Abstract
Background: Use of bacteriophages as antibiofilm agents to tackle multidrug-resistant bacteria has gained importance in recent years.
Materials and Methods: In this study, biofilm formation by Staphylococcus aureus, Pseudomona aeruginosa, Klebsiella pneumoniae, and Escherichia coli under different growth conditions was studied. Furthermore, the ability of bacteriophages to inhibit biofilm formation was analyzed.
Results: Under dynamic growth condition, wherein the medium is renewed for every 12 h, the amount of biomass produced and log10 colony-forming unit counts of all bacterial species studied was highest when compared with other growth conditions tested. Biomass of biofilms produced was drastically reduced when incubated for 2 or 4 h with bacteriophages vB_SAnS_SADP1, vB_PAnP_PADP4, vB_KPnM_KPDP1, and vB_ECnM_ECDP3. Scanning electron microscopy and confocal laser scanning microscopy analyses indicated that the reduction in biomass was due to the lytic action of the bacteriophages.
Conclusions: Results of our study reinforce the concept of developing bacteriophages as alternatives to antibiotics to treat bacterial infections.
Keywords: bacteriophages, multidrug resistance, antibiotic resistance, alternative antimicrobials, biomass, colony-forming units
Introduction
Bacteriophage-based therapy was in practice from as early as in 1915, but its sheen declined by the 1940s when antibiotics were discovered. It was used to treat general bacterial diseases such as dysentery, typhoid fever, urinary tract infections, and sepsis caused by surgical wounds.1 A large number of studies in Russia, Poland, and Georgia demonstrated the use of bacteriophages for treating a variety of diseases.2 However, due to the advent of antibiotic resistance, phage therapy was considered as an alternative from the 1980s and has seen a steady rise. It still continues to be a promising alternative to treat bacterial infections in light of the uncontrolled antibiotic resistance. Various aspects of phage therapy—historical perspective, potential to be the alternative strategy and the challenges to apply it in the postantibiotic era are elegantly reviewed.3 In the postantibiotic era, clinical trials for phage therapy were conducted to treat vascularized ulcers,4 chronic otitis,5 burn wounds,6 diarrheal disease,7–9 gastrointestinal disorders,10 and other multidrug-resistant (MDR) bacterial infections.11–14 Thus, a re-emergence of phage therapy is taking shape.
The conventional phage therapy uses only bacteriophages as treatment agents. When delivered at the infection site, bacteriophages attach to their receptors (membrane receptors, pili, and flagella), eject their genetic material into the bacterial cells, replicate to produce a large number of active progeny, which in turn produce phage-derived enzymes (depolymerases to target capsules and biofilm structures) and cell wall degrading endolysins to target biofilm structures, thereby leading to bacterial lysis.3,15–17 The use of bacteriophages to remove biofilms is a well-standardized concept and a variety of strategies have been used.18 Biofilms formed by Pseudomonas aeruginosa, Enterococcus faecalis, Fusobacterium nucleatum, and Streptococcus species were successfully treated by phage therapy.19,20 Although many studies have shown bacteriophage-mediated mechanism of biofilm degradation, very few studies have used biofilms formed by clinical isolates.21–23 Furthermore, cocktails of bacteriophages have been used to treat biofilms caused by different bacteria.24–28 Owing to the active investigation in the area of phage therapy, commercial products based on bacteriophages have emerged to remove biofilms for the treatment of foot ulcers and septic29,30 wounds and as disinfecting agents in food industry.31–33
Wounds (burn or diabetic) are infected by an array of microbial flora. The presence of P. aeruginosa, Escherichia coli, Klebsiella pneumoniae, and Staphylococcus aureus in wounds is reported.34,35 Furthermore, the use of bacteriophages for wound healing is being explored actively and these attempts have reached clinical trials stage.36 Hence, in this study, we characterized bacteriophages that target P. aeruginosa, E. coli, K. pneumoniae, and S. aureus for their potential application in treating burn wounds. Since the pH of the burn wounds play a crucial role in healing,37 the stability of the bacteriophages were analyzed at different pH. Their thermal stability was also analyzed since it is an important aspect of characterization protocol. Biofilm formation in different growth conditions and the ability of bacteriophages to act on these biofilms was analyzed. We report that the structural and stability of bacteriophages are in conformity with previous studies. Furthermore, the bacteriophages exhibited potent biofilm clearing ability as determined by spectrophotometric, transmission, and fluorescence microscopy.
Materials and Methods
Ethical statement
This experimental study was approved by the Institutional Ethics Committee for Human Research (IECHR).
Bacterial cultures
To determine the characteristics of biofilm formation and the ability of bacteriophages on the biofilms, isolation and screening of MDR bacteria were carried out as described previously.38 P. aeruginosa yvu1 (GenBank: KY018605.1), S. aureus yvu2 (GenBank: KY496615.1), K. pneumoniae yvu3 (GenBank: KY496614.1), and E. coli yvu4 were grown on Luria agar at 37°C.
Characterization of bacteriophages
To determine the biofilm inhibition ability of the bacteriophages vB_PAnP_PADP4, vB_ECnM_ECDP3, vB_KPnM_KPDP1, and vB_SAnS_SADP1 that infect P. aeruginosa, E. coli, K. pneumoniae, and S. aureus, respectively, they were isolated as described in our previous study.38 They were stored in salts of magnesium (SM) buffer (0.1 M NaCl, 0.01 M MgSO4.7H2O, 1M Tris HCl, pH 7.5) at 4°C.
