ABSTRACT
Ammonia-oxidizing archaea (AOA) and bacteria (AOB) perform key steps in the global nitrogen cycle, the oxidation of ammonia to nitrite. While the ammonia oxidation pathway is well characterized in AOB, many knowledge gaps remain about the metabolism of AOA. Hydroxylamine is an intermediate in both AOB and AOA, but homologues of hydroxylamine dehydrogenase (HAO), catalyzing bacterial hydroxylamine oxidation, are absent in AOA. Hydrazine is a substrate for bacterial HAO, while phenylhydrazine is a suicide inhibitor of HAO. Here, we examine the effect of hydrazines in AOA to gain insights into the archaeal ammonia oxidation pathway. We show that hydrazine is both a substrate and an inhibitor for AOA and that phenylhydrazine irreversibly inhibits archaeal hydroxylamine oxidation. Both hydrazine and phenylhydrazine interfered with ammonia and hydroxylamine oxidation in AOA. Furthermore, the AOA “Candidatus Nitrosocosmicus franklandus” C13 oxidized hydrazine into dinitrogen (N2), coupling this reaction to ATP production and O2 uptake. This study expands the known substrates of AOA and suggests that despite differences in enzymology, the ammonia oxidation pathways of AOB and AOA are functionally surprisingly similar. These results demonstrate that hydrazines are valuable tools for studying the archaeal ammonia oxidation pathway.
IMPORTANCE Ammonia-oxidizing archaea (AOA) are among the most numerous living organisms on Earth, and they play a pivotal role in the global biogeochemical nitrogen cycle. Despite this, little is known about the physiology and metabolism of AOA. We demonstrate in this study that hydrazines are inhibitors of AOA. Furthermore, we demonstrate that the model soil AOA “Ca. Nitrosocosmicus franklandus” C13 oxidizes hydrazine to dinitrogen gas, and this reaction yields ATP. This provides an important advance in our understanding of the metabolism of AOA and expands the short list of energy-yielding compounds that AOA can use. This study also provides evidence that hydrazines can be useful tools for studying the metabolism of AOA, as they have been for the bacterial ammonia oxidizers.
KEYWORDS: ammonia-oxidizing archaea, hydrazines
INTRODUCTION
Aerobic oxidation of ammonia (NH3) to nitrite (NO2−) is the first step in nitrification and is carried out by ammonia-oxidizing archaea (AOA) and bacteria (AOB) along with complete ammonia-oxidizing (comammox) bacteria. Comammox bacteria and nitrite-oxidizing bacteria then further oxidize NO2− to nitrate (NO3−). Nitrifiers play a central role in the global nitrogen cycle, with important consequences for greenhouse gas emission and leaching of nitrate from terrestrial environments. AOA are ubiquitous and significantly contribute to nitrification in many ecosystems, including acidic soils, unfertilized soils, and the oligotrophic open ocean. Ammonia monooxygenase (AMO), a member of the copper membrane monooxygenase (CuMMO) superfamily, is found in ammonia-oxidizing archaea and bacteria and initiates the nitrification process through the conversion of NH3 to hydroxylamine (NH2OH) (1, 2). Catalysis by AMO requires the input of two electrons, which are supplied by the downstream oxidation of hydroxylamine to NO2−, leaving two net electrons to enter the respiratory electron transport chain and making the oxidation of hydroxylamine the first energy-yielding reaction in the pathway (1, 3).
The functional and structural understanding of the archaeal AMO has improved through work exploring substrate analogues (4, 5). This has led to applications such as the use of octyne to distinguish between archaeal and bacterial ammonia oxidation in soil microcosms (4) and the use of alkadiynes in combination with click chemistry to label the AMO (6). AMO has never been purified in its active form, and virtually all knowledge about this enzyme comes from work on substrate analogues, highlighting the importance and potential of substrate analogues as tools in nitrification research. Very little is known about how hydroxylamine, produced from the initial oxidation of ammonia by the AMO, is converted to nitrite in archaea.
In AOB, hydroxylamine is converted to nitric oxide (NO) by hydroxylamine dehydrogenase (HAO) (7, 8), and NO is then further oxidized to NO2− by an unknown mechanism (3). HAO is a homotrimer, with each subunit containing eight c-type hemes, and one of these, the active site, is a P460 cofactor (9). In the AOA, no genetic HAO homologue has been identified, and the genetic inventory for the production of c-type hemes is incomplete (10), suggesting that a fundamentally different enzyme system for hydroxylamine oxidation is required. Based on proteomics and comparative genomics, several candidate enzymes have been identified (11), including F420-dependent enzymes and multicopper oxidases. However, no candidate enzymes have been experimentally verified.
Hydrazine (N2H4) is an alternative substrate for HAO from AOB. It competes with hydroxylamine for access to the HAO active site and thus can be described as a competitive inhibitor (12). However, hydrazine can be used as an external source of reductants to supply the bacterial AMO with the electrons required for activity, making it possible to study the oxidation of a wide range of compounds by the AMO (13–19). The product of hydrazine oxidation by the HAO is dinitrogen gas (N2) (20). Anaerobic ammonia-oxidizing (anammox) bacteria also have an HAO homologue, hydrazine dehydrogenase, which catalyzes the conversion of hydrazine to N2 gas as part of their ammonia oxidation pathway (21).
Organohydrazines, on the other hand, are irreversible suicide inhibitors of the HAO, covalently modifying the P460 active site (22). Phenylhydrazine has been used to characterize the HAOs of different groups of AOB, differential responses between AOB groups (23). An attempt was made to use organohydrazines (phenylhydrazine, methylhydrazine, and 2-hydroxyethylhydrazine) to distinguish between bacterial and archaeal ammonia oxidation in soil microcosms (24), relying on the absence of a genetic HAO homologue in the AOA. However, the authors of that study found that the abundances of both AOA and AOB were affected. The inhibition of both AOA and AOB by organohydrazines was confirmed later in a different soil microcosm study (25). However, the effect of organohydrazines on AOA cultures has not been studied, and the inhibition of AOA by hydrazines warrants further investigation. If hydrazines inhibit AOA, they would be valuable tools for investigating the archaeal ammonia oxidation pathway.
The objectives of this study were to investigate the effect of hydrazines on ammonia and hydroxylamine oxidation using three strains of soil AOA and to compare the hydrazine metabolism of AOA and AOB. Specifically, we aimed to address the following questions. (i) Do hydrazines inhibit archaeal hydroxylamine and ammonia oxidation? (ii) Are hydrazines reversible or irreversible inhibitors in AOA? (iii) Are hydrazines oxidized, and do they yield ATP in AOA? (iv) Can AOA oxidize hydrazine to N2 as AOB do?
RESULTS
Effect of phenylhydrazine on NH3- and hydroxylamine-dependent nitrite production by ammonia oxidizers.
To investigate the effect of phenylhydrazine on NH3 and hydroxylamine oxidation by three different AOA strains and Nitrosomonas europaea, levels of NO2− production, as a proxy for activity, were compared after exposure to different concentrations of phenylhydrazine (Fig. 1). Both NH3-dependent (Fig. 1) and hydroxylamine-dependent (Fig. S1) NO2− production were used to characterize and compare the inhibitory thresholds of the AOA and N. europaea.
