostaglandins (2) or placebo (1). Methods identified in the future will be added to the end of the list. The current list is as follows:
placebo/no treatment;
vaginal prostaglandins (Kelly 2003);
intracervical prostaglandins (Boulvain 2008);
intravenous oxytocin (Kelly 2001c);
amniotomy (Bricker 2000);
intravenous oxytocin with amniotomy (Howarth 2001);
vaginal misoprostol (Hofmeyr 2003);
oral misoprostol (Alfirevic 2006);
mechanical methods including extra‐amniotic Foley catheter (Boulvain 2001);
membrane sweeping (Boulvain 2005);
extra‐amniotic prostaglandins (Hutton 2001);
intravenous prostaglandins (Luckas 2000);
oral prostaglandins (French 2001);
mifepristone (Neilson 2000);
oestrogens with/without amniotomy (Thomas 2001);
corticosteroids (Kavanagh 2006a);
relaxin (Kelly 2001a);
hyaluronidase (Kavanagh 2006b);
castor oil, bath, and/or enema (Kelly 2001b);
acupuncture (Smith 2004);
breast stimulation (Kavanagh 2005);
sexual intercourse (Kavanagh 2001);
homoeopathic methods;
nitric oxide (Kelly 2008);
buccal or sublingual misoprostol (Muzonzini 2004)
hypnosis
other methods for induction of labour.
The reviews are analysed by the following subgroups:
previous caesarean section or not;
nulliparity or multiparity;
membranes intact or ruptured;
cervix favourable, unfavourable or undefined.
The trials included in the reviews were extracted from an initial set of trials covering all interventions used in induction of labour (see above for details of search strategy). The data extraction process was conducted centrally. This was co‐ordinated from the Clinical Effectiveness Support Unit (CESU) at the Royal College of Obstetricians and Gynaecologists, UK, in co‐operation with the Pregnancy and Childbirth Group of The Cochrane Collaboration. This process allowed the data extraction process to be standardised across all the reviews.
The trials were initially reviewed on eligibility criteria, using a standardised form and the basic selection criteria specified above. Following this, data were extracted to a standardised data extraction form which was piloted for consistency and completeness. The pilot process involved the researchers at the CESU and previous review authors in the area of induction of labour.
Information was extracted regarding the methodological quality of trials on a number of levels. This process was completed without consideration of trial results. Assessment of selection bias examined the process involved in the generation of the random sequence and the method of allocation concealment separately. These were then judged as adequate or inadequate using the criteria described in Appendix 1 for the purpose of the reviews.
Performance bias was examined with regards to whom was blinded in the trials, i.e. patient, caregiver, outcome assessor or analyst. In many trials the caregiver, assessor and analyst were the same party. Details of the feasibility and appropriateness of blinding at all levels were sought.
Predefined subgroup analyses are: previous caesarean section or not; nulliparity or multiparity; membranes intact or ruptured, and cervix unfavourable, favourable or undefined. Only those outcomes with data will appear in the analysis tables.
Individual outcome data were included in the analysis if they met the pre stated criteria in Types of outcome measures. Included trial data were processed as described in the Cochrane Reviewers' Handbook (Clarke 2002). Data extracted from the trials were analysed on an intention‐to‐treat basis (when this was not done in the original report, re‐analysis is performed if possible). Where data were missing, clarification was sought from the original authors. If the attrition was such that it might significantly affect the results, these data are excluded from the analysis. This decision rests with the review authors of primary reviews and is clearly documented. Once missing data become available, they will be included in the analyses.
Data were extracted from all eligible trials to examine how issues of quality influence effect size in a sensitivity analysis. In trials where reporting was poor, methodological issues were reported as unclear or clarification sought.
Due to the large number of trials, double data extraction was not feasible and agreement between the three data extractors was therefore assessed on a random sample of trials.
Once the data had been extracted, they were distributed to individual review authors for entry onto the Review Manager computer software (RevMan 2003), checked for accuracy, and analysed as above using the RevMan software. For dichotomous data, risk ratios and 95% confidence intervals were calculated, and in the absence of heterogeneity, results were pooled using a fixed‐effect model.
The predefined criteria for sensitivity analysis included all aspects of quality assessment as mentioned above, including aspects of selection, performance and attrition bias. Letters are used to indicate the quality of the included trials as described by Clarke 2002. The sensitivity analysis explores the influence of high‐quality trials (defined as "A"), versus moderate quality trials (defined as "B"), and high‐quality trials (defined as "A") versus low‐quality trials (defined as "C"), as well as the effects of analysing by intention to treat on the effect size.