Single-step growth analysis
One-step or single-step growth curves were generated to determine the latent period and burst size of the bacteriophages.39 In brief, 50 mL of selected MDR bacterial cultures were grown up to mid-exponential phase (A600 = 0.6) and harvested by centrifugation at 10,000 g for 30 s at 4°C. The pellets were resuspended in 0.5 mL of Luria-Bertani (LB) media (at a density of 1 × 109) and mixed with 1 × 109 PFU of phages in 0.5 mL so that the multiplicity of infection is 1. This mixture was allowed to stand under motion (130 rpm) for 3 min at 37°C to facilitate phage adsorption on to the host cells. The mixture was then centrifuged at 13,000 g for 2 min to remove the free phage particles. The pellet was resuspended in 100 mL of LB medium and cultured at 37°C with shaking at 150 rpm. Samples (1 mL) were collected with 5-min intervals up to 70 min. They were centrifuged at 13,000 g for 1 min and the phage titer determined by double-layer agar method. The assay was performed in triplicates. The latent period is the time interval between the adsorption and the beginning of the initial rise in the phage count. The burst size of respective phages was calculated as the ratio of the final phage titer to the initial count of infected bacterial cells during the latent period.
Thermal and pH stability of phages
Stability of phages at varying ranges of temperature and pH is crucial for their ability to act at different physiological conditions. To determine the stability of the phages isolated in this study, phages (1 × 109 PFU suspended in 0.5 mL) were added to microtubes containing 0.5 mL of SM buffer and placed at different temperatures (37°C, 40°C, 50°C, 60°C, and 70°C) for 0, 30, 60, and 90 min as described earlier.40 Bacteriophage grown at 37°C was considered as control. For the pH stability assay, phages (1 × 109 PFU/mL) was inoculated in a series of tubes containing SM buffer at pH 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, and 11.0 and incubated at 37°C for 4 h as described earlier.41 Bacteriophage grown at pH 7.0 was considered as control. After incubation, the lytic ability was analyzed by double-layer agar method. All assays were performed in triplicates.
Characterization and quantitation of biofilms
Biofilm formation by bacteria depends on a variety of physiological factors. To determine as to which growth conditions favor the biofilm formation, it is important to determine the qualitative and quantitative aspects. Biofilms were qualitatively characterized by the standard test tube, Congo red agar, and microtiter plate methods.42 For quantitation of the biofilm formed, overnight cultures of P. aeruginosa, S. aureus, E. coli, or K. pneumonia were diluted to 106 CFU/mL into fresh brain heart infusion (BHI) broth supplemented with 1% glucose. One hundred microliters of each culture was diluted with 100 μL of the same medium and added to 800 μL of BHI broth placed into each well of 24-well plates (Greiner Bio-one CELLSTAR cell culture multiwall microplates with lid; Catalog No. 07-000-030). Three different experimental conditions were applied: (1) static renewal (SR) with renewal of media for every 12 h without shaking; (2) dynamic nonrenewal (DNR) condition without renewal of media and shaking at 130 rpm; and (3) dynamic renewal (DR) with renewal of media for every 12 h of incubation and shaking at 130 rpm in an orbital shaker (Rotech, MKSI-115-Series). For the renewal of media in SR and DR conditions, half the volume of old medium in the culture was replaced with fresh medium. To avoid setting of anaerobic conditions under DNR conditions, the assay container was opened once in 12 h as was done for SR and DR conditions. The development of biofilms was monitored up to 96 h. Biofilms formed were fixed with 200 μL of methanol for 15 min, followed by the addition of crystal violet and incubated for 15 min. The wells were then washed with water and dried for 2 h at room temperature. Two hundred microliters of ethanol (95%) was added to dissolve the stain. The absorbance of eluted stain was measured at 570 nm in a spectrophotometer. The absorbance at 570 nm is a direct indication of the amount of biofilm formed. Hence, biomass is represented as O.D. at 570 nm. All the measurements were made with samples obtained from triplicate experiments. For the determination of colony-forming units (CFUs), the adhered cells were collected by scratching the bottom and sides of the wells (n = 6) from each bacterial sample, with a sterile swab and subsequently suspended in 9 mL of phosphate-buffered saline (PBS) buffer by vigorous vortexing for 1 min. Serial dilutions of these suspensions were plated on BHI agar medium, and the colonies formed were counted and expressed as log10 CFU/mL by taking into consideration the dilution factor.
Bacteriophage treatments
In this study, the ability of bacteriophages to act on biofilms is determined. To accomplish this, biofilms formed by P. aeruginosa, S. aureus, K. pneumoniae, or E. coli under SR, DR, and DNR conditions after 24, 48, 72, and 96 h were infected with vB_PAnP_PADP4, vB_SAnS_SADP1, vB_KPnM_KPDP1, and vB_ECnM_ECDP3, respectively. In brief, 1 × 109 phage particles suspended in 0.5 mL were added to the biofilms and incubated for 2 or 4 h. Immediately after incubation with the phages, the biofilms were quantitated by staining with crystal violet and measuring the absorbance at 570 nm.
Transmission electron microscopy
Bacteriophages are known to affect the structural aspects of bacteria. In this study, we attempt to analyze the morphological changes that may occur consequently to the action of bacteriophages on the biofilms. To facilitate this, the morphological features of bacteriophages used in this study were characterized by transmission electron microscopy (TEM) as described earlier.43 The isolated bacteriophage filtrate was passed through 0.45-μm filters and concentrated by centrifugation at 30,000 g for 60 min and the pellet reconstituted with 5 mL of SM buffer. Five microliters of phage concentrate was placed on Formvar coated 200 × 200 copper grids. Five microliters of 0.5% uranyl acetate was then applied to the grids and air-dried. Samples were viewed with the FEI Tecnai G2 S-Twin Transmission Electron at an operating voltage at 80 kV.
