Abstract
Background:
Gestational diabetes mellitus (GDM) is a common pregnancy complication that increases the risk of adverse maternal and perinatal outcomes. While experts recommend universal GDM screening, there is no consensus about which of two clinically recommended screening approaches to use.
Methods:
We performed a pragmatic randomized trial comparing 1-step fasting 75g oral glucose tolerance testing (OGTT) with 2-step screening (non-fasting 50g glucose challenge, followed by 100g OGTT if positive) among all pregnant women treated in 2 health systems. Primary outcomes were GDM diagnosis, large-for-gestational-age infants; a perinatal composite consisting of stillbirth, neonatal death, shoulder dystocia, bone fracture, or any upper extremity nerve palsy related to birth injury; gestational hypertension/preeclampsia; and primary cesarean section.
Results:
A total of 23,792 women were randomized. Adherence to randomization was 66% in the 1-step arm and 92% in the 2-step arm. GDM incidence was 16.5% among women randomized to the 1-step approach, versus 8.5% with the 2-step approach [unadjusted relative risk (RR)=1.94, 95% CI 1.79-2.11]. In intention to treat analyses, there were no significant differences between groups in any primary outcome [large for gestational age: 8.9% vs. 9.2%, RR(95%CI) 0.95 (0.87-1.05); perinatal composite: 3.1% vs. 3.0%, 1.04 (0.88-1.23); gestational hypertension/preeclampsia: 13.6% vs. 13.5%, 1.00 (0.93-1.08); primary c-section: 24.0% vs. 24.7%, 0.98 (0.93-1.02)]. Results were materially unchanged in inverse-probability weighted intention-to-treat analyses accounting for differential adherence to screening approaches.
Conclusions:
Despite a doubling in the incidence of GDM diagnosis with the 1-step approach, there were no significant between-group differences in the risks of any primary outcome.
INTRODUCTION
Gestational diabetes (GDM), one of the most common complications of pregnancy,1,2 affects 6-25% of pregnant women (depending on diagnostic criteria)3,4 and is associated with increased risk of stillbirth and neonatal death, as well as multiple serious morbidities for both the mother and baby.1 Fetal overgrowth from GDM is associated with increased risk for birth trauma (e.g., brachial plexus injury or clavicular fracture) and of cesarean section (c-section) to avoid such trauma.1,5 Universal GDM screening is recommended at 24-28 weeks’ gestation,6 as there is randomized controlled trial evidence that GDM treatment improves maternal and perinatal outcomes.7,8
There is no scientific consensus on how best to diagnose GDM. Expert professional organizations acknowledge two acceptable options: the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) 1-step screening approach (currently preferred by the American Diabetes Association), and the 2-step Carpenter-Coustan screening approach (recommended by the American College of Obstetricians and Gynecologists); both organizations note the need for additional evidence related to outcomes.1,9 Each approach has advantages and disadvantages.1,9 The 1-step approach involves a 2-hour oral glucose tolerance test (OGTT) for all participants: while screening and diagnosis can be completed in a single visit, all women must fast before screening and make time for a 2-hour visit. The 2-step approach includes an initial non-fasting 1-hour glucose challenge test, which is logistically simpler for patients, and can easily be done as part of a scheduled prenatal visit; most women do not require further screening. However, approximately 20% of patient fail this screening and must return for a 3-hour fasting diagnostic OGTT.10,11 The methods also have different diagnostic cutoffs: the 1-step approach identifies women with milder hyperglycemia as having GDM. Although there is a clear linear relationship between maternal hyperglycemia and maternal and perinatal outcomes,12 the effects of identifying and treating milder cases of GDM on these outcomes are not known.1,2,10 The National Institutes of Health 2013 GDM consensus conference recommended that a randomized trial compare these approaches with respect to clinically important outcomes.10
We conducted a pragmatic randomized clinical trial (RCT), ScreenR2GDM, among pregnant women receiving care at Kaiser Permanente Northwest and Hawaii , to compare rates of GDM diagnosis and maternal and neonatal outcomes with the 1-step versus 2-step approach to screening and diagnosis of GDM.
METHODS
Trial Design and Oversight
The ScreenR2GDM design and population characteristics were published previously.13 All pregnant women treated at Kaiser Permanente Northwest and Hawaii were randomized to either 1-step or 2-step GDM screening and diagnosis. Institutional Review Boards at both institutions approved the RCT including waivers for individual consent; the rationale was that both approaches are minimal risk and clinically recommended, and thus waiving consent would not adversely affect patients’ rights or welfare, as long as providers could retain clinical judgment to decide whether to adhere to randomization. A Data Safety Monitoring Board provided study oversight (See Supplemental Appendix [SA] Section S2.1). and conducted one mid-trial data review.13 All authors were involved in implementing the trial, gathering data, the decision to publish, and gave critical intellectual feedback interpreting analyses and revising the manuscript; the second author (KP) analyzed the data; the first and second authors (TH and KP) vouch for the accuracy of all data and analyses including the fidelity of the report to the protocol and statistical analysis plan. The protocol and statistical analysis plan are available at NEJM.org.
