Abstract
Frog metamorphosis, the development of an air-breathing froglet from an aquatic tadpole, is controlled by thyroid hormone (TH) and glucocorticoids (GC). Metamorphosis is susceptible to disruption by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), an aryl hydrocarbon receptor (AHR) agonist. Krüppel-like factor 9 (klf9), an immediate early gene in the endocrine-controlled cascade of expression changes governing metamorphosis, can be synergistically induced by both hormones. This process is mediated by an upstream enhancer cluster, the klf9 synergy module (KSM). klf9 is also an AHR target. We measured klf9 mRNA following exposures to triiodothyronine (T3), corticosterone (CORT), and TCDD in the Xenopus laevis cell line XLK-WG. klf9 was induced 6-fold by 50 nM T3, 4-fold by 100 nM CORT, and 3-fold by 175 nM TCDD. Cotreatments of CORT and TCDD or T3 and TCDD induced klf9 7- and 11-fold, respectively, whereas treatment with all 3 agents induced a 15-fold increase. Transactivation assays examined enhancers from the Xenopus tropicalis klf9 upstream region. KSM-containing segments mediated a strong T3 response and a larger T3/CORT response, whereas induction by TCDD was mediated by a region ∼1 kb farther upstream containing 5 AHR response elements (AHREs). This region also supported a CORT response in the absence of readily identifiable GC responsive elements, suggesting mediation by protein-protein interactions. A functional AHRE cluster is positionally conserved in the human genome, and klf9 was induced by TCDD and TH in HepG2 cells. These results indicate that AHR binding to upstream AHREs represents an early key event in TCDD’s disruption of endocrine-regulated klf9 expression and metamorphosis.
Keywords: endocrine disruption, aryl hydrocarbon receptor, metamorphosis, dioxin, thyroid, glucocorticoid
Frog metamorphosis, the developmental transition from an aquatic tadpole to a terrestrial froglet, involves substantial, widespread tissue remodeling, including tail resorption, limb growth, intestinal shortening, alterations to the central nervous system, and conversion of the respiratory system to enable air breathing (reviewed throughout Shi [2000]). Metamorphosis starts and proceeds under endocrine control, mediated primarily by thyroid hormones (TH) with substantial modulation by glucocorticoids (GC; Sachs and Buchholz, 2019). The tractable physical, chemical, and genetic manipulation of Xenopus embryos, larvae, and tadpoles (X. laevis and X. tropicalis) has enabled their emergence as a model system for examining the basic mechanisms of endocrine control of development and its disruption by xenobiotics to yield adverse outcomes, particularly the TH- and GC-controlled events supporting metamorphosis (eg, Helbing et al., 2007a, b; Mengeling et al., 2017; Miyata and Ose, 2012; OECD, 2009; Thambirajah et al., 2019; US EPA, 2011).
Dioxin-like chemicals (DLCs), including chlorinated dioxins and furans and planar polychlorinated biphenyls, are environmental contaminants that can disrupt the endocrine control of metamorphosis in Xenopus (Taft et al., 2018). 2,3,7,8 Tetrachlorodibenzo-p-dioxin (TCDD) exposure speeds resorption of cultured tail explants while slowing tadpole limb growth. These morphological effects are likely related to the altered expression of several genes that drive the earliest molecular events in metamorphosis, including klf9, which is also a target of both TH and GC (Bagamasbad et al., 2015; Taft et al., 2018). The effects of TCDD and other DLCs are mediated by the aryl hydrocarbon receptor (AHR), a ligand-activated transcription factor. DLC agonists bind the cytoplasmic receptor, triggering its translocation to the nucleus, where it forms a heterodimer with the ARNT protein and binds cognate enhancer elements (AHREs, also known as XREs or DREs), affecting the transcription of target genes (reviewed in Gasiewicz and Henry [2012]).
TH and GC also exert their effects through specific binding to their respective nuclear receptors. In the context of tadpole metamorphosis, each hormone directly or indirectly affects hundreds of tadpole genes individually or in concert (Kulkarni and Buchholz, 2012). The most potent form of TH, T3 (triiodothyronine; converted by deiodinases from thyroxine [T4] at target tissues [Bianco et al., 2019]), initially binds the constitutively expressed thyroid receptor alpha (TRα) and subsequently the T3-induced TRβ. Each TR can form a functional dimeric transcription factor in concert with the retinoid X receptor (RXR), binding any of several sequences that define a thyroid-responsive element (TRE). The TRα:RXR heterodimer binds constitutively to DNA, acting as a transcriptional repressor in the unbound state and an activator following T3 binding. These “dual functions” serve to regulate the timing and inducibility of metamorphic events, inhibiting them prior to TH production and stimulating them thereafter as a result of receptor binding (reviewed in Buchholz et al. [2006] and Buchholz and Shi [2018]). Glucocorticoids, widely associated with the stress response, play important roles in vertebrate development, often speeding processes that define transitions between life stages (Wada, 2008). These adrenal corticosteroids, which include corticosterone (CORT) and cortisol, act by binding the glucocorticoid receptor (GR). Ligand binding triggers GR translocation from the cytoplasm to the nucleus, where it interacts directly with DNA (classically as a homodimer) at a glucocorticoid response element (GRE) or through interactions mediated by additional transcription factors or cofactors (reviewed in Scheschowitsch et al. [2017]).
The klf9 gene, encoding the basic leucine zipper protein krüppel-like factor 9 (a.k.a. “BTEB”), is among the earliest targets for transcriptional induction by TH and GC in the cascade of molecular events that results in metamorphosis. Klf9 protein in turn acts as a key regulator of subsequent transcriptional changes that drive this process (Furlow and Kanamori, 2002). Recent studies have highlighted the synergistic nature of klf9 induction by TH and GC, identifying a ∼180-bp cluster of enhancers known as the “klf9 synergy module” (KSM) that regulates klf9 induction. The KSM, well conserved among tetrapods, contains functional versions of both GRE and TRE motifs (Bagamasbad et al., 2015).
Our own previous studies in multiple X. laevis cell lines and whole tadpoles demonstrate that klf9 mRNA expression is induced directly by TCDD in AHR-dependent fashion, whereas coexposure to TCDD and T3 can boost klf9 expression in apparently synergistic fashion (Taft et al., 2018). This phenomenon likely contributes substantially to the morphological perturbations TCDD elicits in developing tadpole tissues. In the present study, we probed the mechanism by which TCDD disrupts the endocrine control of klf9 expression, seeking to identify enhancers that mediate the role of AHR and their relationship with the KSM in the altered transcriptional response to both T3 and CORT. We report the identification of a functional cluster of AHREs in both the frog and human genomes as well as a region upstream from the KSM that supports GC-induced klf9 transcription.
MATERIALS AND METHODS
Chemicals
3,3′,5-Triiodo-l-thyronine sodium salt (T3), CORT, and TCDD were purchased from Sigma-Aldrich (St Louis, Missouri). Solutions of T3, CORT, and TCDD were prepared in dimethyl sulfoxide (DMSO) obtained from Sigma Aldrich. MS-222 was obtained from Western Chemical Company (Ferndale, Washington).
Cell Culture
Frog cells
The X. laevis adult renal epithelial cell line, XLK-WG (ATCC; Manassas, Virginia), is a longstanding and well-characterized frog cell line for studying cellular responsiveness to AHR agonists (Freeburg et al., 2017; Iwamoto et al., 2012; Laub et al., 2010; Taft et al., 2018). XLK-WG cells were cultured as directed by the supplier at 29°C/5% CO2 in RPMI-1640 medium supplemented with 20% fetal bovine serum (FBS; ThermoFisher) and passaged at ∼70% confluence using a 0.25%-trypsin EDTA solution in 1× PBS. Media were used and stored with care to minimize formation of 6-formylindolo[3,2b]carbazole (FICZ) and related AHR agonists resulting from light exposure (Oberg et al., 2005).
Human cells
The HepG2 cell line (ATCC) was used to interrogate klf9 induction by TCDD and T3 cotreatment. Cells were maintained as directed by the supplier in ATCC-formulated Eagle’s minimum essential medium (EMEM) supplemented with 10% FBS.
Exposures and RNA purification
For experiments measuring klf9 mRNA in X. laevis, XLK-WG cells were cultured to 70% confluence in T-25 flasks. To facilitate direct comparison, cells were treated as described in previously published studies with 100 nM CORT, 50 nM T3 (high hormone concentrations to maximize response; Bonett et al., 2010), 175 nM TCDD (Taft et al., 2018), or combinations thereof for 24 h. This high concentration of TCDD represents the lower-bound EC50 for cyp1a6 induction in this cell line (Laub et al., 2010), reflecting the relatively low affinity of frog AHRs for TCDD (Lavine et al. 2005). All exposure groups, including controls, held the concentration of DMSO vehicle at 0.25% in RPMI-1640 supplemented with 10% charcoal-stripped FBS. Similarly, concentrations of exposure agents were chosen for HepG2 cells to facilitate direct comparisons with previously published work, using high concentrations to maximize the response. Cells were exposed as described previously to TCDD (10 nM; Dere et al., 2011; Jennen et al., 2011), T3 (100 nM; Cvoro et al., 2015), and/or DMSO vehicle (0.25%) for 24 h.