Scanning electron microscopy
To gain further insight into the microarchitecture of biofilms, scanning microscopy was performed as described earlier.44 Biofilms were grown on borosilicate glass coverslips previously placed into the wells of a 24-well microtiter plate and washed twice with PBS and dried in an incubator for 20 h at 37°C. The biofilms coated on glass slides were fixed with glutaraldehyde (2.5%) and dehydrated through a series of graded ethanol (30–100%) for 5 min in each. They were then sputtered with gold after critical point drying, and the aggregated biofilms were examined using scanning electron microscopy (SEM; FEI, Tecnai G-2S Twin).23
Confocal laser scanning microscopy
The effect of bacteriophages on bacterial cell death can be visualized by the fluorescent dyes propidium iodide (PI) and fluorescent dyes that stain live or dead cells (SYTO) that are specific to live and dead cells, respectively. To determine the ability of bacteriophages to induce killing, biofilms of MDR bacteria were stained with SYTO or PI. In brief, 3 μL of SYTO® and 3 μL of PI stock solutions were added to 1 mL of filter-sterilized water to prepare a working solution. Two hundred microliters of it was on the biofilms and incubated in dark for 15 min at room temperature and washed with sterile water to remove excess stain.45 Damaged bacteria in the biofilm take up PI and appear red, whereas the intact bacteria that take up the SYTO dye appear green.
Statistical analysis
The data were analyzed using the GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA). All the values were expressed as mean ± standard deviation. Significant difference between variations denoted by p-value <0.05 was estimated by analysis of variance (ANOVA) and Dunn's and Bonferroni's multiple comparison test.
Results
Characterization of bacteriophages
For practical purposes, the phages were named based on Ackerman classification, which relies on the morphology of tail features. Morphological features of phages vB_PAnP_PADP4, vB_ECnM_ECDP3, vB_KPnM_KPDP1, and vB_SAnS_SADP1 as observed by TEM are shown in Supplementary Figure S1A–D, respectively. The morphological features observed were hexagonal head and a tail that is akin to most bacteriophages. The one-step growth curve of vB_PAnP_PADP4, vB_ECnM_ECDP3, vB_KPnM_KPDP1, and vB_SAnS_SADP1 are shown in Supplementary Figure S1E–H, respectively. All the phages displayed an initial log phase followed by exponential and stationary phases of growth. The capsid size, tail length, latent period, and burst size obtained basing on TEM and the one-step growth curve analyses are presented in Supplementary Table S1. The latent period/burst size for PADP4, SADP1, KPDP1, and ECDP3 was found to be 20 min/102, 30 min/126, 20 min/76, and 15 min/144, respectively.
Stability of phages
The stability of vB_PAnP_PADP4, vB_ECnM_ECDP3, vB_KPnM_KPDP1, and vB_SAnS_SADP1was investigated under different thermal and pH conditions. The percentage of phages able to produce infection were reduced by one order of magnitude after 60 and 90 min incubation periods at 40°C, 50°C, and 60°C (Supplementary Fig. S2A–D), and the stability of phages at different range of pH showed that the phages were stable within the pH range 6–8 and showed about 90% activity (Supplementary Fig. S2E–H). For all the phages tested, incubation at pH 5 and 9 caused 1 log decrease in the plaque-forming units (PFUs) after 1 h, and incubation at pH 4 and 10 caused a 10-fold decrease in the PFUs after 1 h. Inactivation was observed at pH 2.0 and 11.0 for all the phages. The results suggest that extreme temperatures and pH conditions affect the stability of phages.
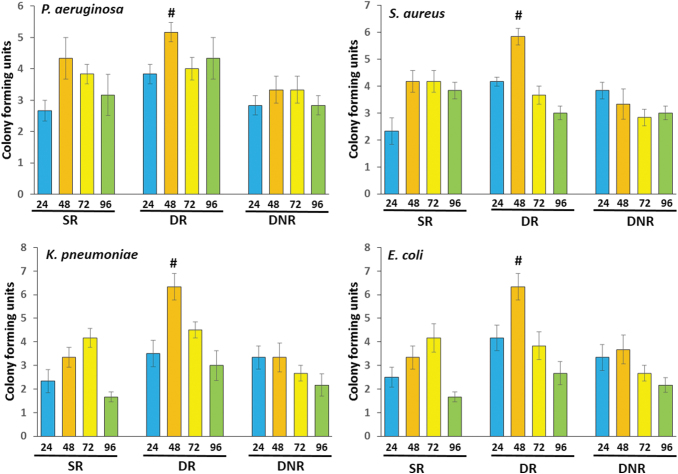
CFU count and biomasses of the bacterial biofilms under various growth conditions
The representative biofilm formation in Congo red agar plates, 96-well culture plates, and test tubes are shown in Supplementary Figure S3A–C, respectively. To determine as to which of the growth conditions allow biofilm formation to the maximum extent, bacterial cultures were grown in SR, DR, and DNR conditions. In general, highest CFU count was observed in the DR condition at the 48 h time point for all the bacteria tested (Fig. 1). The CFU counts for P. aeruginosa, S. aureus, K. pneumoniae, and E. coli after 48-h incubation in DR conditions were 5.16 ± 0.30 × 106, 5.8 ± 0.30 × 106, 6.3 ± 0.55 × 106, and 6.3 ± 0.50 × 106, respectively, whereas the same were 4.3 ± 0.66 × 106, 4.16 ± 0.4 × 106, 3.33 ± 0.42 × 106, and 3.33 ± 0.49 × 106 in SR conditions; 3.33 ± 0.43 × 106, 3.33 ± 0.55 × 106, 3.33 ± 0.61 × 106, and 3.66 ± 0.61 × 106 in DNR conditions.
FIG. 1.
CFU counts formed by MDR bacterial isolates under static and DRs. Four MDR bacterial isolates were independently cultured for 24, 48, 72, and 96 h under SR, DR, and DNR conditions. CFU formed were hand counted. All assays were conducted in triplicates. Values presented are mean ± SD. #Indicates the condition at which highest CFU was observed. CFU, colony-forming unit; DNR, dynamic nonrenewal; DR, dynamic renewal; MDR, multidrug resistant; SD, standard deviation; SR, static renewal.
