Abstract
Background
Retinal abnormalities are being increasingly reported in COVID-19, in addition to the well-known symptoms of this disease accounting for the neurological involvement. In this study, we aimed to investigate whether ganglion cell layer thickness (GCLT) was different in recovered COVID-19 patients compared to controls in the subacute stage and to determine whether it correlated with COVID-19-related neurological symptoms or pneumonia.
Methods
This study involved 40 patients who had recovered from COVID-19 and 40 age- and sex-matched healthy controls. All the participants underwent ophthalmological examination, spectral domain optical coherence tomography and neurological examination. The clinical and biochemical properties of the patients were noted and their correlations with GCLT were sought.
Results
The duration after COVID-19 infection was 113 ± 62 (mean ± SD) days. At this subacute stage, there was no significant difference between the GCLT measurements of the COVID-19 patients and the controls (14 ± 4.0 µm [median ± IQR] vs 16 ± 4.8 µm, respectively). When we analyzed the relationships with neurological symptoms in the patient group, we found that patients with cognitive symptoms had lower GCLT values compared to those without (13 ± 3 µm vs. 16 ± 4 µm, respectively; p = 0.002). Patients who suffered headache during the acute infection also had lower GCLT values compared to those without (14 ± 4 µm vs. 18 ± 5 µm, respectively; p = 0.015). The GCLT values did not differ significantly with respect to anosmia, ageusia, sleep disturbances, having had COVID-19 pneumonia, or smoking status. Age, duration after COVID-19, and blood levels of thyroid stimulating hormone, glucose, vitamin D and vitamin B12 were not in correlation with GCLT in our study.
Conclusion
Our findings highlight an association between GCLT values and neurological symptoms such as cognitive disturbance (brain fog) and headache in patients who had recovered after non-severe COVID-19 infection. Neuroretinal involvement by SARS-CoV2 might be linked to central neurological symptoms. The patients with lower GCLT values may benefit from close monitoring for neurological problems.
Keywords: COVID-19, Optical coherence tomography, Ganglion cell layer, Brain fog, Headache
1. Introduction
SARS-CoV-2 enters host cells via angiotensin-converting enzyme 2 (ACE2) and TMPRSS2, a cell surface-associated protease that promotes viral uptake [1]. ACE2 expression is high in the epithelium of nasal mucosa and also alveolar pneumocytes [2], [3]. Recently, ocular cells have been demonstrated to express ACE2 and TMPRSS2 [4], [5]. SARS-CoV-2 itself was detected in the conjunctival secretions of some patients, suggesting possible transmission of the disease through ocular secretions in the absence of conjunctivitis [6]. In a recent paper, researchers were able to show the expression of ACE2 and TMPRSS2 in human retina via immunohistochemistry and immunoprecipitation [7]. Expression was identified in multiple non-vascular neuroretinal cells, including the ganglion cell layer, endothelial cells and pericytes. Araujo-Silva et al. investigated the eyes of three severe COVID-19 patients and observed presumed viral particles morphologically within endothelial cells and also the inner and outer nuclear layers by electron microscopy. They also stained for viral proteins, and showed their intracellular presence via immunofluorescence in these enucleated eyes. The patients were severe COVID-19 cases who needed mechanical ventilation and had a fatal course [8].
These findings are important from a neurological perspective, since retina can be described as an outgrowth of the brain, and the fact that transsynaptic spread is proposed to be a dissemination pathway for SARS-CoV-2 [9]. Studies showing retinal abnormalities in COVID-19 patients are being increasingly reported in the literature [10], [11], [12], [13]. Some of these abnormalities are of vascular or microvascular origin and others may be related to coagulopathy or systemic inflammation; however, direct demonstration of SARS-CoV-2 receptors on many types of ocular cells (surface and neuronal cells) and identification of viral proteins in neuroretinal cells indicate the possibility that the retina may be a gateway for viral entry to the brain, and therefore, retinal involvement might serve as a marker for central neurological involvement. This is particularly important, because ACE2 is expressed not only by vascular cells in the brain but also by glia and neurons in a widespread manner [14], [15], [16], [17]. With respect to the various neurological manifestations of COVID-19, this route of viral propagation warrants further investigation.
