Abstract
Background
Among systemic abnormalities caused by the novel coronavirus, little is known about the critical attack on the central nervous system (CNS). Few studies have shown cerebrovascular pathologies that indicate CNS involvement in acute patients. However, replication studies are necessary to verify if these effects persist in COVID-19 survivors more conclusively. Furthermore, recent studies indicate fatigue is highly prevalent among ‘long-COVID’ patients. How morphometry in each group relate to work-related fatigue need to be investigated.
Method
COVID survivors were MRI scanned two weeks after hospital discharge. We hypothesized, these survivors will demonstrate altered gray matter volume (GMV) and experience higher fatigue levels when compared to healthy controls, leading to stronger correlation of GMV with fatigue. Voxel-based morphometry was performed on T1-weighted MRI images between 46 survivors and 30 controls. Unpaired two-sample t-test and multiple linear regression were performed to observe group differences and correlation of fatigue with GMV.
Results
The COVID group experienced significantly higher fatigue levels and GMV of this group was significantly higher within the Limbic System and Basal Ganglia when compared to healthy controls. Moreover, while a significant positive correlation was observed across the whole group between GMV and self-reported fatigue, COVID subjects showed stronger effects within the Posterior Cingulate, Precuneus and Superior Parietal Lobule.
Conclusion
Brain regions with GMV alterations in our analysis align with both single case acute patient reports and current group level neuroimaging findings. We also newly report a stronger positive correlation of GMV with fatigue among COVID survivors within brain regions associated with fatigue, indicating a link between structural abnormality and brain function in this cohort.
Keywords: COVID-19, VBM, Limbic, Basal ganglia, Fatigue
1. Introduction
SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) is a highly contagious novel coronavirus that started a global pandemic causing more than 400 million confirmed cases of COVID-19 and nearly 6 million deaths world-wide((WHO) WHO, 2022). Mass vaccinations have mitigated cases in USA, but infections continue to rise – especially in India. The literature shows evidence of structural brain abnormalities but exactly where and how long these effects persist in acute patients during recovery, remains unclear.
Attack on the central nervous system (CNS) in acutely ill COVID-19 patients can cause a broad range of pathology including ischemic strokes, encephalitis (Kremer et al., 2020), inflammatory vascular pathologies in cerebral vessels (Keller et al., 2020; Nicholson et al., 2020), and microhemorrhages (Radmanesh et al., 2020) among many others. Ischemic strokes (27%), encephalitis (13%), confusion (53%), impaired consciousness (39%) along with agitation (31%) and headaches (16%) were also reported from patients across multiple sites (11 hospitals, n = 64) (Kremer et al., 2020). Moreover, brain lesions and hyperintensities were observed from fluid-attenuated inversion recovery (FLAIR) imaging in multiple brain regions (Kandemirli et al., 2020) along with autoimmune and hemorrhagic encephalitis (Paterson et al., 2020). But these were mostly case studies, and the current literature needs to move from individual cases to conclusive group level estimates delineating structural brain alterations between COVID-19 patients and healthy controls (HCs).
A few recent neuroimaging studies have emerged to address this gap with moderate (Duan et al., 2021; Qin et al., 2021) to large sample sizes (Douaud et al., 2021), including follow-up (Lu et al., 2020; Tu et al., 2021) and longitudinal designs (Douaud et al., 2021). Lu et al., 2020 (Lu et al., 2020) had reported neurological symptoms in over 68% (41/60) of hospitalized patients that persisted after a 3 month follow up (55%). They had also performed structural MRI and showed significantly higher gray matter volume (GMV) in several regions of interest (ROIs) – Rolandic operculum, bilateral olfactory, insular, and hippocampal regions, as well as in the right cingulate gyrus and left Heschl's gyrus (Lu et al., 2020). An alarming number of survivors are now undergoing a sequela of symptoms (Logue et al., 2021; Tabacof et al., 2020; Peluso et al., 2021) which converge to the brain as the responsible organ. Therefore, changes in brain structure could correlate to the severity of these symptoms. In regard to that, another follow-up study (Tu et al., 2021) assessed structural and functional changes in COVID-19 patients in two consecutive time points - 3 and 6 months and evaluated their relation to post-traumatic stress symptoms (PTSS). They showed increased GMV in bilateral hippocampus and amygdala, which also correlated negatively with self-reported Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5) scores in COVID subjects. This could also relate to the interval after hospital discharge and the severity of symptoms can alter with time. For example, Tu et al., also show that the PCL-5 scores from Session1 (3 months) correlated with the time after discharge and the total PCL-5 scores from these survivors increased by ∼20% at Session2 (6 months).
It is possible that the COVID and control groups do not demonstrate any global GMV changes, rather, GMV could be modulated locally due to fever and hypoxemic conditions. For instance, a recent study used source-based morphometry (SBM) on Computed Tomography (CT) scans to show that the fronto-temporal network is more susceptible to fever and reduced oxygen levels in COVID patients, despite no global difference in GMV (Duan et al., 2021). Furthermore, the modified Rankin Scale (mRS), a clinical disability score, significantly correlated with lower GMV in the frontal gyrus both during discharge and after a 6 month-follow-up. Interestingly, COVID survivors can also develop neurological symptoms during recovery, despite no such manifestations in the acute stage. For example, a recent study reported reduced cortical thickness in the left insula, hippocampus, and superior temporal gyrus, after a 3 month-follow-up MRI scan in two 2 sub-types (mild and severe), who had no signs of neurological manifestations during the acute stage (Qin et al., 2021).
Another important question is how these neurological changes develop in individuals before and after infection with COVID-19? A recent longitudinal study (Douaud et al., 2021) used a large pool of patients (N=785, nCOVID = 401) from the UK Biobank COVID-19 reimaging study, to show reduced GM thickness and contrast in the orbitofrontal and parahippocampal gyrus, as well as, in insula, amygdala and the anterior cingulate cortex. In addition, they report increased tissue damage in brain regions functionally associated with the piriform cortex and the olfactory system, as well as higher volumes in CSF. Therefore, the literature shows mixed evidence of increased GMV and contrarily, reduced GM thickness from cross-sectional, follow-up and longitudinal designs, nevertheless, in quite consistent anatomical locations. Our goal was to first assess, if the participants from our study, scanned after a much shorter interval from hospital discharge (2 weeks), demonstrated altered GMV in regions that are consistent with both acute stage single case reports and more recent group level neuroimaging reports from lengthy recovering (3–6 months) patients. Moreover, since fatigue is the highest reported symptom from surviving patients (Logue et al., 2021; Tabacof et al., 2020; Peluso et al., 2021), we wanted to ask, if self-reported fatigue (during work) independently correlated to voxel-wise GMV in regions, known to be functionally associated with fatigue.
