Abstract
Background: Poultry products are the largest food category linked to salmonellosis in Canada. Bacteriophages (phages) have been proposed as a novel antimicrobial in the poultry industry due to their documented ubiquity, efficacy, and safety benefits.
Materials and Methods: A library of 78 lytic phages was rapidly screened against 50 prominent poultry-associated Salmonella enterica isolates procured from British Columbia, Canada.
Results: A phage cocktail was successfully formulated using only three sewage-isolated phages (SE4, SE13, and SE20) to achieve broad-spectrum antimicrobial efficacy across all S. enterica serovars. Highly promising phages were also characterized using one-step growth curves and transmission electron microscopy.
Conclusion: Relative host efficiency is a new agar-based semiquantitative metric developed here for the rapid comparison of different phages against a panel of known bacterial targets.
Keywords: Salmonella enterica, poultry, bacteriophage cocktail, relative host efficiency
Introduction
Nontyphoidal Salmonella enterica accounts for ∼94 million enteric infections annually, of which 80.3 million are considered foodborne, resulting in 155,000 human deaths globally.1 Poultry products are the largest food category linked to salmonellosis in Canada, with serovars of S. enterica as the most prevalent sources of human disease.2 The occurrence of Salmonella on poultry farms has been found to range from 5% to 100% based on fecal and ecological sampling.3 Broilers harboring these common poultry-associated S. enterica serotypes at typical concentrations tend to display no disease symptoms, making on-farm diagnosis difficult.
Salmonella enterica serotype Enteritidis is the most predominant source of poultry product-related contamination resulting in foodborne human illness.4 Salmonella Enteritidis has been an unrelenting challenge in the poultry industry despite numerous biosecurity and antimicrobial countermeasures ranging from vaccinations and stringent hygiene requirements to segregation of production zones, constant monitoring, and established disease response plans throughout the production chain.5
The overusage of antibiotics in human medicine and agriculture has been connected to the emergence of widespread antimicrobial resistance in many pathogens, including Salmonella.6 In particular, the emergence of Salmonella isolates resistant to third-generation cephalosporins, fluoroquinolones, and quinolones has notably increased in recent years.7 In Canada, these concerns have already motivated the elimination of Categories I (those most important to human health) and II antibiotics in poultry production, with Category III set for removal by the end of 2020.8 Therefore, research for alternative antimicrobials to supplement antibiotic use is essential to sustain high yields in poultry production.9
Bacteriophages, which are bacterial viruses, have shown promise as alternative antimicrobial agents for combatting pathogens associated with agriculture.10 The ability of a phage to target and infect a bacterium is dependent on surface-associated receptors, such as lipopolysaccharides, fimbriae, or proteins.11 Phages offer numerous advantages over current antibiotics including natural abundance, nontoxicity to host cells and microbiome, high specificity to target bacteria, and affordability.12 Early attempts at poultry-based phage therapy has shown antimicrobial efficacy against pathogens including Salmonella,13 Escherichia coli,14 and Campylobacter.15 Despite the ostensible potential of phage treatments, shortcomings still exist that must be investigated before the adoption of phages as reliable antimicrobials in any large industry.
Phages can either reproduce through the lytic or lysogenic cycles depending on their genetic composition. Virulent phages undergo the lytic cycle meaning they simply infect bacterial cells and multiply rapidly leading to host death. However, temperate phages utilize the lysogenic cycle by integrating into the bacterial genome and lying dormant until a stressor triggers the lytic cycle.11 Only phages containing annotated genomes identified as strictly lytic by Fong et al. (2019) were used in this study to formulate phage cocktails since phages containing integrative or toxic-encoding genes must be excluded for antimicrobial use.16 Phage bactericidal efficacy should then be screened in vitro to identify the best cocktail candidates for broad-spectrum activity against defined bacterial targets within a niche. Since individual phage characteristics can also impact infection efficacy differently between liquid and agar media, a treatment phage should be effective against a target pathogen in both models. By investigating activity in a variety of models, we can better understand their efficacy in diverse environments. Application parameters can then be optimized to achieve ideal treatment procedures within real-world settings.17
This study evaluated the in vitro efficacy of strictly virulent phages from assorted environments in British Columbia (BC), Canada, against a panel of poultry-associated S. enterica serotypes. We hypothesize that a viable phage cocktail can be formulated from our Salmonella phage library (n = 78) that will achieve broad-spectrum coverage across S. enterica serovars (n = 50) implicated with poultry in BC.