Optical coherence tomography (OCT) is a simple and non-invasive imaging modality that enables the assessment of retinal changes. Ganglion cells are the final output neurons of retina, hence ganglion cell layer thickness may be used as a single measure of neuroretinal involvement. In this study, we aimed (i) to investigate whether ganglion cell layer thickness (GCLT; measured by OCT) was different in recovered COVID-19 patients compared to controls in the subacute stage (≥4 weeks after recovery of acute infection) and, (ii) to determine whether GCLT correlated with COVID-19-related neurological symptoms (headache, cognitive symptoms and other cranial nerve involvements–like anosmia and ageusia) or pneumonia.
2. Methods
2.1. Patients
This study involved 40 patients who had recovered from COVID-19 and 40 healthy controls. Sample size was decided after power calculation with the preliminary data assuming alpha = 0.05 and beta = 0.15. The patient group was comprised of subjects who were admitted to our outpatient clinics between March 1, 2021 and June 1, 2021 with a prior diagnosis of COVID-19. Inclusion criteria for the patient group were, being aged between 18 and 70 years and having undergone a polymerase chain reaction test confirming COVID-19 diagnosis at the acute stage of disease. All the patients who met these criteria were recruited to the study consecutively. The control group was comprised of age- and sex-matched subjects without prior diagnosis of COVID-19. Individuals were excluded from the study if they had previously documented retinal disease(s), glaucoma or high refractive disorder. All patients with COVID-19 had been treated with favipiravir after diagnosis due to official health regulations. Demographic characteristics, smoking status, comorbidities (hypertension, diabetes mellitus, endocrinopathies etc.), duration since recovery from COVID-19 and biochemical parameters (thyroid stimulating hormone, glucose, vitamin D and vitamin B12 levels) were recorded. Family history for any neurodegenerative or ocular degenerative disease was noted. We also recorded whether patients had been diagnosed with pneumonia and whether hospitalization was needed during their acute infection. The ‘recovery’ was accepted as the cessation of the acute symptoms of infection (fever, respiratory symptoms, arthralgia, myalgia etc.) or the end of quarantine period and getting back to work -as in the cases with minimal symptoms. The study was approved by the local Clinical Research Ethics Committee (KAEK 118/095) of our hospital, and individuals were included in the study only after obtaining written informed consent from each participant. All steps and procedures of the study conformed to the World Medical Association Declaration of Helsinki.
2.2. Ophthalmological evaluation
All participants (N = 80) underwent slit-lamp evaluation, best corrected visual acuity (BCVA) assessment and spectral domain OCT (Heidelberg Engineering, Inc., Heidelberg, Germany). Two ophthalmologists examined COVID-19 patients for the presence of papilledema, and any retinal or choroidal pathology. OCT studies were performed by two experienced technicians without prior pupil dilatation. The minimum signal strength for acceptable OCT image quality was defined as > 7/10. The GCLT values of both eyes were measured from the macula via spectral domain OCT, and subjects in which the two eyes showed a GCLT difference greater than 2 micrometers were planned to be excluded from the study with respect to the possibility of unknown ocular confounders. However, none of the patients were excluded according to this criterion. For comparative analyses, we used the GCLT values of the right eye of each participant.
2.3. Neurological evaluation
The patient group was inquired about the following COVID-related neurological symptoms: cranial nerve involvement during acute infection (anosmia, parosmia, ageusia), headache, central symptoms (sleep disturbance, cognitive complaints/disturbance–brain fog). Analyses of GCLT values within patient subgroups were performed with respect to these characteristics. All patients had undergone neurological examination.
2.4. Statistical analysis
Before conducting analyses in continuous variables, the normality of distribution of each variable was assessed by evaluation of skewness, kurtosis and coefficient of variation, and application of normality test (Shapiro-Wilk). Continuous variables showing normal distribution were described with mean ± standard deviation (SD) values, while non-normally distributed variables were described with median ± interquartile range (IQR). The Mann-Whitney U test was utilized for group comparisons of GCLT. Correlations between GCLT, duration since recovery, age and biochemical parameters were analyzed by calculating Spearman correlation coefficients. Chi-square tests were used for the comparison of the distribution of categorical variables between groups. Statistical analysis was performed using the IBM SPSS version 20 software.
3. Results
The mean age of the patient group was 35.9 ± 10.8 (SD) and the group included 24 females and 16 males. The mean age of the control group was 33.4 ± 7.8 (SD) and the group consisted of 26 females and 14 males. The age and sex distributions were similar between the cases and controls (p = 0.25, student’s t-test; p = 0.64, Chi-square test, respectively). Mean duration after COVID-19 infection was 113 ± 62 (SD) days ( Table 1).