Primary analysis was limited to the prespecified outcomes and subgroup analyses. In the event of differences in unspecified outcomes or sub‐groups being found, these were analysed post hoc, but clearly identified as such to avoid drawing unjustified conclusions.
Results
Description of studies
Four studies were identified. Two were included and two were excluded. The search strategy identified two randomised controlled trials for inclusion in this review (Beer 1999; Dorfman 1987).
The study by Beer et al (Beer 1999) compared caulophyllum in a placebo double blind controlled trial and was undertaken in Germany. The authors of this trial examined the efficacy and tolerability of the homoeopathic remedy caulophyllum D4 in 40 women at term with prelabour rupture of membranes (PROM) at term and not in labour. The trial examined the effect of caulophyllum on the time interval from entry to the onset of regular uterine contractions. Other outcomes examined the effect on the duration of labour, oxytocin requirements, mode of delivery and the rate of maternal and neonatal infection. Women were administered caulophyllum or a placebo hourly for seven hours. Each active tablet consisted of 250 mg caulophyllum trituration D4, a mixture of magnesium stearate and a wheat starch mixture. The placebo contained no active ingredients but contained the magnesium stearate and a wheat starch mixture.
The trial presented information on the baseline characteristics between the two randomised groups. No differences in age, weight, height, cervical score at trial entry and time since PROM were found between study groups.
The study by Dorfman et al (Dorfman 1987) was undertaken in France. The trial compared five homoeopathic therapies with placebo in 93 women from 36 weeks' pregnant; 53 women were randomised to the treatment group and 40 to the placebo group. The trial examined the effect of the homoeopathic therapy on length of labour and the proportion of women experiencing a difficult labour. No details were provided on the placebo. The groups were comparable with respect to parity.
Excluded studies
The use of caulophyllum for the preparation of labour (Arnal‐Laserre 1986) and for use in false labour and dystocia (Coudert‐Deguillaume 1981) have been examined in two research theses. Extensive efforts have been made to contact the authors and we do not have enough information to make a decision whether the trial meets the inclusion criteria.
Risk of bias in included studies
The method of randomisation in both trials was not described and was therefore unclear. Both trials were reported as double blind although there was no information as to whether caregivers and the outcome assessors were blind to the women's group allocation. There were no withdrawals from the trials. The authors of the trials did not state they performed an intention‐to‐treat analysis, although the studies did analyse outcome data from the same number of women who were reported as having been randomised. The sample size was small in both trials and there was no description of the sample size calculation or if a calculation was undertaken. The information on any side effects arising from caulophyllum was unclear and it was unclear as to how women assessed the tolerability of caulophyllum. No data were provided on side effects from the Dorfman 1987 trial.
Effects of interventions
Two trials involving 133 women were included in the review.
Caulophyllum versus placebo
Forty women with a singleton pregnancy and prelabour rupture of membranes were randomised to caulophyllum or placebo (Beer 1999).
Primary outcomes
Vaginal delivery not achieved within 24 hours was reported for one woman in the control group (1/20) and no women in the treatment group (risk ratio (RR) 0.33, 95% confidence interval (CI) 0.01 to 7.72). Data on uterine hyperstimulation were not recorded. Two women in the group given caulophyllum had caesarean sections compared with no women in the placebo group (RR 5.0, 95% CI 0.26 to 98.00). No data were presented on fetal heart rate changes although the author describes that slight but not significant differences were noted. No data were reported on serious maternal or neonatal morbidity such as; meconium‐stained liquor; Apgar score less than seven at five minutes; neonatal intensive care unit admission; postpartum haemorrhage; or serious maternal complications (e.g. intensive care unit admission, septicaemia).
Secondary outcomes
No data were presented on cervical change, however the author reported minor differences between groups. Oxytocin augmentation was administered to nine women (45%) in each group, no differences were found (RR 1.0, 95% CI 0.50 to 1.98). There was no difference in the rate of instrumental delivery between the two groups (RR 1.0 95%CI 0.54 to 1.86). No differences were found in Apgar scores between groups. Women's and midwives' views on this method were sought and all described the method as tolerable.
Additional data
The difference in the interval between administration of the intervention and regular uterine contractions was 13 hours in the treatment group and 13.4 hours in the control group (mean difference ‐0.40, 95% CI ‐7.21 to 6.41).
