Highlights
-
•
Buprenorphine retention improved with less restrictive policies during COVID-19.
-
•
Risk of discontinuing buprenorphine was lowest among patients using telemedicine.
-
•
Low-threshold access to buprenorphine should continue to be expanded.
Keywords: Opioid use disorder, Retention, Adherence, People who use drugs, Buprenorphine, COVID-19, Telemedicine
Abstract
Background
Medications such as buprenorphine are considered the gold standard for the treatment of opioid use disorders. This study aimed to determine whether less restrictive buprenorphine prescribing practices during the COVID-19 pandemic impacted retention in and adherence to buprenorphine among patients accessing treatment from 2018-2020 at a community-based syringe services program.
Methods
In this retrospective cohort study, we compared retention in treatment before and during the COVID-19 pandemic. Then, with relaxed restrictions acting as the intervention in a natural experiment, we conducted a sub-analysis of “continuity participants” who accessed treatment services both before and during the COVID-19 period. Records of 418 historical control patients treated with buprenorphine before COVID-19 were compared to 88 patients enrolled during COVID-19 (n=43 remote telemedicine and n=45 remote provider with patient on-site). Cox proportional hazards regressions were used to assess risk factors for treatment discontinuation. The sub-analysis used proportion of days covered (PDC) differences before and during COVID-19 (n=164) for a paired analysis in a nonparametric bootstrap test.
Results
The risk of discontinuation was 71% lower in those accessing remote telemedicine during COVID-19 (HR=0.29; CI: 0.18, 0.47) and 51% lower in those accessing their remote provider onsite during COVID-19 (HR=0.49; CI:0.31, 0.77), compared to the historical control group. The average PDC did not significantly differ before and during COVID-19 (difference=2.4%; CI:-0.6%, 5.3%).
Conclusions
The risk of discontinuing treatment was lower in both COVID-19 treatment groups compared to historical controls. Less restrictive buprenorphine prescribing guidelines during COVID-19 led to improved retention in care over 6-months.
1. Introduction
In the United States, approximately 1.6 million people are living with an opioid use disorder (OUD) (Substance Abuse and Mental Health Services Administration, 2020a). Currently, there are Food and Drug Administration (FDA) approved medications for opioid use disorder (MOUD), including buprenorphine and methadone. These medications are effective treatments for OUD, decreasing mortality by over 50% in the first year following initiation (Larochelle et al., 2018; Substance Abuse and Mental Health Services Administration, 2020b). Those with untreated OUD remain at an increased risk of overdose death (Larochelle et al., 2018; Santo et al., 2021), as well as exposure to HIV and Hepatitis C through injection drug use (Hodder et al., 2021; Holtzman et al., 2021; Powell et al., 2019). Despite significant increases in MOUD prescribing between 2009 and 2018, still only a small percentage of people with OUD initiate and continue these life-saving medications for at least 180 days (Olfson et al., 2020; Williams et al., 2019). Before the SARS-CoV-2 (COVID-19) pandemic, the United States strictly regulated MOUD with high-threshold approaches such as mandatory in-person clinic visits and urine drug screenings (UDS) that may have prevented patients from initiating or continuing treatment (Gryczynski et al., 2014; Khatri and Aronowitz, 2021; Stringer et al., 2021).
The COVID-19 pandemic has created many challenges for MOUD programs to provide safe, high-quality service, but it also provided an opportunity to study new modes of service delivery. As required by the Drug Enforcement Agency (DEA), standard of care prior to March 2020 (U.S. Department of Justice Drug Enforcement Administration, 2021) included face-to-face appointments with providers for induction and maintenance on buprenorphine. While routine urine drug screenings to assess for treatment adherence were not mandated prior to the COVID-19 pandemic, they were a common feature of clinical practice and recommended by the American Society for Addiction Medicine (ASAM) (American Society of Addiction Medicine, 2020). In March 2020, as part of COVID-19 safety measures, the DEA relaxed its regulations and ASAM recommended pausing routine urine screenings (American Society of Addiction Medicine, 2020; U.S. Department of Justice Drug Enforcement Administration, 2021). Additionally, COVID-19 saw the widespread implementation of telemedicine to conduct healthcare appointments, including in buprenorphine programs (Nordeck et al., 2021; Tofighi et al., 2021). Telemedicine has eliminated barriers and improved retention to MOUD in rural settings but less is known about its impact in more urban areas (Gilmore et al., 2017; Weintraub et al., 2018). In this retrospective cohort study, we examine the effect of less restrictive prescribing guidelines during the COVID-19 pandemic on retention of patients in a low-threshold MOUD program based within Prevention Point Philadelphia (PPP), a large multi-service harm reduction services organization. We compared treatment records of a historical control group (treated with buprenorphine pre-COVID-19) to a group of participants in the less restrictive service regulation era ushered in by COIVD-19. We hypothesized relaxed restrictions would improve rates of retention in treatment over the 6-month follow-up period. Then, with relaxed restrictions acting as the intervention in a natural experiment, we conducted a sub-analysis of “continuity participants” who accessed MOUD services before and during the COVID-19 period. We hypothesized within-patient adherence to MOUD would improve in the less restrictive post-COVID treatment era.
2. Methods
2.1. Program model
In 2008, Prevention Point Philadelphia, a large syringe services program and harm reduction organization in the Kensington neighborhood of Philadelphia, Pennsylvania initiated a buprenorphine maintenance treatment program called the Stabilization, Treatment, and Engagement Program (STEP). This program has been described in detail and evaluated elsewhere (Bachhuber et al., 2018). As of February 2020, before widespread COVID-19 travel and physical distancing restrictions in the United States, the STEP program operated 6 days a week on-site at PPP's main building, and 3 days a week from a mobile unit, with a staff of 8 providers and 8 case managers that served approximately 250 patients across all sites.
