Abstract
Objective
This study examined the functional outcomes and safety of endovascular treatment (EVT) in acute ischemic stroke (AIS) patients owing to large vessel occlusion of the anterior circulation, presented during a late-time window (6–24 hours after last seen well (LSW)) in a real-world practice.
Methods
This was a retrospective analysis from a bi-center prospective cohort. According to the stroke treatment, patients with continuous Alberta Stroke Plan Early Aspect score (ASPECTS) ≥6 on non-contrast CT (NCCT) and moderate to good collateral state on CT angiography (CTA) were divided into EVT group and standard medical treatment (SMT) group. The primary outcome was the rate of functional independence (90-day mRS ≤2). Safety outcomes were the occurrence of symptomatic intracranial hemorrhage (sICH) and the 90-day mortality.
Results
Among the 288 enrolled patients (53.5% male, median age 64 years), there were 167 patients in the EVT group and 121 in the SMT group. After multivariable adjustments for potential confounders, EVT was associated with functional independence (adjusted OR: 3.052; 95% confidence interval (CI): 1.553–5.997; p = 0.001). In the PSM cohort, 44.2% (42/95) of patients in the EVT group versus 18.9% (18/95) in the SMT group achieved functional independence (OR: 3.39, 95% CI: 1.763–6.517), and there was a significant difference favoring EVT over the SMT in the overall distribution of mRS (OR: 2.170, 95% CI: 1.302–3.618) at 90 days. The rate of sICH did not differ between the EVT and SMT groups (10.5% vs 8.4%, p = 0.804) nor did 90-day mortality (18.9% vs 22.1%, p = 0.719). No interaction was found in p-values with statistical significance in subgroup analysis.
Conclusion
This real-world experience suggests that EVT for late-presenting stroke patients, based on small core on NCCT and moderate to good collaterals on CTA, is associated with better outcomes than SMT alone, with no increase in sICH and 90-day mortality rates.
Keywords: endovascular treatment, late time window, propensity score matching, real-world study
Introduction
The DEFUSE-31 and DAWN2 trials demonstrated that when the neuroimaging evaluation based on computed tomography perfusion (CTP) or magnetic resonance imaging by automated imaging software showed the existence of a salvageable penumbra or a mismatch, the time window of EVT for acute ischemic stroke with large vessel occlusion (AIS-LVO) could be extended to 16 or 24 hours. Hence, a shift from a time-based paradigm toward a tissue-based paradigm of stroke treatment has occurred, whereby baseline imaging features can be used to select patients receiving reperfusion therapy.
The updated American Heart Association/American Stroke Association (AHA/ASA) guidelines3 for 2018 suggested that the eligibility criteria defined by the EVT trials could be extended to the late-window period. However, recent non-randomized clinical studies4–7 have shown that a larger portion of the stroke patients who are considered ineligible for late EVT, could still be benefited, as per the criteria of DAWN and DEFUSE-3. Besides, the high costs of imaging hardware, software, and annual maintenance have remained a roadblock for the widespread application of perfusion inspection techniques and post-processing analysis, especially in developing countries.8 In contrast, the non-contrast CT (NCCT) and CT angiography (CTA) of the head are routinely applied at most emergency departments. Acknowledging this disparity and choosing an alternative clinical and imaging approach that is more practical to identify appropriate EVT candidates outside the traditional window would be helpful in real-world treatment decision-making. The Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke (ESCAPE) trial9 revealed that an imaging triage by evaluation the status of CTA collaterals at the distal end of occlusive vessel was capable of selecting AIS-LVO patients who would benefit from EVT up to 12 hours. Recently, several comparative studies have revealed that application of relatively simpler imaging criteria to screen for EVT-eligible patients beyond 6 hours also achieved similar therapeutic effects as EVT during 6 hours.10–13 However, evidence for clinical benefits might be relatively weaker when compared to patients within 6 hours, without a control group of non-EVT population under the same selection conditions.
Therefore, we aimed to evaluate the functional outcomes and safety of EVT by comparing with standard medical treatment (SMT) using propensity score matching (PSM) for AIS-LVO patients presenting within 6–24 hours from the time of last seen well (LSW) in real-world practice. In this study, we selected a combined triage composed of the Alberta Stroke Plan Early Aspect score (ASPECTS) ≥6 on NCCT and moderate to good collateral status on single-phase CTA (Tan score of 2 to 3) as the imaging criterion, in comparison to DAWN and DEFUSE-3 trials, which has recently been demonstrated as a promising way to select slow progressors for reperfusion therapies.14
Methods
Study Population
This was a retrospective analysis of an observational, bi-center, prospective cohort between January 1st, 2017 to October 31st, 2018. Anterior circulation LVO patients who were admitted or transferred to two advanced stroke centers during a late-time window were prospectively enrolled into the database. The inclusion criteria were as follows: (1) age ≥18 years old; (2) pre-stroke modified Rankin Scale (mRS) ≤2; (3) time from LSW to admission was within 6–24 hours; (4) ASPECTS on NCCT ≥6; (5) single-phase CTA collateral score was moderate to good (Tan score of 2–3);15 and (6) presence of LVO in the anterior circulation (internal carotid artery and middle cerebral artery [MCA], M1 or proximal M2 segment) on CTA/ digital subtraction angiography. Exclusion criteria included: (1) poor-quality imaging; (2) intra-arterial thrombolysis alone; (3) no follow-up data at 90 days; and (4) missing study-related important baseline information (National Institutes of Health Stroke Scale [NIHSS] score). Patients were assigned to the EVT and SMT groups according to the treatment received. Treatment recommendation was made by neuro-interventionalists through the evaluation of the ASPECTS on NCCT and the collateral state on CTA images. Besides the doctors’ advice, the final decision on EVT depended on the patients’ will as well. The most common reasons for patients refusing EVT in the control arm were advanced age (62/121), co-morbidities of vascular risk factors (45/121), and longer time interval from LSW to admission (14/121). The definition of SMT included secondary preventive measures, hospitalization to a stroke ward or intensive care unit, and rehabilitation. The current study and relevant analyses were approved by the ethics committees of the two hospitals (West China Hospital and Affiliated Hospital of Southwest Medical University). Participating patients or their close relatives provided written informed consent for the prospective stroke database.
