Abstract
Background
As several vaccines for SARS-CoV-2 have been developed, a large proportion of individuals have been vaccinated worldwide so far. The rapid and accurate immunoassays are urgently needed for detecting the specific virus-neutralizing antibody (NAb), which reflect the protective effect of the vaccines among different populations.
Methods
In this study, we designed a quantum dot lateral flow immunoassay strip (QD-LFIA) for smartphones for the detection of specific IgG or neutralizing antibodies in SARS-CoV-2 in human serum or whole blood samples. The recombinant receptor binding domain of the SARS-CoV-2 spike protein was used as the antigen to combine with NAb or angiotensin-converting enzyme 2.
Results
Among 81 patients who recovered from COVID-19 who were diagnosed using the nucleic acid test initially, 98.8% (80/81) were positive for IgG and 88.9% (72/81) were positive for NAb by QD-LFIA. Among 64 individuals inoculated with inactivated vaccines and six subunit vaccines, 90% (63/70) were positive for IgG and 82.9% (58/70) were positive for NAb by QD-LFIA, whereas no cross-reaction was found in 150 healthy blood donors, two patients with influenza B, and three patients with common cold.
Conclusion
The established platform could achieve a rapid and accurate detection of NAb specific to SARS-CoV-2, which could be used for detecting the protective effect of the vaccines in areas of world that currently affected by the pandemic.
Key words: SARS-CoV-2, Neutralizing antibody, IgG, Quantum dot, Smartphone
Introduction
In December 2019, COVID-19, caused by SARS-CoV-2, was first discovered in Wuhan, China and was then reported worldwide within a few months (Wang et al., 2020; Zhou et al., 2020). Some patients rapidly developed acute complications such as acute respiratory distress syndrome and acute respiratory failure (Chen et al., 2020). The main routes of SARS-CoV-2 transmission in humans were respiratory droplets and virus exposure between individuals. By the end of May 2021, more than 170 million people were diagnosed with COVID-19 by viral nucleic acid test (NAT), and over 3 million deaths were reported worldwide, with more than 4000 deaths being reported in China.
SARS-CoV-2 is a single-stranded sense RNA virus that is over 30,000 nucleotides in length and is a member of the β genus of the coronavirus family (Chan et al., 2020; Chu et al., 2020; Phelan et al., 2020). Confirmation of the genomic viral sequence (GenBank MN908947) allowed for testing for SARS-CoV-2 RNA to determine whether individuals were infected with the virus. Due to the samples’ collection time, storage, and transportation conditions, some NATs could not detect SARS-CoV-2 from nasal or pharynx swabs (Tanner et al., 2015; Lim et al., 2018; Kim et al., 2019; Patra et al., 2019). Many patients had to be tested repeatedly before they were finally diagnosed (Corman et al., 2020; Zhang et al., 2020). However, testing for antibodies specific to SARS-CoV-2 could be a helpful complementary approach to confirm infection. Suspected cases, asymptomatic patients, or resolvers of COVID-19 can be detected by the antibodies specific to SARS-CoV-2.
Several vaccines for SARS-CoV-2 have been developed and are currently approved for emerging use in vaccination worldwide (Gao et al., 2020; van Doremalen et al., 2020; Yu et al., 2020). It is generally believed that the vaccination can induce virus-neutralizing antibody (NAb), which will reduce the likelihood of (re)infection and the development of severe disease (Jackson et al., 2020; Krammer, 2020; Xia et al., 2020). As there are individual differences after vaccination, the levels of NAb titers could be measured to predict the protective effect of the vaccines (Dagotto et al., 2020). There are several virus neutralization assays (VNTs) and pseudovirus neutralization assays (pVNT), which could be used to evaluate the NAb titers, but they required the use of live viruses and cells (Crawford et al., 2020; Manenti et al., 2020). A surrogate virus neutralization assay mimicking the virus–host interaction in an ELISA plate well was established but needed several hours to be completed (Tan et al., 2020).
Although there are several neutralizing antibody methods, more rapid and simpler tests are still required strongly by the vaccinated individuals. In this study, we designed a smartphone-based quantum dot lateral flow immunoassay strip (QD-LFIA) for the detection of specific IgG and NAb to SARS-CoV-2 in human serum or whole blood samples. The testing can be carried out within 30 minutes and this novel assay is ultrasensitive, cost-effective, rapid, and simple, which can be used for on-site detection of specific IgG and NAb in SARS-CoV-2 infection in humans.
Materials and methods
Blood specimen
Serum samples from 81 convalescent patients who had COVID-19, 49 Sinopharm recipients (inactivated vaccines, one month after the vaccination with two doses of vaccines), 15 Sinovac recipients (inactivated vaccine), and six Zhifei recipients (recombinant subunit vaccine, one month after vaccination with three doses of vaccines) were obtained from the Shenzhen Center for Disease Control and Prevention (CDC). The patients were initially confirmed positive for SARS-CoV-2 RNA using real-time reverse transcriptase-quantitative polymerase chain reaction. A total of 150 plasma samples from healthy blood donors were used as the negative controls that were collected from Guangzhou or Shenzhen blood center during the period between August and October of 2019 before the COVID-19 outbreak in Wuhan, China. Serum samples from two patients with influenza B and three patients with the common cold were obtained from the Shenzhen and Guangzhou CDC. All samples were transported in cold chain to the laboratory within 12 hours and stored at –20°C before using. This study was approved by the Medical Ethics Committees of Shenzhen CDC and Southern Medical University and adhered to the ethical guidelines established by the 1975 Declaration of Helsinki.