In accordance with COVID-19 safety measures, PPP switched from in-person to exclusively virtual appointments for new and existing STEP patients in March 2020. During this period, in-person attendance and urine drug screening requirements were removed and standard intervals between appointments were extended to 1 month. Patients with phones were able to schedule and make calls to providers. Patients without phones could return to the PPP building and access a virtual appointment with assistance from a case manager, while providers remained off-site.
2.2. Participants
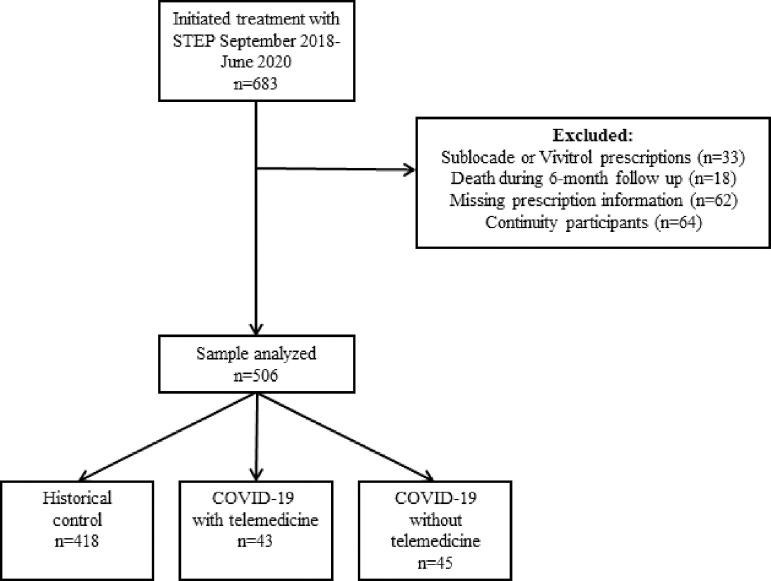
Patients of the STEP program who initiated treatment from September 2018 through June 2020 were considered for inclusion in the retrospective cohort (n=683) (Fig. 1). To be eligible for STEP, patients must have had a history of participation in PPP's syringe or social services program and been willing to participate in case management and attend all scheduled STEP appointments. Exclusion criteria for the analyses were prescriptions for Sublocade or Vivitrol (n = 33), as the logistics of treatment differ for these medications, and death during the 6-month follow-up period (n=18). An additional 56 participants were removed from the analysis due to missing initiation dates and prescription data in the Pennsylvania Prescription Drug Monitoring Program (PDMP) database (historical controls, n=48 and initiated during COVID-19 group, n=6) as well as unavailable treatment modality data (initiated during COVID-19 group, n=2).
Fig. 1.
Participant flow diagram for analyses of buprenorphine treatment retention.
For the primary analysis, participants (n=506) were divided into three treatment groups based on the date they enrolled in STEP as follows: Pre-COVID-19 treatment group (historical controls), new COVID-19 intakes who used telemedicine remotely, and new COVID-19 intakes who used telemedicine on-site. Historical controls were all participants who enrolled in STEP on or after September 1, 2018 whose six-month follow-up period ended before the implementation of program changes for COVID-19 on March 13, 2020. New COVID-19 participants included individuals with intakes on or after March 13, 2020.
For the “natural experiment” sub-analysis, we defined a group of participants in the STEP database as “continuity patients” (n=170). Six participants were removed from this sub-analysis due to missing prescription data in PDMP. Participants included: (1) those whose six months of treatment follow-up included telemedicine, but their intake date was before the implementation of program changes for COVID-19 (n=64) as well as (2) historical controls who continued treatment past >6 months and their treatment trajectory crossed into the COVID-19 era (n=100). In other words, continuity patients are defined as individuals with experience in PPP's buprenorphine treatment program pre- and post- the COVID-19 restrictions. We report adherence on these continuity participants. Of note, the six participants removed from the sub-analysis for missing data plus the group of 64 participants described above are not included in the primary analysis (n=506) due to their unique treatment trajectory.
2.3. Measures
Covariates were extracted from PPP electronic medical records. These included participant sociodemographic characteristics (e.g., age, gender, race/ethnicity, insurance status, and housing status). Housing status at enrollment was categorized into permanent housing, unstable housing (i.e., emergency shelter, transitional housing, with family/friends), and street homelessness. Self-reported substance use characteristics included history of injection drug use, polysubstance use (cocaine, benzodiazepine, amphetamine, and/or methamphetamine use at intake), and prior MOUD treatment experience. We also collected enrollment date in the STEP program, and service modality for each visit during the follow-up period (in-person visit with an in-person clinician, in-person visit with telehealth clinician, and remote visit with telehealth clinician). After reviewing the distribution of visits by service modality, we created two treatment groups: (1) “COVID-19 telemedicine” participants who had remote visits with a telehealth clinician (both parties off-site) and (2) “COVID-19 without telemedicine” participants who had visits on-site, but the provider was remote.
Outcome data for all participants were extracted from the Pennsylvania Prescription Drug Monitoring Program and included the date each prescription was written, the date each prescription was filled, the number of prescriptions filled during the 6-month follow-up period, and the number of doses in each fill. The primary outcome for this analysis was retention, defined as the time between treatment initiation and treatment discontinuation, up to and including six months. Based on previous literature, treatment discontinuation was defined as having no active prescription or appointment attendance for a period of two months (Bachhuber et al., 2018). We used the dates extracted from PDMP to identify discontinuation dates, with the “date each prescription was written” used as a proxy for attendance dates. We report the proportion of participants retained at 180 days by treatment group.