Randomization
All pregnancies were randomly assigned to the 1-step or 2-step approach (1:1 ratio) at their first prenatal visit using an electronically-generated random assignment procedure; this assignment was presented to the provider within the electronic medical record at the time of ordering GDM screening (typically 24-28 weeks’ gestation).13 If screening was ordered more than once, the same assigned test was presented to providers each time. Randomization was implemented independently within each region’s EMR system on May 28, 2014 in Kaiser Permanente Northwest (first patient enrolled June 3, 2014) and on July 7, 2014 in Kaiser Permanente Hawaii. Randomization continued in both regions through December 31, 2017; outcomes were collected through delivery (2014-2018).
Owing to the pragmatic trial design, providers could not be blinded to randomization. All investigators and study staff, except statisticians, were blinded to all trial data except overall adherence rates until randomization was completed for all patients.
GDM Screening and Diagnosis Approaches
The 1-step approach consisted of a fasting 75g 2-hour OGTT. Women were diagnosed with GDM if any of the following glucose thresholds were met: fasting ≥ 92 mg/dl; 1hr ≥ 180 mg/dl; 2hr ≥ 153 mg/dl.9 In the 2-step approach, the first (screening) step was a nonfasting 50g, 1-hour glucose challenge test (GCT).1 Women with GCT ≥ 200 mg/dL are considered to have GDM and do not undergo further testing.11 Women with a positive GCT (≥130 mg/dl at Kaiser Permanente Northwest; ≥140mg/dl at Kaiser Permanente Hawaii) below 200 mg/dl underwent diagnostic 100g 3-hour OGTT. GDM was diagnosed if two or more of four glucose thresholds were met: fasting ≥ 95 mg/dl; 1hr ≥ 180 mg/dl; 2hr ≥ 155 mg/dl; and/or 3hr ≥ 140 mg/dl).1 GDM treatment was based on the same national practice guidelines regardless of screening approach1,14 (see SA Section S2.5).
Study Outcomes
We prespecified 5 primary outcomes (not listed in order of importance) based on prior research.7,8,12 These included : GDM diagnosis; large-for-gestational age (LGA; birthweight > 90th percentile) infants;7,8,12,15 a composite measure of perinatal outcomes (stillbirth, neonatal death, shoulder dystocia, bone fracture, and/or any upper extremity nerve palsy related to birth injury);8 primary c-section;7,8,12 and gestational hypertension/preeclampsia1,7,8,16 (see Table S1 for variable definitions).
Secondary outcomes were incidence of macrosomia (>4,000g);7,8 small for gestational age (SGA; birthweight ≤ 10th percentile) infants;7,8,15 maternal GDM requiring insulin or oral hypoglycemic treatment;1 neonatal respiratory distress;7,8 neonatal jaundice requiring treatment;7 neonatal hypoglycemia;7,8 and the individual components of the composite perinatal outcome8 (See Table S1 for definitions). Neonatal hypoglycemia screening practices were consistent with the American Academy of Pediatrics guidelines that advise screening neonates with risk factors for hypoglycemia within 24 hours of birth;17,18 newborn screening in both regions is done by a heel stick with point of care glucose testing in the delivery room or nursery. Safety outcomes were neonatal sepsis, neonatal intensive care unit (NICU) admission, pre-term birth (<37 and <32 weeks’ gestation), and induction of labor (See Table S1 for definitions). Primary, secondary and safety outcomes were assessed for subgroups of participants diagnosed with GDM (prespecified) and unscreened (post hoc analyses).
Statistical Methods
We originally estimated a sample size of 17,626 pregnancies in order to provide 80% power to detect a relative between-group difference of 20% for all primary outcomes except the composite perinatal outcome, for which we would be powered to detect a 40% difference, at a two-sided significance level of 0.05. However, early monitoring of randomization fidelity revealed that at both sites, a higher proportion of those randomized to 1-step screening received 2-step screening than the reverse.13 Providers reported that this was partly due to efforts to ensure screening by conducting the non-fasting 2-step GCT at a prenatal visit. Given the pragmatic nature of this trial, the research team was unable to enforce strict adherence to randomization. Accordingly, we modified our protocol to continue the trial until adequate sample size had been achieved among those receiving the 1-step approach,13 and to include additional statistical analyses to account for non-adherence (see below and SA Section S2.11.1).13,19,20
We estimated relative risks of each primary outcome between the two study arms using generalized linear log-binomial models with adjustment for correlated errors due to multiple pregnancies per woman. The Quasi-likelihood information criterion was used to confirm working correlation structure and variable selection.21
Planned ITT analyses used an unadjusted model comparing pregnancy outcomes between randomly assigned groups, as well as models adjusting for GDM diagnosis, group-by-diagnosis interaction, and other covariates that may modify the relationship of group with each outcome including excessive gestational weight gain based on National Academy of Medicine guidelines,22,23 as this is independently related to several outcomes.24–26 The GDM by group interaction was not significant for any outcome. Thus final adjusted models included GDM, pre-planned covariates, and factors related to non-adherence. To further account for non-adherence to randomized assignment, we conducted inverse probability (IP) weighted ITT analyses,19,20 in which pregnancies were assigned stabilized weights based on modeled probability of adhering to the assigned approach (See Figure 1).13
Figure 1: Consort Diagram of Randomization of GDM Screening Methods and Analytical Comparison Groups.