Reagents and equipment
Bovine serum albumin (BSA) and goat antimouse polyclonal immunoglobulin G (G-mIgG) were purchased commercially (Sigma-Aldrich, St. Louis, USA). Mouse antihuman IgG (M-hIgG) were purchased from Beijing Bersee Science and Technology Co. Ltd (Beijing, China). Recombinant spike protein receptor binding domain (RBD) fused with His-tag (RBD-His) of SARS-CoV-2, angiotensin-converting enzyme 2 (ACE2), and mouse monoclonal antibody to His-tag (anti-His mAb) were purchased from Bioeast Biotech Co. Ltd (Hangzhou, China). Polystyrene-coated quantum dot nanoparticles (QDs) with a 160 nanometer diameter were purchased from Huge Biotech Ltd (Shanghai, China). Nitrocellulose (NC) membranes, sample pads, and absorbent pads were purchased from Millipore Corporation (Bedford, MA, USA). A portable fluorescence strip reader was designed by this study. For control assays, SARS-CoV-2 Ab Rapid Test Kits (AuNPs-LFIA) were purchased from Wondfo Biotech Co., Ltd. (Guangzhou, China), SARS-CoV-2 NAb test strips (AuNPs-LFIA) were purchased from Shihuier Co., Ltd. (Yangzhou, China), and ELISA kits were purchased from WANTAI BioPharm Co., Ltd. (Beijing, China).
Preparation of QD-LFIA strips
QD-LFIA strips consisted of a 25-millimeter sample pad, a 20-millimeter NC membrane, and a 15-millimeter absorbent pad; all of these were pasted on an adhesive backing card. The conjugate pad was saturated with treatment buffer (10 mM phosphate-buffered saline [PBS], pH 7.4 containing 4% sucrose, 0.5% Tween-20, 0.5% polyvinylpyrrolidone, and 1% BSA) for 1 hour and then dried at 37°C for 24 hours.
An aliquot of QDs were conjugated with a specific amount of anti-His mAb via covalent attachment. Briefly, 1.5 μL of EDC (1 mg/mL) and 1.5 μL of N-hydroxysulfosuccinimide (sulfo-NHS) (2 mg/mL) solutions were mixed and initially added into 25 μL of QDs suspended in 475 μL activating buffer (25 mM MES, pH 6.1). The mixture was incubated for 30 minutes at room temperature with gentle stirring. After the NHS-ester was formed, the QDs was washed two times with binding buffer (10 mM phosphate buffer, pH 7.0) by centrifugation at 10,000 × g for 15 minutes to remove unreacted sulfo-NHS and EDC and was resuspended in 475 μL binding buffer. A total of 20 μg of anti-His antibody was added to the activated QDs and the mixture was incubated for two hours with gentle rotation. The washing process was repeated two times to remove unbound chemicals or proteins and were suspended in blocking buffer and incubated for another 30 minutes with gentle mixing to cover any remaining unbound hydrophobic sites on the surface of the QDs. Finally, the QD anti-His mAb were rinsed two times using washing buffer and suspended in 250 μL storing buffer.
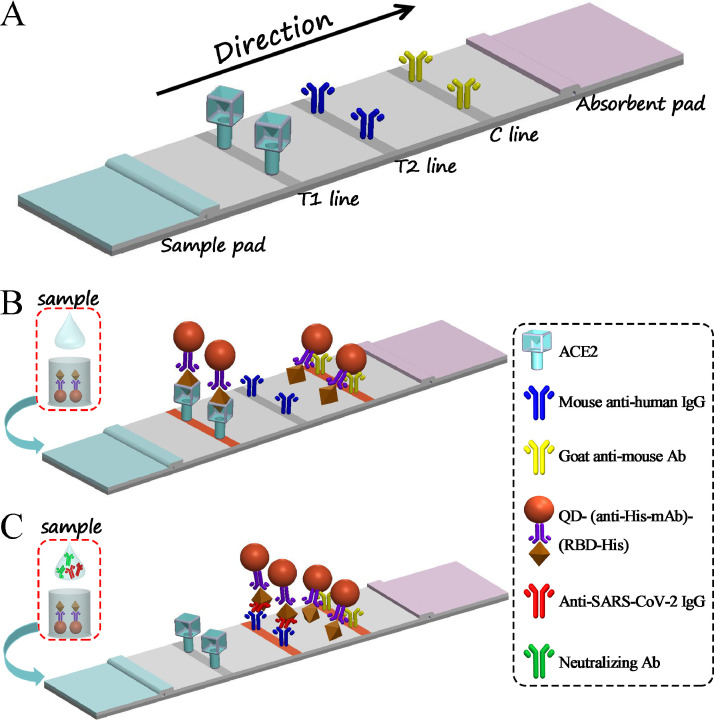
The G-mIgG (1 mg/mL), M-hIgG (1 mg/mL), and ACE2 (0.2 mg/mL) were spotted onto the NC membrane with dispensing parameter of 1 μL/cm (from the absorbent pad to the sample pad) to generate the C, T1, and T2 lines, respectively (Figure 1 A). The coated NC was dried at 37°C for 30 minutes. The sample pad was pretreated with an optimized treatment buffer and then dried at 37°C overnight.