Materials and Methods
Bacterial strains and culture conditions
Salmonella enterica isolates (n = 50) representing seven serotypes were isolated by the BC Ministry of Agriculture from various environmental and diagnostic poultry samples in 2018 (Table 1). All Salmonella strains were kept at −80°C in tryptic soy broth (TSB; Becton, Dickson and Company, Sparks, MD) supplemented with 25% glycerol for long-term storage. Working stocks were made by quadrant-streaking glycerol stocks onto tryptic soy agar (TSA; Becton, Dickson and Company) plates, incubating for 18 h at 37°C and then stored at 4°C. Phages (n = 78) were previously isolated from a variety of environments in BC, including sewage, chicken feces, various water sources, and sediment.18 Phages were propagated according to Adams,19 and pure phage lysates were stored at 4°C in TSB.
Table 1.
Poultry-Associated Salmonella Isolates from British Columbia Ministry of Agriculture from the Last Quarter of 2018 Representing the Percentage of Observed Positive Serotypes
| Isolate no. | Source | Serogroup | Serotype |
|---|---|---|---|
| 1 | Environmental | B | Brandenburg |
| 2 | Environmental | B | I: 4, 5, 12: i: — |
| 3–4 | Diagnostic | B | Reading |
| 5–6 | Diagnostic | B | Typhimurium |
| 7 | Environmental | C1/C4 | Rissen |
| 8–25 | Environmental | C2/C3 | Kentucky |
| 26–50 | Diagnostic/environmental | D1 | Enteritidis |
Bacteriophage host range characterization
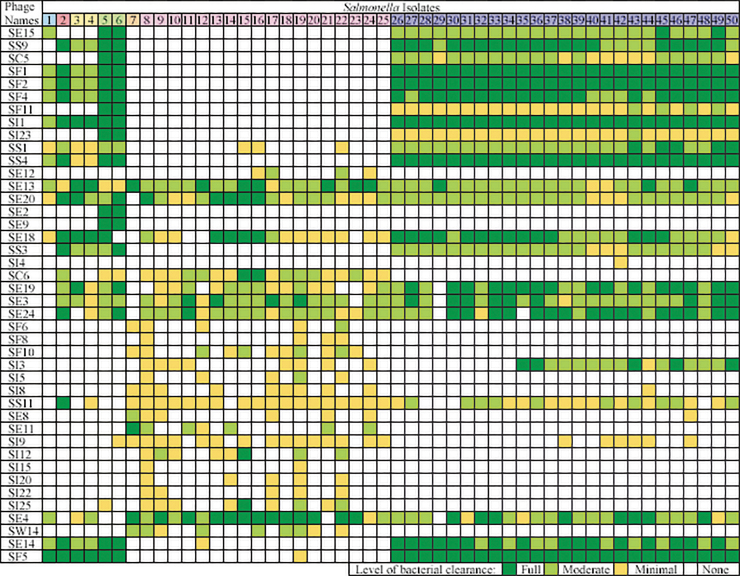
Host range spot tests were conducted to assess the bactericidal efficacy of individual phages (n = 78) against each poultry S. enterica isolate (n = 50). Salmonella isolates were grown in 5 mL of TSB for 16–18 h at 37°C with shaking at 170 rpm. Next, 300 μL of a 1/10 dilution of the bacterial culture (optical density at 600 nm [OD600] = 0.6) was added to 4 mL of 50°C TSB with 0.7% agar (VWR) and poured onto a TSA plate. After allowing top agar to solidify for ∼10 min, 5 μL of undiluted phage lysate was spotted onto the bacterial lawn in duplicate and incubated for 18 ± 2 h at 37°C. The laboratory phage titers ranged from 107 to 1011 plaque forming units (PFU)/mL. The phage spots were categorized from 0 to 3 based on clarity (Fig. 1), with 3 representing a clear plaque and 0 delineating no clearing according to Kutter.20
FIG. 1.
Truncated heat map indicating the qualitative level of clearing produced by the best plaque-forming phages spotted onto bacterial lawns. The first letter in the phage name denotes the isolation host (S = Salmonella), and the second letter denotes the isolation source (B = beach samples, C = chicken samples, E = sewage effluent, F = cattle feces, I = irrigation water, S = sediment, and W = other water samples).
Efficiency of plating assay
A subset of phages (n = 18) selected for their broad host ranges (Supplementary Table S1) were subsequently screened at a series of concentrations to test for lysis from without and better quantify phage bactericidal efficacy against each poultry Salmonella isolate (n = 50) according to Kutter.20 Salmonella enterica cultures and 0.7% top agars (VWR) were prepared and poured as already described. Phages were serially diluted to produce sequential 10-fold dilutions from 100 to 10−8. Each dilution was spotted in duplicate onto bacterial lawns and incubated for 16–18 h at 37°C.