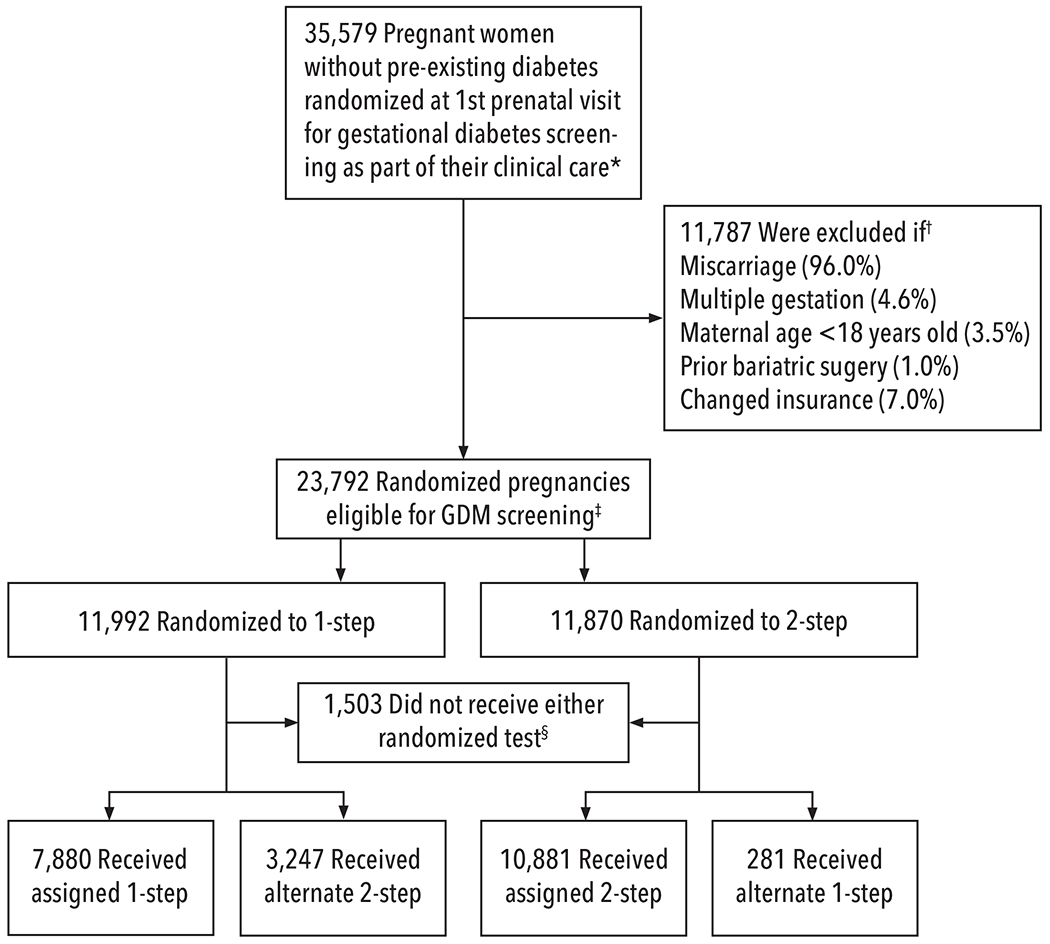
☆All pregnancies were randomized to 1-step or 2-step GDM screening strategies within the EMR as part of clinical care at their first prenatal visit. The 1 step (75g 2 hour OGTT) approach diagnosed GDM based on IADPSG criteria,9 and the 2-step screening approach by Carpenter and Coustan criteria.1
†Percentages do not add up to 100% as some pregnancies met multiple exclusion criteria. The major reason for exclusion was miscarriage (as randomization occurred at the 1st prenatal visit, in many cases this visit also determined non-viability, or miscarriage, on the same day of randomization and before any GDM screening was ordered); terminations were also included in this exclusion category. Change of insurance during pregnancy was an exclusion as we were unable to evaluate outcomes in these pregnancies.
‡Intention-to-treat analyses (ITT) were planned. Due to unanticipated lower adherence to the fasting 1-step at both sites, we continued randomizing until we enrolled enough pregnancies with 1-step screening to have adequate statistical power, and conducted additional analyses - inverse probability (IP) weighted ITT - both with and without adjustment for factors related to non-adherence.13,19,20 Factors related to lower adherence included both maternal and provider characteristics as well as provider reliance on non-fasting tests to ensure that GDM screening was completed at a visit.13 These pragmatic barriers to adherence could not be adequately addressed without putting patients at risk of not receiving GDM screening.
