ABSTRACT
The emergence of several new variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in recent months has raised concerns around the potential impact on ongoing vaccination programs. Data from clinical trials and real-world evidence suggest that current vaccines remain highly effective against the alpha variant (B.1.1.7), while some vaccines have reduced efficacy and effectiveness against symptomatic disease caused by the beta variant (B.1.351) and the delta variant (B.1.617.2); however, effectiveness against severe disease and hospitalization caused by delta remains high. Although data on the effectiveness of the primary regimen against omicron (B.1.1.529) are limited, booster programs using mRNA vaccines have been shown to restore protection against infection and symptomatic disease (regardless of the vaccine used for the primary regimen) and maintain high effectiveness against hospitalization. However, effectiveness against infection and symptomatic disease wanes with time after the booster dose. Studies have demonstrated reductions of varying magnitude in neutralizing activity of vaccine-elicited antibodies against a range of SARS-CoV-2 variants, with the omicron variant in particular exhibiting partial immune escape. However, evidence suggests that T-cell responses are preserved across vaccine platforms, regardless of variant of concern. Nevertheless, various mitigation strategies are under investigation to address the potential for reduced efficacy or effectiveness against current and future SARS-CoV-2 variants, including modification of vaccines for certain variants (including omicron), multivalent vaccine formulations, and different delivery mechanisms.
KEYWORDS: SARS-CoV-2, COVID-19, vaccines, mutation, variant
INTRODUCTION
Since the first reports of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in humans in December 2019, numerous genetically distinct lineages have evolved (1). Recently, the emergence of several variants carrying mutations with phenotypic implications has raised concerns, as variants with increased transmissibility, disease severity, or ability to escape from antibodies have potential to negatively impact pandemic management strategies. In this article, we review the evolutionary mechanisms underpinning alterations in the genome of SARS-CoV-2 compared with other coronaviruses and RNA viruses; summarize current data on the impact of new variants on authorized mRNA, vector-based, subunit, and inactivated coronavirus disease 2019 (COVID-19) vaccines; and discuss potential mitigation strategies in response to current and future variants of SARS-CoV-2. The search strategy and selection criteria used to identify references for this review are summarized in Text S1 in the supplemental material.
Search strategy and selection criteria. Download Text S1, DOCX file, 0.1 MB (55.1KB, docx) .
Copyright © 2022 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
SARS-COV-2 EVOLUTIONARY MECHANISMS
Genetic variation in the SARS-CoV-2 genome can arise through two mechanisms: randomly occurring mutations followed by selection, and recombination (2). Random nucleotide sequence errors (substitutions or short deletions/insertions) that occur during replication can alter the amino acid composition of viral proteins (2, 3). In general, RNA viruses accumulate point mutations owing to the low fidelity of the RNA-dependent RNA polymerase (3), but coronaviruses carry a 3′→5′ exoribonuclease that provides proofreading ability, resulting in slower acquisition of mutations (4, 5). Nevertheless, point mutations seem to be major contributors to SARS-CoV-2 evolution (6). The high incidence of mutations detected in the S gene (7), particularly in the receptor-binding domain (RBD) and N-terminal domain (NTD) (8, 9), is likely due to selection for substitutions that improve viral fitness; i.e., through changes in the structure of the spike protein that lead to improved binding to the host receptor or escape from antibody recognition (8, 9). In addition, deletions, which cannot be corrected by the proofreading enzyme (10), are recurrently detected at particular sites in the S gene, primarily located within the NTD, and may contribute to the acquisition of genetic variance by SARS-CoV-2. The increased frequency of these deletions is also likely due to selection for resistance to neutralizing antibodies (10). Rarely, short insertions of a few nucleotides have also been observed (1). Mutations in other genes may also be the result of selection; for example, mutations in the N/Orf9b region have been implicated in enhanced immune escape through suppression of the host innate immune response (11). An unusually high incidence of parallel amino acid substitutions between the RBDs of the spike proteins of SARS-CoV-2- and SARS-CoV-1-related clades suggests the occurrence of evolutionary convergence, possibly as a mechanism of adaptation to the same host cell receptor (12).
RNA recombination occurs at a high rate in coronaviruses (13–16) and has an important role in their evolution. Phylogenetic analyses suggest that recombination events between SARS-CoVs and bat coronaviruses are frequent (17). Although the exact mechanisms by which recombination occurs are unknown, the proofreading exoribonuclease from nonstructural protein 14 (nsp14-ExoN) may be required (18). During replication of coronaviruses, including SARS-CoV-2, a set of subgenomic RNAs is generated, which is thought to increase the homologous recombination rate among closely related genes from different lineages of coronaviruses by template switching (15, 19, 20). Although recombination-mediated changes can theoretically occur at any location, they are detected more frequently in the S gene due to selection favoring changes in the spike glycoprotein (13).
Coronaviruses have the largest known genomes of RNA viruses, allowing for additional plasticity for mutation and recombination relative to viruses with smaller genomes (15). Unlike segmented RNA viruses, such as influenza A, the nonsegmented nature of the coronavirus genome does not allow for evolution via reassortment (3), but conversely, influenza A viruses do not undergo homologous recombination (21). As such, the mutation rate of coronaviruses is considered moderate to high compared with other single-stranded RNA viruses (15). The mutation rate of SARS-CoV-2 has been estimated at a median 1.12 × 10−3 mutations per site-year (22), which is similar to those of the related viruses SARS-CoV-1 and Middle East respiratory syndrome coronavirus (MERS-CoV), estimated at 0.80–2.38 × 10−3 and 1.12 × 10−3 nucleotide substitutions per site-year, respectively (23, 24).
SARS-CoV-2 is assumed to be of zoonotic origin, although the exact zoonotic source of the parental virus and the circumstances behind its emergence in humans in late 2019 remain unknown (25, 26). SARS-CoV-2 shares approximately 96% homology with bat sarbecovirus RaTG13, and specific genes are highly conserved across SARS-CoV-2 and other bat coronaviruses (e.g., SARS-CoV-2 open reading frame 8 [ORF8] shares 94% identity with ZC45 and ZXC21), which suggests a probable bat origin (27, 28). A recent publication reported on bat sarbecoviruses with an RBD of the S protein even more closely related to SARS-CoV-2, one of which could be isolated in human cell cultures, in contrast to RaTG13, for which only a nucleotide sequence is available (29). SARS-CoV-2 has been suggested to be derived from a viral lineage that has been circulating in horseshoe bats for decades (25, 26), and recombination events may have had a role in the origin of the virus (16, 17, 30).
As SARS-CoV-2 circulated globally, the viral genome continued to acquire new mutations, some of which have become widespread. Until late 2020, the most notable was the spike protein mutation D614G. The G614 variant was rare before March 2020, but quickly became dominant, occurring in around three-quarters of all published sequences by June 2020 (31, 32). This rapid spread seems to have been due to increased infectivity, stability, and transmissibility over the ancestral D614 form (32, 33), resulting from a shift to the open configuration of the spike protein trimer, which is required for binding to the host angiotensin-converting enzyme 2 (ACE2) receptor (31) and host cell entry (27).
As of January 25, 2022, 11 global clades of SARS-CoV-2 according to GISAID nomenclature (34) and 25 according to NextStrain nomenclature (35) have been identified. There are several possible contributing factors to the evolution of these different clades. First, the virus is likely still adapting to the new host, as it has been circulating in humans for about 2 years. Second, many countries are experiencing multiple waves of COVID-19 with high infection incidence rates, increasing the probability of advantageous mutations occurring through the sheer number of viral replication events. In addition, as more people recover from SARS-CoV-2 infection or are vaccinated, and population immunity levels increase, selection favors adaptations that evade neutralization by antibodies (36). Increased sequencing coverage in recent months may also have affected the number of variants detected.
Multiple studies have reported long-term shedding of SARS-CoV-2 over several months in immunocompromised individuals (37–41), promoting viral evolution within a single host (37–39, 41). In case reports of immunocompromised patients with COVID-19, treatments such as antivirals (including remdesivir), monoclonal antibody cocktails, or convalescent plasma (37, 39, 41) may have exerted selection pressure, contributing to increased prevalence of antibody escape mutants.
While it is possible that the roll-out of COVID-19 vaccination programs may contribute to viral evolution by increasing selection for immune-escape variants, the reduction in viral circulation resulting from vaccination is expected to result in an overall reduction in the rate of viral adaptation (42).
SARS-COV-2 VARIANTS
SARS-CoV-2 variants of proven or suspected clinical or epidemiological relevance are designated as a variant of concern (VOC) or variant of interest (VOI) based on criteria such as increased transmissibility and ability to escape immunity (43, 44). Increased transmissibility is of particular concern, as it increases infection rates and can require the introduction of more stringent public health measures. Variant escape from antibody neutralization can reduce the effectiveness of vaccination programs and necessitate the development of modified vaccines or administration of booster doses. Variants with a combination of these characteristics have a significant impact on pandemic management.
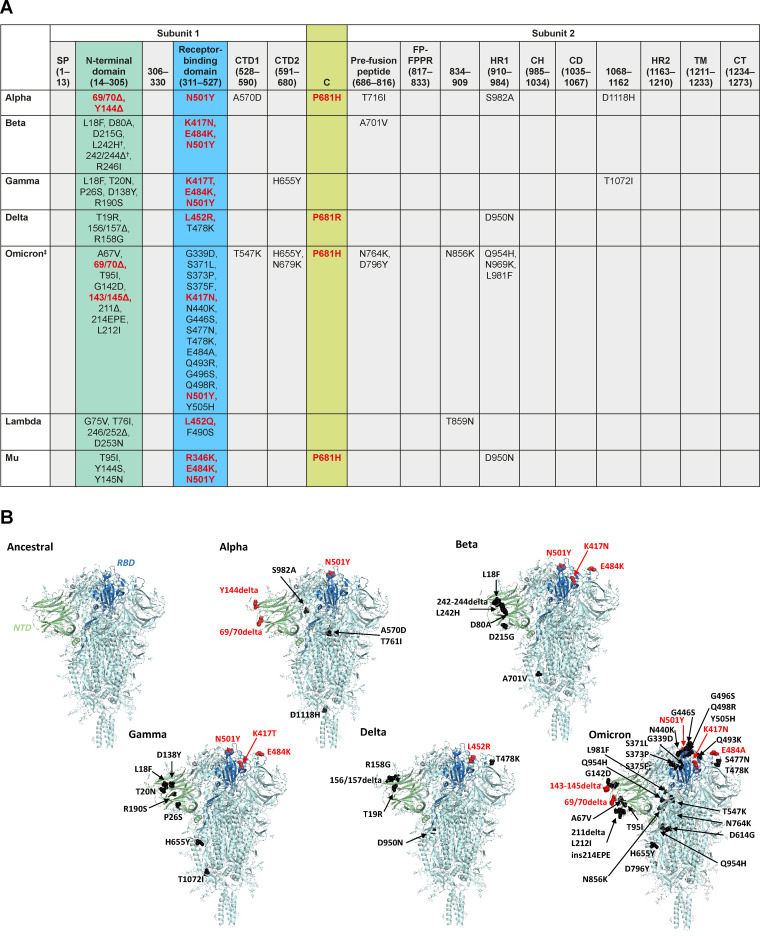
Five recently emerged SARS-COV-2 variants have been designated VOCs by the World Health Organization (WHO), as well as other regional agencies (45–47) (Table S1). Notably, all five VOCs exhibit two clusters of S gene mutations—one at the NTD and one at the RBD (1, 48)—both of which are domains targeted by neutralizing antibodies.
Characteristics of SARS-CoV-2 variants of concern and variants of interest (1). SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VOC, variant of concern; VOI, variant of interest. *Exact position and number of mutations may differ according to source; †Excluding D614G; ‡Disputed with mutation at same site (2); §Mutations with known or proposed biological significance shown in red. ¶The larger group of omicron sequences (B.1.1.529/21M) includes BA.1 (21K) and BA.2 (21L). Data shown here are based on BA.1 as the dominant sequence at the time of writing. Data based on sequences retrieved on 14 September 2021, except for omicron, which was retrieved on 14 January 2022. Download Table S1, DOCX file, 0.06 MB (64.7KB, docx) .
Copyright © 2022 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The alpha variant (also known as B.1.1.7, VOC202012/01, or GRY) (1, 46, 49, 50) was initially detected in the United Kingdom in September 2020 (49) and is hypothesized to have emerged from a prolonged infection of an immunocompromised host (51). Alpha has a large number of mutations (27 in total, excluding the now dominant mutation D614G) (1). Of the 27 mutations, 20 (17 nonsynonymous substitutions and three deletions) are amino acid-altering. Eight of these mutations are in the S gene (1) (Table S1), of which four have known biological effects. Mutation N501Y lies within the RBD (49); mutations at this position have previously been shown to affect binding affinity to the ACE2 receptor (52). The deletion at position 69–70 (69/70Δ) has occurred in several other lineages of SARS-CoV-2 in association with RBD changes and may be linked with immune evasion or with infectivity (10, 49, 53). The deletion at position 144 has also been detected in other lineages and may affect the orientation and stability of the spike glycoprotein (54), and confer resistance to NTD-directed neutralizing antibodies (55). Mutation P681H is adjacent to the furin cleavage site at the junction between the S1 and S2 domains of the spike protein and has been shown to promote entry into human lung cells and improve transmissibility in an animal model (49, 56, 57). Recent evidence suggests that the frequency of mutations at position 681 is increasing exponentially worldwide (58).
The beta variant (also known as B.1.351 or GH/501Y.V2) (1, 46, 59, 60) was first detected in South Africa in October 2020 (59). Beta carries 19 mutations (excluding D614G), including eight nonsynonymous mutations in the S gene (1), in addition to variable changes at position L242 (deletion or another nonsynonymous substitution) (59) (Table S1). Three of these mutations are at key sites in the RBD that are associated with immune evasion: N501Y (shared with alpha), E484K, and K417N (59).
The gamma variant (also known as P.1 or GR/501Y.V3) was first detected in Brazil in December 2020 (1, 46, 61). Gamma carries 31 mutations, of which 21 are amino acid-altering (Table S1) (1). These include 20 nonsynonymous substitutions and one deletion (1). Ten mutations affect the spike protein, including two shared with beta (N501Y and E484K), as well as a different mutation at position 417 (K417T) (61).
The delta variant (also known as B.1.617.2 or G/478K.V1) was first documented in India in October 2020 (46). Delta has 21 nonsynonymous mutations, one deletion, and five synonymous mutations (Table S1) (1). Six point mutations affect the spike protein, including P681R (a mutation position shared with alpha and adjacent to the furin cleavage site), and L452R, which is in the RBD and has been linked with increased binding to ACE2 (1, 49, 62) and neutralizing antibody resistance (63). There is also a deletion in the spike protein at position 156/157 (1).
The omicron variant (also known as B.1.1.529 or GRA) was first documented in multiple countries in November 2021 (46). Although it has some mutations in common with the other VOCs, the overall number of mutations is significantly larger than has been seen with any previous variant. Omicron has 45 nonsynonymous mutations, seven deletions, one insertion, and 10 synonymous mutations, with the majority of nonsynonymous mutations located in the S gene at the NTD and RBD (1). Key mutations shared with other VOCs include the deletion at position 69–70 (shared with alpha), K417N (shared with beta), N501Y (shared with alpha), and P681H (shared with alpha) (1). Three subvariants of omicron exist: BA.1 currently dominates in most countries in which omicron is prevalent, but BA.2 seems to have become more common in some countries since January 2022 (64). BA.2 is also referred to as “stealth omicron,” as it lacks the deletion at position 69/70 in the S protein, a mutation characteristic for alpha and BA.1 that is used in mutation-specific PCR assays to differentiate BA.1 from delta. BA.3 is currently still rare (64).
These five VOCs have circulated globally (60, 65) and have become the dominant variants in the geographic regions where they were first identified. As of January 14, 2022, alpha has been reported in 179 countries, beta in 120 countries, gamma in 92 countries, delta in 188 countries, and omicron in 119 countries, each spreading across multiple continents, with omicron currently being the most prevalent VOC in many countries, including the United Kingdom, the United States, and many European countries (65). The rapid spread of alpha, delta, and omicron in particular strongly suggests that these variants have transmission advantages over the ancestral viruses. Based on modeling data, alpha has been estimated to be 43–90% more transmissible than previously circulating variants (66), and delta is thought to be approximately 60% more transmissible than alpha (67). Omicron is highly transmissible, with early estimates suggesting that it may be around 100% more transmissible than delta (68). Some preliminary studies have suggested that omicron is associated with a reduced risk of hospitalization and disease severity compared with delta, although it is not known how much of this is due to increasing population immunity over time (69, 70). Nevertheless, omicron should not be considered to generally cause only mild disease, and its rapid spread is leading to health care systems becoming overwhelmed.
Several other lineages have been classed as VOIs by the WHO, despite not having spread as widely as the five variants described above (46). However, many of these have been reclassified and are no longer being monitored. Currently, only lambda (C.37; GR/452Q.V1) and mu (B1.621; GH) are classed as VOIs, with sporadic transmission (Table S1). Mu has a high accumulation of spike protein mutations seen independently in several other VOIs and VOCs (e.g., E484K, N501Y, and P681H) in addition to the insertion of N at position 146 in the NTD (1, 71). This insertion could potentially affect the S1 closed–open conformation and subsequent binding to ACE2, although the impact on transmissibility and severity of disease is still unknown (71).