Relative host efficiency (RHE) is a comparative score that we developed for this stage of screening to account for initial phage titer variations usually overlooked by classic efficiency of plating (EOP) analysis. Normally, phages would be diluted to equal concentrations before EOP testing. However, since our goal is to identify the best overall phage candidates against this panel of real-world S. enterica serovars, phage amplification potential should not be dismissed. RHE is a logarithmic scoring metric that provides the magnitude difference between the maximum phage titer and the phage spot dilution that results in visible plaques during EOP against each bacterial host (Calculation 1). We believe that this is important to distinguish for the practical application of phage treatments since amplification potential could add to the overall antibacterial value of a phage candidate.
Calculation 1: RHE score calculation for one phage against one bacterial host. C = highest phage concentration achieved with library amplification host (PFU/mL); C0 = lowest phage concentration producing visually detectable plaques during EOP; Pn = phage number.
In vitro infectivity assay
The phages with the highest RHE scores (n = 6) were tested at multiplicity of infection (MOI) = 10 against one representative poultry Salmonella isolate for each serotype (n = 7). First, 96-well round-bottomed plates were inoculated with 100 μL of each phage dilution in triplicate. The plate was then left for 30 min to equilibrate to room temperature. Salmonella enterica cultures were prepared as already described. One milliliter of bacterial culture was then transferred to 9 mL of TSB and incubated (37°C, 170 rpm) until OD600 = 0.6 was obtained (∼5 × 108 colony forming units [CFU]/mL in log phase). Next, 100 μL of bacterial culture was added to all wells of the equilibrated plate, excluding negative control wells. The plate was then placed in a plate reader (SpectraMax M2; Molecular Devices, Sunnyvale, CA) set to 25°C. Readings were recorded at OD600 every 30 min for 48 h with 5 s of shaking before each reading to determine bacterial cell abundance. Each phage was measured in triplicate against representative poultry-associated serotypes. This assay was then repeated with 11 different phage cocktail formulations based on single phage broth-based results. The positive (+) control wells contained Salmonella with no phage, and the negative (−) control wells had 200 μL of TSB. Since the growth rates differed between the representative S. enterica serovars, phage antibacterial efficacy was compared by converting phage treatment growth curves into a percentage of the positive (+) control for each serovar using Calculation 2.
Calculation 2: To quantitatively compare the inhibition effect of phages against representative S. enterica serotypes, triplicate OD600 readings for each treatment were averaged and then converted to a percentage of the untreated positive control average after subtracting the mean of the negative control wells.
One-step growth curves
One-step growth curves were generated for the best putative phages from EOP (n = 6) to determine the burst sizes and burst times, with modifications based on Park et al.21 Bacterial cultures corresponding to phage amplification hosts were grown for 16 h in TSB at 37°C with shaking at 170 rpm. One milliliter of bacterial culture was then transferred to 9 mL of TSB and incubated (37°C, 170 rpm) until an OD600 of 1.0 was observed (∼1 × 109 CFU/mL). Phages were added to their corresponding bacterial culture at MOI = 0.01 and incubated at room temperature for 5 min to allow phage adsorption. Next, cocultures were centrifuged at 4000 × g for 10 min at 4°C and supernatant was discarded to remove unadsorbed phages. The pellets were resuspended with 10 mL of TSB, then 45 μL aliquots were taken in 10 min intervals for 150 min. Aliquots were immediately serial diluted (from 100 to 10−8) in SM buffer and 5 μL of each dilution was spotted onto the 0.7% TSA overlay in duplicates. This protocol was repeated independently for each phage in three replicates. Salmonella enterica cultures and 0.7% top agars (VWR) were prepared and poured as already described in the preceding section. The spotted plates were incubated at 37°C for 18 ± 2 h and imaged for plaque formation.
Transmission electron microscopy
Concentrated phages were diluted to 1.0 × 108 PFU/mL in filter sterilized 0.1 M ammonium acetate (Amresco, Solon, OH) for SE4, SE11, SE13, SE14, SE19, and SE20. Copper grids (Ted Pella, Redding, CA) were negatively charged by exposure to glow discharge, then 10 μL of diluted phage preparation was pipetted onto the grid and allowed to sit at room temperature for 1 min. Next, excess fluid was carefully absorbed from the edge of the sample using filter paper. Then, 20 μL of uranyl acetate 2% was pipetted onto the grid and incubated at room temperature for 20 s. Excess fluid was absorbed from the edge of the sample using filter paper and the grid was air dried for 3 min. A Hitachi H-7600 transmission electron microscope (TEM) was used to image phage samples at the University of British Columbia Bioimaging Facility. Accelerating voltage was 80.0 kV.
Results
Host range spot test analysis of bacteriophages against S. enterica poultry isolates
Spot tests were conducted to identify the host range of phages across the poultry-associated Salmonella library (Table 1). Phages resulting in clear plaques (scored 2–3) against target Salmonella serovars were selected for further testing, whereas phages resulting in weak or no plaques (scored 0–1) were omitted. This assay indicated that 18/78 phages tested were promising based on their perceived antimicrobial efficacy at the serotype level (Fig. 1).