Figure 1.
Schematic of the QD-LFIA for detection of IgG/NAb produced in response to SARS-CoV-2 infection in humans. (A) The components of QD-LFIA strip. (B, C) The working principle of QD-LFIA strip.
This QD-LFIA assay was performed by mixing a drop (approximately 10 μL) of serum or whole blood sample with 100 μL of sample buffer (10 mM PBS, pH 7.4 containing 0.5% Tween-20, 1% S9, and 1% BSA), 3 μL of QD@anti-His mAb conjugates (20 μg of anti-His antibody conjugated with 25 μL of QDs in 250 μL storing buffer), and 1 μL of RBD-His (25 μg/mL). The sample solution was reacted at room temperature for 10 minutes and then added to the sample pad of testing strip for immunochromatography. The results could be shown in 15 minutes. Fluorescence signals on the T1/T2 and C lines of the strip were captured using a self-produced portable fluorescence strip reader and were uploaded to a smartphone via Wi-Fi for data processing.
Enzyme-linked immunosorbent assay (ELISA)
The ELISA tests for serum samples from 81 convalescent patients who had COVID-19 and 70 serum samples from vaccine recipients were performed following the instruction of Wantai SARS-CoV-2 IgG ELISA kit (Wantai Biological Pharmacy, Beijing, China). All samples were tested in serial dilutions beginning at 1:50, and endpoint titers were defined as the highest plasma dilution that yielded an absorbance > cutoff value. The cutoff value was calculated as the mean of three negative controls plus 3 SD (mean + 3SD).
Pseudovirus neutralization assay (pVNT)
The SARS-CoV-2 pseudoviruses expressing a luciferase reporter gene were generated as described previously (Yang et al., 2004). Briefly, the packaging construct psPAX2 and luciferase reporter plasmid pLenti-CMV Puro-Luc (Miaoling Biotechnology Co., Ltd) and spike protein expressing pcDNA3.1-SARS-CoV-2 SΔCT were cotransfected into HEK293T cells with calcium phosphate. After 48 hours post-transfection, the supernatants containing the pseudotype viruses were collected and purified by 0.45 µm filter. HEK293T-hACE2 cells were seeded in 96-well tissue culture plates at a density of 2 × 104 cells/well overnight. The serum samples were prepared in two-fold serial dilutions and mixed with 50 µL of pseudovirus and then incubated at 37°C for 1 hour before adding to cells; cells were lysed after 48 hours. SARS-CoV-2 neutralization titers were defined as the sample dilution at which a 50% inhibition rate (IC50) was observed relative to the average of the virus control wells. Inhibition rate (%) = (1 – sample relative light unit [RLU]/virus control RLU) × 100%.
Statistical analysis
All data were analyzed using the statistical package SPSS v.16.0. All experiments were performed at least three times independently. The results of skewness and kurtosis tests are listed in the Supplemental Table 1, and the results of different methods are presented as the median/IQR and analyzed by the nonparametric tests (McNemar test). The correlation of IgG and NAb titers between assays were analyzed by using linear regression, and a P-value < 0.05 was considered statistically significant.
