Abstract
Background: Pantoea is a genus within the Enterobacterales whose members encompass free-living and host-associated lifestyles. Despite our growing understanding of the role of mobile genetic elements in the biology, ecology, and evolution of this bacterial group, few Pantoea bacteriophages have been identified and characterized.
Materials and Methods: A bacteriophage that could infect Pantoea agglomerans was isolated from barnyard soil. We used electron microscopy and complete genome sequencing to identify the viral family, and evaluated its host range across 10 different Pantoea species groups using both bacterial lawn and phage lawn assays. The latter assays were carried out using a scalable microplate assay to increase throughput and enable spectrophotometric quantitation. We also performed a phylogenetic analysis to determine the closest relatives of our phage.
Results: Phage vB_PagP-SK1 belongs to the genus Teseptimavirus of the Podoviridae family in the order Caudovirales. The 39,938 bp genome has a modular structure with early, middle, and late genes, along with the characteristic direct terminal repeats of 172 bp. Genome composition and synteny were similar to that of the Erwinia amylovora phage, vB_EamP-L1, with the exception of a few loci that are most similar to genes of phage infecting other members of the Enterobacteriaceae. A total of 94 Pantoea strains were surveyed and vB_PagP-SK1 was found to infect 15 Pantoea strains across three species, predominantly P. agglomerans, along with one Erwinia billingiae strain.
Conclusions: vB_PagP-SK1 belongs to the Teseptimavirus genus and has a host range that spans multiple species groups, and is most closely related to the E. amylovora phage, vB_EamP-L1. The presence of xenologous genes in its genome indicates that the genome is a mosaic of multiple Teseptimavirus phages that infect members of the Enterobacteriaceae.
Keywords: Teseptimavirus, Pantoea, Podoviridae, host range, Erwinia, lytic
Introduction
Pantoea is a genus within the Enterobacterales whose members frequently form associations with plants and animals, often leading to disease in plant and animal hosts, as well as opportunistic infections in humans.1–3 Strains of Pantoea have also been harnessed for a variety of biotechnological applications, including biocontrol, bioremediation, and therapeutic products.4 Many of the genetic factors contributing to these capabilities, including specific genetic determinants as well as plasmids, have been identified and characterized.5–7 Bacteriophages capable of infecting Pantoea, however, remain underexplored despite the importance of these mobile genetic elements in shaping the general biology, ecology, and evolution of bacteria.8,9
To date, very few bacteriophages capable of infecting species of Pantoea have been described and characterized. LIMEzero and LIMElight were isolated using Pantoea agglomerans as the host, and were assigned to the genus Phikmvvirus (PhiKMV-like viruses) in the Podoviridae family using both imaging approaches and genome analysis.10 Host range assays of these phages using a selection of Pantoea and Erwinia strains showed that LIMEzero was only able to infect the P. agglomerans strain from which it was isolated, while LIMElight also infected a second P. agglomerans strain, LMG 2660.10 No plaque formation was observed for either phage on overlays of Erwinia amylovora strain GBBC 403, Erwinia mallotivora strain LMG 1271, and Pantoea stewartii strains LMG 2717 and LMG 2719; however, some Erwinia phages have been reported to infect strains of Pantoea. Phage L1 and S2 (Podoviridae), identified as E. amylovora phage, are able to also infect representative strains of P. agglomerans and Pantoea ananatis.11 The E. amylovora phage S10 and M7 (Myoviridae) infected only P. ananatis or P. agglomerans, respectively, while phage S7 (Podoviridae) exhibited an even broader host range, infecting not only E. amylovora but also Erwinia billingiae, P. agglomerans, Pantoea vagans, and P. ananatis.11
Here we report the isolation, characterization, and complete genome sequence of vB_PagP-SK1; a lytic bacteriophage that was identified as a member of the genus Teseptimavirus of the Podoviridae family in the order Caudovirales. vB_PagP-SK1 has a genome size of 39,938 bp and is most closely related to the E. amylovora phage vB_EamP-L1 despite having been isolated on a strain of P. agglomerans. Our host range assays revealed that vB_PagP-SK1 is capable of infecting 15 strains of Pantoea across three species groups along with one E. billingiae strain, suggesting that vB_PagP-SK1 is a broad host range phage.
Materials and Methods
Bacterial strains and culturing conditions
Bacterial strains (Table 1) were revived from −80°C glycerol stocks and cultured on lysogeny broth (LB) agar plates. Plates were incubated aerobically at 30°C for 24–48 h after which they were transferred to a 4°C fridge for storage. Log-phase liquid cultures were prepared by inoculating 5 mL LB tubes with a single colony and placing in a 220 rpm shaking incubator at 30°C for 12–18 h.
Table 1.
Susceptibility of Bacterial Strains to vB_PagP-SK1 Using Both the Bacterial and Phage Lawn Methods
| Genus/species | Isolate | Host/localea | Location | Source | Bacterial lawnb | Phage lawnc | Control (OD600) | Phage (OD600) | Difference |
|---|---|---|---|---|---|---|---|---|---|
| Aeromonas sp. | SM02150 | Lake water | Regina, SK, Canada | 1 | − | − | 1.17 | 1.17 | 0.00 |
| Erwinia amylovora | EA321 | Hawthorn | George Sundin, Michigan State | − | na | Na | na | na | |
| Erwinia billingiae | EhWHF18 | Unidentified | Gwyn Beattie, Iowa State | + | + | 1.25 | 0.48 | −0.61 | |
| Escherichia coli | B/r | B strain, UV resistance | − | na | na | na | na | ||
| E. coli | OP50 | B strain, uracil auxotroph | − | na | na | na | na | ||
| Kosakonia sp. | 12202 | Melon | ICMP | − | na | na | na | na | |
| Mixta calida | B021323 | Human, 28-year-old female, urine midstream | Saskatchewan, Canada | Roy Romanow Provincial Laboratory | − | − | 1.34 | 1.35 | 0.01 |