EOP assay
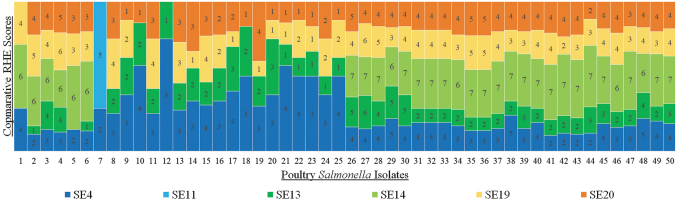
EOP was performed to more clearly differentiate the antimicrobial efficacy of the most promising phages identified from host range spot testing (n = 18). This was done by diluting the best stock phages from their maximum concentrations, spotting them on bacterial lawns and once again observing subsequent plaquing patterns. EOP testing narrowed down the best phage candidates from 18 to 6 based on observed serotype inactivation efficacy. RHE scores were then generated for each phage–host combination with Calculation 1 to compare the overall antimicrobial efficacy of each phage, taking host amplification potential into consideration. RHE scores indicate the magnitude difference between the maximum phage titer and the greatest phage dilution that still produces visible plaques.
As depicted by the comparative RHE profiles in Figure 2, phages SE4, SE11, SE13, SE14, SE19, and SE20, all isolated from sewage, had high scores across target bacteria. Therefore, we decided to proceed with these six phages for cocktail formulation. These phages were selected because their RHE profiles (Fig. 2) indicated that their bactericidal efficacies would provide complementary broad-spectrum coverage when combined across all prevalent S. enterica isolates. Phage SE4 displayed bactericidal activity across all bacterial hosts, especially against S. Kentucky isolates. Phage SE11 was only bactericidal against S. Rissen, however, it was most able to inhibit this isolate. Phages SE13, SE14, SE19, and SE20 each displayed broad-spectrum bactericidal activity; however, SE14 did not exhibit inhibition of S. Kentucky.
FIG. 2.
Comparative RHE profiles developed from efficiency of plating results for the subset of six sewage-isolated phages. RHE scores appeared optimal for the generation of a broad-spectrum cocktail against all Salmonella enterica isolates. Higher RHE scores and larger corresponding bars indicate that a phage is more effective against poultry Salmonella isolates when greatly diluted. RHE, relative host efficiency.
RHE scores are displayed in Figure 2 to compare phage efficacy against each poultry-associated bacterial isolate. RHE scores provided a magnitude-based approximation of phage efficacy to narrow down putative therapeutic phage candidates before proceeding with broth-based infectivity assays.
In vitro infectivity assays
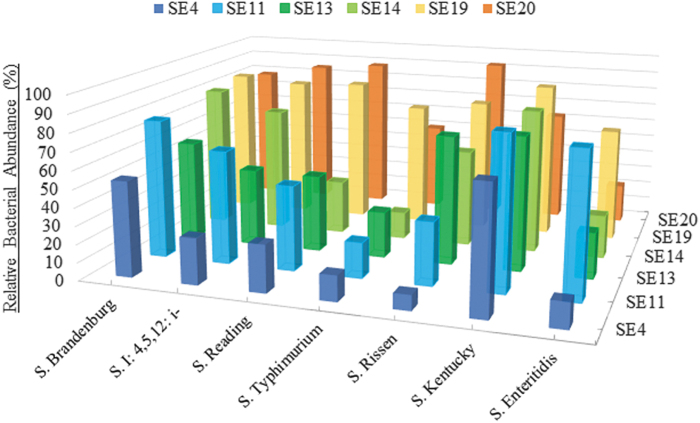
To observe real-time infection dynamics, a broth-based in vitro infectivity assay was conducted (Fig. 3) for each putative phage (n = 6) against one representative target bacteria for each S. enterica serotype (n = 7). To quantitatively compare the inhibition effect of single phages between the different representative bacterial growth curves, triplicate OD600 readings were averaged and then converted to a percentage of the untreated positive control with Calculation 2. Based on the quantitative profiles observed in Figure 3, 11 phage cocktail formulations were proposed. Each cocktail contained three to five phages with complementary infectivity profiles so that bactericidal activity could be achieved across all Salmonella strains under consideration. Broth-based in vitro infectivity assays were then repeated for the proposed phage cocktail formulations (n = 11) against the representative S. enterica serotypes (n = 7).
FIG. 3.
Individual phage inhibition efficacy against each S. enterica representative serotype after T = 48 h at 25°C. At T = 0, 100 μL of undiluted phage was added to 100 μL of bacterial culture (OD600 = 0.6). OD600, optical density at 600 nm.
