A biofilm is a population of cells growing on a surface and enclosed in an exopolysaccharide matrix. Biofilms are notoriously difficult to eradicate and are a source of many recalcitrant infections. The nature of bacterial biofilm resistance to antimicrobials is the subject of the present minireview. Pathogenic yeast such as Candida albicans also form recalcitrant biofilms, and this topic has recently been reviewed (5).
Resistance is an ability of a microorganism to grow in the presence of an elevated level of an antimicrobial. In short, a strain for which the MIC is increased is resistant. By this conventional criterion, biofilm cells do not necessarily show increased resistance. With some exceptions, biofilm cells do not grow better than planktonic cells in the presence of a broad range of antimicrobials. This is evident from examination of susceptibility data in the biofilm literature (33). However, in most biofilm susceptibility studies, only survival of cells in a preformed biofilm rather than the ability of a biofilm to grow is recorded. Accordingly, the reported “resistance” describes an increased resistance of cells to killing. This is indeed what biofilms are good at: they are not easily eradicated by cidal antimicrobials. The ability of antimicrobials to inhibit biofilm growth indicates that they are able to diffuse through the biofilm and act normally against their targets. Why, then, do biofilm cells not die? This is the crux of the problem and the riddle that needs to be solved.
THE USUAL SUSPECTS
One can find a list of factors considered to be responsible for biofilm resistance in papers and recent reviews on the subject (15, 22, 36). These include restricted penetration of antimicrobials into a biofilm, decreased growth rate, and expression of possible resistance genes. Alone or in combination, these factors are useful in explaining biofilm survival in a number of cases.
Restricted penetration.
Biofilms are enclosed within an exopolymer matrix that can restrict the diffusion of substances and bind antimicrobials. This will provide effective resistance for biofilm cells against large molecules such as antimicrobial proteins lysozyme and complement. The diffusion barrier is also probably effective against smaller antimicrobial peptides—the numerous defensins and their analogs. The negatively charged exopolysaccharide is very effective in protecting cells from positively charged aminoglycoside antibiotics by restricting their permeation, possibly through binding (26, 59).
In most cases involving small antimicrobial molecules, the barrier of the polysaccharide matrix should only postpone the death of cells rather than afford useful protection. A case in point are fluoroquinolone antibiotics, which readily equilibrate across the biofilm (3, 26, 59, 67). Fluoroquinolones are indeed very effective in stopping the growth of a biofilm (11). At the same time, restricted diffusion can protect the biofilm from a degradable antimicrobial. Retarded diffusion will decrease the concentration of the antibiotic entering the biofilm, helping an enzyme like β-lactamase destroy the incoming antibiotic. This synergy between retarded diffusion and degradation provides effective resistance to Pseudomonas aeruginosa biofilms expressing a β-lactamase (23). The synergistic relationship between diffusion retardation and degradation has been convincingly analyzed in a mathematical model based on these experimental observations (63).
Another interesting case of a diffusion barrier that helps protect the cells was described for hydrogen peroxide. Unlike planktonic cells of P. aeruginosa that were very sensitive to killing by 50 mM H2O2, biofilm cells that actually had lower levels of catalase (KatA) were effectively protected (18, 24). A restricted penetration of this small molecule coupled to its destruction by the microbial cells was apparently responsible for resistance. It can be expected that any mechanism of antibiotic destruction or modification (like acetylation of aminoglycosides) will be especially effective when coupled with a diffusion barrier of the biofilm. It is surprising, however, that bacteria did not come up with a general mechanism for detoxifying antibiotics like the cytochrome P450 oxidation system of animals. Humans are no doubt the fortunate beneficiaries of this limitation.
The synergistic arrangement between the diffusion barrier and an enzyme destroying an incoming antimicrobial is analogous to the effective synergy between the outer membrane and multidrug resistance (MDR) pumps that transport antimicrobials across this permeability barrier (35, 44). It was recently discovered that a transenvelope AcrAB-TolC MDR pump of Escherichia coli acts in synergy with a chloramphenicol efflux pump (CmlA) located in the cytoplasmic membrane (32). Apparently, chloramphenicol is transported into the periplasm by CmlA, where it is picked up by a Mex pump and extruded out of the cell. One can envision that in a biofilm the effectiveness of this resistance mechanism could be further improved by retarded diffusion of chloramphenicol and a cellular chloramphenicol acetyltransferase. Biofilm resistance might literally be multilayered.
In all of the cases described above, one would expect the biofilm cells to be able to grow in the presence of antimicrobials; that is, the MIC for biofilm cells is higher than that for planktonic cells. Whether this is indeed the case remains to be determined; as mentioned above, in most biofilm susceptibility experiments, the killing effectiveness of the antimicrobials rather than growth inhibition was measured.
The number of studies on antimicrobial diffusion through biofilms is small, no doubt reflecting the technical difficulties involved. Even when these measurements are performed, the results are not entirely conclusive. One cannot exclude the possibility that a given biofilm is heterogeneous and contains pockets of material through which diffusion is strongly restricted. A functional test for growth seems to be a simpler and better way to assess diffusion of antimicrobials through a biofilm. Growth can be measured in the same way that killing is: by counting the numbers of CFU from a growing biofilm after it is dislodged. By this measure, an antibiotic (not inactivated by the cells) that has an MIC for biofilm cells similar to the one for planktonic cells diffuses well throughout the biofilm.
Decreased growth rate.
Virtually all antimicrobials are more effective in killing rapidly growing cells. Some antibiotics have an absolute requirement for cell growth in order to kill. Penicillin and ampicillin do not kill nongrowing cells at all, and the rate of killing is proportional to the rate of growth. Some of the more advanced β-lactams, cephalosporins, aminoglycosides, and fluoroquinolones can kill nongrowing cells, but they are distinctly more effective in killing rapidly dividing cells. Slow growth undoubtedly contributes to biofilm resistance to killing (15). Similarly, slow growth is a major factor in the increased resistance of stationary planktonic cells to killing.
Expression of possible biofilm-specific resistance genes.
Since biofilms are not usually more resistant than planktonic cells to growth inhibition by antimicrobials, there does not seem to be a need to invoke special drug resistance mechanisms operating in the biofilm. β-Galactosidase was found to be expressed in response to imipenem and piperacillin in biofilms of P. aeruginosa (23); however, the level of expression was lower than that in induced planktonic cells. MDR pumps play a role in biofilm resistance at low antibiotic concentrations (11, 36), and there is reason to believe that unknown MDR pumps might be overexpressed in P. aeruginosa biofilms (11). I must caution, however, against concluding that a certain mechanism is specifically overexpressed in a biofilm until a broad range of conditions that planktonic cells grow under has been examined.
A more interesting question is whether biofilms express a specific survival mechanism that explains their remarkable resistance to killing by a broad range of factors. This will be considered in the next section.
UNUSUAL SUSPECTS
The factors that I analyzed above do not explain the resistance of biofilms to killing by at least one important group of antimicrobial agents: the fluoroquinolone antibiotics. It has been shown that fluoroquinolones equilibrate across bacterial biofilms. A decreased growth rate contributes to quinolone resistance, but quinolones killed nongrowing planktonic cells of P. aeruginosa in a simple phosphate buffer, while biofilm cells in a fresh rich medium were more resistant to killing (11).