Table 1.
Clinical characteristics of the study population. SD: standard deviation.
| Cases of COVID-19 N = 40 | Controls N = 40 | |
|---|---|---|
| Age (mean ± SD) | 35.9 ± 10.8 | 33.4 ± 7.8 |
| Sex | 24 Female/16 Male | 26 Female/14 Male |
| Duration (mean ± SD) | 113 ± 62 days | – |
| Treatment | N = 40 | – |
| Hospitalization | N = 1 | – |
| Comorbidity Hypertension Diabetes Mellitus Thyroid disorder |
N = 1 N = 1 N = 4 |
N = 3 N = 1 |
| Having pneumonia | N = 8 | – |
Only one of the patients had been hospitalized during the COVID-19 infection and needed nasal oxygen supply. The other patients had been quarantined at home and all had received favipiravir therapy. Comorbidities were identified in 15% of the patient group, including diabetes mellitus (n = 1), hypertension (n = 1), and thyroid disorder(s) (n = 4). None of the patients were found to have neurological deficit on examination. Ophthalmological evaluation did not reveal papilledema, any retinal or choroidal pathology in any subject. Slit-lamp examinations were unremarkable in all. There was no significant difference in BCVA measurements of the cases and controls. Interestingly, one patient reported having ocular pain during the acute stage of infection and another one reported suffering from flashes of light in both eyes in the relatively later stages of disease. Their fundoscopic examinations were unremarkable, as well.
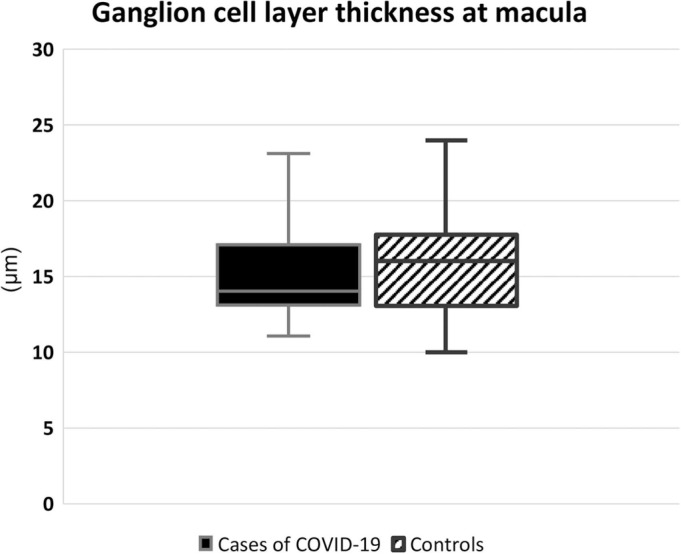
The GCLT value of patients who had suffered from COVID-19 was 14 ± 4.0 µm (median ± IQR), while the value was 16 ± 4.8 µm (median ± IQR) in the control group. The difference between the groups was not statistically significant (p = 0.332) ( Fig. 1).
Fig. 1.
Ganglion cell layer thickness measurements at macula via OCT did not show a significant difference between cases of COVID-19 and controls.
When we analyzed the relationships between GCLT values and neurological symptoms in the patient group ( Table 2), we found that there were significant differences with respect to the presence/absence of cognitive symptoms and headache. Patients with cognitive symptoms (n = 15) had significantly lower GCLT values compared to those without (13 ± 3 µm vs. 16 ± 4 µm, respectively; p = 0.002). Patients who suffered headache during the acute infection (n = 29) also had significantly lower GCLT values compared to those without (14 ± 4 µm vs. 18 ± 5 µm, respectively; p = 0.015). The GCLT values did not differ significantly with respect to anosmia, ageusia, sleep disturbances, having had COVID-19 pneumonia, or smoking status.
Table 2.
