Abstract
Salmonellosis is an infection that significantly impacts chicken and humans who consume it; it is a burden on public health and a contributor to commercial losses in the chicken industry worldwide. To tackle chicken meat-related bacterial infections, significant quantities of antibiotics alongside several infection prevention measures are used worldwide. However, chemical additives, such as organic acids, and chlorine-based interventions all have different limitations. These include feed refusal due to a change of taste, and incompatibility between organic acids and other inoculated preservative agents such as antimicrobial agents. Phages are host-specific viruses that interact with bacteria in a specific manner. Therefore, they possess unique biological and therapeutic features that can be used to reduce bacterial contamination, leading to improved food safety and quality. This systematic review examines the current evidence regarding the effectiveness of various phages on Salmonella colonization in chicken meat. This review summarizes findings from 17 studies that were conducted in vitro with similar experimental conditions (temperature and incubation parameters) to test the efficacy of isolated and commercially available phages on chicken raw meat samples. The current evidence suggests that most of the in vitro studies that used phages as a biocontrol to eradicate Salmonella contamination in chicken meat were successful. This indicates that phages constitute a promising solution worldwide for tackling foodborne bacteria, including Salmonella.
Keywords: chickens, poultry, Salmonella, phages, in vitro
Introduction
Salmonellosis is one of the most significant infections that impacts chicken, posing a threat to public health and it is a cause of commercial losses within the chicken industry worldwide.1 Salmonellosis is caused by the Salmonella spp., which are Gram-negative, rod-shaped, facultative anaerobic bacteria, and are one of the most common causative agents of gastroenteritis.2
Fecal-oral is the main transmission route of foodborne pathogens by the ingestion of water and food contaminated with the feces of chronic carriers such as chicken.3 Within humans, one of the most common clinical manifestations is gastroenteritis, which is characterized by a sudden onset of diarrhea, abdominal cramps, fever, and vomiting.4 Out of the 93.8 million cases reported worldwide, there are an estimated 155,000 deaths per year. There were 1.2 million cases of human-related Salmonellosis reported in the United States in 2011 alone, resulting in a significant financial cost of $365 million.5
The Salmonella genus is classified into two species, Salmonella enterica and Salmonella bongori. The S. enterica subspecies enterica is responsible for the majority of clinical Salmonella infections and it is known to have >2600 serovars. The most important serovars that infect humans are S. enterica serovar Enteritidis and S. enterica serovar Typhimurium.6 In addition, S. enterica serovar Gallinarum biovars Pullorum (S. Pullorum) and Gallinarum (S. Gallinarum) can infect chicken and turkeys, respectively, causing widespread septicemic diseases such as fowl typhoid and pullorum disease.7
The consumption of chicken meat is extremely high in Saudi Arabia. The statistics show that 28.6 kg of chicken meat was consumed per capita per year between 1996 and 1998, increasing consumption between 2000 and 2004 to 37.7 kg per capita8 .According to the Food and Agriculture Organization (FAO), the consumption of chicken meat in Saudi Arabia was ranked among the highest globally and was predicted to increase.9 In the United States in 2018, 65.2 pounds (453.6 g/lb; 365.2 d/year) of chicken per person were available for Americans to eat compared with 54.6 pounds of beef, indicating the high consumption of chicken meat in the United States.10
To tackle bacterial infections within the poultry field, a large number of antimicrobials and antimicrobial technologies are used worldwide. Antibiotics are used in food-producing animals for the prevention and treatment of infections. However, the use of such agents has been linked to the development and spread of antibiotic-resistant bacteria.11
Salmonella in poultry
Chicken meat products are among the highest consumed products worldwide and many essential antibiotics are used during chicken production in places such as the United States, China, Brazil, Poland, the United Kingdom, Germany, France, and Spain.12 Nonetheless, food animal growth-promoting antibiotics have been banned in several countries. This is because the antibiotic use in broiler chicken farming can risk the effectiveness of the antibiotics by inadvertently increasing the possibility of bacteria developing resistance, and the subsequent spread of antibiotic-related resistance genes within the chicken environment.13
The extensive use of antimicrobials has tremendously increased internationally, along with the value of veterinary drugs such as antimicrobials.14 Antibiotics such as bacitracins, tylosins, and different tetracyclines are used to treat or prevent infections in chicken meat in North America.13 In the European Union, tetracyclines represent 37% of antimicrobials administered to animal farms. In intensive animal farming, 1.5 million kgs of antimicrobials were used in Canada in 2014.15 This excessive antibiotic consumption plays a crucial role in the development of antibiotic resistance in pathogens, leading to serious consequences such as treatment failure and economic losses16 Importantly, antibiotic consumption is not fully monitored in many countries around the world; therefore, the true extent of antibiotic usage and the downstream negative effects cannot be known.13
The chicken meat industry uses different chemical additives such as organic acids, essential oils, and chlorine-based interventions to mitigate the presence of pathogens.17 However, using these additives can result in a number of issues such as feed refusal due to a change of taste, incompatibility between organic acids and other inoculated preservative agents such as antimicrobials, and the alteration of microbial activity and behavior.18 Therefore, chicken meat processors urgently need to find alternatives to comply with the performance standards while meeting consumers' demands of wholesome, clean label, and safe products that are readily available.19 Bacteriophage (phage)-based products have been shown to be promising antibacterial intervention tools, and these are currently available and approved for commercial use in the United States and Europe.20
Bacteriophages as novel therapeutics
Phages are viruses that infect bacteria, and they are considered to be the most abundant entity on earth.21 Phages are very sensitive in relation to the hosts that they bind to, with some phages being able to infect a broad host range, and others probably only infecting a single host, both of which can be beneficial and advantageous.22 Their unique biological and therapeutic features can be utilized and manipulated to reduce bacterial contamination within the environment and industrial setting, leading to improved food safety.23
Phages can infect their host via two main lifecycles, these being lytic and temperate.21 In the lytic infection cycle, phages inject their DNA into the bacterial cells, hijacking the host and inducing the replication of phage DNA and production of mature phage particles, before releasing lysis enzymes to lyse the host cell, allowing for the release of progeny phages.21 Temperate phages release their DNA into the host, which integrates with the host DNA, resulting in the formation of prophages. These integrated phages replicate each time the host cell undergoes replication, and under moments of stress, can excise and enter the lytic cycle.
The integration and excision of phages to and from the bacterial cell can result in the infected bacteria acquiring phage genetic matter.21 It is important to assess the phage lifecycle genomically and experimentally before choosing a phage for therapeutic and biotechnological purposes, because temperate phages can transfer toxin-related and antibiotic resistance genes to their hosts, potentially making them more virulent.24
The survival of phages in experimental settings can be affected by different factors such as the physical characteristics of the phage itself, along with the matrix composition of the food or medium in question, and external factors influencing the phage such as temperature, pH, and the acidity of the environment.18,25 All these aspects must be investigated and well characterized before an active phage biocontrol agent can be introduced in the market.