As shown in Fig. 1, mutations in the SARS-CoV-2 spike protein in currently circulating variants are concentrated around the NTD, RBD, and furin cleavage site, suggesting a potential for further mutations to arise in these areas. Although the appearance of the same or similar mutations in multiple variants suggests that they have been driven by evolutionary pressures, the pressures exerted on the virus may change as vaccine programs continue to roll out and new therapeutics are introduced, potentially affecting the specific mutations that will arise in the future.
FIG 1.
(A) Location of spike protein mutations in SARS-CoV-2 variants alpha, beta, gamma, delta, lambda, mu, and omicron (1); (B) 3D structure of the spike protein of SARS-CoV-2 variants alpha, beta, gamma, delta, and omicron in comparison with an ancestral virus created using PyMOL molecular graphic system version 2.3.2 (https://pymol.org). C, cleavage site (residues 681–685); CD, connector domain; CH, central helix; CT, cytoplasmic domain fusion; CTD, C-terminal domain; FP, fusion protein; HR, heptad repeat; SP, signal peptide; TM, transmembrane domain. †Disputed with mutation at same site. ‡Based on BA.1 lineage, which is part of the larger group of omicron/B.1.1.529 sequences. Exact position and number of mutations may differ according to source. Structure of spike glycoprotein in A based on Cai et al. (186). Mutations with known or proposed biological significance shown in red. Images in B are based on protein data bank (PDB) entry 6XR8 (https://www.rcsb.org/structure/6xr8). The structure contains D614 but has better solved sequence coverage than other entries although residue P681 is missing and not labeled where mutated in a VOC. Beta: R226I is missing and is not labeled. Gamma: T1072I (in seq is E1072). Omicron: N679K and N969K are missing and not labeled. Domain coloring of spike subunit as follows: NTD: green; RBD: blue; all others including entire subunits 2 and 3: cyan. Mutations are shown in color of function (red: known; black: unknown) and are shown in a single subunit for clarity.
IMPACT OF SARS-COV-2 VARIANTS AND MUTATIONS ON IMMUNITY
In vitro studies assessing escape from neutralizing antibodies.
In light of concerns about the potential of new SARS-CoV-2 variants to escape antibodies elicited by vaccination or previous infection, and to resist antibody-based therapeutics (such as monoclonal antibodies or convalescent plasma), numerous studies have evaluated the impact of SARS-CoV-2 variants and mutations on neutralizing antibody activity (55, 72–117). As neutralizing antibody titers represent only one component of the immune response, and correlates of protection are still being established (118, 119), these studies cannot be used to draw conclusions on vaccine efficacy or effectiveness. In addition, as most COVID-19 vaccines elicit very high neutralizing antibody titers (considerably greater than those found in convalescent-phase sera), dramatic fold decreases in neutralizing activity are not necessarily meaningful given the high starting point. Furthermore, titer is not the only indicator of a robust neutralizing antibody response; the nature and quality of antibodies are also important. For example, clonal evolution of SARS-CoV-2 RBD-specific memory B cells over time can result in antibodies with greater resistance to RBD mutations and increased potency (120).
Neutralizing antibody escape studies are valuable for characterization purposes, but comparisons across studies should be made with caution, owing to considerable variations in assay techniques, use of pseudoviruses (of varying construction) versus live virus isolates, vaccine dosing intervals/time since infection, and participant age and immune status, among other factors. In particular, the cell line used to perform neutralization assays can have a considerable effect on results; for example, some studies have used Vero E6 cells, which lack transmembrane protease serine 2 (TMPRSS2), forcing the virus to enter the host cell via the endosomal pathway (121). Antibodies to the NTD of the spike protein are known to be particularly sensitive to pH and may detach in the endosomal environment, leading to an apparent reduction in neutralizing activity. Therefore, findings in Vero E6 cells may be substantially different to those in cell lines that carry TMPRSS2 and, therefore, allow entry at the cell surface.
Nevertheless, some clear patterns have emerged. Reductions in neutralizing activity against new SARS-CoV-2 variants and mutations compared with other circulating lineages have been observed for both convalescent-phase sera and monoclonal antibodies (55, 72, 76, 77, 91–95, 99–103, 105, 108), with beta, omicron, and combinations of mutations including E484K generally resulting in greater reductions than alpha in studies that compared these variants/mutations (55, 72, 77, 92, 102, 109, 114, 115). In addition to beta, E484K may also contribute to antibody escape by gamma and mu, which also carry this mutation (Table S2). Notably, a recent study found that the spike protein of mu escapes neutralization to a degree similar to beta, which had shown the greatest degree of antibody escape prior to the emergence of omicron (122).
Studies assessing escape from antibody neutralization by novel variants from sera of COVID-19-vaccinated individuals. Comparisons across these studies should be made with caution, owing to considerable variations in assay techniques, use of pseudoviruses (of varying construction) versus true isolates, vaccine dosing intervals/time since infection, and participant ages and immune status, among other factors. ACE2, angiotensin-converting enzyme 2; CI, confidence interval; GMT, geometric mean titer; IC50, half maximal inhibitory concentration; MAb, monoclonal antibody; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMPRSS2, transmembrane protease serine 2; VSV, vesicular stomatitis virus. Download Table S2, DOCX file, 0.2 MB (194.3KB, docx) .
Copyright © 2022 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Studies assessing neutralization of SARS-CoV-2 variants by mRNA vaccine-elicited sera have yielded a similar pattern, although neutralizing activity has generally been retained due to high antibody titers (Table S2). Reductions in neutralizing activity of varying magnitude have been observed against alpha (55, 72, 75–78, 80–83, 85–88, 96, 100, 102), beta (55, 72, 74, 77–79, 84, 85, 87, 88, 96, 100, 102), gamma (72, 79, 85, 89, 90, 102), delta (96, 108), mu (122, 123), and omicron (109–117), when compared with other lineages, with reductions against beta and omicron being notably high (Table S2). Despite the high fold-reductions in neutralization of beta, cell entry was still inhibited at low dilutions (79, 102). Reductions in neutralizing titers have also been observed against viruses carrying the E484K mutation (74, 78, 79, 81, 100, 101, 105), which were generally of greater magnitude than non-E484K mutation combinations (81, 100, 105), suggesting that E484K is a key driver of neutralization escape. Of note, for the lambda variant, which does not carry the E484K mutation, there was no reduction in neutralization relative to that of wild-type SARS-CoV-2 in sera of individuals fully vaccinated with BNT162b2 (124). Reductions in neutralizing titers against variants with mutations at the E484 position (beta, gamma, and mu) were notably smaller or absent in sera from subjects previously exposed to the E484K mutation, suggesting cross-neutralization can occur between variants sharing some or all of the same spike mutations (124). However, reductions in the neutralization of omicron, which carries E484A, have been reported to be greater than with beta in sera from individuals vaccinated with a primary regimen of BNT162b2 (110, 117). A restoration of neutralizing activity against omicron has been reported in sera from individuals who received a booster dose of mRNA vaccine, with even higher titers against delta demonstrated (111, 112, 114–117). Similarly, in a clinical trial of a BNT162b2 primary regimen and booster, the booster dose increased neutralizing antibody titers against the beta variant by 15–20 times compared with post-dose 2, reducing the difference between neutralizing activity against the beta variant and wild-type virus (125).
Fewer studies have assessed escape from vector-based vaccine-elicited antibodies. Reductions in neutralizing activity of post-ChAdOx1 nCoV-19 sera against alpha (85, 86, 104), beta (73), gamma (85), delta (108), and omicron (113) have been observed, with an undetectable neutralization response to beta in eight of 13 samples in one study (73) and to omicron in 20 of 20 samples in another (113). Similarly, in a study of people vaccinated with the vector-based Sputnik V Ad26/Ad5 vaccine (Gamaleya Research Institute of Epidemiology and Microbiology, Moscow, Russian Federation), neutralizing titers against alpha were similar to the D614G control, but 50% of samples did not achieve the IC80 threshold for neutralization of beta (106). In a study in which a primary regimen of mRNA vaccines or ChAdOx1 nCoV-19 exhibited reduced neutralizing activity against omicron, sera from all individuals vaccinated with a primary regimen of Sputnik V and all but one vaccinated with a single dose of Ad26.COV2.S had reduced activity against this variant (109). Reduced neutralizing antibody titers against beta and gamma have also been reported in Ad26.COV2.S recipients (98, 126).
One study assessed neutralization activity of sera from people vaccinated with NVX-CoV2373 (a recombinant spike protein subunit vaccine), demonstrating that alpha was neutralized by all samples with modestly diminished activity compared with the control virus (76). In a study assessing neutralization activity of sera from individuals vaccinated with the inactivated vaccine Coronavac, there was no neutralizing activity against omicron after two doses, or after a third CoronaVac dose; however, nine of 10 samples from individuals who received a primary Coronavac regimen and a BNT162b2 booster exhibited neutralizing activity, implying that heterologous boosting with an mRNA vaccine following a vector-based primary regimen results in a broader immune response than a homologous vector-based regimen (112). A primary regimen of the inactivated vaccine BBiBP-CoRV has also been shown to have no neutralizing activity against omicron for the majority of recipients (109). Data on neutralization of other variants and mutations with other vector-based or subunit vaccines are limited.
POTENTIAL IMPORTANCE OF THE T-CELL RESPONSE
Pharmaceutical research on vaccine-elicited immunity to respiratory viruses tends to focus on the neutralizing antibody response, which is a key element of sterilizing immunity. Neutralizing antibody correlates of protection against symptomatic COVID-19 have been proposed, although evidence of a correlate for asymptomatic infection is lacking (118, 119). However, antibodies represent only one aspect of the immune response to SARS-CoV-2. The T-cell response is important for complete protective immunity, as demonstrated by reports of SARS-CoV-2-exposed individuals with positive T-cell responses but no detectable antibodies (127). Variations in SARS-CoV-2-specific T-cell responses as a function of disease severity have been observed (128, 129), with a coordinated CD4+ and CD8+ T-cell response associated with milder disease, suggesting a role in protective immunity against COVID-19 (130). Consistent with this, recent studies suggest that the T-cell response may provide protection against SARS-CoV-2 variants. An analysis of T cells from individuals previously infected with SARS-CoV-2 showed that both CD4+ and CD8+ T cells can recognize multiple epitopes across the SARS-CoV-2 proteome, suggesting that new variants may not easily escape T-cell recognition after natural infection (131). While SARS-CoV-2 may have the capacity to subvert CD8+ T-cell surveillance through escape mutations (132), neither alpha nor beta escape CD4+ T-cell-mediated vaccine-elicited immunity to the wild-type spike protein (133). In people infected with earlier circulating lineages of SARS-CoV-2, the T-cell response to beta was preserved, despite a loss of CD4+ epitope recognition in mutated regions of the spike protein (134).
Vaccines that induce a robust and poly-epitopic cellular response may provide greater protection against novel variants, as a portion of the epitopes recognized by the vaccine-induced T cell repertoire will remain conserved upon further evolution of the virus. Moreover, owing to the highly polymorphic nature of major histocompatibility complex (MHC) class I and II molecules, virus-antigen-specific T-cell responses, in contrast to vaccine-induced antibodies, are not likely to be subject to immune escape on a population level (135, 136).
BNT162b2 has been shown to elicit robust CD8+ and CD4+ T-cell responses (137), and data from individuals vaccinated with BNT162b2 have shown that the majority of the T-cell response is directed against epitopes conserved across beta, alpha, and the original B lineage (77). Similarly, ChAdOx1 nCoV-19 has been shown to cause expansion of CD4+ and CD8+ T cells to specific SARS-CoV-2 spike protein epitopes; of 87 epitopes identified, 75 were unaffected by beta mutations (73). A study of cross-recognition of SARS-CoV-2 variants across vaccine platforms, including mRNA-1273, BNT162b2, Ad26.CoV2.S, and NVX-CoV2373, showed preservation of at least 83% and 85% of CD4+ and CD8+ responses, respectively, regardless of variant (including omicron) (138). In individuals vaccinated with BNT162b2 or Ad26.CoV2.S, the magnitude of T cells with cross-reactivity to omicron was similar to that of beta and delta, despite the significantly larger number of mutations in the omicron variant (139).
VACCINE EFFICACY AGAINST SARS-COV-2 VARIANTS: EVIDENCE FROM CLINICAL TRIALS
Clinical trials in regions where SARS-CoV-2 variants are prevalent have provided data on COVID-19 vaccine efficacy against those variants (Table S3). A phase 2/3 trial indicated that the efficacy of the ChAdOx1 nCoV-19 vaccine against symptomatic alpha infection is similar to that against previously circulating nonalpha lineages, despite the 9-fold reduction in neutralization activity observed in vitro (104). Similarly, the protein-based NVX-CoV2373 vaccine (Novavax) has been shown to have high efficacy (86.3%) against confirmed symptomatic COVID-19 caused by alpha in a clinical trial in the United Kingdom, compared with 95.6% against nonalpha disease (140).
Efficacy of COVID-19 vaccines against SARS-CoV-2 variants (1–6). CI, confidence interval; COVID-19, coronavirus disease 2019; HIV, human immunodeficiency virus; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. Download Table S3, DOCX file, 0.08 MB (85.4KB, docx) .
Copyright © 2022 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
In a phase 1b/2 clinical trial in a limited number of participants without human immunodeficiency virus (HIV) infection in South Africa, the ChAdOx1 nCoV-19 vaccine only provided minimal protection against mild-to-moderate COVID-19 infection from beta (39 cases, vaccine efficacy 10.4%) (73). However, efficacy against severe disease could not be assessed, as the population was low risk (median age 30 years) and the trial was relatively small (73). Efficacy of NVX-CoV2373 in a phase 2 trial in South Africa was 60% in participants without HIV; of 41 COVID-19 events with available sequencing data, 92.7% (38 events) were due to beta (141). Similarly, in the phase 3 ENSEMBLE study, efficacy of a single dose of the Ad26.COV2.S vaccine (Janssen Vaccines & Prevention) against moderate-to-severe COVID-19 was 57% in South Africa, where 95% of COVID-19 events were due to beta (142). Notably, these efficacy values remain above the 50% threshold established by the U.S. Food and Drug Administration (FDA) for COVID-19 vaccine approval (143). In the South African cohort of the phase 3 trial of BNT162b2, in which eight of the nine events were caused by beta and one was of undetermined lineage, efficacy against symptomatic disease was 100% (144).
Efficacy data against the delta variant are limited, as this variant emerged after the primary clinical trials were complete. However, a randomized clinical trial assessing the efficacy of a primary regimen and booster dose of BNT162b2 compared with a primary BNT162b2 regimen and placebo booster has demonstrated vaccine efficacy of 95.6% during a period when delta was the dominant strain (145).
Efficacy data are limited to short duration and early variants, owing to the nature and timing of the clinical trials. Real-world data from ongoing vaccination programs provide further insights into effectiveness against later variants, duration of protection, and need for booster doses.
VACCINE EFFECTIVENESS AGAINST SARS-COV-2 VARIANTS: EVIDENCE FROM ONGOING VACCINATION PROGRAMS
Ongoing vaccination programs have provided data on COVID-19 vaccine effectiveness against several SARS-CoV-2 variants in a “real-life” setting (Table 1). Data from the mass vaccination campaign in Israel suggest that, consistent with in vitro studies, effectiveness of the BNT162b2 mRNA vaccine against alpha is high, with studies carried out during the alpha-dominant period demonstrating vaccine effectiveness after the primary regimen of BNT162b2 up to 94.5% against SARS-CoV-2 infection (146, 147). Similarly, results obtained during the mass vaccination campaign in Scotland also indicate effectiveness against confirmed alpha infections of 92% for BNT162b2 and 73% for ChAdOx1 nCoV-19 after the primary regimen (148). In England, effectiveness of the primary regimen of BNT162b2 or ChAdOx1 nCoV-19 was 93.7% and 74.5%, respectively, against symptomatic COVID-19 (149). Similarly, during the vaccine roll-out in Qatar, the primary regimen of BNT162b2 provided 89.5% protection against confirmed alpha infections and 100% protection against severe, critical, or fatal disease caused by alpha (150). A Canadian study showed that a primary regimen of BNT162b2, mRNA-1273, or ChAdOx1 nCoV-19 was 89%, 91%, and 75% effective against symptomatic infection from alpha (151). Together, these data demonstrate that vaccine effectiveness against alpha after a primary regimen of mRNA- or vector-based vaccines remains high, suggesting that immune escape is unlikely with alpha.
