Antibiotic resistance (ABR) is highly prevalent in Latin America where it threatens to increase mortality and poverty in low-income countries. Many research groups in Latin America have explored the use of phage-based biocontrol as an alternative to the use of antibiotics with very positive results, some of which are described here. Evidence presented in these studies demonstrates the great potential that phages have as biocontrol agents in the poultry and aquaculture industries. The recent testing of a commercial phage cocktail in a poultry farm provided a pioneering example of integration between scientists and food production companies in Latin America. This type of collaborative effort is incredibly valuable to help move phages from bench to a wider use.
According to the European Centre for Disease Prevention and Control, antibiotic resistant bacteria currently cause death of 33,000 people every year1 and 10 million people could die by 2050.2 These figures illustrate a looming scenario for public health services worldwide, in which the lack of effective antibiotics could result in once readily treatable infections becoming life threatening.
In addition to highlighting the evident risk that ABR poses on people's lives globally, the World Health Organization (WHO) and the World Bank have also expressed concern upon the burden that ABR poses on the world's economy. A recent report predicted that ABR could cause a drop of 1.1 and 3.8% in the gross domestic product in countries with low- and high-impact ABR scenarios, respectively.3 It also highlights that the economic impact of ABR would be more severe in countries classified as low income, based on the World Bank's country classification into income groups. Thus, unless effective containment strategies are widely put in place worldwide, ABR threatens to increase the extent of poverty in low-income countries and curtail all efforts to achieve the Sustainable Development Goals for 2030 related to poverty reduction.
Several epidemiological surveys have shown that ABR is prevalent and significantly higher in Latin America than in the United States and Europe. For example, extended-spectrum beta-lactamases (ESBL) rates in the United States, Europe, and Latin America were 2.8%, 6.4%, and 12.0% for Escherichia coli isolates, 5.3%, 8.8%, and 27.6% for Klebsiella spp., and 25.3%, 11.8%, and 31.1% for Enterobacter spp.4 Furthermore, a recent report revealed that rates of ABR between 36% and 89% were reported for clinical isolates of critical pathogens such as Klebsiella pneumoniae, Acinetobacter baumannii, methicillin-resistant Staphylococcus aureus, and Pseudomonas aeruginosa.5 Similarly, high ABR rates have been observed in farm animals and products, for example, 98% of Salmonella isolates from Colombian poultry meat were resistant to at least one antibiotic,6 and 70.6% of Campylobacter spp. isolates from chickens in Ecuador and Brazil demonstrated ABR.7,8
Increasing ABR rates are primarily a consequence of the systematic misuse and overuse of antibiotics in human medicine and food production, where they have been used to treat infected animals and as growth promoters. Strikingly, 73% of all antibiotics consumed each year are used for food animals.9 In 2015, the WHO launched the Global Action Plan on Antimicrobial Resistance that recommended all member states to implement a series of strategies aimed at containing the spread of ABR and safeguarding access to effective antibiotics.10 In Latin America, this plan has been coordinated by the Pan American Health Organization and resulted in the introduction of policies to promote sensible antibiotic stewardship, including restrictions to antibiotics use in food production.11,12 Although these actions are necessary to contain ABR spread, they leave a gap in the resources available to treat bacterial infections in livestock and there is a demand for sustainable alternative strategies.
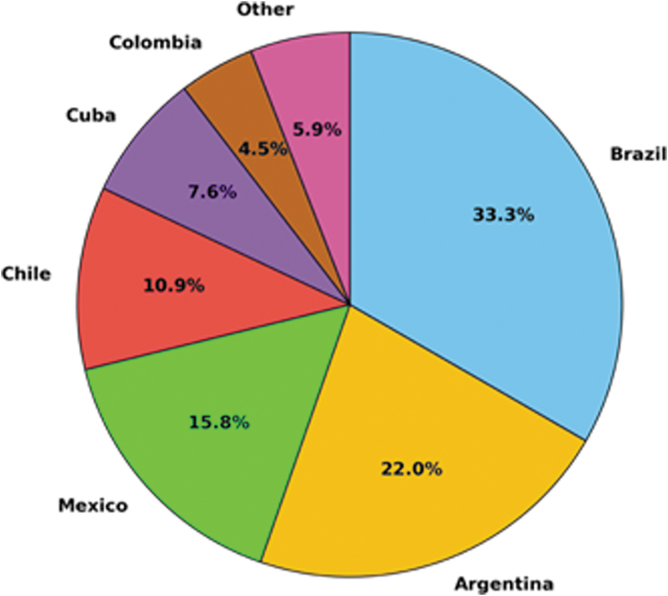
The increase and spread of ABR have prompted research from across the globe to explore and evaluate the use of phages as a feasible and effective strategy to control bacterial infections on farm animals, or those carried by animals that infect humans. At the time of this writing, 407 out of 36,663 phage-related publications in PubMed included at least one author with an affiliation to a Latin American institution (Fig. 1).