In Vivo Experiments
Animals
Xenopus laevis tadpoles (NF 52-54; Nieuwkoop and Faber, 1994) were obtained from Nasco (Fort Atkinson, Wisconsin). Protocols for tadpole experiments were approved by the Kenyon College Institutional Animal Care and Use Committee.
Exposures
During exposures, tadpoles were kept at a density of 3 individuals per 250 ml FETAX solution, a widely used medium containing dilute ions that is optimized for toxicological studies involving Xenopus embryos and tadpoles (ASTM, 2019; Dawson and Bantle, 1987; Taft et al., 2018), under a 12L:12D photoperiod at 23°C. To facilitate direct comparison, tadpoles were treated as described for previously published studies with 100 nM CORT, 50 nM T3 (high hormone concentrations to maximize response; Bonett et al., 2010), 50 nM TCDD (Taft et al., 2018), or combinations thereof for 24 h. All exposures, including controls, held the concentration of DMSO vehicle at 0.25%. At the end of the exposure period, tadpoles were sacrificed by rapid anesthetic overdose in bicarbonate-buffered MS-222 (0.2%) and immediately flash frozen in liquid nitrogen.
klf9 Enhancer Analysis
Reporter plasmids
To determine the positions of response element sequences relative to klf9 in X. tropicalis and human genomes, we obtained their respective upstream sequences from the Xenbase genome browser (X. tropicalis v9.1; Karimi et al., 2018) or the UCSC human genome browser (GRCh38/hg38; Kent et al., 2002). Conserved aryl hydrocarbon response elements, GRE, and TH response elements within 10 kb of the annotated transcription start site were identified by transcription factor subsequence search in MacVector 18.1, which includes the tfsites collection (http://www.ifti.org; last accessed February 5, 2022) as well as additional curated sequences. This effort was augmented by manual searches.
Dr Pia Bagamasbad and Dr Robert Denver (University of Michigan) generously provided plasmid constructs spanning 1 kb intervals up to position −7000 of X. tropicalis klf9 in the pGL4.23 firefly luciferase reporter vector. We designed additional constructs containing human or X. tropicalis sequences (indicated in the figures) for synthesis by Epoch Life Sciences (Sugar Land, Texas). Reporter plasmids were transformed into chemically competent JM109 E. coli (Promega, Madison, Wisconsin) and isolated in large quantities using a ZymoPURE-EndoZero Maxiprep kit (Zymo Research, Irvine, California) or a HiSpeed Plasmid Maxi Kit (Qiagen). To examine the functionality of select putative AHR or GR binding sites, elements were mutagenized to abrogate receptor binding in the pGL4.23 construct containing elements from −5.7 to −7.7 kb. Mutant constructs containing point mutations of 5 putative AHREs (singly or collectively; 5′CACGC3′ > 5′CATGC3′) (Karchner et al., 1999; Powell et al., 1999) and 1 GRE (5′AGAACAGT3′’ > 5′TAGCATCT3′) (Ren and Stiles, 1999) were generated by site-directed mutagenesis (Epoch Life Sciences).
Transactivation assays
To characterize putative response elements of the X. tropicalis klf9 upstream region, we conducted a series of transactivation assays. We seeded 48-well plates with XLK-WG cells at a density of 10 000 or 15 000 cells per well prior to overnight incubation. Each well was then transfected with 188–220 ng of a pGL4.23 firefly luciferase reporter vector containing a segment of the X. tropicalis klf9 upstream region. Cells were also cotransfected with 15 ng pTK-Renilla (Promega) plasmids using Lipofectamine 3000 reagent (Thermo Fisher Scientific). Cells were incubated for 24–48 h in the transfection solution. Following transfection, populations of plated cells were dosed with DMSO vehicle (0.25%), CORT, T3, and/or TCDD for 24 h as indicated in the figure legends. Reporter gene expression was assessed in 20 µl of cell lysates using the Dual Luciferase Assay and a GloMax luminometer (Promega). To control for transfection efficiency, the degree of transactivation was expressed as relative luciferase units (RLU), the ratio of reporter-driven firefly luciferase activity to constitutive Renilla luciferase activity (driven by pTK-Renilla). The mean RLU value for each treatment group was calculated for each plasmid relative to the corresponding vehicle control, resulting in the plotted fold change values. At least 3 biological replicates were conducted for each experiment, each with 3 repeated measures. Assays in HepG2 cells were performed similarly to examine KLF9 enhancers in the human genome.
Data were plotted and analyzed statistically using Prism 8.4.3 (GraphPad). Dual luciferase assay data were log-transformed prior to statistical analysis, facilitating direct comparison with a previously published workflow for klf9 enhancer analysis (Bagamasbad et al., 2015). We followed a similar protocol for our HepG2 cell line transactivation assays, modifying only the exposure concentrations and media as described above.
mRNA Expression Analysis
RNA purification from cultured cells
Total RNA was purified using QIAshredder and RNeasy kits (Qiagen), with on-column DNase treatment as directed to remove residual genomic DNA.
RNA purification from tadpoles
Whole X. laevis tadpoles (NF 52-54) were homogenized in RNA STAT-60 (Tel-Test, Inc., Friendswood, Texas) and total RNA purified using a Direct-zol RNA miniprep kit (Zymo Research). We used an RNA Clean and Concentrator Kit (Zymo Research) with RNase-Free DNase (QIAGEN, Germantown, Maryland) and/or DNAfree TURBO (ThermoFisher) to remove residual genomic DNA.
Quantitative RT-PCR
mRNA measurements were performed essentially as described previously (Freeburg et al., 2017; Laub et al., 2010; Taft et al., 2018; Zimmermann et al., 2008). For both X. laevis and human RNA, cDNA synthesis reactions were performed using the TaqMan Reverse Transcription Reagent kit (Thermo Fisher Scientific) with random hexamer primers. Reaction conditions were as prescribed by the manufacturer: 25°C/10 min, 37°C/30 min, 95°C/10 min. Quantitative real-time PCR (RT-PCR) was performed using PowerupSYBR Green 2X PCR Master Mix (Thermo Fisher Scientific) and oligonucleotide primers (Eurofins Genomics, Louisville, Kentucky; Tables 1 and 2) on a 7500 Real-Time PCR system (Thermo Fisher Scientific). For X. laevis tadpole and XLK-WG samples, induction of the target gene klf9 (klf9.L) was quantified relative to expression of the actb endogenous control (Freeburg et al., 2017; Laub et al., 2010; Taft et al., 2018; Zimmermann et al., 2008). For human cell line experiments, we measured the relative amplification of KLF9 and CYP1A1 using the widely employed endogenous control GAPDH. Cycling parameters: 95°C/10ʹʹ; [95°C/15ʹʹ, 60°C/60ʹʹ] × 40 cycles. To verify the amplification of a single target, melting point analysis was performed after each reaction. Target gene induction was quantified by the ΔΔCt method using SDS 1.4 software (Thermo Fisher Scientific). Data were plotted and statistical analyses were conducted using Prism 8.4.3 (GraphPad).
Table 1.
RT-PCR Primer Sequences Used With XLK-WG Cells
| Gene (X. laevis) | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| Actb | CGAGCCGCATAGAAAGGAGA | TGTGATCTGAGGGTTGGACG |
| klf9 (klf9.L) | GCCGCTGTTTTGTGTCTTTG | CTGCGCCCCTCCTGTGT |
| cyp1a6 (cyp1a1.S) | GCTTGGTTGGTGATGGGAAG | TCTGCGTCGAGCTCTCCAC |
Table 2.
RT-PCR Primer Sequences Used With HepG2 Cells
| Gene (Human) | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| KLF9 | CAGAGTGCATACAGGTGAACGG | CTTCTCACACAGCGGACAGC |
| CYP1A1 | GGTCAAGGAGCACTACAAAACC | TGGACATTGGCGTTCTCAT |
| GAPDH | AAGGTGAAGGTCGGAGTCA | GGAAGATGGTGATGGGATTT |
RESULTS
klf9 Is Induced by Thyroid Hormone, Corticosterone, and TCDD
Previously published work characterized the combined effects of T3 and CORT (Bagamasbad et al., 2015) or T3 and TCDD (Taft et al., 2018) on klf9 induction in Xenopus. This study extends previous studies by exploring the mechanisms underlying the intersecting functions of both hormones and the AHR pathway, which have not been simultaneously examined. To probe the interactions of T3, CORT, and TCDD in altering klf9 expression in the frog model, we used the XLK-WG cell line, the most extensively characterized model of frog AHR signaling (Freeburg et al., 2017; Iwamoto et al., 2012; Laub et al., 2010; Taft et al., 2018), derived from X. laevis renal epithelium.
