Abstract
Background
Data on patients with COVID-19 who have pulmonary tuberculosis (TB) are limited. In this study, we compared the clinical characteristics of patients with COVID-19/TB and patients with COVID-19 only. In addition, we analyzed the links between the severity of COVID-19 disease and the clinical characteristics of patients with COVID-19/TB.
Methods
We conducted a retrospective, anonymized, cross-sectional study of 111 patients who met inclusion criteria for analysis (75 patients with COVID-19/TB and 36 patients with COVID-19).
Results
Patients in both groups (COVID-19/TB vs COVID-19) mainly suffered from fever (72.0% vs 100%, p < 0.001), fatigue (76.0% vs 94.4%, p = 0.018), chest pain (72.0% vs 36.1%, p < 0.001), followed by cough (60.0% vs 97.2%, p < 0.001) and dyspnea (44.0% vs 63.9%, p = 0.05). In group COVID-19/TB the most frequently reported co-morbidities were chronic liver disease (17 [22.7%]), cardiovascular diseases (25 [33.3%]), and diseases of the nervous system (13 [17.3%]).
Female gender, fever, dyspnea, pulmonary bilateral TB lesion, and three or more co-morbidities have a statistically significant positive effect on the severity of the disease among patients with COVID-19/TB.
Conclusion
It is important to perform rapid molecular testing and computed tomography to correctly distinguish COVID-19 and TB because of the similar clinical characteristics of both diseases. Bilateral pulmonary TB lesion and co-morbidity should be considered risk factors for severe COVID-19.
Keywords: tuberculosis, COVID-19, disease severity, comorbidity, clinical characteristics, co-infection
Introduction
The global spread of COVID-19 may affect the epidemiology and clinical course of other infectious diseases such as tuberculosis (TB). Moreover, TB is epidemic in many parts of the world (Singh et al., 2020). The problems of co-infection can be associated with a decrease in the quality of routine medical care for patients with TB because of forced restrictive measures (McQuaid et al., 2021) and an increased risk of an atypical or more severe course of the disease against the background of COVID-19 (Antonio-Arques et al., 2021; Yang et al., 2020).
At present, there is no clear understanding of the interaction between COVID-19 and TB. Most researchers regard pulmonary TB as a risk factor for the severe course of a new coronavirus infection (Chen et al., 2020; Gupta et al., 2020; Khayat et al., 2021). The reverse negative interaction of these diseases has also been described – for example, an increased risk of a latent infection turning into an active form of TB against the background of COVID-19 because of the depletion of CD4 + T cells (Elziny et al., 2021; Starshinova and Dovgalyuk, 2021; Visca et al., 2021). Several studies have noted an aggravation of the course of both diseases in their mutual existence because of common social, epidemiological, and clinical determinants (Mousquer et al., 2021; Ritacco and Kantor, 2020; Yang and Lu, 2020).
In addition, lung damage because of fibrosis and cavitation because of active TB with superimposed viral infection in patients with COVID-19 lead to further deterioration of already impaired lung function (Gupta et al., 2020).
According to numerous forecasts, an increase in cases of active pulmonary TB is expected soon (Kozińska and Augustynowicz-Kopeć, 2021; Saunders and Evans, 2020; Sumner et al., 2020). Although this can be considered a consequence of the COVID-19 pandemic, it is necessary to continue studying the specifics of interaction between these diseases to improve prevention, diagnosis, and therapy in patients co-infected with COVID-19/TB. Therefore, this study analyzed the clinical characteristics of patients with a combination of TB and COVID-19 and identified factors that determine the severity of COVID-19 in this cohort of patients.
Methods
Study design
We performed an observational, retrospective, two-center cross-sectional study based on the original data collection to compare the clinical characteristics of COVID-19/TB and COVID-19 only and identify the links between the severity of COVID-19 disease and clinical characteristics of patients with COVID-19/TB.
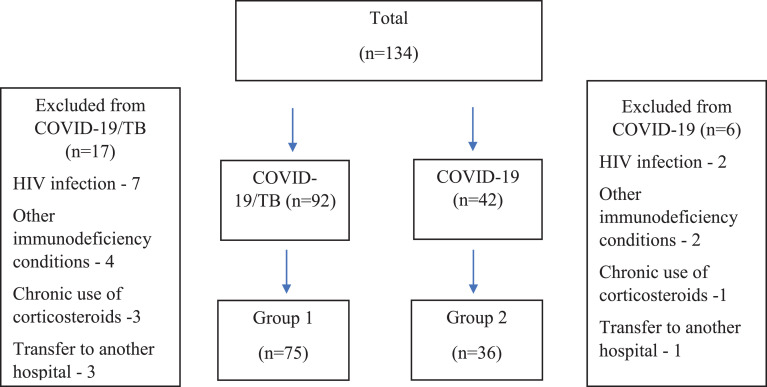
From October 2020 to August 2021, 134 patients were hospitalized for the treatment of either COVID-19 and TB combination (COVID-19/TB; 92 patients) or COVID-19 without TB (COVID-19; 42 patients). Data were collected between October 15, 2021, and December 1, 2021, from TB hospitals in Khabarovsk (patients with COVID-19/TB; COVID-19) and Moscow (patients with COVID-19), Russia.