In this study, we try to address this by recruiting a group of patients, hospitalized due to a positive PCR test for COVID-19. We imaged them two weeks after hospital discharge after converting to be PCR negative. One expectation is that changes in brain tissue structure in COVID survivors can still cause changes in compartmental volumes that remain shortly after hospital discharge. Specifically, we hypothesized that these surviving COVID-negative patients would demonstrate GMV differences with the HC group and show significant correlation of altered GMV with self-reported fatigue scores. T1-weighted MRI images are sensitive to such changes and can be used to estimate GMV differences between two groups using voxel-based morphometry (VBM) (Ashburner and Friston, 2000).
2. Materials and methods
Participants: 47 COVID-negative patients and 35 HCs were recruited by the Indian Institute of Technology (IIT), Delhi, India where they were imaged following all Institutional Review Board (IRB) guidelines. Please note, these subjects were recruited from a much larger pool of patients. The patients were initially classified based on illness severity data derived from a database of 2538 COVID patients admitted to the Metro Hospital in Delhi from May to December 2020. 24% of this sample did not require O2, 40% required O2, 22% required Continuous Positive Airway Pressure (CPAP); and 14% were intubated. This 14% of intubated patients were excluded from the recruitment process in the current study. Patients were studied two weeks after discharge, after becoming PCR negative [333 who needed CPAP to raise O2 levels; 333 who needed nasal O2 to raise O2 levels; and 334 who were admitted but did not need supplemental O2]. The sample of 47 COVID subjects constituting the COVID group in this study were collected from this cohort (those who agreed to participate in this ongoing study so far). Of these 47, 36.17% (17/47) patients were reported to be ‘mild’, 8.51% (4/47) to be ‘moderate’ and 36.17% (17/47) were between ‘moderate’ to ‘severe’. Information from the rest of the 19.15% (9/47) was not provided by the hospital because those patients did not give consent to sharing their medical symptoms. Among the ‘moderate’ to ‘severe’ patients, 58.82% (10/17) were given Remdesivir and 29.41% (5/17) required additional oxygenation. One patient (1/17) was given a mix of Dexamethasone, Ceftriaxone and Clexane injections and another (1/17) was put in an Antibiotic and Steroid regime for 4 weeks (Progressively reduced). One other patient (1/17) was given Actemra 2 times, who was also administered Bilevel Positive Air Pressure (BiPap) for 4–5 days. On average these 47 patients stayed in the hospital for approximately 11 ± 3.30[SD] days. Before data analysis, six subjects (1 COVID and 5 HC) were removed during quality control assessment. Effectively, T1-weighted images from 46 (31 males) COVID and 30 (23 males) HCs were included in the study with mean age 33.5 years ±9.74[SD] years (HC) and 34.63 years ± 11.54[SD] years (COVID). Please see Table 1 for more details on demographics.
Table 1.
Group level statistics on participant demographics and global VBM metrics of each tissue type in HC and COVID group. The first three rows represents the test results from participant demographics including age, sex and fatigue. The last four rows represents the global VBM metrics of compartmental and total volumes. Keys: GMV = Gray Matter Volume, WMV = White Matter Volume, CSFV = Cerebrospinal Fluid Volume, TIV = Total Intracranial Volume, p = p-value,stat = test statistics,t = two-sample t-test statistic,= Chi-Squared statistic, T = Wilcoxon Rank Sum test score, ml = milliliter, M = Male, F = Female.
| Measures | p | stat | HC, mean (SD) | COVID, mean (SD) |
|---|---|---|---|---|
| Age (years) | 0.66 | −0.44 (t) | 33.50 (9.74) | 34.63 (11.54) |
| Sex | 0.38 | 0.76 () | 23M, 7F | 31M, 15F |
| Fatigue | 2.86e-07 | 1093 (T) | 0.61 (0.78) | 2.67 (1.27) |
| GMV (ml) | 0.57 | −0.57 (t) | 629.59 (44.17) | 638.30 (76.39) |
| WMV (ml) | 0.20 | −1.30 (t) | 394.44 (42.51) | 407.64 (43.93) |
| CSFV (ml) | 0.20 | −1.29 (t) | 246.49 (59.72) | 263.48 (54.02) |
| TIV (ml) | 0.20 | −1.30 (t) | 1270.52 (106.92) | 1309.42 (139.03) |
Clinical Assessment: The most commonly reported symptoms from the participants during hospitalization were - fever, cough, body ache, chills, difficulty breathing, bowel irritation, nausea, loss of sense of smell and loss of consciousness. We also assessed if they were having any ongoing/new symptoms from day of discharge to the day of scan – fatigue, anxiety, lack of attention, body ache, headache, memory loss, delayed recovery of sense of taste and/or smell, bowel irritation and interestingly, hair loss were commonly reported. Particularly, since we were interested in fatigue related correlates of GMV, a subset of each group (nCOVID = 33, nHC = 18), successfully reported their fatigue levels on a scale of 0–5, with 0 representing no fatigue and 5 representing the highest fatigue levels observed during work. The average fatigue score in the sub-set of COVID participants was 2.67/5 ± 1.27 [SD] and that of the HC group was 0.61 ± 0.78 [SD].
Imaging: High-resolution structural images were acquired using a 3T GE scanner with a 32-channel head coil in 3D imaging mode with a fast BRAVO sequence. The imaging parameters were TI = 450 ms; 244 x 200 matrix; Flip angle = 12 and FOV = 256 mm, number of slices = 152 (sagittal), slice thickness = 1.00 mm and spatial resolution of 1.0 mm x 1.0 mm x 1.0 mm.
