Since the first detailed pathological description by Brinton in 1950,1 the importance of pulmonary arterial hypertension (PAH) to excess mortality has been reproduced widely. Initial studies testing PAH pharmacotherapies aimed to salvage end-stage disease; however, progress in clinical phenotyping and widened availability of medical and surgical interventions over the ensuing decades have transformed PAH into a contemporary and treatable disease. Nevertheless, modern era epidemiological trends suggest that long-term survival has improved incrementally,2 driven in part by late detection of incident disease. In 2018 the mean pulmonary artery pressure (mPAP) diagnostic threshold was lowered from ≥25 to >20 mmHg, which provided the first evidence-based hemodynamic definition for pulmonary hypertension while offering an opportunity to capture PAH patients earlier and thereby mitigate longitudinal disease burden.3
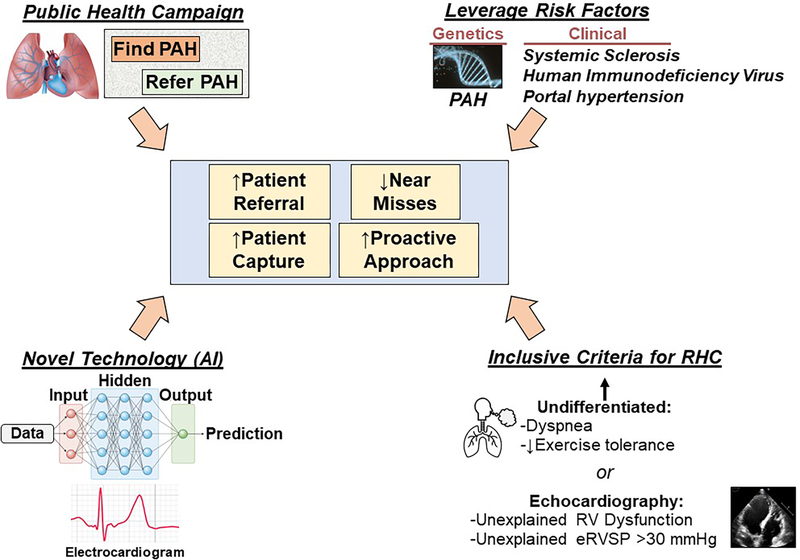
Shifting focus to earlier disease detection amplifies challenges known already to complicate PAH diagnosis: substantial pulmonary vascular remodeling occurs before gross hemodynamic abnormalities, non-specific symptoms are common and hence often misattributed to other processes, the clinical trajectory is indolent prior to advanced stage disease, and, unlike systemic hypertension, definitively profiling central cardiopulmonary hemodynamics non-invasively is not possible. Indeed, newly diagnosed PAH patients present with mPAP of ~45 mmHg,4 well above 20 mmHg that is the upper normal limit and level linked to adverse outcome in referral populations. Thus, finding early-stage PAH has emerged as a new chapter in the diagnostic dilemma of this highly morbid disease. Here, we propose that addressing this problem requires a strategic overhaul leveraging public health campaigns, technological advances that automate PAH discovery at point-of-care, exploitation of unique genetic risk factors, and expanded clinical indications for right heart catheterization (RHC) (Figure).
Figure. A next-generation strategy identifying early-stage pulmonary arterial hypertension (PAH).
We propose a strategic overhaul focusing on early PAH diagnosis by increasing awareness, utilizing genetic and clinical risk factors, integrating artificial intelligence (AI) technologies at point-of-care, and contemporizing right heart catheterization (RHC) indications. eRVSP, estimated right ventricular systolic pressure.
Delayed PAH diagnosis by more than 2 years following symptom onset is common. This is ascribed to a low index of clinical suspicion among non-specialists, driven by non-specific symptoms (i.e., dyspnea) and compounded by low disease prevalence. This problem is likely modifiable through simplified (and clearer) indications for patient referral to Expert Centers, improved community outreach by experts, and implementation of programs that promote PAH awareness. In addition to physical examination, biochemical, or other diagnostic data suggesting PAH, additional criteria warranting expert PAH consultation should include undifferentiated dyspnea or an incremental change in exercise capacity occurring without a clear reason. In this regard, much opportunity exists for medical branding, proven to raise awareness in other morbid disease (e.g., “Heart Truth”, cardiovascular disease in women), as a method by which to promote the evaluation of patients at risk for PAH by pulmonary circulatory disease specialists.
Approximately 600 million electrocardiograms (ECG) are performed annually worldwide, making ECG among the most widely used and standardized clinical tools in medicine. Indeed, ECG findings consistent with right ventricular (RV) strain, right atrial enlargement, and right axis deviation may suggest moderate or severe PAH. However, automated rule-based computational algorithms, which are capable of processing vast amounts of data, can optimize the selection of ECG features that predict a clinical phenotype. This strategy has already been explored with preliminary success in other forms of pulmonary hypertension.5 Artificial intelligence (AI) is well-positioned to detect subtle 12-lead ECG patterns that may identify mild PAH and owing to the frequency by which this test is used in general practices offers an important path forward for expanding the range of patients requiring further evaluation. Computational strategies that link ECG data with coded information in the electronic health record could, in turn, arbitrate the sensitivity and specificity of ECG criteria deduced from AI. Adding PAH to the computer-generated differential diagnosis guiding interpretation of the ECG may provide a key entry point into the diagnostic pipeline for clinicians.