TABLE 1.
| Variant | Vaccine | Design and setting | Key outcomes | Vaccine effectiveness, % (95% CI) | Reference |
|---|---|---|---|---|---|
| Alpha | BNT162b2 | Israel; ≥7 days post-dose 2, up to 80% isolates during study period were alpha | PCR-confirmed documented infection | 92 (88 to 95) | 146 |
| PCR-confirmed symptomatic COVID-19 | 94 (87 to 98) | ||||
| Hospitalization due to COVID-19 | 87 (55 to 100) | ||||
| Severe COVID-19 | 92 (75 to 100) | ||||
| Israel; ≥7 days post-dose 2, during alpha-dominant period; HCPs | PCR-confirmed infection | 94.5 (82.6 to 98.2) | 147 | ||
| PCR-confirmed symptomatic infection | 97.0 (72.0 to 99.7) | ||||
| England; ≥14 days post-dose 2 | PCR-confirmed symptomatic COVID-19 caused by alphab | 93.7 (91.6 to 95.3) | 149 | ||
| Qatar, ≥14 days post-dose 2 | Any PCR-confirmed infection caused by alpha | 89.5 (85.9 to 92.3) | 150 | ||
| Severe, critical, or fatal disease caused by alpha | 100 (81.7 to 100) | ||||
| Scotland, ≥14 days post-dose 2 | PCR-confirmed infection caused by alphab | 92 (90 to 93) | 148 | ||
| Canada, ≥14 days post-dose 2 | PCR-confirmed symptomatic infection | 89 (87 to 91) | 151 | ||
| BNT162b2 and/or mRNA-1273 and/or ChAdOx1 nCoV-19c | Norway, ≥7 days post-dose 2 | PCR- or whole genome sequencing-confirmed infection by alpha or delta | 84.4 (81.8 to 86.5) | 187 | |
| mRNA-1273 | Canada, ≥14 days post-dose 2 | PCR-confirmed symptomatic infection | 91 (84 to 95) | 151 | |
| ChAdOx1 nCoV-19 | England, ≥14 days post-dose 2 | PCR-confirmed symptomatic COVID-19 caused by alphab | 74.5 (68.4 to 79.4) | 149 | |
| Scotland, ≥14 days post-dose 2 | PCR-confirmed infection caused by alphab | 73 (66 to 78) | 148 | ||
| Canada, ≥14 days post-dose 2 | PCR-confirmed symptomatic infection | 75 (−98 to 97) | 151 | ||
| Beta | BNT162b2 | Qatar, ≥14 days post-dose 2 | Any PCR-confirmed infection caused by beta | 75.0 (70.5 to 78.9) | 150 |
| Severe, critical, or fatal disease caused by beta | 100 (73.7 to 100) | ||||
| Delta | BNT162b2 | England, ≥14 days post-dose 2 | PCR-confirmed symptomatic COVID-19 caused by deltab | 88.0 (85.3 to 90.1) | 149 |
| Scotland, ≥14 days post-dose 2 | PCR-confirmed infection caused by deltab | 79 (75 to 82) | 148 | ||
| UK, ≥14 days post-dose 2, during delta-dominant period | PCR-confirmed infection | 80 (77 to 83) | 152 | ||
| Canada, ≥14 days post-dose 2 | PCR-confirmed symptomatic infection | 85 (59 to 94) | 151 | ||
| US, ≥14 days post-dose 2, during delta-dominant period | PCR-confirmed COVID-19 associated hospitalization | 80 (73 to 85) | 154 | ||
| PCR-confirmed COVID-19-associated emergency department/urgent care encounters | 77 (74 to 80) | ||||
| US, ≥7 days post-dose 2 | PCR-confirmed infection caused by delta | 75 (71 to 78) | 156 | ||
| US, ≥7 days post-dose 2 | Hospitalization due to delta | 93 (84 to 96) | |||
| England, 2–≥25 wks post-dose 2 and 1–≥2 wks post-BNT162b2 booster | PCR-confirmed symptomatic disease caused by delta | wks post-dose 2: 2–9: 88.2 (86.7 to 89.5) 10–14: 77.7 (76.3 to 79.0) 15–19: 72.2 (71.0 to 73.4) 20–24: 64.8 (62.6 to 66.9) ≥25: 63.5 (61.4 to 65.5) Wks post-booster: 1–2: 92.2 (90.7 to 93.4) ≥2: 92.6 (92.0 to 93.1) |
159 | ||
| Denmark, 1–150 days post-dose 2 and 1–30 days post-booster | PCR-confirmed infection caused by deltae | Days post-dose 2 1–30: 86.7 (84.6 to 88.6) 31–60: 80.9 (79.0 to 82.6) 61–90: 72.8 (71.7 to 73.8) 91–150: 53.8 (52.9 to 54.6) Post-booster: 81.2 (79.2 to 82.9) |
161 | ||
| England, ≥14 days post-dose 2 and ≥14 days post-mRNA vaccine booster | PCR-confirmed symptomatic infection caused by delta | Cohort analysis: Post-dose 2: 55.9 (55.5 to 56.3) Post-booster: 88.6 (88.1 to 89.1) Test-negative case–control: Post-dose 2: 69.8 (69.4 to 70.2) Post-booster: 94.3 (93.9 to 94.6) |
162 | ||
| BNT162b2 or mRNA-1273 | US, ≥14 days post-dose 2, during delta-dominant period | PCR-confirmed infection | 74 (65 to 82) | 153 | |
| mRNA-1273 | US, ≥14 days post-dose 2 and post-booster | PCR-confirmed infection caused by deltad | Days post-dose 2: 14–90: 82.8 (69.6 to 90.3) 91–180: 63.6 (51.8 to 72.5) 181–270: 61.4 (56.8 to 65.5) ≥270: 52.9 (43.7 to 60.5) Post-booster: 95.2 (93.4 to 96.4) |
160 | |
| Hospitalization caused by deltad | Post-dose 2: 98.0 (87.2 to 99.7) | ||||
| Denmark, 1–150 days post-dose 2 and 1–30 days post-booster | PCR-confirmed infection caused by deltae | Days post-dose 2: 1–30: 88.2 (83.1 to 91.8) 31–60: 81.5 (77.7 to 84.6) 61–90: 72.2 (70.4 to 74.0) 91–150: 65.0 (63.6 to 66.3) Post-booster: 82.8 (58.8 to 92.9) |
161 | ||
| ChAdOx1 nCoV-19 | England, ≥14 days post-dose 2 | PCR-confirmed symptomatic COVID-19 caused by deltab | 67.0 (61.3 to 71.8) | 149 | |
| Scotland, ≥14 days post-dose 2 | PCR-confirmed infection caused by deltab | 60 (53 to 66) | 148 | ||
| UK, ≥14 days post-dose 2, during delta-dominant period | PCR-confirmed infection | 67 (62 to 71) | 152 | ||
| England, 2–≥25 wks post-dose 2 and 1–≥2 wks post-ChAdOx1 nCoV-19 booster | PCR-confirmed symptomatic disease caused by delta | wks post-dose 2: 2–9: 76.2 (63.7 to 84.4) 10–14: 64.9 (55.2 to 72.4) 15–19: 48.5 (44.7 to 52.0) 20–24: 45.4 (43.0 to 47.6) ≥25: 41.8 (39.4 to 44.1) Wks post-booster: 1–2: 87.0 (85.5 to 88.4) ≥2: 93.8 (93.2 to 94.3) |
159 | ||
| England, ≥14 days post-dose 2 and ≥14 days post-ChAdOx1 nCoV-19 booster | PCR-confirmed symptomatic infection caused by delta | Cohort analysis: Post-dose 2: 25.0 (24.3 to 25.7) Post-booster: 89.7 (88.9 to 90.4) Test-negative case–control: Post-dose 2: 43.7 (43.0 to 44.4) Post-booster: 93.8 (93.3 to 94.3) |
162 | ||
| BNT162b2 and/or mRNA-1273 and/or ChAdOx1 nCoV-19c | Norway, ≥7 days post-dose 2 | PCR- or whole-genome sequencing-confirmed infection by alpha or delta | 64.4 (60.6 to 68.2) | 187 | |
| Ad26.COV2.S | US, ≥14 days post-dose 1, during delta-dominant period | PCR-confirmed infection | 51 (−2 to 76) | 153 | |
| US, ≥14 days post-dose 1, during delta-dominant period | PCR-confirmed COVID-19-associated hospitalization | 60 (31 to 77) | 154 | ||
| PCR-confirmed COVID-19-associated emergency department/urgent care encounters | 65 (56 to 72) | ||||
| US, ≥14 days post-dose 2, during delta-dominant period | PCR-confirmed COVID-19-associated hospitalization | 95 (92 to 97) | |||
| PCR-confirmed COVID-19-associated emergency department/urgent care encounters | 92 (89 to 93) | ||||
| England, 2–≥25 wks post-dose 2 and ≥2 wks post-BNT162b2 booster | PCR-confirmed symptomatic disease caused by omicron | wks post-dose 2: 2–9: 88.0 (65.9 to 95.8) 10–14: 48.5 (24.3 to 65.0) 15–19: 34.1 (9.7 to 52.0) 20–24: 36.6 (0.4 to 59.6) ≥25: 34.2 (−5.0 to 58.7) Wks post-booster: ≥2: 75.5 (56.1 to 86.3) |
159 | ||
| Omicron | BNT162b2 | Denmark, 1–150 days post-dose 2 and 1–30 days post-booster | PCR-confirmed infection caused by omicrone | Days post-dose 2: 1–30: 55.2 (23.5 to 73.7) 31–60: 16.1 (−20.8 to 41.7) 61–90: 9.8 (−10.0 to 26.1) 91–150: −76.5 (−95.3 to −59.5) Post-booster: 54.6 (30.4 to 70.4) |
161 |
| England, adults aged ≥65 yrs, post-booster | PCR-confirmed symptomatic disease caused by omicron | wks post-BNT162b2 booster: 2–4: 65 5–9: 49 ≥10: 31 Wks post-mRNA-1273 booster: 2–4: 70 5–9: 57 |
163 | ||
| mRNA-1273 | US, ≥14 days post-dose 2 and post-booster | PCR-confirmed infection caused by omicrond | Days post-dose 2: 14–90: 30.4 (5.0 to 49.0) 91–180: 15.2 (0 to 30.7) 181–270: 0 (0 to 1.2) ≥270: 0 (0 to 1.7) Post-booster: 62.5 (56.2 to 67.9) |
160 | |
| Denmark, 1–150 days post-dose 2 | PCR-confirmed infection caused by omicrone | Days post-dose 2: 1–30: 36.7 (−69.9 to 76.4) 31–60: 30.0 (−41.3 to 65.4) 61–90: 4.2 (−30.8 to 29.8) 91–150: −39.3 (−61.6 to −20.0) |
161 | ||
| ChAdOx1 nCoV-19 | England, 2–≥25 wks post-dose 2 and 1–≥2 wks post-BNT162b2 booster | PCR-confirmed symptomatic disease caused by omicron | wks post-dose 2: 15–19: −54.7 (−174.0 to 12.6) 20–24: −13.2 (−60.2 to 20.1) ≥25: 5.9 (−29.7 to 31.7) Wks post-booster: 1–2: 71.9 (9.1 to 91.3) ≥2: 71.4 (41.8 to 86.0) |
159 | |
| England, adults aged ≥65 yrs, post- BNT162b2 or mRNA-1273 booster | PCR-confirmed symptomatic disease caused by omicron | wks post-booster: 2–4: 62–65 5–9: 48–56 ≥10: 32 (BNT162b2 booster only) |
163 | ||
CI, confidence interval; COVID-19, coronavirus disease 2019; HCP, health care professionals; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Based on presence or absence of deletion in S gene at 69–70.
ChAdOx1 nCoV-19 was discontinued in Norway on 11 March, 2021, and those who received their first dose were offered a second dose of either BNT162b2 or mRNA-1273. In Norway, a mixed regimen of the two mRNA doses has been administered. The majority of patients in this study (81.3%) received BNT162b2 (187).
Specimens with S gene target failure were considered to be omicron, and specimens for which S gene was detected were assumed to be delta.
All specimens investigated for omicron by sequencing or variant-specific PCR; cases not identified as omicron assumed to be delta.
Data on the effectiveness of COVID-19 vaccines against beta and gamma are limited. In Qatar, effectiveness of BNT162b2 against any documented infection with beta was 75%, and effectiveness against severe, critical, or fatal disease caused by beta was 100% (150). In a Canadian study, beta and gamma specimens obtained from patients vaccinated with BNT162b2, mRNA-1273, or ChAdOx1 nCoV-19 were grouped together to evaluate vaccine effectiveness, due to the low number of patients with either variant, as both variants carry N501Y and E484K mutations (151). The primary regimen of BNT162b2 was found to be 85% effective against symptomatic infection and 98% effective against hospitalization or death for this beta/gamma group (151).
The rapid spread of delta has allowed analysis of effectiveness against this variant, with vaccine effectiveness against delta generally remaining high following a primary regimen. In England, vaccine effectiveness against delta was 88.0% with BNT162b2 and 67.0% with ChAdOx1 nCoV-19, with similar findings reported in Scotland (BNT162b2, 79%; ChAdOx1 nCoV-19, 60%) and the United Kingdom as a whole (BNT162b2, 80%; ChAdOx1 nCoV-19, 67%) (148, 149, 152). Similarly, a Canadian study showed that BNT162b2 was 85% effective against symptomatic infection from delta (151). Two doses of BNT162b2 or mRNA-1273 were 74% effective against confirmed infections during a delta-dominant period in the United States, while the one-dose Ad26.COV2.S was 51% effective (153). An interim report from the VISION Network in the United States reported overall effectiveness of 86% for BNT162b2, mRNA-1273, or Ad26.COV2.S against COVID-19-associated hospitalizations during a period when delta accounted for >50% of cases; effectiveness was higher with mRNA-1273 (95%) than with BNT162b2 (80%) and Ad26.COV2.S (60%) (154). However, this interim analysis did not assess effectiveness by time since vaccination; thus, the impact of possible waning of antibody levels is not known. A report from Israel suggested decreased effectiveness of BNT162b2 against COVID-19 infection and symptomatic disease during a period of spread of delta (155); however, this may be an effect of longer intervals post-dose 2 leading to waning antibody levels over time, as a large proportion of the population of Israel was vaccinated in early 2021. The VISION Network observed a significantly lower overall effectiveness of BNT162b2, mRNA-1273, or Ad26.COV2.S against hospitalizations among adults ≥75 years of age compared with adults 18–74 years of age, which may also be a result of waning antibody levels, as adults ≥75 years of age were vaccinated earlier in the United States (154). Similarly, a decline in effectiveness over time since second dose in adults 18–64 years of age was reported in the United Kingdom (152). Furthermore, a study from Southern California found that effectiveness of BNT162b2 against delta infections declined from 93% in the first month after full vaccination to 53% at ≥4 months (156). Similar findings were observed with other SARS-CoV-2 variants, suggesting that reductions in vaccine effectiveness against delta and other variants are likely associated with increased time interval since the primary regimen, rather than vaccine escape (156). Effectiveness of BNT162b2 against severe COVID-19 disease and hospitalization in Israel and in the Southern California study remained high (155, 156). Studies from countries that used wider intervals between the first and second dose of BNT162b2, such as the United Kingdom and Canada, have reported higher vaccine effectiveness against delta infection, although the duration of follow-up has been insufficient to assess the effect of waning (148, 151, 156, 157). This may be due to increased time for proliferation of memory T cells and B cells (120, 158). In more recent studies, in countries where a booster vaccination program has been implemented, vaccine effectiveness of mRNA vaccines and ChAdOx1 nCoV-19 primary regimen with an mRNA booster against delta infection or symptomatic disease has been around 90% or more (159, 160).
Initial data on COVID-19 vaccine effectiveness against the omicron variant suggest that a primary mRNA vaccination regimen provides limited protection against infection. Studies from the United Kingdom, United States, and Denmark have shown that mRNA vaccines have limited-to-moderate effectiveness versus omicron during the first 1–2 months after the second dose (159–162), but with a steep decline in effectiveness thereafter (159, 160). In a study that predicted vaccine effectiveness against omicron relative to delta, controlling for age, region, and ethnic group, effectiveness of two doses of BNT162b2 against omicron was significantly lower than against delta (162). However, in line with neutralizing antibody studies, a booster dose of mRNA vaccine has been shown to partially restore protection (159–162). In England, effectiveness of a primary regimen and booster dose of BNT162b2 against symptomatic omicron disease was 76% (159), and in a test-negative study in the United States, effectiveness of a primary regimen and booster of mRNA-1273 against omicron infection was 63% (160). Reported effectiveness against omicron infection for a primary regimen and booster of BNT162b2 in Denmark was 55% 1 to 30 days after the booster dose (161). Data from the United Kingdom from adults ≥65 years of age who received a primary regimen of BNT162b2 have demonstrated effectiveness against symptomatic omicron of 65% 2 to 4 weeks post-BNT162b2 booster and 70% 2 to 4 weeks post-mRNA-1273 booster (163).
Primary regimens of vector-based vaccines are not effective against omicron (159). In England, effectiveness of two doses of ChAdOx1 nCoV-19 ranged from −55% to 6% from 15 weeks after the second dose (159). However, 2 weeks after a BNT162b2 booster, effectiveness increased to 71% (159).
Despite lower effectiveness against omicron infection, combined vaccine effectiveness against severe disease and hospitalization caused by omicron remains high, with initial data from the United Kingdom reporting effectiveness against hospitalization close to 90% (163, 164). However, preliminary evidence suggests that effectiveness against infection and symptomatic disease wanes with time after the booster dose, with effectiveness of a BNT162b2 primary regimen and booster dropping from 65% at 2–4 weeks post-booster to 49% at 5–9 weeks and 31% at 10 weeks (163, 164). Similar waning was observed with a primary BNT162b2 regimen and mRNA-1273 booster, and a primary ChAdOx1 nCoV-19 regimen with mRNA vaccine booster (163).
MITIGATION STRATEGIES TO ADDRESS SARS-COV-2 VARIANTS
As described above, data from vaccination programs have highlighted a need for booster doses to increase vaccine effectiveness against certain variants. Therefore, several clinical trials have been conducted to evaluate the safety and immunogenicity of homologous (165–168) and heterologous boosters (169, 170). The randomized trial of a primary regimen and booster of BNT162b2 remains the only booster trial to report efficacy data (145).
