Abstract
Treatment-resistant bipolar depression (TRBD) has been reported in about one-quarter of patients with bipolar disorders, and few interventions have shown clear and established effectiveness. We conducted a narrative review of the published medical literature to identify papers discussing treatment-resistant depression concepts and novel interventions for bipolar depression that focus on TRBD. We searched for potentially relevant English-language articles published in the last decade. Selected articles (based on the title and abstract) were retrieved for a more detailed evaluation. A number of promising new interventions, both pharmacological and non-pharmacological, are being investigated for TRBD treatment, including ketamine, lurasidone, D-cycloserine, pioglitazone, N-acetylcysteine, angiotensin-converting enzyme inhibitors, angiotensin II type 1 receptor blockers, cyclooxygenase 2 inhibitors, magnetic seizure therapy, intermittent theta-burst stimulation, deep transcranial magnetic stimulation, vagus nerve stimulation therapy, and deep brain stimulation. Although there is no consensus about the concept of TRBD, better clarification of the neurobiology associated with treatment non-response could help identify novel strategies. More research is warranted, mainly focusing on personalizing current treatments to optimize response and remission rates.
Keywords: Bipolar disorder, depression, treatment-resistant bipolar disorder
Introduction
Bipolar depression accounts for most symptomatic periods in bipolar disorder (BD) and is associated with elevated suicide risk and high morbidity and mortality rates.1 Despite a current lack of consensus about its definition, treatment-resistant bipolar depression (TRBD) has been reported in about one-quarter of BD patients.2 No interventions have shown clear and established effectiveness for its treatment.2-4 This review will discuss the concept of TRBD, its associated variables and neurobiology, novel strategies for decreasing its burden, and the potential of new paradigms to identify more precise and effective treatments for psychiatric diseases. We performed a narrative review of the medical literature to identify papers discussing treatment-resistant depression (TRD) and novel interventions for bipolar depression that focus on TRBD. We searched for potentially relevant English-language articles published in the last decade. Selected articles (based on the title and abstract) regarding TRD and TRBD were retrieved for a more detailed evaluation.
The concept of treatment-resistant depression
Traditionally, the concept of TRD is based on the expected outcome and in its measurement. Thus, different studies have approached TRD in distinct forms, which have also been influenced by changes in the definition of depression itself. As Demyttenaere and Van Duppen pointed out, “TRD refers to the failure in obtaining an acceptable outcome, yet what is an acceptable outcome is not a universally agreed-upon definition.”5 Even though this article will address TRD in BD, this concept has been historically discussed in the context of major depression disorder (MDD), and the vast majority of published studies on this topic focus on MDD.6 This paper will consider resistance and refractoriness as similar concepts, since they are frequently used interchangeably, referring to related clinical situations. Although non-adherence, non-psychiatric comorbidities, and a lack of adequate evidence-based interventions can contribute to treatment non-response,7 for the purposes of the present article, we will use the term TRBD to designate cases in which treatment resistance suggests a lack of response to therapeutic interventions per se.
Most studies have considered TRBD as a failure in two or more interventions. This agrees with a recent consensus of internationally recognized experts, which defined TRBD as “failure to reach sustained symptomatic remission for 8 consecutive weeks after two different treatment trials, at adequate therapeutic doses, with at least two recommended monotherapy treatments or at least one monotherapy treatment and another combination treatment.”8 More specifically, multi-therapy resistant bipolar depression was conceptualized like TRBD, with the addition of failure in psychotherapeutic treatment, at least one trial with an antidepressant, and electroconvulsive therapy.8 Hidalgo-Mazzei et al., the authors of the consensus statement, also provided the adequate drug dose range for the main evidence-based drugs and the plasma levels for lithium, which was similar to the approach of Pacchiarotti et al.9 Among the implications for this consensus definition, the authors state that it will help clinicians and researchers decide when to consider novel strategies for TRBD.8
Variables associated with treatment-resistant bipolar depression
Clinical variables
The Group for the Study of Resistant Depression reported demographic and clinical characteristics associated with TRBD, defined as the failure to reach a score of less than 17 in the 17-item Hamilton Depression Rating Scale (HDRS) after at least two consecutive adequate antidepressant treatments (4 weeks or more at the optimal dose of an adequate antidepressant in combination witha well-established mood stabilizer).2 Among the 375 patients with BD type I or II and a history of at least one adequate antidepressant treatment associated with a mood stabilizer, approximately one-quarter (26.4%) met the criteria for TRBD.2 The authors found that higher severity, melancholic temperament, suicide risk, and social phobia were significantly associated with treatment resistance.2 Fornaro et al. found a similar proportion (28.3%) of patients with TRBD (defined as “a lack of response to at least two previously established treatments for bipolar depression”), in whom mixed features according to the DSM-5 specifier, in addition to symptoms of irritability, impulsivity, and distractibility, were considered predictors of TRBD.3 Similarly, cognitive dysfunction has been described as highly frequent in patients with TRBD. Kessler et al. reported global cognitive impairment (processing speed, attention/vigilance, working memory, verbal learning, visual learning, and reasoning and problem solving), including clinically significant dysfunction in two or more cognitive domains, in almost 70% of patients with BD type I and 40% of patients with BD type II with TRD.10
Neurobiological variables
Genetic, biochemical, bioenergetic, inflammatory, and brain activity and connectivity dysfunction have been reported as associated with BD.11,12 These dysfunctional biological processes can help identify effective treatment interventions.11-14 However, the neurobiology could be even harder to disentangle when dealing with specific clinical characteristics and mood states, such as treatment-resistant depression.