| M. calida | BB957621-A1 | Human, male, CAPD dialysate, peritonitis | Winnipeg, Canada | St. Boniface General Hospital | − | − | 1.47 | 1.34 | −0.09 |
| M. calida | BB957621-A2 | Human, male, CAPD dialysate, peritonitis | Winnipeg, Canada | St. Boniface General Hospital | − | − | 0.82 | 0.86 | 0.05 |
| M. calida | BB957621-B1 | Human, male, CAPD dialysate, peritonitis | Winnipeg, Canada | St. Boniface General Hospital | − | − | 1.37 | 1.44 | 0.05 |
| M. calida | BB957621-B2 | Human, male, CAPD dialysate, peritonitis | Winnipeg, Canada | St. Boniface General Hospital | − | − | 1.49 | 1.45 | −0.02 |
| M. calida | BB957621-C1 | Human, male, CAPD dialysate, peritonitis | Winnipeg, Canada | St. Boniface General Hospital | − | − | 1.37 | 1.37 | 0.00 |
| M. calida | BB957621-C2 | Human, male, CAPD dialysate, peritonitis | Winnipeg, Canada | St. Boniface General Hospital | − | − | 1.58 | 1.40 | −0.12 |
| Pantoea agglomerans | 240R | Pear flower | California, USA | Steven Lindow, UC Berkeley | − | − | 0.54 | 0.48 | −0.10 |
| P. agglomerans | 308R | Pear flower | California, USA | Steven Lindow, UC Berkeley | − | − | 0.64 | 0.61 | −0.04 |
| P. agglomerans | 3-770398 | Human, female, blood | Toronto, Canada | Sunnybrook Hospital | + | + | 1.47 | 0.62 | −0.58 |
| P. agglomerans | H42501 | Human, male, blood | Toronto, Canada | Sunnybrook Hospital | − | − | 1.41 | 0.85 | −0.40 |
| P. agglomerans | B015092 | Human, 9-year-old female, urine midstream | Saskatchewan, Canada | Roy Romanow Provincial Laboratory | − | − | 0.83 | 1.23 | 0.49 |
| P. agglomerans | B016395 | Human, 83-year-old female superficial wound | Saskatchewan, Canada | Roy Romanow Provincial Laboratory | − | − | 0.47 | 0.47 | 0.00 |
| P. agglomerans | B025670 | Human, 13-year-old male, superficial wound | Saskatchewan, Canada | Roy Romanow Provincial Laboratory | − | − | 0.53 | 0.52 | −0.04 |
| P. agglomerans | B026440 | Human, female, sputum, aortic aneurysm | Winnipeg, Canada | St. Boniface General Hospital | + | − | 1.23 | 0.82 | −0.34 |
| P. agglomerans | Eh318 | Apple leaf | Brion Duffy | − | − | 0.47 | 0.52 | 0.10 | |
| P. agglomerans | G4032547 | Human, ear | Regina, SK, Canada | Regina General Hospital | + | + | 1.41 | 0.66 | −0.53 |
| P. agglomerans | SN01080 | Slug | Regina, SK, Canada | 1 | − | − | 1.21 | 1.05 | −0.13 |
| P. agglomerans | SN01121 | Bee | Regina, SK, Canada | 1 | + | + | 1.27 | 0.68 | −0.47 |
| P. agglomerans | SN01122 | Bee | Regina, SK, Canada | 1 | − | − | 0.64 | 0.78 | 0.22 |
| P. agglomerans | SN01170 | Caterpillar | Regina, SK, Canada | 1 | − | − | 1.32 | 0.83 | −0.37 |
| P. agglomerans | SP00202 | Apple | Regina, SK, Canada | 1 | − | − | 0.79 | 0.78 | −0.01 |
| P. agglomerans | SP00303 | Raspberry | Regina, SK, Canada | 1 | + | + | 1.28 | 0.72 | −0.44 |
| P. agglomerans | SP01202 | Strawberry leaf and stem | Regina, SK, Canada | 1 | − | + | 1.42 | 0.59 | −0.58 |
| P. agglomerans | SP01230 | Virginia creeper leaves and stem | Regina, SK, Canada | 1 | − | − | 1.41 | 0.84 | −0.41 |
| P. agglomerans | SP02022 | Thistle | Regina, SK, Canada | 1 | + | − | 0.50 | 0.46 | −0.07 |
| P. agglomerans | SP02230 | Diseased tree leaf | Regina, SK, Canada | 1 | + | − | 0.60 | 0.55 | −0.08 |
| P. agglomerans | SP02243 | Tree | Regina, SK, Canada | 1 | − | + | 1.12 | 0.62 | −0.45 |
| P. agglomerans | SP03310 | Diseased maize leaf | Regina, SK, Canada | 1 | − | + | 1.18 | 0.66 | −0.44 |
| P. agglomerans | SP03383 | Diseased maize leaf | Regina, SK, Canada | 1 | − | − | 1.32 | 0.89 | −0.33 |
| P. agglomerans | SP04011 | Tomato leaf | Regina, SK, Canada | 1 | − | − | 1.56 | 0.99 | −0.36 |
| P. agglomerans | SP04021 | Tomato leaf | Regina, SK, Canada | 1 | − | − | 1.32 | 1.17 | −0.11 |
| P. agglomerans | SP04022 | Tomato leaf | Regina, SK, Canada | 1 | − | + | 1.71 | 0.79 | −0.54 |
| P. agglomerans | SP05051 | Tomato leaf | Regina, SK, Canada | 1 | + | + | 0.93 | 0.35 | −0.62 |
| P. agglomerans | SP05052 | Tomato leaf | Regina, SK, Canada | 1 | + | − | 0.84 | 0.76 | −0.09 |
| P. agglomerans | SP05092 | Tomato leaf | Regina, SK, Canada | 1 | − | − | 0.58 | 0.53 | −0.09 |
| P. agglomerans | SP05120 | Diseased maize leaf | Regina, SK, Canada | 1 | − | − | 1.37 | 0.86 | −0.38 |
| P. agglomerans | SS02010 | Soil, ground squirrel burrow | Regina, SK, Canada | 1 | − | − | 0.48 | 0.44 | −0.07 |
| P. agglomerans | TX10 | Human, sputum | Houston, Texas | Texas Children's Hospital | − | − | 1.34 | 1.30 | −0.03 |
| P. agglomerans | DB522094 | Human, elbow sore | Winnipeg, Canada | St. Boniface General Hospital | − | − | 0.50 | 0.47 | −0.06 |
| P. agglomerans | M41864 | Human, female, blood | Toronto, Canada | Sunnybrook Hospital | − | − | 0.71 | 0.72 | 0.01 |
| P. agglomerans | SM03214 | Goose feces | Regina, SK, Canada | 1 | − | − | 0.54 | 0.48 | −0.12 |
| P. agglomerans | BC594466A | Subhepatic abscess | Winnipeg, Canada | St. Boniface General Hospital | − | − | 0.95 | 0.91 | −0.04 |
| P. agglomerans | BE528629 | Human, peritoneal dialysis | Winnipeg, Canada | St. Boniface General Hospital | − | − | 0.72 | 0.76 | 0.05 |
| P. agglomerans | G0063668 | Clinical | Regina, SK, Canada | Regina General Hospital | − | − | 0.74 | 0.96 | 0.29 |
| P. agglomerans | BB834250 | Human, female, sputum, aortic aneurysm | Winnipeg, Canada | St. Boniface General Hospital | − | − | 0.72 | 0.75 | 0.05 |
| P. agglomerans | 13301 | Golden delicious apple | New Zealand | ICMP | + | − | 0.44 | 0.43 | −0.02 |
| P. agglomerans | SP03412 | Diseased bean leaf | Regina, SK, Canada | 1 | + | − | 0.75 | 0.59 | −0.21 |
| P. agglomerans | BB350028B | Human, female, blood culture, fever | Winnipeg, Canada | St. Boniface General Hospital | + | − | 0.67 | 0.59 | −0.12 |
| P. agglomerans | 7373 | Onion | South Africa | ICMP | + | − | 0.48 | 0.39 | −0.20 |
| P. agglomerans | DC432 | Maize | David Coplin, Ohio State | − | − | 0.50 | 0.46 | −0.08 | |
| P. agglomerans | DC556 | Gypsophila (baby's breath) | David Coplin, Ohio State | − | − | 0.57 | 0.46 | −0.18 | |
| P. agglomerans | 1574 | Unidentified | ICMP | − | − | 0.66 | 0.58 | −0.12 | |