In parallel to evaluating booster doses with the original vaccines, the emergence of VOCs led some manufacturers to begin development of variant-specific vaccines in 2021 (165, 171). Since the omicron outbreak, the majority of manufacturers have announced development of omicron-specific vaccines. Currently, BioNTech/Pfizer are exploring beta-, delta-, and omicron-specific BNT162b2 vaccines, as well as a multivalent alpha and delta candidate (172). Initial data suggest that alpha- and delta-specific vaccines, and the multivalent candidate, elicit higher neutralizing activity against omicron than the original vaccine when administered as boosters (173). Moderna is also evaluating delta- and omicron-specific mRNA-1273 vaccines, and is focusing on a multivalent candidate (174). Preliminary analyses show that boosters of modified mRNA-1273 can increase neutralizing antibody titers against beta and gamma (165). AstraZeneca/University of Oxford are reportedly developing modified versions of ChAdOx1 nCoV-19 to target beta and omicron (175, 176), and omicron-specific versions of the Sputnik V Ad26/Ad5 and NVX-CoV2373 vaccines are also in development (177, 178).
Regulatory bodies have issued guidance to vaccine manufacturers on data requirements and processes for approval of modified vaccines (179–182). The WHO Technical Advisory Group on COVID-19 Vaccine Composition (TAG-CO-VAC) has encouraged vaccine developers to gather small-scale data on the breadth and magnitude of immune responses to modified and multivalent vaccines against VOCs (182). Informed by experience with other continuously evolving infectious diseases, such as influenza, approval of modified vaccines will be primarily based on immunogenicity bridging studies to expedite development and review (179–181). Important considerations will include immunogenicity in vaccine-naive and vaccine-experienced individuals, optimal booster dosing, and potential value of multivalent candidates, including both original and modified sequences. An alternative approach to the modification of existing vaccines is the development of new vaccines targeting combinations of viral proteins (e.g., spike, nucleocapsid, and envelope proteins), or with different delivery mechanisms, such as oral or intranasal, to improve the mucosal immune response (183). The TAG-CO-VAC also notes that a pan SARS-CoV-2 vaccine would be a more sustainable long-term option that would effectively be variant-proof (182).
It is worth noting the importance of transmissibility in addition to immune escape features. Beta and omicron both exhibit immune escape, but while the prevalence of beta has remained relatively stable in most regions in recent months, limiting its impact, omicron, which is highly transmissible, has become highly prevalent (65). A risk remains of future emergence of SARS-CoV-2 variants with both increased immune escape and greater replicative and transmission fitness. However, there are no established criteria to indicate whether vaccine adaptation will be required for new variants. Furthermore, this may be dependent on the specific vaccine. For variants without immune escape capability, a vaccine with high efficacy may only require a booster dose to protect against a new variant, while a lower-efficacy vaccine may require adaptation; however, variants with significant immune escape ability are likely to require adaptation for all vaccines. Monitoring of viral mutations by the WHO and international experts, in combination with early warning systems combining structural and computational modeling, will continue to have an essential role in the early identification of high-risk variants as the pandemic continues (46, 184). It is possible that variant-specific vaccines, such as those adapted to beta, delta, or omicron, may elicit cross-reactive neutralizing antibody responses that protect against several different variants (172, 185). T-cell responses induced by each vaccine are also likely to have a role.
CONCLUSIONS
Current evidence on COVID-19 vaccine performance against SARS-CoV-2 variants is reassuring, demonstrating that alpha has a limited impact on effectiveness and that some vaccine platforms may have the potential to provide at least partial protection against beta and delta infection, likely due to the high levels of neutralizing antibodies elicited and the robust and broad nature of the T-cell response elicited by several of the vaccines. Data from booster vaccination programs suggest that mRNA vaccine boosters can provide some protection against omicron. In addition, vaccine effectiveness against severe disease and hospitalization remains high, suggesting that vaccination has weakened the link between infection and severity of disease. Although vaccine adaptation for existing variants may be unnecessary, strategies are in place to modify vaccines, including different delivery mechanisms, should future emerging variants result in reduced effectiveness. The lower vaccine effectiveness reported against the omicron variant may also be a result of waning immunity over time, underscoring the importance of continuing preventative measures to reduce transmission regardless of vaccination status, as currently recommended by several countries (particularly in the light of the high transmissibility rate of omicron).
Several data gaps remain to be addressed to fully understand the impact of novel variants of SARS-CoV-2 on vaccines. For example, correlates of protective efficacy other than neutralizing antibodies need to be established, the role of other immune effector functions mediated by antibody binding, and the contributions of T-cell responses to protective immunity from vaccination over longer periods of time should be ascertained, to better elucidate the potential risks posed by both existing and future variants.
ACKNOWLEDGMENTS
Medical writing support, including assisting authors with the development of the initial draft and incorporation of comments, was provided by Rachel Wright, and editorial support, including formatting, proofreading, and submission, was provided by Ian Norton, both of Scion, London, supported by BioNTech according to Good Publication Practice guidelines (https://www.acpjournals.org/doi/10.7326/M15-0288). P.L.M. is supported by the South African Research Chairs Initiative of the Department of Science and Innovation and the National Research Foundation (Grant No. 9834) and the South African Medical Research Council. J.P.K. is supported by grants from the Rockefeller Foundation (2021 HTH 010 GA-S), Emergent Ventures at the Mercatus Center (FastGrants #2239), and the U.S. National Institutes of Health (3P20GM121307-04S1). T.F.S. is supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy—EXC 2155 “RESIST”—Project ID 39087428, and the Lower Saxony Ministry of Science and Culture (project 14-76103-184).
G.R.M., T.F.S., B.L., and P.L.M. have no conflicts of interest to report. Ö.T. and U.S. are management board members and employees at BioNTech SE (Mainz, Germany). J.P.K. has a grant from the Rockefeller Foundation to increase equity and representativeness in SARS-CoV-2 sequencing, has a role on an NIH grant to carry out SARS-CoV-2 sequencing and detection of viral variants, served on a BioNTech advisory panel, and holds stock in BioNTech and Pfizer, who manufacture COVID-19 vaccines. A.M. and S.P. are employees at BioNTech SE. A.M., Ö.T., and U.S. are inventors on patents and patent applications related to RNA technology and COVID-19 vaccines. A.M., Ö.T., and U.S. have securities from BioNTech SE.
All authors contributed to the manuscript conception, writing, and review process, and approved the final version for submission.
Contributor Information
Shanti Pather, Email: Shanti.Pather@biontech.de.
Vinayaka R. Prasad, Albert Einstein College of Medicine
REFERENCES
- 1.CoVariants. 2022. CoVariants. https://covariants.org/. Accessed 14 January, 2022.
- 2.Rahimi A, Mirzazadeh A, Tavakolpour S. 2021. Genetics and genomics of SARS-CoV-2: A review of the literature with the special focus on genetic diversity and SARS-CoV-2 genome detection. Genomics 113:1221–1232. doi: 10.1016/j.ygeno.2020.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rehman SU, Shafique L, Ihsan A, Liu Q. 2020. Evolutionary trajectory for the emergence of novel coronavirus SARS-CoV-2. Pathogens 9:240. doi: 10.3390/pathogens9030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minskaia E, Hertzig T, Gorbalenya AE, Campanacci V, Cambillau C, Canard B, Ziebuhr J. 2006. Discovery of an RNA virus 3'→5' exoribonuclease that is critically involved in coronavirus RNA synthesis. Proc Natl Acad Sci USA 103:5108–5113. doi: 10.1073/pnas.0508200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denison MR, Graham RL, Donaldson EF, Eckerle LD, Baric RS. 2011. Coronaviruses: an RNA proofreading machine regulates replication fidelity and diversity. RNA Biol 8:270–279. doi: 10.4161/rna.8.2.15013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mercatelli D, Giorgi FM. 2020. Geographic and genomic distribution of SARS-CoV-2 mutations. Front Microbiol 11:1800. doi: 10.3389/fmicb.2020.01800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vilar S, Isom DG. 2021. One year of SARS-CoV-2: how much has the virus changed? Biology (Basel) 10:91. doi: 10.3390/biology10020091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson EC, Rosen LE, Shepherd JG, Spreafico R, da Silva Filipe A, Wojcechowskyj JA, Davis C, Piccoli L, Pascall DJ, Dillen J, Lytras S, Czudnochowski N, Shah R, Meury M, Jesudason N, De Marco A, Li K, Bassi J, O'Toole A, Pinto D, Colquhoun RM, Culap K, Jackson B, Zatta F, Rambaut A, Jaconi S, Sreenu VB, Nix J, Zhang I, Jarrett RF, Glass WG, Beltramello M, Nomikou K, Pizzuto M, Tong L, Cameroni E, Croll TI, Johnson N, Di Iulio J, Wickenhagen A, Ceschi A, Harbison AM, Mair D, Ferrari P, Smollett K, Sallusto F, Carmichael S, Garzoni C, Nichols J, Galli M, COVID-19 Genomics UK (COG-UK) Consortium, et al. 2021. Circulating SARS-CoV-2 spike N439K variants maintain fitness while evading antibody-mediated immunity. Cell 184:1171–1187.e20. doi: 10.1016/j.cell.2021.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qing E, Kicmal T, Kumar B, Hawkins GM, Timm E, Perlman S, Gallagher T. 2021. Dynamics of SARS-CoV-2 spike proteins in cell entry: control elements in the amino-terminal domains. mBio 12:e0159021. doi: 10.1128/mBio.01590-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCarthy KR, Rennick LJ, Nambulli S, Robinson-McCarthy LR, Bain WG, Haidar G, Duprex WP. 2021. Recurrent deletions in the SARS-CoV-2 spike glycoprotein drive antibody escape. Science 371:1139–1142. doi: 10.1126/science.abf6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thorne LG, Bouhaddou M, Reuschl AK, Zuliani-Alvarez L, Polacco B, Pelin A, Batra J, Whelan MVX, Hosmillo M, Fossati A, Ragazzini R, Jungreis I, Ummadi M, Rojc A, Turner J, Bischof ML, Obernier K, Braberg H, Soucheray M, Richards A, Chen KH, Harjai B, Memon D, Hiatt J, Rosales R, McGovern BL, Jahun A, Fabius JM, White K, Goodfellow IG, Takeuchi Y, Bonfanti P, Shokat K, Jura N, Verba K, Noursadeghi M, Beltrao P, Kellis M, Swaney DL, Garcia-Sastre A, Jolly C, Towers GJ, Krogan NJ. 2021. Evolution of enhanced innate immune evasion by SARS-CoV-2. Nature 602:487–495. doi: 10.1038/s41586-021-04352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y. 2020. Strong evolutionary convergence of receptor-binding protein spike between COVID-19 and SARS-related coronaviruses. bioRxiv doi: 10.1101/2020.03.04.975995. [DOI]
- 13.Bobay LM, O'Donnell AC, Ochman H. 2020. Recombination events are concentrated in the spike protein region of Betacoronaviruses. PLoS Genet 16:e1009272. doi: 10.1371/journal.pgen.1009272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai MM, Cavanagh D. 1997. The molecular biology of coronaviruses. Adv Virus Res 48:1–100. doi: 10.1016/S0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, Liu W, Bi Y, Gao GF. 2016. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol 24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lytras S, Hughes J, Martin D, de Klerk A, Lourens R, Kosakovsky Pond SL, Xia W, Jiang X, Robertson DL. 2021. Exploring the natural origins of SARS-CoV-2 in the light of recombination. bioRxiv doi: 10.1093/gbe/evac018. [DOI] [PMC free article] [PubMed]
- 17.Li X, Giorgi EE, Marichannegowda MH, Foley B, Xiao C, Kong XP, Chen Y, Gnanakaran S, Korber B, Gao F. 2020. Emergence of SARS-CoV-2 through recombination and strong purifying selection. Sci Adv 6:eabb9153. doi: 10.1126/sciadv.abb9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gribble J, Stevens LJ, Agostini ML, Anderson-Daniels J, Chappell JD, Lu X, Pruijssers AJ, Routh AL, Denison MR. 2021. The coronavirus proofreading exoribonuclease mediates extensive viral recombination. PLoS Pathog 17:e1009226. doi: 10.1371/journal.ppat.1009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker MM, Graham RL, Donaldson EF, Rockx B, Sims AC, Sheahan T, Pickles RJ, Corti D, Johnston RE, Baric RS, Denison MR. 2008. Synthetic recombinant bat SARS-like coronavirus is infectious in cultured cells and in mice. Proc Natl Acad Sci USA 105:19944–19949. doi: 10.1073/pnas.0808116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexandersen S, Chamings A, Bhatta TR. 2020. SARS-CoV-2 genomic and subgenomic RNAs in diagnostic samples are not an indicator of active replication. Nat Commun 11:6059. doi: 10.1038/s41467-020-19883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boni MF, Zhou Y, Taubenberger JK, Holmes EC. 2008. Homologous recombination is very rare or absent in human influenza A virus. J Virol 82:4807–4811. doi: 10.1128/JVI.02683-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koyama T, Platt D, Parida L. 2020. Variant analysis of SARS-CoV-2 genomes. Bull World Health Organ 98:495–504. doi: 10.2471/BLT.20.253591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Z, Li H, Wu X, Zhong Y, Zhang K, Zhang YP, Boerwinkle E, Fu YX. 2004. Moderate mutation rate in the SARS coronavirus genome and its implications. BMC Evol Biol 4:21. doi: 10.1186/1471-2148-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cotten M, Watson SJ, Zumla AI, Makhdoom HQ, Palser AL, Ong SH, Al Rabeeah AA, Alhakeem RF, Assiri A, Al-Tawfiq JA, Albarrak A, Barry M, Shibl A, Alrabiah FA, Hajjar S, Balkhy HH, Flemban H, Rambaut A, Kellam P, Memish ZA. 2014. Spread, circulation, and evolution of the Middle East respiratory syndrome coronavirus. mBio 5:e01062-13. doi: 10.1128/mBio.01062-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boni MF, Lemey P, Jiang X, Lam TT, Perry BW, Castoe TA, Rambaut A, Robertson DL. 2020. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat Microbiol 5:1408–1417. doi: 10.1038/s41564-020-0771-4. [DOI] [PubMed] [Google Scholar]
- 26.MacLean OA, Lytras S, Weaver S, Singer JB, Boni MF, Lemey P, Kosakovsky Pond SL, Robertson DL. 2021. Natural selection in the evolution of SARS-CoV-2 in bats created a generalist virus and highly capable human pathogen. PLoS Biol 19:e3001115. doi: 10.1371/journal.pbio.3001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellis P, Somogyvari F, Virok DP, Noseda M, McLean GR. 2021. Decoding Covid-19 with the SARS-CoV-2 genome. Curr Genet Med Rep 9:1–12. doi: 10.1007/s40142-020-00197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Temmam S, Vongphayloth K, Salazar E, Munier S, Bonomi M, Régnault B, Douangboubpha B, Karami Y, Chretien D, Sanamxay D, Xayaphet V, Paphaphanh P, Lacoste V, Somlor S, Lakeomany K, Phommavanh N, Pérot P, Donati F, Bigot T, Nilges M, Rey F, Werf S, Brey P, Eloit M. 2022. Coronaviruses with a SARS-CoV-2-like receptor-binding domain allowing ACE2-mediated entry into human cells isolated from bats of Indochinese peninsula. Nature Portfolio doi: 10.21203/rs.3.rs-871965/v1. [DOI] [Google Scholar]
- 30.Zhu Z, Meng K, Meng G. 2020. Genomic recombination events may reveal the evolution of coronavirus and the origin of SARS-CoV-2. Sci Rep 10:21617. doi: 10.1038/s41598-020-78703-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yurkovetskiy L, Wang X, Pascal KE, Tomkins-Tinch C, Nyalile TP, Wang Y, Baum A, Diehl WE, Dauphin A, Carbone C, Veinotte K, Egri SB, Schaffner SF, Lemieux JE, Munro JB, Rafique A, Barve A, Sabeti PC, Kyratsous CA, Dudkina NV, Shen K, Luban J. 2020. Structural and functional analysis of the D614G SARS-CoV-2 spike protein variant. Cell 183:739–751.e8. doi: 10.1016/j.cell.2020.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, Hengartner N, Giorgi EE, Bhattacharya T, Foley B, Hastie KM, Parker MD, Partridge DG, Evans CM, Freeman TM, de Silva TI, Sheffield C-GG, McDanal C, Perez LG, Tang H, Moon-Walker A, Whelan SP, LaBranche CC, Saphire EO, Montefiori DC, Sheffield COVID-19 Genomics Group. 2020. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell 182:812–827.e19. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plante JA, Liu Y, Liu J, Xia H, Johnson BA, Lokugamage KG, Zhang X, Muruato AE, Zou J, Fontes-Garfias CR, Mirchandani D, Scharton D, Bilello JP, Ku Z, An Z, Kalveram B, Freiberg AN, Menachery VD, Xie X, Plante KS, Weaver SC, Shi PY. 2021. Spike mutation D614G alters SARS-CoV-2 fitness. Nature 592:116–121. doi: 10.1038/s41586-020-2895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.GISAID. 2021. https://www.gisaid.org/phylodynamics/global/nextstrain/. Accessed 14 July, 2021.
- 35.Nextclade. 2021. Nextstrain/ncov. https://github.com/nextstrain/ncov/blob/master/defaults/clades.tsv. Accessed 14 July, 2021.