The biomasses of bacterial biofilms at SR, DNR, and DR when grown for 24–92 h are shown in Figure 2. The intensity of color (O.D. measured at 570 nm) generated by the biofilms with crystal violet, is a direct measure of the biomass formed. In general, it was observed that the biomass formed is more in the DR condition compared with other conditions. All the bacteria tested produced highest biomass at the 72 h time point in the DR conditions, except S. aureus. The biomass in terms of O.D. of crystal violet was 0.47 ± 0.02, 0.51 ± 0.057, 0.62 ± 0.057, and 0.74 ± 0.04 for P. aeruginosa, S. aureus, K. pneumoniae, and E. coli, respectively. In the SR condition, biomass was highest for all the bacteria at 72 h time point (0.40 ± 0.02, 0.38 ± 0.01, 0.48 ± 0.04, and 0.68 ± 0.01 for P. aeruginosa, S. aureus, K. pneumoniae, and E. coli, respectively). In the DNR condition, S. aureus and E. coli formed the highest amount of biomass at the 48 h time point (0.3 ± 0.19 and 0.57 ± 0.04), whereas it was at 72 h for P. aeruginosa (0. 24 ± 0.03) and at 24 h for K. pneumoniae (0.35 ± 0.02). However, it is not noted that the highest biomass formed by all the four bacteria in SR and DNR conditions was less than that is formed under DR condition at 72 h.
FIG. 2.
Biomasses of biofilms by MDR bacterial isolates under static and DRs. Four MDR bacterial isolates were independently cultured for 24, 48, 72, and 96 h under SR, DR, or DNR conditions and stained with crystal violet. The intensity of color formed measured at 570 nm is a direct indication of biomass formed. All assays were conducted in triplicates. Values presented are mean ± SD. #Indicates the condition at which highest biomass was observed.
Effect of phage treatment on the biomass of bacterial biofilms
The lytic activity of vB_PAnP_PADP4, vB_ECnM_ECDP3, vB_KPnM_KPDP1, and vB_SAnS_SADP1 on biofilms formed by P. aeruginosa, E. coli, K. pneumoniae, and S. aureus, respectively, were evaluated. Biofilms obtained under SR, DR, and DNR conditions for all the bacteria were treated with the phages for 2–4 h. Immediately after incubation with respective phage, the biofilms were stained by crystal violet assay and the biomass is determined by measuring the O.D. at 570 nm. All the phages reduced the biomass of their respective bacterial biofilms in a time-dependent manner (Figs. 3 and 4), irrespective of the condition under which the biofilm was formed. In the case of P. aeruginosa grown under SR conditions, upon phage treatment for 4 h the O.D. decreased in biofilms of 24 h (0.68 ± 0.033 to 0.26 ± 0.032), 48 h (1.00 ± 0.00 to 0.25 ± 0.02), 72 h (0.95 ± 0.037 to 0.27 ± 0.017), and 96 h (1.00 ± 0.00 to 0.23 ± 0.026). When grown under DR conditions, for the biofilms obtained at 24, 48, 72, and 96 h, the decrease in O.D. upon phage treatment for 4 h was from 0.503 ± 0.039 to 0.16 ± 0.015, 0.86 ± 0.038 to 0.191 ± 0.026, 0.83 ± 0.033 to 0.18 ± 0.016, and 0.47 ± 0.035 to 0.17 ± 0.024, respectively. When biofilms grown under DNR conditions and obtained at 24, 48, 72, and 96 h were treated with the phage for 4 h, the decrease in the O.D. was 0.76 ± 0.031 to 0.16 ± 0.016, 1.00 ± 0.00 to 0.221 ± 0.018, 1.00 ± 0.01 to 0.33 ± 0.022, and 1.00 ± 0.01 to 0.29 ± 0.034, respectively.
FIG. 3.
Lytic action of bacteriophages on bacterial biofilms. Biofilms formed by Pseudomonas aeruginosa and Staphylococcus aureus under SR, DR, and DNR conditions after 24, 48, 72, and 96 h were treated for 2–4 h with bacteriophages vB_PAnP_PADP4 and vB_SAnS_SADP1, respectively. Immediately after incubation with the phages, the biofilms were stained with crystal violet and the intensity of color, which is a direct indication of the biomass, was read at 570 nm. All assays were conducted in triplicates. Values presented are mean ± SD. *Indicates p < 0.05 compared with the respective control (0 h).
FIG. 4.
Lytic action of bacteriophages on bacterial biofilms. Biofilms formed by Klebsiella pneumoniae and Escherichia coli under SR, DR, and DNR conditions after 24, 48, 72, and 96 h were treated for 2–4 h with bacteriophages vB_KPnM_KPDP1and vB _ECnM_ECDP3, respectively. Immediately after incubation with the phages, the biofilms were stained with crystal violet and the intensity of color, which is a direct indication of the biomass, was read at 570 nm. All assays were conducted in triplicates. Values presented are mean ± SD. *Indicates p < 0.05 compared with the respective control (0 h).
In the case of S. aureus grown under SR conditions, upon phage treatment for 4 h, the O.D. decreased in biofilms of 24 h (0.78 ± 0.033 to 0.18 ± 0.028), 48 h (1.00 ± 0.00 to 0.15 ± 0.018), 72 h (1.00 ± 0.001 to 0.24 ± 0.031), and 96 h (0.96 ± 0.02 to 0.20 ± 0.028). When grown under DR conditions, for the biofilms obtained at 24, 48, 72, and 96 h, the decrease in O.D. upon phage treatment for 4 h was from 0.68 ± 0.06 to 0.16 ± 0.017, 0.89 ± 0.035 to 0.2 ± 0.027, 0.82 ± 0.048 to 0.15 ± 0.033, and 0.47 ± 0.036 to 0.16 ± 0.032, respectively. When biofilms grown under DNR conditions and obtained at 24, 48, 72, and 96 h were treated with the phage for 4 h, the decrease in the O.D. was 0.85 ± 0.055 to 0.15 ± 0.018, 1.00 ± 0.01 to 0.17 ± 0.015, 0.90 ± 0.02 to 0.14 ± 0.015, and 0.98 ± 0.01 to 0.12 ± 0.08, respectively.