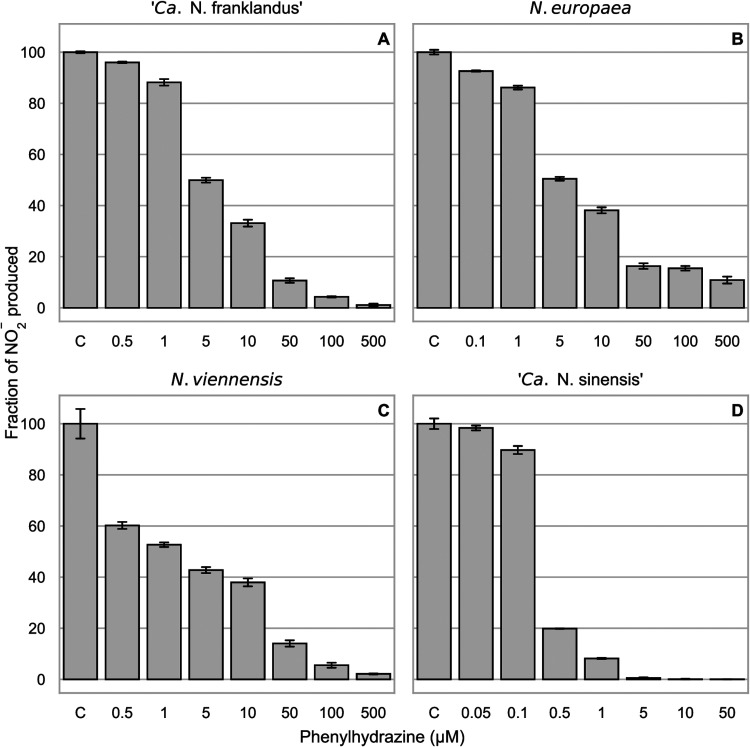
FIG 1.
Percentage of NO2− production compared to the uninhibited control (C) after 1 h of incubation with different concentrations of phenylhydrazine using 100 μM NH4+ as the substrate. NO2− was measured 1 h after the addition of the substrate. Nitrite accumulation in the uninhibited control treatment represents 100% activity, and the treatments with phenylhydrazine are shown as percentages of activity compared to this control. Error bars represent standard deviations (n = 3). One hundred percent activity corresponded to 100 μM, 58 μM, 53 μM, and 61 μM nitrite accumulated after 1 h in (A) “Ca. Nitrosocosmicus franklandus,” (B) N. europaea, (C) N. viennensis, and (D) “Ca. Nitrosotalea sinensis,” respectively.
The inhibition thresholds of “Candidatus Nitrosocosmicus franklandus” (Fig. 1A) and N. europaea (Fig. 1B) were similar, but “Ca. Nitrosocosmicus franklandus” was less sensitive to phenylhydrazine inhibition than Nitrososphaera viennensis (Fig. 1A and C). The acidophilic AOA “Ca. Nitrosotalea sinensis” was more sensitive than the other AOA tested, and 5 μM phenylhydrazine inhibited NH3-dependent NO2− production completely (Fig. 1D).
The inhibitory ranges of phenylhydrazine were similar between NH3-dependent and hydroxylamine-dependent NO2− accumulation (Fig. 1; Fig. S1 in the supplemental material), although the threshold for phenylhydrazine inhibition was higher when hydroxylamine was used as the substrate in N. europaea and N. viennensis. From these results, 100 μM phenylhydrazine was chosen to inhibit all strains in subsequent experiments, except for “Ca. Nitrosotalea sinensis,” where 10 μM phenylhydrazine was used. The lowest concentrations that resulted in nearly full inhibition were chosen to minimize abiotic interactions and toxic effects (26). In addition, hydroxylamine is reactive and potentially toxic and may participate in abiotic reactions. To mitigate toxic effects and abiotic reactions, a suitable hydroxylamine concentration (200 μM for “Ca. Nitrosocosmicus franklandus” and N. viennensis and 100 μM for N. europaea and “Ca. Nitrosotalea sinensis”) was chosen by prescreening a range of hydroxylamine concentrations.
Effect of hydrazine on NH3- and hydroxylamine-dependent NO2− production.
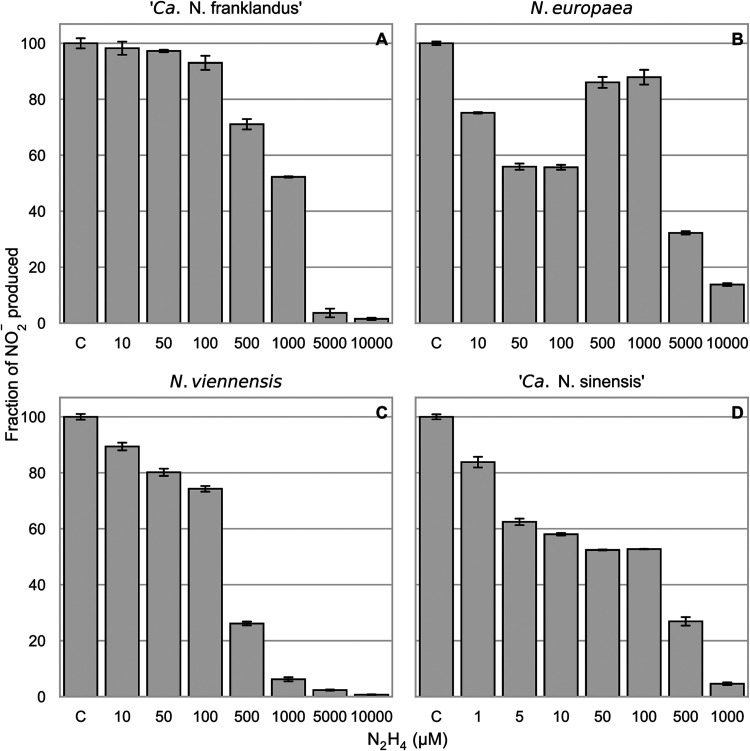
Hydrazine was tested to determine whether it inhibits NH3-dependent (Fig. 2) and hydroxylamine-dependent (Fig. S2) NO2− production. It was hypothesized that hydrazine would compete with hydroxylamine as a substrate due to its similar chemical properties (27). Higher concentrations of hydrazine than phenylhydrazine were required to inhibit NO2− production in all ammonia oxidizers (Fig. 2). In N. europaea hydrazine is known to be a competitive inhibitor of the HAO (12), and increasing hydrazine concentrations inhibited the ammonia oxidation activity to a greater extent (Fig. 2B). Interestingly, 500 and 1,000 μM phenylhydrazine inhibited NO2− production less than lower concentrations in N. europaea. This profile was less pronounced but also apparent when hydroxylamine was used as the substrate (Fig. S2B). When supplied with NH3 as the substrate, the sensitivities of N. viennensis and “Ca. Nitrosotalea sinensis” to 500 μM N2H4 were very similar, but in comparison, “Ca. Nitrosocosmicus franklandus” was inhibited only slightly (Fig. 2A, C, and D). As with phenylhydrazine, “Ca. Nitrosotalea sinensis” was more sensitive to hydrazine than the other strains (Fig. 2D). The highest concentrations tested, 5,000 μM and 10,000 μM N2H4, strongly inhibited all the ammonia oxidizer strains tested and were likely toxic.
FIG 2.
Percentage of NO2− production compared to the uninhibited control (C) after 1 h of incubation with different concentrations of hydrazine using 100 μM NH4+ as the substrate. NO2− was measured 1 h after the addition of the substrate. Nitrite accumulation in the uninhibited control treatment represents 100% activity, and the treatments with hydrazine are shown as percentages of activity compared to this control. Error bars represent standard deviations (n = 3). One hundred percent activity corresponded to 100 μM, 59 μM, 54 μM, and 63 μM nitrite accumulated after 1 h in (A) “Ca. Nitrosocosmicus franklandus,” (B) N. europaea, (C) N. viennensis, and (D) “Ca. Nitrosotalea sinensis,” respectively.