FIG. 1.
Contribution of different Latin American countries to the currently available phage scientific literature.
Phages for the Control of Salmonella in the Poultry Industry
Since the 1990s, the production of poultry meat in Latin America continuously increased and Brazil in particular has emerged as a major producer and exporter.13 Despite the well-documented benefits that the production and consumption of poultry products bring to the economy and people's nutrition in developing countries, some of the most common foodborne pathogens are transmitted through the consumption of these products, particularly Salmonella spp. and Campylobacter jejuni.14,15 To achieve the goals of the Global Action Plan on Antimicrobial Resistance in Latin America and support a sustainable management of its poultry industry, the application of phage-based biocontrol of foodborne pathogens might be a promising alternative to the use of antibiotics.
One of the earliest documented phage applications in the Latin American poultry industry was the use of phage typing in epidemiological surveys of Salmonella spp. outbreaks due to consumption of meat and eggs.16,17 Phage typing refers to the use of standard sets of phages to type bacterial isolates based on their susceptibility patterns, determined using standard plating techniques.18 Shortly after the turn of the 21st century, the first studies of the use of phages to control bacterial pathogens in poultry were published.
In 2007, a Brazilian study reported that two phage cocktails significantly reduced the concentration of Salmonella Enteritidis in the feed (up to the 6th h postphage treatment) and in the cecal tonsils of broiler chicks (up to 24 h after phage treatment).19 However, the difference in the concentration of Salmonella Enteritidis in the chicks' cecal tonsils between the treated and control groups was not significantly different 48 h post-treatment, presumably due to the selection of phage-resistant variants in the Salmonella population.19
Two studies conducted at the Department of Preventive Animal Medicine of the University of Chile provided further evidence of the effectiveness of phage-based biocontrol against Salmonella Enteritidis in chickens. The first of these described the isolation of three Salmonella Enteritidis lytic phages from the sewage system of commercial chicken flocks and tested the effect of the prophylactic use of a cocktail of these phages on Salmonella Enteritidis in chicks. The results demonstrated that the phage cocktail significantly reduced the incidence and intestinal bacterial counts in chicks up to 10 days after bacterial challenge.20 In the second study, a similar experiment was conducted, and the effectiveness of the same phage cocktail was compared with the use of the commercial probiotic “Broilact.” The results extended the effect previously observed with the phage cocktail and revealed that its prophylactic use in combination with the probiotic resulted in a significantly sharper reduction in the incidence and intestinal counts of Salmonella Enteritidis than when phages alone were used.21
Even though the aforementioned studies provided compelling evidence for the effective control of Salmonella using phage-based biocontrol, all were conducted in tightly controlled settings that do not closely reflect the conditions in a chicken farm. In 2019, the phage cocktail SalmoFREE® was used to control the incidence of Salmonella in a commercial broiler farm in Colombia. SalmoFREE is a patented cocktail of six characterized lytic Salmonella phages, whose safety had been tested in chickens reared in battery cages.22
The results of the study conducted in a Colombian broiler farm revealed that treating chickens with SalmoFREE through drinking water did not affect the farm's productivity, as measured by monitoring the chickens' weight gain, mortality rate, feed conversion index, and weight homogeneity.23 In addition, a decrease in the incidence of Salmonella in the chickens was observed during the course of the experiment. However, due to some events in the experimental farmhouses that were out of the authors' control, it was not possible to attribute the observed incidence reduction to the use of SalmoFREE.23 Nonetheless, as the first Latin American study of phage-based biocontrol of Salmonella performed in broiler farms, it provides a relevant baseline and reference for future studies of this kind.