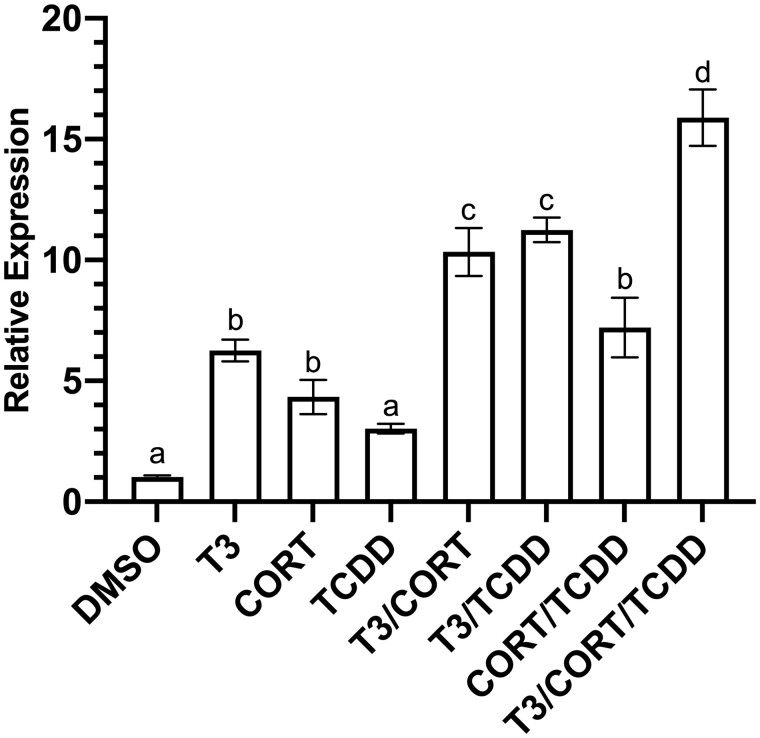
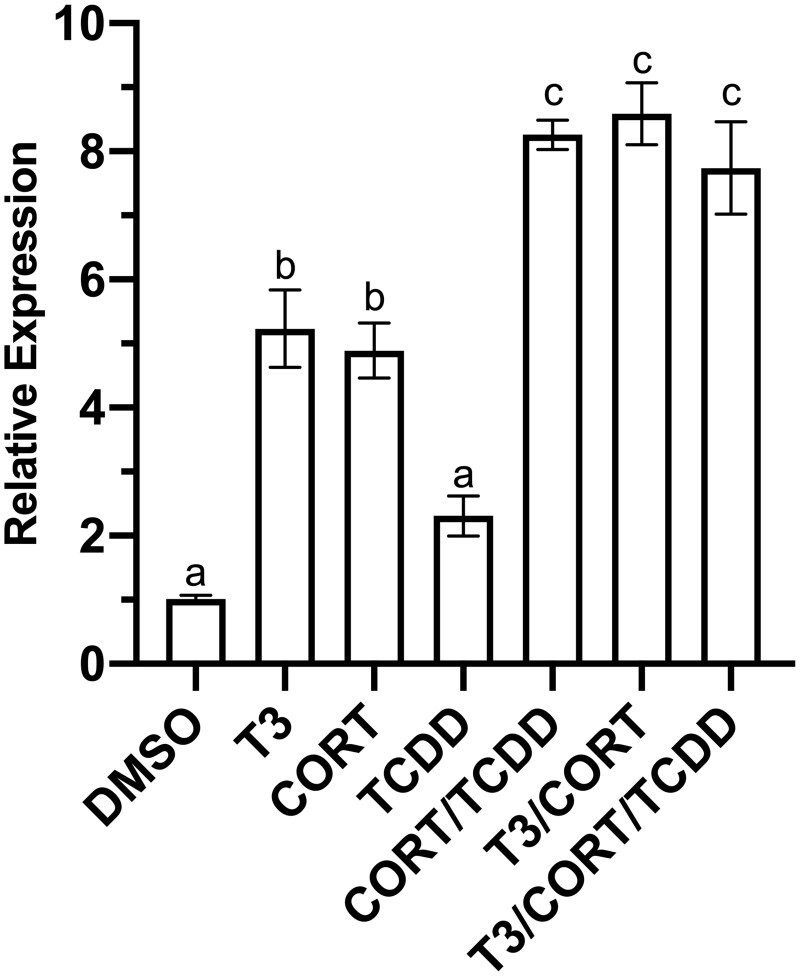
Relative to vehicle control, XLK-WG klf9 mRNA expression was elevated 6-fold by 50 nM T3, 4-fold by 100 nM CORT, and 3-fold by 175 nM TCDD following 24-h exposures (Figure 1). Combined exposures drove further increases in klf9 induction. Cotreatment with T3 and TCDD induced klf9 mRNA 11-fold, whereas CORT and TCDD together yielded a 7-fold relative increase. Simultaneous exposure to T3, CORT, and TCDD increased expression 16-fold (Figure 1). A similar induction pattern was observed for klf9 mRNA in whole tadpoles (NF 52–54) exposed for 24 h to 50 nM T3, 100 nM CORT, and 50 nM TCDD, individually or in combination. Mean klf9 mRNA displayed apparent increases of 2- to 5-fold to single agents, and exposure to pairwise combinations (CORT + TCDD or CORT + T3) boosted expression further. Coexposure to all 3 compounds did not elicit an increase greater than either pairwise combination (Figure 2). Nonetheless, each of the three chemicals was in some fashion associated with elevated klf9 mRNA in vivo.
Figure 1.
Induction of klf9 mRNA in XLK-WG cells. Cells were incubated for 24 h with 0.25% DMSO vehicle, 50 nM T3, 100 nM CORT, 175 nM TCDD, and all possible combinations in RPMI-1640 media supplemented with 20% charcoal-stripped FBS. Mean klf9 mRNA induction relative to actb endogenous control was determined by RT-qPCR using the ΔΔCt method. n = 3 biological replicates per treatment group, 9 replicate measures in total. Error bars represent SEM. Statistical significance of differences between treatment groups were assessed by 1-way ANOVA with Holm-Sidak’s multiple comparisons in GraphPad Prism 8.4.3. Values sharing a letter designation are not significantly different.
Figure 2.
Induction of klf9 mRNA in Xenopus laevis tadpoles. Tadpoles (NF 52–54) were treated for 24 h with 0.25% DMSO vehicle, 50 nM T3, 100 nM CORT, 175 nM TCDD, singly and in combination for 24 h under a 12 L:12D photoperiod. Mean klf9 mRNA induction relative to actb endogenous control was determined by RT-qPCR using the ΔΔCt method. n = 3 tadpoles per treatment, 9 replicate measures in total. Error bars represent SEM. Statistical significance of differences between treatment groups and DMSO control were assessed by one-way ANOVA with Holm-Sidak’s multiple comparisons test in GraphPad Prism 8.4.3. Values sharing a letter designation are not significantly different.
klf9 Enhancer Analysis
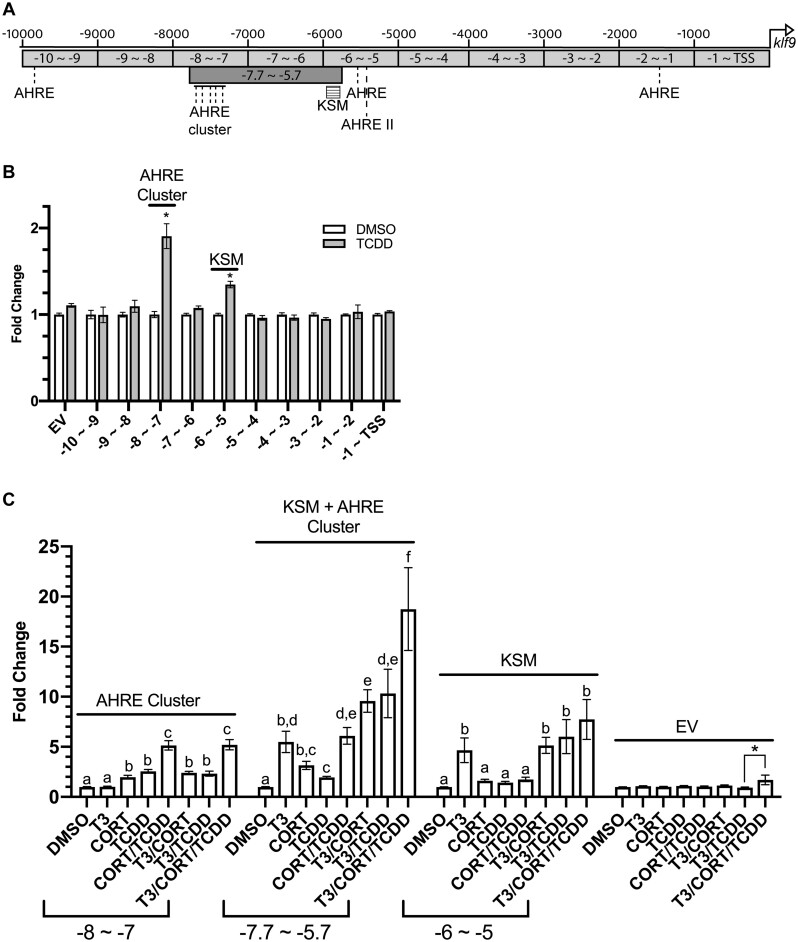
To determine the molecular basis for the interactive effects of T3, CORT, and TCDD on klf9 mRNA induction, we used segments of the upstream flanking region of the X. tropicalis klf9 promoter to conduct transactivation assays as described previously (Bagamasbad et al., 2015). Locations of these segments relative to the TSS are denoted by their names (Figure 3A). Each segment was cloned into pGL4.23 a firefly luciferase reporter vector. Reporter constructs bearing 1 kb segments up to −7 kb were a gift from Dr Robert Denver (University of Michigan), whereas our group generated additional constructs to extend the range of analyzed sequences to −10 kb, deriving sequence information from the X. tropicalis genome browser (v9.2; Karimi et al., 2018).