Most of the studies that have addressed the neurobiology of TRD have approached it presumably in the context of unipolar depression. For instance, studies by the Group for the Study of Resistant Depression have identified genes associated with treatment resistance that have a role in the serotoninergic system, neuroplasticity, and neuronal cell adhesion, such as BDNF, 5HTR2A, CREB1, and GAP43.15,16 Immune system dysfunction has also been extensively reported as associated with TRD. The findings of Clark et al., who investigated DNA methylation signatures associated with depressive status, suggest that TRD has an immune signature.17 In a review, Yang et al. found that higher interleukin-6 and C-reactive protein /high-sensitivity-C-reactive protein could predict treatment resistance, and a study with patients with and without TRD reported a relationship between overall activation of the inflammatory system and non-response to antidepressants.18,19 Fabbri et al. compared genes linked to pathways associated with TRD to genes associated with the targets of drugs in development to identify potential pharmacological options for the treatment of resistant-depression (with the purpose of drug repositioning).20 Several mechanisms of action have been found for compounds in TRD-related pathways, including those involved in monoamine and glutamatergic neurotransmission, inflammation/immune response, peroxisome proliferator-activated receptor-gamma (PPAR-γ) agonists, oxidoreductase reaction modulators, angiotensin signaling, and GSK3 modulation, the most promising of which are modulators of neural plasticity and inflammation.20 One of the most remarkable medications treating resistant depression is ketamine, an N-methyl-D-aspartate receptor antagonist, which suggests that glutamatergic neurotransmission dysfunction has an important role in the pathophysiology of BD. This finding has stimulated the investigation of novel glutamatergic agents for TRBD.4,21,22 The administration of ketamine has also been associated with changes in the kynurenine system, a pathway that intersects immune response and the glutamatergic system.23
Increased amygdala activity, decreased ventromedial prefrontal cortex (PFC) activity, and connectivity dysfunction between these regions during emotional tasks have been reported in patients with BD. Moreover, increased activity has been found in the ventral striatum, the orbitofrontal cortex, and the ventrolateral PFC, and dysfunctional connectivity between them during reward anticipation.13 However, few studies have investigated these patterns in the context of TRBD. Downar et al. applied repetitive transcranial magnetic stimulation (TMS) over the dorsomedial PFC in patients with bipolar or unipolar TRD. At baseline, non-responders presented significantly lower connectivity than responders in regions related to reward-behavior, such as the striatum, the ventral tegmental area, and the ventromedial PFC.24 The brain circuitry pattern of mood regulating regions in TRD patients who responded to deep brain stimulation (DBS) of subcallosal cingulate white matter was distinct from that of non-responders. For instance, among treatment responders, there were bilateral pathways between the activated region and the medial frontal cortex, the rostral and dorsal cingulate cortex, and the subcortical nuclei; among non-responders, these connections were inconsistent.25
These neurobiological pathways could lead to the identification of novel treatments for TRBD and should be further investigated. We will describe some promising interventions for TRBD that have demonstrated positive results or whose mechanism of action suggests potential benefits.