Medication adherence was measured using a modified proportion of days covered (PDC) calculation (Benner et al., 2002). The denominator for PDC was the length of retention (1-180 days). The numerator, total number of days for which medication is available, was calculated using a “coverage matrix” of time between prescription fill dates and the days of medication supplied in each fill. For example, a participant that has 14 pills prescribed over 14 days and is retained in care for only a total of 14 days will have a PDC of 1. This is an individual-level measure that reports medication availability during the time a patient was retained in care. Because stockpiling to avoid unnecessary in-person appointments was common during the pandemic, excess medications were carried over into the next fill period, when applicable.
2.4. Analysis
Baseline characteristics are reported across the three treatment groups and across the two retention groups (retained in treatment or discontinued treatment, as defined above). Descriptive analyses were conducted using the Kruskal-Wallis rank sum test for continuous variables and the Pearson's chi-square test or Fisher's exact test for categorical variables. Kaplan-Meier curves for time to discontinuation were created with participants stratified into the three treatment groups.
The primary analysis includes the 506 pre- and post-COVID-19 treatment participants. Cox proportional hazards regressions were used to estimate the time to discontinuation and assess risk factors for discontinuation. Bivariable and multivariable models for retention were created. For all models, the proportional hazards assumption was evaluated by calculating correlations between time and Schoenfeld residuals for each predictor, and these were assessed using a chi-squared test (Grambsch and Therneau, 1994). For the analysis of medication adherence, both bivariable and multivariable linear regressions were used to compare PDC (Andrade et al., 2006) between historical controls and new intakes during COVID-19. The multivariable regression model was constructed to examine the relative contribution of the significant explanatory variables to predicting adherence. For both analyses, variables that were associated with the outcome at p<0.20 in the bivariable models were included in multivariable models.
For the sub-analysis of continuity participants, PDC was calculated for both the pre-COVID-19 phase and the COVID-19 phase of treatment. Differences between PDC in each treatment period were calculated and used for paired analysis in a nonparametric bootstrap test. PDC differences were resampled with replacement 1000 times and a 95% confidence interval of the bootstrapped means was calculated to determine the significance of results.
All analyses were conducted using SAS software, Version 9.4 (SAS Institute Inc., Cary, NC, USA). This research was approved by the Drexel University Institutional Review Board.
3. Results
A total of 506 STEP patients were categorized into three treatment groups: historical control (n=418), COVID-19 intakes with telemedicine (n=43), and COVID-19 intakes without telemedicine (n=45) (Table 1). The majority of participants were male (72.9%), and non-Hispanic White (53.5%) or Hispanic (24.4%) with an average age of 37.9 years. Most participants were either unstably housed or homeless (81.2%). Injection drug use (70.1%), polysubstance use (64.2%), and previous experience with MOUD (74.3%) were common. Participants in the three different treatment periods significantly differed in terms of race/ethnicity (p<.01), insurance status (p=0.02), housing status (p=0.05), and polysubstance use (p<.01). There were no significant differences between the two COVID-19 treatment groups when analyzed separately (results not shown).
Table 1.
Sociodemographic and substance use characteristics by treatment group.
| Total(n=506) | Historical control(n=418) | COVID-19 treatment with telemedicine(n=43) | COVID-19 treatment without telemedicine (n=45) | p-value | |
|---|---|---|---|---|---|
| Age (years) | 37.9 (31.4-44.8) | 37.7 (31.1-44.4) | 38.8 (32.4-44.9) | 38.6 (32.4-49.3) | 0.50 |
| Sex | 0.37 | ||||
| Male | 369 (72.9%) | 300 (71.8%) | 35 (81.4%) | 34 (75.6%) | |
| Female | 137 (27.1%) | 118 (28.2%) | 8 (18.6%) | 11 (24.4%) | |
| Race/Ethnicity1 | <.01 | ||||
| White | 268 (53.5%) | 230 (55.6%) | 18 (41.9%) | 20 (45.5%) | |
| Black | 103 (20.6%) | 79 (19.1%) | 11 (25.6%) | 13 (29.6%) | |
| Hispanic/ Latinx |
122 (24.4%) | 97 (23.4%) | 14 (32.6%) | 11 (25.0%) | |
| Other2 | 8 (1.6%) | 8 (1.9%) | 0 (0.0%) | 0 (0.0%) | |
| Housing status3 | 0.05 | ||||
| Permanent | 91 (18.7%) | 66 (16.5%) | 15 (34.9%) | 10 (23.3%) | |
| Unstable | 233 (47.9%) | 198 (49.5%) | 16 (37.2%) | 19 (44.2%) | |
| Street homeless | 162 (33.3%) | 136 (34.0%) | 12 (27.9%) | 14 (32.6%) | |
| Insurance status | 0.02 | ||||
| Insured | 414 (81.8%) | 333 (79.7%) | 39 (90.7%) | 42 (93.3%) | |
| Uninsured | 92 (18.2%) | 85 (20.3%) | 4 (9.3%) | 3 (6.7%) | |
| Injection drug use history4 | 0.26 | ||||
| No | 141 (29.9%) | 123 (31.1%) | 11 (29.0%) | 7 (18.4%) | |
| Yes | 330 (70.1%) | 272 (68.9%) | 27 (71.1%) | 31 (81.6%) | |
| Polysubstance use5 | <0.01 | ||||
| No | 175 (35.8%) | 130 (31.9%) | 24 (58.5%) | 21 (52.5%) | |
| Yes | 314 (64.2%) | 278 (68.1%) | 17 (41.5%) | 19 (47.5%) | |
| Previous MOUD experience6 | 0.43 | ||||
| No | 128 (25.8%) | 109 (26.7%) | 11 (25.6%) | 8 (17.8%) | |
| Yes | 369 (74.3%) | 300 (73.4%) | 32 (74.4%) | 37 (82.2%) |
Note: Values represent median with interquartile range for continuous variables and frequency with percentage for categorical variables.