§Among the 1,503 pregnancies that did not receive either 1-step or 2-step screening, 1,450 (6.1%) were unscreened (778 [6.5%] randomized to 1-step and 672 (5.7%) randomized to 2-step), and these pregnancies presented on average at a mean of 18.9 weeks’ gestation compared to 10.5 weeks for pregnancies with screening. There were also 53 pregnancies that had other clinically recommended screening in the first trimester (HbA1c or FPG)9 but did not have either randomized GDM screening approach.
We also conducted sensitivity analyses, including multiple imputation, to account for missing data (Section S2.11.3 and Tables S3–S4). Our statistical analysis plan (SAP) pre-specified 97.5% confidence intervals for relative risks of primary outcomes; these are reported here. For secondary or other outcomes, we report 95% confidence intervals. Because the widths of confidence intervals have not been adjusted to account for the multiplicity of outcomes assessed, these should not be used to infer definitive effects of one versus the other screening approach. All analyses were performed using SAS Statistical Analysis System version 9.4 (SAS Institute, Cary, NC).
RESULTS
Trial Population
Overall, 23,792 eligible pregnancies were randomized to 1-step or 2-step GDM screening (see Figure 1); 94% of those eligible completed GDM screening. Adherence to the randomized arm was 66% in the 1-step arm and 92% in the 2-step arm. Baseline characteristics of the 2 groups are presented in Table 1.
Table 1.
Characteristics of Study Population
| Randomized Group☆ | ||
|---|---|---|
| Characteristic | 1-step | 2-step |
| n=11922 | n=11870 | |
| Maternal Age in years: Mean (SD) | 29.4 (5.5) | 29.3 (5.5) |
| Body Mass Index at 1st prenatal visit: mean (SD) † | 27.4 (6.7) | 27.6 (7.0) |
| Pre-pregnancy Obesity† (BMI≥30 kg/m2): n (%) | 2527 (26.6) | 2615 (27.7) |
| Medicaid: n (%) | 1914 (16.1) | 1810 (15.3) |
| Race/Ethnicity: n (%) ‡ | ||
| Caucasian | 6608 (55.4) | 6586 (55.5) |
| Asian | 1789 (15.0) | 1782 (15.0) |
| Native Hawaiian/Pacific Islander | 623 (5.2) | 619 (5.2) |
| Black | 329 (2.8) | 328 (2.8) |
| American Indian | 49 (0.4) | 50 (0.4) |
| Multiple Races | 1317 (11.1) | 1310 (11.0) |
| Other | 40 (0.3) | 42 (0.4) |
| Unknown | 1167 (9.8) | 1153 (9.7) |
| Study Site Region: n (%) | ||
| Northwest | 8203 (68.8) | 8140 (68.6) |
| Hawaii | 3719 (31.2) | 3730 (31.4) |
| Nulliparous: n (%) | 3642 (30.6) | 3616 (30.5) |
| Prior Hypertension: n (%) | 948 (8.0) | 976 (8.2) |
| Prior GDM: n (%) | 636 (5.3) | 637 (5.4) |
| Gestational Weight Gain exceeding NAM guidelines: n (%) § | 4160 (45.0) | 4255 (46.6) |
Randomized Group - compares pregnancies randomized to 1-step vs those randomized to 2-step
BMI was assessed at first OB visit (mean gestation = 10.9 weeks); six pregnancies did not have these measurements; Pre-pregnancy obesity is BMI≥30kg/m2 based on weight. Pre-pregnancy weight was most recent pre-pregnancy weight measurement up to 3 months prior to estimated date of conception, or (if not available) the earliest weight measurement during pregnancy (up to 12 weeks of gestation)
Hispanic ethnicity was reported for 11,859 pregnancies (49.8%); 1,343 (22.4%) pregnancies randomized to 1-step and 1,280 (21.8%) pregnancies randomized to 2-step reported Hispanic ethnicity
5420 patients were missing either pre-pregnancy weight or weight at delivery needed to calculate this measure
Primary Outcomes
GDM was diagnosed in 16.5% of pregnancies randomized to the 1-step approach and 8.5% randomized to the 2-step approach (RR=1.94, 95% CI 1.79-2.11). In ITT analyses, the incidences of other primary outcomes did not significantly differ between those randomized to 1-step vs. 2-step [LGA: 8.9% vs. 9.2%, RR(95%CI) 0.95 (0.87-1.05) ; perinatal composite: 3.1% vs. 3.0%, 1.04 (0.88-1.23) ; gestational hypertension/preeclampsia: 13.6% vs. 13.5%, 1.00 (0.93-1.08); primary c-section: 24.0% vs. 24.7%, 0.98 (0.93-1.02)], even after adjustment for GDM, pre-planned, and adherence-related covariates (Table 2). Results of the IP-weighted analyses were similar to those of the ITT analyses (Table 2).