- 36.Atlani-Duault L, Lina B, Chauvin F, Delfraissy JF, Malvy D. 2021. Immune evasion means we need a new COVID-19 social contract. Lancet Public Health 6:e199–e200. doi: 10.1016/S2468-2667(21)00036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi B, Choudhary MC, Regan J, Sparks JA, Padera RF, Qiu X, Solomon IH, Kuo HH, Boucau J, Bowman K, Adhikari UD, Winkler ML, Mueller AA, Hsu TY, Desjardins M, Baden LR, Chan BT, Walker BD, Lichterfeld M, Brigl M, Kwon DS, Kanjilal S, Richardson ET, Jonsson AH, Alter G, Barczak AK, Hanage WP, Yu XG, Gaiha GD, Seaman MS, Cernadas M, Li JZ. 2020. Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N Engl J Med 383:2291–2293. doi: 10.1056/NEJMc2031364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hensley MK, Bain WG, Jacobs J, Nambulli S, Parikh U, Cillo A, Staines B, Heaps A, Sobolewski MD, Rennick LJ, Macatangay BJC, Klamar-Blain C, Kitsios GD, Methe B, Somasundaram A, Bruno TC, Cardello C, Shan F, Workman C, Ray P, Ray A, Lee J, Sethi R, Schwarzmann WE, Ladinsky MS, Bjorkman PJ, Vignali DA, Duprex WP, Agha ME, Mellors JW, McCormick KD, Morris A, Haidar G. 2021. Intractable coronavirus disease 2019 (COVID-19) and prolonged severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication in a chimeric antigen receptor-modified T-cell therapy recipient: A case study. Clin Infect Dis 73:e815–e821. doi: 10.1093/cid/ciab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avanzato VA, Matson MJ, Seifert SN, Pryce R, Williamson BN, Anzick SL, Barbian K, Judson SD, Fischer ER, Martens C, Bowden TA, de Wit E, Riedo FX, Munster VJ. 2020. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell 183:1901–1912.e9. doi: 10.1016/j.cell.2020.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aydillo T, Gonzalez-Reiche AS, Aslam S, van de Guchte A, Khan Z, Obla A, Dutta J, van Bakel H, Aberg J, Garcia-Sastre A, Shah G, Hohl T, Papanicolaou G, Perales MA, Sepkowitz K, Babady NE, Kamboj M. 2020. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med 383:2586–2588. doi: 10.1056/NEJMc2031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kemp SA, Collier DA, Datir RP, Ferreira I, Gayed S, Jahun A, Hosmillo M, Rees-Spear C, Mlcochova P, Lumb IU, Roberts DJ, Chandra A, Temperton N, Collaboration C-N, Consortium C-GU, Sharrocks K, Blane E, Modis Y, Leigh KE, Briggs JAG, van Gils MJ, Smith KGC, Bradley JR, Smith C, Doffinger R, Ceron-Gutierrez L, Barcenas-Morales G, Pollock DD, Goldstein RA, Smielewska A, Skittrall JP, Gouliouris T, Goodfellow IG, Gkrania-Klotsas E, Illingworth CJR, McCoy LE, Gupta RK, COVID-19 Genomics UK (COG-UK) Consortium. 2021. SARS-CoV-2 evolution during treatment of chronic infection. Nature 592:277–282. doi: 10.1038/s41586-021-03291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cobey S, Larremore DB, Grad YH, Lipsitch M. 2021. Concerns about SARS-CoV-2 evolution should not hold back efforts to expand vaccination. Nat Rev Immunol 21:330–335. doi: 10.1038/s41577-021-00544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization. 2021. COVID-19 Weekly Epidemiological Update: special edition: Proposed working definitions of SARS-CoV-2 variants of interest and variants of concern.
- 44.Public Health England. 2021. SARS-CoV-2 variants of concern and variants under investigation in England: technical briefing 7.
- 45.Public Health England. 2022. Variants: distribution of case data, 14 January 2022. https://www.gov.uk/government/publications/covid-19-variants-genomically-confirmed-case-numbers/variants-distribution-of-case-data-14-january-2022. Accessed 14 January, 2022.
- 46.World Health Organization. 2022. Tracking SARS-CoV-2 variants. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/. Accessed 14 January, 2022.
- 47.European Centre for Disease Prevention and Control (ECDC). 2022. SARS-CoV-2 variants of concern as of 13 January 2022.
- 48.Outbreak.info. 2021. https://outbreak.info/. Accessed 14 January, 2022.
- 49.Rambaut A, Loman N, Pybus OG, Barclay W, Barrett J, Carabelli A, Connor T, Peacock T, Robertson DL, Volz E, COVID-19 Genomics Consortium UK (CoG-UK). 2021. Preliminary genomic characterisation of an emergent SARS-CoV-2 lineage in the UK defined by a novel set of spike mutations. https://virological.org/t/preliminary-genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-the-uk-defined-by-a-novel-set-of-spike-mutations/563. Accessed 14 September, 2021.
- 50.Public Health England. 2020. Investigation of novel SARS-COV-2 variant: Variant of Concern 202012/01: technical briefing 1.
- 51.Khatamzas E, Rehn A, Muenchhoff M, Hellmuth J, Gaitzsch E, Weiglein T, Georgi E, Scherer C, Stecher S, Weigert O, Girl P, Zange S, Keppler OT, Stemmler J, von Bergwelt-Baildon M, Wölfel R, Antwerpen M. 2021. Emergence of multiple SARS-CoV-2 mutations in an immunocompromised host. medRxiv doi: 10.1101/2021.01.10.20248871. [DOI] [Google Scholar]
- 52.Starr TN, Greaney AJ, Hilton SK, Ellis D, Crawford KHD, Dingens AS, Navarro MJ, Bowen JE, Tortorici MA, Walls AC, King NP, Veesler D, Bloom JD. 2020. Deep mutational scanning of SARS-CoV-2 receptor binding domain reveals constraints on folding and ACE2 binding. Cell 182:1295–1310.e20. doi: 10.1016/j.cell.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meng B, Kemp SA, Papa G, Datir R, Ferreira I, Marelli S, Harvey WT, Lytras S, Mohamed A, Gallo G, Thakur N, Collier DA, Mlcochova P, Consortium C-GU, Duncan LM, Carabelli AM, Kenyon JC, Lever AM, De Marco A, Saliba C, Culap K, Cameroni E, Matheson NJ, Piccoli L, Corti D, James LC, Robertson DL, Bailey D, Gupta RK, COVID-19 Genomics UK (COG-UK) Consortium. 2021. Recurrent emergence of SARS-CoV-2 spike deletion H69/V70 and its role in the Alpha variant B.1.1.7. Cell Rep 35:109292. doi: 10.1016/j.celrep.2021.109292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saha P, Banerjee AK, Tripathi PP, Srivastava AK, Ray U. 2020. A virus that has gone viral: amino acid mutation in S protein of Indian isolate of Coronavirus COVID-19 might impact receptor binding, and thus, infectivity. Biosci Rep 40:BSR20201312. doi: 10.1042/BSR20201312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang P, Nair MS, Liu L, Iketani S, Luo Y, Guo Y, Wang M, Yu J, Zhang B, Kwong PD, Graham BS, Mascola JR, Chang JY, Yin MT, Sobieszczyk M, Kyratsous CA, Shapiro L, Sheng Z, Huang Y, Ho DD. 2021. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 593:130–135. doi: 10.1038/s41586-021-03398-2. [DOI] [PubMed] [Google Scholar]
- 56.Hoffmann M, Kleine-Weber H, Pohlmann S. 2020. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell 78:779–784.e5. doi: 10.1016/j.molcel.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peacock TP, Goldhill DH, Zhou J, Baillon L, Frise R, Swann OC, Kugathasan R, Penn R, Brown JC, Sanchez-David RY, Braga L, Williamson MK, Hassard JA, Staller E, Hanley B, Osborn M, Giacca M, Davidson AD, Matthews DA, Barclay WS. 2021. The furin cleavage site in the SARS-CoV-2 spike protein is required for transmission in ferrets. Nat Microbiol 6:899–909. doi: 10.1038/s41564-021-00908-w. [DOI] [PubMed] [Google Scholar]
- 58.Maison DP, Ching LL, Shikuma CM, Nerurkar VR. 2021. Genetic characteristics and phylogeny of 969-bp S gene sequence of SARS-CoV-2 from Hawai'i reveals the worldwide emerging P681H mutation. Hawaii J Health Soc Welf 80:52–61. [PMC free article] [PubMed] [Google Scholar]
- 59.Tegally H, Wilkinson E, Giovanetti M, Iranzadeh A, Fonseca V, Giandhari J, Doolabh D, Pillay S, San EJ, Msomi N, Mlisana K, von Gottberg A, Walaza S, Allam M, Ismail A, Mohale T, Glass AJ, Engelbrecht S, Van Zyl G, Preiser W, Petruccione F, Sigal A, Hardie D, Marais G, Hsiao NY, Korsman S, Davies MA, Tyers L, Mudau I, York D, Maslo C, Goedhals D, Abrahams S, Laguda-Akingba O, Alisoltani-Dehkordi A, Godzik A, Wibmer CK, Sewell BT, Lourenco J, Alcantara LCJ, Kosakovsky Pond SL, Weaver S, Martin D, Lessells RJ, Bhiman JN, Williamson C, de Oliveira T. 2021. Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 592:438–443. doi: 10.1038/s41586-021-03402-9. [DOI] [PubMed] [Google Scholar]
- 60.PANGO. 2022. PANGO lineages: global report investigating novel coronavirus haplotypes. https://cov-lineages.org/global_report.html. Accessed 14 January, 2022.
- 61.Faria NR, Claro IM, Candido D, Moyses Franco LA, Andrade PS, Coletti TM, Silva CAM, Sales FC, Manuli ER, Aguiar RS, Gaburo N, Camilo C, Fraiji NA, Esashika Crispim MA, Carvalho M, Rambaut A, Loman N, Pybus OG, Sabino EC, CADDE Genomic Network. 2021. Genomic characterisation of an emergent SARS-CoV-2 lineage in Manaus: preliminary findings. https://virological.org/t/genomic-characterisation-of-an-emergent-sars-cov-2-lineage-in-manaus-preliminary-findings/586. Accessed 18 February, 2021
- 62.Cherian S, Potdar V, Jadhav S, Yadav P, Gupta N, Das M, Rakshit P, Singh S, Abraham P, Panda S, NIC Team. 2021. SARS-CoV-2 spike mutations, L452R, T478K, E484Q and P681R, in the second wave of COVID-19 in Maharashtra, India. Microorganisms 9:1542. doi: 10.3390/microorganisms9071542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Q, Wu J, Nie J, Zhang L, Hao H, Liu S, Zhao C, Zhang Q, Liu H, Nie L, Qin H, Wang M, Lu Q, Li X, Sun Q, Liu J, Zhang L, Li X, Huang W, Wang Y. 2020. The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell 182:1284–1294.e9. doi: 10.1016/j.cell.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.World Health Organization. 2022. Enhancing response to Omicron SARS-CoV-2 variant. https://www.who.int/publications/m/item/enhancing-readiness-for-omicron-(b.1.1.529)-technical-brief-and-priority-actions-for-member-states. Accessed 25 January, 2022.
- 65.GISAID. 2022. Tracking of variants. https://www.gisaid.org/hcov19-variants/. Accessed 14 January, 2022.
- 66.Davies NG, Abbott S, Barnard RC, Jarvis CI, Kucharski AJ, Munday JD, Pearson CAB, Russell TW, Tully DC, Washburne AD, Wenseleers T, Gimma A, Waites W, Wong KLM, van Zandvoort K, Silverman JD, Diaz-Ordaz K, Keogh R, Eggo RM, Funk S, Jit M, Atkins KE, Edmunds WJ. 2021. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science 372:eabg3055. doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scientific Advisory Group for Emergencies (SAGE). 2021. SPI-M-O: consensus statement on COVID-19, 3 June 2021. https://www.gov.uk/government/publications/spi-m-o-consensus-statement-on-covid-19-3-june-2021. Accessed 16 September, 2021
- 68.Sofonea MT, Roquebert B, Foulongne V, Verdurme L, Trombert-Paolantoni S, Roussel M, Haim-Boukobza S, Alizon S. 2022. From Delta to Omicron: analysing the SARS-CoV-2 epidemic in France using variant-specific screening tests (September 1 to December 18, 2021). medRxiv doi: 10.1101/2021.12.31.21268583. [DOI] [Google Scholar]
- 69.Wolter N, Jassat W, Walaza S, Welch R, Moultrie H, Groome M, Amoako DG, Everatt J, Bhiman JN, Scheepers C, Tebeila N, Chiwandire N, Du Plessis M, Govender N, Ismail A, Glass A, Mlisana K, Stevens W, Treurnicht FK, Makatini Z, Hsiao N-y, Parboosing R, Wadula J, Hussey H, Davies M-A, Boulle A, von Gottberg A, Cohen C. 2021. Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa. medRxiv doi: 10.1101/2021.12.21.21268116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheikh A, Kerr S, Woolhouse M, McMenamin J, Robertson C. 2021. Severity of Omicron variant of concern and vaccine effectiveness against symptomatic disease: national cohort with nested test negative design study in Scotland. Epub ahead of print. [DOI] [PMC free article] [PubMed]
- 71.Laiton-Donato K, Franco-Munoz C, Alvarez-Diaz DA, Ruiz-Moreno HA, Usme-Ciro JA, Prada DA, Reales-Gonzalez J, Corchuelo S, Herrera-Sepulveda MT, Naizaque J, Santamaria G, Rivera J, Rojas P, Ortiz JH, Cardona A, Malo D, Prieto-Alvarado F, Gomez FR, Wiesner M, Martinez MLO, Mercado-Reyes M. 2021. Characterization of the emerging B.1.621 variant of interest of SARS-CoV-2. Infect Genet Evol 95:105038. doi: 10.1016/j.meegid.2021.105038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen RE, Zhang X, Case JB, Winkler ES, Liu Y, VanBlargan LA, Liu J, Errico JM, Xie X, Suryadevara N, Gilchuk P, Zost SJ, Tahan S, Droit L, Turner JS, Kim W, Schmitz AJ, Thapa M, Wang D, Boon ACM, Presti RM, O'Halloran JA, Kim AHJ, Deepak P, Pinto D, Fremont DH, Crowe JE, Jr, Corti D, Virgin HW, Ellebedy AH, Shi PY, Diamond MS. 2021. Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nat Med 27:717–726. doi: 10.1038/s41591-021-01294-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Madhi SA, Baillie V, Cutland CL, Voysey M, Koen AL, Fairlie L, Padayachee SD, Dheda K, Barnabas SL, Bhorat QE, Briner C, Kwatra G, Ahmed K, Aley P, Bhikha S, Bhiman JN, Bhorat AE, Du Plessis J, Esmail A, Groenewald M, Horne E, Hwa SH, Jose A, Lambe T, Laubscher M, Malahleha M, Masenya M, Masilela M, McKenzie S, Molapo K, Moultrie A, Oelofse S, Patel F, Pillay S, Rhead S, Rodel H, Rossouw L, Taoushanis C, Tegally H, Thombrayil A, van Eck S, Wibmer CK, Durham NM, Kelly EJ, Villafana TL, Gilbert S, Pollard AJ, de Oliveira T, Moore PL, Sigal A, et al. 2021. Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med 384:1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y, Liu J, Xia H, Zhang X, Fontes-Garfias CR, Swanson KA, Cai H, Sarkar R, Chen W, Cutler M, Cooper D, Weaver SC, Muik A, Sahin U, Jansen KU, Xie X, Dormitzer PR, Shi PY. 2021. Neutralizing activity of BNT162b2-elicited serum. N Engl J Med 384:1466–1468. doi: 10.1056/NEJMc2102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muik A, Wallisch A-K, Sänger B, Swanson KA, Mühl J, Chen W, Cai H, Maurus D, Sarkar R, Türeci Ö, Dormitzer PR, Şahin U. 2021. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science 371:1152–1153. doi: 10.1126/science.abg6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen X, Tang H, McDanal C, Wagh K, Fischer W, Theiler J, Yoon H, Li D, Haynes BF, Sanders KO, Gnanakaran S, Hengartner N, Pajon R, Smith G, Glenn GM, Korber B, Montefiori DC. 2021. SARS-CoV-2 variant B.1.1.7 is susceptible to neutralizing antibodies elicited by ancestral spike vaccines. Cell Host Microbe 29:529–539.e3. doi: 10.1016/j.chom.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Skelly DT, Harding AC, Gilbert-Jaramillo J, Knight ML, Longet S, Brown A, Adele S, Adland E, Brown H, Tipton T, Stafford L, Mentzer AJ, Johnson SA, Amini A, Tan TK, Schimanski L, Huang KA, Rijal P, Frater J, Goulder P, Conlon CP, Jeffery K, Dold C, Pollard AJ, Sigal A, de Oliveira T, Townsend AR, Klenerman P, Dunachie SJ, Barnes E, Carroll MW, James WS. 2021. Two doses of SARS-CoV-2 vaccination induce robust immune responses to emerging SARS-CoV-2 variants of concern. Nat Commun 12:5061. doi: 10.1038/s41467-021-25167-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu K, Werner AP, Moliva JI, Koch M, Choi A, Stewart-Jones GBE, Bennett H, Boyoglu-Barnum S, Shi W, Graham BS, Carfi A, Corbett KS, Seder RA, Edwards DK. 2021. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv doi: 10.1101/2021.01.25.427948. [DOI]