For K. pneumoniae grown under SR conditions, upon phage treatment for 4 h, the O.D decreased in biofilms of 24 h (0.74 ± 0.03 to 0.24 ± 0.021), 48 h (1.00 ± 0.01 to 0.21 ± 0.029), 72 h (0.96 ± 0.001 to 0.24 ± 0.032), and 96 h (0.96 ± 0.02 to 0.21 ± 0.03). When grown under DR conditions, for the biofilms obtained at 24, 48, 72, and 96 h, the decrease in O.D. upon phage treatment for 4 h was from 0.51 ± 0.043 to 0.16 ± 0.019, 0.86 ± 0.038 to 0.16 ± 0.031, 0.84 ± 0.040 to 0.16 ± 0.031, and 0.47 ± 0.035 to 0.16 ± 0.022, respectively. When biofilms grown under DNR conditions and obtained at 24, 48, 72, and 96 h were treated with the phage for 4 h, the decrease in the O.D. was 0.76 ± 0.030 to 0.13 ± 0.015, 1.00 ± 0.01 to 0.19 ± 0.028, 1.00 ± 0.02 to 0.54 to 0.032, and 0.98 ± 0.01 to 0.12 ± 0.05, respectively.
For E. coli grown under SR conditions, upon phage treatment for 4 h, the O.D. decreased in biofilms of 24 h (0.81 ± 0.047 to 0.21 ± 0.041), 48 h (1.00 ± 0.01 to 0.21 ± 0.025), 72 h (1.00 ± 0.001 to 0.24 ± 0.032), and 96 h (1.00 ± 0.02 to 0.20 ± 0.031). When grown under DR conditions, for the biofilms obtained at 24, 48, 72, and 96 h, the decrease in O.D. upon phage treatment for 4 h was from 0.63 ± 0.059 to 0.16 ± 0.017, 0.82 ± 0.028 to 0.17 ± 0.029, 0.92 ± 0.030 to 0.18 ± 0.016, and 0.47 ± 0.035 to 0.15 ± 0.026, respectively. When biofilms grown under DNR conditions and obtained at 24, 48, 72, and 96 h were treated with the phage for 4 h, the decrease in the O.D. was 0.79 ± 0.016 to 0.17 ± 0.015, 1.00 ± 0.01 to 0.19 ± 0.013, 0.96 ± 0.02 to 0.27 to 0.045, and 1.0 ± 0.01 to 0.24 ± 0.034, respectively.
Microscopic characterization of action of bacteriophages
Since biofilms incubated for 2 or 4 h with phages showed decreased biomass and lysis, we used SEM and confocal laser scanning microscopy (CLSM) to gather more evidence on the morphological changes that occur during this process. Biofilm formation was clearly evident in the untreated P. aeruginosa, S. aureus, K. pneumoniae, and E. coli under dynamic conditions (Fig. 5A–D). Treatment with respective phages for 4 h resulted in lesser biofilm content when compared with untreated control (Fig. 5A1–D1). The ability of bacteriophages to inhibit biofilm formation was evaluated by confocal microscopy (Fig. 6). Predominant numbers of dead cells (red) in the biofilm were evident due to bacteriophage treatment for 4 h of incubation (Fig. 6). These results indicate that phages effectively inhibit the biomasses of biofilms in vitro.
FIG. 5.
Scanning electron microscopic analyses of the lytic action of bacteriophages. Biofilms formed by Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella pneumoniae, and Escherichia coli under DR condition were treated for 4 h with vB_PAnP_PADP4, vB_SAnS_SADP1, vB_KPnM_KPDP1, and vB _ECnM_ECDP3, respectively. Panels (A–D) show biofilms formed by P. aeruginosa, S. aureus, K. pneumoniae, and E. coli, respectively, without phage treatment, whereas panels (A1–D1) are for the biofilms formed by corresponding bacteria that received phage treatment.
FIG. 6.
Confocal microscopic analyses of the lytic action of bacteriophages. Biofilms of Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella pneumoniae, and Escherichia coli formed under DR condition were treated for 4 h with vB_PAnP_PADP4, vB_SAnS_SADP1, vB_KPnM_KPDP1, and vB _ECnM_ECDP3, respectively, were stained with SYTO ® 9 or PI. Cells with red stain are dead, whereas those with green stain are alive. Scale bars: 20–100 μM.