Results
Structure and working principle of smartphone-based QD-LFIA
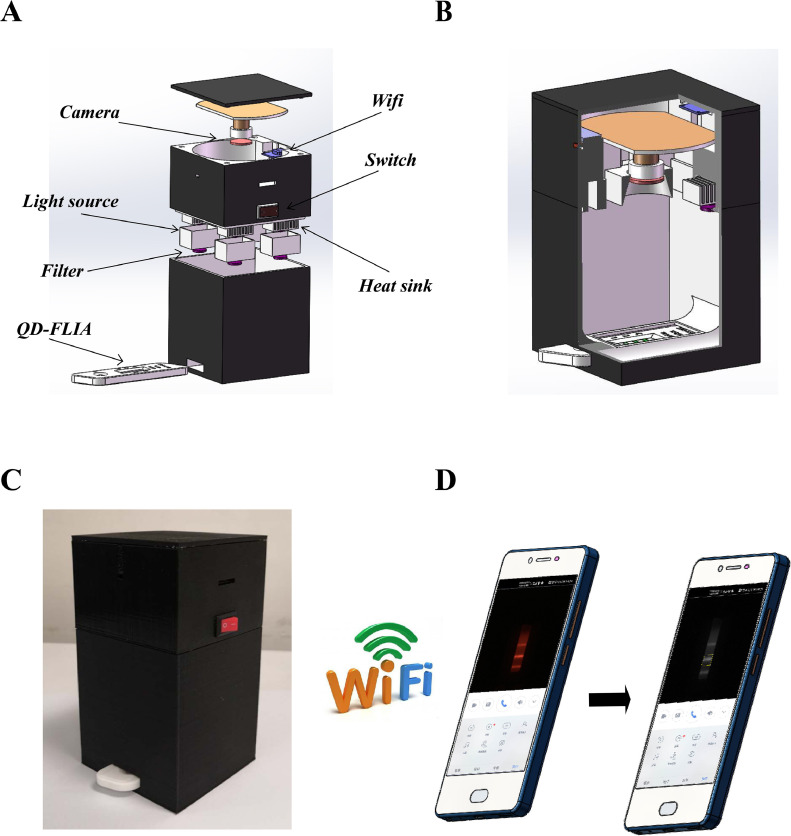
This system included a QD-LFIA strip and a portable fluorescence strip reader connected to a smartphone platform. The QD-LFIA strip contained a sample pad, NC membrane with C and T1/T2 lines, and an absorbent pad (Figure 1A). The working principle was shown in Figure 1B and 1C when a sample was added to the sample pad. To report the testing results conveniently, a portable fluorescence strip reader connected to a smartphone platform was invented for QD-LFIA strip (Figure 2 ).
Figure 2.
Schematic of the self-produced portable fluorescence real-time camera reader. (A, B) The components of QD-LFIA strip. (C) The appearance of this device. (D) This device could be connected with smartphone through Wi-Fi.
As shown in Figure 1B, if the sample did not contain the IgG or NAb specific to SARS-CoV-2, the RBD-His would react with QD@anti-His mAb, migrate, and bind to ACE2 on the T2 line of NC membrane. The excessive complexes would continue to move along the membrane until they are captured on the C line by secondary goat antimouse antibodies. The C line was always visible, which indicated that the test strip and procedure worked well.
As shown in Figure 1C, if the sample contained the IgG or NAb specific to SARS-CoV-2, the IgG or NAb would combine with RBD-His, then react with QD@anti-His mAb. When the complexes migrated on the NC membrane, the NAb saturated RBD complexes could not bind to the immobilized ACE2 on the T2 line, whereas IgG saturated RBD complexes would bind to the mouse antihuman IgG (T1). The more NAb molecules or titers were presented in the sample, the lower density of T2 line was appeared.
Finally, the strip was inserted into the self-produced fluorescence reader, and fluorescent images of the T2/T1/C lines were captured and transferred to a smartphone through Wi-Fi (Figure 2). The fluorescence lines were then selected and the fluorescence intensity of the T1, T2, or C lines was converted to peak areas. The value of the T1/C or T2/C was then calculated, and the sample was determined to be negative or positive after comparing the result with the cutoff value.
Optimization of QD-LFIA
To enhance the performance of this assay, the amount of anti-His mAb-labeled quantum-dots (QDs) was optimized. A total of 20 μL of 1 mg/mL anti-His mAb was used for labeling 25 μL of QDs (Supplementary Figure 1). The QD@anti-His mAb and RBD-His (25 μg/mL) were comparatively tested for reacting with sample solution, of which 3μL of QD@anti-His mAb and 1μL of RBD-His were optimally selected, respectively (Supplementary Figure 2 and 3). The amount of 1 μL/cm ACE2 (0.2 mg/ml) and 1 μL/cm M-hIgG (1mg/mL) was optimized for spotting the T lines on NC membrane of strip (Supplementary Figure 4).
Three types of sample pads were compared (Supplementary Figure 5), of which glass fiber (type G-2) was used for the sample pad. We also tested the time curve of this assay. As shown in Supplementary Figure 6, 10 minutes were sufficient for the reaction of the sample solution incubated in microwells, and 15 minutes were satisfactory to read the results.
To reduce nonspecific interactions, we optimized the sample buffer and treatment buffer used for the QD-LFIA (Supplementary Figure 7 and 8). An optimized sample buffer (PBS + 1% BSA + 0.1% Triton X-100, pH 7.4) was chosen for this assay and a mixed treatment buffer (PBST with 1% BSA and 0.1% gelatin, pH 7.4) was optimized for blocking the sample pad.
Sensitivity of QD-LFIA
A total of 50 healthy blood donor plasma samples (provided by Guangzhou Blood Center) were tested negative for specific IgG or NAb to SARS-CoV-2 by ELISA or pVNT, respectively. By using these negative control samples, the appropriate cutoff values of specific IgG or NAb to SARS-CoV-2 in QD-FLIA were defined as 0.021 (T1/C, mean plus 3 SD) or 2.783 (T2/C, mean minus 3 SD), respectively.
