Abstract
Given the role of comorbid conditions in the pathophysiology of HFpEF, we aimed to identify and rank the importance of comorbid conditions associated with post-hospitalization outcomes of older adults hospitalized for HFpEF. We examined data from 4605 Medicare beneficiaries hospitalized in 2007–2014 for HFpEF based on ICD-9-CM codes for acute diastolic heart failure (428.31 or 428.33). To identify characteristics with high importance for prediction of mortality, all-cause rehospitalization, rehospitalization for heart failure, and composite outcome of mortality or all-cause rehospitalization up to 1 year, we developed boosted decision tree ensembles for each outcome, separately. For interpretability, we estimated hazard ratios (HRs) and 95% confidence intervals (CI) using Cox proportional hazards models. Age and frailty were the most important characteristics for prediction of mortality. Frailty was the most important characteristic for prediction of rehospitalization, rehospitalization for heart failure, and the composite outcome of mortality or all-cause rehospitalization. In Cox proportional hazards models, a 1-SD higher frailty score (0.1 on theoretical range of 0–1) was associated with a HR of 1.27 (1.06–1.52) for mortality, 1.16 (1.07–1.25) for all-cause rehospitalization, 1.24 (1.14–1.35) for HF rehospitalization, and 1.15 (1.07–1.25) for the composite outcome of mortality or all-cause rehospitalization. In conclusion, frailty is an important predictor of mortality and rehospitalization in adults aged 66 years with HFpEF.
Keywords: heart failure, frailty
Heart failure with preserved ejection fraction (HFpEF) comprises 50% of heart failure across the United States1 and is a condition that can lead to dyspnea, peripheral edema, and fatigue despite a preserved, or normal, left ventricular ejection fraction. Although many clinical trials have been conducted over the past decade, none have identified pharmacologic agents that improve outcomes in patients with HFpEF.2 The leading hypothesis for these failed clinical trials is that HFpEF is a highly heterogeneous condition,3 with a diverse set of abnormalities that span across cardiovascular and non-cardiovascular systems. In response to this heterogeneity, the field has embraced a “phenotype-matching” approach to developing and studying therapeutics for HFpEF, whereby treatment can be personalized based on the presence (or absence) of specific clinical characteristics.3 By identifying and describing unique phenotypes within HFpEF, pharmacologic therapeutics that target unique features of each phenotype can be developed, and ultimately improve outcomes in this complex population. To date, there are many HFpEF phenotypes that have been described in the literature—some based on physiologic parameters,3 and some based on empiric clinical impression.4 Given the role of comorbid conditions in the pathophysiology of HFpEF5 and their association with adverse outcomes,6 there is strong rationale to incorporate comorbidity into the phenotyping of HFpEF. We accordingly aimed to identify and rank the importance of common comorbid conditions that predict mortality and rehospitalization among older adults hospitalized for HFpEF.
METHODS
The study population included beneficiaries aged 66–110 years in the United States Medicare program hospitalized for HFpEF. Medicare provides medical and prescription drug insurance for older adults, as well as individuals with disabilities and end-stage renal disease. For this study, we first identified individuals in the national Medicare 5% sample who had a hospitalization between January 1, 2007 and December 31, 2014 with a discharge diagnosis of heart failure with diastolic dysfunction (International Classification of Diseases, Ninth Revision [ICD-9] 428.31 or 428.33, acute diastolic heart failure). This definition has been used in prior studies, and individuals identified using this approach have characteristics similar to populations with HFpEF studied in randomized controlled trials and population-based studies.7 We included hospitalizations that were at least overnight and up to 30 days in duration. Similar to prior studies examining data from Medicare8, we included hospitalizations for beneficiaries that live in the United States and have continuous fee-for-service medical and prescription drug coverage (Medicare Part A, B, and D) in the 365 days prior to the HFpEF hospitalization, have consistent demographic data, and were hospitalized at an institution with data reported in the 2014 Medicare Hospital Compare and American Hospital Association survey datasets. We selected the first eligible hospitalization for each individual. The study sample included 4,605 Medicare beneficiaries with a hospitalization for HFpEF (Figure 1). This study was reviewed by the University of Alabama at Birmingham Institutional Review Board and Center for Medicare and Medicaid Services Privacy Board.
Figure 1.

Population flow-chart
We ascertained beneficiary demographics (age, sex, race/ethnicity, low income status based on eligibility for Medicaid or Medicare Part D subsidies, and region of residence) from Medicare enrollment information. We examined comorbid conditions and geriatric conditions based on Medicare claims during the 365 days prior to the HFpEF hospitalizations. We opted to examine several comorbid conditions (coronary heart disease, stroke, peripheral vascular disease, diabetes, obesity, anemia, sleep apnea, hypertension, dyslipidemia, atrial fibrillation, chronic obstructive pulmonary disease, chronic kidney disease, cancer, liver disease, rheumatologic disease, prior diagnosis of heart failure, tobacco use, osteoporosis, and depression) given their hypothesized role in the pathophysiology of HFpEF;5 and geriatric conditions given the link between aging processes and HFpEF.9 For geriatric conditions, we examined frailty, dementia, and history of falls. Frailty was defined based on claims using the deficit accumulation approach with scores on a continuum between 0 and 1—this approach has been validated in Medicare.10 We also examined prior healthcare utilization (nursing home residence using a previously validated algorithm,11 number of outpatient visits, cardiologist care, hospitalization, and skilled nursing facility stay) using Medicare claims during the 365 days prior to the index HFpEF hospitalization. We used Medicare claims data to identify characteristics of the hospitalization which including calendar year, intensive care unit stay, and length of stay. We used the American Hospital Association 2014 survey to determine hospital ownership, size, region, urban/rural location, teaching status, and presence of a cardiac critical care unit. Lastly, we used the Center for Medicare and Medicaid Services 2014 Hospital Compare datasets to determine hospital-level heart failure 30-day readmission and mortality rates, overall rating, and patient star rating. Further details are provided in the Supplemental Methods.