n=501 (missing n=4 in Historical control; n=1 in COVID-19 treatment without telemedicine group)
Race/ethnicity “other” includes mixed race and Asian
n=486 (missing n=18 in Historical control; n=2 in COVID-19 treatment without telemedicine)
n=471 (missing n=23 in Historical control; n=5 in COVID-19 treatment with telemedicine; n=7 in COVID-19 treatment without telemedicine)
Polysubstance use indicated cocaine, benzodiazepine, amphetamine, and/or methamphetamine use at intake; n=489 (missing n=10 in Historical control; n=2 in COVID-19 treatment with telemedicine; n=5 in COVID-19 treatment without telemedicine)
Medication for opioid use disorder (MOUD)=Ever prescribed methadone or buprenorphine from other community-based programs, during incarceration, and from emergency department visits; n=497 (missing n=9 in Historical control)
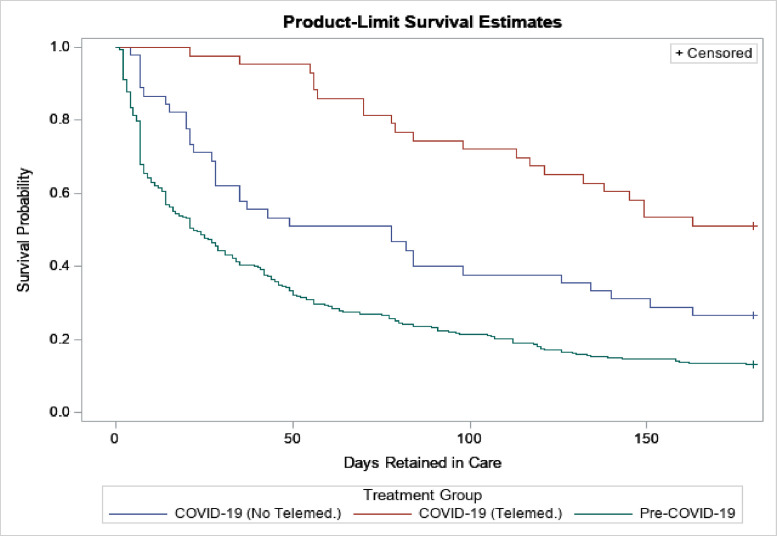
Length of retention significantly differed between the treatment groups over the course of the follow-up period (p<.01) (Fig. 2). The historical control group had an average retention period of 22.5 days, considerably lower compared to the COVID-19 group with telemedicine (180.0 days) and without telemedicine (78.0 days). Retention increased during the COVID-19 pandemic, and it was highest among individuals who had remote telemedicine appointments during this time (Fig. 2). Further, the average number of days of medication supplied for the patients during COVID-19 with telemedicine (14.5 days) was double that of historical controls (7.0 days). However, adherence during treatment, measured as the proportion of days covered, did not differ significantly between the groups (p=0.39) (Supplemental Table 1).
Fig. 2.
Retention in treatment with buprenorphine by treatment era and treatment group.
Bivariable Cox regression showed that the risk of discontinuing treatment was significantly different between treatment groups (Table 2). After controlling for age, race/ethnicity, housing status, insurance status, injection drug use, polysubstance use, and previous MOUD experience, multivariable Cox regression showed that the risk of discontinuing treatment was 71% lower in those accessing telemedicine compared to the historical control group (HR=0.29; CI: 0.18, 0.47). The risk of discontinuing treatment was also 51% lower in those not accessing telemedicine (HR=0.49; CI:0.31, 0.77) compared to the historical control group. Street homelessness (HR=1.54, CI:1.09, 2.20) and injection drug use (HR=1.29, CI:0.99, 1.67) were also associated with a 54% and 29% higher risk of treatment discontinuation in the multivariable model, respectively.
Table 2.
Factors associated with discontinuation of buprenorphine treatment at 6 months.