Table 2.
Primary Outcomes: Incidence and Relative Risk for 1-step vs 2-step GDM Screening
| Randomized Group | Pre-Planned Intention to Treat Analyses☆ | IP Weighted ITT† Analyses | ||||
|---|---|---|---|---|---|---|
| 1-step N=11922 | 2-step N=11870 | Unadjusted | Adjusted for GDM | Adjusted for GDM, pre-planned covariates and factors related to non-adherence‡ | Adjusted for GDM, pre-planned covariates and factors related to non-adherence‡ | |
| n (%) | n (%) | RR (97.5% CI)§ | RR (97.5% CI)§ | RR (97.5% CI)§ | RR (97.5% CI)§ | |
| Gestational diabetes ¶ ‖ | 1837 (16.5) | 945 (8.5) | 1.94 (1.79 - 2.11) | NA (NA) | 1.93 (1.77 - 2.11) | 1.93 (1.76 - 2.12) |
| Large for gestational age ☆☆ ‖ | 977 (8.9) | 1015 (9.2) | 0.95 (0.87 - 1.05) | 0.93 (0.84 - 1.03) | 0.94 (0.85 - 1.04) | 0.92 (0.83 - 1.02) |
| Perinatal composite †† ‖ | 351 (3.1) | 337 (3.0) | 1.04 (0.88 - 1.23) | 1.08 (0.90 - 1.30) | 1.08 (0.89 - 1.31) | 1.10 (0.91 - 1.35) |
| Gestational hypertension/preeclampsia ‡‡ ‖ | 1490 (13.6) | 1472 (13.5) | 1.00 (0.93 - 1.08) | 0.96 (0.88 - 1.03) | 0.98 (0.90 - 1.06) | 0.98 (0.90 - 1.06) |
| Primary cesarean section §§ ‖ | 2826 (24.0) | 2887 (24.7) | 0.98 (0.93 - 1.02) | 0.95 (0.91 - 1.00) | 0.96 (0.91 - 1.02) | 0.96 (0.91 - 1.02) |
Intention to Treat - compares pregnancies randomized to 1-step vs those randomized to 2-step
IP Weighted analyses were conducted to account for the non-adherence to randomized screening test. Stabilized weights were derived from modeling the probability of adhering to the randomly assigned screening test (details in SA Section S2.11)
Pre-planned covariates include race/ethnicity, pre-pregnancy obesity and exceeding NAM weight gain guidelines.22,23 Factors related to non-adherence include maternal age, nulliparity, race, Medicaid insurance, prior GDM, pre-existing hypertension, site, maternal obesity at 1st prenatal visit, provider type, and randomized group
Widths of confidence intervals have not been adjusted to account for multiplicity and cannot be used to infer treatment effects
Denominator is 11,127 for 1-step and 11,162 for 2-step. GDM is diagnosed by the 1-step approach (fasting 75g OGTT; IADPSG criteria), 9 and by Carpenter & Coustan criteria for the 2-step approach.1 Pregnancies that did not receive either screening approach were 795 for 1-step and 708 for 2-step
Denominators vary based on ascertainment method for each outcome. GDM includes only those pregnancies that received one of the two screening strategies. The maternal outcome of gestational hypertension/preeclampsia includes women without pre-existing hypertension prior to pregnancy. Primary cesarean section excludes women who left the health plan prior to delivery (167 for 1-step and 156 for 2-step). The perinatal outcomes include pregnancies for which the information was available in the maternal record (stillbirth and shoulder dystocia) or in newborn records that were matched to maternal records. Reasons for unmatched newborn records include: adopted infants, deliveries within and outside the health-plan where newborn is covered by other insurance, deliveries outside the health-plan and no reimbursement for newborn care is requested, mother left the health plan prior to delivery and no information is available for newborn. Total number of unmatched newborn records were 702 for 1-step and 709 for 2-step. Of the pregnancies with a diagnosis of shoulder dystocia in the maternal record, but no matching newborn record, 30 were 1-step and 21 were 2-step. Of the pregnancies with a diagnosis of stillbirth in the maternal record, but no matching newborn record, 32 were 1-step and 31 were 2-step. For LGA, in addition to excluding unmatched newborn records, birthweight was not available for 192 of 1-step and 175 of 2-step
Denominator is 11,028 for 1-step and 10,986 for 2-step
Denominator is 11,281 for 1-step and 11,213 for 2-step. Perinatal composite was any of the following: stillbirth, neonatal death, shoulder dystocia, bone fracture or nerve palsy.8
Denominator is 10,974 for 1-step and 10,894 for 2-step
Denominator is 11,755 for 1-step and 11,714 for 2-step
Secondary, Safety, and Subgroup Outcomes
There were no significant differences between groups in any secondary or safety outcomes. (Table 3).