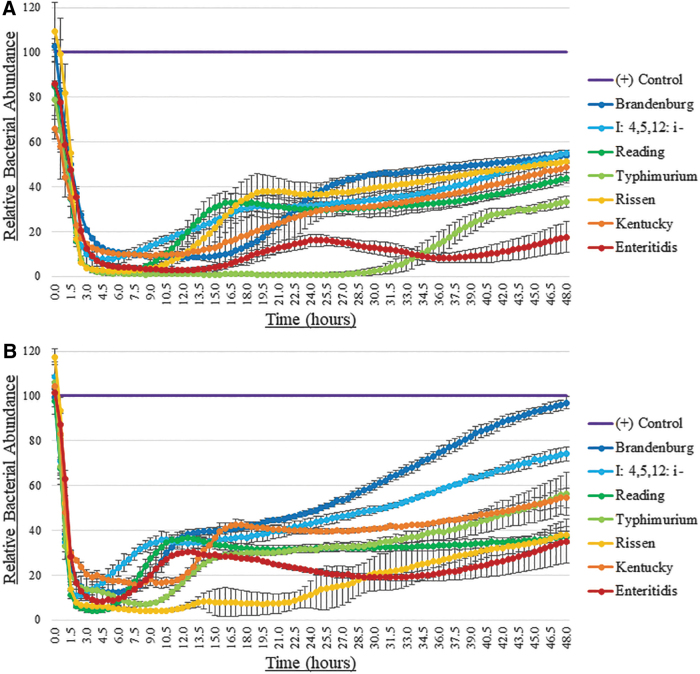
Growth inhibition of representative S. enterica serotypes by the selected phage cocktail formulation (SE4, SE13, and SE20) was monitored at OD600 for 48 h at 25°C and 37°C (Fig. 4). Phage cocktail antimicrobial efficacy was observed for all representative target serotypes at both temperatures tested. Greater inhibition was observed overall at 25°C than at 37°C.
FIG. 4.
Relative bacterial abundance for representative S. enterica isolates from each of the seven serotypes against the best formulated phage cocktail for 48 h at (A) 25°C and (B) 37°C. Lower values designate a greater decrease in OD600 readings than positive controls, indicating better cocktail bactericidal activity for a serotype.
One-step growth curves
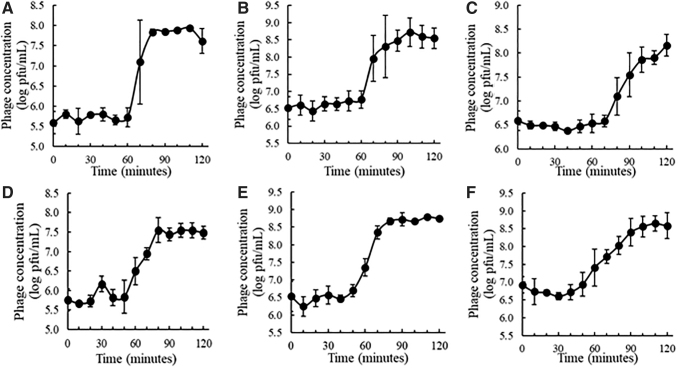
One-step growth curves were generated with phage amplification hosts to observe infection parameters for the most promising phages identified for broad-spectrum cocktail formulation (Fig. 5). The latent period is the time it takes for phages to infect and propagate within their host before cellular lysis. Observed latent periods were ∼40 min for SE19 and SE20, ∼50 min for SE14, ∼60 min for SE4 and SE11, and ∼70 min for SE13. After the latent period, there is a sharp increase in phage titer as phages burst from host cells, then phage titers plateau once again. The difference between phage titers before and after lysis, or the burst size, indicates the approximate number of phages ejected from each bacterial cell. The burst sizes, or phages produced per infected cell, were ∼135 for SE19, ∼129 for SE4, ∼91 for SE11, ∼65 for SE20, ∼45 for SE14, and ∼35 for SE13.
FIG. 5.
One-step growth curves for bacteriophages: (A) SE4, (B) SE11, (C) SE13, (D) SE14, (E) SE19, and (F) SE20. Bacteriophages were added to bacterial culture at multiplicity of infection = 0.01. Three replicates were conducted for each phage.