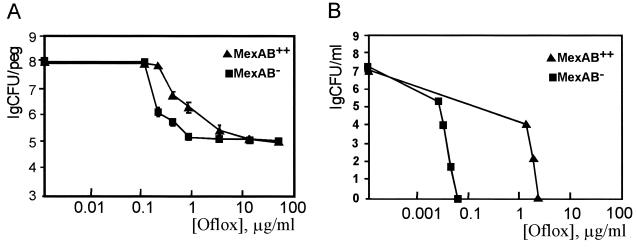
Biofilm resistance to killing has generally been assumed to be a feature shared by the bulk of biofilm cells or at least to be present in a sizable part of the population, such as cells in the deeper layers of a thick biofilm, which have less access to nutrients and which will grow more slowly (15). Resistance to killing by aminoglycosides which have trouble penetrating the biofilm is indeed a shared feature of the bulk of biofilm cells. This, however, is more of an exception rather than the rule. A study of a dose-response killing of P. aeruginosa biofilms by the quinolones ofloxacin and ciprofloxacin showed that the majority of cells are effectively eliminated by low concentrations of antibiotics, which is not much different from what is observed with planktonic cells. Importantly, the majority of biofilm cells were killed within a clinically achievable range of concentrations (≤5 μg/ml) (11). However, after an initial 3- to 4-log drop, a further increase in the antibiotic concentration had no effect on killing (Fig. 1). This experiment shows that a small fraction of persister cells is ultimately responsible for the very high level of resistance of the biofilm to killing.
FIG. 1.
P. aeruginosa persisters surviving in a biofilm treated with ofloxacin (Oflox). (A) Biofilms were formed on pegs of a Calgary Biofilm Device (14) and were then treated with a given concentration of antibiotic in Mueller-Hinton broth for 6 h, rinsed, and dislodged by sonication. Live cells were then counted by plating. The number of live cells recovered from a single peg is expressed as the number of CFU per peg. A strain that overexpressed the main MDR pump that extrudes fluoroquinolones (MexAB++) and a strain that lacked the pump (34) were used in this experiment. The contribution of the pump to resistance is evident at low concentrations of the antibiotic but has little effect on the survival of persisters. (B) Planktonic cells were treated similarly with ofloxacin and plated for determination of the cell count. The apparent absence of persisters is due to the low density of the population and the detection limit of the experiment; at higher densities, persisters are evident at low levels in a planktonic population (A. Spoering and K. Lewis, unpublished data). Adopted from reference 12, with permission.
This simple observation suggests a new paradigm for explaining, at least in principle, the phenomenon of biofilm resistance to killing by a wide range of antimicrobials. The majority of cells in a biofilm are not necessarily more resistant to killing than planktonic cells and die rapidly when treated with a cidal antibiotic that can kill slowly growing cells. Persisters survive and are actually preserved by the presence of an antibiotic that inhibits their growth. Paradoxically, the antibiotic helps persisters persevere.
The role of persisters in biofilm resistance to killing has not been considered in the literature prior to our study (11, 33), but numerous reports over the years show similar biphasic dose-dependent or time-dependent killing of planktonic microbial cells. For example, in E. coli, increasing concentrations of ciprofloxacin or imipenem caused an initial decrease in the number of live cells of a biofilm by 2 to 3 orders of magnitude, while the remaining small population was essentially insensitive to a further increase in the drug concentration (4). This pattern was also observed with amoxicillin and clindamycin in Lactobacillus acidophilus and with erythromycin and metronidazole in the case of Gardnerella vaginalis biofilms, in which initial rapid killing was followed by a plateau of resistant cells (43).
It is possible that biofilms produce more persisters than planktonic populations. Increased numbers of persisters, however, are not the main factor responsible for the vastly better survival of biofilms than planktonic cells in vivo. Consider the empirically derived minimal bactericidal concentration (MBC): according to NCCLS guidelines, MBC is the concentration of an antimicrobial agent that results in the lowering of the number of live cells by ≤99.9% after an overnight incubation under growth conditions. The practical reasoning behind this useful but rather arbitrary measure is that killing a majority of pathogens in many cases is just as good as killing them all. This is often the case, because the immune system collaborates with the antibiotics and probably “mops up” the remaining persisters. Persisters do become a problem when the immune system is not operating. For example, Streptococcus pneumoniae causes recalcitrant meningitis because of the inaccessibility of the cerebrospinal fluid to the components of the immune system. The infection was more pronounced when it was caused by vncS mutants with increased persistence (tolerance) to a range of antibiotics (46), and vncS mutants were isolated from animals with persistent S. pneumoniae meningitis poorly responding to vancomycin therapy. The gastric environment is similarly devoid of immune factors, and Helicobacter pylori infection, which causes peptic ulcers, is famously recalcitrant. The gastric environment requires complete sterilization in order to prevent relapses. The presence of persisters that can rebound when the antibiotic concentration drops would explain the necessity of therapy with a combination of unrelated drugs that together probably eradicate persisters (60; Lynn Silver, personal communication).
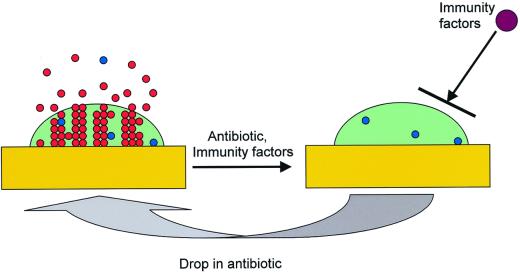
Biofilm infections are in a sense very similar to planktonic infections in the absence of an immune response. The biofilm exopolymer physically protects the cells from the components of the immune system (25, 66). One can envision a biofilm survival dynamic in vivo in which an initial application of a cidal antibiotic eradicates most of the population, leaving a small fraction of surviving persisters (Fig. 2). If the concentration of the antibiotic temporarily drops or if symptoms disappear due to the eradication of planktonic cells and therapy is discontinued, the persisters will reform the biofilm, which will begin to shed off new planktonic cells. This dynamic explains the relapsing nature of biofilm infections and the need for a lengthy antibiotic therapy. This view of a biofilm infection suggests, somewhat counterintuitively, that the recalcitrance of biofilms does not necessarily rely on their higher levels of intrinsic resistance to killing by antibiotics than the level of intrinsic resistance of planktonic cells. Indeed, if a biofilm of a particular species under given conditions in vivo happens to be just as sensitive or even more sensitive to killing by antibiotics than a planktonic population (say, that this biofilm produces fewer persisters than a planktonic population), it will still survive better than planktonic cells, since it is invulnerable to immune attack. This view of biofilm resistance should also alter the current operational definition of an in vitro biofilm that, at least in antimicrobial susceptibility studies, has meant that a cell aggregate on some surface shows increased resistance to killing than a planktonic population. One can suggest, instead, that any cell aggregate that potentially restricts access of host defense components and that produces at least some persister cells can be viewed as a model of a recalcitrant biofilm infection. The proposed criteria will require a susceptibility test to determine if any persisters are present and whether the biofilm under study is capable of restricting penetration across the exopolymer surface. A simple assay for the functionality of the diffusion barrier would be to test the ability of the bulk of the biofilm cells to survive in the presence of an aminoglycoside antibiotic whose penetration is strongly restricted by the matrix, as discussed above. Then a test for susceptibility to killing by a fluoroquinolone like ciprofloxacin to test for the presence of persisters and a test for resistance to killing by an aminoglycoside like tobramycin to test for the diffusion barrier could serve as a straightforward dual-functionality biofilm test for any in vitro biofilm model.