Novel treatment strategies
In a randomized trial, which was part of the Systematic Treatment Enhancement Program for Bipolar Disorder, Nierenberg et al. compared the recovery rates of patients with TRBD after treatment augmentation with inositol, lamotrigine, or risperidone.26 This was the first randomized study of TRBD to compare adjunctive interventions. The results showed that the three medications did not significantly differ concerning recovery rates, which were relatively low, ranging from 12.5-37.5% (inositol) to 16.7-26.7% (lamotrigine) to 7.7-9.1% (risperidone).26 More recently, a systematic review and meta-analysis of randomized controlled trials addressing TRBD management showed that ketamine was associated with a significant response rate one day after infusion. Simultaneously, electroconvulsive therapy presented similar efficacy for TRBD and treatment-resistant unipolar depression.4 Some results also suggested a potential as an augmentation treatment for pramipexole, a dopaminergic agonist, and modafinil/armodafinil, a psychostimulant, in the reduction of depressive symptoms in patients with TRBD.4
The high frequency of TRD in patients with BD, in addition to the narrow range of effective interventions, clearly requires both novel therapeutic strategies and a different paradigm for approaching TRBD. In the present paper, we considered novel strategies to be any therapeutic interventions with a new mechanism of action, a new target for pharmacological or neuromodulatory intervention, or an intervention that was first tested for TRBD.
Pharmacological strategies
Lurasidone
Lurasidone is an atypical antipsychotic agent with a high antagonist affinity for 5-HT2A, 5-HT7, and D2 receptors, moderate partial agonist affinity for 5-HT1A receptors, and small affinity for H1 and M1 receptors.27 In 2014, a double-blind, randomized-controlled trial of treatment augmentation with lurasidone compared to placebo was published. The inclusion criteria were a diagnosis of BD type I with a current major depressive episode of moderate severity (at least four weeks but less than 12 months). All patients were already receiving a mood stabilizer (lithium or valproate) and had not achieved remission with mood stabilizers at appropriate serum levels. A total of 348 participants were included.28 The primary outcome was the mean change from baseline to week 6 in total MADRS score, which was significantly higher in the lurasidone group than the placebo group (-17.1 vs. -13.5, p = 0.005, effect size = 0.34). The treatment group’s MADRS scores surpassed those of the placebo group at week three and continued to do so throughout all subsequent study visits. A significantly higher proportion of the lurasidone group met the response criteria than the placebo group after six weeks of treatment (57% vs. 42%, p = 0.008, number needed to treat [NNT] = 7), and the median time to response was significantly shorter for the lurasidone group (28 vs. 42 days, log-rank p < 0.001). Finally, the proportion of patients who achieved remission by the endpoint was significantly higher in the lurasidone group (50% vs. 35%, p = 0.008, NNT = 7), and the median time to remission was considerably shorter for the lurasidone group (35 vs. 43 days, p = 0.001).
In 2016, Schaffer et al. published an open-label trial of lurasidone.29 Unlike the previous study, this was conducted in a clinical setting in a more naturalistic way. It had minimal exclusion criteria and included participants with BD type I or II. All participants were non-responders or partial, but inadequate, responders to numerous previous trials of standard BD medications (both approved and not approved by the U.S. Food and Drug Administration); thus, they would fulfill the criteria for TRBD. Over the course of the eight-week open-label trial, the observed response rate was 45%. Responders had a lower rate of medication failure than non-responders (15.9 vs. 21.6).
Aripiprazole
Aripiprazole is a partial agonist of D2, D3, and D4 receptors, as well as serotonin receptors 5-HT1A, 5-HT2C, 5-HT7.30 A retrospective study of aripiprazole augmentation31 in 85 patients with bipolar or unipolar TRD found that the aripiprazole intervention remission rate was 36.5% after 6 weeks. Multiple logistic regression analysis indicated that aripiprazole was significantly more effective for bipolar depression than MDD. A lack of comorbid anxiety disorders and a current episode longer than three months were both significantly associated with the efficacy of aripiprazole augmentation. However, a recent systematic review and network analysis of pharmacological treatments for acute BD depression did not report significant differences between aripiprazole and placebo. In addition, aripiprazole was associated with a higher discontinuation rate due to adverse events than placebo.32
Ketamine
An open-label trial with multi-infusion ketamine augmentation showed that ketamine infusions were associated with improved depressive symptoms after one week for 38 patients with TRBD and suicidal ideation (a mean HDRS score reduction of 49.8%). Nonetheless, relapse occurred during the second week after treatment, and the authors suggested that the clinical efficacy of ketamine augmentation is transient.33 Furthermore, a double-blind, randomized, crossover, placebo-controlled study34 on the effects of ketamine in TRBD (18 patients with a current major depressive episode of at least four weeks who had previously failed at least one adequate antidepressant trial and did not respond to either valproate or lithium for a minimum period of 4 weeks at appropriate levels) found that 56% of the participants receiving ketamine responded, with only two (13%) showing remission 40 minutes after ketamine application; the rates of response and remission after one day were 44% and 31%, respectively.