- 79.Wu K, Werner AP, Koch M, Choi A, Narayanan E, Stewart-Jones GBE, Colpitts T, Bennett H, Boyoglu-Barnum S, Shi W, Moliva JI, Sullivan NJ, Graham BS, Carfi A, Corbett KS, Seder RA, Edwards DK. 2021. Serum neutralizing activity elicited by mRNA-1273 vaccine. N Engl J Med 384:1468–1470. doi: 10.1056/NEJMc2102179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xie X, Zou J, Fontes-Garfias CR, Xia H, Swanson KA, Cutler M, Cooper D, Menachery VD, Weaver S, Dormitzer PR, Shi PY. 2021. Neutralization of N501Y mutant SARS-CoV-2 by BNT162b2 vaccine-elicited sera. bioRxiv doi: 10.1101/2021.01.07.425740. [DOI] [PubMed]
- 81.Xie X, Liu Y, Liu J, Zhang X, Zou J, Fontes-Garfias CR, Xia H, Swanson KA, Cutler M, Cooper D, Menachery VD, Weaver S, Dormitzer PR, Shi PY. 2021. Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K, and N501Y variants by BNT162b2 vaccine-elicited sera. bioRxiv doi: 10.1101/2021.01.27.427998. [DOI] [PubMed]
- 82.Collier DA, De Marco A, Ferreira I, Meng B, Datir RP, Walls AC, Kemp SA, Bassi J, Pinto D, Silacci-Fregni C, Bianchi S, Tortorici MA, Bowen J, Culap K, Jaconi S, Cameroni E, Snell G, Pizzuto MS, Pellanda AF, Garzoni C, Riva A, Collaboration C-N, Elmer A, Kingston N, Graves B, McCoy LE, Smith KGC, Bradley JR, Temperton N, Ceron-Gutierrez L, Barcenas-Morales G, Consortium C-GU, Harvey W, Virgin HW, Lanzavecchia A, Piccoli L, Doffinger R, Wills M, Veesler D, Corti D, Gupta RK, COVID-19 Genomics UK (COG-UK) Consortium. 2021. Sensitivity of SARS-CoV-2 B.1.1.7 to mRNA vaccine-elicited antibodies. Nature 593:136–141. doi: 10.1038/s41586-021-03412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Edara VV, Hudson WH, Xie X, Ahmed R, Suthar MS. 2021. Neutralizing antibodies against SARS-CoV-2 variants after infection and vaccination. JAMA 325:1896–1898. doi: 10.1001/jama.2021.4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Edara VV, Norwood C, Floyd K, Lai L, Davis-Gardner ME, Hudson WH, Mantus G, Nyhoff LE, Adelman MW, Fineman R, Patel S, Byram R, Gomes DN, Michael G, Abdullahi H, Beydoun N, Panganiban B, McNair N, Hellmeister K, Pitts J, Winters J, Kleinhenz J, Usher J, O'Keefe JB, Piantadosi A, Waggoner JJ, Babiker A, Stephens DS, Anderson EJ, Edupuganti S, Rouphael N, Ahmed R, Wrammert J, Suthar MS. 2021. Infection- and vaccine-induced antibody binding and neutralization of the B.1.351 SARS-CoV-2 variant. Cell Host Microbe 29:516–521.e3. doi: 10.1016/j.chom.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dejnirattisai W, Zhou D, Supasa P, Liu C, Mentzer AJ, Ginn HM, Zhao Y, Duyvesteyn HME, Tuekprakhon A, Nutalai R, Wang B, Lopez-Camacho C, Slon-Campos J, Walter TS, Skelly D, Costa Clemens SA, Naveca FG, Nascimento V, Nascimento F, Fernandes da Costa C, Resende PC, Pauvolid-Correa A, Siqueira MM, Dold C, Levin R, Dong T, Pollard AJ, Knight JC, Crook D, Lambe T, Clutterbuck E, Bibi S, Flaxman A, Bittaye M, Belij-Rammerstorfer S, Gilbert SC, Carroll MW, Klenerman P, Barnes E, Dunachie SJ, Paterson NG, Williams MA, Hall DR, Hulswit RJG, Bowden TA, Fry EE, Mongkolsapaya J, Ren J, Stuart DI, Screaton GR. 2021. Antibody evasion by the P.1 strain of SARS-CoV-2. Cell 184:2939–2954 e9. doi: 10.1016/j.cell.2021.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Supasa P, Zhou D, Dejnirattisai W, Liu C, Mentzer AJ, Ginn HM, Zhao Y, Duyvesteyn HME, Nutalai R, Tuekprakhon A, Wang B, Paesen GC, Slon-Campos J, Lopez-Camacho C, Hallis B, Coombes N, Bewley KR, Charlton S, Walter TS, Barnes E, Dunachie SJ, Skelly D, Lumley SF, Baker N, Shaik I, Humphries HE, Godwin K, Gent N, Sienkiewicz A, Dold C, Levin R, Dong T, Pollard AJ, Knight JC, Klenerman P, Crook D, Lambe T, Clutterbuck E, Bibi S, Flaxman A, Bittaye M, Belij-Rammerstorfer S, Gilbert S, Hall DR, Williams MA, Paterson NG, James W, Carroll MW, Fry EE, Mongkolsapaya J, et al. 2021. Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera. Cell 184:2201–2211.e7. doi: 10.1016/j.cell.2021.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Planas D, Bruel T, Grzelak L, Guivel-Benhassine F, Staropoli I, Porrot F, Planchais C, Buchrieser J, Rajah MM, Bishop E, Albert M, Donati F, Prot M, Behillil S, Enouf V, Maquart M, Smati-Lafarge M, Varon E, Schortgen F, Yahyaoui L, Gonzalez M, De Seze J, Pere H, Veyer D, Seve A, Simon-Loriere E, Fafi-Kremer S, Stefic K, Mouquet H, Hocqueloux L, van der Werf S, Prazuck T, Schwartz O. 2021. Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies. Nat Med 27:917–924. doi: 10.1038/s41591-021-01318-5. [DOI] [PubMed] [Google Scholar]
- 88.Bates TA, Leier HC, Lyski ZL, McBride SK, Coulter FJ, Weinstein JB, Goodman JR, Lu Z, Siegel SAR, Sullivan P, Strnad M, Brunton AE, Lee DX, Curlin ME, Messer WB, Tafesse FG. 2021. Neutralization of SARS-CoV-2 variants by convalescent and vaccinated serum. medRxiv doi: 10.1101/2021.04.04.21254881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang P, Casner RG, Nair MS, Wang M, Yu J, Cerutti G, Liu L, Kwong PD, Huang Y, Shapiro L, Ho DD. 2021. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. Cell Host Microbe 29:747–751.e4. doi: 10.1016/j.chom.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Parry HM, Tut G, Faustini S, Stephens C, Saunders P, Bentley C, Hilyard K, Brown K, Amirthalingam G, Charlton S, Leung S, Chiplin E, Coombes NS, Bewley KR, Penn EJ, Rowe C, Otter A, Watts R, D’Arcangelo S, Hallis B, Makin A, Richter AG, Zuo J. 2021. BNT162b2 vaccination in people over 80 years of age induces strong humoral immune responses with cross neutralisation of P.1 Brazilian variant. https://ssrn.com/abstract=3816840. Accessed 14 June, 2021 [DOI] [PMC free article] [PubMed]
- 91.Kim YJ, Jang US, Soh SM, Lee JY, Lee HR. 2021. The impact on infectivity and neutralization efficiency of SARS-CoV-2 lineage B.1.351 pseudovirus. Viruses 13:633. doi: 10.3390/v13040633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Betton M, Livrozet M, Planas D, Fayol A, Monel B, Vedie B, Bruel T, Tartour E, Robillard N, Manuguerra JC, Blanchard A, Ghosn J, Visseaux B, Pere H, Lebeaux D, Schwartz O, Veyer D, Hulot JS, French Covid Cohort Study Group. 2021. Sera neutralizing activities against severe acute respiratory syndrome coronavirus 2 and multiple variants 6 months after hospitalization for coronavirus disease 2019. Clin Infect Dis 73:e1337–e1344. doi: 10.1093/cid/ciab308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cheng L, Song S, Zhou B, Ge X, Yu J, Zhang M, Ju B, Zhang Z. 2021. Impact of the N501Y substitution of SARS-CoV-2 Spike on neutralizing monoclonal antibodies targeting diverse epitopes. Virol J 18:87. doi: 10.1186/s12985-021-01554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Graham C, Seow J, Huettner I, Khan H, Kouphou N, Acors S, Winstone H, Pickering S, Galao RP, Dupont L, Lista MJ, Jimenez-Guardeno JM, Laing AG, Wu Y, Joseph M, Muir L, van Gils MJ, Ng WM, Duyvesteyn HME, Zhao Y, Bowden TA, Shankar-Hari M, Rosa A, Cherepanov P, McCoy LE, Hayday AC, Neil SJD, Malim MH, Doores KJ. 2021. Neutralization potency of monoclonal antibodies recognizing dominant and subdominant epitopes on SARS-CoV-2 Spike is impacted by the B.1.1.7 variant. Immunity 54:1276–1289.e6. doi: 10.1016/j.immuni.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Q, Nie J, Wu J, Zhang L, Ding R, Wang H, Zhang Y, Li T, Liu S, Zhang M, Zhao C, Liu H, Nie L, Qin H, Wang M, Lu Q, Li X, Liu J, Liang H, Shi Y, Shen Y, Xie L, Zhang L, Qu X, Xu W, Huang W, Wang Y. 2021. SARS-CoV-2 501Y.V2 variants lack higher infectivity but do have immune escape. Cell 184:2362–2371.e9. doi: 10.1016/j.cell.2021.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wall EC, Wu M, Harvey R, Kelly G, Warchal S, Sawyer C, Daniels R, Hobson P, Hatipoglu E, Ngai Y, Hussain S, Nicod J, Goldstone R, Ambrose K, Hindmarsh S, Beale R, Riddell A, Gamblin S, Howell M, Kassiotis G, Libri V, Williams B, Swanton C, Gandhi S, Bauer DL. 2021. Neutralising antibody activity against SARS-CoV-2 VOCs B.1.617.2 and B.1.351 by BNT162b2 vaccination. Lancet 397:2331–2333. doi: 10.1016/S0140-6736(21)01290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Y, Liu J, Xia H, Zhang X, Zou J, Fontes-Garfias CR, Weaver SC, Swanson KA, Cai H, Sarkar R, Chen W, Cutler M, Cooper D, Muik A, Sahin U, Jansen KU, Xie X, Dormitzer PR, Shi PY. 2021. BNT162b2-elicited neutralization against new SARS-CoV-2 spike variants. N Engl J Med 385:472–474. doi: 10.1056/NEJMc2106083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Alter G, Yu J, Liu J, Chandrashekar A, Borducchi EN, Tostanoski LH, McMahan K, Jacob-Dolan C, Martinez DR, Chang A, Anioke T, Lifton M, Nkolola J, Stephenson KE, Atyeo C, Shin S, Fields P, Kaplan I, Robins H, Amanat F, Krammer F, Baric RS, Le Gars M, Sadoff J, de Groot AM, Heerwegh D, Struyf F, Douoguih M, van Hoof J, Schuitemaker H, Barouch DH. 2021. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature 596:268–272. doi: 10.1038/s41586-021-03681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wibmer CK, Ayres F, Hermanus T, Madzivhandila M, Kgagudi P, Oosthuysen B, Lambson BE, de Oliveira T, Vermeulen M, van der Berg K, Rossouw T, Boswell M, Ueckermann V, Meiring S, von Gottberg A, Cohen C, Morris L, Bhiman JN, Moore PL. 2021. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat Med 27:622–625. doi: 10.1038/s41591-021-01285-x. [DOI] [PubMed] [Google Scholar]
- 100.Tada T, Dcosta BM, Samanovic MI, Herati RS, Cornelius A, Zhou H, Vaill A, Kazmierski W, Mulligan MJ, Landau NR. 2021. Convalescent-phase sera and vaccine-elicited antibodies largely maintain neutralizing titer against global SARS-CoV-2 variant spikes. mBio 12:e0069621. doi: 10.1128/mBio.00696-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jangra S, Ye C, Rathnasinghe R, Stadlbauer D, Krammer F, Simon V, Martinez-Sobrido L, Garcia-Sastre A, Schotsaert M, Personalized Virology Initiative study group. 2021. SARS-CoV-2 spike E484K mutation reduces antibody neutralisation. Lancet Microbe 2:e283–e284. doi: 10.1016/S2666-5247(21)00068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoffmann M, Arora P, Groß R, Seidel A, Hörnich BF, Hahn AS, Krüger N, Graichen L, Hofmann-Winkler H, Kempf A, Winkler MS, Schulz S, Jäck H-M, Jahrsdörfer B, Schrezenmeier H, Müller M, Kleger A, Münch J, Pöhlmann S. 2021. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell 184:2384–2393.e12. doi: 10.1016/j.cell.2021.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cele S, Gazy I, Jackson L, Hwa SH, Tegally H, Lustig G, Giandhari J, Pillay S, Wilkinson E, Naidoo Y, Karim F, Ganga Y, Khan K, Bernstein M, Balazs AB, Gosnell BI, Hanekom W, Moosa MS, Team C-K, Lessells RJ, de Oliveira T, Sigal A, Network for Genomic Surveillance in South Africa. 2021. Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma. Nature 593:142–146. doi: 10.1038/s41586-021-03471-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Emary KRW, Golubchik T, Aley PK, Ariani CV, Angus B, Bibi S, Blane B, Bonsall D, Cicconi P, Charlton S, Clutterbuck EA, Collins AM, Cox T, Darton TC, Dold C, Douglas AD, Duncan CJA, Ewer KJ, Flaxman AL, Faust SN, Ferreira DM, Feng S, Finn A, Folegatti PM, Fuskova M, Galiza E, Goodman AL, Green CM, Green CA, Greenland M, Hallis B, Heath PT, Hay J, Hill HC, Jenkin D, Kerridge S, Lazarus R, Libri V, Lillie PJ, Ludden C, Marchevsky NG, Minassian AM, McGregor AC, Mujadidi YF, Phillips DJ, Plested E, Pollock KM, Robinson H, Smith A, Song R, Oxford COVID-19 Vaccine Trial Group, et al. 2021. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet 397:1351–1362. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang Z, Schmidt F, Weisblum Y, Muecksch F, Barnes CO, Finkin S, Schaefer-Babajew D, Cipolla M, Gaebler C, Lieberman JA, Oliveira TY, Yang Z, Abernathy ME, Huey-Tubman KE, Hurley A, Turroja M, West KA, Gordon K, Millard KG, Ramos V, Da Silva J, Xu J, Colbert RA, Patel R, Dizon J, Unson-O'Brien C, Shimeliovich I, Gazumyan A, Caskey M, Bjorkman PJ, Casellas R, Hatziioannou T, Bieniasz PD, Nussenzweig MC. 2021. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 592:616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ikegame S, Siddiquey MNA, Hung CT, Haas G, Brambilla L, Oguntuyo KY, Kowdle S, Chiu HP, Stevens CS, Vilardo AE, Edelstein A, Perandones C, Kamil JP, Lee B. 2021. Neutralizing activity of Sputnik V vaccine sera against SARS-CoV-2 variants. Nat Commun 12:4598. doi: 10.1038/s41467-021-24909-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhou H, Dcosta BM, Samanovic MI, Mulligan MJ, Landau NR, Tada T. 2021. B.1.526 SARS-CoV-2 variants identified in New York City are neutralized by vaccine-elicited and therapeutic monoclonal antibodies. mBio 12:e0138621. doi: 10.1128/mBio.01386-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, Planchais C, Porrot F, Robillard N, Puech J, Prot M, Gallais F, Gantner P, Velay A, Le Guen J, Kassis-Chikhani N, Edriss D, Belec L, Seve A, Courtellemont L, Pere H, Hocqueloux L, Fafi-Kremer S, Prazuck T, Mouquet H, Bruel T, Simon-Loriere E, Rey FA, Schwartz O. 2021. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 109.Cameroni E, Saliba C, Bowen JE, Rosen LE, Culap K, Pinto D, VanBlargan LA, De Marco A, Zepeda SK, Iulio J, Zatta F, Kaiser H, Noack J, Farhat N, Czudnochowski N, Havenar-Daughton C, Sprouse KR, Dillen JR, Powell AE, Chen A, Maher C, Yin L, Sun D, Soriaga L, Bassi J, Silacci-Fregni C, Gustafsson C, Franko NM, Logue J, Iqbal NT, Mazzitelli I, Geffner J, Grifantini R, Chu H, Gori A, Riva A, Giannini O, Ceschi A, Ferrari P, Cippà P, Franzetti-Pellanda A, Garzoni C, Halfmann PJ, Kawaoka Y, Hebner C, Purcell LA, Piccoli L, Pizzuto MS, Walls AC, Diamond MS, et al. 2021. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. bioRxiv doi: 10.1101/2021.12.12.472269. [DOI] [PMC free article] [PubMed]
- 110.Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H, San JE, Cromer D, Scheepers C, Amoako D, Karim F, Bernstein M, Lustig G, Archary D, Smith M, Ganga Y, Jule Z, Reedoy K, Hwa S-H, Giandhari J, Blackburn JM, Gosnell BI, Karim SSA, Hanekom W, von Gottberg A, Bhiman J, Lessells RJ, Moosa M-YS, Davenport MP, de Oliveira T, Moore PL, Sigal A, NGS-SA, Team C-K. 2021. SARS-CoV-2 Omicron has extensive but incomplete escape of Pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv doi: 10.1101/2021.12.08.21267417. [DOI] [Google Scholar]