Figure 3.
Reporter scan of the Xenopus tropicalis klf9 upstream region for CORT, T3, and TCDD responsiveness. A, A map of relevant enhancers in the klf9 upstream region. B, Luciferase reporter gene expression induced by TCDD. C, Luciferase reporter gene expression induced by T3, CORT, and/or TCDD. Transfected XLK-WG cells were incubated for 24 h singly and in combination with DMSO vehicle, 50 nM T3, 100 nM CORT, 175 nM TCDD, in RPMI-1640 with 20% charcoal-stripped FBS. Reporter expression in lysates was measured by a dual luciferase assay. The EV construct lacks any klf9 segment (“empty vector”). Values represent the mean fold change values of 3 independent biological replicates, each with 3 replicate measures. Error bars represent SEM. In panel B, the statistical significance of response to TCDD exposure was assessed for each vector using Student’s t test. An asterisk (*) indicates significant difference from DMSO control. In panel C, statistical significance of response to each treatment was assessed for each treatment by 1-way ANOVA with Holm-Sidak’s multiple comparison test in GraphPad Prism 8.4.3, using log10-transformed values (Bagamasbad et al., 2015). Values sharing a letter designation are not significantly different. An additional significant difference between treatments in the empty vector group is denoted with an asterisk (*).
We first sought to identify enhancers underlying elevated klf9 mRNA expression by TCDD. We identified a cluster of 5 putative AHREs (Swanson, 2011) between −7 and −8 kb, as well as single AHREs in each of 3 segments located between −1 and −2 kb, −5 and −6 kb, and −9 and −10 kb. A potential AHRE II element (Boutros et al., 2004; Sogawa et al., 2004) was also noted in the −5 to −6 kb region (Figure 3A). The newly discovered distal AHRE cluster (−7 ∼ −8) supported the strongest response to TCDD, a ∼2-fold increase, and appears to be the primary driver of AHR-mediated induction. In addition, the −6 to −5 kb construct drove reporter expression to a smaller degree (∼40% increase; Figure 3B). No other segments of the klf9 upstream region supported TCDD-responsive luciferase induction, regardless of the presence of putative AHREs.
We next examined the interactive effects of T3, CORT, and TCDD on reporter gene expression in the most transcriptionally active regions. Consistent with studies published previously (Bagamasbad et al., 2015), the response to T3 alone was driven exclusively by the KSM, located between −6 and −5 kb and downstream of the AHRE cluster (Figure 3C and data not shown). The KSM also supported a small but statistically insignificant degree of responsiveness to CORT alone, whereas the −7 to −8 kb segment mediated a statistically significant response to CORT exposure (Figure 3C).
To enable simultaneous assessment of both the KSM and the upstream AHRE cluster, an additional reporter plasmid was constructed to include both elements, encompassing the region between −5.7 and −7.7 kb (Figure 3A). Simultaneous exposure to T3 and TCDD elicited a greater response than the CORT/TCDD coexposure and rivaled the combination of T3 and CORT. The 3 agents together induced reporter expression over 18-fold, nearly double the value for the most efficacious combinations of two inducers (Figure 3C).
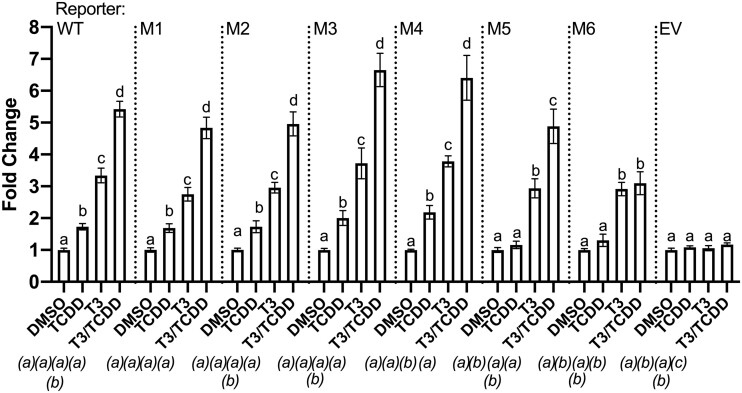
klf9 Upstream AHREs Vary in Strength
To investigate the functionality of each AHRE in the upstream cluster, we introduced a loss-of-function point mutation to each (5′CACGC3′ > 5′CATGC3′) in the context of the −5.7 to −7.7 kb construct. The AHREs are denoted numerically relative to the TSS, with the most distal as “AHRE 1” and the nearest “AHRE 5.” The plasmids carrying nonfunctional AHREs are named M1 through M5 to denote which AHRE is mutated. The M6 construct contains mutations in all 5 AHREs. Wholesale mutation of all 5 AHREs completely abrogated TCDD responsiveness (Figure 4), indicating that the putative AHRE and AHREII located downstream of the KSM (see Figure 3A) are not functional, at least in this heterologous assay. The M5 construct drove TCDD-induced reporter gene expression with comparably low efficacy to M6 (Figure 4), suggesting that most transcriptional regulation of klf9 by AHR involves the AHRE on the downstream end of the cluster. Individual mutations of the remaining AHREs (constructs M1–M4) had insignificant effects on TCDD responsiveness (Figure 4).
Figure 4.
Functional assessment of individual AHREs within the predicted cluster upstream of Xenopus tropicalis klf9. A transactivation assay compared the wild-type sequence and 6 AHRE mutant variants of the −5.7 to −7.7 kb segment of the X. tropicalis klf9 upstream region cloned into pGL4.23 reporter vector. The 5 AHREs are numbered in order of their distance from the TSS, with the mutated AHRE farthest upstream denoted as “M1.” The M6 construct carries nonfunctional mutant versions of all 5 AHREs in the cluster. Transfected XLK-WG cells were treated for 24 h with 0.25% DMSO vehicle, 50 nM T3, 175 nM TCDD, or a cotreatment of TCDD and T3 in RPMI-1640 + 20% FBS. Reporter expression in lysates was measured by a dual luciferase assay. The EV construct lacks any klf9 segment (“empty vector”). Plotted values represent the mean fold change values of 3 independent biological replicates, each with 3 replicate measures. Error bars represent SEM. For each construct, statistical significance of response to each treatment was assessed for log10-transformed values (Bagamasbad et al., 2015) by 1-way ANOVA with Holm-Sidak’s multiple comparison test in GraphPad Prism 8.4.3. Values sharing a letter designation above each bar are not significantly different. Values for each treatment group were also statistically compared between vectors, with symbols to denote statistical significance appearing below each treatment group, italicized and in parentheses. Values sharing the same letter do not differ significantly from the same treatment in different reporter vectors.
CORT Responsiveness from a Distal Enhancer?
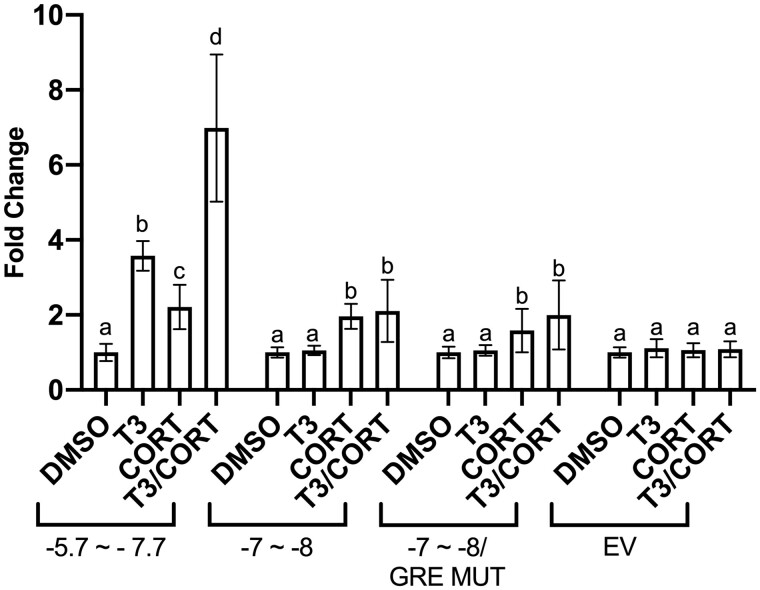
The −7 to −8 kb segment drove an unexpected response to CORT exposure in the absence of TCDD and a greater-than-additive response to treatment by both agents (Figure 3C), suggesting the existence of a novel basis for klf9 regulation by GC outside the KSM. In a search of this region for conserved GRE sequences, we found no apparent local GR homodimeric binding sites on the sense strand in the 5′–3′ direction, nor any instance of the consensus GRE downstream half-site (TGTTCT), which may occur in combination with a second response element associated with a transcription factor able to complex with monomeric GR, such as XGRAF (Morin et al., 2000). However, we identified a previously characterized GRE sequence, a GRE_CS8 (Okret et al., 1986), within the −7 to −8 kb region. To test the hypothesis that the sequence underlies CORT responsiveness, we generated a mutant −7 to −8 kb construct with the GRE_CS8 sequence, AGAACAGT, changed to TAGCATCT (Ren and Stiles, 1999). Compared with the wild-type construct, the GRE mutation conferred no observable decrease in the CORT-induced luciferase expression (Figure 5), indicating that the consensus GRE-CS8 is not directly involved with CORT signaling.
