Abstract
Sulfone-substituted bicyclo[1.1.0]butanes and housanes have found widespread application in organic synthesis due to their bench stability and high reactivity in strain-releasing processes in the presence of nucleophiles or radical species. Despite their increasing utility, their preparation typically requires multiple steps in low overall yield. In this work, we report an expedient and general one-pot procedure for the synthesis of 1-sulfonylbicyclo[1.1.0]butanes from readily available methyl sulfones and inexpensive epichlorohydrin via the dialkylmagnesium-mediated formation of 3-sulfonylcyclobutanol intermediates. Furthermore, the process was extended to the formation of 1-sulfonylbicyclo[2.1.0]pentane (housane) analogues when 4-chloro-1,2-epoxybutane was used as the electrophile instead of epichlorohydrin. Both procedures could be applied on a gram scale with similar efficiency and are shown to be fully stereospecific in the case of housanes when an enantiopure epoxide was employed, leading to a streamlined access to highly valuable optically active strain-release reagents.
The use of highly strained cyclic substrates in ring-expansion or ring-opening processes constitutes a powerful tool for the elaboration of complex and biologically relevant molecules.1 In particular, “strain-release” (also known as “spring-loaded”) reagents, defined as highly strained (>50 kcal/mol) bi- or tricyclic molecules that react via cleavage of their bridging bond resulting in the release of strain,2,3 have become increasingly prominent in recent years. Those include bicyclo[1.1.0]butanes (BCB),2b,4 bicyclo[2.1.0]pentanes (housanes),5 tricyclo[1.1.1.01,3]pentanes (TCP or propellanes),6 and 1-azabicyclo[1.1.0]butanes (ABB),7 leading to the formation of functionalized cyclobutanes, cyclopentanes, bicyclo[1.1.1]pentanes, and azetidines, respectively, following the strain-release process. Some of these moieties are also found directly in natural products8 or utilized in medicinal chemistry for the introduction of bioisosteres.9 More specifically, sulfonyl-substituted BCB and housanes are exceptionally bench-stable and have found widespread applicability in strain-release cycloalkylation with either nucleophiles10 or radical species11 as well as in formal cycloaddition,12 where the resulting sulfonyl group serves as a handle for further derivatization (Scheme 1a, A).13–15 Moreover, in the case of BCB, their structure can be readily diversified via sulfone-directed C–H functionalization (B).10b,c,16,17 While their superior versatility as strain-release reagents is well established, the general access to 1-sulfonylbicyclo[1.1.0]butanes and housanes from inexpensive and readily available materials remains a challenge, typically requiring six steps and two purifications from sulfonyl chlorides in the case of BCB (Scheme 1b).10d,e,18,19 As part of our research program directed at the elaboration and use of reagents of extreme strain such as cyclopropanones,20 we discovered that these strain-release reagents could be accessed in a streamlined manner via the formation of 3-sulfonylcyclobutanol intermediates susceptible to transannular ring-closure upon activation (vide infra). Herein, we report a general procedure for the preparation of 1-sulfonyl-bicyclo[1.1.0]butanes in a single pot from readily available methyl sulfones and inexpensive epichlorohydrin (Scheme 1c). Key to the success of this transformation is the use of a commercially available dialkylmagnesium solution as a “double base” in the initial stage, as well as the careful choice of activating reagent for the 3-sulfonylcyclobutanol intermediate.
Scheme 1.

Syntheses and Applications of Sulfonyl-Substituted BCB and Housane Reagents
Through modification of the reaction conditions, a second procedure was developed allowing extension of the process to 1-sulfonylbicyclo[2.1.0]pentanes (housanes) when 4-chloro-1,2-epoxybutane was used as the electrophile instead, which is shown to be fully stereospecific and thus furnish a streamlined access to enantioenriched housanes.10e Both one-pot procedures can be applied on a gram scale with similar efficiency and utilize minimal amounts of readily available reagents. Considering the importance of 1-sulfonylbicyclo[1.1.0]butanes and housanes as reagents in organic synthesis, the accelerated routes described herein should find widespread use in a variety of fields relying on strain-release processes for the rapid and efficient elaboration of biologically relevant molecules.
Related to our previous work involving the formation of chiral 1-sulfonylcyclopropanols as modular precursors of cyclopropanones,20a our initial studies targeted the formation of a 2-chloromethyl-substituted sulfonylcyclopropane from a methyl sulfone and enantioenriched epichlorohydrin (eq 1).
 |
(1) |
In addition to the expected cyclopropane, the corresponding bicyclo[1.1.0]butane was observed in 24% yield via a 3-sulfonylcyclobutanol21 capable of transannular nucleophilic substitution upon activation with MsCl. A similar cyclobutanol intermediate was previously reported to be accessible from epichlorohydrin using n-BuLi in excess to form an α,α-dilithiated sulfone as effective nucleophile.21a,22 To ensure the generality of the method, readily available and electron neutral phenyl methyl sulfone (1a) was selected as a model substrate for further optimization studies, leading to BCB 2a (Table 1).
Table 1.
Optimization of the One-Pot Synthesis of Bicyclobutanes

| ||||
|---|---|---|---|---|
|
| ||||
| entry | base 1 (equiv) | RSO2Cl (equiv) | base 2 (equiv) | yield (%)a |
| 1 | n-BuLi (1) | MsCl (1) | n-BuLi (1) | 14 |
| 2 | Bu2Mg (1) | MsCl (1) | n-BuLi (1) | 32 |
| 3 | Bu2Mg (1) | NsClb (1) | n-BuLi (1) | <5 |
| 4 | Bu2Mg (1) | PhSO2Cl (1) | n-BuLi (1) | 62 |
| 5c | Bu2Mg (1) | PhSO2Cl (1) | KOt-Bu (3) | 45 |
| 6c | Bu2Mg (1) | PhSO2Cl (1) | NaH (1.2) | <5 |
| 7 | Bu2Mg (1) | PhSO2Cl (1.3) | n-BuLi (1.2) | 77d |
Yield determined by 1H NMR using 1,3,5-trimethoxybenzene as internal standard.
NsCl: 4-O2N–C6H4SO2Cl.
Third stage run at 0 °C to rt.
Isolated yield = 77%.