Baseline Data Collection
The demographics and clinical data were retrieved from the prospective stroke database, including age, gender, vascular risks, pre-antithrombotic therapy, intravenous thrombolysis (IVT), site of occlusion (intracranial ICA, MCA1, proximal MCA2, proximal ICA or tandem lesion), mode of presentation (witnessed, wake-up, unwitnessed), the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification (large artery atherosclerosis (LAA), cardio-embolic (CE), or other), the time interval from LSW to admission, baseline NIHSS score, baseline ASPECTS score, collateral scores, and successful recanalization. Tandem lesion was defined as severe stenosis or occlusion of the ICA involving distal ipsilateral and MCA occlusion. Cerebral recanalization was evaluated by modified thrombolysis in cerebral infarction (mTICI) scale through postoperative cerebral angiography, ranging from 0 to 3, with grade 2b or grade 3 indicating successful recanalization. All available imaging data were stored in an independent image post-processing center and scored by consensus of two experienced neuroradiologists (D.T; L.C) blinded to all clinical data (except the side of stroke), therapeutic methods, and outcome data. In separate sessions and using random patient ordering, the same two raters scored the NCCT-ASPECTS and the collateral score on single-phase CTA.
Imaging Techniques and Data Processing
CT scans were performed on the 256 slice scanners (Philips iCT 256) in both centers. The CTA was collected by injecting 40–60mL of iodinated contrast agent first, followed by 40mL saline, 5mm MIP reconstruction with 1mm increment, 0.993 pitch, iDose4, 0.625mm collimation. The collateral status assessment on single-phase CTA was performed through the Tan score system15 to evaluate pia mater artery status in the whole ischemic area of the MCA, ranging from 0=no anterograde reperfusion of the occluded vascular territory to 3=complete reperfusion, and a score of 2–3 was defined as moderate to good collateral circulation.
Outcomes
The primary outcome was the rate of functional independence (90-day modified Rankin Scale (mRS) ≤2), which was prospectively collected by assessors blinded to the clinical data and therapies through a structured telephone interview. The safety outcomes were 90-day mortality and the ratio of symptomatic intracranial hemorrhage (sICH) which met the European Cooperative Acute Stroke Study III (ECASS-III) criteria.16
Statistical Analysis
Continuous variables were compared by independent sample t test or Mann–Whitney test, while categorical variables were compared with chi-squared test or Fisher’s exact test when appropriate. Inter-rater reliability was evaluated by weighted κ for the ASPECT score and the Tan score (pia mater collaterals on CTA). Tolerance and variance inflation factors (VIF) were used to diagnose multicollinearity among confounders. Multivariable binary logistic regression was completed to compare the primary outcome of patients between the EVT and SMT groups after adjusting for potential confounders from univariable analysis with p <0.1 and clinically important variables. In addition, propensity score matching (PSM) with matching ratio 1:1 was also used to adjust non-randomized treatment allocation with all baseline variables by nearest principle within 0.2*SD of the propensity score index.17 Besides, subgroup analyses for functional independence were reported based on predetermined variation of age (<65 or ≥65), baseline NIHSS (<15 or ≥15), the time interval from LSW to admission (6–12 h or 12–24 h), mode of presentation (witnessed, wake-up, unwitnessed), and baseline ASPECTS (6–7 or 8–10) in the PSM cohort. A p-value of <0.05 was considered significant. Data were analyzed with the SPSS 25 statistical package (IBM Corp, Armonk, NY, USA), R version 3.6.5 (MatchIt package) and MedCalc Statistical Software version 19.6 (MedCalc, Ostend, Belgium; https://www.medcalc.org).