Currently, there are commercially available ready-to-use lytic phage preparations being sold worldwide,26 such as Listex P100 and SalmoFresh™, which are used in the food manufacturing industry in Europe.27 The EBI Food Safety has approved Listex P100 for controlling Listeria in different products such as meat and dairy (cheese). The Lister P100 mix of Listeria-targeting phages was approved by the US-FDA in August 2006.28 SalmoFresh is a phage-based product that targets S. enterica, and it was granted the GRAS (Generally Recognize As Safe) status by the FDA in 2015.29
This review focuses on phage use within chicken meat, not other poultry meat, because it is the most consumed food product in Saudi Arabia, according to the FAO of the United Nations.8 This systematic review summarizes the current evidence regarding the effectiveness of various phages in reducing Salmonella colonization in chicken meat.
Methods
Search strategy
This systematic review was conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses methodology. Databases searched included PubMed, Google Scholar, SCOPUS, Web of Science Core Collection, and the Virtual Health Library. The keywords and search strategy for the main objective included: (Chickens or Poultries OR poult OR poults) OR (Chicken or Broiler or Duck* Geese OR Goose *Turkeys OR Meleagridinae OR Meleagrididae Fowl* AND Domestic Gallus AND (domesticus) fowlphage* Phage* Viruses* Salmonella [Mesh] Salmonella* Salmonellosis [Mesh] Salmonellosis OR Salmonelloses). Two authors (M.A., N.A.) independently conducted this process in January 2021.
Eligibility criteria
Salmonella phage articles were included if they provided an original study investigating the impact of phage therapy in reducing Salmonella-associated infections in chicken meat. We focused on in vitro studies tracing the impact of Salmonella on chicken meat, and the efficacy of phages in countering the presence of Salmonella within chicken meat-related samples. All species and strains of Salmonella and all types of phages were included. This review included studies that were published between January 2009 and May 2021.
The search was extended to 2009 to cover all the experimental studies from that period till 2021.30 Reviews, book chapters, commentaries, and studies conducted on live chicken meat (in vivo) were excluded. Studies conducted on biofilms or any species other than chicken meat were also excluded. Letters, editorials, conference abstracts, and non-English publications were excluded too.
Methods of review
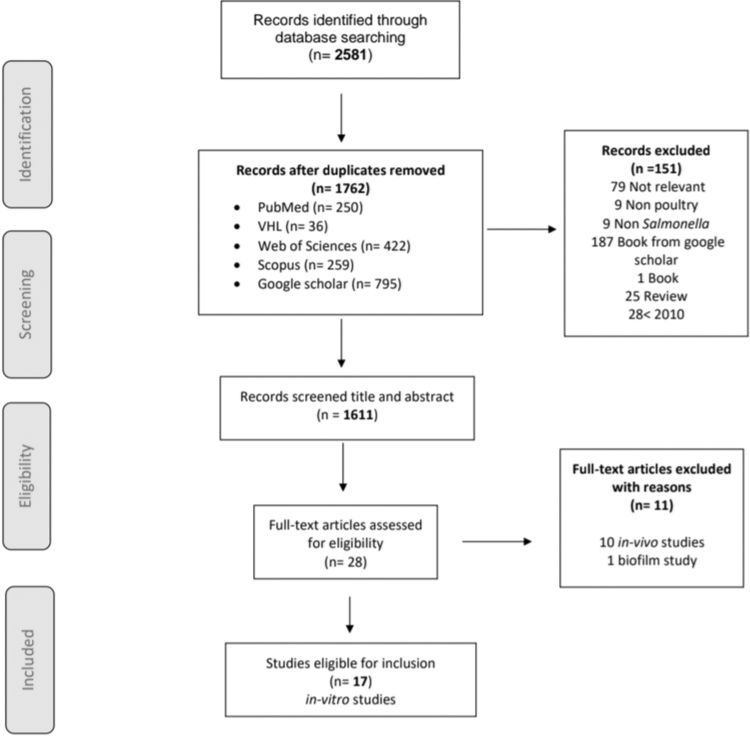
Titles and abstracts of the retrieved studies were collected into an Endnote® library. Overall, 2581 records were identified via database searches, of which 1446 were scientific articles. After screening titles and abstracts, the studies were selected and reduced to 180 articles. After analyzing and reviewing the full texts, 151 studies were found to be irrelevant; therefore, these were excluded. Thereafter, the full texts of the 29 potential articles were assessed extensively to ensure they met the eligibility criteria. From the 29 articles, 13 in vivo studies were excluded, with the remaining 17 studies included and selected for this review. The selected articles were identified by the last name of the first author. It was ensured that the publications met the inclusion criteria, as displayed in the flowchart diagram (Fig. 1).
FIG. 1.
Flowchart diagram of study selection.
The main objective of this work was to investigate the efficacy of phages on reducing Salmonella contamination in raw chicken meat. The outcome measure was a reduction in the viable counts of Salmonella colonization in chicken meat.
Data extraction
Two authors independently derived the data from the final pertinent publications. An excel file was created to collect the following: author name, year of publication, country, study design, type of Salmonella species, types of phage therapy, the dose of phage therapy, sources of phage isolation, and extent of Salmonella reduction. Any conflicts between the two authors were resolved after a discussion with a third author.
Study evaluation
The scoring method was adapted from the Campden BRI Company31 and modified to fit with the variables in the included studies (Tables 1 and 2). Each study was scored based on the following criteria; (Method of phage Propagation and purification, phage characterization, appropriate organisms for food type, inoculation method, number of organisms used [source, culture collection, or food isolates], application method, phage dose calculation, method of phage enumeration, number of time points, number of samples/replicates, the inclusion of relevant controls, statistical analysis performed on the raw data, analysis of data performed—log reduction calculated, conclusions on the efficacy of the phage/suggested, and comparison with other data).
Table 1.