| P. agglomerans | 12531 | Gypsophila (baby's breath) | Netherlands | ICMP | − | − | 0.77 | 0.55 | −0.28 |
| P. agglomerans | 17124 | Olive | Italy | ICMP | − | − | 0.79 | 0.63 | −0.20 |
| P. agglomerans | 83 | Wheat | ICMP | − | − | 0.79 | 0.71 | −0.10 | |
| P. agglomerans | 5565 | Soybean | New Zealand | ICMP | − | − | 0.63 | 0.56 | −0.10 |
| P. agglomerans | 1512 | Green bean | ICMP | − | − | 0.50 | 0.50 | 0.00 | |
| P. agglomerans | 1373 | Balsam | India | ICMP | − | − | 0.65 | 0.52 | −0.19 |
| P. agglomerans | SP05130 | Diseased maize stamen | Regina, SK, Canada | 1 | − | − | 0.86 | 0.71 | −0.18 |
| P. agglomerans | SP03190 | Healthy tree leaf | Regina, SK, Canada | 1 | − | − | 0.94 | 0.93 | −0.02 |
| P. agglomerans | SP03392 | Maize | Regina, SK, Canada | 1 | − | − | 1.04 | 0.99 | −0.05 |
| P. agglomerans | 09-1957-a | Human, renal failure | Winnipeg, Canada | St. Boniface General Hospital | − | − | 0.45 | 0.49 | 0.10 |
| Pantoea ananatis | 15320 | Rice | Australia | ICMP | − | − | na | na | na |
| P. ananatis | B7 | Maize, rifR derivative of M232A | Wisconsin, USA | Steven Lindow, UC Berkeley | − | − | na | na | na |
| P. ananatis | BRT175 | Strawberry | Brentwood, California | Gwyn Beattie, Iowa State | − | − | 0.68 | 0.68 | 0.00 |
| P. ananatis | BRT98 | Strawberry | Brentwood, California | Gwyn Beattie, Iowa State | − | − | 0.57 | 0.55 | −0.03 |
| P. ananatis | DC434 | Maize | David Coplin, Ohio State | − | − | na | na | na | |
| P. ananatis | 17671 | Rice | Cambodia | ICMP | − | − | 0.68 | 0.64 | −0.07 |
| P. ananatis | LMG5342 | Human, wound | Georgia | Teresa Coutinho, University of Pretoria | − | − | 0.66 | 0.70 | 0.07 |
| P. ananatis | LMG20103 | Eucalyptus | South Africa | Teresa Coutinho, University of Pretoria | − | − | 0.91 | 0.59 | −0.35 |
| P. ananatis | Cit30-11R | Naval orange leaf | California, USA | Steven Lindow, UC Berkeley | − | − | 0.48 | 0.46 | −0.04 |
| P. ananatis | M232A | Maize | Wisconsin, USA | Steven Lindow, UC Berkeley | − | − | 0.61 | 0.54 | −0.12 |
| Pantoea brenneri | 09-1151 | Human, posthemicholectomy | Winnipeg, Canada | St. Boniface General Hospital | − | − | 1.32 | 1.30 | −0.02 |
| P. brenneri | B014130 | Human, 11-year-old male superficial wound | Saskatchewan, Canada | Roy Romanow Provincial Laboratory | + | + | 1.28 | 0.63 | −0.50 |
| P. brenneri | B024858 | Human, 26-year-old female, breast abscess | Saskatchewan, Canada | Roy Romanow Provincial Laboratory | − | − | 0.59 | 0.53 | −0.10 |
| Pantoea conspicua | B011017 | Human, 11-year-old female superficial wound | Saskatchewan, Canada | Roy Romanow Provincial Laboratory | − | − | 0.68 | 0.63 | −0.07 |
| Pantoea dispersa | 2-M11657A | Human, male, blood | Toronto, Canada | Sunnybrook Hospital | − | − | 0.67 | 0.67 | 0.01 |
| P. dispersa | 2-M11657B | Human, male, blood | Toronto, Canada | Sunnybrook Hospital | − | − | 0.45 | 0.46 | 0.02 |
| P. dispersa | 625 | Sorghum | India | ICMP | − | − | 0.48 | 0.49 | 0.02 |
| Pantoea eucalypti | 299R | Pear flower | California, USA | Steven Lindow, UC Berkeley | − | − | na | na | na |
| P. eucalypti | 5-F9026 | Human, male, blood | Toronto, Canada | Sunnybrook Hospital | − | − | 0.83 | 0.89 | 0.07 |
| P. eucalypti | B011489 | Human, 52-year-old female, superficial wound | Saskatchewan, Canada | Roy Romanow Provincial Laboratory | − | − | 0.57 | 0.59 | 0.03 |
| P. eucalypti | SP02021 | Thistle leaf | Regina, SK, Canada | 1 | − | − | 1.34 | 0.83 | −0.38 |
| P. eucalypti | SP03372 | Diseased maize leaf | Regina, SK, Canada | 1 | − | − | 1.39 | 1.37 | −0.01 |
| P. eucalypti | SP03391 | Diseased bean leaf | Regina, SK, Canada | 1 | − | − | 0.54 | 0.54 | 0.01 |
| P. eucalypti | SP04013 | Tomato leaf | Regina, SK, Canada | 1 | − | + | 1.38 | 0.76 | −0.45 |
| Pantoea eucrina | 06-868 | Human | Winnipeg, Canada | St. Boniface General Hospital | − | − | 0.91 | 0.92 | 0.01 |
| P. eucrina | TX5 | Human, blood | Houston, Texas | Texas Children's Hospital | − | − | 0.76 | 0.93 | 0.22 |
| P. eucrina | TX6 | Human, blood | Houston, Texas | Texas Children's Hospital | − | − | 0.73 | 0.89 | 0.21 |
| Pantoea latae | TX3 | Human, blood | Houston, Texas | Texas Children's Hospital | − | − | 0.58 | 0.46 | −0.20 |
| Pantoea septica | 06-2465-a | Human, cerebellar (stroke) | Winnipeg, Canada | St. Boniface General Hospital | − | − | 1.23 | 1.09 | −0.11 |
| P. septica | 06-2465-b | Human, cerebellar (stroke) | Winnipeg, Canada | St. Boniface General Hospital | − | − | 1.35 | 1.30 | −0.03 |
| P. septica | 10-1150 | Human | Winnipeg, Canada | St. Boniface General Hospital | − | − | 1.28 | 1.16 | −0.09 |
| P. septica | 1-X44686 | Human, female, blood | Toronto, Canada | Sunnybrook Hospital | − | − | 1.35 | 1.38 | 0.02 |
| P. septica | M1517 | Human, female, blood | Toronto, Canada | Sunnybrook Hospital | − | − | 0.69 | 0.66 | −0.04 |
| P. septica | B016375 | Human, female, finger | Regina, SK, Canada | Roy Romanow Provincial Laboratory | − | − | 1.31 | 1.05 | −0.20 |
| P. septica | BB350028A | Human, female, blood culture, fever | Winnipeg, Canada | St. Boniface General Hospital | − | − | 0.99 | 0.86 | −0.13 |
| P. septica | G2291404 | Human | Regina, SK, Canada | Regina General Hospital | − | − | 1.35 | 1.37 | 0.02 |
| P. septica | G4071105 | Human, urine | Regina, SK, Canada | Regina General Hospital | + | − | 0.86 | 0.63 | −0.26 |
| Pantoea stewartii | 626 | Maize | India | ICMP | − | − | 0.73 | 0.73 | 0.01 |
| Pseudomonas syringae | UnB647 | Kidney bean | David Guttman, University of Toronto | − | na | na | na | na | |
| P. syringae | TLP2 | Nonpathogenic, healthy potato leaf | David Guttman, University of Toronto | − | na | na | na | na | |
| Staphylococcus aureus | K1-7 | Chris Yost, University of Regina | − | na | na | na | na | ||
| Streptococcus mutans | UAIS9:wt | Heather Dietz, University of Regina | − | na | na | na | na |
CAPD.
Scored as susceptible (+) if plaque formation was observed on bacterial lawn.
Scored as susceptible (+) if % change OD600 between Control (no phage) and +Phage was −2σ (less than −0.413).
CAPD, continuous ambulatory peritoneal dialysis.