Both hospitals had departments for newly diagnosed patients with TB and separate diagnostic departments, where patients without established TB were located. During the time at the hospitals, some patients were diagnosed with COVID-19, which allowed us to form the COVID-19/TB and COVID-19 groups. For the COVID-19/TB group, the median duration (IQR) of anti-TB treatment before the diagnosis of COVID-19 was 33 days (14-90). For the COVID-19 group, coming from the differential diagnostic department, the median duration of hospitalization before the diagnosis of COVID-19 was 13 days (6-21). Among patients in whom TB was ruled out, 14 patients had fibrotic changes in the lungs and pleura; eight patients had upper lobe segmental bacterial pneumonia in the stage of incomplete resolution, seven patients had pleurisy of nontuberculous etiology, three patients had sarcoid intrathoracic inflammation of the nodes, one person had echinococcosis.
Data were collected on all available patients, and all patients were tested for HIV infection following the requirements for hospitalization in the Russian Federation. Previously, considered patients did not have TB.
A total of 23 patients (17 from the COVID-19/TB group and six from the COVID-19 group) were excluded from the study. Exclusion criteria included a diagnosis of HIV infection (seven among patients with COVID-19/TB and three among patients with COVID-19), other immunodeficiency conditions (four patients receiving genetically engineered drugs - systemic lupus erythematosus, Crohn's disease; one - skin cancer; one - invasive aspergillosis; one - candidiasis among patients with COVID-19/TB and two patients with lung cancer among patients with COVID-19), chronic use of corticosteroids (two patients with bronchial asthma, one with chronic obstructive pulmonary disease among patients with COVID-19/TB and one among patients with COVID-19), lack of a completed course of treatment for COVID-19 in the institution (three among patients with COVID-19/TB and one among patients with COVID-19 were transferred to another hospital because of the bed load).
Inclusion criteria were: gender, age, hospital admission, one or more co-morbidities, data on the date of onset of the disease, laboratory tests at admission and discharge: erythrocytes, hemoglobin, leukocytes, neutrophils, lymphocytes, aspartate aminotransferase, alanine aminotransferase, creatinine, urea, C-reactive protein, fibrinogen, computed tomography (CT) study based on a visual scale for assessing the extent of the lesion (CT0 = no lesion, CT1 < 25%, CT2 = 25-50%, CT3 = 50-75%, CT4 > 75% involvement).
As a result, 111 patients met the inclusion criteria for our analysis (75 patients with COVID-19/TB and 36 patients with COVID-19). For the summary, see Figure. 1 .
Figure 1.
Patient inclusion flowchart.
All patients were examined for COVID-19 by the polymerase chain reaction (PCR) test in a TB hospital (in the department for patients with TB and the diagnostic department). "AmpliSens® Cov-Bat-FL" RT-PCR was used to detect SARS-CoV-2 RNA in swabs from the nasal/oropharyngeal mucosa (the manufacturer is the Central Research Institute of Epidemiology of Rospotrebnadzor, Russia).
On the basis of the clinical picture, the presence of COVID-19 pneumonia, and test results, the patients were transferred to other departments, and hospitals specialized in the combined pathology of COVID-19/TB or COVID-19 without TB. A total of 105 patients (94.6%) had a laboratory-confirmed SARS-CoV-2 infection, and the remaining patients' diagnosis of COVID-19 was based on clinical and radiological (CT) criteria.
In accordance with the classification of TB in Russia, TB cases were divided into the following forms of newly diagnosed pulmonary TB: Infiltrative TB - 43 [57.3%], Disseminated TB - 15 [20.0%], Focal TB - 2 [2.7%], Tuberculomas - 9 [12.0%], Fibrous-cavernous TB - 6 [8.0%]. Infiltrative TB was characterized by pulmonary infiltrate in one lung (without a cavity or with few cavities). Disseminated TB was characterized by a bilateral pulmonary lesion (without a cavity or with few cavities). Fibrous-cavernous TB was characterized by unilateral or bilateral pulmonary lesions with one or more cavities and with fibrosis.
The Median SpO2 % level at admission was 97% (Q1-Q3 76-98) among patients with COVID-19/TB and 96% (Q1-Q3 90-98) among patients with COVID-19).
Study variables
The following clinical characteristics of the study groups were recorded: the severity of COVID-19 during hospitalization, the presence of confirmed close contact with a patient with COVID-19, the presence of co-morbidities and their number, the presence of symptoms of COVID-19, the results of chest CT, oxygen saturation, laboratory parameters, the presence of complications of COVID-19, ongoing oxygen therapy. The data also included additional explanatory variables such as age, sex, smoking status, temperature, shortness of breath, and pulmonary TB status.
The severity of the condition was assessed following the "Interim Guidelines for Prevention, Diagnosis, and Treatment of the Novel Coronavirus Infection (COVID-19). Version 11 (05/07/2021)" (approved by the Ministry of Health of Russia).
A severe course of COVID-19 was defined through a complex of factors: body temperature > 38°C; respiratory rate > 22/min; shortness of breath during physical exertion; changes in CT (radiography) typical of a viral lesion; SpO2 < 95%; serum CRP > 10 mg/l.
Statistical analysis
Demographic, clinical, and laboratory data were used to compare the groups using the independent samples t-tests. Differences in clinical characteristics between the groups were analyzed using chi-square analysis and Fisher's exact test for categorical variables. The odds ratio (OR) and 95% confidence interval (CI) were calculated. A significance level of 5% (0.05) was used to indicate statistically significant results. The data were analyzed using SPSS software (version 22.0; IBM Corp.).
Based on the obtained data, the factors significant for developing severe COVID-19 in patients with TB were identified. Then, a predictive model was constructed using logistic regression to determine the likelihood of developing a severe COVID-19 condition in patients with TB, depending on the demographic and clinical characteristics of patients.