Regarding the large-scale production of SalmoFREE, a bioprocess model coupled with economic analyses predicted that the most cost-effective process configuration involved a single 156 L bioreactor for producing the six phages, followed by sequential filtration of the phage lysate with 0.45 and 0.2 μm filters, to supply 35% of the Colombian broiler farm market. The model suggested that using this configuration would result in a production cost of $0.02 per chicken.24
Phages for the Control of Fish Pathogens in Aquaculture
Fish catches in Latin America have drastically decreased in recent years, mainly as a result of decaying pelagic fisheries from Peru and Chile.25 This is evidenced by a reduction in wild yearly catches that fell from 19.8 to 10.8 million tonnes between 2000 and 2014. By contrast, aquaculture has substantially evolved during the same period, with an increase in production from 0.8 to 2.8 million tonnes per year. In 2014 (most recent dates available), Latin America contributed 3.8% to the world's aquaculture production.25 Marine diseases are a natural part of ocean ecosystems, and many have economic consequences for fisheries and aquaculture. Of the marine disease agents known to cause impact on the industry's economy, 34% are bacterial and include Flavobacterium psychrophilum, Photobacterium damselae, and Vibrio spp.26
F. psychrophilum is the causative agent of bacterial cold water disease (CWD) and rainbow trout fry syndrome (RTFS) in juvenile salmonids.27 In a study conducted in Chile, phages targeting F. psychrophilum were isolated from aquaculture sites, their host range was determined, and their protective effect was assessed under experimental infection. The results revealed that, despite the very narrow host range observed for the isolated phages, treatment with phages 1H and 6H at a multiplicity of infection (MOI) of 10 significantly reduced the mortality by F. psychrophilum in salmon and trout, respectively.28 This study provided the first observation that phages could provide protection against CWD and RTFS, considering that the use of a phage cocktail would be the best approach in light of the narrow host range observed for the phages.28
V. anguillarum is the etiologic agent of vibriosis, a fatal hemorrhagic septicemia disease that affects >50 fresh and salt-water fish species, including several species that are of economic importance to the aquaculture industry, such as the Atlantic salmon, rainbow trout, turbot, sea bass, and sea bream.29 Isolates of V. anguillarum that cause vibriosis are generally of serotypes O1, O2, and O3, being this last serotype the one most frequently isolated from infected salmon in Chile.30 Although there are multiple commercial vaccines that protect fish against outbreaks caused by serotypes O1 and O2, the outbreaks caused by serotype O3 cannot be completely prevented.31
CHOED, a V. anguillarum phage isolated from bivalve samples in Chile, was tested both in laboratory and farming conditions to determine whether it was capable of preventing infection by V. anguillarum in salmon. Under laboratory conditions, the addition of phage CHOED at a MOI of one led to the survival of 70% of the fish at 10 days postinfection, whereas in the control tanks where no phage was added, only 7% of the fish survived.30 In the experiments conducted under farming conditions, phage CHOED added at an MOI of 10 resulted in the survival of 100% of the fish at 20 days postinfection, whereas in the control tanks, 60% of the fish remained alive during the same period.30 Overall, these results provided compelling evidence of the potential that phage-based biocontrol has for the treatment of vibriosis in salmon.
P. damselae subsp. damselae can infect wounds and cause hemorrhagic septicemia in a wide variety of farmed fish, including Australian longfin eel, European seabass, gilthead seabream, meagre, ovate pompano, rainbow trout, redbanded seabream, turbot, white seabream, and yellowtail.32 In a study conducted in Mexico, phage vB_Pd_PDCC_1 was isolated from the gastrointestinal tract of a lollipop catshark.33 Host range analysis of phage vB_Pd_PDCC_1 revealed that apart from infecting P. damselae subsp. damselae, it could also infect a set of strains from several Vibrio spp. at a high efficiency of plating. Furthermore, treatment of fish with phage vB_Pd_PDCC_1 did not significantly alter the hatching rate of longfin yellowtail eggs and provided them with a protective effect against Vibrio spp. In a challenge assay with P. damselae subsp. damselae, the results demonstrated that 48 h after adding phage vB_Pd_PDCC_1 at an MOI of 100, the hatching rate of longfin yellowtail eggs was close to 80%, which was significantly higher than the 50% hatching rate observed in the control treatment.33
To summarize, evidence to date suggests that phage-based biocontrol has a great potential in the Latin American food production industry and that it represents a promising and feasible alternative to antibiotics that could help to tackle the growing rates of ABR seen in the region. The potential implementation of phage-based biocontrol in Latin America is of utmost importance, as restrictions to the use of antibiotics in food production industries are expected to become tougher and more widespread, and existing AMR is problematic. Therefore, food producers require affordable and effective alternatives that help to prevent bacterial infections in livestock and transmission of foodborne pathogens to human populations.