FIG. 2.
Model of biofilm resistance based on persister survival. An initial treatment with antibiotic kills planktonic cells and the majority of biofilm cells. The immune system kills planktonic persisters, but the biofilm persister cells are protected from host defenses by the exopolysaccharide matrix. After the antibiotic concentration drops, persisters resurrect the biofilm and the infection relapses (33).
METHODS TO STUDY BIOFILMS
A single standard method for the study of biofilm susceptibility is not available, and this is certainly impeding progress in the field. It is very difficult if not impossible to compare the results obtained with biofilms of even the same species cultured and assayed under vastly different conditions. It is hoped that a unified method will emerge. For now, several methods are available, each with its own advantages and shortcomings, and these will be briefly reviewed. For details, see a recent issue of Methods in Enzymology (volume 310, 1999). The volume also contains descriptions of many useful methods for the study of the biology of biofilms.
A popular method used to study biofilms is the Robbins device (Tyler Instruments, Calgary, Alberta, Canada) that is based on passing a bacterial suspension through a flow cell that has 24 detachable coupons to which cells adhere and grow into a biofilm (28). Once a biofilm is formed, the feeding liquid can be switched to a culture medium that contains test compounds. After a period of incubation, the device is taken apart and the cells are dislodged by sonication and plated. This method enables reproducible biofilm formation and the observation of biofilm dynamics. The coupons can also be used for microscopic observations of biofilm structure. The strengths of this approach are in the well-controlled conditions that emulate in vivo biofilm formation and in the ability to characterize the formed biofilm by a variety of techniques. However, this method is ill suited for susceptibility studies, which require hundreds and often thousands of samples to be examined.
A microtiter plate-based method has been introduced for the study of biofilm development. The method was successfully used to search for genes participating in the biofilm development of several gram-negative species (21, 50). Wells of microtiter plates are inoculated with a bacterial suspension, and biofilms form on the surface of the wells. After a 24- to 48-h incubation, planktonic cells are removed by rinsing the wells. A solution of crystal violet is then added to the wells and stains the cells. The wells are then rinsed, and the bound dye is extracted with acetone-ethanol and quantified spectrophotometrically. This provides a quantitative measure of the mass of biofilm cells. It would be very useful to adapt this simple method to antimicrobial susceptibility measurements.
A promising apparatus for susceptibility testing is the Calgary Biofilm Device (14). This disposable apparatus ingeniously combines a shearing force that makes a robust biofilm and a microtiter plate capability. The device looks like a 96-prong replicator with plastic pins. It inserts into a grooved tray that is filled with growth medium inoculated with cells. The apparatus is then placed on a tilting shaker platform, and the growing cell suspension washes the pins, on which biofilms grow. Importantly, any cell or cell mass that is not clinging well to the pin is washed away. As a result, one can form a robust biofilm that can be rinsed without loosing its integrity. After the biofilm is formed, the lid with pins can be placed into a microtiter plate for susceptibility testing. After a period of incubation with antibiotics, the cells can be dislodged from the pins by mild sonication and plated for determination of colony counts. The round pins do not make it easy to perform microscopic observations of the biofilms. One can envision a simple modification in which the pins are made flat and thin with a perforated or thinned base for easy detachment.
INFECTIONS CAUSED BY BIOFILMS
According to a recent public announcement from the National Institutes of Health, “more than 60% of all microbial infections are caused by biofilms.” This seems high, but then if one recalls that such common infections as urinary tract infections (caused by E. coli and other pathogens), catheter infections (caused by Staphylococcus aureus and other gram-positive pathogens), child middle-ear infections (caused by Haemophilus influenzae, for example), common dental plaque formation, and gingivitis, all of which are caused by biofilms, are hard to treat or frequently relapsing, this figure appears realistic. The less common but certainly more threatening are biofilm infections that cause serious morbidity and mortality. These are endocarditis caused by S. aureus; infections of permanent indwelling devices such as joint prostheses and heart valves, also caused by S. aureus; and infections in cystic fibrosis patients caused by P. aeruginosa.
NATURE OF PERSISTERS
In 1944, Joseph W. Bigger of Trinity College (Dublin, Ireland), working in the command laboratory at York, United Kingdom, published a paper in The Lancet that reported on two important discoveries (7). According to Bigger, penicillin is a cidal rather than a bacteriostatic antibiotic, contrary to the prevailing opinion at the time; and treatment of a population of staphylococci with penicillin failed to sterilize the culture, leaving a small portion of cells that he aptly named “persisters”. Bigger estimated the incidence of persisters to be about 10−6. In that pioneering but largely forgotten study, Bigger considered two main hypotheses: (i) persisters have a higher heritable resistance to growth inhibition by penicillin, and (ii) persisters are variants that have the same susceptibility to growth inhibition by penicillin as the bulk of the cells but are insensitive to killing by penicillin. Bigger showed that upon regrowth, persisters that survived treatment with penicillin produce populations indistinguishable from the original strain; they are similarly sensitive to growth inhibition and produce new persisters.
The nature of persistence is unknown, and not much has been done over the past half a century to study these very interesting cells that apparently play a crucial role in population survival. Lack of appreciation of the clinical significance of persisters is in part responsible for the paucity of knowledge. Difficulties in studying a very small part of a population that has transient peculiarities have also contributed to the current state of ignorance. Perhaps the realization that persisters are essentially responsible for the resistance of biofilms to killing will stimulate studies into the nature of persistence. At present, considerably more is known about what persisters are not rather than what they are. Persisters are not mutants. It can also be concluded that persisters do not represent a special stage in the cell cycle, which is the current popular explanation (8, 19). At a rate of 10−6, however, this distinct stage of the cell cycle should occupy 1.8 ms (30 min/106), which would call for an unrealistic synchronization of processes throughout the cell on a time scale comparable to that of a single turnover of an enzymatic reaction. Persisters are most likely not cells in a special dormant state of no growth, which has also been suggested (29) (see below).
Obtaining empirical data on persisters should be relatively straightforward. It will be important to learn such basic facts as species-specific variations in persister prevalence rates, the dependence of the rate of persisters on growth conditions, the number of divisions required for the loss of persistence, whether or not persistence can be lost without undergoing division, how long persisters survive in the presence of antibiotics, and whether persisters are made of one type of cells or, rather, whether there are subpopulations of different kinds of persisters, each more resistant to killing by a particular type or subset of factors. It would be especially important to learn how the growth rate affects the resistance of persisters. For example, it is possible that these two factors act in synergy and that the resistance of persisters to killing is aided by conditions of slow growth.
GENES AFFECTING PERSISTENCE AND TOLERANCE
Even though the most basic facts about the physiology of persisters are not known, several genes strongly affecting the rate of persistence have been described (see reference 33 for a detailed review).