Using Medicare claims, beneficiaries were followed from hospital discharge for the index HFpEF hospitalization for up to 1 year; outcomes collected included mortality, all-cause rehospitalization, rehospitalization for heart failure, and the composite outcome of all-cause rehospitalization or mortality. Mortality was based on Medicare enrollment information; rehospitalization was defined as a claim for any inpatient care; and rehospitalization for heart failure was defined as a claim for inpatient care with a primary discharge diagnosis of heart failure. Follow-up time for beneficiaries was censored if they moved out of the United States or lost Medicare fee-for-service coverage.
We first calculated means and standard deviations or number and percentages for continuous and categorical variables, respectively, to describe beneficiary and hospital characteristics. To identify the most important characteristics associated with mortality, all-cause rehospitalization, rehospitalization for heart failure, and the composite outcome of mortality or all-cause rehospitalization, we developed ensembles of boosted decision trees, a machine learning algorithm that approximates the importance of predictors based on how much they contribute to the algorithm’s prediction function, using the extreme gradient boosting algorithm,12 which is implemented in the xgboost R package (version 0.82.1; https://CRAN.R-project.org/package=xgboost). Ensembles of boosted decision trees combine predictions across multiple decision trees, where each tree is developed to correct errors made by its predecessor, resulting in predictions that generalize to external populations more accurately than those from a single decision tree. These models can incorporate non-linear associations and complex interactions between characteristics. Parameters were tuned by repeated (20 times) 10-fold cross-validation, using the negative log-likelihood as the tuning criteria. Because of their high correlation with other included predictors, the variables hospital region, hospital star rating, hospital cardiac critical care unit, non-profit hospital, and hospital mortality rate were excluded from the modeling procedures. To rank the importance of the beneficiary and hospital characteristics for predicting outcomes following HFpEF hospitalization, we calculated the gain, which is defined as the degree to which the characteristics reduces the loss function when included the decision tree. To describe the magnitude and direction of associations, we calculated hazard ratios (HR) and associated 95% confidence intervals (CI) using Cox proportional hazards models that included the variables with the highest gain values using the XGBoost algorithm. While the HR and CI have the benefit of interpretability, the characteristics associated with the largest gain in ensembles of boosted decision trees may not always be those with the narrowest CI in Cox proportional hazards models. There are two primary reasons for this. First, gain incorporates both the strength of the association between the characteristic and the outcome, and how commonly the characteristic is observed. Second, unlike the ensembles of boosted decision trees, the Cox models only examined linear associations and assumed no interaction between characteristics. Data management and analyses were conducted using SAS version 9.4 and R version 3.6.1.
Data needed to replicate these analyses are available from the Center for Medicare and Medicaid Services and the American Hospital Association. Statistical code is available from the authors.
RESULTS
The average age of Medicare beneficiaries with a HFpEF hospitalization in this cohort was 80.3 years (SD 8.4 years), 70.7% were women, 84.6% were white, and 39.4% had low income as measured by eligibility for Medicare or Medicare Part D subsidies (Table 1). Comorbidities were common; 73.6% had hypertension, 51.1% had coronary heart disease, 46.1% had atrial fibrillation, 45.0% had chronic kidney disease, 42.0% had anemia, and 40.9% had diabetes. Regarding geriatric conditions, the mean frailty score was 0.2 (SD 0.1) and 41.0% had a frailty score of at least 0.25 indicating presence of frailty; 12.8% had dementia, and 1.1% had a history of falls. The average length of stay was 7.3 days and more than a third (37.2%) of hospitalizations included time spent in an intensive care unit (Table 2). Of the 4,605 beneficiaries included in this cohort, 11.6% (n = 536) experienced mortality, 53.9% (n = 2,484) experienced rehospitalization for any cause, 16.9% (n = 777) experienced rehospitalization for heart failure, and 56.1% (n = 2,582) experienced rehospitalization or mortality in the year following discharge.
Table 1.
Characteristics of Medicare beneficiaries in the national 5% random sample with hospitalizations for heart failure with preserved ejection fraction in 2007–2014
| N=4,605 | |
|---|---|
| Variable | |
| Age (years) mean (SD) | 80.3 (8.4) |
| Women | 3255 (70.7%) |
| Race/ethnicity | |
| White | 3895 (84.6%) |
| Black | 410 (8.9%) |
| Asian | 107 (2.3%) |
| Hispanic/Latino | 100 (2.2%) |
| Other | 93 (2%) |
| Low income based on Medicaid/Medicare subsidies eligibility | 1815 (39.4%) |
| Comorbid conditions | |
| Coronary heart disease | 2351 (51.1%) |
| Stroke | 254 (5.5%) |
| Peripheral vascular disease | 474 (10.3%) |
| Diabetes | 1884 (40.9%) |
| Obesity | 855 (18.6%) |
| Anemia | 1932 (42%) |
| Sleep Apnea | 427 (9.3%) |
| Hypertension | 3387 (73.6%) |
| Dyslipidemia | 2639 (57.3%) |
| Atrial fibrillation | 2124 (46.1%) |
| Chronic obstructive pulmonary disease | 1779 (38.6%) |
| Chronic kidney disease | 2073 (45%) |
| Cancer | 859 (18.7%) |
| Liver disease | 119 (2.6%) |
| Rheumatologic disease | 244 (5.3%) |
| Prior diagnosis of heart failure | 1675 (36.4%) |
| Tobacco use | 1039 (22.6%) |
| Osteoporosis | 547 (11.9%) |
| Depression | 1379 (29.9%) |
| Geriatric Conditions | |
| Frailty score, mean (SD) | 0.2 (0.1) |
| Dementia | 588 (12.8%) |
| History of falls | 51 (1.1%) |
| Region of residency | |
| New England | 414 (9%) |
| Middle Atlantic | 781 (17%) |
| East North Central | 834 (18.1%) |
| West North Central | 364 (7.9%) |
| South Atlantic | 866 (18.8%) |
| East South Central | 222 (4.8%) |
| West South Central | 457 (9.9%) |
| Mountain | 154 (3.3%) |
| Pacific | 513 (11.1%) |
| Healthcare utilization in the year prior to hospitalization | |
| Nursing home residence | 378 (8.2%) |
| Number of outpatient evaluations, mean (SD) | 12.3 (9.1) |
| Hospitalization for any cause | 226 (4.9%) |
| Cardiologist care | 1891 (41.1%) |
| Skilled nursing facility stay | 1116 (24.2%) |
Table 2.