Figure 5.
Functional assessment of a candidate GRE. Transactivation assay examined CORT responsiveness of indicated Xenopus tropicalis klf9 upstream regions. The GRE MUT construct contains the −7 to −8 kb region in which the putative GRE contains a mutation to block GR binding. The −5.7 to −7.7 kb segment (a positive control for CORT responsiveness) includes the KSM but lacks the upstream putative GRE site. Transfected XLK-WG cells were incubated for 24 h with 0.25% DMSO vehicle, 50 nM T3, 100 nM CORT, or a cotreatment in RPMI-1640 with 20% charcoal-stripped FBS. Reporter expression in lysates was measured by a dual luciferase assay. Bars represent the mean fold change values of 3 independent biological replicates, each with 3 replicate measures. Error bars represent SEM. For each construct, statistical significance of response to each treatment was assessed for log10-transformed values (Bagamasbad et al., 2015) by 1-way ANOVA with Holm-Sidak’s multiple comparison test in GraphPad Prism 8.4.3. Values sharing a letter designation are not significantly different.
Conservation of Frog klf9 Enhancers in Humans
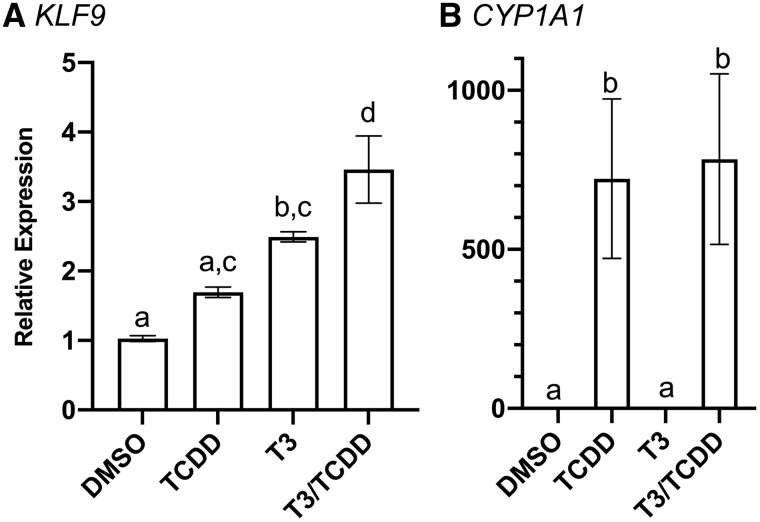
Frog metamorphosis represents a widely used model for the study of the endocrinology and toxicology of human development, especially relating to TH (Buchholz, 2015). To establish the human health relevance of TCDD’s disruption of endocrine regulation of klf9 expression in tadpoles, we sought to investigate the degree of conservation of the sequence and function of the klf9 upstream region shared between these species. We first tested the hypothesis that TCDD alters T3 induction of KLF9, as it does in the frog system (Figs. 1–3 and Taft et al., 2018). mRNA abundance was measured in HepG2 cells (human liver hepatocellular carcinoma), a widely used toxicological model in the study of xenobiotic metabolism, including the role of AHR. HepG2 cells exhibited clear KLF9 mRNA induction by T3 (100 nM) and TCDD (10 nM), and cotreatment with TCDD boosted the response nearly 35% (Figure 6). Notably, TCDD alone induced mean CYP1A1 expression by ∼700-fold, demonstrating the efficacy of this concentration in these assays (Figure 6B).
Figure 6.
Induction of KLF9 mRNA in HepG2 cells. Cells were incubated for 24 h with 0.25% DMSO vehicle, 10 nM TCDD, 100 nM T3, or a cotreatment in EMEM supplemented with 10% charcoal-stripped FBS. Mean KLF9 induction (panel A) was calculated relative to GAPDH endogenous control by RT-qPCR using the ΔΔCt method. CYP1A1 induction (panel B) was also included as a positive control of TCDD responsiveness. n = 3 biological replicates per treatment group, each with 3 replicate measures. Error bars represent SEM. Statistical significance of differences between treatment groups were assessed by 1-way ANOVA with a Holm-Sidak’s multiple comparisons test in GraphPad Prism 8.4.3. Values sharing a letter designation are not significantly different.
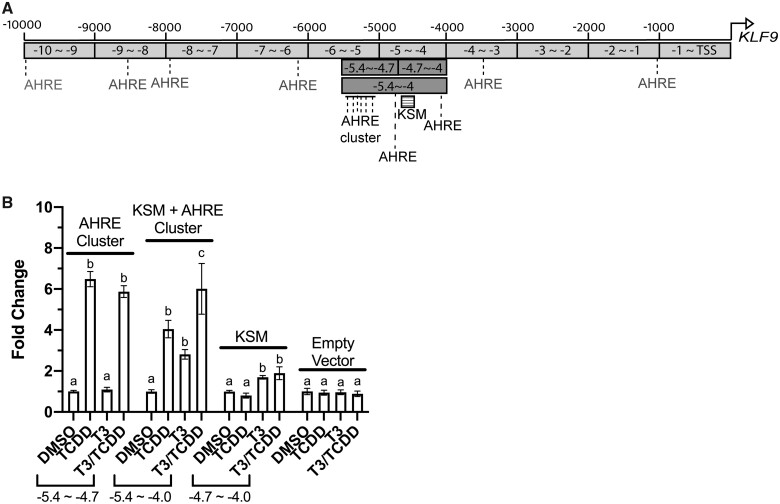
We next explored the organization of enhancers driving human KLF9 expression, revealing a pattern associated with the human gene that is strongly analogous to its counterpart in the frog genome. TRE and GRE elements are situated within the highly conserved KSM (Bagamasbad et al., 2015), ∼4.5 kb upstream to the promoter. In addition, a cluster of 6 core AHREs lies upstream of the KSM, although much closer (less than 1 kb) than in the frog genome. Additional solitary AHREs flank the KSM in both nearby and distant positions (Figure 7A). To assess response element functionality, we next performed luciferase-reporter assays in HepG2 cells, examining the response to T3 and TCDD. Reporter constructs contained either the 6-AHRE cluster (−4.7 to −5.4 kb), the KSM (−4.0 to −4.7 kb), or both groups of enhancers (−4.0 to −5.4 kb). Again, the induction pattern bore striking resemblance to that seen in the frog cells (Figure 7B). The AHRE cluster (−5.4 to −4.7 kb) supported only TCDD responsiveness. The KSM reporter (−4.7 to −4 kb) was induced 2-fold by T3, but TCDD induced no detectable response and did not potentiate T3-driven transactivation. The reporter containing both the KSM and the AHRE cluster was responsive to both agents and exhibited a greater T3 response than did the shorter KSM construct. We conclude that at least in HepG2 cells, TCDD can disrupt T3-induced KLF9 through a mechanism parallel to that of the frog system.
Figure 7.
Enhancer analysis of 3 segments of the human KLF9 upstream region. Transfected HepG2 cells were incubated for 24 h with 0.25% DMSO vehicle, 10 nM TCDD, 100 nM T3, or a cotreatment in EMEM supplemented with 10% charcoal-stripped FBS. Reporter expression in lysates was measured by a dual luciferase assay. Bars represent the mean fold change values of 3 independent biological replicates, each with 3 repeated measures. Error bars represent SEM. For each construct, statistical significance of response to each treatment was assessed for log10-transformed values (Bagamasbad et al., 2015) by 1-way ANOVA with Holm-Sidak’s multiple comparison test in GraphPad Prism 8.4.3. Values sharing a letter designation are not significantly different.
DISCUSSION
Xenopus metamorphosis represents a widely used model system for studying post-embryonic development, including the disruption of endocrine regulation by xenobiotics (US EPA, 2011). TH plays a central role in the activation and repression of gene expression that directs the metamorphosis of tadpoles into frogs (Buchholz et al., 2006; Sachs et al., 2000; Shi, 2009), and expression of either TRα or TRβ is required for survival beyond the metamorphic climax (NF 61; Shibata et al., 2020). Glucocorticoids represent an ancillary endocrine influence on metamorphosis, regulating the rate at which it proceeds by altering sensitivity of target tissues to TH and modulating the expression of TH-regulated genes (Sachs and Buchholz, 2019). The significance of the role of GCs is underscored by the requirement of both CORT and GR for survival and completion of metamorphosis in Xenopus (Shewade et al., 2020; Sterner et al., 2020). The endocrine balance of this system is subject to toxicological disruption by DLCs. Expression of several TR target genes can be induced by TCDD and other AHR agonists, and TCDD exposure can alter tail resorption and limb growth during metamorphosis (Taft et al., 2018). In this study, we sought to characterize upstream enhancers that underlie the AHR contribution to the regulation of klf9, an immediate early gene in the cascade of expression changes in the TH- and GC-mediated control of metamorphosis (Bonett et al., 2009, 2010; Furlow and Kanamori, 2002). We demonstrate the importance of a novel upstream cluster of AHREs in the regulation of klf9 mRNA expression. Conservation of this AHRE cluster in the human genome suggests its importance in klf9-mediated developmental and physiological processes common to people and frogs as well as the potential for cross-talk with and disruption of TR and GR activity.