| Retained in treatment (n=89) | Discontinued treatment (n=417) | Unadjusted hazard ratio (95% confidence interval) | Adjusted hazard ratio (95% confidence interval) | |
|---|---|---|---|---|
| Treatment group | ||||
| Historical control |
55 (13.2%) | 363 (86.8%) | Ref. | Ref. |
| COVID-19 treatment with telemedicine |
22 (51.2%) | 21 (48.8%) | 0.27 (0.18, 0.43)⁎⁎⁎ | 0.29 (0.18, 0.47)⁎⁎⁎ |
| COVID-19 treatment w/o telemedicine |
12 (26.7%) | 33 (73.3%) | 0.58 (0.41, 0.83)⁎⁎⁎ | 0.49 (0.31, 0.77)⁎⁎⁎ |
| Age (years) | 40.6 (34.4-48.9) | 37.0 (30.1-44.2) | 0.99 (0.98, 1.00)⁎⁎⁎ | 0.99 (0.98,1.00)⁎⁎ |
| Sex | ||||
| Male | 67 (18.2%) | 302 (81.8%) | Ref. | – |
| Female | 22 (16.1%%) | 115 (83.9%) | 0.89 (0.72, 1.10) | – |
| Race/Ethnicity | ||||
| White | 45 (16.8) | 223 (83.2%) | Ref. | Ref. |
| Black | 25 (24.3%) | 78 (75.7%) | 0.88 (0.69, 1.15) | 1.12 (0.83, 1.52) |
| Hispanic/ Latinx |
19 (15.6%) | 103 (84.4%) | 1.00 (0.80, 1.17) | 1.13 (0.87, 1.46) |
| Other1 | 0 (0%) | 8 (100%) | 1.86 (0.92, 3.76)⁎⁎ | 1.27 (0.56, 2.88) |
| Housing status | ||||
| Permanent | 29 (31.9%) | 62 (68.1%) | Ref. | Ref. |
| Unstable | 24 (14.8%) | 138 (85.2%) | 1.44 (1.08, 1.92)⁎⁎⁎ | 1.30 (0.93, 1.82)* |
| Street homeless | 36 (15.5%) | 197 (84.6%) | 1.67 (1.24, 2.26)⁎⁎⁎ | 1.55 (1.09, 2.20)⁎⁎⁎ |
| Insurance status | ||||
| Insured | 77 (18.6%) | 337 (81.4%) | Ref. | Ref. |
| Uninsured | 12 (13.0%) | 80 (87.0%) | 1.41 (1.10, 1.80)⁎⁎⁎ | 1.08 (0.81, 1.43) |
| Injection drug use history | ||||
| No | 35 (24.8%) | 106 (75.2%) | Ref. | Ref. |
| Yes | 49 (14.9%) | 281 (85.2%) | 1.24 (0.99, 1.55)⁎⁎ | 1.29 (0.99, 1.67)⁎⁎ |
| Polysubstance use2 | ||||
| No | 38 (21.7%) | 137 (78.3%) | Ref. | Ref. |
| Yes | 50 (15.9%) | 264 (84.1%) | 1.15 (0.94,1.42)* | 0.97 (0.77, 1.24) |
| Previous MOUD experience3 | ||||
| No | 22 (17.2%) | 106 (82.8%) | Ref. | Ref. |
| Yes | 66 (17.9%) | 303 (82.1%) | 0.84 (0.67, 1.05)⁎⁎ | 0.87 (0.68, 1.12) |
Note: Values represent median with interquartile range for continuous variables and frequency with percentage for categorical variables.
Race/ethnicity “other” includes mixed race and Asian
Polysubstance use indicated combinations cocaine, benzodiazepine, amphetamine, and/or methamphetamine use at intake
Medication for opioid use disorder (MOUD)=Ever prescribed methadone or buprenorphine from other community-based programs, during incarceration, and from emergency department visits
p<0.2
p<0.1
p<0.05
In unadjusted linear regression models, COVID-19 intakes with and without telemedicine had a higher proportion of days covered (PDC) compared to historical controls (Beta [B]=0.02, CI:-0.04, 0.07; B=0.04, CI:-0.01, 0.08, respectively). However, this relationship did not reach statistical significance (Supplemental Table 2). Additionally, the PDC adherence measure decreased significantly for each increase in the number of substances reported (B=-0.04, CI: -0.07, -0.01). Lastly, compared to non-Hispanic White participants, those in the “Other” race/ethnicity category had a lower PDC (B=-0.13, CI:-0.24, -0.02).
When treatment group, sex, race/ethnicity, housing status, and polysubstance use were controlled in the multivariable regression model, the significant relationships between adherence and “other” race/ethnicity (B=-0.13, CI:-0.24, -0.01) and polysubstance use (B=-0.04, CI:-0.06, -0.01) remained (Supplemental Table 2). Due to the small sample size in the “Other” category of race/ethnicity, these results should be interpreted with caution. Though non-significant, COVID-19 intakes with and without telemedicine continued to have a higher proportion of days covered (PDC) compared to historical controls (B=0.03, CI:-0.04, 0.07; B=0.03, CI:-0.02, 0.08, respectively).
Finally, the sub-analysis of the continuity participants (n=164) examined within-patient adherence differences among those participants who had prescriptions pre- and post-COVID-19 restrictions. On average, these patients were enrolled in care for 7.5 months before the pandemic (Interquartile Range [IQR]:2-12). Like the primary analysis sample, most participants were male (74.4%), and non-Hispanic White (51.2%) or Hispanic (26.2%) with an average age of 39.4 years. Proportionally less participants were unstably housed or homeless (72.6%), reported injection drug use (54.9%), polysubstance use (61.6%), and had a previous experience with MOUD (60.1%) compared to the primary analysis sample. The median pre-COVID PDC was 98.1% (IQR:86.3%-100.0%) while the average PDC during the COIVD-19 period was 96.4% (IQR:88.7%-100.0%). The difference in average PDC did not significantly differ over time (difference = 2.4% (CI: -0.6%, 5.3%); skewness = 0.70 (CI: -0.3, 1.6)).
4. Discussion
While COVID-19 has introduced several challenges throughout the healthcare system (Chalasani et al., 2021), it has also provided an opportunity to dramatically reimagine service delivery to promote the safety of staff and patients. Standard buprenorphine medication management before COVID-19 typically included mandatory in-person inductions and weekly clinic-based urine drug toxicology screenings. However, the relaxed DEA regulations meant that many outpatient clinics could integrate efficient telemedicine appointments into the standard of care (Bokolo Anthony, 2020) along with longer prescriptions, and the removal of urine drug screening protocols (U.S. Department of Justice Drug Enforcement Administration, 2021).