Discussion
Bacteriophages against MDR P. aeruginosa, S. aureus, K. pneumonia, and K. pneumoniae were named vB_SAnS_SADP1, vB_PAnP_PADP4, vB_KPnM_KPDP1, and vB_ECnM_ECDP3, respectively, were used in this study. Isolation and characterization of bacteriophages against K. pneumoniae,46 E. coli,47 S. aureus,48 and P. aeruginosa49 were reported earlier. The latent period/burst size observed for the four phages in this study are in agreement with previous reports.50–52 Physiological factors such as temperature and pH play a crucial role in phage–bacterial interactions. We observed that the isolated phages were thermally active even at 40–60°C and were viable up to 60°C, whereas the maximum and minimum infectivity was observed at 37°C and 60°C, respectively. These observations are in agreement with previous reports.49,53,54 In this study, phages displayed maximum infectivity at pH 7 and their optimum pH range was 6–8, which is similar to the observations made in earlier studies.55
Phage therapy has been used to treat infections by E. faecalis.28 The environmental bacteriophages extensively reduced the biofilm formation by Vibrio cholera.56 Clinical trials using phage therapy for the treatment of a variety of disorders and infections are documented.5–11,13,14,30 Furthermore, clinical trials for healing of burn wounds using phage therapy are proposed (https://clinicaltrials.gov/ct2/show/NCT04323475). The use of phages for burn wound treatments is extensively reviewed.36 Burn wounds are colonized by a variety of microbes, including the four bacterial species analyzed in this study. We report the lytic action of vB_PAnP_PADP4, vB_SAnS_SADP1, vB_ECnM_ECDP3, and vB_KPnM_KPDP1, which act on P. aeruginosa, S. aureus, E. coli, and K. pneumoniae, respectively. Our results add much more evidence to the possible application of bacteriophages for the treatment of wounds infected with MDR pathogenic bacteria.
We observed that the biomass of single-species biofilms of P. aeruginosa, S. aureus, K. pneumoniae, and E. coli were reduced by over 50–80% in a time-dependent manner by VB_PAnP_PADP4, VB_SAnS_SADP1, VB_KPnM_KPDP1, and VB_ECnM_ECDP3, respectively. Such a reduction in biomass was reported with other phages, wherein the maximum reduction was obtained within 2–5 h, depending on the type of biofilm studied.25,57,58 A previous study indicated that decreasing the density of biofilms requires both antibiotics and bacteriophages.59 Furthermore, it is suggested that combinational therapy with bacteriophages along with DNase enzymes degrades the biofilm matrix efficiently.60 However, our studies demonstrate that the biofilm can be reduced to a maximum extent solely by the bacteriophage itself, which is a significant development in the search for newer agents in place of antibiotics. It would also be interesting to study the effect of a combination of antibiotics and the bacteriophages used in the study.
Previous studies that focused on characterization of biofilms under different physical conditions such as static, dynamic renewal, and dynamic nonrenewal were reported.44,61 Most of the studies concluded that dynamic conditions are more favorable than the other conditions to access the biofilms. Sillankorva et al. reported that the high phage concentration ØIBB-PF7A bacteriophage could be highly efficient in removing Pseudomonas fluorescens biofilms within 4 h of incubation.62 They also demonstrated that the cell lysis starts faster under dynamic than that of the static conditions; interestingly, it was noted that the total relative biofilm reduction was not significant during 4 h in dynamic biofilms as compared with static biofilms. In principle, under static conditions, the released progeny will attach to the neighboring bacteria within the biofilms, whereas in case of dynamic conditions, the progeny of virus can target and lyse the entire span of bacterial colonies within the biofilms because of agitation in the experimental setup.44 However, in our study, bacteriophages were effective on the biofilms in all the growth conditions tested, indicating that the bacteriophages we isolated are versatile in nature.
In conclusion, we report the characterization of bacteriophages and the bacterial biofilm formation by P. aeruginosa, S. aureus, K. pneumoniae, and E. coli. The best condition for biofilm formation was dynamic growth with media renewal. The single species biofilms were effectively inhibited by the corresponding bacteriophages characterized by a concomitant reduction in biomass and CFU. SEM and CLSM analyses indicated physical changes that occur to the biofilm due to the action of bacteriophage-mediated lysis. These findings support the possible use of bacteriophages for the development of alternatives to antibiotics for the treatment of wounds infected with MDR bacteria.
Supplementary Material
Acknowledgments
Assistance from the central facilities of Yogi Vemana University is highly acknowledged. The help received at Center for Nanotechnology, University of Hyderabad for CLSM and SEM is acknowledged. R.R.P. gratefully acknowledges University Grants Commission for financial support in the form of junior and senior research fellowships.
Authors' Contributions
R.R.P. and V.R.P.D. conceived and designed the experiments. R.R.P. performed the experiments and data collection. R.R.P., V.L.D., V.R.N., K.K.V., S.Y., and V.R.P.D. conducted analysis and interpretation of findings. R.R.P., S.Y., and V.R.P.D. prepared the article and figures.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No specific funding was received.