In the Dorfman 1987 trial only two outcomes were reported. The mean length of labour for women receiving the homoeopathic therapy was 5.1 hours compared with 8.48 hours in the placebo group (P less than 0.001). Data could not be entered into the meta‐analysis due to the absence of data on standard deviation. A difficult labour was reported for six women (11.3%) in the treatment group and 16 (40%) in the placebo group (RR 0.28, 95% CI 0.12 to 0.66). Mode of delivery was not reported by study group.
Discussion
This review included two trials. There were no differences seen in any of the primary outcome measures described in this review. Unfortunately, the quality of the trials was difficult to assess because of insufficient detail in the research papers, and the small sample sizes provide inadequate power.
There is little research to assess the effectiveness of remedies in stimulating the onset of labour. The lack of data in this area is compounded by a lack of relevant clinical outcome data which could be included into this review. The use of caulophyllum may not represent common homoeopathic practice, where the prescribing of a therapy would be more individualised.
Authors' conclusions
Implications for practice.
There is insufficient evidence to recommend the use of any homoeopathic therapies as a method of induction of labour.
Implications for research.
Given that some women are likely to continue to seek homoeopathic therapies for induction of labour, there is a need for rigorous, adequately powered trials that assess clinically meaningful outcomes. Such trials should include assessment of uterine hyperstimulation as well as indicators of maternal and neonatal morbidity. Further research using classical homoeopathy might be more relevant to assessing both the effectiveness and safety of homoeopathy.
What's new
| Date | Event | Description |
|---|---|---|
| 12 January 2010 | New search has been performed | Search updated. No new trials identified. Two trials, previously identified, have been translated and are now excluded. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 4, 2001
| Date | Event | Description |
|---|---|---|
| 3 September 2008 | Amended | Converted to new review format. |
| 29 July 2003 | New citation required and conclusions have changed | Substantive amendment. |
| 13 May 2003 | New search has been performed | This update includes one new trial. Two further trials have been identified and will be included in a future update when they have been translated. The Implications for research section has also been updated. |
Acknowledgements
The author would like to thank Bettina Hinger for the German to English translation (Beer 1999) and Alison Ledward for the French to English translation (Dorfman 1987).
Appendices
Appendix 1. Methodological quality of trials
| Methodological item | Adequate | Inadequate |
| Generation of random sequence | Computer‐generated sequence, random number tables, lot drawing, coin tossing, shuffling cards, throwing dice. | Case number, date of birth, date of admission, alternation. |
| Concealment of allocation | Central randomisation, coded drug boxes, sequentially sealed opaque envelopes. | Open allocation sequence, any procedure based on inadequate generation. |
Data and analyses
Comparison 1. Homoeopathy versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Caesarean section | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 98.00] |
1.3. Analysis.

Comparison 1 Homoeopathy versus placebo, Outcome 3 Caesarean section.
Comparison 2. Homoeopathy versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
2.1. Analysis.

Comparison 2 Homoeopathy versus placebo, Outcome 1 Vaginal delivery not achieved within 24 hours.
Comparison 3. Homoeopathy versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Augmentation with oxytocin | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.50, 1.98] |
3.1. Analysis.
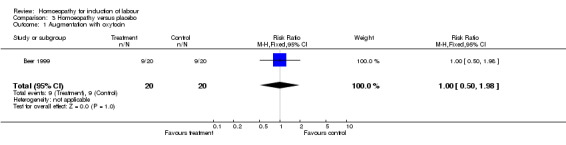
Comparison 3 Homoeopathy versus placebo, Outcome 1 Augmentation with oxytocin.
Comparison 4. Homoeopathy versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Instrumental delivery | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.54, 1.86] |
4.1. Analysis.

Comparison 4 Homoeopathy versus placebo, Outcome 1 Instrumental delivery.
Comparison 5. Homoeopathy versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Length of labour | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐7.21, 6.41] |
5.2. Analysis.

Comparison 5 Homoeopathy versus placebo, Outcome 2 Length of labour.
Comparison 6. Homoeopathy versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Difficult labour | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.12, 0.66] |
6.1. Analysis.
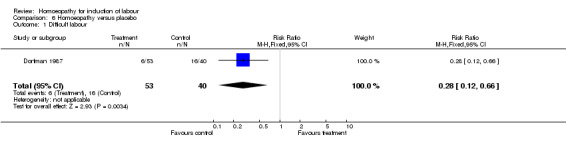
Comparison 6 Homoeopathy versus placebo, Outcome 1 Difficult labour.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beer 1999.