Results
Baseline Characteristics
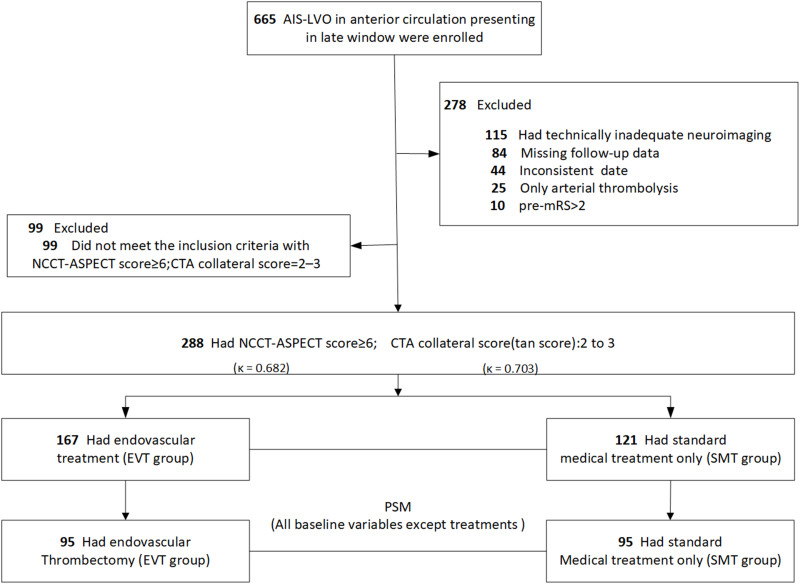
From January 1st, 2017 to October 31st, 2018, among the 665 patients with anterior circulation AIS-LVO who were admitted or transferred to the two advanced stroke centers (West China Hospital and The Affiliated Hospital of Southwest Medical University) within the late-time window, 377 patients did not meet the research criteria and were excluded (Figure 1). In total, there were 288 (43.3%) patients (154 male [53.5%]; median [IQR] age, 64 [54–73] years; median NIHSS, 13 [10–15]; median LSW to admission time, 630 [476–900] minutes) included in the final analysis. One third (96/288) of the patients were transferred from primary stroke centers, and 60.4% (58/96) of them received intravenous thrombolysis within 4.5 h after stroke onset. These in the EVT group (n=167) were significantly younger (median age 61 vs 67 years, p=0.014) than those in the SMT group (n=121), with a higher ASPECTS (median 9 vs 8, p=0.014), higher proportions of hyperlipidemia (28.7% vs 13.2%, p=0.003), higher pre-antithrombotic therapy ratio (35.3% vs 23.1%, p=0.036), and higher intravenous thrombolysis (25.7% vs 12.4%, p=0.008) (Table 1). After PSM analysis, baseline demographics and clinical imaging characteristics between the two groups (n=95 in each group) achieved an excellent balance (Table 1). Inter-rater reliability was acceptable for the ASPECTS (κ=0.682; 95% confidence interval [CI], 0.537–0.827) and the Tan score (κ=0.703; 95% CI, 0.512–0.893) (Figure 1).
Figure 1.
The flow chart.
Note: Reasons for refusing EVT in the SMT group were mainly advanced age (n=62), co-morbidities of vascular risk factors (n=45), and time interval from LSW to admission (n=14).
Abbreviations: ASPECTS, Alberta Stroke Program Early CT Score; CTA, CT angiography; EVT, endovascular treatment; PSM, Propensity Score Matching; SMT, standard medical treatment; NCCT, no-contrast CT; NIHSS, National Institutes of Health Stroke Scale.
Table 1.
Demographic and Clinical Imaging Characteristics of Patients in EVT Group and SMT Group
| Variables | Unmatched Data Set | p value | Propensity Score-Matched Data Set | p value | ||
|---|---|---|---|---|---|---|
| EVT (n=167) | SMT (n=121) | EVT (n=95) | SMT (n=95) | |||
| Demographics | ||||||
| Gender, male, n (%) | 94 (56.3) | 60 (49.6) | 0.315 | 55 (57.9) | 49 (51.6) | 0.466 |
| Age, Mean ± SD | 61 (53, 73) | 67 (57, 74) | 0.014 | 62 (53, 75) | 66 (56, 73) | 0.559 |
| Transferred patients, n (%) | 61 (36.5) | 35 (28.9) | 0.221 | 29 (30.5) | 25 (26.3) | 0.629 |
| History, n (%) | ||||||
| Diabetes | 30 (18.0) | 26 (21.5) | 0.552 | 18 (18.9) | 19 (20.0) | 1 |
| Hypertension | 83 (49.7) | 63 (52.1) | 0.782 | 48 (50.5) | 49 (51.6) | 1 |
| Hyperlipidemia | 48 (28.7) | 16 (13.2) | 0.003 | 21 (22.1) | 16 (16.8) | 0.464 |
| Coronary heart disease | 21 (12.6) | 12 (9.9) | 0.609 | 10 (10.5) | 9 (9.5) | 1 |
| Atrial fibrillation | 52 (31.1) | 28 (23.1) | 0.173 | 30 (31.6) | 22 (23.2) | 0.255 |
| Pre-stroke | 23 (13.8) | 16 (13.2) | 1 | 13 (13.7) | 13 (13.7) | 1 |
| Pre-antithrombotic therapy | 59 (35.3) | 28 (23.1) | 0.036 | 27 (28.4) | 25 (26.3) | 0.871 |
| Smoker | 46 (27.5) | 30 (24.8) | 0.698 | 27 (28.4) | 29 (30.5) | 0.874 |
| Drinker | 25 (15.0) | 16 (13.2) | 0.787 | 12 (12.6) | 18 (18.9) | 0.32 |
| Clinical characteristics | ||||||
| Systolic blood pressure (SBP), mmHg, Median (IQR) | 144 ± 27 | 149 ± 23 | 0.197 | 146 ± 28 | 147 ± 24 | 0.813 |
| Baseline NIHSS, Median (IQR) | 12 (10, 15) | 13 (10, 16) | 0.407 | 12 (10, 15) | 13 (10, 15) | 0.534 |
| Baseline ASPECTS, Median (IQR) | 9 (8, 10) | 8 (8, 9) | 0.014 | 9 (8, 9) | 9 (8, 9) | 0.568 |
| Intravenous thrombolysis, n (%) | 43 (25.7) | 15 (12.4) | 0.008 | 14 (14.7) | 15 (15.8) | 1 |
| Time interval from last-seen-well (LSW) to admission, Median (IQR) min | 630 (494, 852) | 634 (468, 950) | 0.875 | 630 (499, 895) | 654 (475, 964) | 0.956 |
