Abstract
Patients suffering from autoimmune hepatitis, a chronic immune-mediated liver disease with an incidence of 0.9 to 2 per 100,000 population per year in Europe, are considered to have a particularly increased risk for coronavirus disease 2019 (Covid-19)-associated hospitalization and death.1,2 Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) vaccination provides an essential tool to reduce morbidity and mortality in this cohort. However, a large multicenter study in China has shown a lower immunogenic response to inactivated whole-virion SARS-CoV-2 vaccines of chronic liver disease patients in comparison with the healthy population.3 Furthermore, reports from inflammatory bowel diseases or rheumatic disorders showed a reduced serologic response in patients taking glucocorticoids or thiopurine.4,5 The decrease in vaccine-induced antibodies over time, as well as the emergence of variants of concern, led to the recommendation of an additional vaccination in immunocompromised patients.
Abbreviations used in this paper: AIH, autoimmune hepatitis; BAU/mL, binding antibody units per milliliter; Covid-19, coronavirus disease 2019; HC, healthy control; ISO, international organization for standartization; mRNA, messenger RNA; S, spike; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2
Patients suffering from autoimmune hepatitis, a chronic immune-mediated liver disease with an incidence of 0.9 to 2 per 100,000 population per year in Europe, are considered to have a particularly increased risk for coronavirus disease 2019 (Covid-19)-associated hospitalization and death.1 , 2 Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) vaccination provides an essential tool to reduce morbidity and mortality in this cohort. However, a large multicenter study in China has shown a lower immunogenic response to inactivated whole-virion SARS-CoV-2 vaccines of chronic liver disease patients in comparison with the healthy population.3 Furthermore, reports from inflammatory bowel diseases or rheumatic disorders showed a reduced serologic response in patients taking glucocorticoids or thiopurine.4 , 5 The decrease in vaccine-induced antibodies over time, as well as the emergence of variants of concern, led to the recommendation of an additional vaccination in immunocompromised patients.
In this prospective study, performed at the Medical University of Vienna (European Union Drug Regulating Authorities Clinical Trials number 2021-000291-11), we evaluated vaccine response in 12 patients with autoimmune hepatitis and/or primary biliary cholangitis (AIH) and compared it with a group of 24 age- and gender-matched healthy controls. All participants were primarily vaccinated twice with a SARS-CoV-2 messenger RNA (mRNA) vaccine (BNT162b2; BioNTech/Pfizer, Mainz, Germany or mRNA-1273; Moderna, Cambridge, MA). Eight AIH patients and 24 healthy controls (HCs) received a third dose of Covid-19 mRNA vaccine after the 6-month follow-up evaluation. Previous SARS-CoV-2 infection was determined by measuring nucleocapsid-specific antibodies before vaccination and at the 6-month follow-up evaluation. Antibodies to the receptor-binding domain of the viral spike (S) protein were measured before the vaccination, 2 to 3 weeks after the first vaccine dose, 4 to 6 weeks and 5 to 7 months after the second dose, and 4 to 6 weeks after the third dose.