Supplementary Material
References
- 1. Wittebole X, De Roock S, Opal SM. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence. 2014(1);15:226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sulakvelidze A, Alavidze Z, Morris J. Bacteriophage therapy. Antimicrob Agents Chemother. 2001;45(3):649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gordillo Altamirano FL, Barr JJ. Phage therapy in the postantibiotic era. Clin Microbiol Rev. 2019;32(2):e00066–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jikia D, Chkhaidze N, Imedashvili E, et al. The use of a novel biodegradable preparation capable of the sustained release of bacteriophages and ciprofloxacin, in the complex treatment of multidrug-resistant Staphylococcus aureus-infected local radiation injuries caused by exposure to Sr90. Clin Exp Dermatol. 2005;30(1):23–26. [DOI] [PubMed] [Google Scholar]
- 5. Wright A, Hawkins CH, Änggård EE, et al. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; A preliminary report of efficacy. Clin Otolaryngol. 2009;34(4):349–357. [DOI] [PubMed] [Google Scholar]
- 6. Jault P, Leclerc T, Jennes S, et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect Dis. 2019;19(1):35–45. [DOI] [PubMed] [Google Scholar]
- 7. Bruttin A, Brüssow H. Human volunteers receiving Escherichia coli phage T4 orally: A safety test of phage therapy. Antimicrob Agents Chemother. 2005;49(7):2874–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sarker SA, Berger B, Deng Y, et al. Oral application of Escherichia coli bacteriophage: Safety tests in healthy and diarrheal children from Bangladesh. Environ Microbiol. 2017;19(1):237–250. [DOI] [PubMed] [Google Scholar]
- 9. Sarker SA, McCallin S, Barretto C, et al. Oral T4-like phage cocktail application to healthy adult volunteers from Bangladesh. Virology. 2012;434(2):222–232. [DOI] [PubMed] [Google Scholar]
- 10. Gindin M, Febvre HP, Rao S, et al. Bacteriophage for Gastrointestinal Health (PHAGE) Study: Evaluating the safety and tolerability of supplemental bacteriophage consumption. J Am Coll Nutr. 2019;38(1):68–75. [DOI] [PubMed] [Google Scholar]
- 11. Schooley RT, Biswas B, Gill JJ, et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother. 2017;61(10):e00954-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sangha KK, Kumar BVS, Agrawal RK, et al. Proteomic characterization of lytic bacteriophages of Staphylococcus aureus isolated from Sewage Affluent of India. Int Sch Res Notes. 2014;2014:265298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan BK, Turner PE, Kim S, et al. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol Med Public Health. 2018;2018(1):60–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jennes S, Merabishvili M, Soentjens P, et al. Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury—A case report. Crit Care. 2017;21(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bertozzi Silva J, Storms Z, Sauvageau D. Host receptors for bacteriophage adsorption. FEMS Microbiol Lett. 2016;363(4):fnw002. [DOI] [PubMed] [Google Scholar]
- 16. Zhu J, Miller MB, Vance RE, et al. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci. 2002;99(5):3129–3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lin DM, Koskella B, Lin HC. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J Gastrointest Pharmacol Ther. 2017;8(3):162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferriol-González C, Domingo-Calap P. Phages for biofilm removal. Antibiotics. 2020;9(5):268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waters EM, Neill DR, Kaman B, et al. Phage therapy is highly effective against chronic lung infections with Pseudomonas aeruginosa. Thorax. 2017;72(7):666–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szafrański SP, Winkel A, Stiesch M. The use of bacteriophages to biocontrol oral biofilms. J Biotechnol. 2017;250:29–44. [DOI] [PubMed] [Google Scholar]
- 21. Danis-Wlodarczyk K, Olszak T, Arabski M, et al. Characterization of the newly isolated lytic bacteriophages KTN6 and KT28 and their efficacy against pseudomonas aeruginosa biofilm. PLoS One. 2015;10(5):e0127603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oliveira A, Ribeiro HG, Silva AC, et al. Synergistic antimicrobial interaction between honey and phage against Escherichia coli biofilms. Front Microbiol. 2017;8:2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ribeiro KVG, Ribeiro C, Dias RS, et al. Bacteriophage isolated from sewage eliminates and prevents the establishment of Escherichia coli biofilm. Adv Pharm Bull. 2018;8(1):85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maszewska A, Zygmunt M, Grzejdziak I, et al. Use of polyvalent bacteriophages to combat biofilm of Proteus mirabilis causing catheter-associated urinary tract infections. J Appl Microbiol. 2018;125(5):1253–1265. [DOI] [PubMed] [Google Scholar]
- 25. Alves DR, Gaudion A, Bean JE, et al. Combined use of bacteriophage K and a novel bacteriophage to reduce Staphylococcus aureus biofilm formation. Appl Environ Microbiol. 2014;80:6694–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alves DR, Perez-Esteban P, Kot W, et al. A novel bacteriophage cocktail reduces and disperses Pseudomonas aeruginosa biofilms under static and flow conditions. Microb Biotechnol. 2016;9(1):61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Forti F, Roach DR, Cafora M, et al. Design of a broad-range bacteriophage cocktail that reduces pseudomonas aeruginosa biofilms and treats acute infections in two animal models. Antimicrob Agents Chemother. 2018;62(6):e02573-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khalifa L, Gelman D, Shlezinger M, et al. Defeating antibiotic- and phage-resistant Enterococcus faecalis using a phage Cocktail in vitro and in a clot model. Front Microbiol. 2018;9:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fish R, Kutter E, Wheat G, et al. Bacteriophage treatment of intransigent diabetic toe ulcers: A case series. J Wound Care. 2016;25(suppl 7):S27–S33.26949862 [Google Scholar]
- 30. Markoishvili K, Tsitlanadze G, et al. A novel sustained-release matrix based on biodegradable poly(ester amide)s and impregnated with bacteriophages and an antibiotic shows promise in management of infected venous stasis ulcers and other poorly healing wounds. Int J Dermatol. 2002;41(7):453–458. [DOI] [PubMed] [Google Scholar]
- 31. PhageGuard Listeria. 2020 [cited June 12, 2020]. https://phageguard.com/es/solucion-listeria/ (accessed August 7, 2021).
- 32. Intralytix, Inc. www.intralytix.com/index.php?page=prod&id=1 (accessed August 7, 2021).