In our analysis of 506 patients with an OUD from a community-based syringe services program in Philadelphia, implementation of the less restrictive buprenorphine treatment policies was associated with improved retention in care over a 6-month follow-up period, compared to a historical control group that underwent treatment before COVID-19. While all patients enrolled during the less restrictive period had higher retention than those in the control group, retention was highest among individuals who ever accessed telemedicine appointments remotely. This finding is consistent with recent data from other low-threshold drug treatment programs that were implemented during COVID-19 (Harris et al., 2020; Tofighi et al., 2021; Wang et al., 2021).
One program in Baltimore that shifted to telemedicine during COVID-19 found that remote treatment was effective in retaining vulnerable patients in care for a second visit. Although new COVID-19 patients in the Baltimore study did not differ significantly on retention at 30 days compared to a cohort of patients who enrolled before COVID-19, the percentage of patients engaged with follow-up visits remained consistent (62% pre-COVID-19 v. 64% during COVID-19) (Nordeck et al., 2021). Overall, reports suggest telemedicine eliminates many traditional barriers to treatment, at the patient, provider, and system level (Krawczyk et al., 2019; Nordeck et al., 2021; Weinstein et al., 2020).
Despite the removal of some barriers to treatment, individuals in our study who were experiencing street homelessness had a 54% higher risk of treatment discontinuation compared to stably housed persons. People experiencing homelessness face increased barriers to telemedicine treatment, including less consistent access to mobile phones and digital technologies (Humphry, 2019; Zhai, 2021). To improve treatment outcomes for people experiencing homelessness, more research is needed to mitigate disparities in telemedicine access (Nouri et al., 2020; Ortega et al., 2020). For example, treatment programs could expand a pilot program that used mobile technology in combination with case management to increase access to buprenorphine for veterans experiencing homelessness. This program was found to be feasible with moderately high treatment retention, at an average of 19 months (Iheanacho et al., 2020).
Of note is the finding that adherence suffered among people reporting injection drug use and polysubstance use. This indicates that while the COVID-19 buprenorphine policy changes aided in retention in care over six months for many, the removal of face-to-face contact with providers impacted people who inject drugs and polysubstance users differently. More research is needed to understand how low-threshold services such as SSPs providing MOUD can incorporate additional adherence supports for PWID and polysubstance using patients into their standard of care. Services that screen for substance use disorders broadly may be helpful if they do not disqualify patients from initiating or remaining in care (Cunningham et al., 2013; Payne et al., 2019).
In addition to our retrospective cohort study, we were able to capitalize on the changing COVID-19 restrictions, which acted as a natural experiment that allowed us to examine within-patient MOUD adherence differences. Although there is limited evidence that adherence improved in patients during the pandemic, this analysis suggests adherence did not worsen. In our analysis of continuity patients, the median proportion of days covered were similarly high before and during the COVID-19 era (98% and 96%, respectively). This was consistent with findings from the primary analysis that overall, rates of adherence were high among patients in this retrospective study. Future research could consider more precise measures of adherence through systems like directly observed therapy or wearable biosensors (Chai et al., 2015).
Further, it was not unexpected that the continuity group, who remained engaged in care before and after COVID-19 restrictions, differed from the primary analysis sample in terms of housing stability, injection drug use, polysubstance use, and previous experiences with MOUD. These four factors were also associated with treatment discontinuation in the primary sample, further indicating the importance of screening for and addressing these factors to improve healthcare engagement.
Our study is not without limitations. Participants were enrolled retrospectively without randomization into “control” and “intervention” groups. For this reason, there were demographic differences between the historical control and COVID-19 treatment groups. Although we controlled for demographics in our primary analyses, it is possible the unmeasured differences in the groups may have biased our findings, due to the study design. However, COVID-19 provided the ideal landscape for studying low-threshold buprenorphine treatment without having to unethically restrict patient access to low-threshold services. Additionally, both treatment groups experienced the STEP program, which operates in connection with a syringe services program and is based out of a single site in a single city. Therefore, these results may be difficult to generalize to other populations of marginalized patients in other locations or more traditional clinical settings. Our data are also limited in that we did not capture alcohol use and therefore were unable to include findings about alcohol-involved polysubstance use and retention in care. We were however able to include the most common substances found in opioid-involved deaths (benzodiazepines (Liu et al., 2021) and cocaine (Friedman et al., 2022; Hoots et al., 2020)) in our definition of polysubstance use. Lastly, our measure of adherence, proportion of days covered, is subject to bias, as it is calculated based on reported prescription fill dates, which is not as accurate as direct monitoring of patient adherence. However, direct monitoring typically demands more financial and personnel resources.
5. Conclusions
Policies that improve access and retention to MOUD must remain a priority in a post-COVID-19 pandemic world, especially as opioid-related overdose rates continue to soar in the United States (Friedman and Akre, 2021). For increased impact among the most vulnerable patients, such as those experiencing homelessness, access to digital technologies must be equitably distributed. Overall, making low-threshold access to buprenorphine the standard of care and permanently lifting pre-pandemic regulations could improve retention in care among populations traditionally marginalized from healthcare systems.
Statement of authors' contributions
KMW led manuscript writing, data analysis, and assisted with data interpretation.
AS assisted with conception and design of the study, data interpretation, and provided critical revision of the manuscript.
JW led data collection and preliminary data analysis, and assisted with manuscript writing.
BC assisted with conception and design of the study and data interpretation, and provided critical revision of the manuscript.