Results
The demographic and clinical characteristics of the COVID-19 with TB and COVID-19 without TB groups at the start of hospitalization are listed in Table 1 . The aggregate median age was 52 ± 16 (IQR 49–55), 28 (25.2%) were aged 65 years or older, and 71 (64.0%) patients were male. The COVID-19/TB group had younger patients, with P-value (p) < 0.001, and the number of persons aged 65 and older in this group was 14 (18.7%) compared with 14 (38.9%) in the COVID-19 group. The male/female proportions were not statistically different between the two groups (p = 0.664). In the COVID-19/TB group, a history of cigarette smoking and close contact with patients with COVID-19 were more frequently observed (p < 0.001 for both variables). In the COVID-19/TB group, сhronic liver disease (17 [22.7%]), cardiovascular diseases (25 [33.3%]), and diseases of the nervous system (13 [17.3%]) predominated.
Table 1.
Demographic and clinical characteristics of patients with COVID-19 with and without TB (at the start of hospitalization)
| Characteristics | All patientsN = 111 (%) | COVID-19/TBN = 75 (%) | COVID-19N = 36 (%) | p-value |
|---|---|---|---|---|
| Age (years) | 52 ± 16 (49 – 55) | 48±15 (45-52) | 62±16 (56-66) | < 0.001 |
| Gender (male/female) | 71 (64,0)/40(36,0) | 49 (65.3)/ 26 (34.7) | 22 (61.1)/ 14 (38.9) | 0.664 |
| Contact with COVID-19 | 32 (28.8) | 30 (40.0) | 2 (5.6) | < 0.001 |
| Occupation | ||||
| Unemployed | 55 (49,5) | 47 (62,7) | 8 (22,2) | < 0,001 |
| Employed | 26 (23,5) | 10 (13,3) | 16 (44,5) | |
| Retired | 30 (27,0) | 18 (24,0) | 12 (33,3) | |
| Сigarette smoking | 60 (54.1) | 47 (62.7) | 13 (36.1) | 0.009 |
| Alcohol abuse (≥14 drinks per week in men or ≥7 drinks per week in women) | 28 (25.2) | 24 (32.0) | 4 (11.1) | 0.018 |
| Intravenous drug user | 3 (2.7) | 3 (4.0) | 0 (0,0) | - |
| Residence status | ||||
| Big town (>50000 residents) | 88 (79.3) | 56 (74,7) | 32 (88.9) | 0,047 |
| Rural area | 14 (12.6) | 14 (18.7) | 0 (0,0) | |
| Small town (10-49000 residents) | 9(8.1) | 5 (6.7) | 4 (11.1) | |
| Сomorbidities | ||||
| Cardiovascular disease | 61 (55.0) | 25 (33.3) | 23 (63.9) | <0,001 |
| Chronic respiratory disease | 17 (15.3) | 12 (16.0) | 5 (13.9) | 1.000 |
| Chronic liver disease | 19 (17.1) | 17 (22.7) | 2 (5.6) | 0.025 |
| Diabetes mellitus | 20 (18.0) | 8 (10.7) | 12 (33.3) | 0.004 |
| Chronic renal disease | 14 (12.6) | 5 (6.7) | 9 (25.0) | 0.012 |
| Сhronic gastrointestinal tract disease | 11 (9.9) | 5 (6.7) | 6 (16.7) | 0.171 |
| Hypothyroidism | 4 (3.6) | 0 (0.0) | 4 (11.1) | 0.010 |
| Nervous system diseases | 21 (18.9) | 13 (17.3) | 8 (22.2) | 0.538 |
| Co-morbidity, n = 3 and more (%) | 30 (27.0) | 9 (12%) | 21 (58.0%) | < 0.001 |
| Outcome (death/ hospital discharge) | 23 (20.7)/88 (79.3) | 7 (9.3)/68(90.7) | 16 (44.4)/20 (55.6) | < 0.001 |
In the COVID-19 group, co-morbidities were dominated by cardiovascular diseases (23 [63.9%]), diabetes mellitus (12 [33.3%]), and chronic renal disease (9 [25.0%]). The COVID-19 group had more patients with three or more co-morbidities (p < 0.001) – 21 (58%) versus 9 (12%) in the COVID-19/TB group. Overall, 88 (79.3%) were discharged from the hospital, and 23 patients (20.7%) died. Among patients with COVID-19/TB, more cases of recovery (68 [90.7%] vs 20 [55.6%]) were reported. Sixteen (44.4%) and seven (9.3%) patients died without TB and with TB, respectively. This finding can possibly be linked to the age difference between the groups. As it was observed from the previous data on patients with COVID-19, co-morbidities increase the chances of infection, and also the older patients, especially those in long-term care facilities, and patients of any age with serious underlying medical conditions are at a greater risk of getting COVID-19 (CDC, 2020). Perhaps the older age of patients in the COVID-19 group explains the greater number of co-morbidities and death.
Signs and symptoms of the patients with TB/COVID-19 and patients with COVID-19 are listed in Table 2 .
Table 2.