The first screen for genes that specifically affect persistence was performed by Moyed and Bertrand (41). The rationale was to enrich an ethyl methanesulfonate-mutagenized population of E. coli with cells that survived ampicillin treatment and then screen for colonies that produced larger numbers of ampicillin persisters. Only mutants whose growth was normally inhibited by ampicillin were examined further. This approach led to the identification of three independent hip (high-level persistence) loci. All hip mutants produced about 1,000-fold more persistent cells than the wild type did. One of the loci, hipAB, was cloned and sequenced (9, 42). This was the first report of bacterial genes specifically involved in the regulation of cell death. A knockout mutant of the wild-type hipA gene did not have an apparent phenotype, while a hipB knockout mutant was not obtained, indicating that a null mutant is nonviable. A mutant from which both hipA and hipB were deleted was obtained, and this strain had similar levels of persistence in the presence of penicillin as the wild type (8). Biochemical studies have shown that HipB is a transcriptional repressor that binds to the promoter region of the hip operon. HipB is a 10-kDa helix-turn-helix DNA binding protein that forms a dimer and forms a tight 1:1 complex with HipA. Moderate levels of expression of cloned wild-type HipA produced the same phenotype as the original hip (hipA or hipB) mutation did; the numbers of cells that persisted after treatment with penicillin was increased (19). The fact that a null hipB mutation is likely lethal indicates that overexpression of HipA causes death. Indeed, a high level of expression of HipA from a controllable promoter inhibited cell growth, although cell survival was not examined under these conditions (19). Apparently, the hipAB locus has the potential to act both as an inhibitor of cell death and as a killing factor. It was suggested that hipA or hipB mutants have a decreased affinity for HipA or HipB binding and thus have a higher (and moderate) level of free HipA that protects cells from killing by ampicillin. Most importantly, the hip mutants showed increased levels of resistance to factors unrelated to ampicillin. Mutated cells had a 1,000-fold higher survival rate after thymine starvation, which leads to DNA degradation (58), and were more resistant to quinolone antibiotics, which target DNA gyrase and topoisomerase (19, 70). Even more strikingly, the hip mutation protected htpR cells deficient in induction of heat shock proteins from killing by increased temperature (58). In this case, the hip mutation conferred the highest degree of protection: the number of htpR cells decreased by 2 logs after a short incubation at 42°C, while virtually no decrease was observed for htpR hipA cells. Mutants with mutations in hipAB did not protect cells from kanamycin, another cidal factor that was tested. The fact that a mutation can increase the rate of persisters to 100% (as in the case of temperature resistance) suggests that persisters are not dormant and do not represent a special stage of the cell cycle.
A similar targeted search for persister (tolerance) genes was performed with S. pneumoniae (46). The same dual test that Moyed and colleagues used was applied to screen for mutants, saving those with increased resistance to killing by penicillin and those with unchanged susceptibility to growth inhibition by penicillin. One of the 17 mutants obtained in this screen appeared to have a mutation in a new sensory kinase, which was named VncS. vncS mutants were reported to be resistant not only to cell wall inhibitors but also to aminoglycosides and quinolones. At the same time, growth of the vncS mutant was inhibited by antibiotics as effectively as growth of the wild type, showing that antibiotics were able to act normally against their targets in vncS mutant cells. In S. pneumoniae, an autolysin (LytA) is responsible for autolysis, and lytA mutants are resistant to killing by cell wall inhibitors like penicillin (65) (antibiotics that are not cell wall inhibitors were not tested with the lytA mutant). LytA was normally expressed in the vncS strain. Apparently, VncS does not control the synthesis of LytA but, rather, regulates expression of an unknown factor that activates LytA in response to an antibiotic's action (46). VncS is activated at least in part by an extracellular peptide pheromone (45).
In addition to hipAB, several well-studied E. coli genes have also been reported to strongly affect cell survival in the presence of antibiotics.
DNA damage by mutagens (which include quinolone antibiotics) is sensed by the RecA protein, which becomes activated and induces hydrolysis and inactivation of the LexA repressor (68). This releases LexA from the promoter regions of a number of lex box genes and allows the expression of components of the SOS DNA repair response. LexA repressor inactivation also leads to the synthesis of a rapidly hydrolyzed SulA protein that inhibits cell division by binding to FtsZ, the protein that forms the division ring. SulA therefore acts as a checkpoint: it accumulates after exposure to DNA-damaging agents and inhibits cell division. Subsequently, upon DNA repair SulA is degraded by the Lon protease and cell division proceeds. This scenario suggests that without SulA, DNA will not be properly repaired prior to replication, leading to production of nonviable cells. Interestingly, this is not the case. A sulA mutant of an otherwise normal strain (with a lon+ background) had a 100-fold higher rate of survival against killing by mutagenic quinolones (51). This experiment strongly suggests that the main role of SulA is not to aid repair but to trigger elimination of cells with serious defects in DNA from the population.
Another important locus that affects cell survival is relA. It is well established that tolerance to killing by a wide variety of factors correlates inversely with the growth rate. Slow growth activates the RelA-dependent synthesis of ppGpp, which inhibits anabolic processes in bacterial cells (13). Interestingly, ppGpp suppressed the activity of a major E. coli autolysin, SLT (6), which would make the cells more resistant to autolysis and could explain the mechanism of tolerance to antibiotics in slowly growing cells. While a mutation in relA, the gene coding for ppGpp synthase, did not affect the growth rate, it made nongrowing cells sensitive to killing by antibiotics that inhibit cell wall synthesis (57), although even in this mutant prolonged starvation led to the development of resistance. ppGpp inhibits peptidoglycan synthesis, which would explain the decreased levels of activity of cell wall synthesis inhibitors under starvation conditions. It would be interesting to learn whether relA mutants also become sensitive to killing by other types of lethal factors that do not target the cell wall.
FUNCTION OF PERSISTENCE
The fact that several mutations (hip, vncS, sulA, mar) can dramatically increase the number of surviving cells in a population, apparently without adversely affecting cell functions, is puzzling. Why is the better-surviving sulA, for example, the mutant and sulA+ the wild type, and not the other way around? One interesting possibility is that cells with serious defects undergo programmed cell death (PCD) (33). To put it differently, antibiotics do not kill cells but cause damage that triggers suicide. This reasoning is identical to what is known about the death of animal cells: in most cases, death results from apoptosis induced by damage from toxic factors (39). The ability to eliminate defective cells that would otherwise drain their neighbors of limited resources in a futile attempt at repair might be of significant adaptive value to a clonal population. This might be especially true for a biofilm community, which very much resembles a multicellular organism and which would benefit from “apoptosis” of defective cells, similarly to a metazoan organism. However, an antibiotic that spreads uniformly throughout the population would cause suicide of all cells, which is counterproductive. Persisters could represent cells with disabled PCD, a safety mechanism producing cells that will survive if an antibiotic reaches the entire population. Similarly, cells would need to discriminate between an unrepairable defect and starvation. Development of tolerance to antibiotics in starved cells might result from inhibition of PCD and might be aimed at preventing suicide when nutrients are limiting.