Applying the conditions depicted above to 1a led to a decreased yield of 14% (entry 1), along with the undesired 2-chloromethyl-substituted sulfonylcyclopropane in a 1:1 ratio.23 In order to favor the formation of the desired sulfonylcyclobutanol intermediate, it was reasoned that at least two equivalents of base should be employed in the first stage prior to alcohol activation.21 Commercially available n-Bu2Mg, acting as a “double base”, was rapidly identified as ideal for this purpose when added in stoichiometric amount (entry 2). Benzenesulfonyl chloride proved to be superior as activating agent (entries 2–4), whereas the use of n-BuLi in the final transannular SN2 led to an increased yield compared with other bases evaluated such as KOt-Bu or NaH (entries 4–6). After slight adjustments in the reagents’ stoichiometry, the optimized conditions directly led to 2a in 77% isolated yield in one-pot from 1a (entry 7) and could be applied on a gram scale with similar efficiency (Scheme 2). The procedure is shown to be general for a wide range of methyl sulfones, which are either commercially available or readily prepared in one step by thioanisole oxidation or Cu-catalyzed cross-coupling of aryl iodides with NaSO2Me.23 Notably, racemic epichlorohydrin employed here as a key building block can be purchased at very low cost from most vendors ($0.04/g). In the case of brominated BCB 2j, the amount of n-BuLi used in the last stage had to be reduced to 1 equiv to minimize the formation of debrominated product formed via lithium–halogen exchange. While most bicyclo[1.1.0]butanes synthesized here could be accessed in one-pot, heterocyclic derivatives 2m and 2n and aliphatic analogues 2o and 2p required an aqueous workup after the second stage, with KOt-Bu used as an optimal base for the final transannular nucleophilic substitution.
Scheme 2. Scope of Accessible Bicyclo[1.1.0]butanesa.
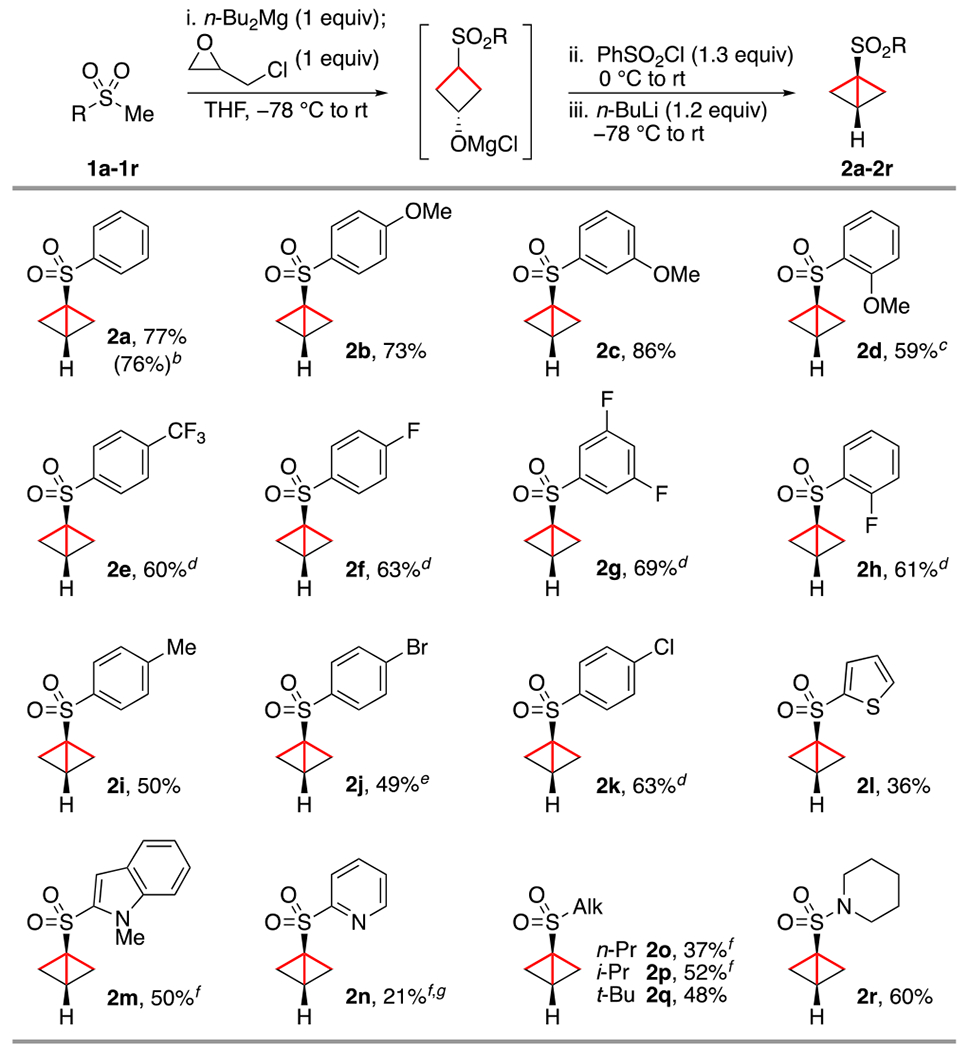
aIsolated yields from 1a–1r on a 0.5 mmol scale. bIsolated yield on a 1 g scale (6.4 mmol) 1a in parentheses. ct-BuLi (1.2 equiv) was used in the third stage. dFirst and third stages were performed at −78 to −20 or 0 °C. e1.0 equiv of n-BuLi was used in the third stage. fKOt-Bu (1.5–2.5 equiv) was used in the third stage after aqueous workup. gTHF/HMPA (9:1) was used as solvent instead of pure THF.
Importantly, application of this procedure to substituted epichlorohydrin derivatives directly led to bicyclobutanes 2s and 2t from methyl sulfone 1a (Scheme 3). 1-Substituted epichlorohydrins, which would have also led to BCB derivatives such as 2t, were found to be incompatible under these conditions due to their poor electrophilicity and lack of regiocontrol in the initial epoxide opening step of the sequence.23
Scheme 3. Direct Access to Substituted BCB Derivativesa.

aIsolated yields from 1a on a 0.5 mmol scale.