Characteristics of the hospitalizations and hospitals of Medicare beneficiaries in the national 5% random sample with hospitalizations for heart failure with preserved ejection fraction in 2007–2014
| N=4,605 | |
|---|---|
| Hospitalization characteristics | |
| Intensive care unit stay | 1713 (37.2%) |
| Length of stay, mean (SD) | 7.3 (4.8) |
| Year of hospitalization | |
| 2007 | 344 (7.5%) |
| 2008 | 622 (13.5%) |
| 2009 | 641 (13.9%) |
| 2010 | 584 (12.7%) |
| 2011 | 567 (12.3%) |
| 2012 | 554 (12.0%) |
| 2013 | 621 (13.5%) |
| 2014 | 672 (14.6%) |
| Hospital characteristics | |
| Hospital ownership | |
| Nonfederal government | 411 (8.9%) |
| Not for profit | 3629 (78.8%) |
| For profit | 565 (12.3%) |
| Bed size | |
| < 100 | 474 (10.3%) |
| 100–199 | 1024 (22.2%) |
| 200–299 | 817 (17.7%) |
| 300–399 | 708 (15.4%) |
| 400–499 | 465 (10.1%) |
| ≥500 | 1117 (24.3%) |
| Geographic region | |
| New England | 364 (7.9%) |
| Middle Atlantic | 284 (6.2%) |
| East North Central | 428 (9.3%) |
| West North Central | 686 (14.9%) |
| South Atlantic | 864 (18.8%) |
| East South Central | 335 (7.3%) |
| West South Central | 725 (15.7%) |
| Mountain | 389 (8.4%) |
| Pacific | 530 (11.5%) |
| Urban/rural location | |
| Metro | 4027 (87.4%) |
| Micro | 464 (10.1%) |
| Rural | 114 (2.5%) |
| Teaching status | 2432 (52.8%) |
| Presence of cardiac critical care unit | 2658 (57.7%) |
| 30-day HF readmission rate, mean (SD) | 21.8 (1.7) |
| 30-day HF mortality rate, mean (SD) | 11.8 (1.6) |
| Hospital overall rating | |
| 1 | 150 (3.3%) |
| 2 | 1050 (22.8%) |
| 3 | 2028 (44%) |
| 4 | 1192 (25.9%) |
| 5 | 140 (3.0%) |
| Not Available | 45 (1.0%) |
| Patient survey star rating | |
| 1 | 93 (2.0%) |
| 2 | 735 (16.0%) |
| 3 | 2679 (58.2%) |
| 4 | 1037 (22.5%) |
| 5 | 28 (0.6%) |
| Not Available | 33 (0.7%) |
| Mortality national comparison | |
| Above the national average | 1246 (27.1%) |
| Same as the national average | 2537 (55.1%) |
| Below the national average | 771 (16.7%) |
| Not Available | 51 (1.1%) |
Abbreviations: HF: Heart failure
In the XGBoost analyses, age and frailty were the most important characteristics related to mortality (Figure 2A). Frailty emerged as the most important characteristic related to all-cause rehospitalization (Figure 3A), rehospitalization for heart failure (Figure 4A), and the composite outcome of mortality or all-cause rehospitalization (Figure 5A). In Cox proportional hazards models, a 1-SD higher frailty score (0.1) was associated with HR 1.27 (1.06–1.52) for mortality (Figure 2B), 1.16 (1.07–1.25) for all-cause rehospitalization (Figure 3B), 1.24 (1.14–1.35) for rehospitalization for heart failure (Figure 4B), and 1.15 (1.07–1.25) for the composite outcome of mortality or all-cause rehospitalization (Figure 5B).
Figure 2. Importance of (A) and Hazard Ratios for (B) beneficiary and hospital characteristics for mortality among Medicare beneficiaries hospitalized for HFpEF.