Comparison of ganglion cell layer thickness measurements of patients with COVID-19 with regard to clinical properties. IQR: interquartile range.
| Ganglion cell layer thickness (median ± IQR) | P value | |
|---|---|---|
| Cases with anosmia | Cases without anosmia | |
| 14.5 ± 5 µm | 14 ± 5 µm | 0.513 |
| Cases with ageusia | Cases without ageusia | |
| 14 ± 5 µm | 14 ± 4.5 µm | 0.595 |
| Cases with headache | Cases without headache | |
| 14 ± 4 µm | 18 ± 5 µm | 0.015 |
| Cases with anosmia or ageusia | Cases with neither anosmia nor ageusia | |
| 15 ± 5 µm | 13 ± 4.5 µm | 0.178 |
| Cases with pneumonia | Cases without pneumonia | |
| 13.5 ± 2.5 µm | 15 ± 5 µm | 0.381 |
| Cases with cognitive symptoms | Cases with no cognitive symptoms | |
| 13 ± 3 µm | 16 ± 4 µm | 0.002 |
| Cases who smoke | Cases who do not smoke | |
| 14 ± 3.5 µm | 14 ± 7 µm | 0.533 |
We also searched for any possible correlations between GCLT and parameters such as age, duration after COVID-19, and some biochemical parameters (thyroid stimulating hormone, glucose, vitamin D and vitamin B12 levels). There were no correlations between these parameters.
4. Discussion
This study revealed that, in the subacute period, the macular GCLT values of recovered COVID-19 patients were similar to that of healthy controls. However, when patients had suffered from cognitive disturbance after COVID-19, their GCLT values were significantly lower than the rest of the patient group that did not suffer cognitive disturbance. A similar trend was seen in patients who had headaches during COVID-19 infection, even though this particular result might be hampered by the low number of subjects without headache during COVID-19.
In this study, we utilized thickness of the ganglion cell layer measured by OCT as an indirect measure of subtle retinal involvement in COVID-19, since the retina is generally normal on fundoscopic examination [18]. The presence of anosmia or ageusia -the signs of other cranial nerve involvements- appear to be unrelated to retinal involvement. Central neurological complaints (headache and cognitive symptoms, but not sleep disturbances), however, showed relationships with GCLT. Direct demonstration of the presence of SARS-CoV2 in neuroretinal tissue is extremely difficult except for postmortem studies, hence, we need to appraise some indirect measures in clinical studies. The association we found between GCLT in the subacute period and the neurological symptoms might imply possible viral access to the central nervous system through ocular structures although we can get no proof at the tissue level. It is evident that further studies are necessary to arrive at definitive conclusions; however, it may be feasible to suggest that patients with lower GCLT values may benefit from close monitoring for neurological problems.
Patients with headaches and cognitive symptoms in this study demonstrated ganglion cell layer thinning. Many OCT studies on COVID-19 patients either indicated no difference compared to controls, or presented some topographical differences in the retinal nerve fiber layer (RNFL) or the ganglion cell layer [19], [20], [21]. These studies had mostly assessed patients during the acute period of infection or the relatively early period after disease. In those periods, it is evident that the systemic inflammatory response could still be active, and thus, could have been affecting many tissues in the body. Nonetheless, a study on retinal findings of COVID-19 patients 1–4 months after the illness revealed no significant change in central macular thickness, RNFL or the foveal avascular zone compared to healthy controls [22]. This study also did not include severe COVID-19 cases, similar to our cohort. They reported no subgroup analysis according to symptoms associated with neurological involvement. In a study by Burgos-Blasco et al., which assessed patients four weeks after COVID-19 diagnosis, they found that patients had significant increases in peripapillary RNFL and decreases in macular RNFL in some quadrants [21]. Besides, the patients who reported anosmia and ageusia during the infection were found to have increased peripapillary RNFL thickness and macular GCLT in some sectors of the retina compared to patients without these symptoms [21]. The authors noted that these alterations may be associated with residual inflammation in some regions of the retina and could lead into atrophy in the long term. In this context, our findings appear to be in support of previous findings, and lend credibility to the notion that viral dissemination into the eye may lead to atrophy –since our measurements were performed at a later stage after COVID-19 (mean of 113 days). We found no significant difference between patients with and without anosmia or ageusia. This may also be related to the time window for neuroretinal assessment (i.e. transition from acute to chronic stage). However, GCLT was already and significantly lower in the patients who suffered from headache and those who had cognitive symptoms after acute illness, implying central neurological involvement. The observed change in the GCLT values of this specific group of COVID-19 patients may support the trans-neuronal route of virus dissemination towards central nervous system [9]. In a recent review, a possible pathophysiological mechanism for long COVID symptoms was proposed to be olfactory sensory neuronal damage leading to glymphatic circulatory failure in the brain [23]. SARS-CoV-2 can cause a decrease in the number of olfactory sensory neurons, which may increase the resistance to cerebrospinal fluid outflow through the cribriform plate. This may, in turn, result in increased intracranial pressure [23]. Interestingly, OCT and retinal nerve fiber layer thinning may reflect this disturbance in glymphatic circulation since the toxic build-up and increased pressure have the potential for damaging neuroretinal cells [24]. Our finding of thinner ganglion cell layer in patients who suffered from headache and those who had cognitive symptoms seems to be in line with this hypothesis. It is important to note that none of our patients had severe acute neurological complications of COVID-19 which could have the potential to affect the nerve layer of the eye. In addition, the scarcity of comorbidities in our patient group was also a positive feature in terms of confounders that could alter GCLT.