Adjustments in the reaction conditions led to extension of the method to the analogous production of sulfonyl-substituted housanes 3a–3l (Scheme 4),23 a class of strain-release reagents previously demonstrated by Baran and co-workers as highly versatile in cyclopentylation reactions.10d,e In this case, the addition of HMPA as a cosolvent was found to significantly improve the yield, with commercially available (n-Bu)(s-Bu)Mg used as the initial base instead of n-Bu2Mg. Moreover, t-BuLi was employed as a base in the last stage instead of n-BuLi to avoid the formation of inseparable impurities.23 In general, the observed yields of housane products 3a–3l were found to be slightly lower than the corresponding BCB, mostly due to incomplete reaction and competitive elimination pathways occurring on the activated 3-sulfonylcyclopentanol intermediates involved.24 Racemic 4-chloro-1,2-epoxybutane used here as the electrophile can be prepared on multigram scale in two simple steps from 3-butenol.23 Kinetic resolution using Jacobsen’s Co(III)-catalyzed hydrolytic procedure25 affords access to a virtually enantiopure epoxide. Employing this chiral reagent in our one-pot procedure leads the rapid production of highly enantioenriched housanes, as demonstrated here with 3e and 3g, previously shown to constitute superior strain-release reagents in stereospecific cyclopentylation reactions (Scheme 5).10e In order to ensure the stereospecificity of the overall process, the procedure had to be slightly modified where a shorter reaction time was employed in the second stage to minimize competitive intermolecular substitution events, leading to partial epimerization of the activated 3-sulfonylcyclopentanol intermediate.
Scheme 4. Scope of Accessible Bicyclo[2.1.0]pentanesa.

aIsolated yields from 1a–1h, 1k–1l, and 1q–1r on a 0.5 mmol scale. bIsolated yield on a 1 g scale (6.4 mmol) 1a in parentheses. cFirst stage was performed at −20 °C (then 0 °C, 1 h) and third stage at −78 to −20 °C. dn-Bu2Mg (1 equiv) was used at −20 °C in the first stage instead of (n-Bu)(s-Bu)Mg (without HMPA).
Scheme 5. Stereospecific Synthesis of Chiral Housanesa.

aIsolated yields from 1e or 1g on a 0.25 mmol scale.
In summary, we report one-pot procedures for the streamlined synthesis of highly valuable 1-sulfonylbicyclo[1.1.0]butanes and housanes from readily available methyl sulfones. Both reactions can be performed on a gram scale with similar efficiency, and the use of enantiopure 4-chloro-1,2-epoxybutane is shown to afford access to highly enantioenriched housanes via a stereospecific pathway. Considering the bench stability and established versatility of sulfonyl-substituted strain-release reagents in a wide variety of processes such as cycloalkylation with nucleophiles10 or radical intermediates,11 this general approach should find widespread utility in the elaboration and functionalization of biologically relevant molecules.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the NIH (R35GM142965). All nuclear magnetic resonance (NMR) spectroscopy and high-resolution mass spectrometry (HRMS) measurements were performed by the Molecular Education, Technology, and Research Innovation Center (METRIC) at NC State University, which is supported by the State of North Carolina.
Funding
National Institutes of Health (R35GM142965).
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/jacs.2c00923
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.2c00923.
Additional experimental details, spectroscopic data, materials and methods; 1H and 13C NMR spectra for all compounds (PDF)
The authors declare no competing financial interest.
Contributor Information
Myunggi Jung, Department of Chemistry, North Carolina State University, Raleigh, North Carolina 27695, United States.
Vincent N. G. Lindsay, Department of Chemistry, North Carolina State University, Raleigh, North Carolina 27695, United States.
REFERENCES
- (1).(a) Liebman JF; Greenberg A A survey of strained organic molecules. Chem. Rev 1976, 76, 311–365. [Google Scholar]; (b) Wiberg KB The Concept of Strain in Organic Chemistry. Angew. Chem., Int. Ed. Engl 1986, 25, 312–322. [Google Scholar]; (c) Liebman JF; Greenberg A Survey of the heats of formation of three-membered-ring species. Chem. Rev 1989, 89, 1225–1246. [Google Scholar]; (d) Advances in Strain in Organic Chemistry; Halton B, Ed.; JAI Press: London, 1997. [Google Scholar]; (e) Dudev T; Lim C Ring Strain Energies from ab Initio Calculations. J. Am. Chem. Soc 1998, 120, 4450–4458. [Google Scholar]; (f) Advances in Strained and Interesting Organic Molecules; Halton B, Ed.; JAI Press: Stamford, CT, 2020. [Google Scholar]; (g) Khoury PR; Goddard JD; Tam W Ring strain energies: substituted rings, norbornanes, norbornenes and norbornadienes. Tetrahedron 2004, 60, 8103–8112. [Google Scholar]; (h) Bach RD; Dmitrenko O The Effect of Carbonyl Substitution on the Strain Energy of Small Ring Compounds and Their Six-Member Ring Reference Compounds. J. Am. Chem. Soc 2006, 128, 4598–4611. [DOI] [PubMed] [Google Scholar]; (i) Schneider TF; Kaschel J; Werz DB A New Golden Age for Donor–Acceptor Cyclopropanes. Angew. Chem., Int. Ed 2014, 53, 5504–5523. [DOI] [PubMed] [Google Scholar]; (j) Pirenne V; Muriel B; Waser J Catalytic Enantioselective Ring-Opening Reactions of Cyclopropanes. Chem. Rev 2021, 121, 227–263. [DOI] [PubMed] [Google Scholar]