Transmission electron microscopy
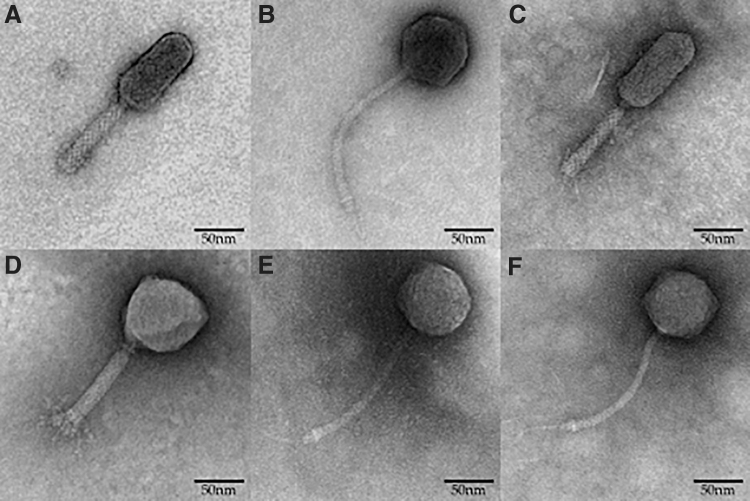
TEM images were captured for top phage candidates (n = 6) identified with RHE scores. Visual inspection revealed that SE4, SE13, and SE14 phages possessed morphologies of the family Myoviridae, and SE11, SE19, and SE20 of the family Siphoviridae (Fig. 6). SE4, SE13, and SE14 appear to be ∼210 nm in length, whereas SE11, SE19, and SE20 appear to be ∼250 nm in length.
FIG. 6.
Transmission electron microscope images of phages: (A) SE4, (B) SE11, (C) SE13, (D) SE14, (E) SE19, and (F) SE20. Phages SE11, SE19, and SE20 display flexible noncontractile tails and icosahedral heads characteristic of the family Siphoviridae. Phages SE4, SE13, and SE14 display contractile tails and icosahedral heads characteristic of the family Myoviridae.
Discussion
A central objective of current phage research is to define the safety and sustainability of treatments within industry-specific niches. In this study, the efficacy of 78 lytic Salmonella phages from various BC environments was screened against 50 S. enterica isolates representing the most prominent poultry-associated serotypes observed by the BC Ministry of Agriculture (Table 1). The goal of this study was to rapidly produce a practical broad-spectrum phage cocktail that proved highly effective in vitro across these S. enterica isolates from a generic collection of environmental Salmonella phages. Such a cocktail would build upon early attempts to reduce pathogenic bacteria harbored within the poultry industry.
Host range spotting and EOP were used as preliminary qualitative measures to eliminate many phages from contention as therapeutic agents for the pathogens of interest. In both agar-based tests, phages were ranked qualitatively by assessing the plaques that formed on bacterial lawns. At the onset of this study, the undiluted bactericidal efficacy of each phage was observed against each S. enterica isolate (Fig. 1).
Importantly, phage–host range is influenced by numerous host-specific factors such as antigenic receptor profiles, restriction endonucleases, and many others. Therefore, phage infection efficiency is not absolute, but rather relative to individual bacterial hosts.22 Since our phage library was propagated and stored with an assortment of Salmonella serovars before this study, EOP was implemented for each phage–host combination after host range spot testing to identify the relative efficiency of each phage with all S. enterica isolates. Phages that showed completely clear plaques at this rapid agar-based phase were noted (n = 18) as this indicates high infection success. Those that did not produce high-quality plaques were not pursued for further testing.
At this stage of screening, a new metric known as RHE was developed to rapidly score and compare lytic phage efficiency within and between all 50 S. enterica isolates (Calculation 1). RHE was helpful for quickly illustrating the approximate comparative performance of phages against each poultry-associated host (Fig. 2) by indicating the magnitude by which a phage could be diluted while still maintaining bactericidal activity. RHE scores were used to narrow down potential phage candidates before more time-consuming broth-based quantification methods. Owing to the highly specific nature of phages, superior phage storage titers did not always indicate the best compatibility for target hosts. Low RHE scores indicated that phages were either causing lysis from without during spot test analysis or that phages had low lytic efficiency. The phage candidates with the highest RHE scores (Fig. 2) included SE4, SE11, SE13, SE14, SE19, and SE20, which all proceeded to additional testing. These phages were all isolated from sewage. Some other phages from EOP could have arguably been carried forward for additional testing; however, the goal of this study was to rapidly create a suitable broad-spectrum cocktail. The phage candidates chosen at this juncture appeared to contain complementary RHE profiles that could achieve this end. Had additional testing revealed inadequacy of these phages, the next best phages from this section could have been revisited.
The best phage candidates identified according to RHE scores appeared to have varying efficacy against representative hosts as shown in Figure 3. Since the RHE scores for each phage appeared to be highly serotype-specific, subsequent broth-based tests were conducted with one representative S. enterica isolate for each serotype from the poultry-associated bacterial library. The OD600 values were converted to a percentage of the untreated positive control (Calculation 2) to compare the relative decrease in bacteria for each representative S. enterica serotype. These quantitative individual phage profiles were compared to predict phage combinations that would be most successful together as a broad-spectrum cocktail. The most effective and efficient cocktail combination (i.e., greatest bactericidal effect and least phages required) contained SE4, SE13, and SE20. Since these phages could effectively target and infect S. Rissen, SE11 was not included in the final concoction. Since phages SE19 and SE20 consistently showed similar profiles during screening, SE19 was left out since SE20 performed mildly better with some representative hosts.