D-cycloserine
As discussed above, maintaining an initial clinical response after ketamine infusion can be challenging. One study analyzed the efficacy of D-cycloserine, a partial agonist of the glycine co-agonist binding site of N-methyl-D-aspartate receptors, as an augmentation treatment in patients with TRD (17 with MDD and 15 with bipolar depression) who initially responded to ketamine. During the 6-week treatment, total HAMD scores did not significantly differ between the D-cycloserine and placebo groups. The results remained consistent when stratified by diagnosis. A mixed model analysis indicated that the D-cycloserine group had lower HAMD scores for item 3 (suicide) than the placebo group throughout the follow-up period (p = 0.01). The superior anti-suicidal effect of ketamine in the D-cycloserine group was maintained, which suggests that D-cycloserine may be therapeutically beneficial for patients with TRD who have a good initial response to ketamine infusion.35
Minocycline and aspirin
Savitz et al.36 tested the efficacy of aspirin and minocycline as an augmentation therapy for bipolar depression. Ninety-nine outpatients with BD were enrolled in a six week, double-blind, placebo-controlled trial and were randomized to one of four groups: active minocycline + active aspirin, active minocycline + placebo aspirin, placebo minocycline + active aspirin, and placebo minocycline + placebo aspirin. The primary outcome was treatment response, which was defined as a decrease of at least 50% in total MADRS score. When all four arms were included in the statistical analysis, the main effect of aspirin on treatment response was driven by the active minocycline + active aspirin and the placebo-minocycline + active aspirin arms (p = 0.019, OR = 3.67, NNT = 4). Additionally, there was a significant 3-way interaction between aspirin, minocycline, and IL-6, indicating that the minocycline response was significantly greater in participants in the minocycline group with higher IL-6 concentrations. Further, participants in the minocycline group who responded to treatment had significantly greater decreases in IL-6 levels between baseline and visit seven vs. non-responders. Neither aspirin nor minocycline had a significant main effect for mean change in MADRS score across visits.
Pioglitazone
Selective agonists of the nuclear transcription factor PPAR-γ, which are also called thiazolidinediones or glitazones, have anti-inflammatory and insulin-sensitizing properties and are widely used to treat type 2 diabetes mellitus.37 An 8-week, double-blind, randomized, placebo-controlled trial assessed pioglitazone’s efficacy in treating bipolar depression, although not necessarily TRBD. Thirty-eight outpatients with BD in a current depressive episode were randomized to pioglitazone (15-45 mg/day) or placebo. The primary outcome measure was the change in total 30-item Inventory of Depressive Symptomatology, Clinician Rated scores from baseline to endpoint. The mean score reduction from baseline to week 8 was -6.59 for pioglitazone and -11.63 for placebo. Mixed-effects modeling showed a borderline significant difference between the two groups (p = 0.056) in favor of placebo. A meta-analysis of pioglitazone as a depression treatment indicated that it has more significant benefits for remission than placebo in patients diagnosed with MDD, as opposed to BD (27% vs. 10%, p = 0.008).38
N-acetylcysteine
N-acetylcysteine is a precursor of reduced glutathione, which has important antioxidant properties in the brain.39 Studies have shown that, by regulating glutathione levels, N-acetylcysteine can decrease the oxidative stress caused by reactive oxygen species.40,41 A meta-analysis42 assessed the efficacy of N-acetylcysteine for depressive symptoms in subjects with psychiatric conditions, including TRBD. Five studies were included, providing data on 574 participants, of whom 291 were randomized to receive N-acetylcysteine and 283 to placebo. Two studies included participants with BD and current depressive symptoms. N-acetylcysteine improved depressive symptoms (according to MADRS and HDRS scores) compared to placebo (standardized mean difference [SMD] = 0.37; 95%CI 0.19-0.55; p < 0.001); participants receiving N-acetylcysteine had better depressive symptom scores on the Clinical Global Impressions- Severity of Illness scale at follow-up than the placebo group (SMD = 0.22; 95%CI 0.03-0.41; p < 0.001).
Angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor blockers
The renin-angiotensin system has been proposed as a new target for depression, both in bipolar and unipolar depression, including TRBD.43 In a retrospective sample of 836 men without a history of depression enrolled in the Geelong Osteoporosis Study, 80 (9.6%) were exposed to angiotensin-converting enzyme inhibitors. No one in this group had a new depressive episode, whereas 40 (5.3%) of the 756 non-exposed participants had a new episode of depression.44
Celecoxib
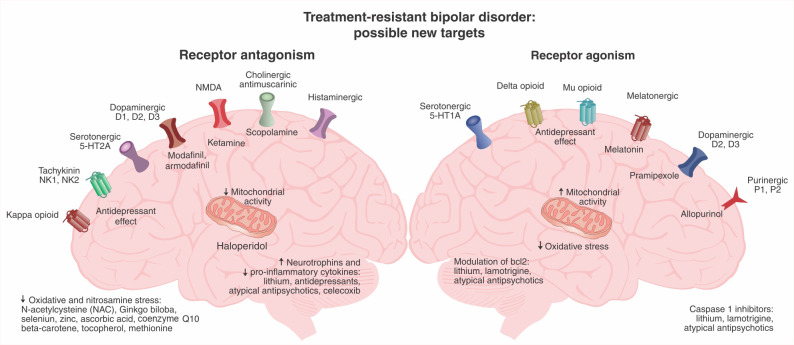
Celecoxib is a cyclooxygenase 2 inhibitor. Halaris et al. investigated the potential of its anti-inflammatory properties as a treatment for TRBD,45 analyzing data from 47 participants with BD who were using escitalopram and were randomized to celecoxib or placebo. The participants were also using a mood stabilizer (other than lithium) and/or an atypical antipsychotic. Treatment-resistance was defined in this study according to the Maudsley Staging Method, and most participants were classified as having moderate to severe treatment-resistance.46 The results showed that adjunctive celecoxib had clear benefits in comparison with placebo: the odds for response and remission rates were 4.13 (95%CI 1.03-18.48; p = 0.02) and 14.34 (95%CI 2.59-153.17; p < 0.001), respectively. Figure 1 provides some potential targets for pharmacological interventions.47
Figure 1. Potential biological targets for novel pharmacological interventions in the treatment of treatment-resistant bipolar disorder (adapted from Dodd et al. with permission)47.
Non-pharmacological strategies: non-invasive treatments
Sleep deprivation and light therapy
One study48 assessed the efficacy of total sleep deprivation with sleep phase advance in TRD, including participants with both unipolar and bipolar depression who were already on antidepressants and mood-stabilizers. The intervention lasted four days and included one night of total sleep deprivation and three consecutive nights with sleep phase advance. Ten participants (50%) fulfilled the criteria for response, defined as a decrease of more than 50% in HDRS score on the 14th day of the intervention. Another study investigated a combination of sleep deprivation and light therapy, called combined chronotherapy, in patients with TRD and its implementation in daily clinical practice.49 The sample comprised 26 individuals with bipolar or unipolar depression who received combined chronotherapy, i.e. three nights of sleep deprivation with alternating recovery nights, light therapy, and continued antidepressant medication. The primary outcome, Inventory of Depressive Symptoms (IDS-C) scores, were determined before chronotherapy and at weeks 1, 2, and 4. The mean scores at pre-treatment, week 2, and week 4 were 39.3±9.6, 28.4±10.2, and 28.6±14.0, respectively. The overall response rate was 34.6% and the remission rate 19.2%. However, this study was open-label and did not include a control group.
Magnetic seizure therapy
One study assessed the efficacy and safety of magnetic seizure therapy for TRBD, a novel intervention still limited to investigation at specialized centers.50 In this open-label trial, 26 patients with TRBD were treated with magnetic seizure therapy for up to 24 sessions or until remission. The primary outcome, total HDRS score, significantly decreased. Adequate trial completers had a remission rate of 23.1% and a response rate of 38.5%. Per-protocol completers had a remission rate of 30% and a response rate of 50%.