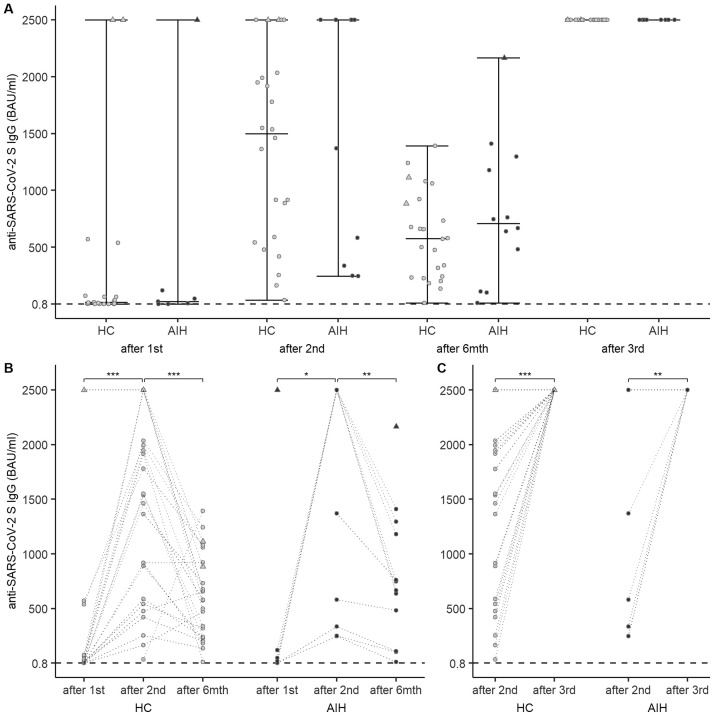
Baseline characteristics, administered vaccines, and treatments of AIH patients are listed in Supplementary Table 1. The median time between therapy onset and the first vaccine dose was 2 years. Humoral responses were analyzed in 26 participants after the first immunization and 35 participants after the second immunization. After the first vaccine dose, 6 of 7 AIH patients and all of the 19 HCs seroconverted. After the second vaccine dose, all AIH patients seroconverted. No significant difference was found in the median SARS-CoV-2 S antibody levels binding antibody units per milliliter (BAU/mL) between AIH patients and HCs after the first (21.9 [Q1–Q3 = 3.61–83.05] vs 14.2 [Q1–Q3 = 1.33–65.03]; P = .890) and second (2500 [Q1–Q3 = 459–2500] vs 1499 [Q1–Q3 = 577.250–2002]; P = .459) immunizations (Figure 1 A). Antibody levels increased significantly after the second vaccine dose in AIH patients (P = .036) and HCs (P < .001) (Figure 1 B). At the 6-month follow-up evaluation, antibody levels decreased significantly in AIH patients (707 [Q1–Q3 = 388.75–1208.25]; P = .006; median decrease, 1089 [Q1–Q3 = 232.1–153]) and HCs (577 [Q1–Q3 = 240–893.25]; P < .001; median decrease, 906 [Q1–Q3 = 653.75–1490]) (Figure 1 B). Eight AIH patients and 24 HCs received a third vaccine dose. Antibody levels increased significantly in AIH patients (2500 [Q1–Q3 = 2500–2500]; P = .059) and HCs (2500 [Q1–Q3 = 2500–2500]; P < .001) (Figure 1 C).
Figure 1.
(A) Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) spike (S) antibody levels binding antibody units per milliliter (BAU/mL) in autoimmune hepatitis (AIH) patients and healthy controls (HCs) after the first and second dose of messenger RNA (mRNA) vaccine, at the 6-month follow-up evaluation, and after the third vaccine dose. (B) Change in SARS-CoV-2 S antibody levels over time. (C) Change in SARS-CoV-2 S antibody levels from the second to the third vaccine dose. Circles indicate individual antibody responses. Participants with antibodies against the SARS-CoV-2 nucleocapsid at baseline are represented as triangles. The dashed horizontal line indicates the cut-off value for seroconversion at 0.8 BAU/mL. Darker lines connecting circles indicate overlapping points.
At baseline, 1 AIH patient and 2 HCs had a history of COVID-19 and showed antibodies against the SARS-CoV-2 nucleocapsid. No breakthrough infections were reported in the HC group. Two breakthrough infections occurred in the AIH group. One in a patient who had Covid-19 before the first vaccination and received 2 vaccine doses (anti–SARS-CoV-2 S IgG 2166 BAU/mL at the last visit before the infection), the other one in a patient after 3 vaccinations (anti–SARS-CoV-2 S IgG ≥ 2500 BAU/mL at the last visit before the infection). Both breakthrough infections occurred between January and February 2022 during the BA.1 omicron wave.
Overall, we show a robust SARS-CoV-2 vaccine response in patients suffering from AIH, comparable with the response in age- and sex-matched HCs. Over the course of 6 months, AIH patients and controls showed a similar decay of antibody levels, which were restored to levels greater than 2500 BAU/mL in all patients after a third vaccination.