- (2).For reviews on the synthesis and applications of strain-release reagents, see:; (a) Turkowska J; Durka J; Gryko D Strain release – an old tool for new transformations. Chem. Commun 2020, 56, 5718–5734. [DOI] [PubMed] [Google Scholar]; (b) Wiberg KB; Lampman GM; Ciula RP; Connor DS; Schertler P; Lavanish J Bicyclo[1.1.0]butane. Tetrahedron 1965, 21, 2749–2769. [Google Scholar]; (c) Hoz S Chapter 19 -Bicyclo[1.1.0]butane. In The Chemistry of the Cyclopropyl Group; Rappoport Z, Ed.; Wiley: New York, 1987; Vol. 1, pp 1121–1191. . [Google Scholar]; (d) Delia EW; Lochert IJ Synthesis of Biudgehead-Substituted Bicyclo[1.1.1]pentanes. A Review. Org. Prep. Proced. Int 1996, 28, 411–441. [Google Scholar]; (e) Bartnik R; Marchand AP Synthesis and Chemistry of Substituted 1-Azabicyclo[1.1.0]butanes. Synlett 1997, 1997, 1029–1039. [Google Scholar]; (f) Levin MD; Kaszynski P; Michl J Bicyclo[1.1.1]pentanes, [n]Staffanes, [1.1.1]Propellanes, and Tricyclo[2.1.0.02,5]pentanes. Chem. Rev 2000, 100, 169–234. [DOI] [PubMed] [Google Scholar]; (g) Ramazanov IR; Yaroslavova AV; Dzhemilev UM Russ. Chem. Rev 2012, 81, 700–728. [Google Scholar]; (h) Walczak MAA; Krainz T; Wipf P Ring-Strain-Enabled Reaction Discovery: New Heterocycles from Bicyclo[1.1.0]butanes. Acc. Chem. Res 2015, 48, 1149–1158. [DOI] [PubMed] [Google Scholar]; (i) Dilmaç AM; Spuling E; de Meijere A; Bräse S Propellanes—From a Chemical Curiosity to “Explosive” Materials and Natural Products. Angew. Chem., Int. Ed 2017, 56, 5684–5718. [DOI] [PubMed] [Google Scholar]; (j) Kanazawa J; Uchiyama M Recent Advances in the Synthetic Chemistry of Bicyclo[1.1.1]pentane. Synlett 2019, 30, 1–11. [Google Scholar]; (k) Ma X; Nhat Pham L Selected Topics in the Syntheses of Bicyclo[1.1.1]Pentane (BCP) Analogues. Asian J. Org. Chem 2020, 9, 8–22. [Google Scholar]; (l) Pramanik MMD; Qian H; Xiao W-J; Chen J-R Photoinduced strategies towards strained molecules. Org. Chem. Front 2020, 7, 2531–2537. [Google Scholar]; (m) Anderson JM; Measom ND; Murphy JA; Poole DL Bridge Functionalisation of Bicyclo[1.1.1]pentane Derivatives. Angew. Chem., Int. Ed 2021, 60, 24754–24769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).For structural analyses of strain-release reagents, see:; (a) Suenram RD; Harmony MD Microwave Spectrum, Structure, and Dipole Moment of Bicyclo[2.1.0]pentane. J. Chem. Phys 1972, 56, 3837–3842. [Google Scholar]; (b) Irngartinger H; Lukas KL Bonding in Bicyclo[1.1.0]butane Derivatives. Angew. Chem., Int. Ed. Engl 1979, 18, 694–695. [Google Scholar]; (c) Gassman PG; Greenlee ML; Dixon DA; Richtsmeier S; Gougoutas JZ X-ray and theoretical analysis of the relationship between substituent steric effects and the structure of bicyclo[1.1.0]butane. The unexpected flexibility of the bicyclo[1.1.0]butane skeleton. J. Am. Chem. Soc 1983, 105, 5865–5874. [Google Scholar]; (d) Ioffe AI; Svyatkin VA; Nefedov OM Molecular-mechanical analysis of the structure of strained organic molecules. 6. [m.n.k]-Propellanes. Russ. Chem. Bull 1988, 37, 1827–1836. [Google Scholar]; (e) Saettel NJ; Wiest O Sterically Crowded Bicyclo[1.1.0]butane Radical Cations. J. Org. Chem 2003, 68, 4549–4552. [DOI] [PubMed] [Google Scholar]; (f) Polo V; Andres J; Silvi B New insights on the bridge carbon–carbon bond in propellanes: A theoretical study based on the analysis of the electron localization function. J. Comput. Chem 2007, 28, 857–864. [DOI] [PubMed] [Google Scholar]; (g) Sterling A; Smith R; Anderson E; Duarte F Beyond strain release: Delocalisation-enabled organic reactivity. ChemRxiv 2021, DOI: 10.26434/chemrxiv-2021-n0xm9-v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).(a) Lemal DM; Menger F; Clark GW Bicyclobutane. J. Am. Chem. Soc 1963, 85, 2529–2530. [Google Scholar]; (b) Srinivasan R A Simple Synthesis of Bicyclo[1.1.0]butane and its Relation to the Internal Conversion of Electronic Energy in 1,3-Butadiene. J. Am. Chem. Soc 1963, 85, 4045–4046. [Google Scholar]; (c) Frey HM; Stevens IDR Carbenoid Decomposition of Cyclopropanecarboxaldehyde Tosylhydrazone: Formation of Bicyclobutane. Proc. Chem. Soc. (London) 1964, 144. [Google Scholar]; (d) Bayless J; Friedman L; Smith JA; Cook FB; Shechter H Intramolecular Reactions of Cyclopropylcarbinyl, Cyclobutyl, and Allylcarbinyl Cationic Systems. J. Am. Chem. Soc 1965, 87, 661–663. [Google Scholar]; (e) Lampman GM; Aumiller JC Bicyclo[1.1.0]butane. Org. Synth 1971, 51, 55. [Google Scholar]; (f) Chang MH; Dougherty DA 2,3-Diazabicyclo-[2.1.1]hex-2-ene. Synthesis and thermal decomposition. J. Org. Chem 1981, 46, 4092–4093. [Google Scholar]