Glucocorticoid Regulation of Xenopus klf9
This toxicological study extends the fundamental understanding of endocrine control of klf9 induction by GC receptors. Although the role of TR and GR binding within the KSM is a well-documented requirement for klf9 induction (Bagamasbad et al., 2015), our results point to a novel role for GR outside the KSM. Combined CORT/TCDD exposure drove transactivation associated with 2 klf9 reporter constructs—the −7 to −8 kb region (containing the AHRE cluster) and the −5.7 to −7.7 kb region (AHRE cluster + KSM)—but not −5 to −6 kb region (KSM alone; Figure 3). Thus, the functional interaction of AHR and GR does not require the well-characterized GRE in the KSM that drives synergistic klf9 induction in conjunction with TR (Bagamasbad et al., 2015). Examining a possible alternative, we tested the functionality of the only readily predicted GRE motif in the −7 to −8 kb region (GRE_CS8, AGAACAGT; Okret et al., 1986), showing that CORT-driven transactivation was unchanged following mutation of this site. This result is not unreasonable. Although rat GR can bind the GRE_CS8 sequence (Okret et al., 1986), mutation of the motif in the upstream region of the human A1AR gene failed to reduce dexamethasone-stimulated transactivation (Ren and Stiles, 1999). In silico identification of functional GREs from nucleotide sequence alone presents a complex challenge. GR binds only a fraction of predicted response elements, and those vary with cell type and physiological context. Functional GREs can be located far from the core promoter, and GR can interact with poorly conserved, nonclassical response elements (Burd and Archer, 2013). The most predictively useful characteristics of functional GREs include chromatin accessibility (Burd and Archer, 2013), evolutionary conservation (So et al., 2008), and the presence of proximal binding sites for additional transcription factors (Datson et al., 2011).
GR is also known to regulate transcription of certain targets via protein: protein interactions with DNA-binding partners like AP-1 and NF-1 (Scheschowitsch et al., 2017). In vivo, many key developmental events do not appear to rely on direct GR:DNA binding. Although GR expression is necessary for survival in mice, mouse strains expressing mutant GR lacking the ability to form the canonical DNA-binding homodimer nonetheless survived birth (Reichardt and Schutz, 1998). The ability of activated AHR and GR to interact directly has been demonstrated by immunoprecipitation, and AHR activation by 3-methylcholanthrene coupled with GR activation by dexamethasone led to recruitment of AHR to a GRE and a synergistic increase in metallothionein 2A (MT2A) mRNA in HeLa cells (Sato et al., 2013). Furthermore, increased transactivation driven by a GR:AHR complex has been observed in GRE-driven reporter gene assays in human cell lines stimulated with dexamethasone and the AHR agonist benzo[a]pyrene (Wang et al., 2009). These effects are gene specific, as cotreatment with dexamethasone and TCDD has been found to increase the recruitment of GR and AHR to select genes in ARPE-19 cells (eg, ANGPLT4 and FKBP5) but to limit AHR association with response elements associated with other genes, including the canonical AHR targets CYP1A1, CYP1B1, and AHRR (Jin et al., 2017). Similar protein:protein interactions may underlie the CORT-induced transactivation driven by the −7 to −8 kb region that lacks an apparent functional GRE motif.
Our examination of the −5 to −6 kb region reinforces previously published evidence that activated GR directly binds the conserved classical GRE in the KSM (Bagamasbad et al., 2015; Mostafa et al., 2021), though the magnitude of our observed CORT response is lower than values reported previously (Bagamasbad et al., 2015). We inconsistently observed transactivation induced solely by CORT in some individual experiments (data not shown), but the largest and most consistent CORT response occurred with the −5.7 to −7.7 kb reporter construct, which includes the KSM but may also overlap with the upstream CORT-responsive segment (−7 to −8 kb). Thus, the KSM GRE alone may not be sufficient for maximal CORT induction of klf9. The conservation of this additional functional GRE locus in humans is not yet clear.
Enhancers Driving Disruption of klf9 Expression by TCDD
These experiments identify a cluster of functional AHREs upstream of the X. tropicalis KSM. This arrangement of enhancers is mirrored by a strong TCDD response proximal to the human KSM in a region containing several predicted AHREs. Mutation of individual AHREs within the X. tropicalis cluster was associated with varying degrees of response to TCDD, whereas the simultaneous mutation of all 5 AHREs abrogated the TCDD response and the additive CORT/TCDD response (Figure 4). These transactivation results are consistent with reports of AHR and AHR Repressor ChIPseq peaks upstream of human KLF9 in MCF-7 cells (Yang et al., 2018 and corresponding data in the GEO, GSE90550). The position of these peaks (−5371) directly corresponds to the cluster of functional AHREs we characterized in HepG2 cells. AHRE clusters have also been identified in the regulatory region of other dioxin-induced genes, most notably mouse Cyp1a1 and Cyp1a2 (Nukaya et al., 2009). The variable degree of functionality of individual AHREs in the X. tropicalis cluster (Figure 4) appears to be common to AHRE clusters in other genes, including mouse Cyp1a2 (Kawasaki et al., 2010) and zebrafish cyp1a (Zeruth and Pollenz, 2007).
In experiments comparing transactivation driven by combined T3/CORT/TCDD exposure associated with plasmids containing the −5.7 to −7.7 kb region and those containing the −5 to −6 kb region, the longer segment supported a greater response (Figure 3C). This effect is likely driven by increased TR and GR binding at the KSM occurring in concert with AHR-driven transcriptional upregulation linked to the upstream AHRE cluster. This discrepancy may also result from differences in plasmid structure. The plasmids used in transactivation assays are necessarily imperfect models of gene regulation, as they lack the capacity for long-range transcription factor interactions and undergo dynamic conformational changes in ways distinct from genomic DNA (Higgins and Vologodskii, 2015). Likewise, altered chromatin accessibility driven by nucleosome alterations, a key aspect of transcription factor function in vivo, may not occur uniformly in all plasmid models. Although nucleosome formation has been reported from supercoiled plasmid DNA (Nakagawa et al., 2001), total plasmid size is a factor in chromatin formation (Jeong et al., 1991). Regardless, previous observations that ligand-bound TR can drive transcription irrespective of promoter chromatinization (Shi, 2000) provide a basis for our observation the that T3-driven transactivation response associated with the −5 to −6 kb region plasmid was of a similar magnitude to that of the −5.7 to −7.7 kb region plasmid while the GR and AHR responses varied more substantially. A full accounting of the mechanisms by which these nuclear receptors interact to alter transcription will require chromatin immunoprecipitation of factors bound within the actual genome, not merely a heterologous system. These experiments, including the development of antibodies amenable to use with the frog orthologs, will be the subject of future studies.
CONCLUSIONS AND TOXICOLOGICAL IMPLICATIONS
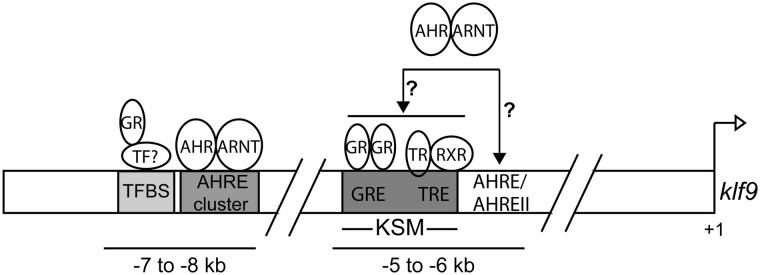
Building on previous experiments that identified the KSM (Bagamasbad et al., 2015), this work provides a molecular basis for dioxin-driven disruption of endocrine-mediated klf9 induction in frogs. It also identifies a second locus of CORT response. We propose an updated model of klf9 regulation by TRs, GR, and AHR (Figure 8). This model includes a CORT-responsive site and an AHRE cluster between −7 and −8 kb that act in concert with the established roles of the evolutionarily conserved GRE and TRE in the KSM. Additionally, 2 potential sources of the modest AHR transactivation mediated by elements proximal to the KSM itself are indicated, which include tethering of the AHR-ARNT complex with DNA-bound transcription factors within the KSM and direct DNA binding at putative AHREs. We propose that the activated TR and GR pathways interact to regulate klf9 induction, and that TCDD can disrupt this process by further increasing klf9 expression through “cross-talk” between AHR and the 2 hormone receptors, disrupting baseline endocrine control. These findings provide new and promising avenues for the study of the TR/GR/AHR regulatory axis not only in Xenopus but also in animal models for developmental toxicology across the tetrapod lineage. They expand the focus on enhancers regulating klf9 transcriptional regulation beyond the well-studied KSM (Bagamasbad et al. 2015). They also define an important early event—binding of AHR to the distal AHRE cluster—in the emerging adverse outcomes pathway underlying the effects of TCDD on limb growth and tail resorption during Xenopus metamorphosis as well as events in human development and physiology that are under control of TR, GR, and KLF9.