The relative bacterial abundance (Fig. 4) for the ideal phage cocktail had variable results at 25°C and 37°C, most likely because phages infect bacteria during active cellular replication. Therefore, bacteria grown at 25°C may have been more effectively inhibited by the cocktail due to the 37°C cultures reaching stationary phase more rapidly. Although there are many complicated environmental factors to consider for a treatment applied to a living animal, bacteria harbored within a broiler chicken, for example, would likely not replicate at the maximum rate observed here at 37°C because of competition with other microbes within the gut, suboptimal pH, and an assortment of naturally present compounds that may also inhibit growth. Notably, in both cases, S. Enteritidis, which is the most common isolate harbored in poultry, remained the most inhibited by the identified phage cocktail.
Burst sizes and latent periods are distinctive for each phage–host interaction within specific environments.23 It is desirable for a therapeutic phage to display a rapid latent period and a high burst size as this would indicate greater potential for biocontrol.24 The latent periods and burst sizes for each of the best phage candidates identified (Fig. 5) appeared to be comparable with other phages deemed useful for application in an agricultural setting.25 All best phage candidates (n = 6) were also observed by TEM to examine their respective morphologies. TEM images indicated that phages SE11, SE19, and SE20 displayed the flexible, noncontractile tails, and icosahedral heads characteristic of the family Siphoviridae. Furthermore, phages SE4, SE13, and SE14 appeared to have contractile tails and icosahedral heads characteristic of the family Myoviridae. Both families are within the order Caudovirales that are defined as tailed phages containing double-stranded DNA genomes and are commonly applied in phage treatment research.19
A phage cocktail treatment was successfully formulated from our generic Salmonella phage library for all S. enterica serotypes identified as prevalent in poultry by the BC Ministry of Agriculture. We have demonstrated that such a generic phage library was sufficient for rapid cocktail development in vitro without implementation of additional timely phage library host optimization measures. If a cocktail had not been successfully identified during this straightforward procedure, then more time-consuming methods could have followed. However, our practical RHE metric appeared to work well as a rapid comparative measurement to narrow down the best phage candidates before broth-based quantitative measurements. In this way, the double-edged sword of incredible host specificity was overcome by the other great aspect of phages, which is their overwhelming ubiquity. A successful in vitro cocktail such as that defined here should be further tested in small scale field trials to identify best application timing and methodology for antimicrobial use in a real-world industrial poultry setting.20
Conclusions
Phage treatments require customization for each environmental niche due to their extraordinary host specificity. Although most Salmonella phages from our generic library had limited effects, a subset of phages was found to be highly efficacious in combination across all target S. enterica poultry isolates. This indicates that phage ubiquity can compensate for challenges associated with extreme host specificity when developing a phage cocktail for a suite of niche-specific bacterial targets, as seen harbored in the Canadian poultry industry. To address the urgent need for alternative antimicrobials in the poultry sector, future research should investigate the real-world efficacy of a cocktail developed in this manner for this specific industrial niche.
Supplementary Material
Acknowledgment
We thank Erin Zabek from Animal Health Centre, British Columbia Ministry of Agriculture for providing the Salmonella strains used in this study.
Authors' Contributions
All authors have provided their approval before submission and agree to the integrity of this study as it has been presented. T.B. and S.W. conceived of the presented idea. T.B. and S.L. carried out the experiment. K.F. provided the samples. T.B. performed the analytic calculations and took the lead in writing the article with input from all authors. S.W. supervised the project.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This research was funded by Canada-BC Agri-Innovation Program (URACP19-210). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the article; or in the decision to publish the results.
Supplementary Material
References
- 1. Majowicz SE, Musto J, Scallan E, et al. The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50(6):882–889. [DOI] [PubMed] [Google Scholar]
- 2. Public Health Agency of Canada. Key findings of the quantitative microbial risk assessment for Salmonella in broiler chicken meat produced and consumed in Canada. 2019. https://www.canada.ca/en/health-canada/services/food-nutrition/reports-publications/food-risk-analysis-publications/key-findings-quantitative-microbial-risk-assessment-salmonella-broiler-chicken.html Last accessed September 10, 2019.