Scoring of the Available Studies Assessing Phages in Reducing Salmonella Serovar in In Vitro Studies
| Title | Scoring | Study (year) |
|---|---|---|
| Reduction of Salmonella contamination on the surface of chicken skin using bacteriophage | 2 | Atterbury et al. (2020)33 |
| Application of a broad range lytic phage LPST94 for biological control of Salmonella in foods | 2 | Islam et al. (2020)35 |
| Bio-control of Salmonella spp. in carrot salad and raw chicken skin using lytic bacteriophages | 2 | Kumar et al. (2020)46 |
| Application of a phage cocktail for control of Salmonella in foods and reducing biofilms | 2 | Islam et al. (2019)32 |
| Lysis profiles of Salmonella phages on Salmonella isolates from various sources and efficiency of a phage cocktail against S. Enteritidis and S. Typhimurium | 1 | Petsong et al. (2019)37 |
| Biocontrol of foodborne Salmonella using bacteriophages | 2 | Abo-Senna et al. (2018)40 |
| Phage applications for improving food safety and infection control in Egypt | 2 | El-Shibiny et al. (2017)43 |
| Reduction of Salmonella in ground chicken using a bacteriophage | 2 | Grant et al. (2017)44 |
| Use of a lytic bacteriophage to control Salmonella Enteritidis in retail food | 2 | Thung et al. (2017)42 |
| Bacteriophage application on red meats and poultry: Effects on Salmonella population in final ground products | 2 | Yeh et al. (2017)45 |
| Isolation, characterization, and bioinformatic analyses of lytic Salmonella Enteritidis phages and tests of their antibacterial activity in food | 2 | Han et al. (2017)41 |
Scoring was based on the following measures: No details provided (0); some details are missing (1), fully available data (2).
Table 2.
Scoring of the Available Studies Assessing Phages in Reducing Salmonella Serovar in In Vitro Studies
| Title | Scoring | Study (year) |
|---|---|---|
| Reduction of Salmonella on chicken breast fillets stored under aerobic or modified atmosphere packaging by the application of lytic bacteriophage preparation SalmoFresh™ | 2 | Sukumaran et al. (2016)39 |
| Biocontrol of Salmonella Enteritidis in spiked chicken cuts by lytic bacteriophages ΦSP-1 and ΦSP-3 | 2 | Augustine and Bhat (2015)47 |
| Bio-control of Salmonella Enteritidis in foods using bacteriophages | 2 | Bao et al. (2015)36 |
| Bacteriophage P22 to challenge Salmonella in foods | 1 | Zinno et al. (2014)34 |
| Use of bacteriophages to reduce Salmonella in chicken skin in comparison with chemical agents | 2 | Hungaro et al. (2013)48 |
| Use of a bacteriophage cocktail to control Salmonella in food and the food industry | 2 | Spricigo et al. (2013)38 |
Scoring was based on the following measures: No details provided (0); some details are missing (1); fully available data (2).
Moreover, scoring was based on the following measures: (0) No details provided; (1) some details are missing; and (2) fully available data.
Two authors independently evaluated the included studies, and any conflicts between the two authors were resolved after a discussion with a third author.
Results
Characteristics and quality of studies
All of the studies included in this preview present the therapeutic efficacy of phages with the use of either phage cocktails or single-phage preparations to reduce Salmonella numbers on chicken meat. Nine studies out of the 17 focused on 2 Salmonella serovars, S. Enteritidis, and S. Typhimurium. Five studies experimented the efficacy of phages on S. Enteritidis alone, whereas three studies were conducted on different S. enterica strains as follows: S. Typhimurium strain (UK-1, ATCC 13311) and strain 41 of S. enterica serovar.32
Two studies were performed on S. Newport, S. Typhimurium, and S. Thompson, as well as other serovars including S. Heidelberg ATCC 8326, S. Enteritidis ATCC 13076, and S. Typhimurium. All these studies evaluated the efficacy of phages on different strains of Salmonella in vitro. The complete systematic review strategy is charted in Figure 1. In total, 17 studies satisfied the inclusion criteria and were included in the final assessment.32–48
All the reviewed studies were conducted in vitro and in all these experiments, similar conditions (temperature and incubation parameters) were used to test the efficacy of isolated and commercially available phages. However, there were differences in the incubation period of phages with the bacterial samples between the studies and the range of the incubation period shown (Tables 3–5).
Table 3.
Efficiency of Phages in Reducing Salmonella Serovar in In Vitro Studies
| Study | Salmonella serovar(s) | Phage/cocktail identification | Source of phage | Method of treatment | Concentration of phage | Incubation conditions | p | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Atterbury et al. (2020)33 |
S. Enteritidis P125109 S. Typhimurium 4/74. |
Eφ151, Tφ7, and Tφ11 | Poultry excreta and abattoir effluent | Spraying on chicken skin | MOI of 10 (9 log10 PFU/mL) | 24 h 37°C | <0.0001 | Significant reduction of Salmonella numbers in skin sections and surfaces after phage treatment compared with untreated samples |
| Islam et al. (2020)35 |
S. Enterica S. Typhimurium |
Phage LPST94 | Environmental water samples | Liquid (incubated with the samples) | MOI of 1000 or MOI of 10,000 | 48 h 4°C or 25°C | Not reported | A significant reduction in Salmonella counts by 3 log10 CFU/mL |
| Islam et al. (2019)32 |
S. Enterica S. Typhimurium |
Phage cocktail (LPSTLL, LPST94 and LPST153) | Environmental water samples | Liquid (spreading over the samples) | MOI of 1000 (6 log10 PFU/mL) or MOI of 10,000 (7 log10 PFU/mL) | 48 h 4°C or 25°C | Not reported | Salmonella contamination was eliminated, and biofilms were eradicated by using phage cocktail |
| Zinno et al. (2014)34 |
S. Enterica S. Typhimurium |
Phage P22 (Temperate phage) | Unknown | Spraying on chicken breast | MOI of 107 (12 log10) | 48 h 4°C | <0.01 | Reduction in Salmonella counts by 2–3 log10 CFU/mL |
| Bao et al. (2015)36 |
S. Enteritidis ATCC13076 S. Enteritidis CVCC2184 |
Phage cocktail (PA13076 and PC2184) | Chicken excretion sewage | Liquid (poured onto the surface) | MOI of 10,000 (8 log10 PFU/mL) | 5 h 4°C or 25°C | <0.05 | The cocktail was able to reduce bacterial number significantly (4 log10 CFU/sample) |
| Petsong et al. (2019)37 |
S. Enterica S. Typhimurium |
Phage cocktail (KP1, KP2, KP4, KP5, KP9, KP34, KP36, KP49, and KP50) | Animal farm | Liquid (spiked to the surface) | MOI of 100 (7 log10 PFU/mL) | 4 days 4°C | <0.05 | Phage cocktail reduced S. Enteritidis and S. Typhimurium by 0.66 log CFU/cm2 and 1.73 log CFU/cm2, respectively |
MOI, multiplicity of infection.
Table 4.