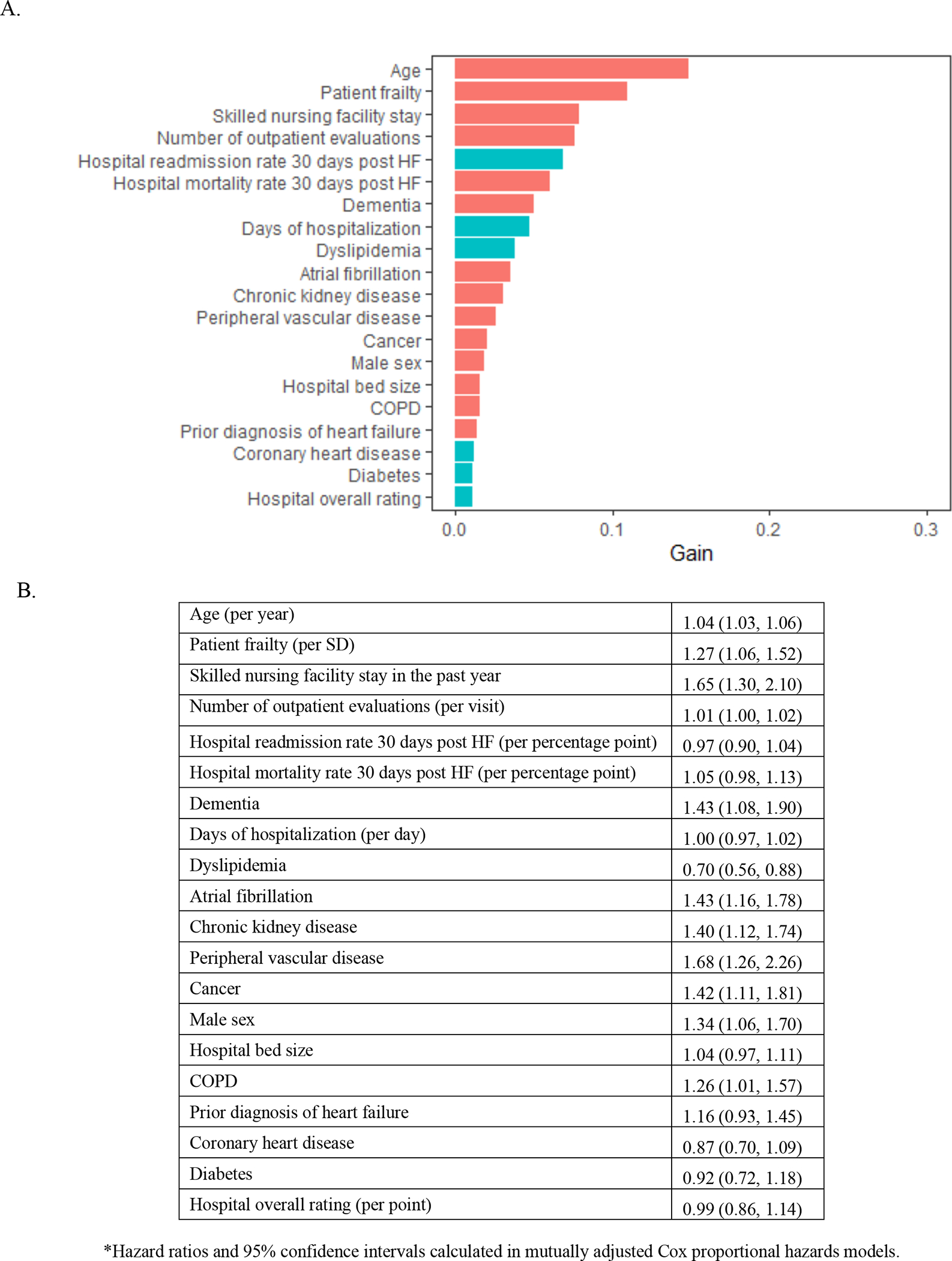
The red bars indicate hazard ratio >1 and turquoise indicate hazard ratio<1. Abbreviations: HFpEF=heart failure with preserved ejection fraction
Figure 3. Importance of (A) and Hazard Ratios for (B) beneficiary and hospital characteristics for all-cause rehospitalization among Medicare beneficiaries hospitalized for HFpEF.
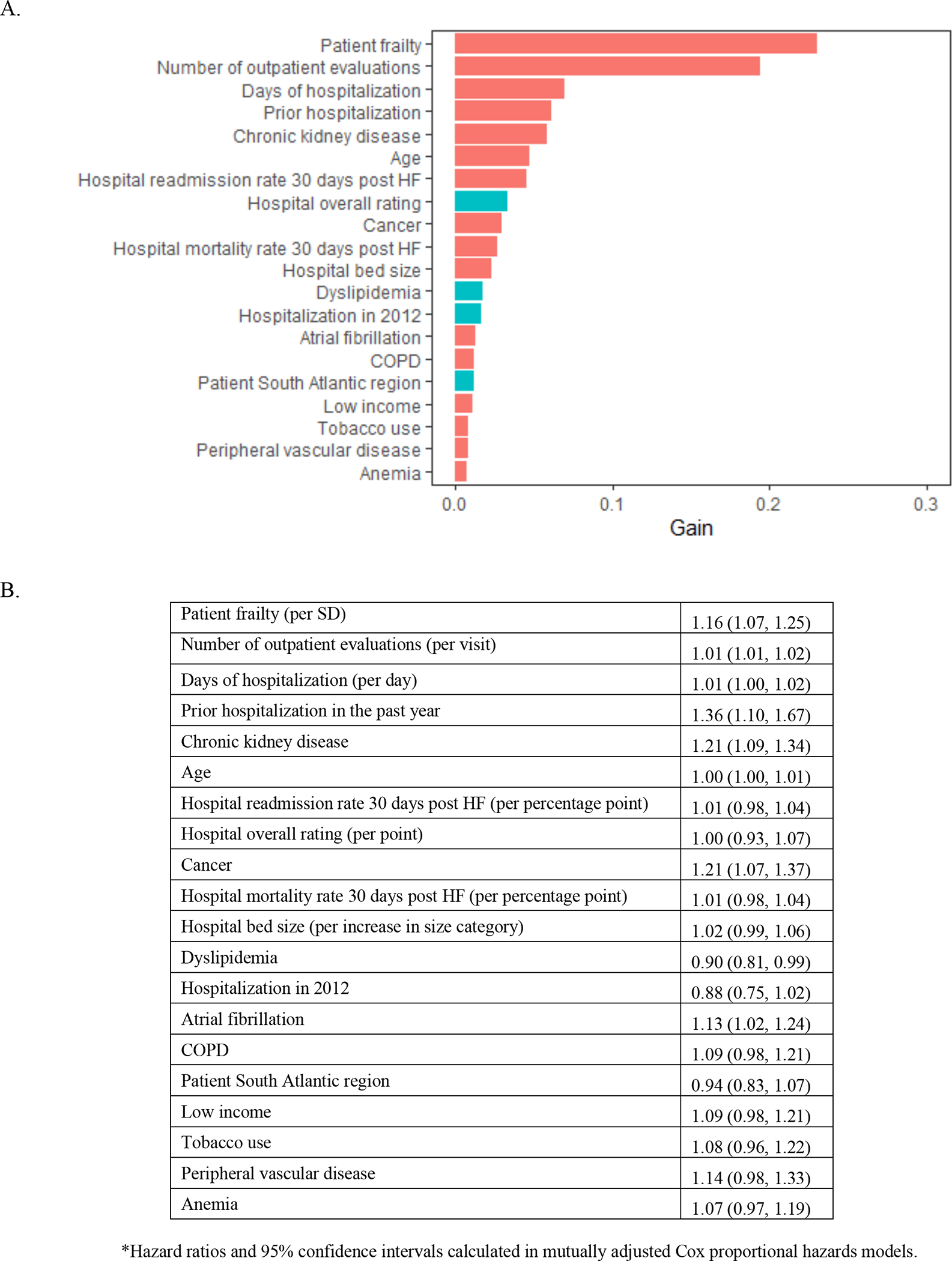
The red bars indicate hazard ratio >1 and turquoise indicate hazard ratio<1. Abbreviations: HFpEF=heart failure with preserved ejection fraction
Figure 4. Importance of (A) and Hazard Ratios for (B) beneficiary and hospital characteristics for HF rehospitalization among Medicare beneficiaries hospitalized for HFpEF.