- 33. Iacumin L, Manzano M, Comi G. Phage inactivation of listeria monocytogenes on san daniele dry-cured ham and elimination of biofilms from equipment and working environments. Microorganisms. 2016;4(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalan LR, Brennan MB. The role of the microbiome in nonhealing diabetic wounds. Ann N Y Acad Sci. 2019;1435(1):79–92. [DOI] [PubMed] [Google Scholar]
- 35. Lachiewicz AM, Hauck CG, Weber DJ, et al. Bacterial infections after burn injuries: Impact of multidrug resistance. Clin Infect Dis. 2017;65(12):2130–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pinto AM, Cerqueira MA, Bañobre-Lópes M, et al. Bacteriophages for chronic wound treatment: From traditional to novel delivery systems. Viruses. 2020;12(2):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ono S, Imai R, Ida Y, et al. Increased wound pH as an indicator of local wound infection in second degree burns. Burns. 2015;41(4):820–824. [DOI] [PubMed] [Google Scholar]
- 38. Pallavali RR, Degati VL, Lomada D, et al. Isolation and in vitro evaluation of bacteriophages against MDR-bacterial isolates from septic wound infections. PLoS One. 2017;12(7):e0179245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhao J, Zhang Z, Tian C, et al. Characterizing the biology of lytic bacteriophage vB_EaeM_ϕEap-3 infecting multidrug-resistant enterobacter aerogenes. Front Microbiol. 2019;10:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Melo LDR, Ferreira R, Costa AR, et al. Efficacy and safety assessment of two enterococci phages in an in vitro biofilm wound model. Sci Rep. 2019;9(1):6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yin Y, Ni P, Deng B, et al. Isolation and characterisation of phages against Pseudomonas syringae pv. actinidiae. Acta Agric Scand Sect B Soil Plant Sci. 2019;69(3):199–208. [Google Scholar]
- 42. Mathur T, Singhal S, Khan S, et al. Detection of biofilm formation among the clinical isolates of staphylococci: An evaluation of three different screening methods. Indian J Med Microbiol. 2006;24(1):25–29. [DOI] [PubMed] [Google Scholar]
- 43. Stalin N, Srinivasan P. Characterization of Vibrio parahaemolyticus and its specific phage from shrimp pond in Palk Strait, South East coast of India. Biologicals. 2016;44(6):526–533. [DOI] [PubMed] [Google Scholar]
- 44. Sillankorva S, Neubauer P, Azeredo J. Phage control of dual species biofilms of Pseudomonas fluorescens and Staphylococcus lentus. Biofouling. 2010;26(5):567–575. [DOI] [PubMed] [Google Scholar]
- 45. González S, Fernández L, Campelo AB, et al. The behavior of Staphylococcus aureus dual-species biofilms treated with bacteriophage phiIPLA-RODI depends on the accompanying microorganism. Appl Environ Microbiol. 2017;83(3):e02821-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kumari S, Harjai K, Chhibber S. Isolation and characterization of Klebsiella pneumoniae specific bacteriophages from sewage samples. Folia Microbiol (Praha). 2010;55(3):221–227. [DOI] [PubMed] [Google Scholar]
- 47. Fan H, Mi Z, Fan J, et al. A fast method for large-scale isolation of phages from hospital sewage using clinical drug-resistant Escherichia coli. African J Biotechnol. 2012;11(22):6143–6148. [Google Scholar]
- 48. Li L, Zhang Z. Isolation and characterization of a virulent bacteriophage SPW specific for Staphylococcus aureus isolated from bovine mastitis of lactating dairy cattle. Mol Biol Rep. 2014;41(9):5829–5838. [DOI] [PubMed] [Google Scholar]
- 49. Piracha ZZ, Saeed U, Khurshid A, et al. Isolation and partial characterization of virulent phage specific against Pseudomonas aeruginosa. Glob J Med Res. 2014;14(1):1–8. [Google Scholar]
- 50. Abedon ST, Herschler TD, Stopar D. Bacteriophage latent-period evolution as a response to resource availability. Appl Environ Microbiol. 2001;67(9):4233–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Al-Yassari IH, Al-Mola GA. Characterization of E. coli phage isolated from sewage. Al-Qadisiyah J Vet Med Sci. 2010;9(2):45. [Google Scholar]
- 52. Eriksson H, Maciejewska B, Latka A, et al. A suggested new bacteriophage genus, “Kp34likevirus,” within the Autographivirinae subfamily of podoviridae. Viruses. 2015;7(4):1804–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ksik-Szeloch A, Drulis-Kawa Z, Weber-Dabrowska B, et al. Characterising the biology of novel lytic bacteriophages infecting multidrug resistant Klebsiella pneumoniae. Virol J. 2013;10:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang Z, Zheng P, Ji W, et al. SLPW: A virulent bacteriophage targeting methicillin-resistant staphylococcus aureus in vitro and in vivo. Front Microbiol. 2016;7:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Taj MK, Ling JX, Bing LL, et al. Effect of dilution, temperature and pH on the lysis activity of t4 phage against E. coli BL21. J Anim Plant Sci. 2014;24(4):1252–1255. [Google Scholar]
- 56. Naser I Bin, Hoque MM, Abdullah A, et al. Environmental bacteriophages active on biofilms and planktonic forms of toxigenic Vibrio cholerae: Potential relevance in cholera epidemiology. PLoS One. 2017;12(7):e0180838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Taha OA, Connerton PL, Connerton IF, et al. Bacteriophage ZCKP1: A potential treatment for Klebsiella pneumoniae isolated from diabetic foot patients. Front Microbiol. 2018;9:2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pires D, Sillankorva S, Faustino A, et al. Use of newly isolated phages for control of Pseudomonas aeruginosa PAO1 and ATCC 10145 biofilms. Res Microbiol. 2011;162(8):798–806. [DOI] [PubMed] [Google Scholar]
- 59. Verma V, Harjai K, Chhibber S. Structural changes induced by a lytic bacteriophage make ciprofloxacin effective against older biofilm of Klebsiella pneumoniae. Biofouling. 2010;26(6):729–737. [DOI] [PubMed] [Google Scholar]
- 60. Hughes KA, Sutherland IW, Jones MV. Biofilm susceptibility to bacteriophage attack: The role of phage-borne polysaccharide depolymerase. Microbiology. 1998;144(pt11):3039–3047. [DOI] [PubMed] [Google Scholar]
- 61. Sagar SS, Kumar R, Kaistha SD. Efficacy of phage and ciprofloxacin co-therapy on the formation and eradication of Pseudomonas aeruginosa biofilms. Arab J Sci Eng. 2017;42:95–103. [Google Scholar]
- 62. Sillankorva S, Neubauer P, Azeredo J. Isolation and characterization of a T7-like lytic phage for Pseudomonas fluorescens. BMC Biotechnol. 2008;8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.