We also checked for some biochemical parameters that may affect retinal measurements. GCLT did not show any correlation with TSH, glucose, vitamin D or vitamin B12 levels of the cases. Smoking may cause toxic effects on the optic nerve and could lead to thinning of retinal layers [25]. Besides, nicotine can stimulate ACE2 expression, and nicotinic acetyl choline receptors have functional interactions with ACE2, the point of viral entry [26]. Based on this knowledge, smoking status of the cases and the whole study population were analyzed; but no differences were found with respect to cigarette smoking in GCLT values. Finally, the age of the subjects might have an effect on GCLT, independently of COVID-19. However, our analyses revealed no association between age and GCLT.
There are several limitations of our study. First of all, the area chosen for GCLT measurements (i.e. macula) may have affected the results. Secondly, the duration since recovery from COVID-19 had a wide range among our patients (mean ± SD: 113 ± 62 days) although all were in the subacute period after the disease (≥4 weeks). This may have caused variability in the results. Also, the sample size of our study group was small, especially for subgroup analysis.
Nevertheless, this study highlights an association between GCLT values measured via OCT and neurological symptoms such as cognitive disturbance (brain fog) and headache in patients who had recovered after non-severe COVID-19 infection. Unlike other cranial nerve involvements (anosmia and ageusia), central neurological symptoms might be linked to retinal viral entry and may warrant higher surveillance.
CRediT authorship contribution statement
Aslıhan Taskiran-Sag: Conceptualization, Investigation, Writing − original draft preparation. Erdal Eroglu: Investigation, Writing − review & editing. Kemal Ozulken: Conceptualization, Resources. Sule Canlar: Investigation, Writing − review & editing. Baris Mustafa Poyraz: Writing − review & editing, Conceptualization. Manolya Berguzar Sekerlisoy: Investigation, Writing − review & editing. Tarkan MUMCUOGLU: Conceptualization, Resources.
Acknowledgments
The authors declare no conflict of interest. The authors thank the technicians of ophthalmology department for their invaluable work and all the participants of the study.
References
- 1.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80 e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osuchowski M.F., Winkler M.S., Skirecki T., et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir. Med. 2021;9(6):622–642. doi: 10.1016/S2213-2600(21)00218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou Y.J., Okuda K., Edwards C.E., et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182(2):429–46 e14. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma D., Chen C.B., Jhanji V., et al. Expression of SARS-CoV-2 receptor ACE2 and TMPRSS2 in human primary conjunctival and pterygium cell lines and in mouse cornea. Eye (Lond. ) 2020;34(7):1212–1219. doi: 10.1038/s41433-020-0939-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou L., Xu Z., Castiglione G.M., Soiberman U.S., Eberhart C.G., Duh E.J. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul. Surf. 2020;18(4):537–544. doi: 10.1016/j.jtos.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaya H., Caliskan A., Okul M., Sari T., Akbudak I.H. Detection of SARS-CoV-2 in the tears and conjunctival secretions of Coronavirus disease 2019 patients. J. Infect. Dev. Ctries. 2020;14(9):977–981. doi: 10.3855/jidc.13224. [DOI] [PubMed] [Google Scholar]