| Mode of presentation, n (%) | 0.085 | 0.485 | ||||
| Witnessed | 114 (68.3) | 70 (57.9) | 54 (56.8) | 58 (61.1) | ||
| Wake-up | 43 (25.7) | 36 (29.8) | 33 (34.7) | 26 (27.4) | ||
| Unwitnessed | 10 (6) | 15 (12.4) | 8 (8.4) | 11 (11.6) | ||
| TOAST classification, n (%) | 0.122 | 0.393 | ||||
| Large artery atherosclerosis (LAA) | 104 (62.3) | 89 (73.6) | 61 (64.2) | 70 (73.7) | ||
| Cardio-embolic (CE) | 53 (31.7) | 28 (23.1) | 30 (31.6) | 23 (24.2) | ||
| Others | 10 (6.0) | 4 (3.3) | 4 (4.2) | 2 (2.1) | ||
| Occlusion site, n (%) | 0.016 | 0.276 | ||||
| Intracranial ICA | 43 (25.7) | 23 (19.0) | 23 (24.2) | 19 (20.0) | ||
| MCA1 | 89 (53.3) | 58 (47.9) | 47 (49.5) | 53 (55.8) | ||
| Proximal MCA2 | 18 (10.8) | 11 (9.1) | 15 (15.8) | 8 (8.4) | ||
| Proximal ICA or Tandem lesion | 17 (10.2) | 29 (24.0) | 10 (10.5) | 15 (15.8) | ||
Note: Propensity score matching (PSM) with matching ratio 1:1 was also used to adjust non-randomized treatment allocation with all baseline variables by nearest principle within 0.2*SD of the propensity score index.
Abbreviations: ASPECTS, Alberta Stroke Program Early CT Score; NIHSS, National Institutes of Health Stroke Scale; ICA, internal carotid artery; MCA, middle cerebral artery; TOAST, Trial of Org 10172 in acute stroke treatment.
Outcomes in the Unmatched Cohort
With respect to the primary outcome, univariable logistic regression indicated that gender, age, diabetes, hyperlipidemia, baseline NIHSS, baseline ASPECTS, IVT, mode of presentation and occlusion site were significant confounders (p <0.1, Table 2). After multivariable adjustments of these confounders and selected clinical character (time interval from LSW to admission), EVT was found to be significantly associated with functional independence (adjusted OR, 3.052; 95% CI, 1.553–5.997; p=0.001) and had a better mRS shift score (adjusted OR, 1.905; 95% CI, 1.217–2.984; p=0.005) at 90 days (Table 3; Figure 2). For the safety outcomes, sICH rates (7.2% EVT vs 8.3% SMT group; p=0.908) and mortality rates at 90-day (16.8% vs 23.1%; p=0.231) were comparable between the two groups (Table 3).
Table 2.
Univariate Analysis for Functional independence in the Unmatched Cohort
| Variables | OR (95% CI) | p |
|---|---|---|
| Demographics | ||
| Gender, male | 1.877(1.132–3.115) | 0.015 |
| Age | 0.938(0.916–0.96) | 0.001 |
| Transferred patients | 1.293(0.772–2.168) | 0.392 |
| History | ||
| Diabetes | 0.499(0.25–0.997) | 0.049 |
| Hypertension | 0.699(0.426–1.147) | 0.156 |
| Hyperlipidemia | 0.562(0.296–1.066) | 0.077 |
| Coronary heart disease | 0.629(0.272–1.453) | 0.278 |
| Atrial fibrillation | 0.845(0.484–1.476) | 0.554 |
| Pre-stroke | 1.037(0.506–2.123) | 0.921 |
| Pre-antithrombotic therapy | 1.127(0.662–1.919) | 0.661 |
| Smoker | 1.629(0.946–2.807) | 0.179 |
| Drinker | 1.427(0.731–2.783) | 0.297 |
| Clinical characteristics | ||
| Systolic pressure (SP) | 0.992(0.982–1.002) | 0.113 |
| Baseline NIHSS | 0.853(0.796–0.915) | 0.001 |
| Baseline ASPECTS | 1.841(1.415–2.395) | 0.001 |
| Intravenous thrombolysis | 2.119(1.176–3.819) | 0.012 |
| Time interval from last-seen-well (LSW) to admission | 1(0.999–1.001) | 0.938 |
| Mode of presentation, n (%) | ||
| Witnessed | Reference | |
| Wake-up | 1.054(0.607–1.832) | 0.851 |
| Unwitnessed | 0.262(0.075–0.909) | 0.035 |
| TOAST classification | ||
| Large artery atherosclerosis (LAA), | Reference | |
| Cardio-embolic (CE) | 1.023(0.586–1.785) | 0.936 |
| Other | 2.164(0.727–6.441) | 0.165 |
| Occlusion site | ||
| Intracranial ICA | Reference | |
| MCA1 | 1.974(1.014–3.844) | 0.045 |
| Proximal MCA2 | 2.078(0.807–5.349) | 0.13 |
| Proximal ICA or Tandem lesion | 1.487(0.635–3.487) | 0.361 |
| EVT | 3.411(1.959–5.937) | <0.001 |
Abbreviations: ASPECTS, Alberta Stroke Program Early CT Score; NIHSS, National Institutes of Health Stroke Scale; ICA, internal carotid artery; MCA, middle cerebral artery; TOAST, Trial of Org 10172 in acute stroke treatment.
Table 3.