Efficiency of Phages in Reducing Salmonella Serovar in In Vitro Studies
| Study | Salmonella serovar | Phage/cocktail identification | Source of phage | Method of treatment | Concentration of phage | Incubation conditions | p | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Spricigo et al. (2013)38 |
S. Enteritidis UA1894 S. Typhimurium UA1872 |
Phage cocktail (UAB_Phi20, UAB_Phi78, and UAB_Phi87) | Animal farm | Liquid (spiked to the surface) | MOI of 1000 (9 log10 PFU/mL) | 5 min at 25°C and then 7 days at 4°C | <0.01 | Significant decrease in S. Typhimurium (2.2 log10 CFU/g) and S. Enteritidis (1.4 log10 CFU/g) |
| Sukumaran et al. (2016)39 |
S. Typhimurium (ATCC 14028) S. Enteritidis (ATCC 4931) S. Heidelberg |
SalmoFresh (SBA-1781, SKML-39, SPT-1, SSE-121, STML-13-1, STML-198) | Commercial | Liquid (surface and dipping treatment) | MOI of 106 (9 log10 PFU/mL) | 7 days 4°C | <0.05 | Higher reduction in bacterial counts for surface-treated samples by 1.2 log CFU/g within the first day of incubation |
| Abo-Senna et al. (2018)40 | S. Typhimurium (ATCC 14028) | Phage cocktail (1A, 1B, 1C, and 1D) | Waste water | Liquid (soaking and spraying treatment) | MOI of 106 (10 log10 PFU/mL) | 7 days 4°C | Not reported | Phage-treated samples showed a reduction in Salmonella counts by 3.2 log/g (soaking) and 2.2 log/g (spraying) within a day. After 4 days of incubation, no bacterial growth was observed. |
| Han et al. (2017)41 | S. Enteritidis | Phage cocktail (BPS2H1, BPS7T1, BPS8H2, BPS11Q3, BPS11T1, BPS11T2, and BPS15Q2) | Sewage (Sanitary sewers and livestock farms) | Liquid (pre-addition) | MOI of 106 (8 log10 PFU/g) | 1, 2, 4, 6, 8, or 10 days at 8, 15, 20, 25, 30, or 35°C | Not reported | S. Enteritidis contamination was completely eliminated within the first day of phage treatment |
| Thung et al. (2017)42 | S. Enteritidis | Phage SE07 | Retail chicken meat sample | Liquid (spraying and dispensing) | MOI of 107 (12 log10 PFU/mL) | 12, 24, 48 h 4°C | <0.01 | Bacterial population was reduced by 1.8 log/mL when treated with SE07 compared with untreated samples in 12 h |
| El-Shibiny et al. (2017)43 | S. Enterica ATCC 25566 | Phage ZCSE1 | Sewage water | Liquid (inoculation) | MOI of 0.1 (7 log10 PFU/mL) | Inoculation for 45 min at 25°C before incubation for 6–8 days at 4°C | <0.005 | Phage treatment reduced Salmonella count by 2 log/mL compared with untreated samples |
Table 5.
Efficiency of Phages in Reducing Salmonella Serovar in In Vitro Studies
| Study | Salmonella serovar | Phage/cocktail identification | Source of phage | Method of treatment | Concentration of phage | Incubation conditions | p | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Grant et al. (2017)44 |
S. Newport, S. Typhimurium, (S. Thompson), strains (S. Heidelberg ATCC 8326, S. Enteritidis ATCC 13076, S. Typhimurium) Nonground chicken isolates S. Heidlburg (ATCC8326), S. Enteritidis (ATCC13076), and S. Typhimurium Ground chicken cocktail: S. Newport, S. Thompson, and S. Typhimurium |
Salmonelex™ (S16 and FO1a), | Commercial | Liquid spreading onto chicken by using sterile tap or filtered water. | MOI of 1000 (7 log10 PFU/cm2) | 30 min or 8 h 4°C and 37°C | <0.05 | Phage mixture diluted in sterile tap water resulted in greater Salmonella reduction than in filtered water (0.39 and 0.23 log CFU/cm2, respectively). Longer incubation time (8 h) showed more bacterial reduction than 30 min (0.90 and 0.71 log CFU/cm2, respectively). |
| Yeh et al. (2017)45 |
S. enterica (ATCC 51741), S. Heidelberg (ATCC 8326), S. Newport (ATCC 27869) S. Enteritidis C Se 13 |
Salmonelex (S16 and Felix-O1a (FO1a) | Commercial | Liquid inoculation | MOI of 1 (7 log10 PFU/g), and MOI of 10 (8 log10 PFU/g) | 30 min and 6 h 4°C | No significant reduction | No significant reduction was observed when phage was added to an MOI of 1 and incubated for 30 min. However, with longer incubation time (6 h) and higher MOI (10), Salmonella populations decreased by 0.8 and 1.1 log CFU/g, respectively. |
| Kumar et al. (2020)46 | S. Enteritidis (ATCC 13076) and Salmonella spp. (MTCC 1162) | Phage NINP13076 and phage NINP1162 | Sewage water | Liquid by pipetting | MOI of 1000 (8 log10 PFU/mL) | 5 h 25°C | Not reported | Treatment of raw chicken skin with bacteriophages showed a significant reduction in Salmonella count (6.7–5.4 log CFU/g) after 3 h of incubation |
| Augustine and Bhat (2015)47 | S. Enteritidis | Phage SP-1 and phage SP-3 | Intestinal content of chicken | Liquid by pipetting | MOI of 10 (4 log10 PFU/mL), or MOI of 1000 (6 log10 PFU/mL) | 72 h 4°C, room temperature, and 37°C | Not reported | When phage cocktail was applied at an MOI of 10, the count decreased by 0.98 log10 CFU/mL after 3 days. At MOI of 1000, the cocktail was able to reduce Salmonella count to beyond detectable levels within 3 days of incubation. |
| Hungaro et al. (2013)48 | S. Enteritidis | Phage cocktail (phiSE1 phiSE2, phiSE3, phiSE4, and phiSE5) | Free-range chicken feces | Liquid by dipping | MOI of 104 (9 log10 PFU/mL) | 16 h 6°C | <0.05 | Reduction in Salmonella count by 1.0 log CFU/cm2 after 16 h of phage treatment |
Bacteriophage in vitro therapy and efficacy on chicken meat
Two studies by Grant et al.44 and Atterbury et al.33 reported the effectiveness of Salmonelex™ by (Intralytix, Inc.) founded in Baltimore, Maryland, United States, a commercially available phage cocktail containing phages S16 and FO1a, on killing a mixture of different Salmonella serovars after similar periods of incubation. The first study by Grant et al.44 was mainly dependent on the water type that was used to dilute the phage lysate, time of treatment, and the susceptibility of Salmonella cells to phages.44 The water used to dilute the phages was either sterile-filtered water (sfH2O) or sterile tap water (stH2O).
The final concentration of phages after being diluted was ∼107 PFU/cm2; later on, phages were spread onto the chicken meat. When the phage was diluted in stH2O, the effectiveness of the Salmonelex in inhibiting the bacterial cells was significant compared with that of the phages diluted using sfH2O, 0.39 log CFU/cm3, and 0.23 log CFU/cm3, respectively (p < 0.05). A study by Yeh et al.45 was conducted to explore the effectiveness of the Salmonelex phages after incubating for either 30 min or 6–8 h with a mixture of the Salmonella serovars. In addition, Salmonella strains and the phage were incubated on a soft Luria-Bertani agar (0.6% agar).