Comparative analysis of signs and symptoms in the COVID-19/TB and COVID-19 groups (n/%)
| Characteristics | All patientsN = 111 (%) | COVID-19/TBN = 75 (%) | COVID-19N = 36 (%) | p-value |
|---|---|---|---|---|
| Severe condition at the start of hospitalization | 74 (66.7) | 38 (50.7) | 36 (100) | < 0.001 |
| Fever | 90 (81.1) | 54 (72.0) | 36 (100.0) | < 0.001 |
| Nasal congestion | 25 (22.5) | 5 (6.7) | 20 (55.6) | < 0.001 |
| Fatigue | 91 (81.9) | 57 (76.0) | 34 (94.4) | 0.018 |
| Dyspnea | 56 (50.5) | 33 (44.0) | 23 (63.9) | 0.050 |
| Cough | 80 (71.7) | 45 (60.0) | 35 (97.2) | < 0.001 |
| Chest pain | 67 (60,4) | 54 (72.0) | 13 (36.1) | < 0.001 |
| Congestion in the chest | 16 (14.4) | 0 (0.0) | 16 (44.4) | < 0.001 |
| Sore throat | 20 (18.0) | 2 (2.7) | 18 (51.4) | < 0.001 |
| Headache | 20 (18.0) | 10 (13.3) | 10 (27.8) | 0.064 |
| General malaise | 41 (36,9) | 18 (24.0) | 23 (63.9) | < 0.001 |
| Olfactory disorders | 20 (18.0) | 1 (1.3) | 19 (52.8) | < 0.001 |
| Taste disorders | 14 (12.6) | 1 (1.3) | 13 (36.1) | < 0.001 |
| CT 3-4 (more than 50% of lungs damaged) | 31 (27.9) | 13 (17.3) | 18 (50.0) | < 0.001 |
| SpO2 % | 96 (93–97) | 96 (93–97) | 95 (94–97) | 0,272 |
| Number of respiratory movements (per minute) | 19 (18–22) | 19 (18–20) | 19 (18–25) | 0,969 |
| Complications | ||||
| DVT/PE (Deep Vein Thrombosis /Pulmonary Embolism) | 14 (12.6) | 1 (1.3) | 13 (36.1) | <0,001 |
| ARDS (Acute Respiratory Distress Syndrome) | 22 (19.8) | 6 (8.0) | 16 (44.4) | <0,001 |
| Respiratory failure | 47 (42.3) | 17 (22.7) | 30 (83.3) | <0,001 |
| Sepsis | 6 (5.4) | 0 (0.0) | 6 (16.7) | - |
| Ventilation and oxygen therapy | ||||
| No ventilation | 78 (70.3) | 62 (82.7) | 16 (44.4) | <0,001 |
| Nasal cannula | 5 (4.5) | 2 (2.7) | 3 (8.3) | <0,001 |
| Oxygen mask | 13 (11.7) | 11 (14.7) | 2 (5.6) | <0,001 |
| Mechanical ventilation | 12 (10.8) | 0 (0.0) | 12 (33.3) | - |
| ECMO | 3 (2.7) | 0 (0.0) | 3 (8.3) | - |
Confirmed and reported cases of COVID-19 have a wide range of symptoms, from mild complaints, such as fever and cough, to more critical cases associated with difficulty in breathing (CDC, 2020). Some of the most common symptoms include cough, fever, chills, shortness of breath, muscle aches, sore throat, unexplained loss of taste or smell, diarrhea, and headache (Maragakis, 2020). In our study, in both groups (COVID-19/TB vs COVID-19), the main complaint on admission to the hospital was fever (72.0% vs 100%, p < 0.001), fatigue (76.0% vs 94.4%, p = 0.018), chest pain (72.0% vs 36.1%, p < 0.001), followed by cough (60.0% vs 97.2%, p < 0.001), and dyspnea (44.0% vs 63.9%, p = 0.05). Other symptoms noted in a minority of patients included sore throat, congestion, headache, nasal congestion, and general malaise. Loss of smell and taste disorders rarely occurred in the COVID-19/TB group (1.3% vs 52.8%, p < 0.001 and 1.3% vs 36.1%, p < 0.001).
Characteristics of pulmonary TB in individuals who fell ill with COVID-19 are listed in Table 3 . All patients (n = 75) had newly diagnosed TB and bacteriologically confirmed disease with pulmonary localization: Mycobacterium tuberculosis was detected in sputum by culture in 44 [59.5%] patients; more than half (57.3%) had pan-susceptible TB. Multidrug-resistant TB occurred in 42 patients [56.0%], of which 10 [23.8%] patients had extensively drug-resistant TB.
Table 3.
Characteristics of pulmonary tuberculosis
| Pulmonary TB | N = 75 (%) |
|---|---|
| TB lesion scale | |
| Less than two segments | 23 (31.5) |
| More than two segments | 50 (68.5) |
| Microbiology | |
| TB microbiology (1 or more tests) | 75 (100.0) |
| Smear microscopy | 36 (48.0) |
| Liquid and solid culture | 44 (59.5) |
| Drug resistance | |
| MDR | 32 (42.7) |
| XDR among MDR | 10 (13.3) |
| Radiology at TB diagnosis | |
| Pulmonary cavitary lesion | 36 (49.3) |
| Disseminated TB with bilateral pulmonary lesion | 15 (20.0) |
| Infiltrated TB without a cavity or with one or more cavities. | 43 (57.3) |
| Tuberculoma | 9 (12.0) |
| Focal TB | 2 (2.7) |
| Fibrous-cavernous TB | 6 (8.0) |
Pulmonary cavitary lesions were found among 36 (49.3%) patients, and involvement of two segments and more in pulmonary TB in 50 (68.5%) patients. Unilateral pulmonary cavitary lesions were found among 21 patients, bilateral pulmonary cavitary lesions in 15 patients, unilateral pulmonary infiltrate (no cavities) in 30 patients, and bilateral pulmonary infiltrate (no cavities) in 7 patients.