| Methods | Double‐blind placebo controlled trial. The method of allocation concealment was unclear. | |
| Participants | 40 women 38‐42 weeks' gestation with PROM. The study was undertaken in Germany. | |
| Interventions | Caulophyllum D4 or a placebo tablet. Doses were repeated hourly for 7 hours or until labour started. | |
| Outcomes | Time to the onset of regular uterine contractions, labour and delivery outcomes. Maternal and neonatal infection. | |
| Notes | No sample size calculation. No losses to follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear. |
Dorfman 1987.
| Methods | Double‐blind placebo controlled trial. The method of concealment was not described. | |
| Participants | 93 women were recruited to the study at 36 weeks' gestation. The study was undertaken in France. Women were excluded from the study if they had a history of a poor obstetric history, a current history of hypertension, diabetes, previous caesarean section or cephalo‐pelvic disproportion. | |
| Interventions | The treatment group received 5 homoeopathic therapies: caulophyllum, arnica, actea racemosa, pulsatilla and gerenium, with three granules administered morning and evening from 36 weeks' gestation. When labour commenced, the same dosage was given every 15 minutes and stopped after 2 hours or sooner if the woman was comfortable. Not details were provided on the placebo or the precise dosage. | |
| Outcomes | Average length of labour and difficult labour. | |
| Notes | No sample size calculation. No losses to follow up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear. |
PROM: prelabour rupture of membranes
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arnal‐Laserre 1986 | We have been unable to contact the authors, and do not have enough information to make a decision whether the trial meets the inclusion criteria. |
| Coudert‐Deguillaume 1981 | We have been unable to contact the authors, and do not have enough information to make a decision whether the trial meets the inclusion criteria. |
Contributions of authors
The review author prepared the review, selected studies for inclusion, extracted the data and prepared the text of the review.
Sources of support
Internal sources
University of Adelaide, Adelaide, Australia.
University of South Australia, Adelaide, Australia.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Beer 1999 {published data only}
- Beer AM, Heiliger F. Randomized, double blind trial of Caulophyllum D4 for induction of labour after premature rupture of membranes at term. Gerburtshilfe und Frauenheilkunde 1999;59:431‐5. [Google Scholar]
Dorfman 1987 {published data only}
- Dorfman P, Lasserre M, Tetau M. Homoeopathic preparation for labour: two fold experiment comparing a less widely known therapy with a placebo. Cahiers de Biotherapie 1987;94:77‐81. [Google Scholar]
References to studies excluded from this review
Arnal‐Laserre 1986 {published data only}
- Arnal‐Laserre MN. Preparation a l'accouchement par homeopathie: experimentation en double insu versus placebo (Dissertation). Paris: Academie de Paris, Universite Rene Descartes, 1986. [Google Scholar]
Coudert‐Deguillaume 1981 {published data only}
- Coudert‐Deguillaume M. Etude l'accouchement par homeopthie: experimentation en double insu versus placebo [Dissertation]. Limoges: Faculte de Medecine et de Pharmacie, Universite de Limoges, 1981. [Google Scholar]
Additional references
Alfirevic 2006
- Alfirevic Z, Weeks A. Oral misoprostol for induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD001338.pub2] [DOI] [PubMed] [Google Scholar]
Allaire 2000
- Allaire AD, Moos M, Wells SR. Complementary and alternative medicine in pregnancy: a survey of North Carolina nurse‐midwives. Obstetrics & Gynecology 2000;95(1):19‐23. [DOI] [PubMed] [Google Scholar]
Boissel 1996
- Boissel JP, Cucherat M, Haugh M, Gauthier E. Critical literature on the effectiveness of homoeopathy: overview of data from homoeopathic medicine trials.. Homoeopathic Medicine Research Group, editors. Report. Brussels: Commission of the European Communities 1996:195‐210.
Boulvain 2001
- Boulvain M, Kelly A, Lohse C, Stan C, Irion O. Mechanical methods for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD001233] [DOI] [PubMed] [Google Scholar]
Boulvain 2005
- Boulvain M, Stan C, Irion O. Membrane sweeping for induction of labour. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD000451.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulvain 2008
- Boulvain M, Kelly AJ, Irion O. Intracervical prostaglandins for induction of labour. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006971] [DOI] [PubMed] [Google Scholar]
Bricker 2000
- Bricker L, Luckas M. Amniotomy alone for induction of labour. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002862] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 2002
- Clarke M, Oxman AD, editors. Cochrane Reviewers’ Handbook 4.2.0 [updated March 2003]. In: The Cochrane Library, Issue 2, 2003. Oxford: Update Software. Updated quarterly.