HOW TO ERADICATE BIOFILMS
The prognosis does not look good for the immediate future: too little is known about persisters to suggest ways of eradicating them. Knowing where to look for the cause of biofilm resistance, however, is a good place to start. Genes responsible for persistence can be identified (in addition to the examples discussed above), and these may serve as targets for drug discovery. Any inhibitor of a factor that causes persistence could then be combined with a conventional antibiotic such as a fluoroquinolone to eradicate a biofilm. Such a dual therapy is logistically similar to the currently used β-lactam–β-lactamase inhibitor combinations or MDR inhibitor-antibiotic combinations which are in development (37, 56, 62). Both types of approaches are based on a combination of an antibiotic and a substance inhibiting the mechanism of resistance to this antibiotic.
Development of drugs disabling the persister phenotype is likely to provide an effective therapy for biofilm infections and other types of infections in which persisters are a problem, but this will take time. Meanwhile, a variety of approaches that can be used to fight or prevent biofilm infections are being tested.
The only specific antibiofilm therapy presently in use is based on the incorporation of antibiotics into the material of indwelling catheters (16, 53, 61, 71). The combination of rifampin and minocycline is especially effective. This approach decreases the probability of colonization and is in essence a prophylactic measure. This seems to be a straightforward and useful approach, although it has obvious limitations. Bacteria resistant to the impregnated antibiotic will colonize the indwelling device; the method is probably limited to relatively short-term catheters and will not be effective for artificial joints or heart valves, nor does it address the issue of biofilm infections unrelated to indwelling devices.
Two interesting physical approaches to the eradication of biofilms are being developed: the use of an electromagnetic field (38) or ultrasound (55), both in conjunction with antibiotic therapy. These promising methods are in preclinical stages of development.
Biofilm development is an area of intense research (see reference 47 for a recent comprehensive review), and the components involved in development have been considered possible targets for therapy. Random transposon insertion libraries were used for a generalized screen for “biofilm genes” by detecting the ability of mutant clones to adhere to the wells of microtiter plates. This approach was pioneered by Pierre Genevaux and coworkers (21) and was originally applied to E. coli. Similar studies were then performed with a number of other species: Pseudomonas fluorescens (49), P. aeruginosa (48), and Vibrio cholerae (69). An independent study of E. coli was done as well (52). In all cases, biofilm formation was impaired in nonmotile mutants. Pili, although different kinds for each species, were found to facilitate initial adherence. Genes coding for the synthesis of exopolysaccharide were found to be necessary for biofilm formation in V. cholerae. A detailed analysis of the dynamics of biofilm formation with some of these insertion mutants showed that motile cells are better at reaching the surface, accounting for the need for flagella. Pili are specialized attachment organelles and, not surprisingly, assist with biofilm formation. These findings confirmed previous observations that motility (30, 31) and pili (64) are needed for biofilm formation. Some other surface adhesion factors as well as regulators of expression of surface compounds were found to be involved in biofilm formation (47).
Is the presence, then, of flagella, pili, and exopolysaccharide sufficient to build a biofilm? Perhaps exopolysaccharide alone is sufficient in species that lack flagella or pili? Interestingly, exopolysaccharide synthesis defects were found to prevent biofilm formation only in V. cholerae and not the other species studied. This might be due to the redundancy of different polysaccharides. For example, alginate is formed copiously in some strains of P. aeruginosa and is believed to contribute to the pathology of cystic fibrosis. However, strains deficient in alginate production use other exopolysaccharides to form biofilms. It is also important that the ability of cells to adhere to a surface strongly depends on the nature of the surface. For example, the presence of pili was found to actually inhibit the attachment of P. aeruginosa to contact lenses (20). It is clear that both the surface of the cell and the surface of the substratum determine the effectiveness of adhesion in biofilm formation. Numerous surface adhesins of pathogens, of which the pilus is only one example, will facilitate binding to host cells (2, 40, 54) and abiotic surfaces (27, 47). These adhesins might or might not play a role in biofilm formation on a particular artificial surface.
One limitation of the transposon insertion screening studies is that they test the mass of the cells making up the biofilm, which will not report defects in biofilm architecture. It was recently found that the quorum-sensing factor N-(3-oxododecanoyl)-l-homoserine lactone (HSL) is required for the formation of a biofilm with a complex “wild-type” architecture: rather loosely packed cell masses with a mushroom appearance with substantial amounts of exopolysaccharide and aqueous channels traversing the entire biofilm (17). A P. aeruginosa lasI mutant defective in HSL production formed thin, dense biofilms on a glass surface that were easily dislodged by sodium dodecyl sulfate (SDS), unlike the wild-type biofilms, which were not affected by SDS. We found that this mutant formed biofilms on polystyrene in the Calgary Biofilm Device that were not affected by SDS (11). More importantly, the mutant biofilm showed the same level of resistance to ofloxacin as the wild type, suggesting that architecture or other properties of this defective biofilm do not affect its ability to produce persister cells and to resist killing by an antibiotic. The “mushroom” architecture of biofilms, with cell columns separated by water channels, evokes function. This would ease delivery of nutrients and release of metabolic products (17). The sophisticated architecture in turn suggests that a dedicated program is in place to build a biofilm (47). However, the usefulness of a well-structured biofilm compared to that of its flattened version has not been experimentally demonstrated.
This analysis brings me to an important question: are development proteins viable targets for antibiofilm drug discovery? Are antagonists of HSL good candidates for drug development? One problem with components like pili or flagella is that targeting of developmental components means that the therapy will provide a prophylaxis rather than a cure for a biofilm infection. The exopolysaccharide synthesis genes seem like a better potential choice as targets since these components are probably required for the maintenance of biofilm formation and not only for the initial steps of biofilm formation. However, redundancy of polysaccharides and the differences between the biosynthesis genes in various species is a serious limitation for possible drug development by use of this pathway as a target. Similarly, quorum-sensing factors vary among different species, and HSL does not appear to be required for the formation of a biofilm resistant to killing.
This analysis of options suggests that the development of a universal antibiofilm therapy, possibly on the basis of targeting of persister proteins, is a long-term project, yet a possible simple solution to biofilm infection follows directly from the dynamics of in vitro biofilm eradication. The rationale is to administer a cidal antibiotic, then withdraw it, and then add it again. The first application of antibiotic will eradicate the bulk of biofilm cells, leaving persisters. In a realistic example, ofloxacin decreases the size of a P. aeruginosa biofilm from 108 cells to 105 persisters (11). Withdrawal of the antibiotic will allow this persister population to start growing. Assume that after two divisions the persistence phenotype is lost. At this point, the new population of 4 × 105 cells will produce 40 persisters. A second application of antibiotic should then completely eradicate the biofilm. This type of a simple cyclical antibiotic regimen was proposed previously by Bigger (7) for eradication of staphylococcal persisters. This approach might work in topical applications, in which the delivery of antibiotics can be well controlled. For example, biofilm infections are common in urinary catheters, into which a desired solution can be instilled. P. aeruginosa biofilm infections of cystic fibrosis patients provide another example in which this approach might work well. Antibiotics can be delivered topically to cystic fibrosis patients as aerosols. The popular medication Tobra (PathoGenesis/Chiron) is a tobramycin aerosol. This antibiotic is very effective in eradicating planktonic cells, which explains the clinical usefulness of the preparation. However, as discussed above, biofilms are resistant to tobramycin. In a cyclical application, one would deliver an aerosol of a fluoroquinolone antibiotic like ciprofloxacin, which would penetrate the biofilm and kill the cells. A second antibiotic application after a minimal period of time that would be necessary for survivors to start growing and loose their persister phenotype could then eradicate the biofilm. The feasibility of a cyclical biofilm eradication approach will depend on the rate with which persisters lose resistance to killing and regenerate new persisters and on the ability to manipulate the antibiotic concentration. Development of resistance in a situation in which the antibiotic concentration is allowed to drop is a concern, but cycling of two different antibiotics could largely eliminate this problem. If this approach works for topical applications, it will encourage an inquiry into the possible use of cyclical treatment of systemic biofilms as well. It is entirely possible that successful cases of antimicrobial therapy of biofilm infections result from a fortuitous optimal cycling of an antibiotic concentration that eliminated first the bulk of the biofilm and then the progeny of the persisters that began to divide.