Table 3.
Pre-planned Secondary and Safety Outcomes
| Randomized Group | Relative Risk (95% CI)☆ for 1-step vs 2-step | ||
|---|---|---|---|
| 1-step | 2-step | ||
| Secondary Outcomes | n (%) | n (%) | |
| Macrosomia (>4,000g)†‡ | 1178 (11.4) | 1186 (11.5) | 0.99 (0.91-1.06) |
| Small for gestational age§‡ | 937 (8.5) | 892 (8.1) | 1.05 (0.96-1.14) |
| Maternal GDM requiring insulin or oral hypoglycemic treatment¶‡ | 783 (42.6) | 431 (45.6) | 0.93 (0.87-1.03) |
| Neonatal respiratory distress‖‡ | 225 (2.0) | 227 (2.0) | 0.99 (0.82-1.18) |
| Neonatal jaundice requiring treatment‖‡ | 478 (4.3) | 476 (4.3) | 1.00 (0.88-1.13) |
| Neonatal hypoglycemia‖‡ | 1034 (9.2) | 838 (7.5) | 1.23 (1.12-1.34) |
| Components of Perinatal Composite Outcome | |||
| Stillbirth☆☆‡ | 56 (0.5) | 64 (0.6) | 0.87 (0.61-1.25) |
| Neonatal Death‖‡ | 7 (0.1) | 12 (0.1) | 0.58 (0.23-1.47) |
| Shoulder Dystocia††‡ | 239 (2.1) | 223 (2.0) | 1.07 (0.89-1.28) |
| Bone Fracture‖‡ | 59 (0.5) | 42 (0.4) | 1.40 (0.94-2.07) |
| Nerve Palsy‖‡ | 14 (0.1) | 15 (0.1) | 0.93 (0.45-1.92) |
| Safety Outcomes | |||
| Neonatal Sepsis‖‡ | 46 (0.4) | 38 (0.3) | 1.20 (0.78-1.85) |
| NICU Admits‖‡ | 526 (4.7) | 473 (4.2) | 1.11 (0.98-1.25) |
| Pre-term Birth (<37 weeks)‖‡ | 716 (6.4) | 711 (6.4) | 1.00 (0.91-1.11) |
| Pre-term Birth (<32 weeks)‖‡ | 118 (1.1) | 125 (1.1) | 0.94 (0.73-1.21) |
| Induction of Labor‡ ‡‡ | 3675 (31.3) | 3670 (31.3) | 1.00 (0.96-1.04) |
Widths of confidence intervals have not been adjusted to account for multiplicity and cannot be used to infer treatment effects
Denominator is 10,312 for 1-step and 10,275 for 2-step
Denominators vary based on ascertainment method for each outcome. The perinatal outcomes include pregnancies for which the information was available in the maternal record (stillbirth and shoulder dystocia) or in newborn records that were matched to maternal records. Reasons for unmatched newborn records include: adopted infants, deliveries within and outside the health-plan where newborn is covered by other insurance, deliveries outside the health-plan and no reimbursement for newborn care is requested, mother left the health plan prior to delivery and no information is available for newborn. Total number of unmatched newborn records were 702 for 1-step and 709 for 2-step. Of the pregnancies with a diagnosis of shoulder dystocia in the maternal record, but no matching newborn record, 30 were 1-step and 21 were 2-step. Of the pregnancies with a diagnosis of stillbirth in the maternal record, but no matching newborn record, 32 were 1-step and 31 were 2-step. For macrosomia and SGA, in addition to excluding unmatched newborn records, birthweight was not available for 192 of 1-step and 175 of 2-step. Further, macrosomia is estimated in newborns with gestational age >36 weeks. Maternal GDM requiring insulin includes only those pregnancies with GDM. Induction of labor excludes women who left the health plan prior to delivery (167 for 1-step and 156 for 2-step)
Denominator is 11,028 for 1-step and 10,986 for 2-step
Denominator is 1837 for 1-step and 945 for 2-step. Of the 1214 GDM pregnancies with A2class GDM requiring medication, 1092 (90.0%) received insulin and 64 (5.3%) received oral medication; 58 (4.8%) received both
Denominator is 11,220 for 1-step and 11,161 for 2-step
Denominator is 11,252 for 1-step and 11,192 for 2-step
Denominator is 11,250 for 1-step and 11,182 for 2-step
Denominator is 11,755 for 1-step and 11,714 for 2-step
There were no significant differences in outcomes between groups in a prespecified analysis limited to women diagnosed with GDM (Table S7). In 39% of 1-step GDM cases, diagnosis was based on isolated fasting plasma glucose (FPG) alone, and half of these cases met criteria by an isolated FPG in the 92-94 mg/dl range, meaning that their glucose levels at diagnosis were within treatment goals (FPG <95 mg/dl)1. Among women with GDM, percentages of women treated with insulin or hypoglycemic medication were similar for the 1-step versus 2-step approach (42.6% vs 45.6%, respectively; Table 3).