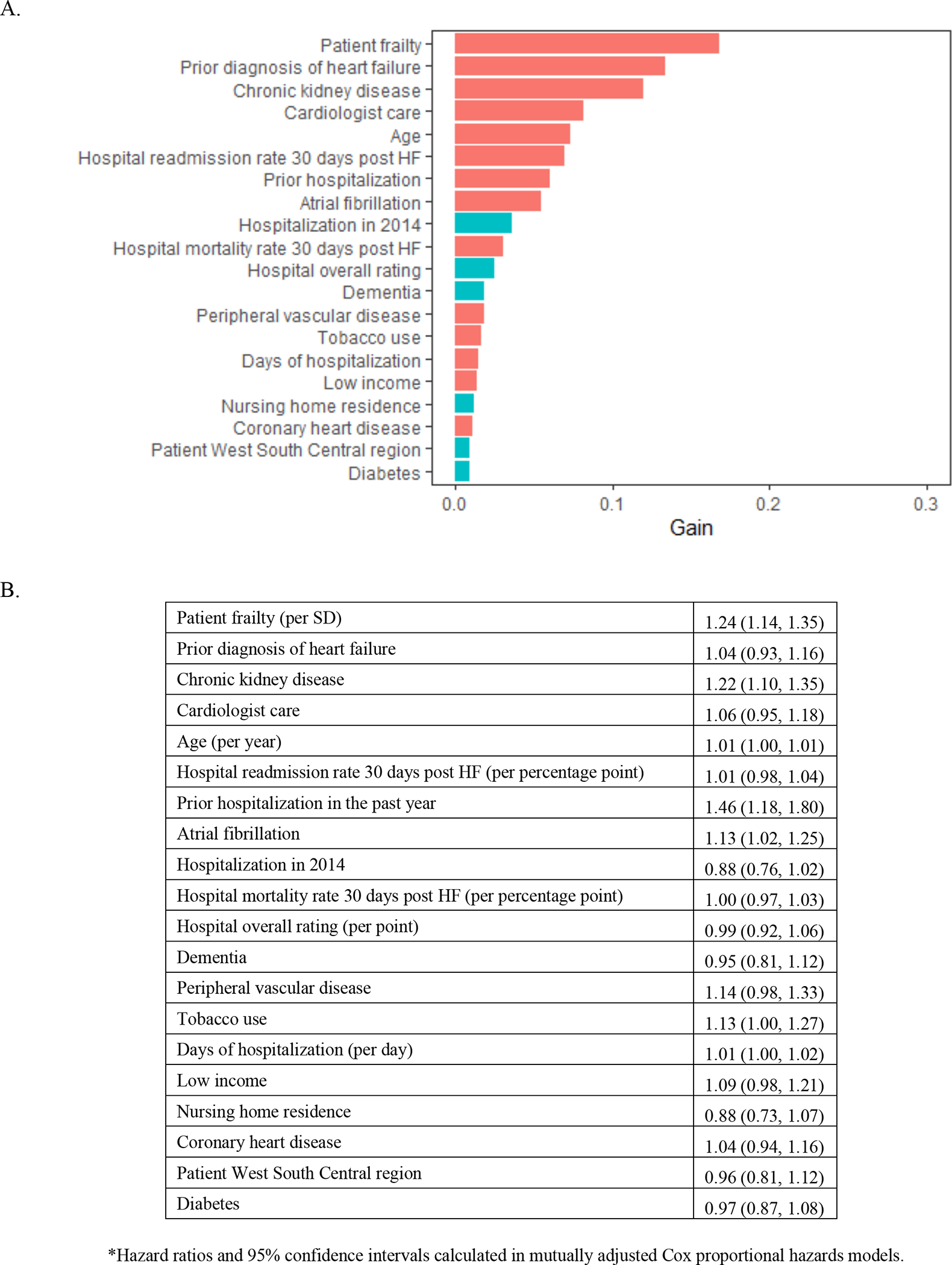
The red bars indicate hazard ratio >1 and turquoise indicate hazard ratio<1. Abbreviations: HF = heart failure, HFpEF=heart failure with preserved ejection fraction
Figure 5. Importance of (A) and Hazard Ratios for (B) beneficiary and hospital characteristics for composite of mortality and rehospitalization among Medicare beneficiaries hospitalized for HFpEF.