- 7.Zhou L., Xu Z., Guerra J., et al. Expression of the SARS-CoV-2 Receptor ACE2 in Human Retina and Diabetes-Implications for Retinopathy. Invest Ophthalmol. Vis. Sci. 2021;62(7):6. doi: 10.1167/iovs.62.7.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araujo-Silva C.A., Marcos A.A.A., Marinho P.M., et al. Presumed SARS-CoV-2 viral particles in the human retina of patients with COVID-19. JAMA Ophthalmol. 2021 doi: 10.1001/jamaophthalmol.2021.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newcombe V.F.J., Dangayach N.S., Sonneville R. Neurological complications of COVID-19. Intensive Care Med. 2021 doi: 10.1007/s00134-021-06439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marinho P.M., Marcos A.A.A., Romano A.C., Nascimento H., Belfort R., Jr. Retinal findings in patients with COVID-19. Lancet. 2020;395(10237):1610. doi: 10.1016/S0140-6736(20)31014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Invernizzi A., Torre A., Parrulli S., et al. Retinal findings in patients with COVID-19: Results from the SERPICO-19 study. EClinicalMedicine. 2020;27 doi: 10.1016/j.eclinm.2020.100550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lani-Louzada R., Ramos C., Cordeiro R.M., Sadun A.A. Retinal changes in COVID-19 hospitalized cases. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0243346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zapata M.A., Banderas Garcia S., Sanchez-Moltalva A., et al. Retinal microvascular abnormalities in patients after COVID-19 depending on disease severity. Br. J. Ophthalmol. 2020 doi: 10.1136/bjophthalmol-2020-317953. [DOI] [PubMed] [Google Scholar]
- 14.Xu J., Lazartigues E. Expression of ACE2 in human neurons supports the neuro-invasive potential of COVID-19 virus. Cell Mol. Neurobiol. 2020 doi: 10.1007/s10571-020-00915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallagher P.E., Chappell M.C., Ferrario C.M., Tallant E.A. Distinct roles for ANG II and ANG-(1-7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am. J. Physiol. Cell Physiol. 2006;290(2):C420–C426. doi: 10.1152/ajpcell.00409.2004. [DOI] [PubMed] [Google Scholar]
- 16.Doobay M.F., Talman L.S., Obr T.D., Tian X., Davisson R.L., Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292(1):R373–R381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atlas AB. 2021. 〈http://human.brain-map.org/microarray/search/show?exact_match=true&search_term=ACE2&search_type=gene&donors=10021,15496,14380,12876,9861,15697〉 (accessed 4 september 2021).
- 18.Mehta S., Prabhudesai P. Fundus Lesions in Patients Hospitalized With COVID-19 Infection in Mumbai, India: A Retrospective Review. Cureus. 2020;12(11) doi: 10.7759/cureus.11512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oren B., Aksoy Aydemir G., Aydemir E., et al. Quantitative assessment of retinal changes in COVID-19 patients. Clin. Exp. Optom. 2021;104(6):717–722. doi: 10.1080/08164622.2021.1916389. [DOI] [PubMed] [Google Scholar]
- 20.Cetinkaya T., Kurt M.M., Akpolat C. Assessment of Retinal Neurodegeneration and Choroidal Thickness in COVID-19 Patients Using Swept-Source OCT Technology. Klin. Monbl Augenheilkd. 2021 doi: 10.1055/a-1340-0066. [DOI] [PubMed] [Google Scholar]
- 21.Burgos-Blasco B., Guemes-Villahoz N., Vidal-Villegas B., et al. Optic nerve and macular optical coherence tomography in recovered COVID-19 patients. Eur. J. Ophthalmol. 2021 doi: 10.1177/11206721211001019. [DOI] [PubMed] [Google Scholar]
- 22.Szkodny D., Wylęgała E., Sujka-Franczak P., et al. Retinal OCT Findings in Patients after COVID Infection. J. Clin. Med. 2021;10(15) doi: 10.3390/jcm10153233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wostyn P. COVID-19 and chronic fatigue syndrome: Is the worst yet to come? Med Hypotheses. 2021;146 doi: 10.1016/j.mehy.2020.110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wostyn P., De, Deyn P.P. The retinal nerve fiber layer as a window to the glymphatic system. Clin. Neurol. Neurosurg. 2020;188 doi: 10.1016/j.clineuro.2019.105593. [DOI] [PubMed] [Google Scholar]
- 25.Dervişoğulları M.S., Totan Y., Tenlik A., Yüce A., Güler E. Effect of smoking on retina nerve fiber layer and ganglion cell-inner plexiform layer complex. Cutan. Ocul. Toxicol. 2015;34(4):282–285. doi: 10.3109/15569527.2014.975240. [DOI] [PubMed] [Google Scholar]
- 26.Kabbani N., Olds J.L. Does COVID19 infect the brain? If so, smokers might be at a higher risk. Mol. Pharm. 2020;97(5):351–353. doi: 10.1124/molpharm.120.000014. [DOI] [PMC free article] [PubMed] [Google Scholar]