Data Pre-Processing: We performed pre-processing using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/) within the MATLAB environment (Mathworks Inc, Massachusetts, USA). All anatomical images were visually inspected for artifacts, re-centered and reoriented to the anterior-posterior commissure (ac-pc) line. Each brain compartment was segmented into specific tissue classes mainly - gray matter (GM), white matter (WM), and cerebro-spinal fluid (CSF). A study-specific template was first generated using the fast diffeomorphic image registration algorithm (DARTEL) (Ashburner, 2007) which is representative of the average across all the participants included in the study (Ashburner and Friston, 2009; Yassa and Stark, 2009). Subject level maps were non-linearly warped to this reference template for relatively higher specificity and accuracy (Yassa and Stark, 2009). Finally, each map was normalized to the Montreal Neurological Institute (MNI) space using affine transformation and resampled to an isotropic voxel dimension of 1.5 mm x 1.5 mm x 1.5 mm. Modulated images were obtained for each subject, which account for contractions and expansions from non-linear spatial transformations. The normalized modulated images were then spatially smoothed with a gaussian kernel of 8 mm. VBM is a volumetric computational method that can quantify voxel-wise changes in tissue volume in the gray matter (GM)(Ashburner and Friston, 2000). It is a useful method to report group level differences in tissue volume between patients and healthy controls (HCs), using T1-weighted anatomical images. VBM can be estimated from gray matter probability maps obtained from the segmentation stage. Each value in a tissue specific probability map represents the likelihood of the voxel belonging to a brain compartment and tissue volumes can estimated by summing over the product of each voxel's dimension and the corresponding probabilities (L ü ders et al., 2002). Total Intracranial Volume (TIV) is the sum of volumes from each major compartment in the brain – GM, WM, and CSF, with TIV = GMV + WMV + CSFV; where GMV = Gray Matter Volume, WMV = White Matter Volume and CSFV = Cerebro-Spinal Fluid Volume. These quantities were used to assess central tendency measures in each group. To visualize the probability distributions and group average compartmental and total brain volumes, we customized and adopted a script in RStudio (RStudio. RStudio Team, 2021) to generate a ‘raincloud’ figure, as depicted in a recent publication (Allen et al., 2021).
Statistical Analysis: To assess differences in participant demographics, we performed two sample t-test on age, GMV, WMV, CSFV and TIV and chi-squared test for sex differences between the two groups. Since the fatigue scores deviated from normality (Shapiro Wilk: p < 0.05), we used Wilcoxon's ranksum test to assess group difference between HCs and COVID subjects. To determine group level differences in GMV, we performed a two-sample t-test using the smoothed, modulated, and normalized GM tissue maps from the two groups. TIV of each subject was group-mean centered and added as a covariate along with age and sex to account for confounding effects. An implicit mask with absolute threshold of 20% above the group mean was set to exclude unwanted voxel quantities from the smoothed GM maps. Regions with significant difference in GMV between the two groups were identified by first applying a height threshold of p unc < 0.001 and then family wise error (FWE) corrected at p FWE < 0.05, for multiple comparisons. Cluster-based thresholding can be a problem when using VBM since residuals tend to vary in spatial smoothness. Therefore, we used a non-stationary cluster-based correction available in SPM to evaluate significant results.
To evaluate the relationship between GMV and fatigue among COVID and HC participants, we performed a multiple linear regression analysis, with voxel-wise GMV as the response variable and the fatigue scores as the covariate of interest, while, age, sex and TIV were included as covariates of no interest. Regions with significant correlation between GMV and fatigue score were identified using non-stationary cluster-based thresholding at height threshold p unc < 0.01 and FWE corrected at p FWE < 0.05, for multiple comparisons. To visualize any significant linear relationship between the two variables, the average GMV within the significant cluster was obtained from each subject across both groups. These average GMV values were then linearly regressed against the fatigue scores and age, sex and TIV of each participant were regressed out during this step. The mean GMV across the participants were then added back to the residuals and the correlation with fatigue scores was computed and visualized within a scatter plot and a line of best fit with 95% confidence interval. The correlation analysis and the graphical plotting was done using ‘inhouse’ scripts prepared in RStudio (RStudio. RStudio Team, 2021).
3. Results
We assessed group level differences in demographics (age, sex and fatigue), as well as in global VBM metrics including GMV, WMV, CSFV and TIV. We observed no significant differences in age and sex between the two groups (p > 0.05). However, the COVID group demonstrated significantly higher fatigue levels compared to HC group (T = 1093, p = 2.86e-07). No significant difference was observed in any global morphometry metrics (p > 0.05). The central tendency measures for each measure along with statistical results are listed in Table 1 (see Fig. S1 for a visual assessment of group-wise distributions of GMV, WMV, CSFV and TIV).
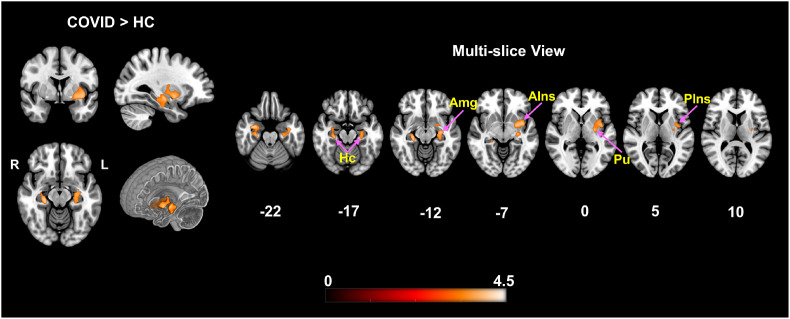
The COVID survivors demonstrated significantly higher GMV compared to HCs. Fig. 1 (left) shows two significant clusters exhibiting GMV differences between the groups in axial, sagittal and coronal planes. The clusters comprised of anatomical regions majorly from the Limbic System and Basal Ganglia. The multi-slice axial view (Fig. 1, right) is provided with labels and arrows identifying some of these regions. More specifically, bilateral hippocampus (Hc), left amygdala (Amg), insula (AIns, PIns), right entorhinal area (Ent.) and Parahippocampal gyrus (PHG) from the Limbic System can be observed with higher GMV in the COVID group. From the Basal Ganglia – left putamen and pallidum also demonstrated higher GMV among the survivors compared to the HC group. The clusters survived a non-stationary cluster extent threshold of k E = 1000 voxels with maximum peak voxel t-statistic values of t peak = 4.42 and 4.03, respectively.
Fig. 1.