Comparing the scale of typical ground-glass opacity on CT in the two groups, it was found that during hospitalization, the scale of pulmonary lesions of more than 50% was recorded less frequently in patients with TB (17.3% vs 50.0%, p < 0.001). The extent of the lesion may have positively affected the incidence of complications in the non-TB group, which had more cases with thromboembolic syndrome (1 [1.3%] vs 13 [36.1%]), acute respiratory distress syndrome (6 [8.0%] vs 16 [44.4%]), and respiratory failure (17 [22.7%] vs 30 [83.3%]). Importantly, there were no cases of sepsis in the COVID-19/TB group versus six (16.7%) in the non-TB group. Oxygen therapy was also used less frequently in COVID-19/TB individuals (62 [82.7%] vs 16 [44.4%]).
Laboratory parameters also differed between the two groups (Table 4 ). Patients with TB had higher platelet counts (marginal errors (ME) are 276 vs 185, p = 0.006), whereas patients without TB had higher levels of abnormalities in urea (p < 0.001), creatinine (p < 0.001), C-reactive protein (p < 0.001), and alanine aminotransferase (p < 0.001).
Table 4.
Laboratory results of patients with COVID-19 with and without TB (at the start of hospitalization)
| Laboratory indicators | COVID-19/TBN = 75 | COVID-19N = 36 | p-value |
|---|---|---|---|
| MEQ₁ – Q₃ | MEQ₁ – Q₃ | ||
| Hemoglobin (g/l) | 114.00 99.50–130.50 |
134.00 17.75–143.00 |
0.001 |
| Erythrocytes (×1012/l) |
3.77 3.43 – 4.30 |
4.31 3.76–4.60 |
0.013 |
| Leukocytes (×109/l) | 6 4–9 |
6 5–8 |
0.848 |
| Platelets (×109/l) | 276 167–366 |
185 151 – 231 |
0.006 |
| Neutrophils % |
62 49-77 |
78 63–87 |
< 0.001 |
| Lymphocytes (%) |
30 13-40 |
20 14-35 |
0.313 |
| Urea | 5 4-7 |
7 5–11 |
< 0.001 |
| Creatinine (µmol/l) | 75 62–86 |
102 87–116 |
< 0.001 |
| ALT (U/l) | 15 9-30 |
28 19–47 |
< 0.001 |
| AST (U/l) | 29 19-45 |
28 20-43 |
0.853 |
| C-reactive protein (mg/l) | 34 6–87 |
47 19–106 |
0.171 |
| Fibrinogen (g/l) | 4 3–7 |
5 4–6 |
0.616 |
We analyzed the need for oxygen (Table 2). A total of 29.7% of all patients required oxygenation. At the same time, the need for oxygen therapy in patients with COVID-19/TB was significantly less than in the group of patients without TB (62 [82.7] vs 16 [44.4], p < 0.001).
The features of the severity of patients in both groups were analyzed (Table 5 ). The median age in the COVID-19/TB group was 48 years (Q1–Q3: 39–66) and 62 in the COVID-19 group (Q1–Q3: 55–68). The complication rate comparisons are: deep vein thrombosis/pulmonary embolism - 2.6% in the COVID-19/TB group versus 36.1% in the COVID-19 group (p < 0.001); respiratory failure - 44.7% versus 83.3% (p < 0.001); acute respiratory distress syndrome - 15.8% versus 44.4% (p = 0.007). No sepsis was recorded in the COVID-19/TB group (0%), compared with 16.7% in the COVID-19 group (p = 0.011). In terms of the complication development, in the group of patients with COVID-19/TB, the development of deep vein thrombosis/pulmonary embolism was 20.913 times less common (OR = 0.048; 95% CI: 0.006–0.390), the development of acute respiratory distress syndrome was 4.267 times lower (OR = 0.234; 95% CI: 0.079 - 0.698), rheumatoid factor - 6.176 times lower (OR = 0.162; 95% CI: 0.055 - 0.479).
Table 5.