Another interesting possibility for biofilm elimination comes from the observations of biofilm self-destruction. P. fluorescens readily forms a biofilm in a well-oxygenated environment, such as near the liquid surface on a glass slide inserted vertically in a beaker. As the oxygen gets depleted by the growing biofilm mass, a specific exopolysaccharide lyase is induced and digests the biofilm matrix, liberating the cells (1). The result is a striking, almost complete disappearance of the biofilm. The authors suggested that the degradation of the matrix serves two functions: it provides nutrients for the starving biofilm and liberates cells, allowing them to seek greener pastures. The nutrient limitation in this experiment comes from oxygen deficiency rather than carbon deficiency, and it remains unclear whether a biofilm will self-destruct in response to any type of energy (or essential nutrient) limitation. It seems reasonable to expect that this dramatic and so obviously useful (to humans as well) ability of a biofilm to self-destruct is not limited to oxygen deficiency. Disassembly of the biofilm could be exploited to treat infections. One approach would be to emulate energy deprivation by providing inhibitors of oxidative phosphorylation. Such substances are usually toxic, but a number of topical antimicrobials are membrane-acting agents. The quaternary ammonium compound benzalkonium chloride or the cationic base chlorhexidine are pertinent examples. Salicylate, widely used in food preservation, is an uncoupler. It might very well appear that some of the topical antimicrobials are causing biofilm self-destruction to a certain extent. However, it must be pointed out that the aim of conventional antimicrobial therapy is to deliver and maintain the drug at the maximally achievable and safe level. A high concentration of an antiseptic like chlorhexidine will simply kill the majority of cells, will probably leave the persisters largely intact, and will not cause biofilm self-destruction. Synthesis and export of lyase are required for biofilm degradation, but these will happen only under conditions that decrease the energy level and that do not completely inhibit protein synthesis. The same logic would apply to industrial biofilm eradication (cooling towers, pipes, etc.): it might appear that an optimal low level of a biocide will be more effective than a high dose for the treatment of biofilms. Another and possibly more productive approach would be to develop specific drugs that interact directly with the components of the biofilm self-destruction pathway. In an experiment that could serve as a model for this approach, expression of alginate lyase from a controllable promoter increased sloughing of cells from a colony of mucoid P. aeruginosa cells that overproduced alginate (10). Genes controlling biofilm self-destruction might appear to be of more use than genes involved in biofilm formation.
ACKNOWLEDGMENTS
The research in my laboratory described in this paper has been supported by NIH grants RO1 GM54412 and RO1 GM61162.
REFERENCES
- 1.Allison D G, Ruiz B, SanJose C, Jaspe A, Gilbert P. Extracellular products as mediators of the formation and detachment of Pseudomonas fluorescens biofilms. FEMS Microbiol Lett. 1998;167:179–184. doi: 10.1111/j.1574-6968.1998.tb13225.x. [DOI] [PubMed] [Google Scholar]
- 2.Amano A, Nakagawa I, Hamada S. Studying initial phase of biofilm formation: molecular interaction of host proteins and bacterial surface components. Methods Enzymol. 1999;310:501–513. doi: 10.1016/s0076-6879(99)10038-7. [DOI] [PubMed] [Google Scholar]
- 3.Anderl J N, Franklin M J, Stewart P S. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother. 2000;44:1818–1824. doi: 10.1128/aac.44.7.1818-1824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashby M J, Neale J E, Knott S J, Critchley I A. Effect of antibiotics on non-growing planktonic cells and biofilms of Escherichia coli. J Antimicrob Chemother. 1994;33:443–452. doi: 10.1093/jac/33.3.443. [DOI] [PubMed] [Google Scholar]
- 5.Baillie G S, Douglas L J. Candida biofilms and their susceptibility to antifungal agents. Methods Enzymol. 1999;310:644–656. doi: 10.1016/s0076-6879(99)10050-8. [DOI] [PubMed] [Google Scholar]
- 6.Betzner A S, Ferreira L C, Holtje J V, Keck W. Control of the activity of the soluble lytic transglycosylase by the stringent response in Escherichia coli. FEMS Microbiol Lett. 1990;55:161–164. doi: 10.1016/0378-1097(90)90187-u. [DOI] [PubMed] [Google Scholar]
- 7.Bigger J W. Treatment of staphylococcal infections with penicillin. Lancet. 1944;ii:497–500. [Google Scholar]
- 8.Black D S, Irwin B, Moyed H S. Autoregulation of hip, an operon that affects lethality due to inhibition of peptidoglycan or DNA synthesis. J Bacteriol. 1994;176:4081–4091. doi: 10.1128/jb.176.13.4081-4091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black D S, Kelly A J, Mardis M J, Moyed H S. Structure and organization of hip, an operon that affects lethality due to inhibition of peptidoglycan or DNA synthesis. J Bacteriol. 1991;173:5732–5739. doi: 10.1128/jb.173.18.5732-5739.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyd A, Chakrabarty A M. Role of alginate lyase in cell detachment of Pseudomonas aeruginosa. Appl Environ Microbiol. 1994;60:2355–2359. doi: 10.1128/aem.60.7.2355-2359.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooun A, Liu S, Lewis K. A dose-response study of antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2000;44:640–646. doi: 10.1128/aac.44.3.640-646.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooun A, Tomashek J J, Lewis K. Purification and ligand binding of EmrR, a regulator of a multidrug transporter. J Bacteriol. 1999;181:5131–5133. doi: 10.1128/jb.181.16.5131-5133.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cashel M, Gentry D R, Hernandez V J, Vinella D. The stringent response. In: Neidhardt F C, Curtiss R I, Ingraham J L, Lin C C L, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C.: ASM Press; 1996. pp. 1400–1416. [Google Scholar]
- 14.Ceri H, Olson M E, Stremick C, Read R R, Morck D, Buret A. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol. 1999;37:1771–1776. doi: 10.1128/jcm.37.6.1771-1776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costerton J W, Stewart P S, Greenberg E P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 16.Darouiche R O, Smith J A, Jr, Hanna H, Dhabuwala C B, Steiner M S, Babaian R J, Boone T B, Scardino P T, Thornby J I, Raad I I. Efficacy of antimicrobial-impregnated bladder catheters in reducing catheter-associated bacteriuria: a prospective, randomized, multicenter clinical trial. Urology. 1999;54:976–981. doi: 10.1016/s0090-4295(99)00288-5. [DOI] [PubMed] [Google Scholar]