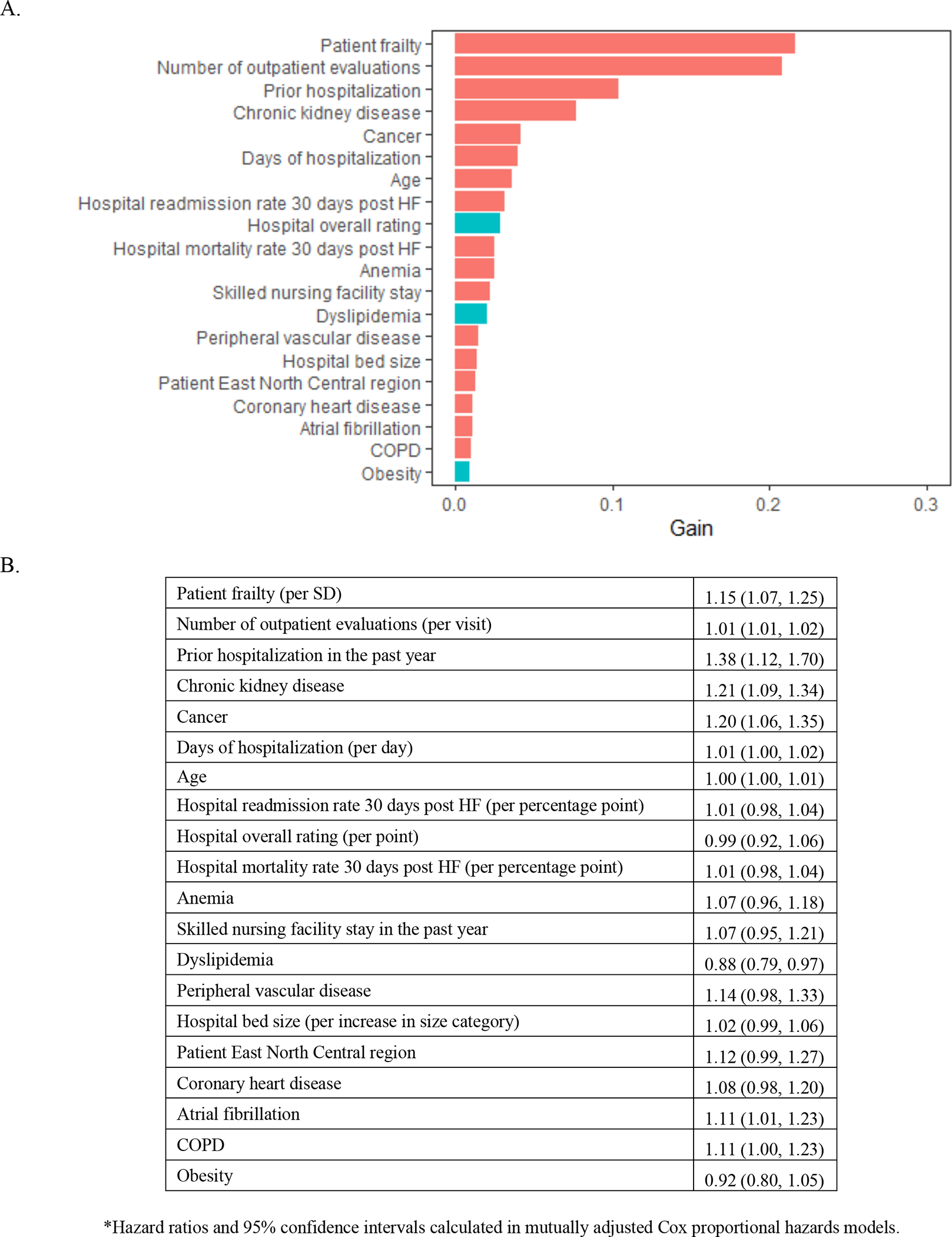
The red bars indicate hazard ratio >1 and turquoise indicate hazard ratio<1. Abbreviations: HFpEF=heart failure with preserved ejection fraction
Other factors that were important across study outcomes included health utilization such as skilled nursing facility stay, number of prior outpatient visits, and prior hospitalization (Figure 2–5).
DISCUSSION
In this study of Medicare beneficiaries hospitalized with HFpEF, we sought to identify key comorbid conditions that were important in the prediction of adverse post-hospitalization outcomes. Our primary finding was that frailty, a clinical marker of biological aging, was the most important predictor of rehospitalization and the second most important predictor of mortality (after age) for Medicare beneficiaries with HFpEF. These findings underscore the importance of considering frailty in HFpEF management; and further suggest the need for future studies to better understand the role of biological aging in HFpEF, and determine whether biological aging could be a novel target for treatment of HFpEF.
HFpEF has been described as the prototypical geriatric syndrome9 that disproportionately affects older adults (mean age >75 years)7 due to a pathophysiology5 that results from age-related changes to the cardiovascular system and common age-associated comorbid conditions implicated in its pathogenesis. Not surprisingly, data suggest that frailty is common in HFpEF patient population, with prevalence over 90% reported in large clinical trials like the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial.13 Furthermore, the presence of frailty in chronic heart failure has been associated with mortality, hospitalization, and worse patient-reported outcomes.14–16 This study now extends findings about the importance of frailty in HFpEF by identifying frailty as the most important factor for predicting post-hospitalization outcomes (in addition to age) using a machine learning-based analysis. Machine-learning has emerged as a set of methodologies that can account for the inherent complexities of real-world phenomena, accounting for high-level interactions of variables frequently present in the real-world which may not be easily discernable to even the most astute clinician and/or scientist.17 Given that these methods a) only require minimal statistical assumptions, b) can model complex multidimensional relationships among a large number of predictors, and c) can provide useful insights by identifying novel risk predictors that may not otherwise be discernable by traditional methods, leveraging the data-driven nature of learning algorithms is particularly well-suited for gaining novel insights on HFpEF, a complex and heterogenous disease.
Post-hospitalization outcomes have been a major focus of quality over the past decade. Accordingly, many groups have developed prediction tools; unfortunately, prediction tools to date have failed to achieve very good discriminatory power.18–20 This could relate to the heterogeneity of HF. Another possibility is that factors included in the risk calculators have incompletely accounted for the many contributors to adverse post-hospitalization outcomes. For example, geriatric conditions are not frequently included in risk calculators despite calls for the routine assessment of geriatric conditions when caring for adults with HFpEF 21,22 Our findings emphasize the key role of geriatric conditions, showing that frailty is an important indicator of risk. This supports the concept that biological age is as important (or more so) than chronological age, and lends further support to that notion that geriatric conditions like frailty merit routine incorporation into clinical assessments21 and also risk prediction models in the future.