Clinical characteristics of the patients with a severe condition of COVID-19
| Characteristics | COVID-19/TBN = 38 (%) | COVID-19N = 36 (%) | p-value |
|---|---|---|---|
| Gender (male/female) | 21 (55,3)/ 17 (44,7) | 22 (61,1)/ 14 (38,9) | 0,610 |
| Contact with COVID-19 | 11 (28,9) | 2 (5,6) | 0,013 |
| Сigarette smoking | 22 (57,9) | 13 (36,1) | 0,061 |
| Signs and Symptoms | |||
| Fever > 38°C | 32 (84,2) | 36 (100,0) | 0,025 |
| Nasal congestion | 3 (7,9) | 20 (55,6) | < 0,001 |
| Fatigue | 12 (31,6) | 23 (63,9) | 0,005 |
| Dyspnea during physical exertion | 22 (57,9) | 23 (63,9) | 0,598 |
| Cough | 27 (71,1) | 35 (97,2) | 0,003 |
| Chest pain | 6 (15,8) | 13 (36,1) | 0,063 |
| Congestion in the chest | 0 (0,0) | 16 (44,4) | < 0,001 |
| Sore throat | 1 (2,6) | 18 (51,4) | < 0,001 |
| Headache | 4 (10,5) | 10 (27,8) | 0,077 |
| Taste disorders | 1 (2,6) | 13 (36,1) | < 0,001 |
| Olfactory disorders | 1 (2,6) | 19 (52,8) | < 0,001 |
| General malaise | 32 (84,2) | 34 (94,4) | 0,263 |
| Rhinorrhea | 0 (0,0) | 1 (2,8) | 0,486 |
| Complications | |||
| DVT/PE (Deep Vein Thrombosis /Pulmonary Embolism) | 1 (2,6) | 13 (36,1) | < 0,001 |
| ARDS (Acute Respiratory Distress Syndrome) | 6 (15,8) | 16 (44,4) | 0,007 |
| Respiratory failure | 17 (44,7) | 30 (83,3) | < 0,001 |
| Sepsis | 0 (0,0) | 6 (16,7) | 0,011 |
| Respiration rate | 23 (22–28) | 22 (20–26) | 0,645 |
| SpO2 | 95 (91–97) | 95 (94–97) | 0,582 |
| Serum CRP | 63 (22–133) | 47 (19–106) | 0,523 |
| CT | |||
| КТ1 | 8 (21.0) | 8 (22.3) | < 0,001 |
| КТ2 | 17 (44,7) | 10 (27,7) | |
| КТ3 | 13(34,3) | 7 (19,5) | |
| КТ4 | 0 (0.0) | 11 (30.5) |
In the COVID-19/TB group, 26 patients (52.5%) had a severity of COVID-19 with more than two segments of the TB process (50 patients). In patients with TB with a pulmonary cavitary lesion, the severity of the illness was observed in 15 (41.7%) cases. Among smokers (46 patients), 21 (45.7%) also had a severity of the illness. It was found that in a subgroup of patients with COVID-19/TB and a serious condition during hospitalization for COVID-19, the levels of C-reactive protein and platelets values were significantly higher (on average) than in the COVID-19 group, where all patients had a severity – (ME 74.9 mg/l [13-213] vs ME 47 mg/l [19-106], p < 0.001) and (226×109/l [167-259] vs 185×109/l [151-231], p = 0.006), respectively.
The severity of patients with COVID-19 without TB may be explained by the older age of the patients and the higher rate of co-morbidities (particularly cardiovascular disease).
In severe cases of COVID-19, oxygen therapy methods were used: nasal cannula - 2.6% in the COVID-19/TB group vs 8.3% in the COVID-19 group; oxygen mask - 28.9% vs 5.6%, mechanical ventilation and extracorporeal membrane oxygenation did not occur in the COVID-19/TB group although being used in the COVID-19 group (33.3% and 8.3%, respectively); oxygen therapy was performed in 31.6% in the COVID-19/TB group vs 53.6% in the COVID-19 group (p < 0.001).
The univariate analysis of the COVID-19/TB data revealed the following statistically significant factors influencing the severity of the disease (Table 6 ). The chances of developing a severe course were: 2.64 times higher in female patients (95% CI: 1.01-7.12) (p = 0.05); 3.52 times higher in patients with fever (95% CI: 1.18-10.51) (p = 0.020); 3.10 times higher in patients with dyspnea (95% CI: 1.19-8.09) (p = 0.019); 9.931 times higher in patients having three or more co-morbidities (95 % CI: 1.17-84.04) (p = 0.013). Statistically insignificant factors were smoking, 0.63 (95% CI: 0.244-1.624) (p = 0.338), disseminated TB, 2.37 (95% CI: 0.722-7.787) (p = 0.148), and patient age 1.02 (95% CI: 0.99-1.06) (p = 0.187).
Table 6.
Logistic regression analysis to assess the relationship between demographic, clinical characteristics, and severity of COVID-19 in the COVID-19/TB group (n = 75)
| Predictor | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| СOR (95% CI) | p-value | АOR (95% CI) | p-value | |
| Age | 1.02 (0.99-1.06) | 0.187 | 1.05 (0.99-1.11) | 0.071 |
| Female | 2. 64(1.01-7.12) | 0.05 | 56.54 (4.35-735.59) | 0.002 |
| Fever | 3.52 (1.18-10.51) | 0.020 | 18.87 (3.21-111.09) | 0.001 |
| Dyspnea | 3.10 (1.19-8.09) | 0.019 | 9.21 (1.90-44.45) | 0.006 |
| Disseminated TB | 2.37 (0.72-7.79) | 0.148 | 5.28 (1.09-25.50) | 0.038 |
| Three or more co-morbidities | 9.931 (1.17-84.04) | 0.013 | 253.55 (2.52-25489.19) | 0.019 |
| Smoking status | 0.63 (0.24-1.62) | 0.338 | 10.90 (1.15-103.33) | 0.37 |
| Diabetes mellitus | 6.97 (0.79-61.07) | 0.047 | ||
We included these factors in a multivariate logistic regression and added three more control variables (disseminated TB, smoking, and age). In the pathogenesis of disseminated TB, small vessels are affected, which may be important in the development of lung damage in COVID-19. Smoking is an important factor influencing the development of TB in an individual. Age was selected, as many studies had shown its effect on COVID-19 outcomes.
The final multivariable logistic regression model included female gender, fever, dyspnea, disseminated TB, three or more co-morbidities, and smoking status as independent contributors to severity. Table 6 lists the connection of each of the parameters.
The odds of a severe course increased 56.54 times (95% CI: 4.35-735.59) (p = 0.002) in female patients, among patients with fever by 18.87 times (95% CI: 3.21- 111.09) (p = 0.001), dyspnea by 9.21 times (95% CI: 1.90-44.45) (p = 0.006), disseminated TB by 5.28 times (95% CI: 1.09 -25.50) (p = 0.038), the presence of more than three co-morbidities - by 253.55 times (95% CI: 2.52-25489.19) (p = 0.019). Age and smoking were not significant, but in multivariate analysis, they influenced other factors, so we left them in the model, whereas diabetes mellitus was not included.