Baseline information and outcomes of pregnancies without any GDM screening (post hoc subgroup, n=1,450) are shown in Tables S8 and S9).
DISCUSSION
In this pragmatic head-to-head RCT of the two clinically recommended GDM screening approaches,1,9 there were no significant differences in maternal or perinatal outcomes between pregnancies (n=23,792) randomized to receive 1-step or 2-step screening as part of their clinical care, despite twice as many women having been diagnosed with GDM by the 1 -step, versus 2-step approach. There was lower adherence screening by the fasting 1-step approach, but results were similar in analyses accounting for differences in adherence.
Our finding that 16.5% of women were diagnosed with GDM by the 1-step approach is consistent with prior research using the same criteria.4,9 RCT evidence showing a benefit of GDM treatment is limited to trials using the 2-step approach;7,8 no previous studies have addressed whether or not treating more women based on the 1-step approach yields better outcomes. While we did not find increased harms associated with diagnosing and treating many more many women with the 1-step approach, some retrospective observational cohort studies have found higher rates of primary cesarean delivery27 and neonatal hypoglycemia28 with 1-step screening following conversion from 2-step protocols, with no substantive improvement in outcomes.27–29 In addition to potential harms, the burden to individual women of GDM diagnoses by these milder criteria, and the burden to the system of treating many more women, should be considered. On the other hand, some studies have found that maternal GDM may be a risk factor for childhood obesity and metabolic sequelae, so treating more women could potentially have long-term benefits.30–32 However, other studies have failed to find associations between maternal GDM and long-term child outcomes.33,34.
Limitations of our trial should be noted. The lower adherence to the 1-step approach resulted in a bias to planned ITT analyses.13 To address this, we extended the trial and conducted additional IP-weighted ITT analyses;19,20 our identification of prognostic factors associated with adherence would be expected to increase validity of the IP-weighted ITT analyses. However, these statistical methods may not fully account for potential differences due to non-adherence. Another potential limitation of our study was that the sites used slightly different GCT thresholds to determine whether 2-step patients should receive OGTT; both thresholds (130 mg dl or 140 mg/dl) are clinically recommended,1,9 see SA Section 2.5 for more details.
We randomized assignment to screening approach as part of clinical care; our research team did not have control over what occurred after the randomized screening test was presented to the clinical provider, including whether the provider would order the test and what clinical care patients would receive following screening. This head-to-head design compares outcomes in a “real-world” clinical setting in which virtually the entire population of these study sites was included, and we would expect results to be generalizable to similar settings. Owing to the overall racial/ethnic makeup of these regions, African American and Native American women are not well-represented in the study sample. Given the pragmatic nature of the trial, we did not blind providers to the approach to screening and diagnosis, and we cannot rule out the possibility that provider awareness of the approach affected some outcomes. An ongoing randomized trial (NCT02309138) involving 921women whose diagnosis of GDM is based on 2-step testing using either IADPSG or Carpenter and Coustan criteria, in which providers remain blinded to the criteria used, is expected to provide more information on outcomes according to diagnostic criteria for GDM).35,36
In summary, in this large randomized trial, 1-step screening, as compared with 2-step screening, doubled the incidence of GDM diagnosis but did not result in lower risks of LGA, adverse perinatal outcomes, primary c-section, or gestational hypertension/preeclampsia.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the volunteer Data Safety & Monitoring Board (DSMB) members for their helpful RCT guidance: Jodi Lapidus, PhD (DSMB Chair); Aaron B. Caughey, MD, MPH, PhD; and Jeanne-Marie Guise, MD, MPH. We also thank Neon Brooks, PhD for editorial support; Robin Daily for administrative support; Stacey Honda, MD, PhD for her role as medical liaison with KP-Hawaii; and Nancy Perrin, PhD for statistical guidance and review during study planning and DSMB data review.
SUPPORT STATEMENT
This work was supported by a grant award R01HD074794 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (to TH). Neither the funding source nor the authors’ institutions (where the study was conducted) had any involvement in the study design; the collection, analysis or interpretation of data; the writing of the report; or in the decision to submit the article for publication.
Footnotes
(ClinicalTrials.gov identifier NCT02266758; Eunice Kennedy Shriver National Institute of Child Health and Human Development)
REFERENCES
- 1.Committee on Practice Bulletins—Obstetrics. ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol 2018;131:e49–e64. [DOI] [PubMed] [Google Scholar]
- 2.Landon MB. Changing the Diagnostic Criteria for Gestational Diabetes Mellitus? Obstet Gynecol 2016;127:3–6. [DOI] [PubMed] [Google Scholar]
- 3.Diabetes During Pregnancy. (Accessed 07/06/2020, at https://www.cdc.gov/reproductivehealth/maternalinfanthealth/diabetes-during-pregnancy.htm.)