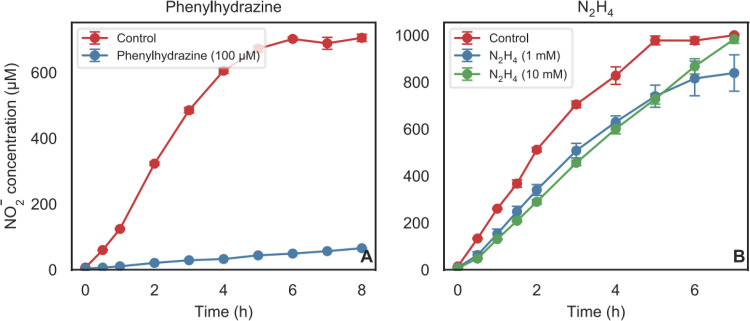
Recovery of NO2− production by “Ca. Nitrosocosmicus franklandus” following inhibition with phenylhydrazine or hydrazine.
Having confirmed that phenylhydrazine and hydrazine inhibited both NH3 oxidation and hydroxylamine oxidation in AOA and AOB, this inhibition was further characterized by testing whether phenylhydrazine and hydrazine act as reversible or irreversible inhibitors. “Ca. Nitrosocosmicus franklandus” was selected as the model AOA for recovery experiments with phenylhydrazine and hydrazine because of its relative ease of growth and high biomass production. Cells were treated with phenylhydrazine (100 μM) or hydrazine (1,000 μM or 10,000 μM) for 1 h, and the inhibitors were subsequently removed by washing. When an enzyme is inhibited with an irreversible inhibitor, de novo protein synthesis is required for the restoration of enzyme activity, resulting in a lag in recovery, as was seen after acetylene inhibition in “Ca. Nitrosocosmicus franklandus” (5). With a reversible inhibitor, recovery is instantaneous, as seen after 1-octyne inhibition in “Ca. Nitrosocosmicus franklandus” (5) and two Nitrososphaera species (28). “Ca. Nitrosocosmicus franklandus” did not recover from inhibition by 100 μM phenylhydrazine (Fig. 3A), indicating that phenylhydrazine is an irreversible inhibitor. Similarly, N. europaea showed no recovery when inhibited with 100 μM phenylhydrazine (Fig. S3). In contrast, inhibition with 1,000 μM and even 10,000 μM N2H4 was readily reversible in “Ca. Nitrosocosmicus franklandus” (Fig. 3B). This also indicates that the inhibition was not due to toxic effects but more likely due to substrate competition.
FIG 3.
Time course of the recovery of NO2− production from 1 mM NH4+ in “Ca. Nitrosocosmicus franklandus” after the removal of 100 μM phenylhydrazine (A) or 1 mM and 10 mM N2H4 (B) by washing. Error bars represent the standard deviations (n = 3).
Hydrazine-dependent O2 consumption in “Ca. Nitrosocosmicus franklandus.”
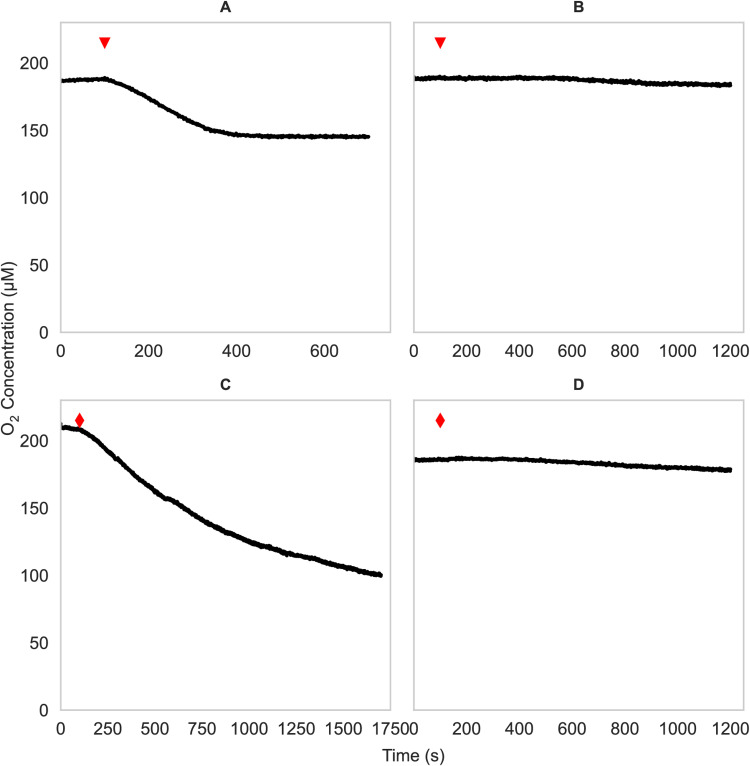
Hydrazine is an alternative substrate for the HAO in AOB and competes with hydroxylamine for the active site (12). To test if hydrazine is also a substrate for the equivalent enzyme in AOA, hydrazine-dependent O2 uptake by “Ca. Nitrosocosmicus franklandus” cells was measured and compared to hydroxylamine-dependent O2 uptake. Additionally, cells were preincubated with phenylhydrazine with the expectation that it would inhibit both hydrazine- and hydroxylamine-dependent O2 uptake. N. europaea was used for comparison as similar experiments have previously been performed with this nitrifier (22).
First, hydroxylamine-induced O2 uptake was investigated. For “Ca. Nitrosocosmicus franklandus,” the optimal concentration of hydroxylamine was 200 μM since a higher concentration reduced the O2 uptake rate, and the induced rate was not linear (Fig. S5A). When cells were given 200 μM NH2OH as the substrate, “Ca. Nitrosocosmicus franklandus” O2 uptake ceased when 49 ± 4 μM O2 had been consumed (Fig. 4A). Subsequent spiking with 200 μM NH2OH caused O2 uptake to resume, and this could be repeated until all O2 was consumed (Fig. S5B), indicating that the cessation of O2 uptake after the reduction of 49 ± 4 μM O2 was due to the depletion of hydroxylamine. The consumption of 49 ± 4 μM O2 coincided with the production of 23 ± 1 μM NO2−, close to a 2:1 O2 to NO2− stoichiometry instead of the 1:1 stoichiometry reported previously for other AOA strains (1, 29).
FIG 4.
Oxygen uptake measurements in cell suspensions of “Ca. Nitrosocosmicus franklandus.” Concentrations of 200 μM NH2OH (A and B, red triangles) or 600 μM N2H4 (C and D, red diamonds) were added. Control cells (A and C) are compared to cells incubated with 100 μM phenylhydrazine (B and D). Experiments were performed at least three times with similar results.
Second, a concentration range of 200 to 1,000 μM N2H4 was tested to determine if higher concentrations were inhibitory like hydroxylamine (Fig. S6). Only a slight increase in the initial rate was observed at concentrations over 600 μM, so for further experiments, “Ca. Nitrosocosmicus franklandus” cells were spiked with 600 μM N2H4, which was also chosen to allow comparison with N. europaea, where 600 μM N2H4 was saturating, and higher concentrations did not increase the O2 uptake rates (19). The initial rate of O2 uptake in the presence of 600 μM N2H4 in “Ca. Nitrosocosmicus franklandus” was 9.51 ± 0.69 μM O2 min−1, similar to the initial rate of hydroxylamine-dependent O2 uptake with 200 μM NH2OH (10.54 ± 0.21 μM O2 min−1). Notably, the hydrazine-induced rate was not linear and started to decrease after 10 min, reaching a steady rate of 1.15 μM O2 min−1 after 20 min, close to the abiotic rate (Fig. 4C). In contrast to hydroxylamine, spiking with more hydrazine did not restore the initial rate (Fig. S5C), although the addition of NH4+ caused oxygen consumption to resume (Fig. S5D). As anticipated, preincubating cells with 100 μM phenylhydrazine inhibited O2 consumption coupled to both hydroxylamine and hydrazine in “Ca. Nitrosocosmicus franklandus” (Fig. 4B and D, respectively), suggesting that the same enzyme oxidizes both substrates, as it does in N. europaea (20) (Fig. S4B and D), or that different enzymes are similarly affected. In contrast to AMO-specific inhibitors (e.g., acetylene (5)), phenylhydrazine inhibited hydroxylamine oxidation activity.