VBM demonstrating significantly higher gray matter volume in COVID-19 subjects compared to HC. Two significant clusters were observed, comprising of several deep brain structures: Right – Hc, Am, Ent, PHG, VDC and Left – Hc, VDC, Pu, Pd, Am, PP, AIns, PIns. The clusters surviving FWE correction, consisted of 1000 and 1968 voxels, with exact corrected p-values of p = 0.017 and 0.023 at MNI coordinates: [34 -4 -24] and [-28 -16 -10], respectively. The orthogonal slices on the left show the difference maps along with a cut-to-depth volume rendered image to better visualize the spatial extent of the anatomical locations comprising the cluster. The multi-slice image on the right shows finer slices (Z-slice gap≥5) to highlight and assess the structural regions with significantly higher GMV. The cluster extends from inferior to superior Z-slices spanning from Hc to AIns, Pu, and PIns regions, respectively. Some of the relevant brain regions with significant differences have been pointed within the figure with purple arrows. The colorbar represents t-score values from the group level contrast. Keys: Hc = Hippocampus, Am = Amygdala, Ent = Entorhinal Area, PHG = Parahippocampal Gyrus, VDC = Ventral Diencephalon, Pu = Putamen, Pd = Pallidum, PP = Planum Polare, AIns = Anterior Insula and PIns = Posterior Insula. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
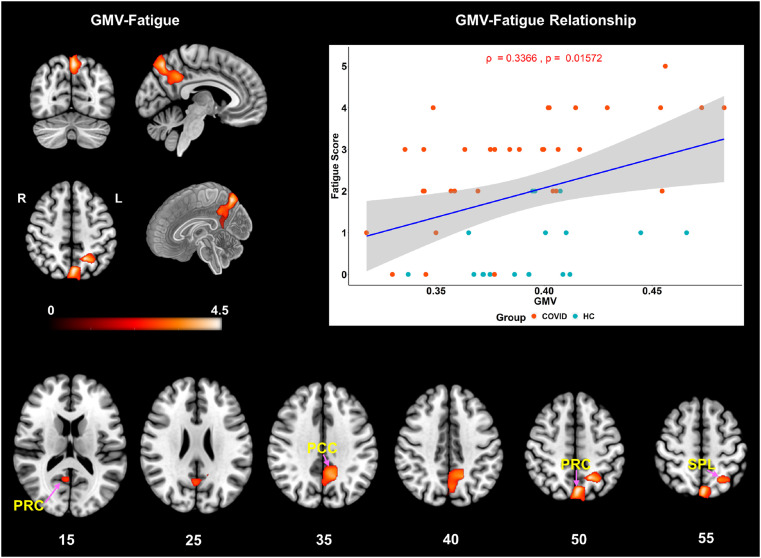
For the correlation analysis using self-reported fatigue scores, the COVID and HC groups (nCOVID = 33, nHC = 18) demonstrated significantly positive correlation with GMV in Posterior Cingulate Cortex (PCC), Precuneus (PRC) and Superior Parietal Lobule (SPL). The top left image in Fig. 2 shows the significant cluster that comprised of these regions (see Fig. 2 bottom row, for a multi-slice view). The scatter plot (Fig. 2, top right) demonstrates the linear relationship (Spearman's ρ = 0.34, p = 0.016) across the whole group between fatigue scores and the GMV of each subject within the cluster. The scatter plot combines data from both groups, and it can be noted that the light pink dots (COVID subjects) have a significantly higher effect compared to the cyan dots (HC subjects). This can also be interpreted more clearly from Fig. S3 in the Supplementary Materials where the linear regression line and correlation values of each group can be assessed separately (ρ_COVID = 0.60, p_COVID = 0.0002 vs. ρ_HC = 0.43, p_HC = 0.07). Therefore, while GMV is positively correlated with fatigue across both groups, the overall trend is primarily driven by the effects from the COVID group.
Fig. 2.
VBM demonstrating significantly positive correlation with fatigue scores across the whole group. The significant cluster (top-left) consisting of 3547 voxels (kE= 3547), comprised of: Left – PCC, PRC, SPL and Right - PRC with peak t-score of 4.74 and exact corrected p-value of pFWE = 0.019 at MNI coordinates: [-16 -54 48]. The axial slices (bottom) show the spatial extent of the same cluster over finer slices. The scatter plot (top-right) with the linear regression line shows significant positive correlation of GMV with self-reported fatigue score across the whole group (ρ = 0.34, p = 0.016, r2= 0.11). Please note, the ρ represents Spearman's rank-order correlation coefficient. The light pink colored dots represent the COVID subjects, and the cyan dots represent the HCs. The COVID group clearly demonstrates higher effects than the HC group. Please note, the GMV in the x-axis represents the residuals plus the mean GMV of the cluster across subjects added back after linear regression. Keys: PCC = Posterior Cingulate Cortex, PRC = Precuneus, SPL = Superior Parietal Lobule. The linear plot (blue) represents the least squares regression line (best fit), and the shaded gray area represents the 95% confidence interval. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
The VBM results support our hypothesis that COVID survivors, now PCR negative, demonstrate altered GMV compared to HCs even 2 weeks after hospital discharge. For the single case reports in the literature – hyperintensity in FLAIR images can arise from several sources including ischemia, micro-hemorrhages, and damage to vasculature, commonly observed in acute COVID patients and these neurological disturbances can modulate tissue volumes. We observed higher GMV in the left Hc and Amg at the group level, which aligns with a case report of hyperintensities in the left Hc and Amg from FLAIR images in an older patient (Male, 56 years) (Kremer et al., 2020). Recent neuroimaging studies have also reported higher GMV among COVID cohorts, in the bilateral Hc after 3 month-follow-up (Lu et al., 2020), as well as, in bilateral Hc and Amg in another follow-up study (3 and 6 months) (Tu et al., 2021). Interestingly, Tu et al., 2021 also report significant correlation of the left Hc and Amg with PCL-5 scores, demonstrating stress related structural changes in these regions. On the contrary, cortical thickness was reported to be reduced in the left Hc after a 3 month-follow-up study (Qin et al., 2021) and in the Amg after infection with COVID-19 in a longitudinal study (Douaud et al., 2021).