- (5).Criegee R; Rimmelin A Darstellung und Eigenschaften von Bicyclo-[0.1.2]-Pentan. Chem. Ber 1957, 90, 414–417. [Google Scholar]
- (6).(a) Wiberg KB; Walker FH [1.1.1]Propellane. J. Am. Chem. Soc 1982, 104, 5239–5240. [Google Scholar]; (b) Semmler K; Szeimies G; Belzner J Tetracyclo[5.1.0.01,6.02,7]octane, a [1.1.1]propellane derivative, and a new route to the parent hydrocarbon. J. Am. Chem. Soc 1985, 107, 6410–6411. [Google Scholar]; (c) Lopchuk JM; Baran PS [1.1.1]Propellane. In Encyclopedia of Reagents for Organic Synthesis, 2nd ed.; Charette AB, Crich D, Fuchs PL, Eds.; John Wiley & Sons Ltd.: Chichester, 2017. [Google Scholar]
- (7).(a) Hortmann AG; Robertson DA Azabicyclobutanes. Synthesis of 3-phenyl-1-azabicyclo[1.1.0]butane. J. Am. Chem. Soc 1967, 89, 5974–5975. [Google Scholar]; (b) Funke W Synthesis and Properties of 1-Azabicyclo[1.1.0]butanes. Angew. Chem., Int. Ed. Engl 1969, 8, 70–71. [Google Scholar]; (c) Funke W Über Synthesen und Reaktionen von 1-Azabicyclo[1.1.0]butanen. Chem. Ber 1969, 102, 3148–3158. [Google Scholar]; (d) Hortmann AG; Robertson DA 1-Azabicyclobutanes. Synthesis and reactions. J. Am. Chem. Soc 1972, 94, 2758–2765. [Google Scholar]
- (8).(a) Schneider C; Niisuke K; Boeglin WE; Voehler M; Stec DF; Porter NA; Brash AR Enzymatic synthesis of a bicyclobutane fatty acid by a hemoprotein–lipoxygenase fusion protein from the cyanobacterium Anabaena PCC 7120. Proc. Nat. Acad. Sci 2007, 104, 18941–18945. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) DeGuire SM; Ma S; Sulikowski GA Synthesis of a Bicyclobutane Fatty Acid Identified from the Cyanobacterium Anabaena PCC 7120. Angew. Chem., Int. Ed 2011, 50, 9940–9942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).(a) Mikhailiuk PK; Afonin S; Chernega AN; Rusanov E; Platonov MO; Dubinina GG; Berditsch M; Ulrich AS; Komarov IV Conformationally Rigid Trifluoromethyl-Substituted α-Amino Acid Designed for Peptide Structure Analysis by Solid-State 19F NMR Spectroscopy. Angew. Chem., Int. Ed 2006, 45, 5659–5661. [DOI] [PubMed] [Google Scholar]; (b) Stepan AF; Subramanyam C; Efremov IV; Dutra JK; O’Sullivan TJ; DiRico KJ; McDonald WS; Won A; Dorff PH; Nolan CE; Becker SL; Pustilnik LR; Riddell DR; Kauffman GW; Kormos BL; Zhang L; Lu Y; Capetta SH; Green ME; Karki K; Sibley E; Atchison KP; Hallgren AJ; Oborski CE; Robshaw AE; Sneed B; O’Donnell CJ Application of the Bicyclo[1.1.1]pentane Motif as a Nonclassical Phenyl Ring Bioisostere in the Design of a Potent and Orally Active γ-Secretase Inhibitor. J. Med. Chem 2012, 55, 3414–3424. [DOI] [PubMed] [Google Scholar]; (c) Measom ND; Down KD; Hirst DJ; Jamieson C; Manas ES; Patel VK; Somers DO Investigation of a Bicyclo[1.1.1]pentane as a Phenyl Replacement within an LpPLA2 Inhibitor. ACS Med. Chem. Lett 2017, 8, 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Locke GM; Bernhard SSR; Senge MO Nonconjugated Hydrocarbons as Rigid-Linear Motifs: Isosteres for Material Sciences and Bioorganic and Medicinal Chemistry. Chem.—Eur. J 2019, 25, 4590–4647. [DOI] [PubMed] [Google Scholar]; (e) Tse EG; Houston SD; Williams CM; Savage GP; Rendina LM; Hallyburton I; Anderson M; Sharma R; Walker GS; Obach RS; Todd MH Nonclassical Phenyl Bioisosteres as Effective Replacements in a Series of Novel Open-Source Antimalarials. J. Med. Chem 2020, 63, 11585–11601 [DOI] [PubMed] [Google Scholar]; (f) Talele TT Opportunities for Tapping into Three-Dimensional Chemical Space through a Quaternary Carbon. J. Med. Chem 2020, 63, 13291–13315. [DOI] [PubMed] [Google Scholar]; For an example of chemoselective bioconjugation using a BCB reagent, see:; Zhang P; Zhuang R; Wang X; Liu H; Li J; Su X; Chen X; Zhang X Highly Efficient and Stable Strain-Release Radioiodination for Thiol Chemoselective Bioconjugation. Bioconjugate Chem. 2018, 29, 467–472. [DOI] [PubMed] [Google Scholar]
- (10).(a) Gaoni Y Conjugate addition of organocopper reagents to 1-arylsulfonylbicyclobutanes. synthesis of the racemic form of the sex pheromone of the citrus mealybug, Planococcus citri (Risso). Tetrahedron Lett. 1982, 23, 5215–5218. [Google Scholar]; (b) Gaoni Y; Tomazic A; Potgieter E Stereochemistry of addition of organocopper reagents and of the hydride ion to 1-(arylsulfonyl)bicyclo[1.1.0]butanes. J. Org. Chem 1985, 50, 2943–2947. [Google Scholar]; (c) Gaoni Y; Tomazic A Bridgehead reactivity, nucleophilic and radical additions, and lithium aluminum hydride reduction of 1-(arylsulfonyl)bicyclobutanes: general access to substituted, functionalized cyclobutanes. Syntheses of (±)-citrilol acetate, (±)-junionone, and the tricyclo[3.3.0.01,4]octane and tricyclo[4.3.0.01,7]nonane ring systems. J. Org. Chem 1985, 50, 2948–2957. [Google Scholar]; (d) Gianatassio R; Lopchuk JM; Wang J; Pan C-M; Malins LR; Prieto L; Brandt TA; Collins MR; Gallego GM; Sach NW; Spangler JE; Zhu H; Zhu J; Baran PS Strain-release amination. Science 2016, 351, 