- 17.Davies G D, Parsek M R, Pearson J P, Iglewski B H, Costerton J W, Greenberg E P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 18.Elkins J G, Hassett D J, Stewart P S, Schweizer H P, McDermott T R. Protective role of catalase in Pseudomonas aeruginosa biofilm resistance to hydrogen peroxide. Appl Environ Microbiol. 1999;65:4594–4600. doi: 10.1128/aem.65.10.4594-4600.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falla T J, Chopra I. Joint tolerance to beta-lactam and fluoroquinolone antibiotics in Escherichia coli results from overexpression of hipA. Antimicrob Agents Chemother. 1998;42:3282–3284. doi: 10.1128/aac.42.12.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fletcher E L, Weissman B A, Efron N, Fleiszig S M, Curcio A J, Brennan N A. The role of pili in the attachment of Pseudomonas aeruginosa to unworn hydrogel contact lenses. Curr Eye Res. 1993;12:1067–1071. doi: 10.3109/02713689309033504. [DOI] [PubMed] [Google Scholar]
- 21.Genevaux P, Muller S, Bauda P. A rapid screening procedure to identify mini-Tn 10 insertion mutants of Escherichia coli K-12 with altered adhesion properties. FEMS Microbiol Lett. 1996;142:27–30. doi: 10.1111/j.1574-6968.1996.tb08402.x. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert P, Das J, Foley I. Biofilm susceptibility to antimicrobials. Adv Dent Res. 1997;11:160–167. doi: 10.1177/08959374970110010701. [DOI] [PubMed] [Google Scholar]
- 23.Giwercman B, Jensen E T T, Hoiby N, Kharazmi A, Costerton J W J W. Induction of β-lactamase production in Pseudomonas aeruginosa biofilm. Antimicrob Agents Chemother. 1991;35:1008–1010. doi: 10.1128/aac.35.5.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassett D J, Ma J F, Elkins J G, McDermott T R, Ochsner U A, West S E, Huang C T, Fredericks J, Burnett S, Stewart P S, McFeters G, Passador L, Iglewski B H. Quorum sensing in Pseudomonas aeruginosa controls expression of catalase and superoxide dismutase genes and mediates biofilm susceptibility to hydrogen peroxide. Mol Microbiol. 1999;34:1082–1093. doi: 10.1046/j.1365-2958.1999.01672.x. [DOI] [PubMed] [Google Scholar]
- 25.Hoyle B D, Jass J, Costerton J W. The biofilm glycocalyx as a resistance factor. J Antimicrob Chemother. 1990;26:1–5. doi: 10.1093/jac/26.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Ishida H, Ishida Y, Kurosaka Y, Otani T, Sato K, Kobayashi H. In vitro and in vivo activities of levofloxacin against biofilm-producing Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:1641–1645. doi: 10.1128/aac.42.7.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kachlany S C, Planet P J, Bhattacharjee M K, Kollia E, DeSalle R, Fine D H, Figurski D H. Nonspecific adherence by Actinobacillus actinomycetemcomitans requires genes widespread in bacteria and archaea. J Bacteriol. 2000;182:6169–6176. doi: 10.1128/jb.182.21.6169-6176.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kharazmi A, Giwercman B, Hoiby N. Robbins device in biofilm research. Methods Enzymol. 1999;310:207–215. doi: 10.1016/s0076-6879(99)10018-1. [DOI] [PubMed] [Google Scholar]
- 29.Koch A L. Similarities and differences of individual bacteria within a clone. In: Neidhardt F C, Curtiss R I, Ingraham J L, Lin C C L, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C.: ASM Press; 1996. pp. 1640–1651. [Google Scholar]
- 30.Korber D R, John R L, Douglas E C. Effect of motility on surface colonization and reproductive success of Pseudomonas fluorescens in dual-dilution continuous culture and batch culture systems. Appl Environ Microbiol. 1994;60:1421–1429. doi: 10.1128/aem.60.5.1421-1429.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korber D R, Lawrence J R, Sutton B, Caldwell D E. Effect of laminar flow velocity on the kinetics of surface recolonization by mot-positive and mot-negative Pseudomonas fluorescens. Microb Ecol. 1989;18:1–20. doi: 10.1007/BF02011692. [DOI] [PubMed] [Google Scholar]
- 32.Lee A, Mao W, Warren M S, Mistry A, Hoshino K, Okumura R, Ishida H, Lomovskaya O. Interplay between efflux pumps may provide either additive or multiplicative effects on drug resistance. J Bacteriol. 2000;182:3142–3150. doi: 10.1128/jb.182.11.3142-3150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewis K. Programmed death in bacteria. Microbiol Mol Biol Rev. 2000;64:503–514. doi: 10.1128/mmbr.64.3.503-514.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X Z, Nikaido H, Poole K. Role of mexA-mexB-oprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1948–1953. doi: 10.1128/aac.39.9.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lomovskaya O, Lewis K. Emr, an Escherichia coli locus for multidrug resistance. Proc Natl Acad Sci USA. 1992;89:8938–8942. doi: 10.1073/pnas.89.19.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maira-Litran T, Allison D G, Gilbert P. An evaluation of the potential of the multiple antibiotic resistance operon (mar) and the multidrug efflux pump acrAB to moderate resistance towards ciprofloxacin in Escherichia coli biofilms. J Antimicrob Chemother. 2000;45:789–795. doi: 10.1093/jac/45.6.789. [DOI] [PubMed] [Google Scholar]
- 37.Markham P N, Westhaus E, Klyachko K, Johnson M E, Neyfakh A A. Multiple novel inhibitors of the NorA multidrug transporter of Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:2404–2408. doi: 10.1128/aac.43.10.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLeod B R, Fortun S, Costerton J W, Stewart P S. Enhanced bacterial biofilm control using electromagnetic fields in combination with antibiotics. Methods Enzymol. 1999;310:656–670. doi: 10.1016/s0076-6879(99)10051-x. [DOI] [PubMed] [Google Scholar]
- 39.Metzstein M M, Stanfield G M, Horvitz H R. Genetics of programmed cell death in C. elegans: past, present and future. Trends Genet. 1998;14:410–416. doi: 10.1016/s0168-9525(98)01573-x. [DOI] [PubMed] [Google Scholar]
- 40.Mittelman M W. Adhesion to biomaterials. In: Fletcher M, editor. The molecular and ecological diversity of bacterial adhesion. New York, N.Y: John Wiley & Sons, Inc.; 1997. pp. 89–127. [Google Scholar]
- 41.Moyed H S, Bertrand K P. hipA, a newly recognized gene of Escherichia coli K-12 that affects frequency of persistence after inhibition of murein synthesis. J Bacteriol. 1983;155:768–775. doi: 10.1128/jb.155.2.768-775.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moyed H S, Broderick S H. Molecular cloning and expression of hipA, a gene of Escherichia coli K- 12 that affects frequency of persistence after inhibition of murein synthesis. J Bacteriol. 1986;166:399–403. doi: 10.1128/jb.166.2.399-403.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muli F W, Struthers J K. The growth of Gardnerella vaginalis and Lactobacillus acidophilus in Sorbarod biofilms. J Med Microbiol. 1998;47:401–405. doi: 10.1099/00222615-47-5-401. [DOI] [PubMed] [Google Scholar]