ATP production in response to hydrazine and phenylhydrazine.
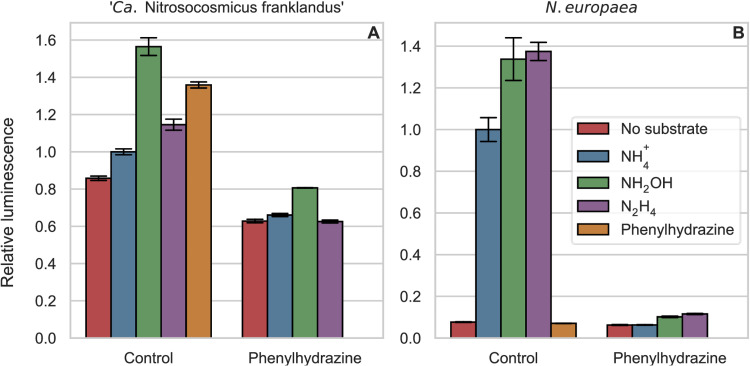
To investigate whether the oxidation of hydrazines is coupled to energy conservation, ATP levels were determined in “Ca. Nitrosocosmicus franklandus” and N. europaea, incubated with known substrates (NH3 or hydroxylamine) or with hydrazine or phenylhydrazine (Fig. 5). We hypothesized that both hydroxylamine and hydrazine would yield more ATP than ammonia. The oxidation of ammonia by AMO consumes two electrons, and these electrons are normally produced from the downstream pathway of hydroxylamine oxidation. Therefore, it is expected that the net yield of electrons and ATP would be higher with hydroxylamine and hydrazine as the substrates. The higher ATP yield from hydroxylamine than from ammonia in the marine AOA Nitrosopumilus maritimus supports this notion (1). Comparisons were made between cells preincubated with and without 100 μM phenylhydrazine for 1 h. All treatments were also performed using heat-killed cells as abiotic controls, which showed no variation in ATP concentrations (data not shown).
FIG 5.
Relative ATP-dependent luminescence, with NH4+ controls normalized to a value of 1. All treatments were added in 100 μM concentrations, and the cells were incubated for 10 min before ATP was measured. Bars on the left and right of each panel show cells preincubated without and with phenylhydrazine, respectively. Error bars represent the standard deviations (n = 3).
In “Ca. Nitrosocosmicus franklandus,” without preincubation with phenylhydrazine, hydroxylamine yielded more ATP than NH3 (Fig. 5A), as was previously reported for the marine AOA N. maritimus (1). Moreover, the addition of hydrazine generated ATP in “Ca. Nitrosocosmicus franklandus,” confirming that hydrazine not only was oxidized but also produced energy in this AOA. The ATP concentration from hydrazine was higher than that from NH3 but lower than that from hydroxylamine. Unexpectedly, short-term incubations with phenylhydrazine caused an increase in ATP in “Ca. Nitrosocosmicus franklandus” (Fig. 5A), whereas preincubation for 1 h with phenylhydrazine depleted ATP to even lower levels than those in cells incubated with no substrate, suggesting that ATP, initially generated, was subsequently consumed and that these cells had an even greater requirement for ATP than control cells. In N. europaea, the amounts of ATP production in response to hydroxylamine and hydrazine were very similar (Fig. 5B). In contrast to “Ca. Nitrosocosmicus franklandus,” N. europaea produced no ATP in response to phenylhydrazine, and ATP values were comparable to those of starved cells.
N2 is a product of hydrazine oxidation in “Ca. Nitrosocosmicus franklandus.”
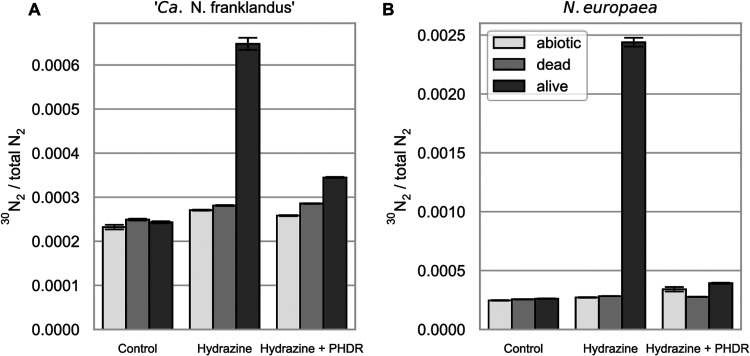
To test if N2 is a product of hydrazine oxidation in the AOA as it is in the AOB (20), 15N-labeled hydrazine was added to cell suspensions of “Ca. Nitrosocosmicus franklandus” and N. europaea. As expected, 29N2/28N2 ratios of the abiotic, killed control, and live-cell incubations did not differ from the natural abundance in the atmosphere. In contrast, there was a clear enrichment of 30N2 with both “Ca. Nitrosocosmicus franklandus” C13 (Fig. 6A) and N. europaea (Fig. 6B), indicating that both organisms produced 30N2 when 15N-hydrazine was added. Additionally, the production of 30N2 from 15N-hydrazine was inhibited when 100 μM phenylhydrazine was included in the incubations. From the total 1,500 nmol of 15N-hydrazine added, 129 (±2) nmol and 1,049 (±23) nmol of 30N2 were produced by “Ca. Nitrosocosmicus franklandus” and N. europaea, respectively, during the 1-h incubation. This is equal to ∼8.8% and 71.4% of the total added 15N-hydrazine being oxidized to 30N2 by “Ca. Nitrosocosmicus franklandus” and N. europaea, respectively. This is consistent with the oxygen electrode data described above (Fig. 4A and C) and suggests that N. europaea was able to consume most of the 15N-hydrazine during the experiment, whereas “Ca. Nitrosocosmicus franklandus” was able to oxidize hydrazine only transiently. It is likely that 71.4%, rather than 100%, of the hydrazine was recovered as N2 in N. europaea due to abiotic degradation of hydrazine.
FIG 6.
30N2/total N2 ratio after 1 h of incubation of cell suspensions of “Ca. Nitrosocosmicus franklandus” (A) and N. europaea (B) with 500 μM 15N-labeled N2H4. Abiotic and dead cells were included as controls. Error bars represent the standard deviations (n = 3); PHDR, phenylhydrazine.
DISCUSSION
Hydrazines as inhibitors of AOA.
While the enzyme that catalyzes hydroxylamine oxidation in AOA has not been identified, hydrazine and phenylhydrazine seem to have similar effects on NH3 and hydroxylamine oxidation by AOA and AOB. Phenylhydrazine and hydrazine inhibit hydroxylamine and NH3 oxidation in AOA as they do in the AOB N. europaea. The variability in inhibition thresholds between different AOA and AOB strains clearly demonstrates the value of using more than one model microorganism, preferably from different clades, when evaluating inhibitors, as has been described previously with AOB (30). A recent study revealed a pattern in the affinity for ammonia similar to the one found for the sensitivity to hydrazines in this study (31), indicating that these different sensitivities may reflect the niche in the environment, with the organisms with higher ammonia affinities being more sensitive to inhibition by hydrazines.
The inhibition of ammonia oxidation by hydrazine has been extensively studied in AOB and, more specifically, N. europaea (2, 7, 32). Hydrazine is a reversible competitive inhibitor of the HAO enzyme (12), which explains the inhibition of NO2− production by N. europaea at higher concentrations (Fig. 2B). However, the inhibition pattern where 500 to 1,000 μM hydrazine inhibited NO2− production in N. europaea less than hydrazine concentrations below 500 μM is difficult to explain and is probably caused by an interplay of hydrazine-driven NO2− reduction (33), the abundance of the reductant (19), and interactions of the HAO and cytochrome P460 with hydrazine (34).