Despite the investigation of several pharmacologic agents, no single therapy has yet to consistently demonstrate improvement in outcomes in HFpEF. This is in part due to the heterogeneity of HFpEF, which will likely require a more phenotype-specific targeted approach for effective treatment.3 Another contributor is that our limited understanding of the molecular mechanisms that drive HFpEF pathophysiology has yet to identify the most effective therapeutic targets for this disease. The importance of frailty in predicting mortality and rehospitalizations in older adults with HFpEF suggests that specifically targeting frailty could be an effective strategy to improving outcomes for this subgroup of HFpEF patients. Preclinical studies have demonstrated that inhibiting catabolic processes involved in both frailty and heart failure pathophysiology can improve cardiac reserve and exercise capacity in animal models of age-related HFpEF.23 Moreover, exercise, which is one of the most effective treatments for frailty in older adults,24 has also been shown to improve HFpEF phenotypes in older animals and humans.25,26 Indeed, prospective randomized controlled trials now show that exercise therapy improves outcomes in older adults with HFpEF.27 Collectively, these data along with our finding linking frailty with adverse outcomes in HFpEF further support the potential role for cardiac rehabilitation, which has yet to be approved specifically for HFpEF by Centers for Medicare & Medicaid Services (CMS). Nutritional interventions for frailty also warrant further investigation given some observed potential to improve physical function, heart failure symptoms, and readmission rates.28
A major strength of this study is the diverse study population broadly representative of HFpEF patients seen in clinical practice. There are also several limitations. Medicare claims data are generated for billing purposes and lack detailed physiologic information and indicators of severity of comorbidities. In particular, these data lack left ventricular ejection fraction—to identify HFpEF, we relied on diagnostic codes for diastolic heart failure. Notably, we did not capture patients with HFpEF who received less specific ICM-9-CM diagnostic codes such as 428.0 or 428.9 which do not specify HF subtype—this in part reflects the sample size of patients identified with HFpEF in this study. Although imperfect, individuals with these codes have similar characteristics as populations with more rigorously defined HFpEF and allow for the inclusion of a large, geographically diverse population.7 Although the frailty score used has been validated against physical performance29 and established frailty metrics,30 we did not have the information needed to calculate gold-standard measures of frailty such as the Fried frailty criteria. It is possible that confounding by unmeasured characteristics could explain part of the association we observed between frailty and post-hospitalization outcomes following a hospitalization with HFpEF. For example, conditions that contribute to frailty and HFpEF, such as cardiac amyloidosis, could explain our observation. Future work is needed to further disentangle shared processes that lead to geriatric conditions like frailty and HFpEF. Finally, generalizability of these findings to younger adults, Medicare Advantage Plan patients, and those outside the United States is uncertain.
In conclusion, we found that frailty is an important predictor of mortality and rehospitalization in older adults with HFpEF, supporting its relevance in the management of HFpEF.
Supplementary Material
Acknowledgments
Grant Support:
This research was supported in part by research funding from Amgen, Inc. The funder did not participate in the study design, analysis, or interpretation.
Disclosures:
Dr. Goyal is supported by the National Institute on Aging grant K76AG064428-01A1. Dr. Goyal has received personal fees for medicolegal consulting on heart failure; and has received honoraria from Akcea inc and Bionest inc.
Dr. Levitan receives research funding from Amgen and has served as a consultant for a research project funded by Novartis.
Dr Jaeger receives support through grant R01HL144773 from the National Heart, Lung, and Blood Institute and grant 15SFRN2390002 from the American Heart Association.
Dr. Roh is supported by the National Institute on Aging grant K76AG064328, Fred and Ines Yeatts Fund for Innovative Research, and the Hassenfeld Research Scholarship.
Dr. Kim is supported by the National Institute on Aging grant R01AG062713.
References
- 1.Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC, Get With the Guidelines Scientific Advisory C, Investigators. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126(1):65–75. [DOI] [PubMed] [Google Scholar]
- 2.Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. 2011;123(18):2006–2013; discussion 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131(3):269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samson R, Jaiswal A, Ennezat PV, Cassidy M, Le Jemtel TH. Clinical Phenotypes in Heart Failure With Preserved Ejection Fraction. J Am Heart Assoc. 2016;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–271. [DOI] [PubMed] [Google Scholar]
- 6.Pandey A, Vaduganathan M, Arora S, Qamar A, Mentz RJ, Shah SJ, Chang PP, Russell SD, Rosamond WD, Caughey MC. Temporal Trends in Prevalence and Prognostic Implications of Comorbidities Among Patients With Acute Decompensated Heart Failure: The ARIC Study Community Surveillance. Circulation. 2020;142(3):230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goyal P, Almarzooq ZI, Horn EM, Karas MG, Sobol I, Swaminathan RV, Feldman DN, Minutello RM, Singh HS, Bergman GW, Wong SC, Kim LK. Characteristics of Hospitalizations for Heart Failure with Preserved Ejection Fraction. Am J Med. 2016;129(6):635 e615–626. [DOI] [PubMed] [Google Scholar]