Intermittent theta-burst stimulation
One small trial investigated the safety and efficacy of neuronavigated intermittent theta-burst stimulation in people with TRBD; 26 patients were randomly allocated to receive either active (n=12) or sham (n=14) stimulation. According to depression and MADRS score changes, the response and remission rates were high following active stimulation (72% and 42%, respectively). Nevertheless, the differences between active treatment and sham stimulation were not significant (42% and 25%).51
Deep transcranial magnetic stimulation
Tavares et al. investigated a modality of transcranial magnetic stimulation (TMS), deep TMS, for patients with TRBD.52 Their criterion for TRBD was failure to achieve remission with two or more interventions approved as first, second, or third-line therapies for BD according to The Canadian Network for Mood and Anxiety Treatments guidelines. They found that deep TMS was superior to sham in decreasing depressive symptoms at weeks 4 and 6, despite no differences regarding response (48% vs. 24%, respectively, at week 4; 32% vs. 24%, respectively, at week 8) or remission (28% vs. 16%, respectively, at week 4; 24% vs. 24%, respectively, at week 8). Another study assessed the efficacy of deep TMS for reducing depressive symptoms in people with treatment-resistant BD or MDD, as well as whether continuing deep TMS after the course of treatment was completed had any influence on sustained remission. After the deep TMS treatment sessions were completed, a significant reduction in mean HDRS score was observed (from 23 to 9). In addition, those who received additional deep TMS sessions as a maintenance treatment had sustained remission at 12-months of follow-up, which did not occur in those who did not received maintenance sessions.53
Non-pharmacological strategies: invasive treatments
Vagus nerve stimulation therapy
A case series described the outcomes of 25 patients with TRBD who were included in acute and long-term early studies of vagus nerve stimulation therapy (VNS) as a treatment for depression.54 The authors reported that the antidepressant efficacy outcomes for these TRBD patients were similar to those of unipolar TRD patients.
The Treatment-Resistant Depression Registry is a long-term, prospective, multicenter, open-label, non-randomized, longitudinal, naturalistic study assessing clinical course and outcomes over five years in two large cohorts of patients with TRD. Registry patients received either treatment as usual or treatment as usual with adjunctive VNS. It was hypothesized that the VNS arm would have better clinical outcomes (long-term depression and mortality rates) than the treatment-as-usual arm.55 The population included 795 patients (494 patients in the VNS arm and 301 patients in the treatment-as-usual arm). About 27% (n=134) of the patients in the VNS arm and 24% (n=71) of the patients in the treatment-as-usual arm were diagnosed with BD type I or II. The mean number of failed depression treatments at baseline was 8.2 in the VNS arm and 7.3 in the treatment-as-usual arm, and the mean lifetime number of attempted suicides was 1.8 in the VNS arm and 1.2 in the treatment-as-usual arm. The response rate was considered the primary outcome, and there was a statistically significant difference between the VNS arm and the treatment-as-usual arm over the 5-year follow-up period (cumulative response rates, 67.6%, 95%CI 63.4-71.7 and 40.9%, 95%CI 35.4-47.1, respectively, p < 0.001). Remission was considered a secondary outcome, based on a total MADRS score < 9 during follow-up. Patients in the VNS arm were significantly more likely to experience remission than those in the treatment-as-usual arm (43.3%, 95%CI 38.9-47.7 and 25.7%, 95%CI 20.7-31.1, respectively, p < 0.001).55
Deep brain stimulation
Deep brain stimulation (DBS) is a neurosurgical technique in which high-frequency stimulation electrodes are placed subcortically or in deep cortical regions to target neural circuits, whose dysfunction is associated with neuropsychiatric manifestations.56 DBS is approved for treating dystonia, treatment-resistant Parkinson's disease, and severe epilepsy and has been investigated as for its role in the management of psychiatric disorders such as MDD,57 obsessive-compulsive disorder,58 and anorexia nervosa.59 In an open-label trial, Holtzheimer et al. reported that DBS had similar efficacy in the bilateral subcallosal cingulate white matter of patients with treatment-resistant unipolar (n=10) and bipolar (n=7) depression, with the latter reporting more lifetime episodes of depression and psychotropic medication use. No patients presented hypomania or mania, and there were no significant changes in hypomanic/manic symptoms during the study, whose primary outcome was measured at 24 weeks of active DBS treatment.60 These authors reported findings from 8 years of follow up, and a sustained treatment response occurred in most of the patients with TRBD.61 Ramasubbu et al. compared long vs. short pulse width subcallosal cingulate stimulation in a sample of 22 individuals (17 with unipolar TRD), five of whom had TRBD. Three (60%) of these responded to treatment during the six-month follow-up.62 Although few studies have assessed DBS as a treatment for patients with TRBD, the available results suggest efficacy. No severe adverse effects have been reported within the follow-up period, which encourages further investigation.63 Box 1 provides a summary of some potential strategies for TRBD and their mechanisms of action or stimulation sites.