In quadruplicate, a volume of 100 μL of diluted overnight cultures of individual strains were prepared. Overall, the phages were applied by liquid inoculation into ground chicken meat, and the application of the phage reduced the amount of Salmonella by 1.1 and 0.9 log CFU/g in ground chicken meat.
The aforementioned studies investigated the effect of incubating the Salmonella cells with the phage cocktail for 30 min. Although this did not result in a significant reduction of viable cell counts, a small reduction in viable Salmonella was detectable after 30 min. However, the maximum reduction of viable Salmonella occurred after 6–8 h of incubation in both studies, with a reduction of ∼1 log CFU/g (Table 2) seen in the study conducted by Atterbury et al.33 and his group, who assessed the effectiveness of two phages (Eφ151 and Tφ10) on two sections of chicken from each bird (72 skin samples total).33 The chickens were first orally inoculated with either 0.3 mL of 8.0 log10 CFU/mL suspension S. Enteritidis P125109 or S. Typhimurium 4/74 to infect the chicken before the in vitro experiment.
After slaughter, chicken skin samples were sprayed with 1 mL (0.5 mL per side) of control solution containing 50 mM Tris-Cl [pH 7.5], 0.1 M NaCl, 8 mM MgSO4.7H2O, and 0.01% w/v gelatin. The treated section of chicken skin was sprayed in an identical manner with either 1 mL of a 9.0 log10 PFU/mL aliquot of phage Eφ151 (group 1) or phage Tφ10 (group 2). After spraying the chicken skin with the phages for 20 min, it was allowed to dry. The phage-treated chicken skin samples resulted in a significant reduction of Salmonella levels by a median of 1.38 log10 per skin section compared with the control (p < 0.0001).33
Impact of atmospheric conditions and phage dosage
Specific conditions or processes can have different influences on the effectiveness of phages to kill Salmonella, one example being the impact of modified atmosphere packaging (MAP). MAP is the process of changing the standard composition of air by altering different percentages of gases (78% nitrogen, 21% oxygen, 0.03% carbon dioxide, and traces of noble gases) to provide an optimum atmosphere for increasing the storage length and quality of food.49 Of relevance, a study conducted by Sukumaran et al.39 showed that the commercially available phage SalmoFresh was able to reduce the cell count under MAP conditions in a 24-h period.39
This study evaluated the efficacy of SalmoFresh under different treatment methods (dipping or surface spraying). The dipping treatment was performed by immersing the chicken breast fillets samples (100 mL of phage solution [109 PFU/mL] for 20 s); this obtained better results than using the phage solution as a surface treatment (0.5 mL of phage solution (109 PFU/mL) for 7 days) on the surface in the treated chicken breast fillets sample, by significantly decreasing the total counts of Salmonella (from 1 log CFU/g to 0.7 log CFU/g and 0.9).
Single phage or phage cocktail comparisons
Another two studies carried out by Islam et al.32,35 tested administering phages as either a single phage (9LPST94) or a cocktail of phages (LPSTLL, LPST94, and LPST153). The results showed that Salmonella could be significantly eliminated from chicken meat with the use of either the single phage or phage cocktail under similar conditions. Both studies showed undetectable counts of Salmonella (<1 log CFU/100 μL) after 6–12 h of incubation with the phages.32 Moreover, a bio-control study reported by using a combination of two-phage strains (P2 and P4) showed a reduction of bacterial presence on chicken breast meat by 1.65 log CFU/sample after 1 h and by 2.5 log CFU/sample after 5 h of incubation.36
Further, two independent studies conducted by Grant et al.,44 and Augustine and Bhat47 determined that phage cocktails administered at different concentrations can significantly reduce bacterial counts more than using a single phage lysate. The studies also showed that at different temperatures (−4°C, 28°C, and 37°C), around 92% of bacterial load was decreased when the samples were treated with phages at 28°C in 72 h. The reduction rates were 79% and 78% at −4°C and 37°C, respectively. Also, results showed that when phage is applied on chicken meat samples using different multiplicity of infection (MOI) values (10 and 1000), where the MOI indicates ratios of phages to bacteria, treatment with high MOI resulted in more reduction in bacterial numbers47 despite using single phage or phage cocktail.
Phages and temperature
Incubation temperature can affect the success of phage treatment by either increasing the reduction of the bacterial count or decreasing it. In the Hungaro et al. study,48 Salmonella counts decreased after phage administration at 6°C temperature and with a short contact period (30 min). In addition, results of another independent study showed a reduction of 2 to 3 log of Salmonella counts after an incubation period of 48 h at 4°C with a single phage application to chicken meat samples (either minced or whole chicken meat breasts).34 Another study showed that phage SE07, a lytic phage, has a strong efficacy in reducing Salmonella concentrations by 2.1 to 2.0 log under similar conditions.42
Impact of delivery on phage efficacy
Different techniques have been experimented to test the efficacy of phages, such as spraying, dipping, and incubating the phages with chicken meat samples.40 Abo-Senna et al40 investigated the effectiveness of using a phage cocktail against different strains of Salmonella by using different treatment methods such as soaking or spraying over a 7 day period. The results showed a gradual reduction of viable Salmonella counts, which is consistent with the findings from the previously mentioned studies. Moreover, the results showed that on the fourth day after spraying the phage cocktail, after an 8-day period, undetectable levels of Salmonella counts were reported; this demonstrated the reduction of Salmonella counts using phage cocktails.37,38,43
Another phage administration method is to use phages prophylactically. In a study where phages were added before the commercially frozen chicken breasts samples, they were cut into pieces (2.5 × 2.5 cm2). Subsequently, such chicken cuts were autoclaved first to ensure no contamination at 121°C for 15 min. Then, all chicken samples were superficially covered with 100 μL of phage cocktail (final concentration of 1 × 109 PFU/g) and stored at 4°C for 24 h. One day later, 50 μL of the Salmonella cocktail was added (final concentration of 1 × 103 CFU/g) to each chicken breast sample.
The samples were incubated for 3 days at these different temperatures: 8°C, 15°C, 20°C, 25°C, 30°C, and 35°C.41 Further, contamination was eliminated within the first day of phage treatment, and the reduction continued in all recorded temperatures and continued to slightly increase until day 4, when there was an observed reduction of the Salmonella counts. With the results obtained, evidence suggests a possible application of phages as a food additive to control secondary contamination in ready-to-eat (RTE) food.41 However, the previous study might indicate a specific period of incubating the phages with chicken meat samples.