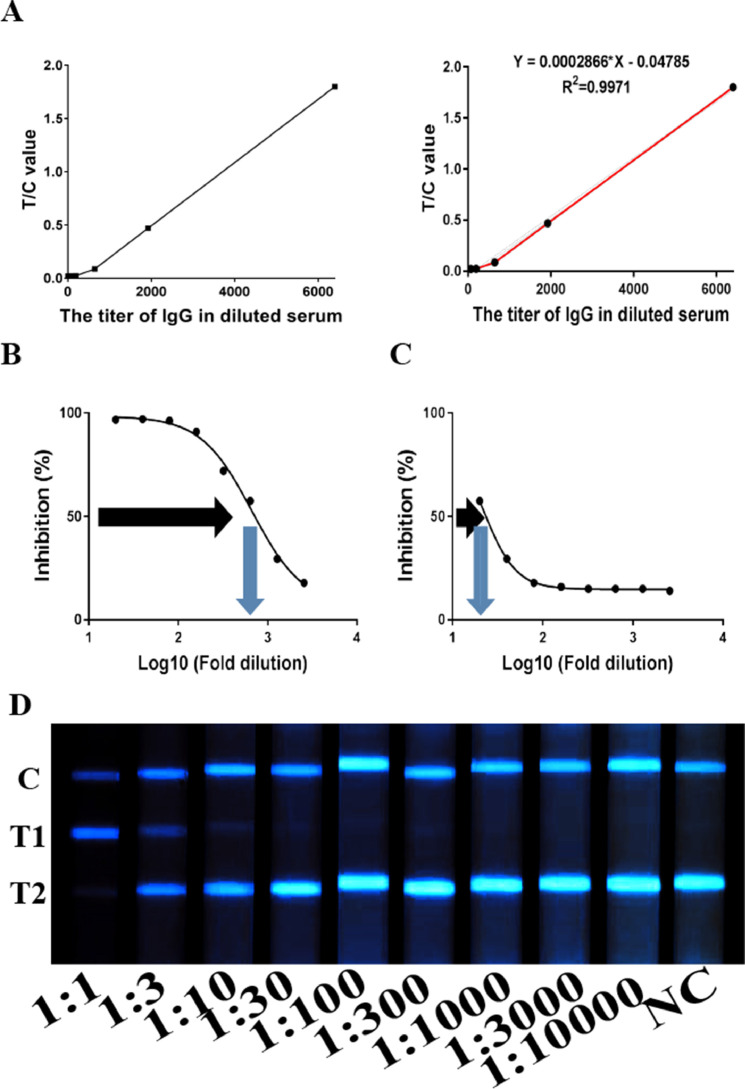
The IgG titer of a convalescent patient who had COVID-19 (No.1 patient in Supplementary Table 2) was 6400 using ELISA, whereas the NAb IC50 was 666 by pVNT, which was highest among all the samples. Thus, the serum sample of this patient was used as reference standard to distinguish positive from negative samples. To establish the standard curve, we used different diluted serum samples of reference standard from 1:1 to 1:10000 and obtained the light intensity for each dilution using the self-produced device. The LOD for detecting the standard was 19.2 (IgG titer) and the linear range was 64–6400 (Figure 3 A). For NAb testing, the reference standard with 1:30 dilution was tested positive using QD-LFIA, of which IC50 was 21 by pVNT (Figure 3B, C). The different diluted reference standard from 1:1 to 1:10000 was listed in Figure 3D. Among the serum samples from 81 convalescent patients who had COVID-19 diagnosed with NAT previously, 98.8% (80/81) were positive for IgG and 88.9% (72/81) were positive for NAb using QD-LFIA (Table 1 ), which were consistent with the results of ELISA or pVNT (Supplementary Table 1). Among the serum samples from 70 vaccine recipeitns, 90% (63/70) were positive for IgG and 82.9% (58/70) were positive for NAb by QD-LFIA, which were also consistent with the results of ELISA or pVNT (Table 1 and Supplementary Table 3). Among all samples from 70 vaccine recipients and 81 convalescent patients who had COVID-19, QD-LFIA had higher sensitivity than AuNPs-LFIA, in which 94.7% (143/151) were positive for IgG by QD-LFIA, whereas 92.7% (140/151) were positive using AuNPs-LFIA. Furthermore, 86.1% (130/151) were positive for NAb using QD-LFIA, whereas 84.1% (127/151) were positive using AuNPs-LFIA (Table 1 and Supplementary Table 3).
Figure 3.
Linear regression of quantitative testing of specific IgG or NAb specific to SARS-CoV-2 in blood samples from COVID-19 patients using the QD-LFIA. (A) Linear regression curves for IgG titers in diluted standard samples. The IC50 of standard sample with 1:1 (B) and 1:30 (C) dilution were tested by pVNT. (D) Representative fluorescent images of reactive C, T1, and T2 lines in diluted standard samples.
Table 1.