Phage isolation, amplification, and visualization
A 50 g soil sample taken from a barnyard near Craven, Saskatchewan, Canada, was gently agitated with 150 mL of deionized water for 30 min at room temperature. The mixture was then vacuum filtered using a Buchner funnel fitted with a glass fiber filter (934AH; Whatman, Reeve Angel). The filtered sample was transferred to sterile 50 mL conical tubes and centrifuged at 4000 g in a Sorvall ST16R centrifuge with a 3655-swinging bucket rotor for 20 min. The supernatant was filtered through a 0.22 μm bottle filter (Nalgene) and 1 mL of the sample was spread onto an LB agar plate with a top agar overlay containing 100 μL P. agglomerans SN01121 (OD600nm = 0.6), 1 mL of 1 × LB, and 4 mL of 0.5% molten agar. The spread plate was incubated at 30°C for 24–72 h before plaque formation was scored. A single plaque was purified as per Sambrook and Russell,12 and suspended in 1 mL of phage buffer (10 mM Tris-HCl pH 8, 10 mM MgSO4, 150 mM NaCl) with 50 μL of CHCl3 and stored overnight at 4°C. The single plaque suspension was then diluted in 100-fold steps from 100 to 10−6 and standard top agar overlays were prepared with 10 μL of phage suspension to determine which dilution reached 80–90% plaque confluence for amplification.
The isolated phage was amplified by preparing 30 top agar plates using the appropriate phage dilution. Plates were incubated at 30°C for 24 h and 5 mL of phage buffer was pipetted onto the surface of the plates. Plates were shaken gently for 1 h at room temperature to allow the phage to diffuse into the buffer. Phage buffer was then transferred from the plates to sterile 50 mL conical tubes and centrifuged at 4000 g for 20 min. Supernatant was then cleared with chloroform in accordance with Sambrook and Russell,12 filtered through a 0.22 μm polyethersulfone syringe filter (VWR International), and phage lysate titered. Aliquots of the high titer lysate were frozen with glycerol at −20°C and −80°C, and the remainder was stored at 4°C. Phage lysate was negatively stained by first applying a small drop of lysate onto carbonized formvar-coated grids (#FF300-CU-50; Electron Microscopy Sciences), and removing the excess liquid by blotting with filter paper. Staining was then performed by adding 2% (wt/vol) phosphotungstic acid (pH 6.8) containing ∼0.01% bovine albumin, and after a 10-s incubation, blotting to remove excess liquid. The phage was then imaged with a JEOL JEM-1011 transmission electron microscope using a Gatan-ES1000W Digital Camera at the Roy Romanow Provincial Laboratory.
DNA extraction
Phage genomic DNA was extracted using a modified zinc chloride phage precipitation protocol described by Santos.13 High titer lysate (>1.0 × 109 PFU/mL) was cleared with chloroform as per Sambrook and Russell,12 and 1.5 mL was added to a sterile 2 mL conical tube. DNAse I and RNAse I were added to final concentrations of 100 μg/mL followed by incubation at 37°C for 30 min. Then, 30 μL of sterile 2.0 M ZnCl2 was added to the reaction and the mixture was incubated at 20°C for 5 min followed by centrifugation at 21,000 g on a Sorvall Legend Micro 21R centrifuge for 1 min. Supernatant was discarded, and the pellet was resuspended in 500 μL of TES solution (0.1 M Tris-HCl pH 8, 0.1 M EDTA, 0.3% SDS) and incubated at 68°C for 20 min.
Subsequently, 90 μL of 3 M potassium acetate pH 4.8 was added and the mixture was vortexed gently for 30 s followed by incubation on ice for 20 min. The debris was pelleted by centrifugation at 21,000 g for 1 min. Supernatant was transferred to a sterile 1.5 mL microfuge tube and an equal volume of absolute isopropanol was added. The solution was gently vortexed for 10 s followed by incubation on ice for 5 min. DNA was pelleted by centrifugation at 21,000 g for 10 min, and the pellet washed twice with 70% ethanol and allowed to air dry. DNA was resuspended in deionized water. Resuspended DNA was further purified by the use of an Omega Bio-tek E.Z.N.A. Cycle-Pure PCR cleanup kit.
Genome sequencing and in silico analysis
Library preparation was performed using the NEBNext Fast DNA Library Prep Set as per the manufacturer's recommended protocols. The phage sequencing library was then sequenced on an Ion PGM (Life Technologies) with 200 bp reads on an Ion 314 v2 chip. Ion Torrent average sequence coverage for vB_PagP-SK1 was 369-fold. The genome was assembled using the MIRA software suite (v3.9) on the Ion PGM server. Putative genes were identified using GeneMark.hmm14 and genome maps generated with VectorNTI Advance v10 (Thermo Fisher). Sequences were compared with the PHASTER prophage and virus database15 using standalone BLASTp, and to the NCBI nr database using BLASTx. Genome comparison was performed using progressiveMauve with default parameters.16 Multilocus sequence alignment (MLSA) was performed using the nucleotide sequence of four core genes; RNA polymerase, DNA polymerase, head-to-tail joining protein, and terminase large subunit. Bacteriophage phiKMV was used as an outgroup. The MLSA was performed with ClustalX2 using iteration after each alignment step.17 A maximum likelihood tree was created in MEGAX using the maximum composite likelihood algorithm with complete gap deletion, 8 gamma rate categories, and 500 bootstrap replicates.18 Pairwise alignments and dot plots were performed with the NCBI BLAST bl2seq tool. The full genome sequence of phage vB_PagP-SK1 has been deposited in GenBank under accession number MN450150.
Host range assays
Plaque formation was assessed using bacterial lawn overlays for each of the 94 strains of Pantoea and 17 strains from other genera. Diluted phage lysate (10 μL) that had been cleared with chloroform was mixed with 100 μL of log phase bacterial culture in 1 mL of 1 × LB and 5 mL of overlay agar at 40°C before being poured onto LB agar plates that had been held at room temperature. The overlay plates were incubated at 30°C for 24 h before plaque formation was scored. Phage lawn overlays were carried out in Greiner flat-bottomed 96-well microplates. Each well contained 250 μL of SM agar (1 L: 10 g glucose, 10 g peptone, 1 g yeast extract, 0.5 g MgSO4 monohydrate, 1.9 g KH2PO4, 0.6 g K2HPO4, and 20 g agar). Once solidified, 3.6 μL of 1.87 × 108 PFU/mL phage lysate was added to each well and excess moisture was allowed to evaporate. The plates were inoculated with 5 μL of liquid bacterial culture at an optical density (OD) 600 nm of 0.6 (∼1.0 × 109 cfu/mL). Controls for each isolate consisted of 3.6 μL of sterile phage buffer instead of phage lysate. Plates were incubated for 24 h at 30°C and then refrigerated for 24–48 h at 4°C. OD for each well on the microplates was read using a Biotek Gen5 microplate reader using endpoint scan at a wavelength of 600 nm, and each reading was standardized using the average OD value from the wells of a 96-well plate containing 250 μL of SM agar only. Strains showing a reduction in OD of ≥2σ relative to control were scored as susceptible.
Results
Phage isolation and morphology
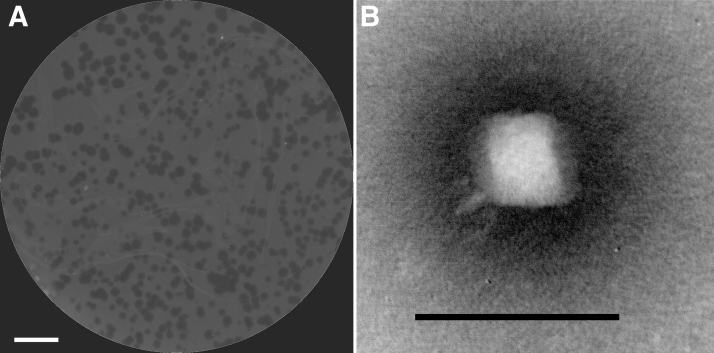
Filtered supernatants taken from washed barnyard soil were mixed with a single target strain, P. agglomerans SN01121 (SN01121), in a standard bacterial lawn overlay assay. A single plaque was then isolated, amplified, and retested on a lawn of SN01121, resulting in the formation of 2 mm clear plaques with no halos (Fig. 1A). Imaging of the phage lysate by transmission electron microscopy revealed a phage that appeared to have an isometric icosahedral capsid ∼60 nm in diameter, a short noncontractile tail, and multiple tail fibers, consistent with the members of the Podoviridae family (Fig. 1B). The phage was named vB_PagP-SK1.