Discussion
Salmonellosis is of public health concern; it is a disease caused by different serovars of Salmonella spp. There is an increasing demand to employ novel, effective, and safe interventions to limit the incidence of foodborne salmonellosis. Conventional control measures such as preheating methods, chemical preservatives, and antibiotics can control the spread and prevalence of pathogens.17 However, these procedures run the risk of negatively affecting the quality of food products, reducing nutritional availability, or exposing the consumer to possibly harmful chemicals.
Although using phages for therapeutic purposes or as biocontrol agents is not a novel idea, in recent years, the need for their use has increased because of the rapid evolution of drug-resistant bacteria.24 Since antibiotics have been commonly used in feed for animal production as a conventional therapy, excessive antibiotic consumption might result in resistance in different bacteria, including Salmonella species.50
In the articles included in this review, most of the in vitro studies used phages as a biocontrol tool was designed to eradicate Salmonella contamination in chicken meat, and the results of these studies were generally positive. This indicates that phages are, indeed, a promising solution to tackle foodborne bacteria, including Salmonella.51 The use of phages as antimicrobial agents in food factories is expanding, and several commercial companies have launched their own phage cocktail products for the purpose of decontaminating foodborne pathogens.52 It is important to state that the wide variety of phage applications have encouraged different industries such as water and food safety, agriculture, and animal health to develop phage-based products.
Risks associated with phages
It is still unclear whether using phages for poultry treatment entails more risk than conventional treatments such as antibiotics and chemical preservatives. Because of this, there is a necessary intense scrutiny to delineate the potential benefits and consequences of using phages. Despite the many advantages of phage therapy, there are certain drawbacks in using phages to eradicate diseases on a large scale. Phages that access the temperate life cycle may transfer genes to the bacterial host, including genes encoding toxic proteins or antibiotic resistance genes. Full genomic characterization of phages used in the treatment should be performed to ensure lytic activity and to check for the presence of toxins and immune-inducing proteins.53
Another hurdle for phage therapy in chicken meat is the current food and safety regulations. Many phage-based commercial products have been approved for use as a feed additive or on RTE food in the United States.30 Other countries need to conduct more research to implement phage applications in their respective chicken meat industries.54 Therefore, more research and scientific opinions are required to overcome the potential hurdles of phage administration in chicken meat.
Impact of environmental conditions and phage composition on efficacy
In this systematic review, we have explored the efficacy of phage in countering the presence of Salmonella in chicken meat by using several studies. Most of the studies mentioned that the main limitation of using phages is their stability under different conditions, such as temperature and pH, which can affect the treatment efficacy. However, a study showed that low temperatures can still be suitable for a specific period, and it could also enhance the activity of the phages on chicken meat samples.36 Moreover, with a more extended period of incubation and treatment with phages at the same temperature, the effectiveness of the phage treatment yielded better results.37
The application of phage cocktails was also more effective in long incubation period, with a significant reduction occurring within 3 h of phage incubation on food such as chicken meat meats.45 This indicates that the incubation time of phages on food samples is an essential factor to consider. Similarities have been noted in studies that experimented with the efficacy of phages in significantly reducing Salmonella in chicken meat samples from between 1 and 10 days. Despite temperature variation, the results showed approximately undetectable levels of Salmonella counts in phage-treated chicken meat samples.38,41
Impact of time and dosage on phage efficacy
Different studies have only assessed the efficacy of phages that were incubated with chicken meat or sprayed on chicken skin samples for 30 min, as this time period was also found to be effective in reducing Salmonella presence in ground chicken meat samples by 1.1 log CFU/g45 and 1 log CFU/cm2 were observed when phage cocktail was sprayed on chicken skin samples.48 These results are consistent with previous studies that used a long incubation period to obtain similar results.45 In the reviewed studies, the most significant reductions in host cell numbers were measured when the contaminated chicken meat samples were incubated with phages for >30 min or even days.
Another factor that has been investigated in the reviewed studies is the concentration of applied phages, with a high concentration of phages found to be more effective in reducing the counts of Salmonella in chicken meat samples compared with lower concentrations of phage preparations.35 Of interest, a higher MOI reduced the viable Salmonella counts below the level of detection. All reviewed studies that used different concentrations showed different trends in reducing bacterial contamination to undetectable levels. Thus, it can be concluded that the effectivity of phage therapy in countering Salmonella contamination is a concentration-dependent process.
Two studies have simulated the industrial process of preparing chicken meat by adding phages during the pre-packaging step or via different techniques.39,41 Further, storing chicken meat samples under aerobic or modified atmospheric conditions reduced the number of Salmonella counts in chicken meat samples treated with phages in comparison to the untreated controls. Phage cocktails significantly reduced the numbers of different Salmonella species when applied to the surface of the chicken meat samples or when the chicken meat samples were dipped into a phage solution.44
Using phage cocktails is a promising approach to maximize the effect of phages in reducing bacterial infections in chicken meat samples.38 Initial studies demonstrated that a Salmonella specific phage cocktail was significantly more effective than a single phage administration in reducing the population of Salmonella.
Further research needs to be conducted to optimize phage application to a mixed population of Salmonella species. Thus, data from the reviewed studies provide convincing evidence for the effectiveness of lytic phage cocktails in limiting the growth of pathogenic Salmonella species in chicken meat.44
Importance of understanding the phage biology to exploitation
Understanding the biology of phages and phage–host interactions is key to understanding the nature of phage life cycles and how this affects the efficacy of phage applications. Phages with lytic life cycles infect and rapidly kill their infected host cells, making them more favorable for phage therapy. This is in comparison to lysogenic (temperate) phages, whose DNA integrates into the bacterial host genome, or exists as a plasmid within their host cells rather than inducing cell lysis.21 Using lysogenic phages has major disadvantages, as they could transfer antibiotic resistance genes and toxin-encoding genes, potentially making the recipient bacteria more resistant to antimicrobial agents and more pathogenic.55
In one of the selected studies, a temperate phage (phage P22) was used to eliminate Salmonella contamination.34 Although P22 is a lysogenic phage, it showed virulent activity against the tested Salmonella strains. It is still not recommended to use lysogenic or temperate phages for phage therapy and biocontrol applications. Therefore, for such cases, strictly lytic phages should be utilized.
Undoubtedly, phage therapy has huge potential and its success depends on a variety of factors such as the administration method, phage concentration, specificity, dose, time of delivery, and environmental conditions.40 Further, a limitation of this review is that the studies only focused on the efficacy of phages. Surprisingly, none of the studies identified in this review commented on the safety of phage therapy. It is important to note that there are a number of factors that might affect phage efficacy and safety, and this should be addressed in further studies.