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Lopchuk JM; Fjelbye K; Kawamata Y; Malins LR; Pan C-M; Gianatassio R; Wang J; Prieto L; Bradow J; Brandt TA; Collins MR; Elleraas J; Ewanicki J; Farrell W; Fadeyi OO; Gallego GM; Mousseau JJ; Oliver R; Sach NW; Smith JK; Spangler JE; Zhu H; Zhu J; Baran PS Strain-Release Heteroatom Functionalization: Development, Scope, and Stereospecificity. J. Am. Chem. Soc 2017, 139, 3209–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Tokunaga K; Sato M; Kuwata K; Miura C; Fuchida H; Matsunaga N; Koyanagi S; Ohdo S; Shindo N; Ojida A Bicyclobutane Carboxylic Amide as a Cysteine-Directed Strained Electrophile for Selective Targeting of Proteins. J. Am. Chem. Soc 2020, 142, 18522–18531. [DOI] [PubMed] [Google Scholar]; (g) Kerner MJ; Wipf P Semipinacol-Type Rearrangements of [3-(Arylsulfonyl)bicyclo[1.1.0]butan-1-yl]alkanols. Org. Lett 2021, 23, 3615–3619. [DOI] [PubMed] [Google Scholar]
- (11).(a) Wu X; Hao W; Ye K-Y; Jiang B; Pombar G; Song Z; Lin S Ti-Catalyzed Radical Alkylation of Secondary and Tertiary Alkyl Chlorides Using Michael Acceptors. J. Am. Chem. Soc 2018, 140. 14836–14843. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Pratt CJ; Aycock RA; King MD; Jui NT Radical α-C–H Cyclobutylation of Aniline Derivatives. Synlett 2020, 31, 51–54. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ociepa M; Wierzba AJ; Turkowska J; Gryko D Polarity-Reversal Strategy for the Functionalization of Electrophilic Strained Molecules via Light-Driven Cobalt Catalysis. J. Am. Chem. Soc 2020, 142, 5355–5361. [DOI] [PubMed] [Google Scholar]; (d) Ernouf G; Chirkin E; Rhyman L; Ramasami P; Cintrat J-C Photochemical Strain-Release-Driven Cyclobutylation of C(sp3)-Centered Radicals. Angew. Chem., Int. Ed 2020, 59, 2618–2622. [DOI] [PubMed] [Google Scholar]; (e) Wierzba AJ; Gryko DT; Gryko D Acylation of electrophilic bicyclo[1.1.0]butanes via Co/Ni-catalyzed reductive cross-coupling. J. Porphyrins Phthalocyanines 2021, 25, 630–638. [Google Scholar]
- (12).(a) Clementson S; Radaelli A; Fjelbye K; Tanner D; Jessing M Strain-Release Driven Cycloadditions for Rapid Construction of Functionalized Pyridines and Amino Alcohols. Org. Lett 2019, 21, 4763–4766. [DOI] [PubMed] [Google Scholar]; For analogous cycloaddition disconnections with carboxylic derivatives, see:; (b) Dhake K; Woelk K; Becica J; Un A; Jenny S; Leitch D Modular Synthesis of Azabicyclohexanes and Cyclobutenyl Amines. September 1, 2021. ChemRxiv 2021, DOI: 10.33774/chemrxiv-2021-p0fzf. [DOI] [PubMed] [Google Scholar]; (c) Schwartz BD; Smyth AP; Nashar PE; Gardiner MG; Malins LR Investigating Bicyclobutane–Triazolinedione Cycloadditions as a Tool for Peptide Modification. Org. Lett 2022, 24, 1268–1273. [DOI] [PubMed] [Google Scholar]
- (13).For strain-release processes using other BCB or housane reagents, see:; (a) Hall HK; Blanchard EP; Cherkofsky SC; Sieja JB; Sheppard WA Synthesis and polymerization of 1-bicyclobutanecarbonitriles. J. Am. Chem. Soc 1971, 93, 110–120. [Google Scholar]; (b) Jamieson C; Walton JC; Ingold KU Radical reactions of bicyclo[2.1.0]pentane. J. Chem. Soc. Perkin Trans 1980, 2, 1366–1371. [Google Scholar]; (c) Hoz S; Livneh M; Cohen D Cyclobutane-bicyclobutane system. 11. Mechanism and stereochemistry of general acid-catalyzed additions to bicyclobutane. J. Org. Chem 1986, 51, 4537–4544. [Google Scholar]; (d) Hoz S; Livneh M; Cohen D Cyclobutane-bicyclobutane system. Part 13. Bromination of bicyclobutanes: a possible case of an electron-transfer mechanism. J. Am. Chem. Soc 1987, 109, 5149–5156. [Google Scholar]; (e) Drujon X; Riess G; Hall HK; Padias AB Synthesis and polymerization of alkyl 1-bicyclobutanecarboxylates. Macromolecules 1993, 26, 1199–1205. [Google Scholar]; (f) Weber J; Haslinger U; Brinker UH 1-Bromobicyclo[1.1.0]butane as an Easily Obtainable C4-Building Block: A Novel Route to Cyclobutanone. J. Org. Chem 1999, 64, 6085–6086. [Google Scholar]; (g) Vasin VA; Petrov PS; Kalyazin VA; Razin VV Chemo-, regio-, and stereoselectivity in acid-catalyzed hydromethoxylation of tricyclo[4.1.0.02,7]hept-1-yl and 7-methyltricyclo[4.1.0.02,7]hept-1-yl phenyl sulfones. Russ. J. Org. Chem 2010, 46, 812–819. [Google Scholar]; (h) Panish R; Chintala SR; Boruta DT; Fang Y; Taylor MT; Fox JM Enantioselective Synthesis of Cyclobutanes via Sequential Rh-catalyzed Bicyclobutanation/Cu-catalyzed Homoconjugate Addition. J. Am. Chem. Soc 2013, 135, 9283–9286. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Panish RA; Chintala SR; Fox JM A Mixed-Ligand Chiral Rhodium(II) Catalyst Enables the Enantioselective Total Synthesis of Piperarborenine B. Angew. Chem., Int. Ed 2016, 55, 4983–4987. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Milligan JA; Busacca CA; Senanayake CH; Wipf P Hydrophosphination of Bicyclo[1.1.0]butane-1-carbonitriles. Org. Lett 2016, 18, 4300–4303. [DOI] [PubMed] [Google Scholar]; (k) Fawcett A; Biberger T; Aggarwal VK Carbopalladation of C–C σ-bonds enabled by strained boronate complexes. Nat. Chem 2019, 11, 117–122. [DOI] [PubMed] [Google Scholar]; (l) Silvi M; Aggarwal VK Radical Addition to Strained σ-Bonds Enables the Stereocontrolled Synthesis of Cyclobutyl Boronic Esters. J. Am. Chem. Soc 2019, 141, 9511–9515. [DOI] [PubMed] [Google Scholar]; (m) Schwartz BD; Zhang MY; Attard RH; Gardiner MG; Malins LR Structurally Diverse Acyl Bicyclobutanes: Valuable Strained Electrophiles. Chem.—Eur. J 2020, 26, 2808–2812. [DOI] [PubMed] [Google Scholar]; (n) Bennett SH; Fawcett A; Denton EH; Biberger T; Fasano V; Winter N; Aggarwal VK Difunctionalization of C–C σ-Bonds Enabled by the Reaction of Bicyclo[1.1.0]butyl Boronate Complexes with Electrophiles: Reaction Development, Scope, and Stereochemical Origins. J. Am. Chem. Soc 2020, 142, 16766–16775. [DOI] [PubMed] [Google Scholar]; (o) Guo L; Noble A; Aggarwal VK α-Selective Ring-Opening Reactions of Bicyclo[1.1.0]butyl Boronic Ester with Nucleophiles. Angew. Chem., Int. Ed 2021, 60, 212–216. [DOI] [PubMed] [Google Scholar]
- (14).For carbene insertions into BCB or housane reagents, see:; (a) Hall HK; Smith CD; Blanchard EP; Cherkofsky SC; Sieja JB Synthesis and polymerization of bridgehead-substituted bicyclobutanes. J. Am. Chem. Soc 1971, 93, 121–130. [Google Scholar]; (b) Applequist DE; Wheeler JW Synthesis of 1,3-disubstituted bicyclo[1.1.1]pentanes. Tetrahedron Lett. 1977, 18, 3411–3412. [Google Scholar]; (c) Applequist DE; Renken TL; Wheeler JW Polar substituent effects in 1,3-disubstituted bicyclo[1.1.1]pentanes. J. Org. Chem 1982, 47, 4985–4995. [Google Scholar]; (d) Rablen PR; Paiz AA; Thuronyi BW; Jones M Computational Investigation of the Mechanism of Addition of Singlet Carbenes to Bicyclobutanes. J. Org. Chem 2009, 74, 4252–4261. [DOI] [PubMed] [Google Scholar]; (e) Bychek RM; Hutskalova V; Bas YP; Zaporozhets OA; Zozulya S; Levterov VV; Mykhailiuk PK Difluoro-Substituted Bicyclo[1.1.1]pentanes for Medicinal Chemistry: Design, Synthesis, and Characterization. J. Org. Chem 2019, 84, 15106–15117. [DOI] [PubMed] [Google Scholar]; (f) Ma X; Sloman DL; Han Y; Bennett DJ A Selective Synthesis of 2,2-Difluorobicyclo[1.1.1]pentane Analogues: “BCP-F2”. Org. Lett 2019, 21, 7199–7203. [DOI] [PubMed] [Google Scholar]; (g) Ma X; Pinto W; Pham LN; Sloman DL; Han Y Synthetic Studies of 2,2-Difluorobicyclo[1.1.1]pentanes (BCP-F2): The Scope and Limitation of Useful Building Blocks for Medicinal Chemists. Eur. J. Org. Chem 2020, 2020, 4581–4605. [Google Scholar]
- (15).For other strain-release rearrangements of BCB or housane reagents, see:; (a) Halberstadt ML; Chesick JP The Kinetics of the Thermal Isomerization of [2.1.0]Bicyclopentane. J. Am. Chem. Soc 1962, 84, 2688–2691. [Google Scholar]; (b) Steel C; Zand R; Hurwitz P; Cohen SG Small Ring Bicyclic Azo Compounds and Bicyclic Hydrocarbons. Isomerization of Bicyclo [2.1.0.] pentane and Bicyclo [2.2.0] hexane. J. Am. Chem. Soc 1964, 86, 679–684. [Google Scholar]; (c) Dauben WG; Kielbania AJ Transition metal catalyzed valence isomerizations of tricyclo [4.1.0.02,7]heptane. Evidence for an organometallic intermediate. J. Am. Chem. Soc 1972, 94, 3669–3671. [Google Scholar]; (d) Gassman P; Atkins T Transition Metal Complex Promoted Rearrangements. Tricyclo-[4.1.0.02,7]heptane and 1-Methyltricyclo[4.1.0.0.2,7]heptane. J. Am. Chem. Soc 1972, 94, 7748–7756. [Google Scholar]; (e) Wipf P; Stephenson CRJ; Okumura K Transition-Metal-Mediated Cascade Reactions: C,C-Dicyclopropylmethylamines by Way of Double C,C-σ-Bond Insertion into Bicyclobutanes. J. Am. Chem. Soc 2003, 125, 14694–14695. [DOI] [PubMed] [Google Scholar]; (f) Wipf P; Walczak MAA Pericyclic Cascade Reactions of (Bicyclo[1.1.0]butylmethyl)amines. Angew. Chem., Int. Ed 2006, 45, 4172–4175. [DOI] [PubMed] [Google Scholar]; (g) Qin C; Davies HML Enantioselective Synthesis of 2-Arylbicyclo[1.1.0]butane Carboxylates. Org. Lett 2013, 15, 310–313. [DOI] [PubMed] [Google Scholar]
- (16).(a) Gaoni Y New bridgehead-substituted 1-(arylsulfonyl)-bicyclo[1.1.0]butanes and some novel addition reactions of the bicyclic system. Tetrahedron 1989, 45, 2819–2840. [Google Scholar]; (b) McNamee RE; Haugland MM; Nugent J; Chan R; Christensen KE; Anderson EA Synthesis of 1,3-disubstituted bicyclo[1.1.0]butanes via directed bridgehead functionalization. Chem. Sci 2021, 12, 7480–7485. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) McNamee RE; Thompson AL; Anderson EA Synthesis and Applications of Polysubstituted Bicyclo[1.1.0]butanes. J. Am. Chem. Soc 2021, 143, 21246–21251. [DOI] [PubMed] [Google Scholar]
- (17).For other examples of functionalization of metallated bicyclo[1.1.0]butane intermediates, see:; (a) Kottirsch G; Szeimies G Nickel(0)-katalysierte Kupplung von (Tricyclo[4.1.0.02,7]hept-1-yl)-magnesiumbromid und verwandten Grignard-Verbindungen mit Aryl-, Vinyl- und Alkinylhalogeniden. Chem. Ber 1990, 123, 1495–1506. [Google Scholar]; (b) Stephen A; Hashmi K; Vollmer A; Szeimies G Nickel-catalyzed cross-coupling reactions of bicyclo[1.1.0]butylmagnesium bromide and bicyclo[1.1.0]butyllithium derivatives with alkynyl chlorides and bromides. Liebigs Ann. 1995, 1995, 471–475. [Google Scholar]