Initial treatment for AIH comprises a glucocorticoid, with or without azathioprine or 6-mercaptopurine, dependent on disease severity and the individual risk of glucocorticoid adverse events.6 A recent analysis of patients with AIH showed seroconversion rates of 97%, but significantly lower antibody titers in comparison with HCs. Interestingly, AIH patients without immunosuppression had comparably low antibody levels with AIH patients under immunosuppression.7 Each patient in this cohort, despite all but 1 being on therapy, showed seroconversion. In contrast to the analysis of Duengelhoef et al,7 we did not find a significant difference in median antibody levels of the AIH patients in comparison with HCs. Feng et al8 showed that SARS-CoV-2 antibody levels greater than 264 BAU/mL correlated with an 80% protection against symptomatic disease mainly caused by the alpha (B.1.1.7) variant. However, new surges of cases first linked to the delta and then superseded by the omicron variant have led to attenuated vaccine effectiveness. In this cohort, we report 2 breakthrough infections during the BA.1 omicron wave. Omicron is known for its increased vaccine-induced humoral immunity evasion properties in comparison with preceding variants.9 Both patients with breakthrough infection had a mild course of illness.
As expected, AIH patients and controls showed a significant decrease in SARS-CoV-2 antibody levels over a period of 6 months. Immunosuppressive treatment in our group of AIH patients was not associated with a diminished humoral response after the third dose of mRNA vaccines. The necessity or timing of an additional booster dose, which has been recommended for a variety of patients with immunosuppressive medication, nevertheless remains to be elucidated in our AIH patients.
Of note, several case reports were published reporting an increased risk of developing immune-mediated AIH secondary to SARS-CoV-2 vaccination. However, a clear correlation between vaccination and AIH development is hard to establish.10 In our cohort, none of the patients reported a worsening of AIH after vaccination.
Key limitations of the study were the small sample size as well as the short follow-up period after the third vaccination. However, our results do not suggest any difference in SARS-CoV-2 antibody kinetics of AIH patients when compared with healthy adults. Therefore, the generally applicable vaccination schedules should be followed in this group of patients.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2022.04.006.
Anti–Severe Acute Respiratory Syndrome Coronavirus Type 2 Testing
The Elecsys anti–SARS-CoV-2 S immunoassay (Roche Diagnostics, Mannheim, Germany) was used to quantify antibodies to the receptor-binding domain of the viral S protein.2 The quantitation range is between 0.4 and 2500.0 BAU/mL. Values greater than 0.8 BAU/mL are considered positive. Results below the lower level of quantification were defined as 0.2 BAU/mL to allow for calculations. Previous SARS-CoV-2 infection was determined by measuring nucleocapsid-specific antibodies with the qualitative Elecsys anti–SARS-CoV-2 assay (Roche Diagnostics, Mannheim, Germany).3 Antibody tests were performed on a Cobas e801 analyzer (Roche Diagnostics, Rotkreuz, Switzerland) at the Department of Laboratory Medicine, Medical University of Vienna (certified according to the international organization for standartization [ISO] 9001:2015 and accredited according to ISO 15189:2012). Ethical approval for this study was granted by the Ethics Committee of the Medical University of Vienna (1291/2021).
Statistical Analysis
The control group was matched to the AIH group in a 2:1 ratio by age, sex, and anti–SARS-CoV-2 nucleocapsid-specific antibodies before the first vaccination using propensity score matching. Controls with 2 or more missing anti–SARS-CoV-2 S values were excluded before control matching. According to the distribution, continuous variables are represented as medians with 25% quartile (Q1) and 75% quartile (Q3). Differences in unpaired groups were assessed using the Wilcoxon rank-sum test. Differences between paired groups were assessed using the Wilcoxon matched-pair rank-sum test. Categoric variables are represented as number and rate in percentage. Statistical analysis was performed using R 4.1.1 (R Core Team (2021), Vienna, Austria). The R packages ggplot2, ggpubr, and viridis were used for graphic representation of the data.
Supplementary Table 1.