Conclusions and Future Prospects
Several approaches are used to improve our food safety and in the elimination of foodborne pathogens, such as pasteurization, high-pressure processing, phage biocontrol, and chemical sanitization.52 The study of phages is an essential tool in the development of biotechnology and could play a role in future as an effective food safety bioagent without any considerable side effects or negatives.
The poultry meat most consumed in Saudi Arabia is chicken meat, and this demand is projected to increase.8 Under the umbrella of the 2030 vision (Saudi Arabia Vision 2030.), the local government of Saudi Arabia is supporting the chicken meat industry for meat production and for economic benefits.56 To fulfill this increasing demand in the future, new safety measures and efficient biocontrol measures need to be in place to prevent foodborne pathogens from causing diseases.
In Saudi Arabia, there are no regulations or control methods aimed at monitoring or surveying phages in poultry. In addition, the efficacy of these naturally present phages to eradicate pathogens should be tested. Bacterial susceptibility to phages, and phage stability and efficiency should be constantly measured and tested during any future phage treatments.
Phages offer many solutions in preventing several issues such as antibiotic resistance, which is a considerable dilemma in the current food safety scene. Many phage-based preparations have been commercialized and registered for use in the United States. However, for their implementation in Saudi Arabia, the due diligence in terms of regulations and scientific opinions is yet to be carried out. Each phage preparation should be assessed individually and the genomic sequences analyzed to ensure the lack of genes encoding for genome integration, antibiotic resistance, and potential virulence factors such as toxins.54
The economic aspects associated with the large-scale phage production needed to supply the poultry industry may be challenging. In addition, it is critical to consider the production costs for phage products at the early stages of development to minimize the investment risk and uncertainty.57 To conclude, the application of phages in the Saudi poultry industry has great potential, with upsides related to health and economic benefits.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
References
- 1. Berchieri AJ, Murphy CK, Marston K, et al. Observations on the persistence and vertical transmission of Salmonella enterica serovars Pullorum and Gallinarum in chickens: Effect of bacterial and host genetic background. Avian Pathol. 2001;30(3):221–231. [DOI] [PubMed] [Google Scholar]
- 2. Özkalp B. Isolation and identification of Salmonellas from different samples. In: Salmonella—A Dangerous Foodborne Pathogen. London: IntechOpen; 2012. [Epub ahead of print]; DOI: 10.5772/28246. [DOI] [Google Scholar]
- 3. Salmonella in the Caribbean-2013 Infection with Salmonella. Retrieved from https://www.cdc.gov/training/SIC_CaseStudy/Infection_Salmonella_ptversion.pdf (accessed June 24, 2021).
- 4. Eng S-K, Pusparajah P, Ab Mutalib N-S, et al. Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front Life Sci. 2015;8(3):284–293. [Google Scholar]
- 5. Phothaworn P, Supokaivanich R, Lim J, et al. Development of a broad-spectrum Salmonella phage cocktail containing Viunalike and Jerseylike viruses isolated from Thailand. Food Microbiol. 2020;92:103586. [DOI] [PubMed] [Google Scholar]
- 6. Saleh S, Van Puyvelde S, Staes A, et al. Salmonella Typhi, paratyphi A, enteritidis and typhimurium core proteomes reveal differentially expressed proteins linked to the cell surface and pathogenicity. PLoS Negl Trop Dis. 2019;13(5):e0007416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xiong D, Song L, Pan Z, et al. Identification and discrimination of Salmonella enterica serovar gallinarum biovars pullorum and gallinarum based on a one-step multiplex PCR assay. Front Microbiol. 2018;9:1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shuaib MA. Poultry sector country review Saudi Arabia FAO Animal Production and Health Division Emergency Centre for Transboundary Animal Diseases Socio Economics, Production and Biodiversity Unit. This review is based on the following report: The Structure and Importance of the Commercial and Village based Poultry Systems in the Kingdom of Saudi Arabia. 2007. [Google Scholar]
- 9. Kornel D. India FAO Animal Production and Health Division Emergency Centre for Transboundary Animal Diseases Socio Economics, Production and Biodiversity Unit Food and Agriculture Organization of the United Nations Poultry sector country review. This review is based on the following report: The structure and importance of the commercial and village based poultry systems in India. 2008. [Google Scholar]
- 10. Daniel CR, Cross AJ, Koebnick C, et al. Trends in meat consumption in the United States. Public Health Nutr. 2011;14(4):575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The WHO Regional Office for Europe. Retrieved from https://www.euro.who.int/en (accessed February 24, 2022).
- 12. Roth N, Käsbohrer A, Mayrhofer S, et al. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: A global overview. Poultry Sci. 2019;98(4):1791–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Agyare C, Boamah VE, Zumbi CN, et al. Antibiotic use in poultry production and its effects on bacterial resistance. Antimicrob Resist Glob Threat. 2018. [Epub ahead of print]; DOI: 10.5772/INTECHOPEN.79371. [DOI] [Google Scholar]
- 14. Global animal health market value 2002-2020 | Statista. Retrieved from https://www.statista.com/statistics/260185/global-animal-health-market/#statisticContainer (accessed February 24, 2022).
- 15. Mehdi Y, Létourneau-Montminy MP, Gaucher ML, et al. Use of antibiotics in broiler production: Global impacts and alternatives. Anim Nutr. 2018;4(2):170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Antimicrobial resistance. Retrieved from https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed July 14, 2021).