KS assisted with data cleaning, analysis, and manuscript writing.
AMR led conception and design of the study, oversaw analysis and manuscript writing, and provided final approval of the version to be published.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was funded by the Fordham University HIV and Drug Abuse Prevention Research Ethics Training Institute/National Institute on Drug Abuse (R25DA031608-01; Director, Celia B. Fisher). The authors thank Prevention Point clients and staff for their support and Kaelee Shepherd for her assistance during data collection and manuscript drafting.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.dadr.2022.100055.
Appendix. Supplementary materials
References
- American Society of Addiction Medicine, 2020. ASAM COVID-19 task force recommendations: caring for patients during the COVID-19 pandemic adjusting drug testing protocols.
- Andrade S.E., Kahler K.H., Frech F., Chan K.A. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol. Drug Saf. 2006;15(8):565–574. doi: 10.1002/pds.1230. [DOI] [PubMed] [Google Scholar]
- Bachhuber M.A., Thompson C., Prybylowski A., Benitez J., Mazzella S., Barclay D. Description and outcomes of a buprenorphine maintenance treatment program integrated within Prevention Point Philadelphia, an urban syringe exchange program. Subst. Abus. 2018;39:167–172. doi: 10.1080/08897077.2018.1443541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner J.S., Glynn R.J., Mogun H., Neumann P.J., Weinstein M.C., Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288(4):455–461. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- Bokolo Anthony J. Use of telemedicine and virtual care for remote treatment in response to COVID-19 pandemic. J. Med. Syst. 2020;44(7):132. doi: 10.1007/s10916-020-01596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai P.R., Castillo-Mancilla J., Buffkin E., Darling C., Rosen R.K., Horvath K.J., Boudreaux E.D., Robbins G.K., Hibberd P.L., Boyer E.W. Utilizing an ingestible biosensor to assess real-time medication adherence. J. Med. Toxicol. 2015;11(4):439–444. doi: 10.1007/s13181-015-0494-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani R., Shinabery J.M., Goetz C.T., Chang C.-C.H., Yang Q., Suda K.J., Gellad W.F. Buprenorphine dispensing in Pennsylvania during the COVID-19 pandemic, January to October 2020. J. Gen. Intern. Med. 2021;36(12):3915–3917. doi: 10.1007/s11606-021-07083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C.O., Giovanniello A., Kunins H.V., Roose R.J., Fox A.D., Sohler N.L. Buprenorphine treatment outcomes among opioid-dependent cocaine users and non-users. Am. J. Addict. 2013;22(4):352–357. doi: 10.1111/j.1521-0391.2013.12032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J., Akre S. COVID-19 and the drug overdose crisis: uncovering the deadliest months in the United States, January‒July 2020. Am. J. Public Health. 2021;111(7):1284–1291. doi: 10.2105/AJPH.2021.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J., Montero F., Bourgois P., Wahbi R., Dye D., Goodman-Meza D., Shover C. Xylazine spreads across the US: a growing component of the increasingly synthetic and polysubstance overdose crisis. Drug Alcohol Depend. 2022;233 doi: 10.1016/j.drugalcdep.2022.109380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore A.K., Wilson S.M., Skopp N.A., Osenbach J.E., Reger G. A systematic review of technology-based interventions for co-occurring substance use and trauma symptoms. J. Telemed. Telecare. 2017;23(8):701–709. doi: 10.1177/1357633X16664205. [DOI] [PubMed] [Google Scholar]
- Grambsch P.M., Therneau T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- Gryczynski J., Mitchell S.G., Jaffe J.H., O’Grady K.E., Olsen Y.K., Schwartz R.P. Leaving buprenorphine treatment: patients’ reasons for cessation of care. J. Subst. Abuse Treat. 2014;46(3):356–361. doi: 10.1016/j.jsat.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M., Johnson S., Mackin S., Saitz R., Walley A.Y., Taylor J.L. Low barrier tele-buprenorphine in the time of COVID-19: a case report. J. Addict. Med. 2020;14(4):e136–e138. doi: 10.1097/ADM.0000000000000682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodder S.L., Feinberg J., Strathdee S.A., Shoptaw S., Altice F.L., Ortenzio L., Beyrer C. The opioid crisis and HIV in the USA: deadly synergies. Lancet. 2021;397(10279):1139–1150. doi: 10.1016/S0140-6736(21)00391-3. [DOI] [PubMed] [Google Scholar]
- Holtzman D., Asher A.K., Schillie S. The changing epidemiology of hepatitis C virus infection in the United States during the years 2010 to 2018. Am. J. Public Health. 2021;111(5):949–955. doi: 10.2105/AJPH.2020.306149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoots B., Vivolo-Kantor A., Seth P. The rise in non-fatal and fatal overdoses involving stimulants with and without opioids in the United States. Addiction. 2020;115(5):946–958. doi: 10.1111/add.14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphry J. Digital First’: homelessness and data use in an online service environment. Commun. Res. Pract. 2019;5(2):172–187. [Google Scholar]
- Iheanacho T., Payne K., Tsai J. Mobile, community-based buprenorphine treatment for veterans experiencing homelessness with opioid use disorder: a pilot, feasibility study. Am. J. Addict. 2020;29(6):485–491. doi: 10.1111/ajad.13055. [DOI] [PubMed] [Google Scholar]