Sensitivity and specificity of QD-LFIA for testing of anti-SARS-CoV-2 IgG or NAb
| Population | Sample (Nb*) | IgG Nb (%) |
NAb Nb (%) |
||||
|---|---|---|---|---|---|---|---|
| ELISA | AuNPs-LFIA | QD-LFIA | pVNT | AuNPs-LFIA | QD-LFIA | ||
| Convalescent patients |
81 | 80(98.8%) | 77(95.1%) | 80(98.8%) | 72(88.9%) | 72(88.9%) | 72(88.9%) |
| Sinopharm vaccinees |
49 | 43(87.6%) | 43(87.6%) | 43(87.6%) | 38(77.6%) | 35(71.4%) | 38(77.6%) |
| Healthy blood donor |
150 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Sinovac vaccinees | 15 | 14%(93.3%) | 14%(93.3%) | 14%(93.3%) | 14%(93.3%) | 14%(93.3%) | 14%(93.3%) |
| Zhifei vaccinees | 6 | 6(100%) | 6(100%) | 6(100%) | 6(100%) | 6(100%) | 6(100%) |
| Flu B | 2 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Common cold serum samples | 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
* Number.
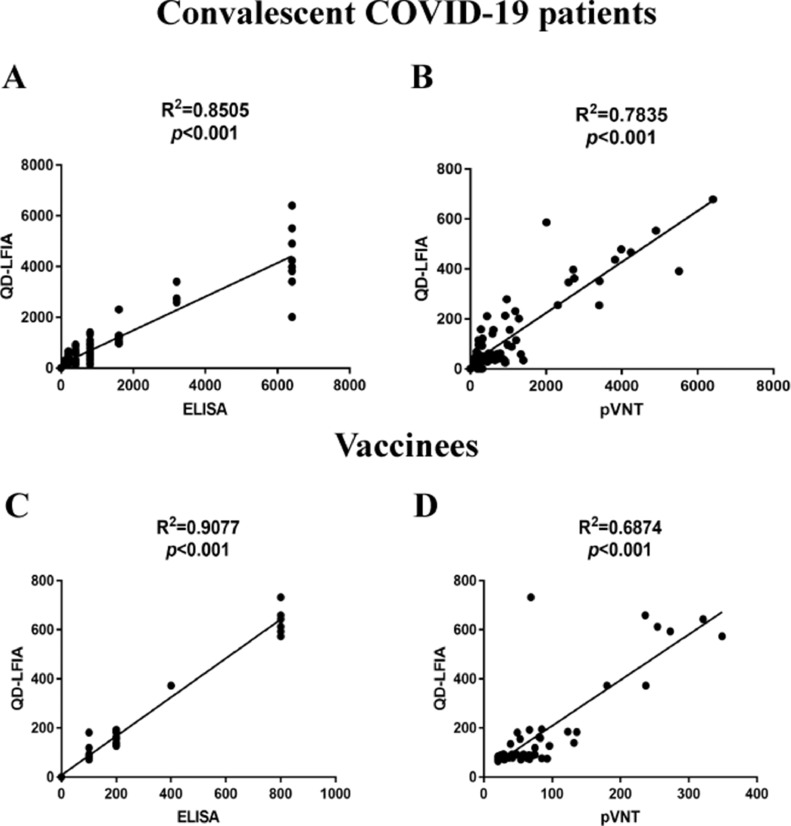
The titers of IgG tested by QD-LFIA had quantitative correlation to the results by ELISA (Figure 4 A, C) and NAb IC50 tested by pVNT (Figure 4Band D), which meant that the titers of IgG tested by QD-LFIA could be a suitable substitute for quantitative detection of NAb. By integrating the quantitative detections of IgG and NAb, this QD-LFIA could accurately determine the immune status of clinical samples and meet clinical sensitivity demands compared with other methods.
Figure 4.
Correlation of IgG and NAb titers in serum samples between QD-LFIA, ELISA and pVNT. (A, B) Correlation between QD-LFIA and ELISA, or QD-LFIA and pVNT for testing of 81 convalescent COVID-19 patients’ samples. (C, D) Correlation between QD-LFIA and ELISA, or QD-LFIA and pVNT for testing of 49 vaccinees’ samples.
Specificity, stability, and accuracy of QD-LFIA
To examine the specificity of QD-LFIA, plasma samples from 150 healthy blood donors and serum samples from two patients with influenza B and three patients with the common cold were tested. All samples were detected negative, which suggested that the specificity of QD-LFIA was 100% for testing of IgG or NAb (Table 1). A blood donor sample (BD5) who was infected with SARS 17 years ago was additionally detected positive by ELISA (Xu et al., 2021) but negative by QD-LFIA (Supplementary Figure 9).
To determine the stability of QD-LFIA, we measured the activity and performance of this assay at different time points and temperatures (Supplementary Figure 10). The results demonstrated that QD-LFIA had constant stability for testing period up to 30 days at room temperature or at temperatures between 10 °C and 50 °C.
The accuracy of QD-LFIA was evaluated by examining the recovery rate and relative standard deviation (RSD). Three different dilutions of standard sample were analyzed, of which the recovery rates and RSD were around 95% and 5%, respectively (Supplementary Table 4). This finding showed that the QD-LFIA performed well in both accuracy and precision.