Clinical Outcomes of Patients in EVT Group and SMT Group
| Unmatched Data Set | EVT (n=167) | SMT (n=121) | Adj OR | 95% CI | P-value |
| mRS shift | 1.905 | 1.217–2.984 | 0.005 | ||
| Functional independence, n (%) | 72 (43.1) | 22 (18.2) | 3.052 | 1.553–5.997 | 0.001 |
| Successful recanalization, *n (%) | 143 (85.6) | NA | NA | ||
| sICH, n (%) | 12 (7.2) | 10 (8.3) | 0.908 | ||
| Mortality, n (%) | 28 (16.8) | 28 (23.1) | 0.231 | ||
| Matched data set after PSM | EVT (n=95) | SMT (n=95) | OR | 95% CI | P-value |
| mRS shift | 2.17 | (1.302–3.618) | <0.001 | ||
| Functional independence, n (%) | 42 (44.2) | 18 (18.9) | 3.39 | (1.763–6.517) | 0.001 |
| Successful recanalization, *n (%) | 83 (87.4) | NA | NA | ||
| sICH, n (%) | 10 (10.5) | 8 (8.4) | 0.804 | ||
| Mortality, n (%) | 18 (18.9) | 21 (22.1) | 0.719 |
Notes: AdjOR denotes odds ratio adjusted for gender, age, diabetes, hyperlipidemia, NIHSS, ASPECTS, IVT, mode of presentation and occlusion site, time interval from LSW to admission. Functional independence was defined as a score of 0, 1, or 2 on the mRS at 90-day; ranges from 0 to 3, with a grade of 2b or 3 indicating successful recanalization (recanalization more than 50% in the affected territory). *Data of successful recanalization were not available for patients in the SMT group for lacking the evaluation of angiography.
Abbreviations: mTICI, modified Thrombolysis in Cerebral Infarction scale; ASPECTS, Alberta Stroke Program Early CT Score; NIHSS, National Institutes of Health Stroke Scale; sICH, symptomatic intracranial hemorrhage.
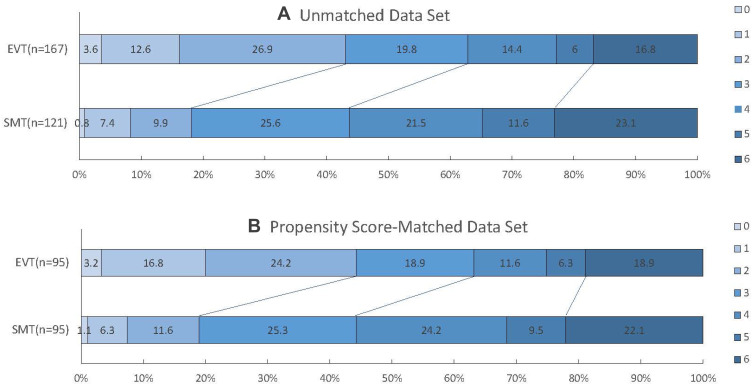
Figure 2.
Score on the modified Rankin Scale at 90 days in both cohorts.
Notes: On the unmatched data set (A) and PSM data set (B), there was a significant difference favoring the EVT group over the SMT group in the overall distribution of mRS scores.
Abbreviations: EVT, endovascular treatment; PSM, Propensity Score Matching; SMT, standard medical treatment. mRS scores on the modified Rankin scale, ranging from 0 to 6.
Outcomes in the PSM Cohort
Similar results were obtained after PSM analysis. Patients in the EVT group showed a significantly higher proportion of functional independence (44.2% vs 18.9%; OR 3.39; 95% CI 1.763–6.517; p=0.001) and a better mRS shift (OR 2.17; 95% CI 1.302–3.618; p<0.001) than those of the SMT group at 90 days (Figure 2). The incidences of sICH (10.5% vs 8.4%; p=0.804) were numerically greater but non-significantly higher in patients treated with EVT than with SMT. Mortality rates (18.9% vs 22.1%; p=0.719) were similar between patients who received EVT and SMT alone (Table 3).
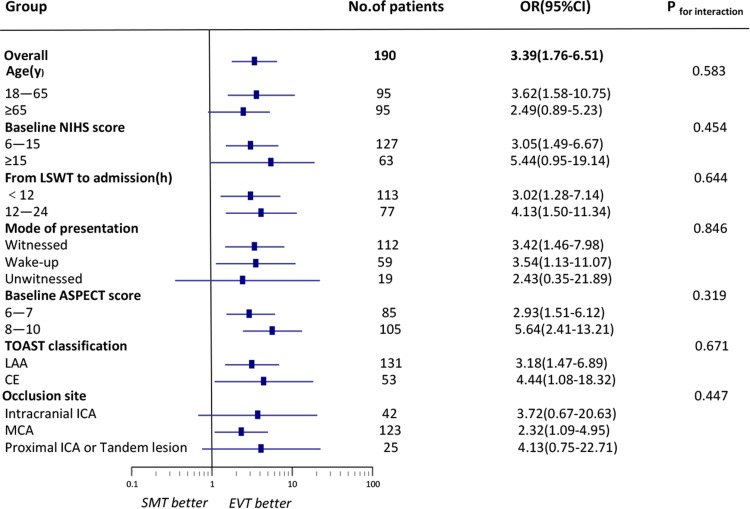
The results from subgroup analyses showed that EVT selected by this combined imaging triage was generally effective in all sub-groups, although the ORs for treatment were not significant in the patients with older age (≥65), higher NIHSS score (≥15), unwitnessed stroke and occlusion site with ICA. No interaction for functional independence was found in p-values with statistical significance (Figure 3). Subgroup analyses for safety outcomes also did not reveal significant interactions (Supplementary Figure A; Supplementary Figure B).