Demographics, Vaccines, and Treatments
| AIH (n = 12) | HC (n = 24) | |
|---|---|---|
| Age, median, y (Q1–Q3) | 53.5 (45.5–57.3) | 49 (39.8–57) |
| Sex, n (%) | ||
| Female | 8 (67) | 16 (67) |
| Male | 4 (33) | 8 (33) |
| Vaccine first dose, n (%) | ||
| BNT162b2 | 10 (83) | 24 (100) |
| mRNA-1273 | 2 (17) | 0 |
| Vaccine second dose, n (%) | ||
| BNT162b2 | 10 (83) | 24 (100) |
| mRNA-1273 | 2 (17) | 0 |
| Vaccine third dose, n (%) | ||
| BNT162b2 | 8/8 | 21/24 (88) |
| mRNA-1273 | 0/8 | 3/24 (13) |
| Anti–SARS-CoV-2 nucleocapsid positive, n (%) | ||
| Baseline | 1 (8) | 2 (8) |
| Six-month follow-up evaluation | 0 | 1 (4) |
| Therapy, n (%) | ||
| Azathioprine | 9 | NA |
| Monotherapy | 6 | |
| + Prednisone | 3 | |
| MMF | 2 | NA |
| Monotherapy | 1 | |
| + Prednisone | 1 | |
| Rituximab | 1 | NA |
AIH, autoimmune hepatitis; HC, healthy control; MMF, mycophenolate mofetil; mRNA, messenger RNA; NA, not applicable; Q, quartile; SARS-CoV-2, severe acute respiratory syndrome coronavirus type 2.
Supplementary Materials and Methods
Patients and Sample Collection
The study is part of a prospective cohort study performed at the Medical University of Vienna (Vienna, Austria) (Eudra CT number 2021-000291-11).
In this substudy, 12 patients with autoimmune hepatitis and/or primary biliary cholangitis prospectively were enrolled from the Clinical Division of Gastroenterology and Hepatology, Medical University of Vienna. As a control group, 24 age- and sex-matched participants were selected from the original cohort of HCs. All participants primarily were vaccinated twice with a SARS-CoV-2 mRNA vaccine (BNT162b2; BioNTech/Pfizer or mRNA-1273; Moderna). Eight AIH patients and 24 HCs received a third dose of Covid-19 mRNA vaccine after the 6-month follow-up period (median, 213.5 d [183–246.3 d] after the second immunization). Antibodies against the SARS-CoV-2 nucleocapsid protein were determined before vaccination and at the 6-month follow-up evaluation. Antibodies against the SARS-CoV-2 receptor-binding domain were measured before the vaccination, 2 to 3 weeks (median, 17.5 d [14–20.3 d]) after the first vaccine dose, 4 to 6 weeks (median, 28.5 d [26–30 d]) and 5 to 7 months (median, 195 d [165–216 d]) after the second dose, and 4 to 6 weeks (median, 31 d [28–35.5 d]) after the third dose. Serum samples for antibody tests were stored at the Biobank of the Medical University of Vienna (MedUni Wien Biobank), a centralized facility for preparing and storing biomaterials with certified quality management (international organization for standartization [ISO] 9001:2015).1 Ethical approval for this study was granted by the Ethics Committee of the Medical University of Vienna (1291/2021). All authors had access to the study data and approved the final manuscript.
References
- 1.Kim D., et al. Clin Gastroenterol Hepatol. 2021;19:1469–1479.e19. doi: 10.1016/j.cgh.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Efe C., et al. Liver Int. 2021;42:607–614. [Google Scholar]
- 3.Ai J., et al. Clin Gastroenterol Hepatol. 2022;20:1516–1524. doi: 10.1016/j.cgh.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deepak P., et al. Ann Intern Med. 2021;174:1572–1585. doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy N.A., et al. Gut. 2021;70:865–875. doi: 10.1136/gutjnl-2021-324388. [DOI] [PubMed] [Google Scholar]
- 6.Lohse A.W., et al. J Hepatol. 2015;63:971–1004. doi: 10.1016/j.jhep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 7.Duengelhoef P., et al. United Eur Gastroenterol J. 2022;10:319–329. doi: 10.1002/ueg2.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng S., et al. Nat Med. 2021:1–9. [Google Scholar]
- 9.Garcia-Beltran W.F., et al. Cell. 2021;184:2372. doi: 10.1016/j.cell.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mungmunpuntipantip R. Hepatology. 2021 [Google Scholar]
References
- 1.Haslacher H., et al. Biopreservation Biobanking. 2018;16:477–482. doi: 10.1089/bio.2018.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higgins V., et al. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.03149-20. e03149–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simon D., et al. Ann Rheum Dis. 2021;80:1312–1316. doi: 10.1136/annrheumdis-2021-220461. [DOI] [PMC free article] [PubMed] [Google Scholar]