We also observed higher GMV in AIns and PIns regions indicating GMV alterations in insular lobes, which were reported to be hyperintense in specific patients (Kandemirli et al., 2020). Similarly, higher GMV was also observed at the group level in the bilateral Ins (Lu et al., 2020), while, on the other hand, others reported reduced cortical thickness in insular lobes (Qin et al., 2021; Douaud et al., 2021). Early reports from severely acute hospitalized patients also mention hyperintense lesions in the brain stem and basal ganglia (Paterson et al., 2020). Our results also show higher GMV in VDC, Pu, and Pd which constitute major parts of the basal ganglia and sub-cortical system. Therefore, the group level estimates from our VBM analysis converge with acutely ill ‘individual’ COVID patient findings and maintain consistency with current neuroimaging reports from moderate to large samples of recovering COVID survivors.
Although not statistically significant (p > 0.05), it is worth mentioning that the mean compartmental CSFV tended to be higher in the COVID group compared to the HCs (see Table 1 and Fig. S1 from Supplementary Materials). This seems to align with the recent UK-Biobank longitudinal study (n = 401), who reported increased CSFV after COVID infection (Douaud et al., 2021). Our data indicates more variation in brain volumes among the COVID patients, with higher standard deviation (SD) in GMV, WMV and TIV (see Table 1). This might arise from varying levels of tissue swelling due to acute infection among surviving patients. However, the underlying neurophysiology that elicit such changes is still unclear. Highly prevalent acute stage neurological damage from CNS viral or vascular pathologies can cause local changes in tissue concentration. Transient reduction in cerebral blood flow (CBF) can also cause gray matter concentration to increase due to change in hydration levels (Ge et al., 2017). Overall, continued brain swelling from neuro-vascular injuries may explain why we observed locally higher GMV in the COVID group even 2 weeks after testing negative.
Our results also support the hypothesis that fatigue levels experienced by the COVID survivors will be higher compared to the HC group and GMV will correlate more strongly with self-reported fatigue in this group. A rising concern with recovering patients has been the manifestation of a sequelae of symptoms that persist several months along the recovery timeline (Logue et al., 2021; Tabacof et al., 2020; Peluso et al., 2021). Fatigue was the highest reported symptom in these studies among others, including lack of attention, delayed recovery of loss of sense of smell and taste. We asked the healthy subjects and surviving patients, what level of fatigue is disrupting their daily work? Based on their reported score, we observed significantly higher levels of fatigue within the COVID group when compared to HCs. We also observe from Fig. 2 that GMV in PCC, PRC and SPL regions are more strongly correlated to fatigue in COVID survivors compared to healthy controls (see Fig. S3 in the Supplementary Materials for effects in each group separately). This could also indicate a link to high percentage of survivors experiencing fatigue during and post recovery, eventually leading to functional or cognitive disruption. This is also typical of neurodegenerative populations and these regions have been found to be related to fatigue. For example, higher metabolic activity within PCC has been shown to be positively correlated with higher fatigue levels in Parkinson's patients (Cho et al., 2017). PCC and SPL have also been shown to be associated with fatigue in patients with chronic fatigue syndrome (CFS) (Boissoneault et al., 2018; Gay et al., 2016). Moreover, atrophy in the parietal lobe has been shown to be associated with fatigue among multiple sclerosis (MS) patients (Calabrese et al., 2010; Pellicano et al., 2010).
Not shown in ‘Results’ is another cluster (did not survive non-stationary clustering threshold, please see Supplementary Material, Fig. S2) that consisted of brain regions from the cholinergic output (BsF, AcA) from the ventral BG and orbitofrontal cortex (MOG, GrE), ACG and MFC from the ventromedial prefrontal cortex (vmPFC). When the cluster GMV was linearly regressed against fatigue, a significant positive correlation (Spearman's ρ = 0.41, p = 0.0028) was observed (see Supplementary Material, Fig. S2). This can also be observed more intuitively in Fig. S4 in the Supplementary Materials, where we show the effects of each group separately (ρ_COVID = 0.52, p_COVID = 0.002 vs. ρ_HC = 0.19, p_HC = 0.45). Interestingly, the BG and vmPFC regions have also been shown to be functionally associated with fatigue (Wylie et al., 2017, 2019; Chaudhuri and Behan, 2000; Dobryakova et al., 2015, 2018, 2020). We observed this effect with 18 HCs and 33 COVID subjects and our expectation is that with a bigger sample size in both groups, this cluster would survive and therefore it may be of relevance to fatigue related effects among survivors.
In addition, we also assessed correlation between GMV with fatigue within the clusters that showed GMV differences between groups. We observed a small effect, but it was not significant (p > 0.05). We have shown these results in Fig. S5 within the Supplementary Materials. We then evaluated any between group differences of GMV within the clusters where significant correlations with fatigue were observed. We found no significant differences in GMV between the two groups within the fatigue-related clusters (p > 0.05). A possible explanation for these observations could be that the neuro invasion of the virus migrates to fatigue-related brain regions where correlations between brain morphometrics and fatigue exist that are independent of any difference in GMV between the groups. A recent study has shown that besides other bodily systems, the novel coronavirus can spread and sustain throughout the brain, which may be causing symptom related pathologies in post-acute sequalae of SARS-CoV-2 infection (PASC) or ‘long-COVID’ patients (Daniel et al., 2022). What is deeply concerning is that they observed these effects even in patients that were asymptomatic during the acute phase. Therefore, the neurophysiology behind structural changes and the relationship with symptom related changes in behavior need further investigation.
In conclusion, our results highlight group level effects in surviving COVID-negative patients that replicate single patient case studies, as well as several neuroimaging studies from surviving COVID-19 cohorts. We have shown significant GMV alterations in multiple brain regions from the limbic system and basal ganglia. We found the COVID group to be more susceptible to fatigue at work, assessed statistically by higher fatigue scores among survivors when compared to the HC group. Moreover, we also noticed significantly stronger positive associations between regional brain volume within the PCC, Precuneus and SPL with self-reported fatigue at work among this group of survivors. Importantly, these brain regions are commonly found to be associated with fatigue in several other neurodegenerative diseases (Cho et al., 2017; Boissoneault et al., 2018; Gay et al., 2016; Calabrese et al., 2010; Pellicano et al., 2010). We also noticed similar effects within regions from the ventral basal ganglia and vmPFC, which are also known to be modulated by neuronal damage and characterized from functional neuroimaging relating to fatigue, pain, emotion, attention, and somatosensory processing (Bornh ö vd et al., 2002; Inagaki et al., 2012; Sergeeva et al., 2015; van Schouwenburg et al., 2015).