In AOA, hydrazine interfered with NO2− production from both NH3 and hydroxylamine, likely due to competition with hydroxylamine for the substrate binding site. It was not possible to unequivocally demonstrate competitive inhibition as hydroxylamine concentrations of >200 μM reduced the hydroxylamine oxidation rate (see Fig. S5A in the supplemental material). The inhibition with hydrazine in AOA was readily reversible in a manner similar to that for AOB.
Organohydrazines were characterized in N. europaea as irreversible suicide substrates of the HAO enzyme using purified protein (22). They have been successfully used to inhibit both archaeal and bacterial nitrification in soil microcosms (24, 25), and here, we provide insight into the archaeal inhibition mechanism. Phenylhydrazine would likely have a short-lived inhibitory effect on nitrification due to its role as an irreversible inhibitor of AOA and AOB. However, the use of phenylhydrazine as an HAO-specific nitrification inhibitor for long-term experiments (>24 h) should be cautioned as it is sensitive to light and unstable in aqueous solutions (26). Nevertheless, it is interesting to consider that hydrazine and its derivatives could potentially enter soil environments either through anthropogenic routes or, to a much lesser extent, via biological production by diazotrophs and some fungi. While the potential for environmental applications and the impacts of hydrazines are unknown, at least in theory, it may be possible that AOA could oxidize hydrazine and gain ATP from this reaction.
While it was not possible to use pure enzymes from AOA, phenylhydrazine was identified as an inhibitor of hydroxylamine oxidation in the three tested AOA strains. The inhibition specific to hydroxylamine/hydrazine oxidation was verified and characterized in “Ca. Nitrosocosmicus franklandus” using several approaches, including NO2− accumulation assays, oxygen consumption, and ATP assays. As in the AOB, inhibition by phenylhydrazine in “Ca. Nitrosocosmicus franklandus” was irreversible, and the cells did not recover, even after several hours (5, 22). Consistent with these approaches, the production of 15N-labeled N2 was also inhibited by phenylhydrazine. One caveat is that it was impossible to verify that phenylhydrazine inhibits the hydroxylamine oxidation enzyme directly, and it could also be affecting downstream enzymes.
Hydrazine as a substrate in AOA.
Oxygen consumption in response to hydrazine confirmed that hydrazine was used as a substrate. However, hydrazine-induced oxygen uptake decreased over time (Fig. 4C), indicating that an unknown mechanism limits the oxidation of hydrazine. The product of hydrazine oxidation is likely N2, as it is in AOB (20), making product inhibition unlikely. The incubations with 15N-labeled hydrazine confirmed that N2 is a product of hydrazine oxidation in the AOA, but it is possible that there are other products such as NO or N2O.
ATP was produced after hydrazine addition in AOA, and future studies should investigate the use of hydrazine in growth experiments and as a source of reductants in physiological experiments. In both N. europaea and “Ca. Nitrosocosmicus franklandus,” hydroxylamine and hydrazine produced higher ATP concentrations than NH3, which was expected. However, in N. europaea, the amounts of ATP produced from both substrates were similar, while in “Ca. Nitrosocosmicus franklandus,” they were vastly different. This could be due to differences in the NH3 oxidation pathways in AOA and AOB or differences in the rates of oxidation, as hydrazine had a lower initial oxidation rate in the AOA, and the rate decreased over time (Fig. 4C). Interestingly, ATP values increased in “Ca. Nitrosocosmicus franklandus” after short-term incubations with phenylhydrazine, which was not observed in the AOB. A possible explanation is that phenylhydrazine can serve as a substrate, but its product, or phenylhydrazine itself, becomes toxic. Hemoproteins such as the HAO of AOB are thought to be inhibited by organohydrazine derivatives by the formation of a cation radical (22). It is likely that a similar radical is formed in the AOA.
Outlook for applications of hydrazines in ammonia oxidation research.
The fact that the hydrazine inhibitors affect AOA and AOB could aid in the development of the next generation of nitrification inhibitors. The availability of crystal structures for the bacterial HAO provides an advantage in the development of new nitrification inhibitors, compared to the AMO, for which no structures are available (9, 23). It is important, however, not to overlook other nitrifiers such as AOA and comammox bacteria in these studies but to investigate further potential inhibitors that target all ammonia oxidizers.
While it is known that organohydrazines inhibit a broad range of enzymes with both oxidative (22, 35) and electrophilic (36) cofactors via a covalent mechanism, it is interesting that they inhibit hydroxylamine oxidation in AOA, which are thought not to have the same heme cofactor as AOB. The inhibition of the archaeal hydroxylamine oxidation mechanism by organohydrazines could be used to finally identify this long-sought-after enzyme by using either 14C-phenylhydrazine (37) or hydrazine probes as activity-based protein profiling probes (38, 39).
In conclusion, this study provides evidence that hydrazine can be oxidized by AOA and that N2 is a product of its oxidation. Phenylhydrazine was shown to be an inhibitor of archaeal hydroxylamine oxidation. The inhibition by hydrazines was tested in several environmentally relevant strains of terrestrial AOA, and further characterization was done in “Ca. Nitrosocosmicus franklandus.” We demonstrate that (i) hydrazine and phenylhydrazine inhibit archaeal NH3 and hydroxylamine oxidation at concentrations comparable to those in AOB and (ii) hydrazine acts as a reversible inhibitor and phenylhydrazine acts as an irreversible inhibitor in both AOA and AOB. Furthermore, we demonstrate that (iii) hydrazine is oxidized by AOA, and this reaction yields ATP, and (iv) N2 is produced from hydrazine oxidation by AOA, as is the case in AOB and anammox bacteria. Despite the profound differences in the enzymology of archaeal and bacterial NH3 oxidation pathways, this study demonstrates that hydrazine metabolism in AOB and AOA is surprisingly similar: both AOA and AOB were inhibited by hydrazines in similar manners and with similar thresholds, and both groups of ammonia oxidizers were able to generate ATP by oxidizing hydrazine to N2. Future studies should focus on identifying the enzymes affected by phenylhydrazine and the use of hydrazine in growth experiments and as a source of reductants.
MATERIALS AND METHODS
Chemicals.
Research-grade phenylhydrazine (C6H5NHNH2), N2H4, and NH2OH (>99%, 97%, and >99%, respectively) were obtained from Sigma-Aldrich (CAS no. 59-88-1, 2644-70-4, and 5470-11-1, respectively). 15N-hydrazine sulfate (>98% purity) was purchased from Cambridge Isotope Laboratories (CAS no. 88491-70-7). Aqueous stock solutions of N2H4, NH2OH, and phenylhydrazine were prepared fresh for every experiment.
Growth of microorganisms.
“Candidatus Nitrosocosmicus franklandus” C13 and Nitrososphaera viennensis EN76T were cultivated in HEPES-buffered (pH 7.5) freshwater medium (FWM) (37) and supplied with 4 mM and 2 mM NH4Cl, respectively. In addition, 0.5 mM sodium pyruvate and 50 mg L−1 kanamycin were added to the N. viennensis FWM (40). The acidophilic AOA “Candidatus Nitrosotalea sinensis” Nd2 was cultivated in morpholineethanesulfonic acid (MES)-buffered FWM (pH 5.3) as previously described (41) and supplied with 400 μM NH4Cl. All three AOA strains were grown in 800-mL volumes in acid-washed 1-L Duran bottles and incubated statically at 37°C in the dark. Nitrosomonas europaea ATCC 19718 was obtained from the University of Aberdeen Culture Collection and cultivated in phosphate-buffered medium (pH 7.8) containing 50 mM NH4+, as described previously (42). N. europaea was incubated with shaking (160 rpm) in 1-L or 100-mL culture volumes in conical flasks (2 L and 250 mL, respectively) at 30°C in the dark. The purity of the cultures was monitored by microscopy and screening for contaminants on R2A agar plates (Oxoid, Basingstoke, UK). Cell counts were performed as described previously (41).