Curtis 1987
- Curtis P, Evans S, Resnick J. Uterine hyperstimulation. The need for standard terminology. Journal of Reproductive Medicine 1987;32:91‐5. [PubMed] [Google Scholar]
Eisenberg 1998
- Eisenberg DA, Davis RB, Ettner SL, Appel S, Wilky S, Rompay M. Trends in alternative medicine use in the United States, 1990‐1997: results of a follow up national survey. JAMA 1998;280:1569‐75. [DOI] [PubMed] [Google Scholar]
French 2001
- French L. Oral prostaglandin E2 for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003098] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmeyr 2003
- Hofmeyr GJ, Gülmezoglu AM. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD000941] [DOI] [PubMed] [Google Scholar]
Hofmeyr 2009
- Hofmeyr GJ, Alfirevic Z, Kelly AJ, Kavanagh J, Thomas J, Neilson JP, Dowswell T. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002074.pub2] [DOI] [Google Scholar]
Howarth 2001
- Howarth GR, Botha DJ. Amniotomy plus intravenous oxytocin for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD003250] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutton 2001
- Hutton E, Mozurkewich E. Extra‐amniotic prostaglandin for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003092] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2001
- Kavanagh J, Kelly AJ, Thomas J. Sexual intercourse for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003093] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2005
- Kavanagh J, Kelly AJ, Thomas J. Breast stimulation for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003392.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2006a
- Kavanagh J, Kelly AJ, Thomas J. Corticosteroids for induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003100.pub2] [DOI] [PubMed] [Google Scholar]
Kavanagh 2006b
- Kavanagh J, Kelly AJ, Thomas J. Hyaluronidase for cervical priming and induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003097.pub2] [DOI] [PubMed] [Google Scholar]
Kelly 2001a
- Kelly AJ, Kavanagh J, Thomas J. Relaxin for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003103] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2001b
- Kelly AJ, Kavanagh J, Thomas J. Castor oil, bath and/or enema for cervical priming and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003099] [DOI] [PubMed] [Google Scholar]
Kelly 2001c
- Kelly AJ, Tan BP. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD003246] [DOI] [PubMed] [Google Scholar]
Kelly 2003
- Kelly AJ, Kavanagh J, Thomas J. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003101] [DOI] [PubMed] [Google Scholar]
Kelly 2008
- Kelly AJ, Kavanagh J. Nitric oxide donors for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006901] [DOI] [PubMed] [Google Scholar]
Linde 1997
- Linde K, Clausius N, Ramirez G, Melchart D, Eitel F, Hedges LV, et al. Are the clinical effects of homoeopathy placebo effects? A meta analysis of placebo controlled trials. Lancet 1997;350:834‐3. [DOI] [PubMed] [Google Scholar]
Luckas 2000
- Luckas M, Bricker L. Intravenous prostaglandin for induction of labour. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002864] [DOI] [PMC free article] [PubMed] [Google Scholar]
MacLennan 2002
- MacLennan AH, Wilson DH, Taylor AW. The escalating cost of alternative medicine. Preventive Medicine 2002;35:166‐73. [DOI] [PubMed] [Google Scholar]
Muzonzini 2004
- Muzonzini G, Hofmeyr GJ. Buccal or sublingual misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004221.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neilson 2000
- Neilson JP. Mifepristone for induction of labour. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002865] [DOI] [PubMed] [Google Scholar]
Priestman 1988
- Priestman KG. A few useful remedies in pregnancy, labour and the first few days of the babies' life. British Homeopathy Journal 1988;77:172‐3. [Google Scholar]
RevMan 2003 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Oxford, England: The Cochrane Collaboration, 2003.
Smith 2004
- Smith CA, Crowther CA. Acupuncture for induction of labour. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002962.pub2] [DOI] [PubMed] [Google Scholar]
Thomas 2001
- Thomas J, Kelly AJ, Kavanagh J. Oestrogens alone or with amniotomy for cervical ripening or induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD003393] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vandenbrouke 1997
- Vandenbroucke JP. Homoeopathy trials: going nowhere. Lancet 1997;350:824. [DOI] [PubMed] [Google Scholar]
Ventoskovskiy 1990
- Ventoskovskiy BM, Popov AV. Homeopathy as a practical alternative to traditional obstetric methods. British Homeopathy Journal 1990;79(4):201‐5. [Google Scholar]
References to other published versions of this review
Smith 2003
- Smith CA. Homoeopathy for induction of labour (Cochrane Review). Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003399] [DOI] [PMC free article] [PubMed] [Google Scholar]