5. Limitations and future directions
Despite our emphasis on group level analysis, we understand that in a clinical setting, it may have little transferability, owing to idiosyncrasies associated with each patient. A possible approach to address this issue would be to compare patient specific VBM against a sufficiently sized control sub-group randomly selected from a larger cohort (Scarpazza et al., 2016). However, it may not be very practical in a clinical setting unless a well-designed control dataset is available for a clinician. Nevertheless, our group level results from a single site seem to match single patient findings quite well, especially when they were collected from several centers across different countries (Gulko et al., 2020). Moreover, currently, we only have 46 COVID subjects. While we do observe significant effects, we still need a larger sample size to verify the main effects more conclusively. Another concern we have is the reversibility or transient nature of some effects. These patients were scanned two weeks after hospital discharge. It is possible some critical effects may have already disappeared through recovery or reversible neurological processes. Therefore, a better approach could be to scan the patients at onset, during and after recovery along with behavioral parameters to assess any possible trends unique to the neurological pathology in COVID patients.
CRediT author statement
Rakibul Hafiz: Methodology, Software, Formal Analysis, Data Curation, Writing – Original Draft, Review and Editing. Tapan K. Gandhi: Conceptualization, Investigation, Resources, Supervision, Writing – Review and Editing. Sapna Mishra: Investigation, Resources, Data Curation, Writing – Review and Editing. Alok Prasad: Writing – Review and Editing. Vidur Mahajan: Writing – Review and Editing. Xin Di: Methodology, Writing – Review and Editing. Benjamin H. Natelson: Writing – Review and Editing. Bharat Biswal: Conceptualization, Resources, Project Administration, Supervision, Writing – Review and Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by NIH grants: R01AT009829 and R01MH131335 and MeitY (Government of India) under Grant No: 4(16)/2019-ITEA.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ynirp.2022.100095.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Allen M., Poggiali D., Whitaker K., Marshall T.R., Van Langen J., Kievit R.A. Raincloud plots: a multi-platform tool for robust data visualization. Wellcome Open Research. 2021;4:63. doi: 10.12688/wellcomeopenres.15191.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Voxel-based morphometry—the methods. Neuroimage. 2000;11(6):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Computing average shaped tissue probability templates. Neuroimage. 2009;45(2):333–341. doi: 10.1016/j.neuroimage.2008.12.008. Epub 2009/01/17, PubMed PMID: 19146961. [DOI] [PubMed] [Google Scholar]
- Boissoneault J., Letzen J., Lai S., Robinson M.E., Staud R. Static and dynamic functional connectivity in patients with chronic fatigue syndrome: use of arterial spin labelling fMRI. Clin. Physiol. Funct. Imag. 2018;38(1):128–137. doi: 10.1111/cpf.12393. Epub 2016/09/28, PubMed PMID: 27678090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornhövd K., Quante M., Glauche V., Bromm B., Weiller C., Büchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain. 2002;125(Pt 6):1326–1336. doi: 10.1093/brain/awf137. Epub 2002/05/23, PubMed PMID: 12023321. [DOI] [PubMed] [Google Scholar]
- Calabrese M., Rinaldi F., Grossi P., Mattisi I., Bernardi V., Favaretto A., Perini P., Gallo P. Basal ganglia and frontal/parietal cortical atrophy is associated with fatigue in relapsing-remitting multiple sclerosis. Mult. Scler. 2010;16(10):1220–1228. doi: 10.1177/1352458510376405. Epub 20100729, PubMed PMID: 20670981. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A., Behan P.O. Fatigue and basal ganglia. J. Neurol. Sci. 2000;179(S 1–2):34–42. doi: 10.1016/s0022-510x(00)00411-1. PubMed PMID: 11054483. [DOI] [PubMed] [Google Scholar]
- Cho S.S., Aminian K., Li C., Lang A.E., Houle S., Strafella A.P. Fatigue in Parkinson's disease: the contribution of cerebral metabolic changes. Hum. Brain Mapp. 2017;38(1):283–292. doi: 10.1002/hbm.23360. Epub 2016/08/29, PubMed PMID: 27571419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel C., Sydney S., Sabrina R., Alison G., Joon-Yong C., Manmeet S., Claude Kwe Y., Clayton W., James D., Kris Y., Sung Hee K., Andrew P., Peter B., Martha Q., Stefania P., Madeleine P., Vincent M., Frida B., Marcos R.-B., Eli B., Daniel H., Joseph R., Kapil S., Ronson M., Ali T., Shahabuddin S., Michael M., Karin P., Jeffrey C., Emmie de W., Kevin V., Stephen H., David K. SARS-CoV-2 infection and persistence throughout the human body and brain. Nature Portfolio. 2022 doi: 10.21203/rs.3.rs-1139035/v1. [DOI] [Google Scholar]
- Dobryakova E., Genova H.M., DeLuca J., Wylie G.R. The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders. Front. Neurol. 2015;6(52) doi: 10.3389/fneur.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobryakova E., Hulst H.E., Spirou A., Chiaravalloti N.D., Genova H.M., Wylie G.R., DeLuca J. Fronto-striatal network activation leads to less fatigue in multiple sclerosis. Mult. Scler. 2018;24(9):1174–1182. doi: 10.1177/1352458517717087. Epub 20170619, PubMed PMID: 28627957. [DOI] [PubMed] [Google Scholar]
- Dobryakova E., Genova H., Schneider V., Chiaravalloti N.D., Spirou A., Wylie G.R., DeLuca J. Reward presentation reduces on-task fatigue in traumatic brain injury. Cortex. 2020;126:16–25. doi: 10.1016/j.cortex.2020.01.003. Epub 20200124, PubMed PMID: 32062140. [DOI] [PubMed] [Google Scholar]
- Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., Lange F., Andersson J.L.R., Griffanti L., Duff E., Jbabdi S., Taschler B., Winkler A., Nichols T.E., Collins R., Matthews P.M., Allen N., Miller K.L., Smith S.M. medRxiv; 2021. Brain Imaging before and after COVID-19 in UK Biobank. Epub 20210620, PubMed PMID: 34189535; PMCID: PMC8240690. [DOI] [Google Scholar]
- Duan K., Premi E., Pilotto A., Cristillo V., Benussi A., Libri I., Giunta M., Bockholt H.J., Liu J., Campora R., Pezzini A., Gasparotti R., Magoni M., Padovani A., Calhoun V.D. Alterations of frontal-temporal gray matter volume associate with clinical measures of older adults with COVID-19. Neurobiol. Stress. 2021;14 doi: 10.1016/j.ynstr.2021.100326. Epub 20210413, PubMed PMID: 33869679; PMCID: PMC8041745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay C.W., Robinson M.E., Lai S., O'Shea A., Craggs J.G., Price D.D., Staud R. Abnormal resting-state functional connectivity in patients with chronic fatigue syndrome: results of seed and data-driven analyses. Brain Connect. 2016;6(1):48–56. doi: 10.1089/brain.2015.0366. Epub 2015/11/10, PubMed PMID: 26449441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Q., Peng W., Zhang J., Weng X., Zhang Y., Liu T., Zang Y.-F., Wang Z. Short-term apparent brain tissue changes are contributed by cerebral blood flow alterations. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0182182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulko E., Oleksk M.L., Gomes W., Ali S., Mehta H., Overby P., Al-Mufti F., Rozenshtein A. MRI brain findings in 126 patients with COVID-19: initial observations from a descriptive literature Review. Am. J. Neuroradiol. 2020 doi: 10.3174/ajnr.A6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T.K., Muscatell K.A., Irwin M.R., Cole S.W., Eisenberger N.I. Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage. 2012;59(4):3222–3226. doi: 10.1016/j.neuroimage.2011.10.090. Epub 2011/11/04, PubMed PMID: 22079507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandemirli S.G., Dogan L., Sarikaya Z.T., Kara S., Akinci C., Kaya D., Kaya Y., Yildirim D., Tuzuner F., Yildirim M.S., Ozluk E., Gucyetmez B., Karaarslan E., Koyluoglu I., Demirel Kaya H.S., Mammadov O., Kisa Ozdemir I., Afsar N., Citci Yalcinkaya B., Rasimoglu S., Guduk D.E., Kedir Jima A., Ilksoz A., Ersoz V., Yonca Eren M., Celtik N., Arslan S., Korkmazer B., Dincer S.S., Gulek E., Dikmen I., Yazici M., Unsal S., Ljama T., Demirel I., Ayyildiz A., Kesimci I., Bolsoy Deveci S., Tutuncu M., Kizilkilic O., Telci L., Zengin R., Dincer A., Akinci I.O., Kocer N. Brain MRI findings in patients in the intensive care unit with COVID-19 infection. Radiology. 2020;297(1):E232–e5. doi: 10.1148/radiol.2020201697. Epub 2020/05/10, PubMed PMID: 32384020; PMCID: PMC7507997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller E., Brandi G., Winklhofer S., Imbach L.L., Kirschenbaum D., Frontzek K., Steiger P., Dietler S., Haeberlin M., Willms J., Porta F., Waeckerlin A., Huber M., Abela I.A., Lutterotti A., Stippich C., Globas C., Varga Z., Jelcic I. Large and small cerebral vessel involvement in severe COVID-19: detailed clinical workup of a case series. Stroke. 2020;51(12):3719–3722. doi: 10.1161/strokeaha.120.031224. Epub 2020/10/16, PubMed PMID: 33054673; PMCID: PMC7678671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer S., Lersy F., Anheim M., Merdji H., Schenck M., Oesterlé H., Bolognini F., Messie J., Khalil A., Gaudemer A., Carré S., Alleg M., Lecocq C., Schmitt E., Anxionnat R., Zhu F., Jager L., Nesser P., Mba Y.T., Hmeydia G., Benzakoun J., Oppenheim C., Ferré J.C., Maamar A., Carsin-Nicol B., Comby P.O., Ricolfi F., Thouant P., Boutet C., Fabre X., Forestier G., de Beaurepaire I., Bornet G., Desal H., Boulouis G., Berge J., Kazémi A., Pyatigorskaya N., Lecler A., Saleme S., Edjlali-Goujon M., Kerleroux B., Constans J.M., Zorn P.E., Mathieu M., Baloglu S., Ardellier F.D., Willaume T., Brisset J.C., Caillard S., Collange O., Mertes P.M., Schneider F., Fafi-Kremer S., Ohana M., Meziani F., Meyer N., Helms J., Cotton F. Neurologic and neuroimaging findings in patients with COVID-19: a retrospective multicenter study. Neurology. 2020;95(13):e1868–e1882. doi: 10.1212/wnl.0000000000010112. Epub 2020/07/19, PubMed PMID: 32680942. [DOI] [PubMed] [Google Scholar]
- Lüders E., Steinmetz H., Jäncke L. Brain size and grey matter volume in the healthy human brain. Neuroreport. 2002;13(17) doi: 10.1097/01.wnr.0000049603.85580.da. https://journals.lww.com/neuroreport/Fulltext/2002/12030/Brain_size_and_grey_matter_volume_in_the_healthy.40.aspx [DOI] [PubMed] [Google Scholar]
- Logue J.K., Franko N.M., McCulloch D.J., McDonald D., Magedson A., Wolf C.R., Chu H.Y. Sequelae in adults at 6 Months after COVID-19 infection. JAMA Netw. Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2021.0830. Epub 2021/02/20, PubMed PMID: 33606031; PMCID: PMC7896197 Merck, Ellume, the Bill and Melinda Gates Foundation, GlaxoSmithKline, and Pfizer; receiving grants from Sanofi-Pasteur; and receiving reagents from Cepheid Research outside the submitted work. No other disclosures were reported. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Li X., Geng D., Mei N., Wu P.-Y., Huang C.-C., Jia T., Zhao Y., Wang D., Xiao A., Yin B. Cerebral micro-structural changes in COVID-19 patients – an MRI-based 3-month follow-up study. EClini. Med. 2020:25. doi: 10.1016/j.eclinm.2020.100484. PubMed PMID: 100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson P., Alshafai L., Krings T. Neuroimaging findings in patients with COVID-19. Am. J. Neuroradiol. 