Figure 8.
A model for frog klf9 regulation by T3, CORT, and TCDD. Enhancer elements demonstrated to be engaged by specific receptors are indicated. Hypothetical interactions are denoted by a question mark. TF, transcription factor; TFBS, transcription factor binding site.
ACKNOWLEDGEMENTS
We gratefully acknowledge Dr Pia Bagamasbad (University of Michigan) for generating multiple reporter gene constructs for transactivation assays and Prof. Robert J. Denver (University of Michigan) for making them available for our studies.
FUNDING
National Institute of Environmental Health Sciences, National Institutes of Health (R15 ES011130); Kenyon College Summer Science Scholars program.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
David T Han, Biology Department, Kenyon College, Gambier, Ohio 43022, USA.
Weichen Zhao, Biology Department, Kenyon College, Gambier, Ohio 43022, USA.
Wade H Powell, Biology Department, Kenyon College, Gambier, Ohio 43022, USA.
REFERENCES
- ASTM (American Society of Testing and Materials) (2019). Standard Guide for Conducting the Frog Embryo Teratogenesis Assay–Xenopus (FETAX). Vol. 11.06, E1439-91, Philadelphia, PA. [Google Scholar]
- Bagamasbad P. D., Bonett R. M., Sachs L., Buisine N., Raj S., Knoedler J. R., Kyono Y., Ruan Y., Ruan X., Denver R. J. (2015). Deciphering the regulatory logic of an ancient, ultraconserved nuclear receptor enhancer module. Mol. Endocrinol. 29, 856–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco A. C., Dumitrescu A., Gereben B., Ribeiro M. O., Fonseca T. L., Fernandes G. W., Bocco B. (2019). Paradigms of dynamic control of thyroid hormone signaling. Endocr. Rev. 40, 1000–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonett R. M., Hoopfer E. D., Denver R. J. (2010). Molecular mechanisms of corticosteroid synergy with thyroid hormone during tadpole metamorphosis. Gen. Comp. Endocrinol. 168, 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonett R. M., Hu F., Bagamasbad P., Denver R. J. (2009). Stressor and glucocorticoid-dependent induction of the immediate early gene Kruppel-Like Factor 9: Implications for neural development and plasticity. Endocrinology 150, 1757–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros P. C., Moffat I. D., Franc M. A., Tijet N., Tuomisto J., Pohjanvirta R., Okey A. B. (2004). Dioxin-responsive AHRE-II gene battery: Identification by phylogenetic footprinting. Biochem. Biophys. Res. Commun. 321, 707–715. [DOI] [PubMed] [Google Scholar]
- Buchholz D. R. (2015). More similar than you think: Frog metamorphosis as a model of human perinatal endocrinology. Dev. Biol. 408, 188–195. [DOI] [PubMed] [Google Scholar]
- Buchholz D. R., Paul B. D., Fu L., Shi Y. B. (2006). Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen. Comp. Endocrinol. 145, 1–19. [DOI] [PubMed] [Google Scholar]
- Buchholz D. R., Shi Y. B. (2018). Dual function model revised by thyroid hormone receptor alpha knockout frogs. Gen. Comp. Endocrinol. 265, 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd C. J., Archer T. K. (2013). Chromatin architecture defines the glucocorticoid response. Mol. Cell Endocrinol. 380, 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvoro A., Devito L., Milton F. A., Noli L., Zhang A., Filippi C., Sakai K., Suh J. H., H Sieglaff D., Dhawan A., et al. (2015). A thyroid hormone receptor/KLF9 axis in human hepatocytes and pluripotent stem cells. Stem Cells 33, 416–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datson N. A., Polman J. A., de Jonge R. T., van Boheemen P. T., van Maanen E. M., Welten J., McEwen B. S., Meiland H. C., Meijer O. C. (2011). Specific regulatory motifs predict glucocorticoid responsiveness of hippocampal gene expression. Endocrinology 152, 3749–3757. [DOI] [PubMed] [Google Scholar]
- Dawson D. A., Bantle J. A. (1987). Development of a reconstituted water medium and preliminary validation of the frog embryo teratogenesis assay–Xenopus (FETAX). J. Appl. Toxicol. 7, 237–244. [DOI] [PubMed] [Google Scholar]
- Dere E., Lee A. W., Burgoon L. D., Zacharewski T. R. (2011). Differences in TCDD-elicited gene expression profiles in human HepG2, mouse Hepa1c1c7 and rat H4IIE hepatoma cells. BMC Genomics. 12, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeburg S. H., Engelbrecht E., Powell W. H. (2017). Subfunctionalization of paralogous aryl hydrocarbon receptors from the frog Xenopus laevis: Distinct target genes and differential responses to specific agonists in a single cell type. Toxicol. Sci. 155, 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlow J. D., Kanamori A. (2002). The transcription factor basic transcription element-binding protein 1 is a direct thyroid hormone response gene in the frog Xenopus laevis. Endocrinology 143, 3295–3305. [DOI] [PubMed] [Google Scholar]
- Gasiewicz T. A., Henry E. C. (2012). History of research on the AHR. In The AH Receptor in Biology and Toxicology (Pohjanvirta R., Ed.), pp. 3–32. John Wiley & Sons, Inc., Hoboken, NJ. [Google Scholar]
- Helbing C. C., Bailey C. M., Ji L., Gunderson M. P., Zhang F., Veldhoen N., Skirrow R. C., Mu R., Lesperance M., Holcombe G. W., et al. (2007a). Identification of gene expression indicators for thyroid axis disruption in a Xenopus laevis metamorphosis screening assay. Part 1. Effects on the brain. Aquat. Toxicol. 82, 227–241. [DOI] [PubMed] [Google Scholar]
- Helbing C. C., Ji L., Bailey C. M., Veldhoen N., Zhang F., Holcombe G. W., Kosian P. A., Tietge J., Korte J. J., Degitz S. J. (2007b). Identification of gene expression indicators for thyroid axis disruption in a Xenopus laevis metamorphosis screening assay. Part 2. Effects on the tail and hindlimb. Aquat. Toxicol. 82, 215–226. [DOI] [PubMed] [Google Scholar]
- Higgins N. P., Vologodskii A. V. (2015). Topological Behavior of Plasmid DNA. Microbiol. Spectr. 3. doi: 10.1128/microbiolspec.PLAS-0036-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto D. V., Kurylo C. M., Schorling K. M., Powell W. H. (2012). Induction of cytochrome P450 family 1 mRNAs and activities in a cell line from the frog Xenopus laevis. Aquat. Toxicol. 114–115, 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennen D., Ruiz-Aracama A., Magkoufopoulou C., Peijnenburg A., Lommen A., van Delft J., Kleinjans J. (2011). Integrating transcriptomics and metabonomics to unravel modes-of-action of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in HepG2 cells. BMC Syst. Biol. 5, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S. W., Lauderdale J. D., Stein A. (1991). Chromatin assembly on plasmid DNA in vitro. Apparent spreading of nucleosome alignment from one region of pBR327 by histone H5. J. Mol. Biol. 222, 1131–1147. [DOI] [PubMed] [Google Scholar]
- Jin H. L., Choi Y., Jeong K. W. (2017). Crosstalk between aryl hydrocarbon receptor and glucocorticoid receptor in human retinal pigment epithelial cells. Int. J. Endocrinol. 2017, 5679517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karchner S. I., Powell W. H., Hahn M. E. (1999). Structural and functional characterization of two highly divergent aryl hydrocarbon receptors (AHR1 and AHR2) in the teleost Fundulus heteroclitus. Evidence for a novel class of ligand-binding basic helix-loop-helix Per-ARNT-Sim (bHLH-PAS) factors. J. Biol. Chem. 274, 33814–33824. [DOI] [PubMed] [Google Scholar]
- Karimi K., Fortriede J. D., Lotay V. S., Burns K. A., Wang D. Z., Fisher M. E., Pells T. J., James-Zorn C., Wang Y., Ponferrada V. G., et al. (2018). Xenbase: A genomic, epigenomic and transcriptomic model organism database. Nucleic Acids Res. 46, D861–D868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y., Sakuma T., Goto Y., Nemoto N. (2010). Regulatory xenobiotic responsive elements in the distal 5′-flanking region of the mouse Cyp1a2 gene required for transcriptional activation by 3-methylcholanthrene and 2,3,7,8-tetrachlorodibenzo-p-dioxin. Drug Metab. Dispos. 38, 1640–1643. [DOI] [PubMed] [Google Scholar]
- Kent W. J., Sugnet C. W., Furey T. S., Roskin K. M., Pringle T. H., Zahler A. M., Haussler D. (2002). The human genome browser at UCSC. Genome Res. 12, 996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S. S., Buchholz D. R. (2012). Beyond synergy: Corticosterone and thyroid hormone have numerous interaction effects on gene regulation in Xenopus tropicalis tadpoles. Endocrinology 153, 5309–5324. [DOI] [PubMed] [Google Scholar]