Figure 3.
Subgroup analyses of the rate of functional Independence using different variables.
Abbreviations: ASPECTS, Alberta Stroke Program Early CT Score; CE, cardio-embolic; ICA, internal carotid artery; LWST, the time of last-seen-well; LAA, large artery atherosclerosis; MCA, middle cerebral artery; NIHSS, National Institutes of Health Stroke Scale.
Discussion
In this study, we found that for AIS-LVO patients selected by small infarction on NCCT and moderate to good collaterals on single-phase CTA, EVT in a late-time window (6–24h from LSW) was feasible and associated with better functional outcomes than SMT alone at 90 days, without significant differences in sICH rates and 90-day mortality.
For all patients in our study, the probability of achieving functional independence in EVT patients was 43.1%, while that in SMT patients was 18.2%. This result was similar to that reported from the most recent pooled meta-analysis of EVT late-window controlled trials (AURORA),18 which identified AIS-LVO patients treated beyond 6 hours either with thrombectomy (n=266) or best medical therapy (n=239), and the functional independence rates of EVT group and control group was 45.9% and 19.3%, respectively. Because the patient selection in our study was based on a combined approach for selecting the extent of at-risk brain tissue, the rate of functional independence was higher than other observational studies19–22 that opted for other strategies to select patients, with functional independence rate ranging from 32% to 36.7%. Furthermore, compared with strict paradigms from EVT trials,1,2 this practical and less stringent criterion might benefit more EVT candidates in clinical practice because it qualified approximately 43.3% (288/665) patients with AIS-LVO in anterior circulation within a late-time window for EVT in the current study. This was relative higher than the previously reported results under the strict trial imaging, in which about 10.4% and 21.3% of patients were qualified.23 After propensity score matching, the functional outcome rate in the EVT group was 25.3 percentage points higher than that in the SMT group, and this was close to the data (28 percentage points improved) from the DEFUSE-3 trial1 which selected late arrivals by using advanced imaging modalities. In addition, in subgroup analysis, our study showed that EVT based on this practical paradigm was generally effective in all populations, although the ORs of treatment were not significant for patients with older age (≥65), higher NIHSS score (≥15) or unwitnessed stroke. This was consistent with previous findings that advanced age and higher baseline NIHSS scores were independently related to unfavorable functional outcomes in AIS patients who received EVT beyond 6 hours,24 and that patients with unwitnessed stroke were reported to be inferior to wake-up stroke in achieving good functional outcomes.25
Here, the difference of sICH rate between the two groups was not statistically significant, nor was the 90-day mortality, which indicated that the safety of EVT was acceptable for patients enrolled with the aforementioned eligibility criterion within a late-time window. Notably, the sICH ratios (10.5% EVT vs 8.4% SMT group) in this PSM cohort were relatively higher than that of the latest AURORA analysis reports (5·3% vs 3·3%, respectively). The different sICH standards in the AURORA mate-analysis may be an explanation.18 In addition, as Asians were reported to be more likely to have hemorrhagic transformation after AIS,26 the relatively higher ratio of sICH in this study probably reflected the real-world setting in Asia.
The selection strategy of EVT in a late-time window should seek to identify AIS-LVO patients, who can benefit most from recanalization penumbra without increasing serious complications of EVT. On one hand, accumulating evidence shows that the larger the infarct size, the lower the possibility of obtaining a good clinical outcome,27,28 and a small core is an important predictor of a favorable outcome following reperfusion therapy.14 Haussen et al29 found a reasonable correlation between NCCT-ASPECTS and CTP-derived core volume, with ASPECTS ≥6 having a median CTP core ≤20 cc. Further data also suggest that the ASPECT score on NCCT is increasingly sensitive to the definition of infarct core on CTP perfusion with increasing time from stroke onset.30,31 On the other hand, the status of collaterals is considered to be a critical determinant for distinguishing between “rapid progressing” and “slow progressing”,32 and robust collaterals can effectively prevent the extension of the core infarct by rescuing the penumbra area.33 Therefore, in the extended time window, patients with a small core, moderate to good collateral, are by definition “slow progressors” who can potentially benefit most from recanalization penumbra,14,34,35 and these are also important factors for achieving good outcomes in our study.
Taken together, the data obtained in the current study revealed the possibility of EVT in a late-time window by using a combined approach based on the ASPECTS on NCCT and CTA collaterals to evaluate the small baseline core volume but a large volume of at-risk tissue. Besides, due to the clinical practicality and universality of this imaging paradigm, it may become a convenient and pragmatic approach to help neuro-interventionists select suitable patients for EVT in the absence of advanced imaging.
There are also certain limitations to our study. Firstly, although the patients were selected continuously from a prospective database, the retrospective analysis has its inherent limitations. Secondly, the most common reasons for patients refusing EVT in the control arm were advanced age, previous co-morbidity and prolonged time interval, which to some extent indicated that patients who expected to evolve better were the ones who tended to treat with EVT, and this may lead to another selection bias for baseline characters. However multivariable logistic regression and PSM were used in this study to reduce this limitation. Thirdly, the use of single-phase CTA for collateral circulation scoring might be another limitation, as multiple-phase CTA can be more dynamic and precise in assessing collateral status. However, single-phase CTA is the most commonly used CTA technique in clinical practice, and it’s now considered as a reliable method to evaluate the baseline collateral status in the ongoing MR CLEAN-LATE trial (ISRCTN19922220).36 Fourthly, since we did not compare our imaging paradigm with that including CTP imaging software, which might represent a drawback of this study, we cannot reach a conclusion that automated software is no longer a key to the decision of EVT for late presenting patients. Finally, the inter-rater reliability of imaging evaluation in our study is moderately acceptable, which may affect the generalization of our findings. Automating the assessment of NCCT-ASPECTS and CTA collaterals with validated algorithms will be a potential solution to this concern.