FIG. 1.
(A) Top agar overlay showing plaque morphology of vB_PagP-SK1 on Pantoea agglomerans SN01121. Scale bar represents 1 cm. (B) Transmission electron micrographs of negatively stained vB_PagP-SK1. Scale bar represents 100 nm.
Genome analysis
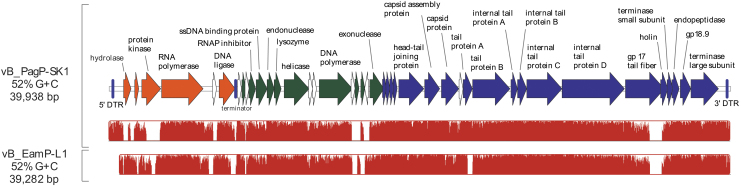
DNA sequencing of vB_PagP-SK1 revealed a genome of 39,938 bp with 44 predicted open reading frames flanked by direct terminal repeats of 172 bp (Table 2). The organization of putative early, middle, and late genes was consistent with that of other members of Teseptimavirus11 (Fig. 2). The early genomic region consists of genes required to initiate an infection,19 and includes an S-adenosyl-l-methionine hydrolase (SAMase), protein kinase, phage RNA polymerase, and phage DNA ligase, which are followed by a predicted T7 early transcription terminator. The middle genomic region consists of bacterial RNA polymerase inhibitor, DNA metabolism genes, and phage DNA replication genes. The late genomic region consists of phage structural proteins, DNA packaging genes, and the holin and endopeptidase lysis-associated genes. Several hypothetical genes are predicted throughout the genome, which have weak hits to phage from other species, including Citrobacter, Cronobacter, Pseudomonas, and Stenotrophomonas (Table 2). vB_PagP-SK1 shares 88% sequence coverage and 94% identity with vB_EamP-L1 at the nucleotide level. A MAUVE comparison of vB_PagP-SK1 and vB_EamP-L1 highlights this high-sequence identity between these phages, with the exception of the SAMase, gp0.65, protein kinase, type II holin, the carboxyl-terminal domain of gp17 (tail fiber/EPS depolymerase), and several of the predicted hypothetical genes that are less than 300 bp (Fig. 2 and Table 2).
Table 2.
Annotation of Predicted Genes of Pantoea Phage vB_PagP-SK1
| Genea | CDS position | Strand | Function | Best blast/PHASTER hit | Accession number | E-value |
|---|---|---|---|---|---|---|
| MF01 | 1..172 | 5′ Direct terminal repeat | ||||
| 1 | 928..1401 | (+) | S-adenosyl-l-methionine hydrolase | Klebsiella phage K5 | NC_028800 | 1.50E-42 |
| 2 | 1794..1976 | (+) | gp0.65 | Erwinia phage vB_EamP-L1 | NC_019510 | 1.00E-16 |
| 3 | 2101..3306 | (+) | Protein kinase | Stenotrophomonas phage IME15 | YP_006990206.1 | 4.16E-68 |
| 4 | 3368..6037 | (+) | RNA polymerase | Erwinia phage vB_EamP-L1 | YP_007005430.1 | 0 |
| 5 | 6843..7000 | (+) | Hypothetical head protein | EBPR podovirus 2 | AEI70915.1 | 1.00E+00 |
| 6 | 7070..8083 | (+) | DNA ligase | Erwinia phage vB_EamP-L1 | YP_007005433.1 | 0 |
| MF02 | 8093..8120 | T7 early terminator | ||||
| 7 | 8106..8243 | (+) | Hypothetical phage protein | Citrobacter phage CR8 | CDM21618.1 | 1.20E-01 |
| 8 | 8773..9093 | (+) | gp1.65 | Erwinia phage vB_EamP-L1 | YP_007005436.1 | 6.78E-57 |
| 9 | 9093..9209 | (+) | Hypothetical protein GAP227 28 | Cronobacter phage Dev_CD_23823 | NC_029070 | 5.47E-05 |
| 10 | 9206..9361 | (+) | Bacterial RNAP inhibitor | Erwinia phage vB_EamP-L1 | YP_007005437.1 | 1.12E-17 |
| 11 | 9444..10154 | (+) | ssDNA binding protein | Erwinia phage vB_EamP-L1 | YP_007005438.1 | 2.61E-129 |
| 12 | 10154..10606 | (+) | Endonuclease | Erwinia phage vB_EamP-L1 | YP_007005439.1 | 4.73E-106 |
| 13 | 10606..11055 | (+) | Lysozyme | Erwinia phage vB_EamP-L1 | YP_007005440.1 | 1.41E-106 |
| 14 | 11247..12836 | (+) | DNA primase/helicase | Erwinia phage vB_EamP-L1 | YP_007005441.1 | 0 |
| 15 | 12948..13160 | (+) | gp4.3 | Erwinia phage vB_EamP-L1 | YP_007005444.1 | 3.94E-38 |
| 16 | 13265..13450 | (+) | gp4.5 | Erwinia phage vB_EamP-L1 | YP_007005445.1 | 1.62E-27 |
| 17 | 13504..15630 | (+) | DNA polymerase | Erwinia phage vB_EamP-L1 | YP_007005446.1 | 0 |
| 18 | 15640..15861 | (+) | Hypothetical protein | Cronobacter phage Dev2 | CDM12546.1 | 4.00E-04 |
| 19 | 15851..16171 | (+) | Hypothetical protein gp5.5 | Klebsiella phage vB_KpnP_KpV289 | NC_028977 | 6.71E-30 |
| 20 | 16234..16377 | (+) | gp5.7 | Erwinia phage vB_EamP-L1 | YP_007005448.1 | 7.01E-22 |
| 21 | 16386..16664 | (−) | Hypothetical protein I7C 035c | Pseudomonas phage MR299-2 | AFD10713.1 | 4.70E+00 |
| 22 | 16744..17652 | (+) | Exonuclease | Erwinia phage vB_EamP-L1 | YP_007005450.1 | 0 |
| 23 | 17649..17753 | (+) | gp6.3 | Erwinia phage vB_EamP-L1 | YP_007005451.1 | 5.29E-07 |
| 24 | 17852..18097 | (+) | gp6.5 | Erwinia phage vB_EamP-L1 | YP_007005452.1 | 1.01E-54 |