Detection of whole blood samples
To verify whether this method could be used for on-site detection in grassroot hospitals or self-check at home, we tried whole blood samples on QD-LFIA. The representative results were presented in Supplementary Figure 11, suggesting that this assay could be applied to whole blood samples.
Discussion
A novel coronavirus from a patient's pharyngeal swab sample was discovered by the Chinese CDC on January 7, 2020, which was temporarily named 2019-nCoV, and subsequently designated as SARS-CoV-2 by the World Health Organization (Cheng and Shan, 2020). SARS-CoV-2 has caused a global pandemic with COVID-19. More than 170 million individuals were diagnosed with COVID-19 and over 3 million deaths were reported to date. This pandemic has made many communities or cities to close down, which caused huge economic losses worldwide. Currently, Asian and European countries where the SARS-CoV-2 infection has been contained are reopening and restarting social activities.
Along with nucleic acid testing of SARS-CoV-2, the antibody tests are largely needed as well. The development of rapid and accurate antibody detection methods is highly welcome. Detection of specific antibody to SARS-CoV-2 can reflect the status of virus infection among different populations and can also provide serological evidence for clinical diagnosis of patients with COVID-19.
Several COVID-19 vaccines have been developed (Keech et al., 2020; Logunov et al., 2020; Poland et al., 2020; Zhu et al., 2020), including mRNA-1273, NVX-CoV2373, BNT162b2, ChAdOx1 nCoV-19, CoronaVac, and two Chinese inactivated virus vaccines.
Billions of individuals have already been vaccinated or will be vaccinated in the near future. Immunity to SARS-CoV-2 induced by these vaccines could protect against reinfection and reduce the risk of becoming critically ill. A critical challenge at present is to identify the vaccine efficacy on the basis of immune protection from SARS-CoV-2 infection and thereby assist in the further development of vaccines. Although antiviral cellular immunity certainly contributes to the protection from SARS-CoV-2 infection, the main protective effect of vaccines was on the basis of neutralizing antibodies (Khoury et al., 2021).
There are conventional live virus neutralization tests (VNTs) or pVNT assays for measuring neutralizing antibody titers, which require virus cell cultures for a few days in a P2 or P3 laboratory. The surrogate VNT on the basis of ELISA can measure the NAb effect to block the virus–host interaction in a microplate, which still needs 2 hours to finish and a microplate reader to read the results. In this study, we developed a QD-LFIA and combined it with a self-produced portable fluorescence real-time camera reader, which could detect specific IgG or NAb to SARS-CoV-2 infection in human serum or whole blood samples. The testing can be carried out within 30 minutes. The results could be read by a self-produced portable fluorescence reader, which is cost-effective compared with the traditional microplate reader. Finally, the results could be sent to a smartphone through Wi-Fi transmission for reporting.
As some of anti-SARS-CoV could neutralize or recognize SARS-CoV-2 (Gavor et al., 2020; Yuan et al., 2020), a serum sample from a blood donor infected with SARS-CoV 17 years ago (BD5, Xu, et al. 2020) was used to test if a false-positive result could be produced by the cross-reaction between SARS-CoV and SARS-CoV-2. As this serum sample tested negative by QD-LFIA, it suggested that this assay might not be cross-reactive with anti-SARS-CoV. Because no new SARS case has been reported over 17 years, it was difficult to collect more anti-SARS-CoV positive samples to make this conclusion. In addition, plasma samples from 150 healthy blood donors and serum samples from two patients with influenza B and three patients with the common cold were tested for the specificity of QD-LFIA, and all samples were negative. These results suggested that this assay was not cross-reactive with anti-SARS-CoV or antibodies to other coronaviruses.
For biosafety, blood samples from patients with COVID-19 were stored only at the Chinese Center for Disease Control and Prevention (CDC). In this study, serum samples 81 patients with COVID-19 and 70 vaccine recipients were provided by Shenzhen and Guangzhou CDC; the sample size was hardly calculated for the corresponding COVID-19 and vaccinated populations. According to previous studies (Chen et al., 2020), however, the size of samples was satisfactory for validating the developed assay, such as this QD-LFIA. According to the results of the skewness and kurtosis tests, the nonparametric test (McNemar test) was used for comparison of the different methods. As shown in Supplemental Table 1, there were no difference between the results of IgG titers tested using ELISA or QD-LFIA or between the results of NAb titers tested using pVNT or QD-LFIA.
In conclusion, this assay will be useful for grassroot hospitals and institutions that are in urgent need for cost-effective, rapid, and simple methods for on-site detection of specific IgG or NAb to SARS-CoV-2 infection in humans.
Declaration of competing interests
The authors have no competing interests to declare.
Acknowledgments
Data availability statement
All data associated with this study are available in the main text or the Supplementary Materials, and are available in the Department of Transfusion Medicine, Southern Medical University, Guangzhou, China.