- Khatri U.G., Aronowitz S.V. Considering the harms of our habits: the reflexive urine drug screen in opioid use disorder treatment. J. Subst. Abuse Treat. 2021;123 doi: 10.1016/j.jsat.2020.108258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk N., Buresh M., Gordon M.S., Blue T.R., Fingerhood M.I., Agus D. Expanding low-threshold buprenorphine to justice-involved individuals through mobile treatment: addressing a critical care gap. J. Subst. Abuse Treat. 2019;103:1–8. doi: 10.1016/j.jsat.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle M.R., Bernson D., Land T., Stopka T.J., Wang N., Xuan Z., Bagley S.M., Liebschutz J.M., Walley A.Y. Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: a cohort study. Ann. Intern. Med. 2018;169(3):137–145. doi: 10.7326/M17-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., O’Donnell J., Gladden R.M., McGlone L., Chowdhury F. Trends in nonfatal and fatal overdoses involving benzodiazepines - 38 States and the District of Columbia, 2019-2020. MMWR Morb. Mortal Wkly. Rep. 2021;70(34):1136–1141. doi: 10.15585/mmwr.mm7034a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeck C.D., Buresh M., Krawczyk N., Fingerhood M., Agus D. Adapting a low-threshold buprenorphine program for vulnerable populations during the COVID-19 pandemic. J. Addict. Med. 2021;15(5):364–369. doi: 10.1097/ADM.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouri S., Khoong E.C., Lyles C.R., Karliner L. Addressing equity in telemedicine for chronic disease management during the Covid-19 pandemic. NEJM Catal. Innov. Care Deliv. 2020;1(3) [Google Scholar]
- Olfson M., Zhang V., Schoenbaum M., King M. Trends in buprenorphine treatment in the United States, 2009-2018. JAMA. 2020;323(3):276–277. doi: 10.1001/jama.2019.18913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega G., Rodriguez J.A., Maurer L.R., Witt E.E., Perez N., Reich A., Bates D.W. Telemedicine, COVID-19, and disparities: policy implications. Health Policy Technol. 2020;9(3):368–371. doi: 10.1016/j.hlpt.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne B.E., Klein J.W., Simon C.B., James J.R., Jackson S.L., Merrill J.O., Zhuang R., Tsui J.I. Effect of lowering initiation thresholds in a primary care-based buprenorphine treatment program. Drug Alcohol Depend. 2019;200:71–77. doi: 10.1016/j.drugalcdep.2019.03.009. [DOI] [PubMed] [Google Scholar]
- Powell D., Alpert A., Pacula R.L. A transitioning epidemic: how the opioid crisis is driving the rise in hepatitis C. Health Aff. 2019;38(2):287–294. doi: 10.1377/hlthaff.2018.05232. [DOI] [PubMed] [Google Scholar]
- Santo T., Jr, Clark B., Hickman M., Grebely J., Campbell G., Sordo L., Chen A., Tran L.T., Bharat C., Padmanathan P., Cousins G., Dupouy J., Kelty E., Muga R., Nosyk B., Min J., Pavarin R., Farrell M., Degenhardt L. Association of opioid agonist treatment with all-cause mortality and specific causes of death among people with opioid dependence: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(9):979–993. doi: 10.1001/jamapsychiatry.2021.0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer K.L., Langdon K.J., McKenzie M., Brockmann B., Marotta P. Leveraging COVID-19 to sustain regulatory flexibility in the treatment of opioid use disorder. J. Subst. Abuse Treat. 2021;123 doi: 10.1016/j.jsat.2020.108263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2019 National Survey on Drug Use and Health; Rockville, MD: 2020. Key Substance Use and Mental Health Indicators in the United States. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2020b. Medications for opioid use disorder, Treatment Improvement Protocol (TIP) Series 63. Rockville, MD.
- Tofighi B., McNeely J., Walzer D., Fansiwala K., Demner A., Chaudhury C.S., Subudhi I., Schatz D., Reed T., Krawczyk N. A telemedicine buprenorphine clinic to serve New York city: initial evaluation of the NYC public hospital system's initiative to expand treatment access during the COVID-19 pandemic. J. Addict. Med. 2021;16(1):e40. doi: 10.1097/ADM.0000000000000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Justice Drug Enforcement Administration, 2021. Diversion control division COVID-19 information page | telemedicine DEA policy. https://www.deadiversion.usdoj.gov/coronavirus.html. (Accessed July 14 2021).
- Wang L., Weiss J., Ryan E.B., Waldman J., Rubin S., Griffin J.L. Telemedicine increases access to buprenorphine initiation during the COVID-19 pandemic. J. Subst. Abuse Treat. 2021;124 doi: 10.1016/j.jsat.2020.108272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein L.C., Iqbal Q., Cunningham A., Debates R., Landistratis G., Doggett P., Silverio A. Retention of patients with multiple vulnerabilities in a federally qualified health center buprenorphine program: Pennsylvania, 2017–2018. Am. J. Public Health. 2020;110(4):580–586. doi: 10.2105/AJPH.2019.305525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub E., Greenblatt A.D., Chang J., Himelhoch S., Welsh C. Expanding access to buprenorphine treatment in rural areas with the use of telemedicine. Am. J. Addict. 2018;27(8):612–617. doi: 10.1111/ajad.12805. [DOI] [PubMed] [Google Scholar]
- Williams A.R., Nunes E.V., Bisaga A., Levin F.R., Olfson M. Development of a cascade of care for responding to the opioid epidemic. Am. J. Drug Alcohol Abuse. 2019;45(1):1–10. doi: 10.1080/00952990.2018.1546862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Y. A call for addressing barriers to telemedicine: health disparities during the COVID-19 pandemic. Psychother. Psychosom. 2021;90(1):64–66. doi: 10.1159/000509000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.