- Laub L. B., Jones B. D., Powell W. H. (2010). Responsiveness of a Xenopus laevis cell line to the aryl hydrocarbon receptor ligands 6-formylindolo[3,2-b]carbazole (FICZ) and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Chem. Biol. Interact. 183, 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine J. A., Rowatt A. J., Klimova T., Whitington A. J., Dengler E., Beck C., Powell W. H. (2005). Aryl hydrocarbon recep- tors in the frog Xenopus laevis: Two AhR1 paralogs exhibit low affinity for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicol. Sci. 88, 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengeling B. J., Wei Y., Dobrawa L. N., Streekstra M., Louisse J., Singh V., Singh L., Lein P. J., Wulff H., Murk A. J., et al. (2017). A multi-tiered, in vivo, quantitative assay suite for environmental disruptors of thyroid hormone signaling. Aquat. Toxicol. 190, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata K., Ose K. (2012). Thyroid hormone-disrupting effects and the amphibian metamorphosis assay. J. Toxicol. Pathol. 25, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin B., Zhu C., Woodcock G. R., Li M., Woodward R. N., Nichols L. A., Holland L. J. (2000). The binding site for Xenopus glucocorticoid receptor accessory factor and a single adjacent half-GRE form an independent glucocorticoid response unit. Biochemistry 39, 12234–12242. [DOI] [PubMed] [Google Scholar]
- Mostafa M. M., Bansal A., Michi A. N., Sasse S. K., Proud D., Gerber A. N., Newton R. (2021). Genomic determinants implicated in the glucocorticoid-mediated induction of KLF9 in pulmonary epithelial cells. J. Biol. Chem. 296, 100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., Bulger M., Muramatsu M., Ito T. (2001). Multistep chromatin assembly on supercoiled plasmid DNA by nucleosome assembly protein-1 and ATP-utilizing chromatin assembly and remodeling factor. J. Biol. Chem. 276, 27384–27391. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P. D., Faber J. (1994). Normal Table of Xenopus laevis (Daudin). Garland Publishing, Inc., New York and London. [Google Scholar]
- Nukaya M., Moran S., Bradfield C. A. (2009). The role of the dioxin-responsive element cluster between the Cyp1a1 and Cyp1a2 loci in aryl hydrocarbon receptor biology. Proc. Natl. Acad. Sci. U.S.A. 106, 4923–4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg M., Bergander L., Hakansson H., Rannug U., Rannug A. (2005). Identification of the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole, in cell culture medium, as a factor that controls the background aryl hydrocarbon receptor activity. Toxicol. Sci. 85, 935–943. [DOI] [PubMed] [Google Scholar]
- OECD (Organization for Economic Cooperation and Development). (2009). Test No. 231: Amphibian Metamorphosis Assay. Paris.
- Okret S., Poellinger L., Dong Y., Gustafsson J. A. (1986). Down-regulation of glucocorticoid receptor mRNA by glucocorticoid hormones and recognition by the receptor of a specific binding sequence within a receptor cDNA clone. Proc. Natl. Acad. Sci. U. S. A. 83, 5899–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell W. H., Karchner S. I., Bright R., Hahn M. E. (1999). Functional diversity of vertebrate ARNT proteins: Identification of ARNT2 as the predominant form of ARNT in the marine teleost, Fundulus heteroclitus. Arch. Biochem. Biophys. 361, 156–163. [DOI] [PubMed] [Google Scholar]
- Reichardt H. M., Schutz G. (1998). Glucocorticoid signalling–multiple variations of a common theme. Mol. Cell. Endocrinol. 146, 1–6. [DOI] [PubMed] [Google Scholar]
- Ren H., Stiles G. L. (1999). Dexamethasone stimulates human A1 adenosine receptor (A1AR) gene expression through multiple regulatory sites in promoter B. Mol. Pharmacol. 55, 309–316. [DOI] [PubMed] [Google Scholar]
- Sachs L. M., Buchholz D. R. (2019). Insufficiency of thyroid hormone in frog metamorphosis and the role of glucocorticoids. Front. Endocrinol. 10, 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs L. M., Damjanovski S., Jones P. L., Li Q., Amano T., Ueda S., Shi Y. B., Ishizuya-Oka A. (2000). Dual functions of thyroid hormone receptors during Xenopus development. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 126, 199–211. [DOI] [PubMed] [Google Scholar]
- Sato S., Shirakawa H., Tomita S., Tohkin M., Gonzalez F. J., Komai M. (2013). The aryl hydrocarbon receptor and glucocorticoid receptor interact to activate human metallothionein 2A. Toxicol. Appl. Pharmacol. 273, 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheschowitsch K., Leite J. A., Assreuy J. (2017). New insights in glucocorticoid receptor signaling-more than just a ligand-binding receptor. Front. Endocrinol. 8, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewade L. H., Schoephoerster J. A., Patmann M. D., Kulkarni S. S., Buchholz D. R. (2020). Corticosterone is essential for survival through frog metamorphosis. Endocrinology 161, bqaa193. [DOI] [PubMed] [Google Scholar]
- Shi Y.-B. (2000). Amphibian Metamorophosis. Wiley-Liss, New York. [Google Scholar]
- Shi Y. B. (2009). Dual functions of thyroid hormone receptors in vertebrate development: The roles of histone-modifying cofactor complexes. Thyroid 19, 987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y., Wen L., Okada M., Shi Y. B. (2020). Organ-specific requirements for thyroid hormone receptor ensure temporal coordination of tissue-specific transformations and completion of Xenopus metamorphosis. Thyroid 30, 300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So A. Y., Cooper S. B., Feldman B. J., Manuchehri M., Yamamoto K. R. (2008). Conservation analysis predicts in vivo occupancy of glucocorticoid receptor-binding sequences at glucocorticoid-induced genes. Proc. Natl. Acad. Sci. U.S.A. 105, 5745–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogawa K., Numayama-Tsuruta K., Takahashi T., Matsushita N., Miura C., Nikawa J., Gotoh O., Kikuchi Y., Fujii-Kuriyama Y. (2004). A novel induction mechanism of the rat CYP1A2 gene mediated by Ah receptor-Arnt heterodimer. Biochem. Biophys. Res. Commun. 318, 746–755. [DOI] [PubMed] [Google Scholar]
- Sterner Z. R., Shewade L. H., Mertz K. M., Sturgeon S. M., Buchholz D. R. (2020). Glucocorticoid receptor is required for survival through metamorphosis in the frog Xenopus tropicalis. Gen. Comp. Endocrinol. 291, 113419. [DOI] [PubMed] [Google Scholar]
- Swanson H. (2011). Dioxin response elements and regulation of gene transcription. In The AH Receptor in Biology and Toxicology (R. Pohjanvirta, Ed.), pp. 81–91. John Wiley & Sons, Inc., Hoboken, NJ. [Google Scholar]
- Taft J. D., Colonnetta M. M., Schafer R. E., Plick N., Powell W. H. (2018). Dioxin exposure alters molecular and morphological responses to thyroid hormone in Xenopus laevis cultured cells and prometamorphic tadpoles. Toxicol. Sci. 161, 196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thambirajah A. A., Koide E. M., Imbery J. J., Helbing C. C. (2019). Contaminant and environmental influences on thyroid hormone action in amphibian metamorphosis. Front. Endocrinol. 10, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA. (2011). Amphibian metamorphosis assay OCSPP guideline 890.1100. US Environmental Protection Agency, Washington, DC.
- Wada H. (2008). Glucocorticoids: Mediators of vertebrate ontogenetic transitions. Gen. Comp. Endocrinol. 156, 441–453. [DOI] [PubMed] [Google Scholar]
- Wang S. H., Liang C. T., Liu Y. W., Huang M. C., Huang S. C., Hong W. F., Su J. G. (2009). Crosstalk between activated forms of the aryl hydrocarbon receptor and glucocorticoid receptor. Toxicology 262, 87–97. [DOI] [PubMed] [Google Scholar]
- Yang S. Y., Ahmed S., Satheesh S. V., Matthews J. (2018). Genome-wide mapping and analysis of aryl hydrocarbon receptor (AHR)- and aryl hydrocarbon receptor repressor (AHRR)-binding sites in human breast cancer cells. Arch. Toxicol. 92, 225–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeruth G., Pollenz R. S. (2007). Functional analysis of cis-regulatory regions within the dioxin-inducible CYP1A promoter/enhancer region from zebrafish (Danio rerio). Chem. Biol. Interact. 170, 100–113. [DOI] [PubMed] [Google Scholar]
- Zimmermann A. L., King E. A., Dengler E., Scogin S. R., Powell W. H. (2008). An aryl hydrocarbon receptor repressor from Xenopus laevis: Function, expression and role in dioxin responsiveness during frog development. Toxicol. Sci. 104, 124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]