Phage-based biocontrol is one such promising alternative, as has been demonstrated in Latin American countries, and its successful implementation would certainly help to mitigate the impact of ABR on Latin America's economy and consequently help to achieve the Sustainable Development Goals for 2030 that are related with poverty reduction. Studies described here demonstrate the necessity and value of a tight connection between academic researchers, companies, and food producers to develop phages for agriculture. The strong connections that exist in Latin America between these sectors may indeed mean that this continent could lead the way in terms of developing phages as our “virus amigos.”
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The author(s) received no specific funding for this work.
References
- 1. European Centre for Disease Prevention and Control. Antibiotic resistance—an increasing threat to human health. European Antibiotic Awareness Day. 2018. Available at https://antibiotic.ecdc.europa.eu/en/publications-data/antibiotic-resistance-increasing-threat-human-health (last accessed February 2, 2021).
- 2. O'Neill J. Review on Antimicrobial Resistance. Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations. 2014. Available at http://www.jpiamr.eu/wp-content/uploads/2014/12/AMR-Review-Paper-Tackling-a-crisis-for-the-health-and-wealth-of-nations_1–2.pdf (last accessed September 30, 2019).
- 3. Jonas OB, Irwin A, Berthe FCJ, et al. Drug-resistant infections: A threat to our economic future (Vol. 2): Final report. 2017. Available at https://documents.worldbank.org/en/publication/documents-reports/documentdetail/323311493396993758/final-report (last accessed February 2, 2021).
- 4. Rossi F, Baquero F, Hsueh P-R, et al. In vitro susceptibilities of aerobic and facultatively anaerobic Gram-negative bacilli isolated from patients with intra-abdominal infections worldwide: 2004 results from SMART (Study for Monitoring Antimicrobial Resistance Trends). J Antimicrob Chemother. 2006;58(1):205–210. [DOI] [PubMed] [Google Scholar]
- 5. Organizacion Panamericana de la Salud. Magnitude and trends of antimicrobial resistance in Latin America [in Spanish]. RELAVRA 2014, 2015, 2016. 2020. Available at https://www.paho.org/es/documentos/magnitud-tendencias-resistencia-antimicrobianos-latinoamerica-relavra-2014-2015-2016 (last accessed February 5, 2021).
- 6. Donado-Godoy P, Byrne BA, León M, et al. Prevalence, resistance patterns, and risk factors for antimicrobial resistance in bacteria from retail chicken meat in Colombia. J Food Prot. 2015;78:751–759. [DOI] [PubMed] [Google Scholar]
- 7. Vinueza-Burgos C, Wautier M, Martiny D, et al. Prevalence, antimicrobial resistance and genetic diversity of Campylobacter coli and Campylobacter jejuni in Ecuadorian broilers at slaughter age. Poult Sci. 2017;96(7):2366–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferro ID, Benetti TM, Oliveira TCRM, et al. Evaluation of antimicrobial resistance of Campylobacter spp. isolated from broiler carcasses. Br Poult Sci. 2015;56(1):66–71. [DOI] [PubMed] [Google Scholar]
- 9. Boeckel TPV, Glennon EE, Chen D, et al. Reducing antimicrobial use in food animals. Science. 2017;357(6358):1350–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. Global action plan on antimicrobial resistance. Available at https://www.who.int/publications-detail-redirect/global-action-plan-on-antimicrobial-resistance (last accessed February 9, 2021).
- 11. Pan American Health Organization. CD54/12 Plan of action on antimicrobial resistance; 2015—PAHO/WHO | Pan American Health Organization. Available at https://www.paho.org/en/documents/cd5412-plan-action-antimicrobial-resistance-2015 (last accessed February 9, 2021).