- 111.Doria-Rose NA, Shen X, Schmidt SD, O’Dell S, McDanal C, Feng W, Tong J, Eaton A, Maglinao M, Tang H, Manning KE, Edara V-V, Lai L, Ellis M, Moore K, Floyd K, Foster SL, Atmar RL, Lyke KE, Zhou T, Wang L, Zhang Y, Gaudinski MR, Black WP, Gordon I, Guech M, Ledgerwood JE, Misasi JN, Widge A, Roberts PC, Beigel J, Korber B, Pajon R, Mascola JR, Suthar MS, Montefiori DC. 2021. Booster of mRNA-1273 Strengthens SARS-CoV-2 Omicron neutralization. medRxiv doi: 10.1101/2021.12.15.21267805. [DOI] [Google Scholar]
- 112.Peiris M, Cheng S, Ka Pun Mok C, Leung Y, Ng S, Chan K, Ko F, Yiu K, Lam B, Lau E, Chan K, Luk L, Li J, Tsang L, Poon L, Chen C, Hui D. 2022. Neutralizing antibody titres to SARS-CoV-2 Omicron variant and wild-type virus in those with past infection or vaccinated or boosted with mRNA BNT162b2 or inactivated CoronaVac vaccines. Nature Portfolio doi: 10.21203/rs.3.rs-1207071/v1. [DOI] [Google Scholar]
- 113.Rössler A, Riepler L, Bante D, Laer D, Kimpel J. 2021. SARS-CoV-2 B.1.1.529 variant (Omicron) evades neutralization by sera from vaccinated and convalescent individuals. medRxiv doi: 10.1101/2021.12.08.21267491. [DOI] [Google Scholar]
- 114.Schmidt F, Muecksch F, Weisblum Y, Silva JD, Bednarski E, Cho A, Wang Z, Gaebler C, Caskey M, Nussenzweig MC, Hatziioannou T, Bieniasz PD. 2021. Plasma neutralization properties of the SARS-CoV-2 Omicron variant. medRxiv doi: 10.1101/2021.12.12.21267646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wilhelm A, Widera M, Grikscheit K, Toptan T, Schenk B, Pallas C, Metzler M, Kohmer N, Hoehl S, Helfritz FA, Wolf T, Goetsch U, Ciesek S. 2021. Reduced neutralization of SARS-CoV-2 Omicron variant by vaccine sera and monoclonal antibodies. medRxiv doi: 10.1101/2021.12.07.21267432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Garcia-Beltran WF, St Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML, Berrios C, Ofoman O, Chang CC, Hauser BM, Feldman J, Roederer AL, Gregory DJ, Poznansky MC, Schmidt AG, Iafrate AJ, Naranbhai V, Balazs AB. 2022. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 185:457–466.e4. doi: 10.1016/j.cell.2021.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Muik A, Lui BG, Wallisch A-K, Bacher M, Mühl J, Reinholz J, Ozhelvaci O, Beckmann N, Caridad Güimil Garcia R, Poran A, Shpyro S, Cai H, Yang Q, Swanson KA, Türeci Ö, Sahin U. 2021. Neutralization of SARS-CoV-2 Omicron pseudovirus by BNT162b2 vaccine-elicited human sera. medRxiv doi: 10.1101/2021.12.22.21268103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Khoury DS, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, Subbarao K, Kent SJ, Triccas JA, Davenport MP. 2021. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 119.Feng S, Phillips DJ, White T, Sayal H, Aley PK, Bibi S, Dold C, Fuskova M, Gilbert SC, Hirsch I, Humphries HE, Jepson B, Kelly EJ, Plested E, Shoemaker K, Thomas KM, Vekemans J, Villafana TL, Lambe T, Pollard AJ, Voysey M. 2021. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. medRxiv doi: 10.1101/2021.06.21.21258528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, Cho A, Jankovic M, Schaefer-Babajew D, Oliveira TY, Cipolla M, Viant C, Barnes CO, Bram Y, Breton G, Hagglof T, Mendoza P, Hurley A, Turroja M, Gordon K, Millard KG, Ramos V, Schmidt F, Weisblum Y, Jha D, Tankelevich M, Martinez-Delgado G, Yee J, Patel R, Dizon J, Unson-O'Brien C, Shimeliovich I, Robbiani DF, Zhao Z, Gazumyan A, Schwartz RE, Hatziioannou T, Bjorkman PJ, Mehandru S, Bieniasz PD, Caskey M, Nussenzweig MC. 2021. Evolution of antibody immunity to SARS-CoV-2. Nature 591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tang T, Bidon M, Jaimes JA, Whittaker GR, Daniel S. 2020. Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antiviral Res 178:104792. doi: 10.1016/j.antiviral.2020.104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Uriu K, Kimura I, Shirakawa K, Takaori-Kondo A, Nakada T-a, Kaneda A, Nakagawa S, Sato K. 2021. Ineffective neutralization of the SARS-CoV-2 Mu variant by convalescent and vaccine sera. bioRxiv doi: 10.1101/2021.09.06.459005. [DOI] [PMC free article] [PubMed]
- 123.Messali S, Bertelli A, Campisi G, Zani A, Ciccozzi M, Caruso A, Caccuri F. 2021. A cluster of the new SARS-CoV-2 B.1.621 lineage in Italy and sensitivity of the viral isolate to the BNT162b2 vaccine. J Med Virol 93:6468–6470. doi: 10.1002/jmv.27247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Laurie MT, Liu J, Sunshine S, Peng J, Black D, Mitchell AM, Mann SA, Pilarowski G, Zorn KC, Rubio L, Bravo S, Marquez C, Petersen M, Havlir D, DeRisi J. 2021. Exposures to different SARS-CoV-2 spike variants elicit neutralizing antibody responses with differential specificity towards established and emerging strains. medRxiv doi: 10.1101/2021.09.08.21263095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Falsey AR, Frenck RW, Walsh EE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Bailey R, Swanson KA, Xu X, Koury K, Kalina W, Cooper D, Zou J, Xie X, Xia H, Türeci Ö, Lagkadinou E, Tompkins KR, Shi P-Y, Jansen KU, Şahin U, Dormitzer PR, Gruber WC. 2021. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N Engl J Med 385:1627–1629. doi: 10.1056/NEJMc2113468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moore PL, Moyo-Gwete T, Hermanus T, Kgagudi P, Ayres F, Makhado Z, Sadoff J, Le Gars M, van Roey G, Crowther C, Garrett N, Bekker L-G, Morris L, Schuitemaker H, Gray G. 2021. Neutralizing antibodies elicited by the Ad26.COV2.S COVID-19 vaccine show reduced activity against 501Y.V2 (B.1.351), despite protection against severe disease by this variant. bioRxiv doi: 10.1101/2021.06.09.447722. [DOI]
- 127.Gallais F, Velay A, Nazon C, Wendling MJ, Partisani M, Sibilia J, Candon S, Fafi-Kremer S. 2021. Intrafamilial exposure to SARS-CoV-2 associated with cellular immune response without seroconversion, France. Emerg Infect Dis 27:113–121. doi: 10.3201/eid2701.203611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Meckiff BJ, Ramirez-Suastegui C, Fajardo V, Chee SJ, Kusnadi A, Simon H, Eschweiler S, Grifoni A, Pelosi E, Weiskopf D, Sette A, Ay F, Seumois G, Ottensmeier CH, Vijayanand P. 2020. Imbalance of regulatory and cytotoxic SARS-CoV-2-reactive CD4(+) T cells in COVID-19. Cell 183:1340–1353.e16. doi: 10.1016/j.cell.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Peng Y, Mentzer AJ, Liu G, Yao X, Yin Z, Dong D, Dejnirattisai W, Rostron T, Supasa P, Liu C, Lopez-Camacho C, Slon-Campos J, Zhao Y, Stuart DI, Paesen GC, Grimes JM, Antson AA, Bayfield OW, Hawkins D, Ker DS, Wang B, Turtle L, Subramaniam K, Thomson P, Zhang P, Dold C, Ratcliff J, Simmonds P, de Silva T, Sopp P, Wellington D, Rajapaksa U, Chen YL, Salio M, Napolitani G, Paes W, Borrow P, Kessler BM, Fry JW, Schwabe NF, Semple MG, Baillie JK, Moore SC, Openshaw PJM, Ansari MA, Dunachie S, Barnes E, Frater J, Kerr G, Goulder P, et al. 2020. Broad and strong memory CD4(+) and CD8(+) T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol 21:1336–1345. doi: 10.1038/s41590-020-0782-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, Belanger S, Abbott RK, Kim C, Choi J, Kato Y, Crotty EG, Kim C, Rawlings SA, Mateus J, Tse LPV, Frazier A, Baric R, Peters B, Greenbaum J, Ollmann Saphire E, Smith DM, Sette A, Crotty S. 2020. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tarke A, Sidney J, Kidd CK, Dan JM, Ramirez SI, Yu ED, Mateus J, da Silva Antunes R, Moore E, Rubiro P, Methot N, Phillips E, Mallal S, Frazier A, Rawlings SA, Greenbaum JA, Peters B, Smith DM, Crotty S, Weiskopf D, Grifoni A, Sette A. 2021. Comprehensive analysis of T cell immunodominance and immunoprevalence of SARS-CoV-2 epitopes in COVID-19 cases. Cell Rep Med 2:100204. doi: 10.1016/j.xcrm.2021.100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Agerer B, Koblischke M, Gudipati V, Montano-Gutierrez LF, Smyth M, Popa A, Genger JW, Endler L, Florian DM, Muhlgrabner V, Graninger M, Aberle SW, Husa AM, Shaw LE, Lercher A, Gattinger P, Torralba-Gombau R, Trapin D, Penz T, Barreca D, Fae I, Wenda S, Traugott M, Walder G, Pickl WF, Thiel V, Allerberger F, Stockinger H, Puchhammer-Stockl E, Weninger W, Fischer G, Hoepler W, Pawelka E, Zoufaly A, Valenta R, Bock C, Paster W, Geyeregger R, Farlik M, Halbritter F, Huppa JB, Aberle JH, Bergthaler A. 2021. SARS-CoV-2 mutations in MHC-I-restricted epitopes evade CD8(+) T cell responses. Sci Immunol 6:eabg6461. doi: 10.1126/sciimmunol.abg6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Geers D, Shamier MC, Bogers S, den Hartog G, Gommers L, Nieuwkoop NN, Schmitz KS, Rijsbergen LC, van Osch JAT, Dijkhuizen E, Smits G, Comvalius A, van Mourik D, Caniels TG, van Gils MJ, Sanders RW, Oude Munnink BB, Molenkamp R, de Jager HJ, Haagmans BL, de Swart RL, Koopmans MPG, van Binnendijk RS, de Vries RD, GeurtsvanKessel CH. 2021. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol 6:eabj1750. doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Riou C, Keeton R, Moyo-Gwete T, Hermanus T, Kgagudi P, Baguma R, Tegally H, Doolabh D, Iranzadeh A, Tyers L, Mutavhatsindi H, Tincho MB, Benede N, Marais G, Chinhoyi LR, Mennen M, Skelem S, Du Bruyn E, Stek C, de Oliveira T, Williamson C, Moore PL, Wilkinson RJ, Ntusi NAB, Burgers WA. 2021. Loss of recognition of SARS-CoV-2 B.1.351 variant spike epitopes but overall preservation of T cell immunity. medRxiv doi: 10.1101/2021.06.03.21258307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Francis JM, Leistritz-Edwards D, Dunn A, Tarr C, Lehman J, Dempsey C, Hamel A, Rayon V, Liu G, Wang Y, Wille M, Durkin M, Hadley K, Sheena A, Roscoe B, Ng M, Rockwell G, Manto M, Gienger E, Nickerson J, Moarefi A, Noble M, Malia T, Bardwell PD, Gordon W, Swain J, Skoberne M, Sauer K, Harris T, Goldrath AW, Shalek AK, Coyle AJ, Benoist C, Pregibon DC. 2021. Allelic variation in Class I HLA determines pre-existing memory responses to SARS-CoV-2 that shape the CD8+ T cell repertoire upon viral exposure. bioRxiv doi: 10.1101/2021.04.29.441258. [DOI] [PMC free article] [PubMed]
- 136.Quadeer AA, Ahmed SF, McKay MR. 2020. Epitopes targeted by T cells in convalescent COVID-19 patients. bioRxiv doi: 10.1101/2020.08.26.267724. [DOI]
- 137.Sahin U, Muik A, Vogler I, Derhovanessian E, Kranz LM, Vormehr M, Quandt J, Bidmon N, Ulges A, Baum A, Pascal KE, Maurus D, Brachtendorf S, Lorks V, Sikorski J, Koch P, Hilker R, Becker D, Eller AK, Grutzner J, Tonigold M, Boesler C, Rosenbaum C, Heesen L, Kuhnle MC, Poran A, Dong JZ, Luxemburger U, Kemmer-Bruck A, Langer D, Bexon M, Bolte S, Palanche T, Schultz A, Baumann S, Mahiny AJ, Boros G, Reinholz J, Szabo GT, Kariko K, Shi PY, Fontes-Garfias C, Perez JL, Cutler M, Cooper D, Kyratsous CA, Dormitzer PR, Jansen KU, Tureci O. 2021. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 595:572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 138.Tarke A, Coelho CH, Zhang Z, Dan JM, Yu ED, Methot N, Bloom NI, Goodwin B, Phillips E, Mallal S, Sidney J, Filaci G, Weiskopf D, da Silva Antunes R, Crotty S, Grifoni A, Sette A. 2021. SARS-CoV-2 vaccination induces immunological memory able to cross-recognize variants from Alpha to Omicron. bioRxiv doi: 10.1101/2021.12.28.474333. [DOI] [PMC free article] [PubMed]
- 139.Keeton R, Tincho MB, Ngomti A, Baguma R, Benede N, Suzuki A, Khan K, Cele S, Bernstein M, Karim F, Madzorera SV, Moyo-Gwete T, Mennen M, Skelem S, Adriaanse M, Mutithu D, Aremu O, Stek C, Bruyn E, Van Der Mescht MA, de Beer Z, de Villiers TR, Bodenstein A, van den Berg G, Mendes A, Strydom A, Venter M, Grifoni A, Weiskopf D, Sette A, Wilkinson RJ, Bekker L-G, Gray G, Ueckermann V, Rossouw T, Boswell MT, Bihman J, Moore PL, Sigal A, Ntusi NAB, Burgers WA, Riou C. 2021. SARS-CoV-2 spike T cell responses induced upon vaccination or infection remain robust against Omicron. medRxiv doi: 10.1101/2021.12.26.21268380. [DOI] [Google Scholar]
- 140.Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, Chadwick DR, Clark R, Cosgrove C, Galloway J, Goodman AL, Heer A, Higham A, Iyengar S, Jamal A, Jeanes C, Kalra PA, Kyriakidou C, McAuley DF, Meyrick A, Minassian AM, Minton J, Moore P, Munsoor I, Nicholls H, Osanlou O, Packham J, Pretswell CH, San Francisco Ramos A, Saralaya D, Sheridan RP, Smith R, Soiza RL, Swift PA, Thomson EC, Turner J, Viljoen ME, Albert G, Cho I, Dubovsky F, Glenn G, Rivers J, Robertson A, Smith K, Toback S. 2021. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med 385:1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shinde V, Bhikha S, Hoosain Z, Archary M, Bhorat Q, Fairlie L, Lalloo U, Masilela MSL, Moodley D, Hanley S, Fouche L, Louw C, Tameris M, Singh N, Goga A, Dheda K, Grobbelaar C, Kruger G, Carrim-Ganey N, Baillie V, de Oliveira T, Lombard Koen A, Lombaard JJ, Mngqibisa R, Bhorat AE, Benade G, Lalloo N, Pitsi A, Vollgraaff PL, Luabeya A, Esmail A, Petrick FG, Oommen-Jose A, Foulkes S, Ahmed K, Thombrayil A, Fries L, Cloney-Clark S, Zhu M, Bennett C, Albert G, Faust E, Plested JS, Robertson A, Neal S, Cho I, Glenn GM, Dubovsky F, Madhi SA. 2021. Efficacy of NVX-CoV2373 Covid-19 vaccine against the B.1.351 variant. N Engl J Med 384:1899–1909. doi: 10.1056/NEJMoa2103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Johnson & Johnson. 2021. Johnson & Johnson announces single-shot Janssen COVID-19 vaccine candidate met primary endpoints in interim analysis of its phase 3 ENSEMBLE trial. https://www.jnj.com/johnson-and-johnson-announces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints-in-interim-analysis-of-its-phase-3-ensemble-trial. Accessed 3 March, 2021.
- 143.U.S. Department of Health and Human Services, U.S. Food and Drug Administration, Center for Biologics Evaluation and Research. 2020. Development and licensure of vaccines to prevent COVID-19: guidance for industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/development-and-licensure-vaccines-prevent-covid-19. Accessed 3 March, 2021
- 144.Thomas SJ, Moreira ED, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, Bailey R, Swanson KA, Xu X, Roychoudhury S, Koury K, Bouguermouh S, Kalina WV, Cooper D, Frenck RW, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Yang Q, Liberator P, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Gruber WC, Jansen KU. 2021. Six month safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. medRxiv doi: 10.1101/2021.07.28.21261159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pfizer, BioNTech. 2021. Pfizer and BioNTech announce phase 3 trial data showing high efficacy of a booster dose of their COVID-19 vaccine. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-phase-3-trial-data-showing. Accessed 14 January, 2022.