| 25 | 18102..18338 | (+) | gp6.7 | Erwinia phage vB_EamP-L1 | YP_007005453.1 | 9.26E-50 |
| 26 | 18325..18597 | (+) | gp7.3 | Erwinia phage vB_EamP-L1 | YP_007005454.1 | 1.46E-55 |
| 27 | 18611..20221 | (+) | Head-to-tail joining protein | Erwinia phage vB_EamP-L1 | YP_007005455.1 | 0 |
| 28 | 20273..21238 | (+) | Capsid assembly protein | Erwinia phage vB_EamP-L1 | YP_007005456.1 | 0 |
| 29 | 21423..22463 | (+) | Capsid protein | Erwinia phage vB_EamP-L1 | YP_007005458.1 | 0 |
| 30 | 22481..22588 | (+) | Hypothetical protein | Stenotrophomonas phage IME15 | YP_006990234.1 | 8.00E-06 |
| 31 | 22751..23335 | (+) | Tail tubular protein A | Erwinia phage vB_EamP-L1 | YP_007005459.1 | 4.23E-142 |
| 32 | 23354..25753 | (+) | Tail tubular protein B | Erwinia phage vB_EamP-L1 | YP_007005461.1 | 0 |
| 33 | 25823..26236 | (+) | Tail internal virion protein A | Erwinia phage vB_EamP-L1 | YP_007005462.1 | 4.51E-100 |
| 34 | 26248..26829 | (+) | Tail internal virion protein B | Erwinia phage vB_EamP-L1 | YP_0070705463.1 | 5.20E-135 |
| 35 | 26841..29102 | (+) | Tail internal virion protein C | Erwinia phage vB_EamP-L1 | YP_007005464.1 | 0 |
| 36 | 29117..33115 | (+) | Tail internal virion protein D | Erwinia phage vB_EamP-L1 | YP_007005465.1 | 0 |
| 37 | 33177..35675 | (+) | gp17 tail fiber - EPS depolymerases | Erwinia phage vB_EamP-L1 | YP_007005466.1 | 0 |
| 38 | 35680..35886 | (+) | gp17.5 (type II holin) | Enterobacteria phage BA14 | YP_002003494.1 | 5.10E-33 |
| 39 | 35879..36136 | (+) | Terminase small subunit | Erwinia phage vB_EamP-L1 | YP_007005468.1 | 2.80E-52 |
| 40 | 36239..36703 | (+) | Endopeptidase | Erwinia phage vB_EamP-L1 | YP_007005469.1 | 2.51E-107 |
| 41 | 36705..37385 | (+) | gp18.9 | Erwinia phage vB_EamP-L1 | YP_007005471.1 | 2.94E-152 |
| 42 | 37397..39157 | (+) | Terminase large subunit | Erwinia phage vB_EamP-L1 | YP_007005472.1 | 0 |
| 43 | 39419..39565 | (+) | gp19.5 | Erwinia phage vB_EamP-L1 | YP_007005475.1 | 1.20E-26 |
| MF03 | 39767..39938 | 3′ Direct terminal repeat |
MF
MF, miscellaneous feature.
FIG. 2.
Genomic organization of vB_PagP-SK1 with predicted early (orange), middle (green), and late (blue) genes. Genes without shading (white) are predicted hypothetical genes with weak hits to other phages, and miscellaneous features are indicated with a purple vertical line. The lower panel shows the results of a Mauve analysis16 comparing the sequence identity between vB_PagP-SK1 and the Erwinia amylovora phage, vB_EamP-L1.
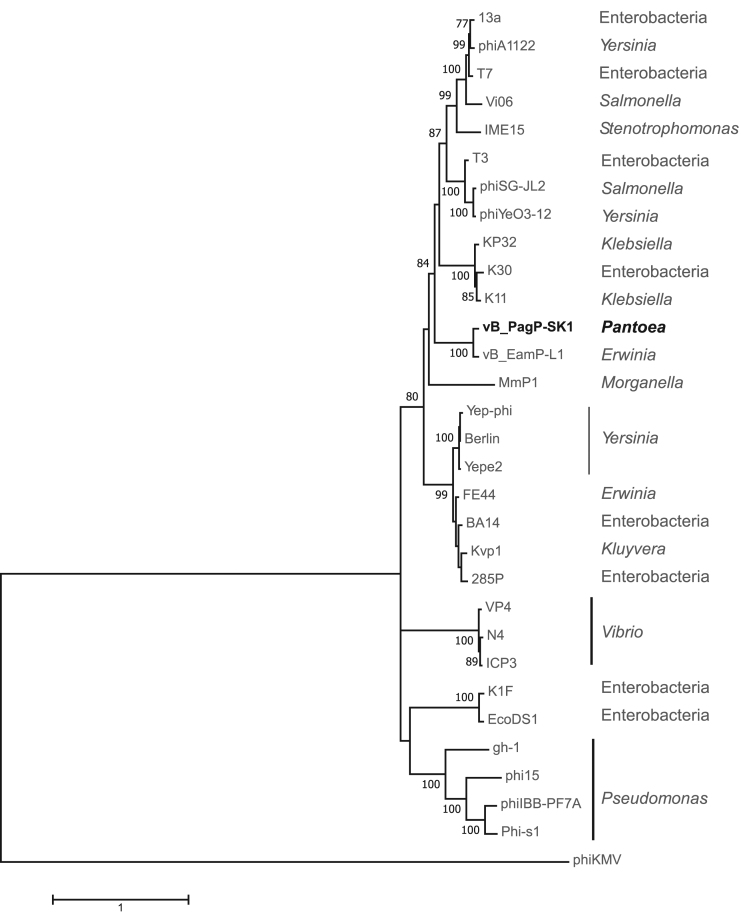
A phylogenetic analysis was carried out on vB_PagP-SK1 and related Teseptimavirus genomes (Table 3) using the concatenated amino acid sequences of the RNA polymerase, DNA polymerase, head-to-tail joining protein, and terminase large subunit (genes 4, 17, 27, 43). The resulting phylogeny, rooted on bacteriophage phiKMV (Phikmvvirus, a sister genus to Teseptimavirus), places vB_PagP-SK1 and vB_EamP-L1 in their own lineage among phages that infect members of mostly the Enterobacterales (Fig. 3).
Table 3.
Teseptimavirus Bacteriophage Genomes Used for Phylogenetic and Comparative Genomic Analyses
| Phage | Accession number |
|---|---|
| Enterobacteria phage 13a | NC_011045.1 |
| Enterobacteria phage 285P | NC_015249.1 |
| Enterobacteria phage BA14 | NC_011040.1 |
| Enterobacteria phage EcoDS1 | NC_011042.1 |
| Enterobacteria phage K1F | NC_007456.1 |
| Enterobacteria phage K30 | NC_015719.1 |
| Enterobacteria phage T7 | NC_001604.1 |
| Erwinia phage FE44 | NC_022744.1 |
| Erwinia phage vB_EamP-L1 | NC_019510.1 |
| Klebsiella phage K11 | NC_011043.1 |
| Klebsiella phage KP32 | NC_013647.1 |
| Kluyvera phage Kvp1 | NC_011534.1 |
| Morganella phage MmP1 | NC_011085.3 |
| Pseudomonas phage gh-1 | NC_004665.1 |
| Pseudomonas phage phi15 | NC_015208.1 |
| Pseudomonas phage phiKMV | NC_005045.1 |
| Pseudomonas phage philBB-PF7A | NC_015264.1 |
| Pseudomonas phage Phi-S1 | NC_021062.1 |
| Salmonella phage phiSG-JL2 | NC_010807.1 |
| Salmonella phage Vi06 | NC_015271.1 |
| Stenotrophomonas phage IME15 | NC_019416.1 |
| Vibrio phage ICP3 | NC_015159.1 |
| Vibrio phage N4 | NC_013651.1 |
| Vibrio phage VP4 | NC_007149.1 |
| Yersinia pestis phage phiA1122 | NC_001604.1 |
| Yersinia phage Berlin | NC_008694.1 |
| Yersinia phage phiYeO3-12 | NC_001271.1 |
| Yersinia phage Yepe2 | NC_011038.1 |
| Yersinia phage Yep-phi | NC_023715.1 |
FIG. 3.