- 8.Goyal P, Loop M, Chen L, Brown TM, Durant RW, Safford MM, Levitan EB. Causes and Temporal Patterns of 30-Day Readmission Among Older Adults Hospitalized With Heart Failure With Preserved or Reduced Ejection Fraction. J Am Heart Assoc. 2018;7(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Upadhya B, Taffet GE, Cheng CP, Kitzman DW. Heart failure with preserved ejection fraction in the elderly: scope of the problem. J Mol Cell Cardiol. 2015;83:73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yun H, Kilgore ML, Curtis JR, Delzell E, Gary LC, Saag KG, Morrisey MA, Becker D, Matthews R, Smith W, Locher JL. Identifying types of nursing facility stays using medicare claims data: an algorithm and validation. Health Services and Outcomes Research Methodology. 2010;10(1):100–110. [Google Scholar]
- 12.Chen T, Guestrin C XGBoost: A Scalable Tree Boosting System. In Krishnapuram Balaji; Shah Mohak; Smola Alexander J.; Aggarwal Charu C.; Shen Dou; Rastogi Rajeev (eds.). Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, August 13–17, 2016. ACM. pp. 785–794. arXiv:1603.02754.. [Google Scholar]
- 13.Sanders NA, Supiano MA, Lewis EF, Liu J, Claggett B, Pfeffer MA, Desai AS, Sweitzer NK, Solomon SD, Fang JC. The frailty syndrome and outcomes in the TOPCAT trial. Eur J Heart Fail. 2018;20(11):1570–1577. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Lupon J, Vidan MT, Ferguson C, Gastelurrutia P, Newton PJ, Macdonald PS, Bueno H, Bayes-Genis A, Woo J, Fung E. Impact of Frailty on Mortality and Hospitalization in Chronic Heart Failure: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2018;7(23):e008251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kundi H, Wadhera RK, Strom JB, Valsdottir LR, Shen C, Kazi DS, Yeh RW. Association of Frailty With 30-Day Outcomes for Acute Myocardial Infarction, Heart Failure, and Pneumonia Among Elderly Adults. JAMA Cardiol. 2019;4(11):1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandey A, Kitzman D, Whellan DJ, Duncan PW, Mentz RJ, Pastva AM, Nelson MB, Upadhya B, Chen H, Reeves GR. Frailty Among Older Decompensated Heart Failure Patients: Prevalence, Association With Patient-Centered Outcomes, and Efficient Detection Methods. JACC Heart Fail. 2019;7(12):1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajkomar A, Dean J, Kohane I. Machine Learning in Medicine. N Engl J Med. 2019;380(14):1347–1358. [DOI] [PubMed] [Google Scholar]
- 18.Ross JS, Mulvey GK, Stauffer B, Patlolla V, Bernheim SM, Keenan PS, Krumholz HM. Statistical models and patient predictors of readmission for heart failure: a systematic review. Arch Intern Med. 2008;168(13):1371–1386. [DOI] [PubMed] [Google Scholar]
- 19.Kansagara D, Englander H, Salanitro A, Kagen D, Theobald C, Freeman M, Kripalani S. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306(15):1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mortazavi BJ, Downing NS, Bucholz EM, Dharmarajan K, Manhapra A, Li SX, Negahban SN, Krumholz HM. Analysis of Machine Learning Techniques for Heart Failure Readmissions. Circ Cardiovasc Qual Outcomes. 2016;9(6):629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorodeski EZ, Goyal P, Hummel SL, Krishnaswami A, Goodlin SJ, Hart LL, Forman DE, Wenger NK, Kirkpatrick JN, Alexander KP, Geriatric Cardiology Section Leadership Council ACoC. Domain Management Approach to Heart Failure in the Geriatric Patient: Present and Future. J Am Coll Cardiol. 2018;71(17):1921–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goyal P, Gorodeski EZ, Flint KM, Goldwater DS, Dodson JA, Afilalo J, Maurer MS, Rich MW, Alexander KP, Hummel SL. Perspectives on Implementing a Multidomain Approach to Caring for Older Adults With Heart Failure. J Am Geriatr Soc. 2019;67(12):2593–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roh JD, Hobson R, Chaudhari V, Quintero P, Yeri A, Benson M, Xiao C, Zlotoff D, Bezzerides V, Houstis N, Platt C, Damilano F, Lindman BR, Elmariah S, Biersmith M, Lee SJ, Seidman CE, Seidman JG, Gerszten RE, Lach-Trifilieff E, Glass DJ, Rosenzweig A. Activin type II receptor signaling in cardiac aging and heart failure. Sci Transl Med. 2019;11(482). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarazona-Santabalbina FJ, Gomez-Cabrera MC, Perez-Ros P, Martinez-Arnau FM, Cabo H, Tsaparas K, Salvador-Pascual A, Rodriguez-Manas L, Vina J. A Multicomponent Exercise Intervention that Reverses Frailty and Improves Cognition, Emotion, and Social Networking in the Community-Dwelling Frail Elderly: A Randomized Clinical Trial. J Am Med Dir Assoc. 2016;17(5):426–433. [DOI] [PubMed] [Google Scholar]
- 25.Roh JD, Houstis N, Yu A, Chang B, Yeri A, Li H, Hobson R, Lerchenmuller C, Vujic A, Chaudhari V, Damilano F, Platt C, Zlotoff D, Lee RT, Shah R, Jerosch-Herold M, Rosenzweig A. Exercise training reverses cardiac aging phenotypes associated with heart failure with preserved ejection fraction in male mice. Aging Cell. 2020;19(6):e13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3(6):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kitzman DW, Brubaker P, Morgan T, Haykowsky M, Hundley G, Kraus WE, Eggebeen J, Nicklas BJ. Effect of Caloric Restriction or Aerobic Exercise Training on Peak Oxygen Consumption and Quality of Life in Obese Older Patients With Heart Failure With Preserved Ejection Fraction: A Randomized Clinical Trial. JAMA. 2016;315(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hummel SL, Karmally W, Gillespie BW, Helmke S, Teruya S, Wells J, Trumble E, Jimenez O, Marolt C, Wessler JD, Cornellier ML, Maurer MS. Home-Delivered Meals Postdischarge From Heart Failure Hospitalization. Circ Heart Fail. 2018;11(8):e004886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim DH, Glynn RJ, Avorn J, Lipsitz LA, Rockwood K, Pawar A, Schneeweiss S. Validation of a Claims-Based Frailty Index Against Physical Performance and Adverse Health Outcomes in the Health and Retirement Study. J Gerontol A Biol Sci Med Sci. 2019;74(8):1271–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim DH, Patorno E, Pawar A, Lee H, Schneeweiss S, Glynn RJ. Measuring Frailty in Administrative Claims Data: Comparative Performance of Four Claims-Based Frailty Measures in the U.S. Medicare Data. J Gerontol A Biol Sci Med Sci. 2020;75(6):1120–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