- 12. Da Silva Jr JB, Espinal M, Ramon-Pardo P. Antimicrobial resistance: Time for action [in Spanish]. Rev Panam Salud Publica. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ventura da Silva M. Poultry and poultry products—Risks for human health. In: Poultry Development Review. Food and Agriculture Organization of the United Nations; 2013: 15. [Google Scholar]
- 14. Farell D. The role of poultry in human nutrition. In: Poultry Development Review. Rome, Italy: Food and Agriculture Organization of the United Nations; 2013: 2. [Google Scholar]
- 15. Bryan FL, Doyle MP. Health risks and consequences of Salmonella and Campylobacter jejuni in raw poultry. J Food Prot. 1995;58(3):326–344. [DOI] [PubMed] [Google Scholar]
- 16. Fernandez J, Fica A, Ebensperger G, et al. Analysis of molecular epidemiology of Chilean Salmonella enterica serotype Enteritidis isolates by pulsed-field gel electrophoresis and bacteriophage typing. J Clin Microbiol. 2003;41(4):1617–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. dos Santos LR, do Nascimento VP, de Oliveira SD, et al. Phage types of Salmonella Enteritidis isolated from clinical and food samples, and from broiler carcasses in Southern Brazil. Rev Inst Med Trop São Paulo. 2003;45(1):1–4. [DOI] [PubMed] [Google Scholar]
- 18. Anderson ES, Williams REO. Bacteriophage typing of enteric pathogens and Staphylococci and its use in epidemiology: A review. J Clin Pathol. 1956;9(2):94–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Andreatti Filho RL, Higgins JP, Higgins SE, et al. Ability of Bacteriophages isolated from different sources to reduce Salmonella enterica Serovar Enteritidis in vitro and in vivo. Poult Sci. 2007;86(9):1904–1909. [DOI] [PubMed] [Google Scholar]
- 20. Borie C, Albala I, Sánchez P, et al. Bacteriophage treatment reduces Salmonella colonization of infected chickens. Avian Dis. 2008;52(1):64–67. [DOI] [PubMed] [Google Scholar]
- 21. Borie C, Sánchez ML, Navarro C, et al. Aerosol spray treatment with bacteriophages and competitive exclusion reduces Salmonella enteritidis infection in chickens. Avian Dis. 2009;53(2):250–254. [DOI] [PubMed] [Google Scholar]
- 22. Holguin-Moreno AV, Vives-Flores MJ, Jimenez-Sanchez AP. Composition comprising bacteriophages for reducing, eliminating and/or preventing Salmonella Enteritidis, Salmonella Typhimurium and Salmonella Paratyphi b [in Spanish]. Universidad de los Andes, assignee. Colombian Pat. No.. 15281747. [Google Scholar]
- 23. Clavijo V, Baquero D, Hernandez S, et al. Phage cocktail SalmoFREE® reduces Salmonella on a commercial broiler farm. Poult Sci. 2019;98(10):5054–5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Torres-Acosta MA, Clavijo V, Vaglio C, et al. Economic evaluation of the development of a phage therapy product for the control of Salmonella in poultry. Biotechnol Prog. 2019;35(5):e2852. [DOI] [PubMed] [Google Scholar]
- 25. Wurmann CG. Regional review on status and trends in aquaculture development in latin america and the caribbean—2015. FAO Fish Aquac Circ. 2017;(C1135/3):III.,IV,V,1–36. [Google Scholar]
- 26. Lafferty KD, Harvell CD, Conrad JM, et al. Infectious diseases affect marine fisheries and aquaculture economics. Annu Rev Mar Sci. 2015;7(1):471–496. [DOI] [PubMed] [Google Scholar]
- 27. Nematollahi A, Decostere A, Pasmans F, et al. Flavobacterium psychrophilum infections in salmonid fish. J Fish Dis. 2003;26(10):563–574. [DOI] [PubMed] [Google Scholar]
- 28. Castillo D, Higuera G, Villa M, et al. Diversity of Flavobacterium psychrophilum and the potential use of its phages for protection against bacterial cold water disease in salmonids. J Fish Dis. 2012;35(3):193–201. [DOI] [PubMed] [Google Scholar]
- 29. Toranzo AE, Magariños B, Romalde JL. A review of the main bacterial fish diseases in mariculture systems. Aquaculture. 2005;246(1):37–61. [Google Scholar]
- 30. Higuera G, Bastías R, Tsertsvadze G, et al. Recently discovered Vibrio anguillarum phages can protect against experimentally induced vibriosis in Atlantic salmon, Salmo salar. Aquaculture. 2013;392–395:128–133. [Google Scholar]
- 31. Mikkelsen H, Lund V, Martinsen L-C, et al. Variability among Vibrio anguillarum O2 isolates from Atlantic cod (Gadus morhua L.): Characterisation and vaccination studies. Aquaculture. 2007;266(1):16–25. [Google Scholar]
- 32. Rivas AJ, Lemos ML, Osorio CR. Photobacterium damselae subsp. damselae, a bacterium pathogenic for marine animals and humans. Front Microbiol. 2013;4. Available at https://www.frontiersin.org/articles/10.3389/fmicb.2013.00283/full (last accessed February 14, 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Veyrand-Quirós B, Gómez-Gil B, Lomeli-Ortega CO, et al. Use of bacteriophage vB_Pd_PDCC-1 as biological control agent of Photobacterium damselae subsp. damselae during hatching of longfin yellowtail (Seriola rivoliana) eggs. J Appl Microbiol. 2020;129(6):1497–1510. [DOI] [PubMed] [Google Scholar]