The predictive model was statistically significant (p <0.001) based on the F-test. In accordance with the coefficient of determination R2 of Nigel Kirk, the predictors included in its composition make up only 55.8% of the factors influencing the dependent variable. The sensitivity of the developed model (1) was 78.4% (29 correct predictions of 37 cases of severe COVID-19/TB), and the specificity was 77.8% (28 correct predictions of 36 cases of no severity in patients with COVID-19 with a history of TB).
Discussion
As a result of the comparative analysis, several findings have arisen. First, patients with TB appear to have a less severe course of COVID-19 and a lower risk of mortality regardless of the form of TB, even with active pulmonary TB, except for pulmonary bilateral disseminated TB. The data obtained are consistent with the results of meta-analysis (Gao et al., 2021), indicating that TB is associated with an increased risk of mortality in patients with COVID-19 (OR = 1.40, 95% CI: 0.10 to 18.93, P = .80; I2 = 31%). In our study, the small number of deaths did not allow us to identify factors influencing COVID-19 mortality in patients with TB.
At the same time, the development of a severe course is more often observed in persons with a lung lesion of more than two segments, with cavities in the lungs, and smokers. Further analysis is needed to confirm this result in the future.
Identification of the relationship between TB and the severity and mortality from COVID-19 is crucial for the development of measures for the prevention and timely diagnosis of COVID-19 in patients with TB. Our study showed that the risk of developing severe COVID-19 in patients with TB was associated with factors such as female gender, smoking, fever, dyspnea, disseminated TB, having three or more co-morbidities, and patient age. When the structure of the lung tissue is affected by TB, resistance to additional infectious agents, such as viruses decreases. In addition, it is known that TB is a secondary immunodeficiency. All this can be the basis for a more severe course of the newly emerged disease. The data obtained in the study suggest that strategies should be developed to reduce the risk of severe COVID-19 in patients with TB.
The second result suggests that the clinical diagnosis of COVID-19 in patients with TB should not be based on taste and smell disorders, which are rare symptoms in patients with TB. Among 538 patients in the global cohort study, they occurred in only 56 (10.4%) and 48 (8.9%) of patients (TB/COVID-19 Global Study Group 2021), compared with 1.3% and 1.3% in our cohort, respectively. These symptoms are significantly more important for the diagnosis of COVID-19 in the absence of TB. At the same time, fever and cough are important for the clinical diagnosis of COVID-19 in patients with TB. The global study by the TB/COVID-19 Global Study Group (2021) found that the dominant symptoms of COVID-19 in patients with TB were fever (386 of 538, 71.7%) and dry cough (311 of 538, 57.8%), compared with our observed 72.0% and 60.0%. Most patients in the COVID-19/TB cohort had symptoms similar to those of patients with COVID-19, making diagnosis difficult.
Thus, during the COVID-19 pandemic, patients with TB should be screened regularly to prevent the spread of COVID-19/TB co-infection. At the same time, it should be remembered that COVID-19 has a rapid clinical manifestation, whereas TB is time-consuming, so the onset of its symptoms takes longer. This feature can help differentiate between the two diseases.
The third result is that patients with TB and co-morbidities appear to be at increased risk of developing COVID-19 and having an adverse disease course. The significance of co-morbidity for mortality and the development of a serious condition in patients with TB with the addition of COVID-19 is widely discussed in the literature. In particular, it has been shown that old age, diabetes, and respiratory diseases are the main factors increasing mortality in patients with COVID-19/TB co-infection (Stochino et al., 2020). In the global study by the TB/COVID-19 Global Study Group (2021), the univariate analysis of mortality showed the statistical significance of having more than one co-morbidity, type 2 diabetes mellitus, cardiovascular disease, chronic respiratory and chronic renal disease. In our study, 69.3% of patients had at least one other disease. At the same time, in patients with COVID-19/TB and patients without TB, the main co-morbidity was cardiovascular disease. Assessing the significance of other co-morbidities for the development of severe COVID-19 requires caution and a larger observation group.
Our study had some limitations. Firstly, our analysis included all cases of COVID-19/TB from only TB hospitals in two regions of Russia, including cases of COVID-19. In other types of hospitals or regions of the country, different results may be obtained. Secondly, although the control group of patients without TB was recruited randomly, its size implies that the results should be interpreted with caution. As more data becomes available, it will be important to identify factors that influence mortality and complications in patients with TB diagnosed with COVID-19. Thirdly, in the retrospective design of the study, analysis of symptoms was limited because not all symptoms could be indicated in the paper history of the disease. In addition, there was a significant age difference between the two groups.
Conclusion
In this study, we compared demographic, clinical, CT, and laboratory parameters of patients with COVID-19/TB and patients without TB. Our results suggest that, in general, patients with TB share the standard clinical signs and manifestations, such as COVID-19, at a younger age and have a milder course in the presence of active TB. However, patients with TB also show weakness and fatigue, with virtually no loss of taste or smell. Because of similar clinical characteristics, diagnostic difficulties arise, which may contribute to the development of severe COVID-19. The data analysis shows the importance of rapid molecular testing and CT to diagnose COVID-19 (the ground-glass opacities).