Box 1 Potential pharmacological and non-pharmacological strategies for treatment-resistant bipolar depression.
| Type of intervention | Mechanism of action or regions of stimulation |
|---|---|
| Pharmacological | |
| Aripiprazole | Partial agonist of D2, D3, D4, 5-HT1A, 5-HT2C, and 5-HT7 receptors31 |
| D-cycloserine | Partial agonist at the glycine recognition of the NMDA receptor35 |
| Ketamine | NMDA receptor antagonist34 |
| Lurasidone | Antagonist of 5-HT2A, 5-HT7, and D2 receptors, agonist of 5-HT1A receptor28 |
| AT1R blocker | Modulator of the renin-angiotensin system43 |
| Minocycline | Anti-microbial with anti-inflammatory and neuroprotective properties36 |
| Pioglitazone | Selective PPAR-γ agonist with anti-inflammatory properties38 |
| N-acetylcysteine | Decreases the oxidative stress caused by ROS through glutathione regulation42 |
| Non-pharmacological | |
| Non-invasive | |
| Deep TMS | Over the left dorsolateral PFC52 |
| iTBS | Over the left dorsolateral PFC51 |
| Magnetic seizure therapy | Over the bilateral PFC or the vertex50 |
| Invasive | |
| DBS | Bilateral subcallosal cingulate white matter60 |
| VNS therapy | Vagus nerve55 |
AT1R = angiotensin II type 1 receptor; DBS = deep brain stimulation; iTBS = intermittent theta-burst stimulation; NMDA = N-methyl-D-aspartate; PFC = prefrontal cortex; PPAR-γ = peroxisome proliferator-activated receptor-gamma; ROS = reactive oxygen species; TMS = transcranial direct-current stimulation; VNS = vagus nerve stimulation.
Precision psychiatry and treatment-resistant bipolar depression
According to DSM criteria, a major depressive episode is a highly heterogeneous clinical concept. Several distinct phenotype combinations are possible in depression, and they could express different etiological and pathophysiological aspects. Identifying neurobiologically-based biotypes and considering environmental and developmental influences could help determine more precise and personalized treatments.64,65 This approach may reduce the response delay and the prolonged suffering associated with a “trial and error” strategy. Rajpurkar et al. evaluated whether machine learning models based on clinical symptoms of depression and electroencephalographic data could predict treatment response to antidepressants. They reported that, by considering most of the evaluated symptoms and the relevance of pre-treatment EEG data, machine learning had satisfactory discriminative performance for treatment response.66 Drysdale et al. used neuroimaging biomarkers defined by resting-state connectivity to identify biotypes capable of predicting treatment response for depression with repetitive TMS. For instance, 82.5% of the individuals categorized as biotype 1 presented a significant improvement to repetitive TMS, compared to 25% of those with biotype 2, even though these two biotypes were associated with similar fatigue and anergia symptomatology.67 Based on a study of patients with a clinical history of psychosis, the Bipolar-Schizophrenia Network for Intermediate Phenotypes study investigated biomarkers that discriminate subgroups with similar biological characteristics.68 Using neuropsychological and sensorimotor reactivity data, Clementz et al. found biotypes that did not match a DSM-based diagnosis. They showed distinct associations with social functioning measures and the frequency of relatives with a history of psychosis.68 They argued that, depending on the biotype, treatments that target specific cognitive dysfunctions would be more efficacious in correcting reduced neural activation or neuronal hyperexcitability.68 With the advance of precision psychiatry and the identification of clinical-neurobiological-based biotypes, the current concept of TRD may be reframed. Moreover, it is expected that more precise and novel strategies for known and new targets could help reduce the burden of TRD in patients with BD.
Conclusion
In 1973, the World Congress of Psychiatry organized a symposium called “Therapy Resistant Depression.”69 Some of the issues discussed at that meeting were the definition, clinical classification, and therapeutic differential criteria of TRD, and its potential association with biochemical and genetic characteristics.69,70 These discussions are still current almost fifty years later, despite a better understanding of the neurobiological mechanisms underlying psychiatric disorders.71,72 The interventions discussed in this article are promising leads for TRD treatment. Considering that all these studies have addressed depression as it is currently classified, clinical trials with more homogenous samples, both in terms of clinical and biological characteristics, with appropriate designs, could optimize intervention efficacy.64
Disclosure
JQ has received clinical research support from LivaNova; has speaker bureau membership with Myriad Neuroscience, Janssen Pharmaceuticals, and Abbvie; has served as a consultant for Eurofarma; is stockholder at Instituto de Neurociencias Dr. Joao Quevedo; and receives copyrights from Artmed Editora, Artmed Panamericana, and Elsevier/Academic Press. JCS has received research grants from Compass, Alkermes, and Allergan; and has served as a consultant for Pfizer, Sunovion, Sanofi, Johnson & Johnson, Livanova, and Boehringer Ingelheim. The other authors report no conflicts of interest.