- (18).Gaoni Y Preparation of ring-substituted (arylsulfonyl)-cyclopropanes and (arylsulfonyl)bicyclobutanes from γ,δ-epoxy sulfones. J. Org. Chem 1982, 47, 2564–2571. [Google Scholar]
- (19).For the synthesis of other types of BCB and housanes, see refs 2b, 4, 5 and:; (a) Wiberg KB; Ciula RP Ethyl Bicyclo[1.1.0]butane-1-carboxylate. J. Am. Chem. Soc 1959, 81, 5261–5262. [Google Scholar]; (b) Rifi MR Electrochemical preparation of bicyclobutanes and other strained cycloalkanes. J. Am. Chem. Soc 1967, 89, 4442–4445. [Google Scholar]; (c) Sieja JB Bicyclo[1.1.0]butanes from ketene and vinyl ethers. J. Am. Chem. Soc 1971, 93, 130–136. [Google Scholar]; (d) Nilsen NO; Skattebøl L; Baird MS; Buxton SR; Slowey PD A simple route to 1-bromobicyclo[1.1.0]butanes by intramolecular trapping of 1-bromo-1-lithiocyclopropanes. Tetrahedron Lett. 1984, 25, 2887–2890. [Google Scholar]; (e) Hoz S; Livneh M Conversion of cyclobutane to bicyclobutane by base-catalyzed 1,3-dehydrohalogenation reaction: a mechanistic study. J. Am. Chem. Soc 1987, 109, 7483–7488. [Google Scholar]; (f) Bentley TW; Engels B; Hupp T; Bogdan E; Christl M Unsubstituted Bicyclo[1.1.0]but-2-ylcarbinyl Cations. J. Org. Chem 2006, 71, 1018–1026. [DOI] [PubMed] [Google Scholar]; (g) Kelly CB; Colthart AM; Constant BD; Corning SR; Dubois LNE; Genovese JT; Radziewicz JL; Sletten EM; Whitaker KR; Tilley LJ Enabling the Synthesis of Perfluoroalkyl Bicyclobutanes via 1,3 γ-Silyl Elimination. Org. Lett 2011, 13, 1646–1649. [DOI] [PubMed] [Google Scholar]; (h) Chen K; Huang X; Kan SBJ; Zhang RK; Arnold FH Enzymatic construction of highly strained carbocycles. Science 2018, 360, 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).(a) Poteat CM; Jang Y; Jung M; Johnson JD; Williams RG; Lindsay VNG Enantioselective Synthesis of Cyclopropanone Equivalents and Application to the Formation of Chiral β-Lactams. Angew. Chem., Int. Ed 2020, 59, 18655–18661. [DOI] [PubMed] [Google Scholar]; (b) Machín Rivera R; Jang Y; Poteat CM; Lindsay VNG General Synthesis of Cyclopropanols via Organometallic Addition to 1-Sulfonylcyclopropanols as Cyclopropanone Precursors. Org. Lett 2020, 22, 6510–6515. [DOI] [PubMed] [Google Scholar]; (c) Jang Y; Lindsay VNG Synthesis of Cyclopentenones with Reverse Pauson–Khand Regiocontrol via Ni-Catalyzed C–C Activation of Cyclopropanone. Org. Lett 2020, 22, 8872–8876. [DOI] [PubMed] [Google Scholar]; (d) Poteat CM; Lindsay VNG Stereospecific Synthesis of Enantioenriched Alkylidenecyclobutanones via Formal Vinylidene Insertion into Cyclopropanone Equivalents. Org. Lett 2021, 23, 6482–6487. [DOI] [PubMed] [Google Scholar]
- (21).(a) Eisch JJ; Dua SK; Behrooz M Sulfone reagents in organic synthesis. Part 7. [(Phenylsulfonyl)methylene]dilithium as a novel cyclizing and homologizing reagent for bifunctional organic substrates. J. Org. Chem 1985, 50, 3674–3676. [Google Scholar]; (b) Decesare JM; Corbel B; Durst T; Blount JF γ- and δ-epoxy sulfones. Formation of different ring-sized products upon reaction with CH3MgI or LiN[CH(CH3)2]2. Can. J. Chem 1981, 59, 1415–1424. [Google Scholar]; (c) Corbel B; Decesare JM; Durst T Preparation of cyclobutenones and cyclopent-2-enones via epoxy sulfone cyclizations. Can. J. Chem 1978, 56, 505–511. [Google Scholar]
- (22).For an analogous disconnection from epichlorohydrin and carboxylic acids instead of a methyl sulfone, see:; (a) Grongsaard P; Bulger PG; Wallace DJ; Tan L; Chen Q; Dolman SJ; Nyrop J; Hoerrner RS; Weisel M; Arredondo J; Itoh T; Xie C; Wen X; Zhao D; Muzzio DJ; Bassan EM; Shultz CS Convergent, Kilogram Scale Synthesis of an Akt Kinase Inhibitor. Org. Process Res. Dev 2012, 16, 1069–1081. [Google Scholar]; (b) Gutekunst WR; Baran PS Applications of C–H Functionalization Logic to Cyclobutane Synthesis. J. Org. Chem 2014, 79, 2430–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).See the Supporting Information for details.
- (24).Jeffery SM; Stirling CJM The strain limit in intramolecular nucleophilic substitution. J. Chem. Soc., Perkin Trans 1993, 2, 1617–1624. [Google Scholar]
- (25).(a) Schaus SE; Brandes BD; Larrow JF; Tokunaga M; Hansen KB; Gould AE; Furrow ME; Jacobsen EN Highly Selective Hydrolytic Kinetic Resolution of Terminal Epoxides Catalyzed by Chiral (salen)CoIII Complexes. Practical Synthesis of Enantioenriched Terminal Epoxides and 1,2-Diols. J. Am. Chem. Soc 2002, 124, 1307–1315. [DOI] [PubMed] [Google Scholar]; (b) Podunavac M; Mailyan AK; Jackson JJ; Lovy A; Farias P; Huerta H; Molgó J; Cardenas C; Zakarian A Scalable Total Synthesis, IP3R Inhibitory Activity of Desmethylxestospongin B, and Effect on Mitochondrial Function and Cancer Cell Survival. Angew. Chem., Int. Ed 2021, 60, 11278–11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