Maximum likelihood MLSA phylogeny of the Teseptimavirus group using concatenated nucleotide sequence of the RNA polymerase, DNA polymerase, head-to-tail joining and terminase genes. The phylogeny was constructed in MEGAX using maximum composite likelihood, 8 gamma categories, and complete gap deletion with 500 bootstrap replicates. MLSA, multilocus sequence alignment.
Host range
The host range of vB_PagP-SK1 was evaluated against 94 strains of Pantoea representing 10 known species using a bacterial lawn overlay method. A total of 15 strains were found to be susceptible (Table 1). In addition to the environmental strain SN01121, which was used as the original strain to identify and enrich the phage, 12 other P. agglomerans strains were found to be susceptible, including 4 clinical and 8 environmental strains (Table 1). Outside of the P. agglomerans group, one of three Pantoea brenneri strains and one of nine P. septica strains tested were also susceptible. The closely related E. billingiae was susceptible, while E. amylovora was found to be resistant. All six tested strains of Mixta calida, another close relative of Pantoea, were resistant (Table 1). Also resistant were the other included enterics, two Escherichia coli strains and a single Kosakonia cowanii strain, along with the nonenteric gram-negative bacteria, Aeromonas and Pseudomonas, and the gram-positive Streptococcus and Staphylococcus strains (Table 1).
We then evaluated host range using a phage lawn, which was used by Luria and Delbruck20 to assess the number of resistant bacteria in their populations. This method has the advantage of being more efficient for identifying host range as many strains can be tested simultaneously, and it was expected to recover similar results as the standard bacterial lawn overlay assay. In this assay, we first applied phage to the agar surface in 96-well microplates, and then applied bacteria over the phage lawn. Susceptibility was scored following a spectrophotometric comparison of bacteria with and without phage. Using this assay, 12 strains had a reduction in OD600 of more than 0.413 (−2σ) between the no-phage bacterial control and bacteria that had been exposed, and were therefore scored as susceptible (Table 1). Of the 16 strains scored as susceptible by the traditional bacterial lawn method, 7 were also susceptible by the phage lawn method (E. billingiae, P. brenneri B014130, and P. agglomerans strains 3-770398, G4032547, SN01121, SP00303, and SP05051) (Table 1).
Discussion
A T7-like phage, vB_PagP-SK1, capable of infecting P. agglomerans SN01121 was isolated from barnyard soil. Imaging using TEM highlighted an icosahedral capsid, short tail, and multiple tail fibers that are characteristic of the members of the Podoviridae (Fig. 1A). Genomic analysis revealed the absence of an integrase or other lysogeny-related genes, suggesting that vB_PagP-SK1 is strictly a lytic phage.21 Our first host range assay used the bacterial lawn overlay method, which identifies those strains in which vB_PagP-SK1 can successfully initiate infection and produce viral progeny. This approach identified 16 susceptible strains, the majority being P. agglomerans, along with 1 P. brenneri, P. septica, and E. billingiae strain (Table 1). Although vB_PagP-SK1 was initially identified as a phage of P. agglomerans, it did not infect most P. agglomerans strains indicating that vB_PagP-SK1 may not be a strict P. agglomerans phage. This is supported by the fact that the genome of vB_PagP-SK1 shared high identity with the Erwinia phage, vB_EamP-L1, and the fact that the host range of vB_PagP-SK1 encompassed E. billingiae. This suggests that vB_PagP-SK1 is a phage of Erwinia that may transiently infect select Pantoea strains. The vB_EamP-L1 phage host range had been shown to span a large number of E. amylovora strains, although the E. billingiae and Erwinia persicina strains tested by the authors were resistant.11 The authors also showed that the host range of vB_EamP-L1 included one P. agglomerans and one P. ananatis strain, although the P. vagans strain was resistant.11
The genomes of vB_PagP-SK1 and vB_EamP-L1 shared extensive conservation, but contained multiple variable regions (Fig. 2). Many of these regions corresponded to genes that have been implicated in host specificity and phage–host interactions, including the SAMase, gp0.65, protein kinase, type II holin, and the carboxyl-terminal domain of gp17 (tail fiber/EPS depolymerase).22–27 SAM hydrolases are responsible for inactivating host restriction enzymes thereby bypassing restriction enzyme-mediated host defence mechanisms.26 Protein kinases (gp0.7) are responsible for inactivation of host RNAse E that can degrade viral mRNA, and for inactivation of the protein CasB of the host CRISPR defence mechanism.24,25 Homologues of the gene, gp5.5 (gene 19), have been shown to affect the nucleoid-associated protein H-NS, which is a bacterial defence mechanism against foreign genetic material, including phage.22
By disrupting H-NS, silencing of exogenous DNA is disrupted allowing transcription of phage genes to continue unrestricted.22 Type II holins (gene 39, gp17.5) are responsible for the timed permeability of the cellular membrane to endopeptidase or other lysis proteins resulting in the degradation of the cell wall and subsequent lysis of the host bacteria.27 The carboxyl-terminal domain of gp17 is responsible for binding with host lipopolysaccharide.23 The variability of these specific regions may modify the host range of vB_PagP-SK1 to encompass other species and/or genera.
We also identified several predicted hypothetical genes in vB_PagP-SK1 that were not found in vB_EamP-L1, but have been identified in phage infecting other members of the Enterobacteriaceae, Xanthomonadaceae, and Pseudomonadaceae,28 including Cronobacter, Citrobacter, Pseudomonas, and Stenotrophomonas (Table 1). This suggests that vB_PagP-SK1 is a mosaic of vB_EamP-L1 and other closely related phage species of Teseptimavirus, having exchanged specific genetic determinants throughout its genome. The host range of this phage may therefore extend to other members of the Enterobacteriaceae. This is consistent with a recent comparative genomics study of 60 Erwiniaceae phage genomes, which found considerable genomic variation and a large proportion of phage proteins with an unknown function.29
The plaque morphologies of vB_PagP-SK1 and vB_EamP-L1 were markedly different. The plaques produced by vB_PagP-SK1 lacked secondary halos that had been described for vB_EamP-L1.11 Halos are usually caused by a diffusible enzyme, such as EPS depolymerase,30,31 although both vB_PagP-SK1 and vB_EamP-L1 carry a predicted EPS depolymerase/tail fiber protein (gp17). The predicted EPS depolymerase/tail fiber protein (gp17) of the two phages share conservation over the first 60% of their ∼2.5 kb nucleotide sequence, with the 3′ end of the gene being divergent. This variability may result in an EPS depolymerase enzyme that has reduced diffusibility or a reduced specificity toward the EPS capsule of the bacterial strains that were evaluated.
We carried out the phage lawn assay of Luria and Delbruck, as carried out in their 1943 landmark article in which they evaluated the number of phage-resistant bacteria in their populations.20 This method has the advantage of being more efficient for identifying host range as many strains can be tested simultaneously on a single phage lawn, as opposed to using a single plate per strain; however, we found that only 7 of the original 16 strains identified as susceptible by the standard bacterial lawn method were susceptible by the phage lawn method, along with four additional P. agglomerans and one Pantoea eucalypti strain that were not identified by the bacterial lawn method (Table 1). These discrepancies may be due to lysis of normally resistant bacteria caused by phage-encoded exopolysaccharide depolymerases in phage lysates, or the presence of enzymes, antibiotics, or bacteriocins in phage lysates, which were produced by the original bacterial host.
It is also possible that in some cases, lysis was caused by “virion-mediated lysis from without,” a phenomenon through which high concentrations of phage adsorbing to bacterial surfaces can induce sufficient damage to the cell wall, even though there is no successful infection.32,33 Lysis of resistant bacteria was also reported in spot testing assays with the phage LIMElight on P. stewartii LMG 2717, P. stewartii LMG 2719, E. amylovora GBBC 403, and E. mallotivora LMG 1271, all of which were resistant in standard bacterial lawn overlay assays.10 Given the relatively small proportion of strains that were scored as susceptible with either method, the host range of this phage within Pantoea is relatively narrow.