- 4.Sacks DA, Hadden DR, Maresh M, et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care 2012;35:526–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moyer VA. Screening for gestational diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:414–20. [DOI] [PubMed] [Google Scholar]
- 6.Final Update Summary: Gestational Diabetes Mellitus, Screening. 2014. (Accessed 02/01/2019, at https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/gestational-diabetes-mellitus-screening.)
- 7.Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009;361:1339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;325:2477–86. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020;43:S14–s31. [DOI] [PubMed] [Google Scholar]
- 10.Vandorsten JP, Dodson WC, Espeland MA, et al. NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements 2013;29:1–31. [PubMed] [Google Scholar]
- 11.Hillier TA, Ogasawara KK, Pedula KL, Vesco KK. Markedly different rates of incident insulin treatment based on universal gestational diabetes mellitus screening in a diverse HMO population. Am J Obstet Gynecol 2013;209:440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002. [DOI] [PubMed] [Google Scholar]
- 13.Pedula KL, Hillier TA, Ogasawara KK, et al. A randomized pragmatic clinical trial of gestational diabetes screening (ScreenR2GDM): Study design, baseline characteristics, and protocol adherence. Contemp Clin Trials 2019;85:105829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Diabetes Association. 14. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020;43:S183–s92. [DOI] [PubMed] [Google Scholar]
- 15.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy. Hypertension in Pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122:1122–31. [DOI] [PubMed] [Google Scholar]
- 17.Committee on Fetus and Newborn, Adamkin DH. Postnatal glucose homeostasis in late-preterm and term infants. Pediatrics 2011;127:575–9. [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Pediatrics. AAP publications reaffirmed or retired. Pediatrics 2015;136:e730. [Google Scholar]
- 19.Hernán MA, Robins JM. Causal Inference: What If. Boca Raton: Chapman & Hall/CRC; 2020. [Google Scholar]
- 20.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–60. [DOI] [PubMed] [Google Scholar]
- 21.Pan W Akaike’s information criterion in generalized estimating equations. Biometrics 2001;57:120–5. [DOI] [PubMed] [Google Scholar]
- 22.Institute of Medicine (IOM) and National Research Council (NRC). Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, D.C.: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 23.American College of Obstetricians and Gynecologists. ACOG Committee opinion no. 548: weight gain during pregnancy. Obstet Gynecol 2013;121:210–2. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein RF, Abell SK, Ranasinha S, et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA 2017;317:2207–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kominiarek MA, Saade G, Mele L, et al. Association Between Gestational Weight Gain and Perinatal Outcomes. Obstet Gynecol 2018;132:875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogozińska E, Zamora J, Marlin N, et al. Gestational weight gain outside the Institute of Medicine recommendations and adverse pregnancy outcomes: analysis using individual participant data from randomised trials. BMC Pregnancy Childbirth 2019;19:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldman RK, Tieu RS, Yasumura L. Gestational Diabetes Screening: The International Association of the Diabetes and Pregnancy Study Groups Compared With Carpenter-Coustan Screening. Obstet Gynecol 2016;127:10–7. [DOI] [PubMed] [Google Scholar]
- 28.Kong JM, Lim K, Thompson DM. Evaluation of the International Association of the Diabetes In Pregnancy Study Group new criteria: gestational diabetes project. Can J Diabetes 2015;39:128–32. [DOI] [PubMed] [Google Scholar]
- 29.Meloncelli NJL, Barnett AG, DʼEmden M, De Jersey SJ. Effects of Changing Diagnostic Criteria for Gestational Diabetes Mellitus in Queensland, Australia. Obstet Gynecol 2020;135:1215–21. [DOI] [PubMed] [Google Scholar]
- 30.Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: The ongoing effects of maternal hyperglycemia. Diabetes Care 2007;30:2287–92. [DOI] [PubMed] [Google Scholar]
- 31.Scholtens DM, Kuang A, Lowe LP, et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Glycemia and Childhood Glucose Metabolism. Diabetes Care 2019;42:381–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowe WL, Jr Scholtens DM, Kuang A, et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): Maternal Gestational Diabetes Mellitus and Childhood Glucose Metabolism. Diabetes Care 2019;42:372–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landon MB, Mele L, Varner MW, et al. The relationship of maternal glycemia to childhood obesity and metabolic dysfunction(‡). J Matern Fetal Neonatal Med 2020;33:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gillman MW, Oakey H, Baghurst PA, Volkmer RE, Robinson JS, Crowther CA. Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diabetes Care 2010;33:964–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scifres CM, Abebe K, Simhan HN, et al. 187-OR: Randomized Clinical Trial of the IADPSG vs. Carpenter Coustan Criteria for Diagnosis of Gestational Diabetes Mellitus Diabetes 2020;69 187-OR. [Google Scholar]
- 36.Comparison of Two Screening Strategies for Gestational Diabetes (GDM2) (GDM2). 2014. (Accessed 06/03/2020, at https://clinicaltrials.gov/ct2/show/NCT02309138)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