Conclusion
The data obtained in this study supported the impression that real-world practice of EVT, based on ASPECTS ≥6 on NCCT and moderate to good collateral on CTA, could obtain better functional outcomes than SMT alone in AIS-LVO patients presenting 6–24 hours after LSW, without increasing the rates of sICH and 90-day mortality.
Funding Statement
This work was supported by the National Natural Science Foundation of China (82071320, 81870937), and the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYGD 18009).
Abbreviations
ASPECTS, Alberta Stroke Plan Early Aspect Score; AIS-LVO, acute ischemic stroke with large vessel occlusion; CI, confidence interval; CT, computed tomography; CTA, CT angiography; EVT, endovascular treatment; IVT, intravenous thrombolysis; MCA, middle cerebral artery; mTICI, modified Thrombolysis in Cerebral Infarction; NCCT, non-contrast CT; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; sICH, symptomatic intracranial hemorrhage; SMT, standard medical treatment; TOAST, the Trial of Org 10172 in Acute Stroke Treatment.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Statement of Ethics
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Biomedical Ethics Committee of West China Hospital and the Affiliated Hospital of Southwest Medical University [2019(881)]; [KY202011].
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. New Engl J Med. 2018;378(8):708–718. doi: 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11–21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 3.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 4.Bouslama M, Haussen DC, Rodrigues G, Barreira C, Frankel M, Nogueira RG. Novel selection paradigms for endovascular stroke treatment in the extended time window. J Neurol Neurosurg Psychiatry. 2021;92(11):1152–1157. doi: 10.1136/jnnp-2020-325284 [DOI] [PubMed] [Google Scholar]
- 5.Desai SM, Haussen DC, Aghaebrahim A, et al. Thrombectomy 24 hours after stroke: beyond Dawn. J Neurointerv Surg. 2018;10(11):1039–1042. doi: 10.1136/neurintsurg-2018-013923 [DOI] [PubMed] [Google Scholar]
- 6.Desai SM, Rocha M, Molyneaux BJ, et al. Thrombectomy 6–24 hours after stroke in trial ineligible patients. J Neurointerv Surg. 2018;10(11):1033–1037. doi: 10.1136/neurintsurg-2018-013915 [DOI] [PubMed] [Google Scholar]
- 7.Herzberg M, Scherling K, Stahl R, et al. Late thrombectomy in clinical practice: retrospective application of dawn/DEFUSE3 criteria within the German stroke registry. Clin Neuroradiol. 2021;31(3):799–810. doi: 10.1007/s00062-021-01033-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanelli PC, Pandya A, Segal AZ, et al. Cost-effectiveness of CT angiography and perfusion imaging for delayed cerebral ischemia and vasospasm in aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2014;35(9):1714–1720. doi: 10.3174/ajnr.A3947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 10.Casetta I, Fainardi E, Saia V, et al. Endovascular thrombectomy for acute ischemic stroke beyond 6 hours from onset: a real-world experience. Stroke. 2020;51(7):2051–2057. doi: 10.1161/STROKEAHA.119.027974 [DOI] [PubMed] [Google Scholar]
- 11.Jung S, Gralla J, Fischer U, et al. Safety of endovascular treatment beyond the 6-h time window in 205 patients. Eur J Neurol. 2013;20(6):865–871. doi: 10.1111/ene.12069 [DOI] [PubMed] [Google Scholar]
- 12.Beaulieu M-C, Nehme A, Fortin F, et al. Non-contrast CT and CT-angiogram for late window ischemic stroke treatment selection. Can J Neurol Sci. 2020;47(3):309–313. doi: 10.1017/cjn.2020.15 [DOI] [PubMed] [Google Scholar]
- 13.Dekker L, Venema E, Pirson FAV, et al. Endovascular treatment in anterior circulation stroke beyond 6.5 hours after onset or time last seen well: results from the MR CLEAN Registry. Stroke Vasc Neurol. 2021;6(4):572–580. doi: 10.1136/svn-2020-000803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rocha M, Jovin TG. Fast versus slow progressors of infarct growth in large vessel occlusion stroke: clinical and research implications. Stroke. 2017;48(9):2621–2627. doi: 10.1161/STROKEAHA.117.017673 [DOI] [PubMed] [Google Scholar]
- 15.Tan IYL, Demchuk AM, Hopyan J, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009;30(3):525–531. doi: 10.3174/ajnr.A1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. doi: 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 17.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jovin TG, Nogueira RG, Lansberg MG, et al. Thrombectomy for anterior circulation stroke beyond 6 h from time last known well (Aurora): a systematic review and individual patient data meta-analysis. Lancet. 2022;399(10321):249–258. doi: 10.1016/S0140-6736(21)01341-6 [DOI] [PubMed] [Google Scholar]