Funding
This work was supported by the grants from the National special funding for COVID-19 prevention and control of China (No. 2020M670013ZX) and the Shenzhen Key Project for Science and Technology (No. JSGG20200519160754005) and the China Postdoctoral Science Foundation (No. 2020M682784).
Author contributions
C.L., Y.K., and B.L. designed study; B.L., J.L., X.T., Z.W., and S.H. performed experiments; J.L., B.L., C.L., and T.L. analyzed data; Y. F. provided materials; and B.L., L.Z., C.L., and Y.K. wrote the paper.
Acknowledgments
The authors thank the Guangzhou and Shenzhen blood centers for providing the healthy blood donor samples used in this study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.04.042.
Appendix. Supplementary materials
References
- Chan JF, Kok KH, Zhu Z, Chu H, To KK, et al. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Zhou M, Dong X, Qu J, Gong F, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng ZJ, Shan J. 2019 Novel coronavirus: where we are and what we know. Infection. 2020;48:155–163. doi: 10.1007/s15010-020-01401-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DKW, Pan Y, Cheng SMS, Hui KPY, Krishnan P, et al. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem. 2020;66:549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford KHD, Eguia R, Dingens AS, Loes AN, Malone KD, et al. Protocol and reagents for pseudotyping lentiviral particles with SARS-CoV-2 spike protein for neutralization assays. Viruses. 2020:12. doi: 10.3390/v12050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagotto G, Yu J, Barouch DH. Approaches and challenges in SARS-CoV-2 vaccine development. Cell Host Microbe. 2020;28:364–370. doi: 10.1016/j.chom.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Bao L, Mao H, Wang L, Xu K, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavor E, Choong YK, Er SY, Sivaraman H, Sivaraman J. Structural basis of SARS-CoV-2 and SARS-CoV antibody interactions. Trends Immunol. 2020;41:1006–1022. doi: 10.1016/j.it.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, et al. An mRNA vaccine against SARS-CoV-2 - preliminary Report [report] N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keech C, Albert G, Cho I, Robertson A, Reed P, et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kang M, Park E, Chung DR, Kim J, et al. A simple and multiplex Loop-Mediated isothermal amplification (LAMP) assay for rapid detection of SARS-CoV. BioChip J. 2019;13:341–351. doi: 10.1007/s13206-019-3404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]
- Lim DR, Kim HR, Park MJ, Chae HG, Ku BK, et al. An improved reverse transcription loop-mediated isothermal amplification assay for sensitive and specific detection of serotype O foot-and-mouth disease virus. J Virol Methods. 2018;260:6–13. doi: 10.1016/j.jviromet.2018.06.017. [DOI] [PubMed] [Google Scholar]
- Logunov DY, Dolzhikova IV, Zubkova OV, Tukhvatullin AI, Shcheblyakov DV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet. 2020;396:887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manenti A, Maggetti M, Casa E, Martinuzzi D, Torelli A, et al. Evaluation of SARS-CoV-2 neutralizing antibodies using a CPE-based colorimetric live virus micro-neutralization assay in human serum samples. J Med Virol. 2020;92:2096–2104. doi: 10.1002/jmv.25986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra S, Tellapragada C, Vandana KE, Mukhopadhyay C. Diagnostic utility of in-house loop-mediated isothermal amplification and real-time PCR targeting virB gene for direct detection of Brucella melitensis from clinical specimens. J Appl Microbiol. 2019;127:230–236. doi: 10.1111/jam.14260. [DOI] [PubMed] [Google Scholar]
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in wuhan, china: challenges for global health governance. JAMA. 2020;323:709–710. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396:1595–1606. doi: 10.1016/S0140-6736(20)32137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CW, Chia WN, Qin X, Liu P, Chen MI, et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- Tanner NA, Zhang Y, Evans TC. Visual detection of isothermal nucleic acid amplification using pH-sensitive dyes. BioTechniques. 2015;58:59–68. doi: 10.2144/000114253. [DOI] [PubMed] [Google Scholar]
- van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham JN, et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Duan K, Zhang Y, Zhao D, Zhang H, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Huang J, Duan C, Liao Q, Shan Z, et al. Low prevalence of antibodies against SARS-CoV-2 among voluntary blood donors in Guangzhou. China. J Med Virol. 2021;93:1743–1747. doi: 10.1002/jmv.26445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZY, Kong WP, Huang Y, Roberts A, Murphy BR, et al. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428:561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Tostanoski LH, Peter L, Mercado NB, McMahan K, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Wu NC, Zhu X, Lee CD, So RTY, et al. A highly conserved cryptic epitope in the receptor binding domains of SARS-CoV-2 and SARS-CoV. Science. 2020;368:630–633. doi: 10.1126/science.abb7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wang L, Deng X, Liang R, Su M, et al. Recent advances in the detection of respiratory virus infection in humans. J Med Virol. 2020;92:408–417. doi: 10.1002/jmv.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu FC, Guan XH, Li YH, Huang JY, Jiang T, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396:479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are available in the main text or the Supplementary Materials, and are available in the Department of Transfusion Medicine, Southern Medical University, Guangzhou, China.