2020;41(8):1380–1383. doi: 10.3174/ajnr.A6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R.W., Brown R.L., Benjamin L., Nortley R., Wiethoff S., Bharucha T., Jayaseelan D.L., Kumar G., Raftopoulos R.E., Zambreanu L., Vivekanandam V., Khoo A., Geraldes R., Chinthapalli K., Boyd E., Tuzlali H., Price G., Christofi G., Morrow J., McNamara P., McLoughlin B., Lim S.T., Mehta P.R., Levee V., Keddie S., Yong W., Trip S.A., Foulkes A.J.M., Hotton G., Miller T.D., Everitt A.D., Carswell C., Davies N.W.S., Yoong M., Attwell D., Sreedharan J., Silber E., Schott J.M., Chandratheva A., Perry R.J., Simister R., Checkley A., Longley N., Farmer S.F., Carletti F., Houlihan C., Thom M., Lunn M.P., Spillane J., Howard R., Vincent A., Werring D.J., Hoskote C., Jäger H.R., Manji H., Zandi M.S. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104–3120. doi: 10.1093/brain/awaa240. Epub 2020/07/09, PubMed PMID: 32637987; PMCID: PMC7454352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicano C., Gallo A., Li X., Ikonomidou V.N., Evangelou I.E., Ohayon J.M., Stern S.K., Ehrmantraut M., Cantor F., McFarland H.F., Bagnato F. Relationship of cortical atrophy to fatigue in patients with multiple sclerosis. Arch. Neurol. 2010;67(4):447–453. doi: 10.1001/archneurol.2010.48. [DOI] [PubMed] [Google Scholar]
- Peluso M.J., Kelly J.D., Lu S., Goldberg S.A., Davidson M.C., Mathur S., Durstenfeld M.S., Spinelli M.A., Hoh R., Tai V., Fehrman E.A., Torres L., Hernandez Y., Williams M.C., Arreguin M.I., Bautista J.A., Ngo L.H., Deswal M., Munter S.E., Martinez E.O., Anglin K.A., Romero M.D., Tavs J., Rugart P.R., Chen J.Y., Sans H.M., Murray V.W., Ellis P.K., Donohue K.C., Massachi J.A., Weiss J.O., Mehdi I., Pineda-Ramirez J., Tang A.F., Wegner M., Assenzio M., Yuan Y., Krone M., Rutishauser R.L., Rodriguez-Barraquer I., Greenhouse B., Sauceda J.A., Gandhi M., Hsue P., Henrich T.J., Deeks S.G., Martin J.N. Rapid implementation of a cohort for the study of post-acute sequelae of SARS-CoV-2 infection/COVID-19. medRxiv. 2021;11 doi: 10.1101/2021.03.11.21252311. 2021.03. [DOI] [Google Scholar]
- Qin Y., Wu J., Chen T., Li J., Zhang G., Wu D., Zhou Y., Zheng N., Cai A., Ning Q., Manyande A., Xu F., Wang J., Zhu W. Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological manifestations. J. Clin. Invest. 2021;131(8) doi: 10.1172/jci147329. PubMed PMID: 33630760; PMCID: PMC8262559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmanesh A., Derman A., Lui Y.W., Raz E., Loh J.P., Hagiwara M., Borja M.J., Zan E., Fatterpekar G.M. COVID-19-associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020;297(1):E223–E227. doi: 10.1148/radiol.2020202040. Epub 2020/05/21, PubMed PMID: 32437314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudio. RStudio Team . RStudio, Inc; Boston, MA: 2021. RStudio: Integrated Development for R.http://www.rstudio.com/ 2021. RStudio. [Google Scholar]
- Scarpazza C., Nichols T.E., Seramondi D., Maumet C., Sartori G., Mechelli A. When the single matters more than the group (II): addressing the problem of high false positive rates in single case voxel based morphometry using non-parametric statistics. Front. Neurosci. 2016;10:6. doi: 10.3389/fnins.2016.00006. PubMed PMID: 26834533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeeva M., Rech J., Schett G., Hess A. Response to peripheral immune stimulation within the brain: magnetic resonance imaging perspective of treatment success. Arthritis Res. Ther. 2015;17(1):268. doi: 10.1186/s13075-015-0783-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabacof L., Tosto-Mancuso J., Wood J., Cortes M., Kontorovich A., McCarthy D., Rizk D., Mohammadi N., Breyman E., Nasr L., Kellner C., Putrino D. Post-acute COVID-19 syndrome negatively impacts health and wellbeing despite less severe acute infection. medRxiv. 2020 doi: 10.1101/2020.11.04.20226126. 2020.11.04.20226126. [DOI] [Google Scholar]
- Tu Y., Zhang Y., Li Y., Zhao Q., Bi Y., Lu X., Kong Y., Wang L., Lu Z., Hu L. Post-traumatic stress symptoms in COVID-19 survivors: a self-report and brain imaging follow-up study. Mol. Psychiatr. 2021 doi: 10.1038/s41380-021-01223-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schouwenburg M.R., den Ouden H.E., Cools R. Selective attentional enhancement and inhibition of fronto-posterior connectivity by the basal ganglia during attention switching. Cerebr. Cortex. 2015;25(6):1527–1534. doi: 10.1093/cercor/bht345. New York, NY : 1991. Epub 2013/12/18, PubMed PMID: 24343891. [DOI] [PubMed] [Google Scholar]
- (WHO) WHO . 2022. WHO Coronavirus (COVID-19) Dashboard. [02/12/2022]. Available from: WHO Coronavirus (COVID-19) Dashboard. [Google Scholar]
- Wylie G.R., Dobryakova E., DeLuca J., Chiaravalloti N., Essad K., Genova H. Cognitive fatigue in individuals with traumatic brain injury is associated with caudate activation. Sci. Rep. 2017;7(1):8973. doi: 10.1038/s41598-017-08846-6. Epub 20170821, PubMed PMID: 28827779; PMCID: PMC5567054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie G.R., Genova H., Dobryakova E., DeLuca J., Chiaravalloti N., Falvo M., Cook D. Fatigue in Gulf War Illness is associated with tonically high activation in the executive control network. NeuroI. Clinic. 2019;21 doi: 10.1016/j.nicl.2018.101641. Epub 20181211, PubMed PMID: 30558870; PMCID: PMC6411905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa M.A., Stark C.E. A quantitative evaluation of cross-participant registration techniques for MRI studies of the medial temporal lobe. Neuroimage. 2009;44(2):319–327. doi: 10.1016/j.neuroimage.2008.09.016. Epub 2008/10/22, PubMed PMID: 18929669. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.