At the same time, some evidence indicates that TB may contribute to the severe course of COVID-19. Thus, in the COVID-19/TB cohort, about half of the patients with pulmonary lesions greater than two segments, with cavities, and smokers had a severe course of the disease. Our results suggest that female patients with TB are more likely to have severe COVID-19. In contrast, the main indicators of severe COVID-19 likelihood in patients with TB are fever, dyspnea, disseminated TB with bilateral pulmonary TB lesion, and the presence of three or more co-morbidities. To better understand the relationship between TB and COVID-19, larger multicenter studies are recommended to determine the set of factors influencing mortality and severity in the COVID-19/TB cohort.
Transparency declaration
This article is part of a supplement entitled Commemorating World Tuberculosis Day March 24th, 2022: “Invest to End TB. Save Lives” published with support from an unrestricted educational grant from QIAGEN Sciences Inc.
Acknowledgments
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical Approval
The study was approved by the local ethics committee of the National Medical Research Center for Phthisiopulmonology and Infectious Diseases by decision No. 61/4 of November 2021, which approved the retrospective collection of data from patient records with anonymization of personal data.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.04.041.
Appendix. Supplementary materials
References
- Antonio-Arques V, Franch-Nadal J, Caylà JA. Diabetes and tuberculosis: A syndemic complicated by COVID-19. Med Clin (Engl Ed) 2021;157:288–293. doi: 10.1016/j.medcle.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (United States) Centers for Disease Control and Prevention; 2020. Coronavirus (COVID-19): symptoms of coronavirus.https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html accessed April 18. [Google Scholar]
- Chen Y, Wang Y, Fleming J, Yu Y, Gu Y, et al. Active or latent tuberculosis increases susceptibility to COVID-19 and disease severity. MedRxiv Preprint posted online March 16, 2020. doi: https://doi.org/10.1101/2020.03.10.20033795.
- Elziny MM, Ghazy A, Elfert KA, Aboukamar M. Case report: development of miliary pulmonary tuberculosis in a patient with peritoneal tuberculosis after COVID-19 upper respiratory tract infection. Am J Trop Med Hyg. 2021;104:1792–1795. doi: 10.4269/ajtmh.20-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Liu M, Chen Y, Shi S, Geng J, Tian J. Association between tuberculosis and COVID-19 severity and mortality: a rapid systematic review and meta-analysis. J Med Virol. 2021;93:194–196. doi: 10.1002/jmv.26311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Ish P, Gupta A, Malhotra N, Caminero JA, et al. A profile of a retrospective cohort of 22 patients with COVID-19 and active/treated tuberculosis. Eur Respir J. 2020;56 doi: 10.1183/13993003.03408-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayat M, Fan H, Vali Y. COVID-19 promoting the development of active tuberculosis in a patient with latent tuberculosis infection: a case report. Respir Med Case Rep. 2021;32 doi: 10.1016/j.rmcr.2021.101344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozińska M, Augustynowicz-Kopeć E. COVID-19 in patients with active tuberculosis. Diagnostics (Basel) 2021;11 doi: 10.3390/diagnostics11101768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragakis LL. Coronavirus symptoms: frequently asked questions. Johns Hopkins Medicine. 2020 https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/coronavirus-symptoms-frequently-asked-questions accessed April 18, 2020. [Google Scholar]
- McQuaid CF, Vassall A, Cohen T, Fiekert K, White RG. The impact of COVID-19 on TB: a review of the data. Int J Tuberc Lung Dis. 2021;25:436–446. doi: 10.5588/ijtld.21.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousquer GT, Peres A, Fiegenbaum M. Pathology of TB/COVID-19 Co-Infection: the phantom menace. Tuberculosis (Edinb) 2021;126 doi: 10.1016/j.tube.2020.102020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritacco V, Kantor IN. Tuberculosis and COVID-19: a dangerous relationship. Medicina (B Aires) 2020;80(Suppl. 6):117–118. [PubMed] [Google Scholar]
- Saunders MJ, Evans CA. COVID-19, tuberculosis and poverty: preventing a perfect storm. Eur Respir J. 2020;56 doi: 10.1183/13993003.01348-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Prasad R, Gupta A, Das K, Gupta N. Severe acute respiratory syndrome coronavirus-2 and pulmonary tuberculosis: convergence can be fatal. Monaldi Arch Chest Dis. 2020:90. doi: 10.4081/monaldi.2020.1368. [DOI] [PubMed] [Google Scholar]
- Starshinova AA, Dovgalyuk IF. Tuberculosis in the structure of COVID-19 patients comorbidities. Pac. Med J. 2021;1:10–14. In Russian. [Google Scholar]
- Stochino C, Villa S, Zucchi P, Parravicini P, Gori A, Raviglione MC. Clinical characteristics of COVID-19 and active tuberculosis co-infection in an Italian reference hospital. Eur Respir J. 2020;56 doi: 10.1183/13993003.01708-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner A, Hoy C, Ortiz-Juarez E. Estimates of the impact of COVID-19 on global poverty. WIDER working paper; 2020.
- TB/COVID-19 Global Study Group Tuberculosis and COVID-19 co-infection: description of the global cohort. Eur Respir J. 2021;59(2) doi: 10.1183/13993003.02538-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visca D, Ong CWM, Tiberi S, Centis R, D'Ambrosio L, et al. Tuberculosis and COVID-19 interaction: a review of biological, clinical and public health effects. Pulmonology. 2021;27:151–165. doi: 10.1016/j.pulmoe.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Lu S. COVID-19 and tuberculosis. J Transl Int Med. 2020;8:59–65. doi: 10.2478/jtim-2020-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zheng Y, Gou X, Pu K, Chen Z, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.