Recently, Montani and colleagues leveraged the French Referral Center for Pulmonary Hypertension program, which is a nationalized system centralizing advanced clinical management of pulmonary circulatory diseases, to annually screen N=55 asymptomatic family members of patients with hereditary PAH that carried a BMPR2 variant.6 Two patients met diagnostic criteria for PAH at enrollment, 12 patients were found to have an abnormal cardiopulmonary hemodynamic response to exercise (consistent with provocable pulmonary vascular disease), and three patients progressed to develop PAH over the 6-year follow-up, overall corresponding to disease capture and incidence rates of 11% and 2.3%/yr, respectively. These data provide critical evidence in support of using genetic data to alter patient surveillance for the purpose of early detection and are in line with effective but underutilized strategies doing the same by focusing on clinical diseases that predispose PAH, particularly systemic sclerosis, human immunodeficiency virus, and portal hypertension, to enhance patient monitoring.
Two-dimensional echocardiography is often the first quantitative test characterizing PAH; in populations, subtle elevations in estimated PAP are associated with pathogenic structural changes to the RV and prognostic for mortality. However, determining the appropriate cardiopulmonary hemodynamic classification, treatment, and prognosis of individual patients requires RHC. Although RHC is safe in experienced hands, most are performed to stage heart failure or pursue a cause of moderate-severe cardiopulmonary symptoms. Undifferentiated dyspnea, asymptomatic or unexplained RV dysfunction, and elevated estimated PAP >30 mmHg on echocardiography are established independent risk factors for clinical morbidity and well-positioned RHC indications to capture mild PAH.
Increased early disease detection is expected to establish the optimal sensitivity and specificity for hemodynamic criteria defining PAH, clarify PAH prevalence, expand clinical trial options testing non-pharmacological and pharmacological interventions, and stimulate novel paths forward for primary and secondary prevention. Importantly, identifying symptomatic PAH patients earlier would allow prompt initiation of dual oral pulmonary vasodilator therapy, which is a standard approach to improve clinical outcomes. We recognize that a universal PAH screening program is not feasible logistically or economically in most instances; however, an organized (and proactive) approach to pursuing pulmonary vascular disease patients focusing on PAH is likely to also capture pulmonary hypertension due to lung disease, left heart disease, or in situ pulmonary vascular thrombotic remodeling. These diseases are more common than PAH but likewise require disease-specific considerations in management; thus, a salutary consequence of efforts to “find” PAH are likely to benefit patients with other forms of pulmonary hypertension.
Success in the next era of PAH will hinge on enhanced monitoring and greater therapeutic opportunities for patients prior to the development of end-stage disease. Accomplishing this end requires forward-thinking strategies that raise PAH awareness among non-specialists driven, in part, by integrating novel technological advances and revamped clinical indications for diagnostic testing focusing on early diagnosis.
Funding
B.A.M.: NIH 1R01HL139613-01, R01HL153502, R01HL155096-01, 2021A007243 BWH/MIT-Broad Institute; McKenzie Family Charitable Trust. M.H.: Assistance Publique Hôpitaux de Paris, Inserm, Université Paris-Saclay.
Footnotes
Conflicts of Interest
B.A.M., Deerfield Corporation (beyond the scope of this work); Actelion Sciences (beyond the scope of this work); Patent #9,605,047 (beyond the scope of this work), PCT/US2019/059890 (beyond the scope of this work), PCT/US2020/066886 (beyond the scope of this work). M.H.: Acceleron, Altavant, Bayer, Janssen, Merck and MorphogenIX (beyond the scope of this work).
References
- 1.Brinton WD. Primary pulmonary hypertension. Br Heart J 1950; 12:305–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010. Jul 13;122(2):156–63. [DOI] [PubMed] [Google Scholar]
- 3.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoeper MM, Pausch C, Grunig E, et al. Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J Heat Lung Transpl 2020;39:1435–1444. [DOI] [PubMed] [Google Scholar]
- 5.Kwon J-M, Kim K-H, Medina-Inojosa J, et al. Artificial intelligence for early prediction of pulmonary hypertension using electrocardiography. J Heart Lung Transpl 2020;39:805–814. [DOI] [PubMed] [Google Scholar]
- 6.Montani D, Girerd B, Jais X, et al. Screening for pulmonary arterial hypertension in adults carrying a BMPR2 mutation. Eur Respir J 2021;58:2004229. [DOI] [PMC free article] [PubMed] [Google Scholar]