Nitrite determination.
The growth and activity of the cultures were monitored by nitrite accumulation. The nitrite concentration was determined using a colorimetric assay with Griess reagent in a 96-well plate format (41). The detection limit was <1 μM. The absorbance was measured at 540 nm using a VersaMax microplate reader (Molecular Devices, CA, USA).
Cell harvesting and preparation.
For “Ca. Nitrosocosmicus franklandus” and N. viennensis, 800 mL of a mid- to late-exponential-phase culture (corresponding to 1,000 to 1,500 μM NO2− accumulation) was harvested via filtration using a 0.22-μm-pore-size polyethersulfone (PES) membrane filter (Millipore). “Ca. Nitrosocosmicus franklandus” cells were washed three times on the filter using 100 mL 10 mM HEPES-buffered FWM salts (pH 7.5) to remove residual nitrite and ammonia. Harvested cells were resuspended by turning over the filter and flushing it with a fresh solution to dislodge the cells. N. viennensis cells adhered more strongly to the filter than the other strains and were scraped from the filter after filtration and subsequently washed three times in 100 mL 10 mM HEPES-buffered FWM salts (pH 7.5) by centrifugation (10 min at 4,000 × g) before finally being resuspended in fresh HEPES-buffered FWM salts. For “Ca. Nitrosotalea sinensis,” 1.6 L of a mid- to late-exponential-phase culture (100 to 200 μM NO2−) was used. The cells were washed three times on the filter with 100 mL 2.5 mM MES-buffered FWM salts (pH 5.3) and resuspended in the same solution. For N. europaea, 100 mL of a mid-exponential-phase culture (15 to 18 mM NO2−) was harvested by filtration, washed three times with 100 mL 50 mM sodium phosphate buffer (pH 7.8) containing 2 mM MgCl2, and resuspended in the same buffer. After harvesting, the cells were incubated at their respective growth temperatures for 1 h to consume any remaining ammonia. Background NO2− was measured prior to commencing the experiments to determine the baseline levels. All subsequent experiments were performed as short-term activity assays (<24 h in duration) rather than growth assays.
Endpoint inhibition assays.
To evaluate the inhibition of ammonia and hydroxylamine oxidation by hydrazine and phenylhydrazine, a 96-well microtiter plate was prepared with 5 μL of the inhibitor from concentrated aqueous stocks or 5 μL of double-distilled water (ddH2O) (controls) in each well. The inhibitors were diluted to final concentrations of 1 to 10,000 μM hydrazine and 0.05 to 500 μM phenylhydrazine with 95 μL of a cell suspension (∼2 × 108, ∼3 × 108, ∼9 × 108, and ∼2 × 108 cells mL−1 for “Ca. Nitrosocosmicus franklandus,” N. europaea, N. viennensis, and “Ca. Nitrosotalea sinensis,” respectively) (see Table S1 in the supplemental material). The plate was preincubated with the inhibitors at the respective growth temperatures for 1 h, before 2 μL of the substrate (either ammonia or hydroxylamine) was added to each well and the plate was incubated for one more hour. Griess reagent was added to stop the reaction and to determine the nitrite concentration. Background nitrite, before substrate addition, was subtracted, and nitrite production was normalized to the control. Hydroxylamine-dependent NO2− accumulation in AOA is not stoichiometric with hydroxylamine consumption (29), and the threshold for hydroxylamine toxicity in AOA is lower than that in AOB, leading to low levels of NO2− accumulation in AOA with this substrate. Therefore, the absolute, rather than relative, values of NO2− accumulation are shown for experiments with hydroxylamine as the substrate. The final ammonia concentration was 100 μM, and the final hydroxylamine concentrations were 100 μM for N. europaea and “Ca. Nitrosotalea sinensis” and 200 μM for “Ca. Nitrosocosmicus franklandus” and N. viennensis. Each assay was done at least two times with similar results with three biological replicates for each treatment.
Oxygen consumption experiments.
A Clark-type electrode (Rank Brothers, Cambridge, UK) was used to determine substrate-induced oxygen consumption. The instrument was comprised of a 3-mL reaction chamber, which was sealed with a stopper containing an injection port. The temperature was maintained by a circulating water bath (Churchill Co. Ltd., Perivale, UK). The temperature was set to the growth temperature of each microorganism, and the electrode was calibrated as described previously (43). The polarizing voltage was set to 0.6 V. Cell suspensions (3 mL) were fully oxygenated by stirring for 5 min without the stopper. The chamber was then sealed, the endogenous rate was established for 2 to 5 min, and the substrate was injected in 15-μL volumes from freshly made concentrated aqueous stocks of NH4+, NH2OH, or N2H4 (Table S2). Endpoint inhibition assays informed the choice of the substrate and inhibitor concentrations for the O2 uptake experiments. Experiments were carried out with either uninhibited cells or cells preincubated with 100 μM phenylhydrazine (∼7 × 108 and ∼3 × 108 cells mL−1 for “Ca. Nitrosocosmicus franklandus” and N. europaea, respectively). Nitrite was measured at the end of each O2 uptake trace. Abiotic controls for all treatments were performed using buffered salts without cells, and oxygen consumption never exceeded 0.5 μM min−1. Reactions were performed in triplicate with similar results.
ATP assays.
To assess the effects of hydrazine and phenylhydrazine on ATP production, cells (∼2 × 108 and ∼3 × 108 cells mL−1 for “Ca. Nitrosocosmicus” and N. europaea, respectively) were first washed and starved without any substrates for 1 h to deplete internal ATP levels. For killed controls, cells were autoclaved at 121°C for 15 min. Pretreatment of cells, where necessary, was performed by incubation for 1 h with 100 μM phenylhydrazine at the respective growth temperatures. Experiments were performed in opaque black 96-well plates, which were prepared with 5 μL of 20-times-concentrated substrate/inhibitor stocks (100 μM final concentration) and 95 μL of a live- or dead-cell suspension. The cells were mixed with the different inhibitors and substrates by pipetting and then incubated for 10 min at the respective growth temperatures. ATP accumulation was then measured using a luminescence assay based on the luciferase enzyme (BacTiter-Glo; Promega, WI, USA). BacTiter-Glo reagent (100 μL) was added, and luminescence was measured every 5 min using a Spectramax ID5 plate reader (Molecular Devices, CA, USA) with a 1-s integration time. The luminescence values reached their maxima after 10 min and remained stable for 5 to 10 min thereafter. Therefore, fluorescence was measured 10 min after the addition of the BacTiter-Glo reagent. The data were normalized against the NH4+ control (100%) (0.1 mM NH4Cl). Killed controls were all very similar and were subtracted from their respective live measurements. Each assay was done at least two times with similar results and with three biological replicates for each treatment.
Recovery assay.
“Ca. Nitrosocosmicus franklandus” and N. europaea were harvested, washed, and starved as described above (∼2 × 108 cells mL−1 and ∼2 × 108 cells mL−1, respectively). Aliquots of 5-mL cell suspensions were added to acid-washed 23-mL glass vials, and inhibitors were added from concentrated aqueous stocks to final concentrations of 100 μM phenylhydrazine and 1,000 μM or 10,000 μM N2H4. The hydrazine concentrations were chosen to include an inhibitory but nontoxic concentration (1,000 μM) and a fully inhibitory concentration that might be toxic (10,000 μM). The vials were sealed with twice-autoclaved butyl rubber seals and incubated for 1 h at the respective growth temperatures of the organisms. After inhibition, the cells were washed by filtration and resuspended in their respective media supplied with 1 mM NH4Cl. The vials were then incubated for 8 h, and NO2− was measured every 30 min for the first 2 h and every 60 min thereafter. All treatments were performed in triplicate.
15N stable isotope analysis.