- 44.Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science. 1994;264:382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- 45.Novak R, Charpentier E, Braun J S, Tuomanen E. Signal transduction by a death signal peptide: uncovering the mechanism of bacterial killing by penicillin. Mol Cell. 2000;5:49–57. doi: 10.1016/s1097-2765(00)80402-5. [DOI] [PubMed] [Google Scholar]
- 46.Novak R, Henriques B, Charpentier E, Normark S, Tuomanen E. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature. 1999;399:590–593. doi: 10.1038/21202. [DOI] [PubMed] [Google Scholar]
- 47.O'Toole G, Kaplan H B, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54:49–79. doi: 10.1146/annurev.micro.54.1.49. [DOI] [PubMed] [Google Scholar]
- 48.O'Toole G A, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 49.O'Toole G A, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Mol Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 50.O'Toole G A, Pratt L A, Watnick P I, Newman D K, Weaver V B, Kolter R. Genetic approaches to study of biofilms. Methods Enzymol. 1999;310:91–109. doi: 10.1016/s0076-6879(99)10008-9. [DOI] [PubMed] [Google Scholar]
- 51.Piddock L J, Walters R N. Bactericidal activities of five quinolones for Escherichia coli strains with mutations in genes encoding the SOS response or cell division. Antimicrob Agents Chemother. 1992;36:819–825. doi: 10.1128/aac.36.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pratt L A, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 53.Raad I, Hanna H. Intravascular catheters impregnated with antimicrobial agents: a milestone in the prevention of bloodstream infections. Support Care Cancer. 1999;7:386–390. doi: 10.1007/s005200050297. [DOI] [PubMed] [Google Scholar]
- 54.Rachid S, Ohlsen K, Witte W, Hacker J, Ziebuhr W. Effect of subinhibitory antibiotic concentrations on polysaccharide intercellular adhesin expression in biofilm-forming Staphylococcus epidermidis. Antimicrob Agents Chemother. 2000;44:3357–3363. doi: 10.1128/aac.44.12.3357-3363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rediske A M, Roeder B L, Nelson J L, Robison R L, Schaalje G B, Robison R A, Pitt W G. Pulsed ultrasound enhances the killing of Escherichia coli biofilms by aminoglycoside antibiotics in vivo. Antimicrob Agents Chemother. 2000;44:771–772. doi: 10.1128/aac.44.3.771-772.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Renau T E, Leger R, Flamme E M, Sangalang J, She M W, Yen R, Gannon C L, Griffith D, Chamberland S, Lomovskaya O, Hecker S J, Lee V J, Ohta T, Nakayama K. Inhibitors of efflux pumps in Pseudomonas aeruginosa potentiate the activity of the fluoroquinolone antibacterial levofloxacin. J Med Chem. 1999;42:4928–4931. doi: 10.1021/jm9904598. [DOI] [PubMed] [Google Scholar]
- 57.Rodionov D G, Ishiguro E E. Direct correlation between overproduction of guanosine 3′,5′-bispyrophosphate (ppGpp) and penicillin tolerance in Escherichia coli. J Bacteriol. 1995;177:4224–4229. doi: 10.1128/jb.177.15.4224-4229.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scherrer R, Moyed H S. Conditional impairment of cell division and altered lethality in hipA mutants of Escherichia coli K-12. J Bacteriol. 1988;170:3321–3326. doi: 10.1128/jb.170.8.3321-3326.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shigeta M, Tanaka G, Komatsuzawa H, Sugai M, Suginaka H, Usui T. Permeation of antimicrobial agents through Pseudomonas aeruginosa biofilms: a simple method. Chemotherapy (Tokyo) 1997;43:340–345. doi: 10.1159/000239587. [DOI] [PubMed] [Google Scholar]
- 60.Smith J G, Kong L, Abruzzo G K, Gill C J, Flattery A M, Scott P M, Silver L, Kropp H, Bartizal K. Evaluation of experimental therapeutics in a new mouse model of Helicobacter felis utilizing 16S rRNA polymerase chain reaction for detection. Scand J Gastroenterol. 1997;32:297–302. doi: 10.3109/00365529709007675. [DOI] [PubMed] [Google Scholar]
- 61.Spencer R C. Novel methods for the prevention of infection of intravascular devices. J Hosp Infect. 1999;43(Suppl.):S127–S135. doi: 10.1016/s0195-6701(99)90075-0. [DOI] [PubMed] [Google Scholar]
- 62.Stermitz F R, Lorenz P, Tawara J N, Zenewicz L, Lewis K. Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Proc Natl Acad Sci USA. 2000;97:1433–1437. doi: 10.1073/pnas.030540597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stewart P S. Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrob Agents Chemother. 1996;40:2517–2522. doi: 10.1128/aac.40.11.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sung J Y, Shaffer E A, Lam K, Rususka I, Costerton J W. Hydrophobic bile salt inhibits bacterial adhesion on biliary stent material. Dig Dis Sci. 1994;39:999–1006. doi: 10.1007/BF02087551. [DOI] [PubMed] [Google Scholar]
- 65.Tomasz A, Albino A, Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970;227:138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- 66.von Eiff C, Heilmann C, Peters G. New aspects in the molecular basis of polymer-associated infections due to staphylococci. Eur J Clin Microbiol Infect Dis. 1999;18:843–846. doi: 10.1007/s100960050417. [DOI] [PubMed] [Google Scholar]
- 67.Vrany J D, Stewart P S, Suci P A. Comparison of recalcitrance to ciprofloxacin and levofloxacin exhibited by Pseudomonas aeruginosa biofilms displaying rapid-transport characteristics. Antimicrob Agents Chemother. 1997;41:1352–1358. doi: 10.1128/aac.41.6.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walker G C. The SOS response of Escherichia coli. In: Neidhardt F C, Curtiss R I, Ingraham J L, Lin C C L, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C.: ASM Press; 1996. pp. 1400–1416. [Google Scholar]
- 69.Watnick P I, Kolter R. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol Microbiol. 1999;34:586–595. doi: 10.1046/j.1365-2958.1999.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolfson J S, Hooper D C, McHugh G L, Bozza M A, Swartz M N. Mutants of Escherichia coli K-12 exhibiting reduced killing by both quinolone and beta-lactam antimicrobial agents. Antimicrob Agents Chemother. 1990;34:1938–1943. doi: 10.1128/aac.34.10.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Woo G L, Mittelman M W, Santerre J P. Synthesis and characterization of a novel biodegradable antimicrobial polymer. Biomaterials. 2000;21:1235–1246. doi: 10.1016/s0142-9612(00)00003-x. [DOI] [PubMed] [Google Scholar]