Conclusion
We have characterized the bacteriophage vB_PagP-SK1, which belongs to the Teseptimavirus genus and was initially purified as a P. agglomerans phage. Our host range analyses suggest that vB_PagP-SK1 is capable of infecting multiple Pantoea species along with strains Erwinia. Our genomic analysis indicated that vB_PagP-SK1 most closely resembles the Erwinia phage vB_EamP-L1, even though the host ranges appear to be slightly different. The presence of xenologous genes in the vB_PagP-SK1 genome originating from phage that infects a breadth of genera indicates that it may be a mosaic of vB_EamP-L1 and other phages that infect members of the Enterobacteriaceae, which may be impacting host range.
Authorship Confirmation Statement
D.L.M., C.D.S., B.J.P., and C.B. performed experiments, acquired data, wrote the early drafts of the manuscript, and revised the final drafts of the manuscript. D.A., C.K.Y., and J.S. were responsible for the conception and direction of the work, analyzing and interpreting data, and writing and revising the final drafts of the manuscript. All coauthors have reviewed and approved of the manuscript before submission, and agree to be accountable for all aspects of the work. This manuscript has been submitted solely to this journal and is not published, in press, or submitted elsewhere.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The authors gratefully acknowledge financial support from the Natural Sciences and Engineering Research Council of Canada (Discovery Grant #2015-06417 to John Stavrinides and Discovery Grant #2016-05670 to Chris Yost).
References
- 1. Nadarasah G, Stavrinides J. Quantitative evaluation of the host-colonizing capabilities of the enteric bacterium Pantoea using plant and insect hosts. Microbiol (United Kingdom). 2014;160:602–615. [DOI] [PubMed] [Google Scholar]
- 2. Cruz AT, Cazacu AC, Allen CH. Pantoea agglomerans, a plant pathogen causing human disease. J Clin Microbiol. 2007;45:1989–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dutkiewicz J, Mackiewicz B, Lemieszek MK, et al. Pantoea agglomerans: A mysterious bacterium of evil and good. Part III. Deleterious effects: Infections of humans, animals and plants. Ann Agric Environ Med. 2016;23:197–205. [DOI] [PubMed] [Google Scholar]
- 4. Walterson AM, Stavrinides J. Pantoea: Insights into a highly versatile and diverse genus within the Enterobacteriaceae. FEMS Microbiol Rev. 2015;39:968–984. [DOI] [PubMed] [Google Scholar]
- 5. Walterson AM, Smith DDN, Stavrinides J. Identification of a Pantoea biosynthetic cluster that directs the synthesis of an antimicrobial natural product. PLoS One. 2014;9:e96208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Maayer P, Chan W-Y, Blom J, et al. The large universal Pantoea plasmid LPP-1 plays a major role in biological and ecological diversification. BMC Genomics. 2012;13:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith DDN, Nickzad A, Déziel E, et al. A novel glycolipid biosurfactant confers grazing resistance upon Pantoea ananatis BRT175 against the social amoeba Dictyostelium discoideum. mSphere. 2016;1:e00075-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frost LS, Leplae R, Summers AO, et al. Mobile genetic elements: The agents of open source evolution. Nat Rev Microbiol. 2005;3:722–732. [DOI] [PubMed] [Google Scholar]
- 9. Fortier L-C, Sekulovic O. Importance of prophages to evolution and virulence of bacterial pathogens. Virulence. 2013;4:354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adriaenssens EM, Ceyssens P-J, Dunon V, et al. Bacteriophages LIMElight and LIMEzero of Pantoea agglomerans, belonging to the “phiKMV-like viruses”. Appl Environ Microbiol. 2011;77:3443–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Born Y, Fieseler L, Marazzi J, et al. Novel virulent and broad-host-range Erwinia amylovora bacteriophages reveal a high degree of mosaicism and a relationship to Enterobacteriaceae phages. Appl Environ Microbiol. 2011;77:5945–5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sambrook J, Russel, DW. Molecular Cloning, 3-Volume Set: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- 13. Santos MA. An improved method for the small scale preparation of bacteriophage DNA based on phage precipitation by zinc chloride. Nucleic Acids Res. 1991;19:5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Besemer J. GeneMarkS: A self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res. 2001;29:2607–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arndt D, Grant JR, Marcu A, et al. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016;44:W16–W21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Darling AE, Mau B, Perna NT. progressiveMauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS One. 2010;5:e11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Larkin MA, Blackshields G, Brown NP. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. [DOI] [PubMed] [Google Scholar]
- 18. Kumar S, Stecher G, Li M, et al. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abedon S. Bacteriophage Ecology: Population Growth, Evolution and Impact of Bacterial Viruses. Advances in Molecular and Cellular Microbiology. Cambridge, UK: Cambridge University Press, 2008. [Google Scholar]
- 20. Luria SE, Delbrück M. Mutations of bacteria from virus sensitivity to virus resistance. Genetics. 1943;28:491–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Groth AC, Calos MP. Phage integrases: Biology and applications. J Mol Biol. 2004;335:667–678. [DOI] [PubMed] [Google Scholar]
- 22. Ali SS, Beckett E, Bae SJ, et al. The 5.5 protein of phage T7 inhibits H-NS through interactions with the central oligomerization domain. J Bacteriol. 2011;193:4881–4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garcia-Doval C, van Raaij MJ. Structure of the receptor-binding carboxy-terminal domain of bacteriophage T7 tail fibers. Proc Natl Acad Sci U S A. 2012;109:9390–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marchand I, Nicholson AW, Dreyfus M. Bacteriophage T7 protein kinase phosphorylates RNase E and stabilizes mRNAs synthesized by T7 RNA polymerase. Mol Microbiol. 2001;42:767–776. [DOI] [PubMed] [Google Scholar]
- 25. Qimron U, Tabor S, Richardson CC. New details about bacteriophage T7-host interactions. Microbe Mag. 2010;5:117–122. [Google Scholar]
- 26. Studier FW, Movva NR. SAMase gene of bacteriophage T3 is responsible for overcoming host restriction. J Virol. 1976;19:136–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang I-N, Smith DL, Young R. Holins: The protein clocks of bacteriophage infections. Annu Rev Microbiol. 2000;54:799–825. [DOI] [PubMed] [Google Scholar]
- 28. Casjens SR. Comparative genomics and evolution of the tailed-bacteriophages. Curr Opin Microbiol. 2005;8:451–458. [DOI] [PubMed] [Google Scholar]
- 29. Thompson DW, Casjens SR, Sharma R, et al. Genomic comparison of 60 completely sequenced bacteriophages that infect Erwinia and/or Pantoea bacteria. Virology. 2019;535:59–73. [DOI] [PubMed] [Google Scholar]
- 30. Hughes KA, Sutherland IW, Clark J, et al. Bacteriophage and associated polysaccharide depolymerases—Novel tools for study of bacterial biofilms. J Appl Microbiol. 1998;85:583–590. [DOI] [PubMed] [Google Scholar]
- 31. Hartung JS. Cloning of a bacteriophage polysaccharide depolymerase gene and its expression in Erwinia amylovora. Mol Plant Microbe Interact. 1988;1:87. [Google Scholar]
- 32. Loessner MJ. Bacteriophage endolysins—Current state of research and applications. Curr Opin Microbiol. 2005;8:480–487. [DOI] [PubMed] [Google Scholar]
- 33. Abedon ST. Lysis from without. Bacteriophage. 2011;1:46–49. [DOI] [PMC free article] [PubMed] [Google Scholar]