- 3. Rodriguez A, Pangloli P, Richards HA, et al. Prevalence of Salmonella in diverse environmental farm samples. J Food Prot. 2006;69(11):2576–2580. [DOI] [PubMed] [Google Scholar]
- 4. Borie C, Albala I, Sánchez P, et al. Bacteriophage treatment reduces Salmonella colonization of infected chickens. Avian Dis. 2008;52(1):64–67. [DOI] [PubMed] [Google Scholar]
- 5. Kim A, Lee YJ, Kang MS, et al. Dissemination and tracking of Salmonella spp. in integrated broiler operation. J Vet Sci. 2007;8(2):155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Su LH, Chiu CH, Chu C, et al. Antimicrobial resistance in nontyphoid Salmonella serotypes: A global challenge. Clin Infect Dis. 2004;9(4):546–551. [DOI] [PubMed] [Google Scholar]
- 7. Martin LJ, Fyfe M, Doré K, et al. Increased burden of illness associated with antimicrobial-resistant Salmonella enterica serotype Typhimurium infections. J Infect Dis. 2004;189(3):377–384. [DOI] [PubMed] [Google Scholar]
- 8. Chicken Farmers of Canada. AMU strategy, a prescription for change. 2018. www.chickenfarmers.ca/wp-content/uploads/2018/01/AMU-Magazine_ENG_web-2.pdf Last accessed January 3, 2020.
- 9. de Oliveira SD, Flores FS, dos Santos LR, et al. Antimicrobial resistance in Salmonella Enteritidis strains isolated from broiler carcasses, food, human and poultry-related samples. Int J Food Microbiol. 2005;97(3):297–305. [DOI] [PubMed] [Google Scholar]
- 10. Summers WC. Bacteriophage research: Early history. In: Kutter E, Sulakvelidze A; eds. Bacteriophages: Biology and applications. Boca Raton, FL: CRC Press; 2005:5–27. [Google Scholar]
- 11. Domingo-Calap P, Georgel P, Bahram S. Back to the future: Bacteriophages as promising therapeutic tools. HLA. 2016;87(3):133–140. [DOI] [PubMed] [Google Scholar]
- 12. Sulakvelidze A, Alavidze Z, Morris JG. Bacteriophage therapy. Antimicrob Agents Chemother. 2001;45(3):649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andreatti Filho RL, Higgins JP, Higgins SE, et al. Ability of bacteriophages isolated from different sources to reduce Salmonella enterica serovar Enteritidis in vitro and in vivo. Poult Sci. 2007;86(9):1904–1909. [DOI] [PubMed] [Google Scholar]
- 14. Huff WE, Huff GR, Rath NC, et al. Prevention of Escherichia coli infection in broiler chickens with a bacteriophage aerosol spray. Poult Sci. 2002;81(10):1486–1491. [DOI] [PubMed] [Google Scholar]
- 15. Goode D, Allen VM, Barrow PA. Reduction of experimental Salmonella and Campylobacter contamination of chicken skin by application of lytic bacteriophages. Appl Environ Microbiol. 2003;69(8):5032–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wernicki A, Nowaczek A, Urban-Chmiel R. Bacteriophage therapy to combat bacterial infections in poultry. Virol J. 2017;14(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lim TH, Kim MS, Lee DH, et al. Y. Use of bacteriophage for biological control of Salmonella Enteritidis infection in chicken. Res Vet Sci. 2012;93(3):1173–1178. [DOI] [PubMed] [Google Scholar]
- 18. Fong K, Tremblay DM, Delaquis P, et al. Diversity and host specificity revealed by biological characterization and whole genome sequencing of bacteriophages infecting Salmonella enterica. Viruses. 2019;11(9):854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adams MH. Bacteriophages. New York, NY: Interscience Publishers; 1959. [Google Scholar]
- 20. Kutter E. Phage host range and efficiency of plating. Methods Mol Biol. 2009;501:141–149. [DOI] [PubMed] [Google Scholar]
- 21. Park M, Lee JH, Shin H, et al. Characterization and comparative genomic analysis of a novel bacteriophage, SFP10, simultaneously inhibiting both Salmonella enterica and Escherichia coli O157: H7. Appl Environ Microbiol. 2012;78(1):58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rakhuba DV, Kolomiets EI, Dey ES, et al. Bacteriophage receptors, mechanisms of phage adsorption and penetration into host cell. Pol J Microbiol. 2010;59(3):145–155. [PubMed] [Google Scholar]
- 23. Kutter E, Sulakvelidze A. Bacteriophages: Biology and Applications Boca Raton, FL: CRC Press; 2004:58. [Google Scholar]
- 24. Abedon ST, Herschler TD, Stopar D. Bacteriophage latent-period evolution as a response to resource availability. Appl Environ Microbiol. 2001;67(9):4233–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kalatzis PG, Bastías R, Kokkari C, et al. Isolation and characterization of two lytic bacteriophages, ϕSt2 and ϕGrn1; phage therapy application for biological control of Vibrio alginolyticus in aquaculture live feeds. PLoS One. 2016;11(3):e0151101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.