- 19.Huang Q, Gu M, Zhou J, et al. Endovascular treatment of acute ischemic stroke due to anterior circulation large vessel occlusion beyond 6 hours: a real-world study in China. BMC Neurol. 2021;21(1):92. doi: 10.1186/s12883-021-02122-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexandre AM, Pedicelli A, Valente I, et al. May endovascular thrombectomy without CT perfusion improve clinical outcome? Clin Neurol Neurosurg. 2020;198:106207. doi: 10.1016/j.clineuro.2020.106207 [DOI] [PubMed] [Google Scholar]
- 21.Tsurukiri J, Ota T, Jimbo H, et al. Thrombectomy for stroke at 6–24 hours without perfusion CT software for patient selection. J Stroke Cerebrovasc Dis. 2019;28(3):774–781. doi: 10.1016/j.jstrokecerebrovasdis.2018.11.022 [DOI] [PubMed] [Google Scholar]
- 22.Beckhauser MT, Castro-Afonso LH, Dias FA, et al. Extended time window mechanical thrombectomy for acute stroke in Brazil. J Stroke Cerebrovasc Dis. 2020;29(10):105134. doi: 10.1016/j.jstrokecerebrovasdis.2020.105134 [DOI] [PubMed] [Google Scholar]
- 23.Nannoni S, Strambo D, Sirimarco G, et al. Eligibility for late endovascular treatment using Dawn, DEFUSE-3, and more liberal selection criteria in a stroke center. J Neurointerv Surg. 2020;12(9):842–847. doi: 10.1136/neurintsurg-2019-015382 [DOI] [PubMed] [Google Scholar]
- 24.Santos T, Carvalho A, Cunha AA, et al. NCCT and CTA-based imaging protocol for endovascular treatment selection in late presenting or wake-up strokes. J Neurointerv Surg. 2019;11(2):200–203. doi: 10.1136/neurintsurg-2018-014051 [DOI] [PubMed] [Google Scholar]
- 25.Bücke P, Pérez MA, Hellstern V, AlMatter M, Bäzner H, Henkes H. Endovascular thrombectomy in wake-up stroke and stroke with unknown symptom onset. AJNR Am J Neuroradiol. 2018;39(3):494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta RH, Cox M, Smith EE, et al. Race/Ethnic differences in the risk of hemorrhagic complications among patients with ischemic stroke receiving thrombolytic therapy. Stroke. 2014;45(8):2263–2269. doi: 10.1161/STROKEAHA.114.005019 [DOI] [PubMed] [Google Scholar]
- 27.Nakajo Y, Zhao Q, Enmi J-I, et al. Early Detection of Cerebral Infarction After Focal Ischemia Using a New MRI Indicator. Mol Neurobiol. 2019;56(1):658–670. doi: 10.1007/s12035-018-1073-1 [DOI] [PubMed] [Google Scholar]
- 28.Zaidi SF, Aghaebrahim A, Urra X, et al. Final infarct volume is a stronger predictor of outcome than recanalization in patients with proximal middle cerebral artery occlusion treated with endovascular therapy. Stroke. 2012;43(12):3238–3244. doi: 10.1161/STROKEAHA.112.671594 [DOI] [PubMed] [Google Scholar]
- 29.Haussen DC, Dehkharghani S, Rangaraju S, et al. Automated CT perfusion ischemic core volume and noncontrast CT ASPECTS (Alberta stroke program early CT score): correlation and clinical outcome prediction in large vessel stroke. Stroke. 2016;47(9):2318–2322. doi: 10.1161/STROKEAHA.116.014117 [DOI] [PubMed] [Google Scholar]
- 30.Voleti S, Vidovich J, Corcoran B, et al. Correlation of Alberta stroke program early computed tomography score with computed tomography perfusion core in large vessel occlusion in delayed time windows. Stroke. 2021;52(2):498–504. doi: 10.1161/STROKEAHA.120.030353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naylor J, Churilov L, Chen Z, Koome M, Rane N, Campbell BCV. Reliability, reproducibility and prognostic accuracy of the Alberta stroke program early CT score on CT perfusion and non-contrast CT in hyperacute stroke. Cerebrovascular Diseases. 2017;44(3–4):195–202. doi: 10.1159/000479707 [DOI] [PubMed] [Google Scholar]
- 32.Kim B, Jung C, Nam HS, et al. Comparison between perfusion- and collateral-based triage for endovascular thrombectomy in a late time window. Stroke. 2019;50(12):3465–3470. doi: 10.1161/STROKEAHA.119.027216 [DOI] [PubMed] [Google Scholar]
- 33.Bonnin P, Kubis N, Charriaut-Marlangue C. Collateral supply in preclinical cerebral stroke models. Transl Stroke Res. 2021. doi: 10.1007/s12975-021-00969-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potreck A, Weyland CS, Seker F, et al. Accuracy and prognostic role of NCCT-ASPECTS depend on time from acute stroke symptom-onset for both human and machine-learning based evaluation. Clin Neuroradiol. 2021. doi: 10.1007/s00062-021-01110-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Regenhardt RW, González RG, He J, Lev MH, Singhal AB. Symmetric CTA collaterals identify patients with slow-progressing stroke likely to benefit from late thrombectomy. Radiology. 2022;302(2):400–407. doi: 10.1148/radiol.2021210455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pirson FAVA, Hinsenveld WH, Goldhoorn R-JB, et al. MR CLEAN-LATE, a multicenter randomized clinical trial of endovascular treatment of acute ischemic stroke in The Netherlands for late arrivals: study protocol for a randomized controlled trial. Trials. 2021;22(1):160. doi: 10.1186/s13063-021-05092-0 [DOI] [PMC free article] [PubMed] [Google Scholar]