To determine if N2 is a product of hydrazine oxidation, 15N-hydrazine sulfate was added to a concentration of 500 μM to cell suspensions as described above, and 29N2 and 30N2 production was investigated. If N2 is produced from 15N-labeled hydrazine, an enrichment in 30N2 is expected as 29N2 cannot be a product of this reaction. “Ca. Nitrosocosmicus franklandus” and N. europaea were harvested, washed, and rested as described above (∼3 × 108 and ∼4 × 108 cells mL−1, respectively). For abiotic controls, samples containing just the medium components as well as heat-killed cells (121°C for 15 min) were included. Additionally, phenylhydrazine-treated samples (100 μM for 1 h) were included to see if this would inhibit 30N2 production. Incubations were carried out in triplicate. 15N-labeled hydrazine sulfate was added from a concentrated aqueous stock, after which the vials were sealed with twice-autoclaved butyl rubber seals and incubated for 1 h at the respective growth temperatures of the organisms. The vials were shaken (180 rpm) to ensure gas exchange between the liquid and the headspace. Headspace gas (10 mL) was then sampled using a gastight syringe fitted with a Luer lock and injected into preevacuated (<0.1 atm) 12-mL exetainers (Labco). Gas samples were analyzed using a Sercon CryoPrep gas concentration system interfaced with a Sercon 20-20 isotope ratio mass spectrometer (Stable Isotope Facility, University of California, Davis, CA, USA). Molar fractions were calculated from the isotope ratios and the N2 concentration in the vials. The amount of 30N2 in the liquid was calculated using Henry’s law (44) and standard conditions and was added to the total 30N2 formed. The background amount of 30N2 from heat-killed cells was subtracted.
ACKNOWLEDGMENTS
A.S. is funded by a Royal Society Dorothy Hodgkin fellowship enhancement award (RGF\EA\180300). C.L.W. is funded by a University of East Anglia-funded Ph.D. studentship. L.E.L.-M. is funded by a Royal Society Dorothy Hodgkin research fellowship (DH150187) and by a European Research Council (ERC) starting grant (UNITY 852993). J.C.M. received funding through the Earth and Life Systems Alliance, Norwich Research Park, and A.T.C. was supported by a Leverhulme Trust early-career fellowship (ECF-2016-626).
We thank the Stable Isotope Facility (University of California, Davis, CA, USA) for the isotope ratio mass spectrometry analysis.
We declare no conflict of interest.
Footnotes
Supplemental material is available online only.
Contributor Information
Laura E. Lehtovirta-Morley, Email: l.lehtovirta-morley@uea.ac.uk.
Laura Villanueva, Royal Netherlands Institute for Sea Research.
REFERENCES
- 1.Vajrala N, Martens-Habbena W, Sayavedra-Soto LA, Schauer A, Bottomley PJ, Stahl DA, Arp DJ. 2013. Hydroxylamine as an intermediate in ammonia oxidation by globally abundant marine archaea. Proc Natl Acad Sci USA 110:1006–1011. 10.1073/pnas.1214272110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshida Y, Alexander M. 1964. Hydroxylamine formation by Nitrosomonas europaea. Can J Microbiol 10:923–926. 10.1139/m64-121. [DOI] [PubMed] [Google Scholar]
- 3.Lancaster KM, Caranto JD, Majer SH, Smith MA. 2018. Alternative bioenergy: updates to and challenges in nitrification metalloenzymology. Joule 2:421–441. 10.1016/j.joule.2018.01.018. [DOI] [Google Scholar]
- 4.Taylor AE, Vajrala N, Giguere AT, Gitelman AI, Arp DJ, Myrold DD, Sayavedra-Soto L, Bottomley PJ. 2013. Use of aliphatic n-alkynes to discriminate soil nitrification activities of ammonia-oxidizing thaumarchaea and bacteria. Appl Environ Microbiol 79:6544–6551. 10.1128/AEM.01928-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright CL, Schatteman A, Crombie AT, Murrell JC, Lehtovirta-Morley LE. 2020. Inhibition of ammonia monooxygenase from ammonia-oxidizing archaea by linear and aromatic alkynes. Appl Environ Microbiol 86:e02388-19. 10.1128/AEM.02388-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakoula D, Smith GJ, Frank J, Mesman RJ, Kop LFM, Blom P, Jetten MSM, van Kessel MAHJ, Lücker S. 2022. Universal activity-based labeling method for ammonia- and alkane-oxidizing bacteria. ISME J 16:958–971. 10.1038/s41396-021-01144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson J. 1964. The metabolism of hydroxylamine to nitrite by Nitrosomonas. Biochem J 91:8–17. 10.1042/bj0910008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caranto JD, Lancaster KM. 2017. Nitric oxide is an obligate bacterial nitrification intermediate produced by hydroxylamine oxidoreductase. Proc Natl Acad Sci USA 114:8217–8222. 10.1073/pnas.1704504114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cedervall P, Hooper AB, Wilmot CM. 2013. Structural studies of hydroxylamine oxidoreductase reveal a unique heme cofactor and a previously unidentified interaction partner. Biochemistry 52:6211–6218. 10.1021/bi400960w. [DOI] [PubMed] [Google Scholar]
- 10.Walker CB, de la Torre JR, Klotz MG, Urakawa H, Pinel N, Arp DJ, Brochier-Armanet C, Chain PSG, Chan PP, Gollabgir A, Hemp J, Hugler M, Karr EA, Konneke M, Shin M, Lawton TJ, Lowe T, Martens-Habbena W, Sayavedra-Soto LA, Lang D, Sievert SM, Rosenzweig AC, Manning G, Stahl DA. 2010. Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc Natl Acad Sci USA 107:8818–8823. 10.1073/pnas.0913533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerou M, Offre P, Valledor L, Abby SS, Melcher M, Nagler M, Weckwerth W, Schleper C. 2016. Proteomics and comparative genomics of Nitrososphaera viennensis reveal the core genome and adaptations of archaeal ammonia oxidizers. Proc Natl Acad Sci USA 113:E7937–E7946. 10.1073/pnas.1601212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hooper AB, Nason A. 1965. Characterization of hydroxylamine-cytochrome c reductase from the chemoautotrophs Nitrosomonas europaea and Nitrosocystis oceanus. J Biol Chem 240:4044–4057. 10.1016/S0021-9258(18)97148-6. [DOI] [PubMed] [Google Scholar]
- 13.Hyman MR, Murton IB, Arp DJ. 1988. Interaction of ammonia monooxygenase from Nitrosomonas europaea with alkanes, alkenes, and alkynes. Appl Environ Microbiol 54:3187–3190. 10.1128/aem.54.12.3187-3190.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keener WK, Arp DJ. 1993. Kinetic studies of ammonia monooxygenase inhibition in Nitrosomonas europaea by hydrocarbons and halogenated hydrocarbons in an optimized whole-cell assay. Appl Environ Microbiol 59:2501–2510. 10.1128/aem.59.8.2501-2510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasche ME, Hyman MR, Arp DJ. 1991. Factors limiting aliphatic chlorocarbon degradation by Nitrosomonas europaea: cometabolic inactivation of ammonia monooxygenase and substrate specificity. Appl Environ Microbiol 57:2986–2994. 10.1128/aem.57.10.2986-2994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keener WK, Arp DJ. 1994. Transformations of aromatic compounds by Nitrosomonas europaea. Appl Environ Microbiol 60:1914–1920. 10.1128/aem.60.6.1914-1920.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyman MR, Kim CY, Arp DJ. 1990. Inhibition of ammonia monooxygenase in Nitrosomonas europaea by carbon disulfide. J Bacteriol 172:4775–4782. 10.1128/jb.172.9.4775-4782.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juliette LY, Hyman MR, Arp DJ. 1993. Inhibition of ammonia oxidation in Nitrosomonas europaea by sulfur compounds: thioethers are oxidized to sulfoxides by ammonia monooxygenase. Appl Environ Microbiol 59:3718–3727. 10.1128/aem.59.11.3718-3727.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hyman MR, Wood PM. 1984. Ethylene oxidation by Nitrosomonas europaea. Arch Microbiol 137:155–158. 10.1007/BF00414458. [DOI] [Google Scholar]