- 17. Yang X, Xin H, Yang C, et al. Impact of essential oils and organic acids on the growth performance, digestive functions and immunity of broiler chickens. Anim Nutr. 2018;4(4):388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ben Braïek O, Smaoui S. Chemistry, safety, and challenges of the use of organic acids and their derivative salts in meat preservation. J Food Qual. 2021;2021:1–20. [Google Scholar]
- 19. Delgado-Pando G, Ekonomou SI, Stratakos AC, et al. Clean label alternatives in meat products. Foods. 2021:10:1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Połaska M, Sokołowska B, Polaska B, et al. Bacteriophages-a new hope or a huge problem in the food industry. Aims Microbiol. 2019;5(4):324–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clokie MRJ, Millard AD, Letarov AV, et al. Phages in nature. Bacteriophage. 2011;1(1):31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hendrix RW. Bacteriophage genomics. Curr Opin Microbiol. 2003;6(5):506–511. [DOI] [PubMed] [Google Scholar]
- 23. Barbu EM, Cady KC, Hubby B. Phage therapy in the era of synthetic biology. Cold Spring Harb Perspect Biol. 2016;8(10):a023879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin DM, Koskella B, Lin HC. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J Gastrointest Pharmacol Ther. 2017;8(3):162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bardina C, Spricigo DA, Cortés P, et al. Significance of the bacteriophage treatment schedule in reducing Salmonella colonization of poultry. Appl Env Microbiol. 2012;78(18):6600–6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cristobal-Cueto P, García-Quintanilla A, Esteban J, et al. Phages in food industry biocontrol and bioremediation. Antibiot. 2021;10(7):786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Evaluation of the safety and efficacy of Listex™ P100 for reduction of pathogens on different ready-to-eat (RTE) food products. EFSA Journal 2016;14(8):4565. [Google Scholar]
- 28. García P, Martínez B, Obeso JM, et al. Bacteriophages and their application in food safety. Lett Appl Microbiol. 2008;47(6):478–485. [DOI] [PubMed] [Google Scholar]
- 29. Zhang X, Niu YD, Nan Y, et al. SalmoFresh™ effectiveness in controlling Salmonella on romaine lettuce, mung bean sprouts and seeds. Int J Food Microbiol. 2019;305:108250. [DOI] [PubMed] [Google Scholar]
- 30. Sarhan WA, Azzazy HME. Phage approved in food, why not as a therapeutic? Expert Rev Anti Infect Ther. 2015;13(1):91–101. [DOI] [PubMed] [Google Scholar]
- 31. FINAL REPORT Systematic and critical review on the potential use of bacteriophage on foods FS102079. 2016. [Google Scholar]
- 32. Islam Y, Liang L, Nime I, et al. Application of a phage cocktail for control of Salmonella in foods and reducing biofilms. Viruses. 2019;11:841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Atterbury RJ, Gigante AM, Rubio Lozano MS, et al. Reduction of Salmonella contamination on the surface of chicken skin using bacteriophage. Virol J. 2020;17(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zinno P, Devirgiliis C, Ercolini D, et al. Bacteriophage P22 to challenge Salmonella in foods. Int J Food Microbiol 2014;191:69–74. [DOI] [PubMed] [Google Scholar]
- 35. Islam MS, Zhou Y, Liang L, et al. Application of a broad range lytic phage LPST94 for biological control of salmonella in foods. Microorganisms. 2020;8(2):247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bao H, Zhang P, Zhang H, et al. Bio-control of Salmonella enteritidis in foods using bacteriophages. Viruses. 2015;7(8):4836–4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petsong K, Benjakul S, Chaturongakul S, et al. Lysis profiles of Salmonella phages on Salmonella isolates from various sources and efficiency of a phage cocktail against S. Enteritidis and S. Typhimurium. Microorganisms. 2019;7(4):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spricigo DA, Bardina C, Cortés P, et al. Use of a bacteriophage cocktail to control Salmonella in food and the food industry. Int J Food Microbiol. 2013;165(2):169–174. [DOI] [PubMed] [Google Scholar]
- 39. Sukumaran AT, Nannapaneni R, Kiess A, et al. Reduction of Salmonella on chicken breast fillets stored under aerobic or modified atmosphere packaging by the application of lytic bacteriophage preparation SalmoFresh™. Poult Sci. 2016;95(3):668–675. [DOI] [PubMed] [Google Scholar]
- 40. Abo-Senna AS, Abd alla NA, El-Fouly MZ, et al. Biocontrol of foodborne Salmonella using bacteriophages. Arab J Nucl Sci Appl. 2018;51(1):100–109. [Google Scholar]
- 41. Han H, Wei X, Wei Y, et al. Isolation, characterization, and bioinformatic analyses of lytic Salmonella Enteritidis phages and tests of their antibacterial activity in food. Curr Microbiol. 2017;74(2):175–183. [DOI] [PubMed] [Google Scholar]
- 42. Thung TY, Jayarukshi K, Premarathne K, et al. Use of a lytic bacteriophage to control Salmonella Enteritidis in retail food. LWT Food Sci Technol. 2017;78:222–225. [Google Scholar]
- 43. El-Shibiny A, El-Sahhar S, Adel M. Phage applications for improving food safety and infection control in Egypt. J Appl Microbiol. 2017;123(2):556–567. [DOI] [PubMed] [Google Scholar]
- 44. Grant Q, Parveen S, Schwarz J, et al. Reduction of Salmonella in ground chicken using a bacteriophage. Poult Sci. 2017;96(8):2845–2852. [DOI] [PubMed] [Google Scholar]
- 45. Yeh Y, Purushothaman P, Gupta N, et al. Bacteriophage application on red meats and poultry: Effects on Salmonella population in final ground products. Meat Sci. 2017;127:30–34. [DOI] [PubMed] [Google Scholar]
- 46. Kumar B, Kumar PU, Ghosh S, et al. Bio-control of Salmonella spp. in carrot salad and raw chicken skin using lytic bacteriophages. LWT Food Sci Technol. 2020;122:109039. [Google Scholar]
- 47. Augustine J, Bhat SG. Biocontrol of Salmonella Enteritidis in spiked chicken cuts by lytic bacteriophages ΦSP-1 and ΦSP-3. J Basic Microbiol 2015;55(4):500–503. [DOI] [PubMed] [Google Scholar]
- 48. Hungaro HM, Mendonça RCS, Gouvêa DM, et al. Use of bacteriophages to reduce Salmonella in chicken skin in comparison with chemical agents. Food Res Int. 2013;52(1):75–81. [Google Scholar]
- 49. Farber JN, et al. Microbiological safety of controlled and modified atmosphere packaging of fresh and fresh-cut produce. Compr Rev Food Sci Food Safety. 2003;2(S1):142–160. [Google Scholar]
- 50. National Research Council. Antibiotics in animal feeds. In The Effects on Human Health of Subtherapeutic Use of Antimicrobials in Animal Feeds. US: National Academies Press; 1980. [PubMed] [Google Scholar]
- 51. Atterbury MA, Ortiz F, Lovell MA, et al. Bacteriophage therapy to reduce salmonella colonization of broiler chickens. Appl Environ Microbiol. 2007;73(14):4543–4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Moye ZD, Woolston J, Sulakvelidze A. Bacteriophage applications for food production and processing. Viruses. 2018;10(4):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wernicki A, Nowaczek A, Urban-Chmiel R. Bacteriophage therapy to combat bacterial infections in poultry. Virol J. 2017;14(1):179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Żbikowska K, Michalczuk M, Dolka B. The use of bacteriophages in the poultry industry. Animals (Basel). 2020;10(5):872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wachino J, Jin W, Kimura K, et al. Intercellular transfer of chromosomal antimicrobial resistance genes between acinetobacter baumannii strains mediated by prophages. Antimicrob Agents Chemother. 2019;63(8):e00334-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vision SA. Saudi Arabia Vision 2030 Homepage—Vision 2030. Retrieved from https://www.vision2030.gov.sa/ (accessed September 9, 2021).
- 57. Torres-Acosta MA, et al. Economic evaluation of the development of a phage therapy product for the control of Salmonella in poultry. Biotechnol Prog. 2019;35(5):e2852. [DOI] [PubMed] [Google Scholar]