- 146.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernan MA, Lipsitch M, Reis B, Balicer RD. 2021. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Katz MA, Harlev EB, Chazan B, Chowers M, Greenberg D, Peretz A, Tshori S, Levy J, Yacobi M, Hirsch A, Amichay D, Weinberger R, Dor AB, Taraday EK, Reznik D, Chayat CB, Sagas D, Zvi HB, Berdinstein R, Rashid G, Avni YS, Mandelboim M, Zuckerman N, Rainy N, Akriv A, Dagan N, Kepten E, Barda N, Balicer RD. 2021. Covid-19 vaccine effectiveness in healthcare personnel in six Israeli hospitals (CoVEHPI). medRxiv doi: 10.1101/2021.08.30.21262465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sheikh A, McMenamin J, Taylor B, Robertson C, Public Health Scotland and the EAVE II Collaborators. 2021. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet 397:2461–2462. doi: 10.1016/S0140-6736(21)01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, Myers R, Campbell CNJ, Amirthalingam G, Edmunds M, Zambon M, Brown KE, Hopkins S, Chand M, Ramsay M. 2021. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med 385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Abu-Raddad LJ, Chemaitelly H, Butt AA, National Study Group for Covid-Vaccination. 2021. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med 385:187–189. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nasreen S, Chung H, He S, Brown KA, Gubbay JB, Buchan SA, Fell DB, Austin PC, Schwartz KL, Sundaram ME, Calzavara A, Chen B, Tadrous M, Wilson K, Wilson SE, Kwong JC. 2021. Effectiveness of COVID-19 vaccines against variants of concern in Ontario. medRxiv doi: 10.1101/2021.06.28.21259420. [DOI] [PubMed] [Google Scholar]
- 152.Pouwels KB, Pritchard E, Matthews PC, Stoesser N, Eyre DW, Vihta K-D, House T, Hay J, Bell JI, Newton JN, Farrar J, Crook D, Cook D, Rourke E, Studley R, Peto T, Diamond I, Walker AS, COVID-19 Infection Survey Team. 2021. Impact of Delta on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. medRxiv doi: 10.1101/2021.08.18.21262237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Barlow RS, Jian K, Larson L, Katz MA, Harlev EB, Chazan B, Chowers M, Greenberg D, Peretz A, Tshori S, Levy J, Yacobi M, Hirsch A, Amichay D, Weinberger R, Dor AB, Taraday EK, Reznik D, Chayat CB, Sagas D, Zvi HB, Berdinstein R, Rashid G, Avni YS, Mandelboim M, Zuckerman N, Rainy N, Akriv A, Dagan N, Kepten E, Barda N, Balicer RD, Levine-Tiefenbrun M, Yelin I, Alapi H, Katz R, Herzel E, Kuint J, Chodick G, Gazit S, Patalon T, Kishony R. 2021. Effectiveness of COVID-19 vaccines against SARS-CoV-2 infection during a delta variant epidemic surge in Multnomah County, Oregon, July 2021. medRxiv doi: 10.1101/2021.08.30.21262446. [DOI] [Google Scholar]
- 154.Grannis SJ, Rowley EA, Ong TC, Stenehjem E, Klein NP, DeSilva MB, Naleway AL, Natarajan K, Thompson MG, VISION Network. 2021. Interim estimates of COVID-19 vaccine effectiveness against COVID-19–associated emergency department or urgent care clinic encounters and hospitalizations among adults during SARS-CoV-2 B.1.617.2 (Delta) variant predominance—nine states, June–August 2021. MMWR Morb Mortal Wkly Rep 70:1291–1293. doi: 10.15585/mmwr.mm7037e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Israel Ministry of Health. 2021. Decline in vaccine effectiveness against infection and symptomatic illness. https://www.gov.il/en/departments/news/05072021-03. Accessed 14 July, 2021
- 156.Tartof SY, Slezak JM, Heidi F, Hong V, Ackerson BK, Ranasinghe ON, Frankland TB, Ogun OA, Zamparo JM, Gray S, Valluri SR, Pan K, Angulo FJ, Jodar L, McLaughlin JM. 2021. Six-month effectiveness of BNT162b2 mRNA COVID-19 vaccine in a large US integrated health system: a retrospective cohort study. SSRN doi: 10.2139/ssrn.3909743. [DOI] [PMC free article] [PubMed]
- 157.Bernal JL, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, Myers R, Campbell C, Amirthalingam G, Edmunds M, Zambon M, Brown K, Hopkins S, Chand M, Ramsay M. 2021. Effectiveness of COVID-19 vaccines against the B.1.617.2 variant. medRxiv doi: 10.1101/2021.05.22.21257658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Payne RP, Longet S, Austin JA, Skelly DT, Dejnirattisai W, Adele S, Meardon N. 2021. Sustained T cell immunity, protection and boosting using extended dosing intervals of BNT162b2 mRNA vaccine. SSRN doi: 10.2139/ssrn.3891065. [DOI] [PMC free article] [PubMed]
- 159.Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O’Connell A-M, Simons D, Blomquist PB, Zaidi A, Nash S, Aziz NIBA, Thelwall S, Dabrera G, Myers R, Amirthalingam G, Gharbia S, Barrett JC, Elson R, Ladhani SN, Ferguson N, Zambon M, Campbell CN, Brown K, Hopkins S, Chand M, Ramsay M, Bernal JL. 2021. Effectiveness of COVID-19 vaccines against the Omicron (B.1.1.529) variant of concern. medRxiv doi: 10.1101/2021.12.14.21267615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tseng HF, Ackerson BK, Luo Y, Sy LS, Talarico CA, Tian Y, Bruxvoort KJ, Tubert JE, Florea A, Ku JH, Lee GS, Choi SK, Takhar HS, Aragones M, Qian L. 2022. Effectiveness of mRNA-1273 against SARS-CoV-2 omicron and delta variants. medRxiv doi: 10.1101/2022.01.07.22268919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hansen CH, Schelde AB, Moustsen-Helm IR, Emborg H-D, Krause TG, Mølbak K, Valentiner-Branth P. 2021. Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: a Danish cohort study. medRxiv doi: 10.1101/2021.12.20.21267966. [DOI] [Google Scholar]
- 162.Ferguson N, Ghani A, Cori A, Hogan A, Hinsley W, Volz E, Imperial College COVID-19 response team. 2021. Report 49—Growth, population distribution and immune escape of the Omicron in England. MRC Centre for Global Infectious Disease Analysis, London, United Kingdom. [Google Scholar]
- 163.UK Health Security Agency. 2021. Effectiveness of 3 doses of COVID-19 vaccines against symptomatic COVID-19 and hospitalisation in adults aged 65 years and older.
- 164.Public Health England. 2021. SARS-CoV-2 variants of concern and variants under investigation in England: technical briefing: update on hospitalisation and vaccine effectiveness for Omicron VOC-21NOV-01 (B.1.1.529).
- 165.Wu K, Choi A, Koch M, Ma L, Hill A, Nunna N, Huang W, Oestreicher J, Colpitts T, Bennett H, Legault H, Paila Y, Nestorova B, Ding B, Pajon R, Miller JM, Leav B, Carfi A, McPhee R, Edwards DK. 2021. Preliminary analysis of safety and immunogenicity of a SARS-CoV-2 variant vaccine booster. medRxiv doi: 10.1101/2021.05.05.21256716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Choi A, Koch M, Wu K, Chu L, Ma L, Hill A, Nunna N, Huang W, Oestreicher J, Colpitts T, Bennett H, Legault H, Paila Y, Nestorova B, Ding B, Montefiori D, Pajon R, Miller JM, Leav B, Carfi A, McPhee R, Edwards DK. 2021. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med 27:2025–2031. doi: 10.1038/s41591-021-01527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Flaxman A, Marchevsky N, Jenkin D, Aboagye J, Aley PK, Angus BJ, Belij-Rammerstorfer S, Bibi S, Bittaye M, Cappuccini F, Cicconi P, Clutterbuck E, Davies S, Dejnirattisai W, Dold C, Ewer K, Folegatti PM, Fowler J, Hill AVS, Kerridge S, Minassian AM, Mongkolspaya J, Farooq Mujadidi Y, Plested E, Ramasamy MN, Robinson H, Sanders H, Sheehan E, Smith H, Snape MD, Song R, Woods D, Screaton GR, Gilbert SC, Voysey M, Pollard A, Lambe T, The Oxford Covid Vaccine Group. 2021. Tolerability and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 (AZD1222). SSRN. doi: 10.2139/ssrn.3873839. [DOI]
- 168.Pan H, Wu Q, Zeng G, Yang J, Jiang D, Deng X, Chu K, Zheng W, Zhu F, Yu H, Yin W. 2021. Immunogenicity and safety of a third dose, and immune persistence of CoronaVac vaccine in healthy adults aged 18–59 years: interim results from a double-blind, randomized, placebo-controlled phase 2 clinical trial. medRxiv doi: 10.1101/2021.07.23.21261026. [DOI] [Google Scholar]
- 169.Tan CS, Collier A-r, Liu J, Yu J, Wan H, McMahan K, He X, Jacob-Dolan C, Chandrashekar A, Sellers D, Stephenson KE, Vidal SJ, Jaegle K, Curran JL, Rowe M, Hemond R, Rivera LB, Anioke T, Barrett J, Chung B, Gardner S, Gebre MS, Lifton M, Powers O, VanWyk H, Wu C, Barouch DH. 2021. Ad26.COV2.S or BNT162b2 boosting of BNT162b2 vaccinated individuals. medRxiv doi: 10.1101/2021.12.02.21267198. [DOI] [Google Scholar]
- 170.Atmar RL, Lyke KE, Deming ME, Jackson LA, Branche AR, El Sahly HM, Rostad CA, Martin JM, Johnston C, Rupp RE, Mulligan MJ, Brady RC, Frenck RW, Bäcker M, Kottkamp AC, Babu TM, Rajakumar K, Edupuganti S, Dobryzynski D, Posavad CM, Archer JI, Crandon S, Nayak SU, Szydlo D, Zemanek J, Islas CPD, Brown ER, Suthar MS, McElrath MJ, McDermott AB, O’Connell SE, Montefiori DC, Eaton A, Neuzil KM, Stephens DS, Roberts PC, Beigel JH, the DMID 21-0012 Study Group. 2021. Heterologous SARS-CoV-2 booster vaccinations—preliminary report. medRxiv doi: 10.1101/2021.10.10.21264827. [DOI] [Google Scholar]
- 171.BioNTech. 2021. BioNTech announces second quarter 2021 financial results and corporate update. https://investors.biontech.de/node/10446/pdf. Accessed 14 September, 2021
- 172.Pfizer, BioNTech. 2021. Pfizer and BioNTech provide update on omicron variant. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-provide-update-omicron-variant. Accessed 14 January, 2022.
- 173.BioNTech. 2021. Update: Omicron variant (B.1.1.529). https://investors.biontech.de/static-files/47b4131a-0545-4a0b-a353-49b3a1d01789. Accessed 20 January, 2022.
- 174.Moderna. 2021. MODERNA announces preliminary booster data and updates strategy to address omicron variant. https://investors.modernatx.com/news/news-details/2021/Moderna-Announces-Preliminary-Booster-Data-and-Updates-Strategy-to-Address-Omicron-Variant/default.aspx. Accessed 14 January, 2022.
- 175.University of Oxford. 2021. ChAdOx1 nCov-19 provides minimal protection against mild-moderate COVID-19 infection from B.1.351 coronavirus variant in young South African adults. https://www.ox.ac.uk/news/2021-02-07-chadox1-ncov-19-provides-minimal-protection-against-mild-moderate-covid-19-infection. Accessed 4 March, 2021.
- 176.Spencer AJ, Morris S, Ulaszewska M, Powers C, Kailath R, Bissett C, Truby A, Thakur N, Newman J, Allen ER, Rudiansyah I, Liu C, Dejnirattisai W, Mongkolsapaya J, Davies H, Donnellan FR, Pulido D, Peacock TP, Barclay WS, Bright H, Ren K, Screaton G, McTamney P, Bailey D, Gilbert SC, Lambe T. 2021. The ChAdOx1 vectored vaccine, AZD2816, induces strong immunogenicity against SARS-CoV-2 Beta (B.1.351) and other variants of concern in preclinical studies. bioRxiv doi: 10.1101/2021.06.08.447308. [DOI] [PMC free article] [PubMed]
- 177.Russian Direct Investment Fund, Gamaleya Institute. 2021. Statement of RDIF and the Gamaleya Institute on omicron variant of COVID. https://sputnikvaccine.com/newsroom/pressreleases/statement-of-rdif-and-the-gamaleya-institute-on-omicron-variant-of-covid/. Accessed 14 January, 2022.
- 178.Novavax. 2021. Novavax announces initial Omicron cross-reactivity data from COVID-19 vaccine booster and adolescent studies. https://ir.novavax.com/2021-12-22-Novavax-Announces-Initial-Omicron-Cross-Reactivity-Data-from-COVID-19-Vaccine-Booster-and-Adolescent-Studies. Accessed 14 January, 2022.
- 179.European Medicines Agency. 2021. Regulatory requirements for vaccines intended to provide protection against variant strain(s) of SARS-CoV-2. https://www.ema.europa.eu/en/regulatory-requirements-vaccines-intended-provide-protection-against-variant-strains-sars-cov-2. Accessed 4 March, 2021.
- 180.U.S. Food and Drug Administration. 2021. Emergency use authorization for vaccines to prevent COVID-19: guidance for industry. https://www.fda.gov/media/142749/download. Accessed 3 March, 2021.
- 181.Medicines and Healthcare products Regulatory Agency. 2021. ACCESS Consortium guidance on strain changes in authorised COVID-19 vaccines. https://www.gov.uk/government/publications/access-consortium-guidance-on-strain-changes-in-authorised-covid-19-vaccines. Accessed 4 March, 2021.
- 182.World Health Organization. 2022. Interim statement on COVID-19 vaccines in the context of the circulation of the Omicron SARS-CoV-2 variant from the WHO Technical Advisory Group on COVID-19 Vaccine Composition (TAG-CO-VAC). https://www.who.int/news/item/11-01-2022-interim-statement-on-covid-19-vaccines-in-the-context-of-the-circulation-of-the-omicron-sars-cov-2-variant-from-the-who-technical-advisory-group-on-covid-19-vaccine-composition. Accessed 14 January, 2022.
- 183.Dai L, Gao GF. 2021. Viral targets for vaccines against COVID-19. Nat Rev Immunol 21:73–82. doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Beguir K, Skwark MJ, Fu Y, Pierrot T, Lopez Carranza N, Laterre A, Kadri I, Lui BG, Sänger B, Liu Y, Poran A, Muik A, Sahin U. 2021. Early computational detection of potential high risk SARS-CoV-2 variants. bioRxiv doi: 10.1101/2021.12.24.474095. [DOI] [PMC free article] [PubMed]
- 185.Moyo-Gwete T, Madzivhandila M, Makhado Z, Ayres F, Mhlanga D, Oosthuysen B, Lambson BE, Kgagudi P, Tegally H, Iranzadeh A, Doolabh D, Tyers L, Chinhoyi LR, Mennen M, Skelem S, Marais G, Wibmer CK, Bhiman JN, Ueckermann V, Rossouw T, Boswell M, de Oliveira T, Williamson C, Burgers WA, Ntusi N, Morris L, Moore PL. 2021. Cross-reactive neutralizing antibody responses elicited by SARS-CoV-2 501Y.V2 (B.1.351). N Engl J Med 384:2161–2163. doi: 10.1056/NEJMc2104192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cai Y, Zhang J, Xiao T, Peng H, Sterling SM, Walsh RM, Jr, Rawson S, Rits-Volloch S, Chen B. 2020. Distinct conformational states of SARS-CoV-2 spike protein. Science 369:1586–1592. doi: 10.1126/science.abd4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Seppala E, Veneti L, Starrfelt J, Danielsen AS, Bragstad K, Hungnes O, Taxt AM, Watle SV, Meijerink H. 2021. Vaccine effectiveness against infection with the Delta (B.1.617.2) variant, Norway, April to August 2021. Euro Surveill 26:2100793. doi: 10.2807/1560-7917.ES.2021.26.35.2100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategy and selection criteria. Download Text S1, DOCX file, 0.1 MB (55.1KB, docx) .
Copyright © 2022 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Characteristics of SARS-CoV-2 variants of concern and variants of interest (1). SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VOC, variant of concern; VOI, variant of interest. *Exact position and number of mutations may differ according to source; †Excluding D614G; ‡Disputed with mutation at same site (2); §Mutations with known or proposed biological significance shown in red. ¶The larger group of omicron sequences (B.1.1.529/21M) includes BA.1 (21K) and BA.2 (21L). Data shown here are based on BA.1 as the dominant sequence at the time of writing. Data based on sequences retrieved on 14 September 2021, except for omicron, which was retrieved on 14 January 2022. Download Table S1, DOCX file, 0.06 MB (64.7KB, docx) .
Copyright © 2022 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Studies assessing escape from antibody neutralization by novel variants from sera of COVID-19-vaccinated individuals. Comparisons across these studies should be made with caution, owing to considerable variations in assay techniques, use of pseudoviruses (of varying construction) versus true isolates, vaccine dosing intervals/time since infection, and participant ages and immune status, among other factors. ACE2, angiotensin-converting enzyme 2; CI, confidence interval; GMT, geometric mean titer; IC50, half maximal inhibitory concentration; MAb, monoclonal antibody; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMPRSS2, transmembrane protease serine 2; VSV, vesicular stomatitis virus. Download Table S2, DOCX file, 0.2 MB (194.3KB, docx) .
Copyright © 2022 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Efficacy of COVID-19 vaccines against SARS-CoV-2 variants (1–6). CI, confidence interval; COVID-19, coronavirus disease 2019; HIV, human immunodeficiency virus; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. Download Table S3, DOCX file, 0.08 MB (85.4KB, docx) .
Copyright © 2022 McLean et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.