- 20.Maalcke WJ, Dietl A, Marritt SJ, Butt JN, Jetten MSM, Keltjens JT, Barends TRM, Kartal B. 2014. Structural basis of biological NO generation by octaheme oxidoreductases. J Biol Chem 289:1228–1242. 10.1074/jbc.M113.525147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schalk J, de Vries S, Kuenen JG, Jetten MSM. 2000. Involvement of a novel hydroxylamine oxidoreductase in anaerobic ammonium oxidation. Biochemistry 39:5405–5412. 10.1021/bi992721k. [DOI] [PubMed] [Google Scholar]
- 22.Logan MSP, Hooper AB. 1995. Suicide inactivation of hydroxylamine oxidoreductase of Nitrosomonas europaea by organohydrazines. Biochemistry 34:9257–9264. 10.1021/bi00028a039. [DOI] [PubMed] [Google Scholar]
- 23.Nishigaya Y, Fujimoto Z, Yamazaki T. 2016. Optimized inhibition assays reveal different inhibitory responses of hydroxylamine oxidoreductases from beta- and gamma-proteobacterial ammonium-oxidizing bacteria. Biochem Biophys Res Commun 476:127–133. 10.1016/j.bbrc.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 24.Wu Y, Guo Y, Lin X, Zhong W, Jia Z. 2012. Inhibition of bacterial ammonia oxidation by organohydrazines in soil microcosms. Front Microbiol 3:10. 10.3389/fmicb.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang W, Wang Y, Tago K, Tokuda S, Hayatsu M. 2017. Comparison of the effects of phenylhydrazine hydrochloride and dicyandiamide on ammonia-oxidizing bacteria and archaea in andosols. Front Microbiol 8:2226. 10.3389/fmicb.2017.02226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misra HP, Fridovich I. 1976. The oxidation of phenylhydrazine: superoxide and mechanism. Biochemistry 15:681–687. 10.1021/bi00648a036. [DOI] [PubMed] [Google Scholar]
- 27.Sakamoto H, Higashimoto Y, Hayashi S, Sugishima M, Fukuyama K, Palmer G, Noguchi M. 2004. Hydroxylamine and hydrazine bind directly to the heme iron of the heme-heme oxygenase-1 complex. J Inorg Biochem 98:1223–1228. 10.1016/j.jinorgbio.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 28.Taylor AE, Taylor K, Tennigkeit B, Palatinszky M, Stieglmeier M, Myrold DD, Schleper C, Wagner M, Bottomley PJ. 2015. Inhibitory effects of C2 to C10 1-alkynes on ammonia oxidation in two Nitrososphaera species. Appl Environ Microbiol 81:1942–1948. 10.1128/AEM.03688-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozlowski JA, Stieglmeier M, Schleper C, Klotz MG, Stein LY. 2016. Pathways and key intermediates required for obligate aerobic ammonia-dependent chemolithotrophy in bacteria and Thaumarchaeota. ISME J 10:1836–1845. 10.1038/ismej.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaur-Bhambra J, Wardak DLR, Prosser JI, Gubry-Rangin C. 2022. Revisiting plant biological nitrification inhibition efficiency using multiple archaeal and bacterial ammonia-oxidising cultures. Biol Fertil Soils 58:241–249. 10.1007/s00374-020-01533-1. [DOI] [Google Scholar]
- 31.Jung M-Y, Sedlacek CJ, Kits KD, Mueller AJ, Rhee S-K, Hink L, Nicol GW, Bayer B, Lehtovirta-Morley L, Wright C, de la Torre JR, Herbold CW, Pjevac P, Daims H, Wagner M. 2022. Ammonia-oxidizing archaea possess a wide range of cellular ammonia affinities. ISME J 16:272–283. 10.1038/s41396-021-01064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kane DA, Williamson KJ. 1983. Bacterial toxicity and metabolism of hydrazine fuels. Arch Environ Contam Toxicol 12:447–453. [Google Scholar]
- 33.Remde A, Conrad R. 1990. Production of nitric oxide in Nitrosomonas europaea by reduction of nitrite. Arch Microbiol 154:187–191. 10.1007/BF00423331. [DOI] [Google Scholar]
- 34.Erickson RH, Hooper AB. 1972. Preliminary characterization of a variant co-binding heme protein from Nitrosomonas. Biochim Biophys Acta 275:231–244. 10.1016/0005-2728(72)90044-8. [DOI] [PubMed] [Google Scholar]
- 35.Binda C, Wang J, Li M, Hubalek F, Mattevi A, Edmondson DE. 2008. Structural and mechanistic studies of arylalkylhydrazine inhibition of human monoamine oxidases A and B. Biochemistry 47:5616–5625. 10.1021/bi8002814. [DOI] [PubMed] [Google Scholar]
- 36.Datta S, Ikeda T, Kano K, Mathews FS. 2003. Structure of the phenylhydrazine adduct of the quinohemoprotein amine dehydrogenase from Paracoccus denitrificans at 1.7 Å resolution. Acta Crystallogr D Biol Crystallogr 59:1551–1556. 10.1107/s090744490301429x. [DOI] [PubMed] [Google Scholar]
- 37.Lehtovirta-Morley LE, Ross J, Hink L, Weber EB, Gubry-Rangin C, Thion C, Prosser JI, Nicol GW. 2016. Isolation of ‘Candidatus Nitrosocosmicus franklandus’, a novel ureolytic soil archaeal ammonia oxidiser with tolerance to high ammonia concentration. FEMS Microbiol Ecol 92:fiw057. 10.1093/femsec/fiw057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matthews ML, He L, Horning BD, Olson EJ, Correia BE, Yates JR, Dawson PE, Cravatt BF. 2017. Chemoproteomic profiling and discovery of protein electrophiles in human cells. Nat Chem 9:234–243. 10.1038/nchem.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett K, Sadler NC, Wright AT, Yeager C, Hyman MR. 2016. Activity-based protein profiling of ammonia monooxygenase in Nitrosomonas europaea. Appl Environ Microbiol 82:2270–2279. 10.1128/AEM.03556-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stieglmeier M, Klingl A, Alves RJE, Rittmann SK-MR, Melcher M, Leisch N, Schleper C. 2014. Nitrososphaera viennensis gen. nov., sp. nov., an aerobic and mesophilic, ammonia-oxidizing archaeon from soil and a member of the archaeal phylum Thaumarchaeota. Int J Syst Evol Microbiol 64:2738–2752. 10.1099/ijs.0.063172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lehtovirta-Morley LE, Ge C, Ross J, Yao H, Nicol GW, Prosser JI. 2014. Characterisation of terrestrial acidophilic archaeal ammonia oxidisers and their inhibition and stimulation by organic compounds. FEMS Microbiol Ecol 89:542–552. 10.1111/1574-6941.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hyman MR, Wood PM. 1985. Suicidal inactivation and labelling of ammonia mono-oxygenase by acetylene. Biochem J 227:719–725. 10.1042/bj2270719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green MJ, Hill HAO. 1984. Chemistry of dioxygen. Methods Enzymol 105:3–22. 10.1016/s0076-6879(84)05004-7. [DOI] [PubMed] [Google Scholar]
- 44.Staudinger J, Roberts PV. 1996. A critical review of Henry’s law constants for environmental applications. Crit Rev Environ Sci Technol 26:205–297. 10.1080/10643389609388492. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S6 and Tables S1 and S2. Download aem.02470-21-s0001.pdf, PDF file, 2.2 MB (2.2MB, pdf)