Acknowledgements
APD is supported by a 2020 NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation.
Footnotes
How to cite this article: Diaz AP, Fernandes BS, Quevedo J, Sanches M, Soares JC. Treatment-resistant bipolar depression: concepts and challenges for novel interventions. Braz J Psychiatry. 2022;44:178-186. http://dx.doi.org/10.1590/1516-4446-2020-1627
References
- 1.Baldessarini RJ, Vazquez GH, Tondo L. Bipolar depression: a major unsolved challenge. Int J Bipolar Disord. 2020;8:1. doi: 10.1186/s40345-019-0160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mendlewicz J, Massat I, Linotte S, Kasper S, Konstantinidis A, Lecrubier Y, et al. Identification of clinical factors associated with resistance to antidepressants in bipolar depression: results from an European Multicentre Study. Int Clin Psychopharmacol. 2010;25:297–301. doi: 10.1097/YIC.0b013e32833c4ceb. [DOI] [PubMed] [Google Scholar]
- 3.Fornaro M, Fusco A, Novello S, Mosca P, Anastasia A, De Blasio A, et al. Predictors of treatment resistance across different clinical subtypes of depression: comparison of unipolar vs. bipolar cases. Front Psychiatry. 2020;11:438. doi: 10.3389/fpsyt.2020.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fornaro M, Carvalho AF, Fusco A, Anastasia A, Solmi M, Berk M, et al. The concept and management of acute episodes of treatment-resistant bipolar disorder: a systematic review and exploratory meta-analysis of randomized controlled trials. J Affect Disord. 2020;276:970–83. doi: 10.1016/j.jad.2020.07.109. [DOI] [PubMed] [Google Scholar]
- 5.Demyttenaere K, Van Duppen Z. The impact of (the concept of) treatment-resistant depression: an opinion review. Int J Neuropsychopharmacol. 2019;22:85–92. doi: 10.1093/ijnp/pyy052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaynes BN, Lux L, Gartlehner G, Asher G, Forman-Hoffman V, Green J, et al. Defining treatment-resistant depression. Depress Anxiety. 2020;37:134–45. doi: 10.1002/da.22968. [DOI] [PubMed] [Google Scholar]
- 7.Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Bond DJ, Frey BN, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20:97–170. doi: 10.1111/bdi.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hidalgo-Mazzei D, Berk M, Cipriani A, Cleare AJ, Di Florio A, Dietch D, et al. Treatment-resistant and multi-therapy-resistant criteria for bipolar depression: consensus definition. Br J Psychiatry. 2019;214:27–35. doi: 10.1192/bjp.2018.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pacchiarotti I, Mazzarini L, Colom F, Sanchez-Moreno J, Girardi P, Kotzalidis GD, et al. Treatment-resistant bipolar depression: towards a new definition. Acta Psychiatr Scand. 2009;120:429–40. doi: 10.1111/j.1600-0447.2009.01471.x. [DOI] [PubMed] [Google Scholar]
- 10.Kessler U, Schoeyen HK, Andreassen OA, Eide GE, Hammar A, Malt UF, et al. Neurocognitive profiles in treatment-resistant bipolar I and bipolar II disorder depression. BMC Psychiatry. 2013;13:105. doi: 10.1186/1471-244X-13-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scaini G, Valvassori SS, Diaz AP, Lima CN, Benevenuto D, Fries GR, et al. Neurobiology of bipolar disorders: a review of genetic components, signaling pathways, biochemical changes, and neuroimaging findings. Braz J Psychiatry. 2020;42:536–51. doi: 10.1590/1516-4446-2019-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diaz AP, Bauer IE, Sanches M, Soares JC. Neuroanatomic and functional neuroimaging findings. In: Young AH, Juruena MF, editors. Current topics in behavioral neurosciences bipolar disorder: from neuroscience to treatment. Berlin/Heidelberg: Springer; 2020. pp. 1–24. [Google Scholar]
- 13.Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171:829–43. doi: 10.1176/appi.ajp.2014.13081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vieta E, Berk M, Schulze TG, Carvalho AF, Suppes T, Calabrese JR, et al. Bipolar disorders. Nat Rev Dis Primers. 2018;4:18008. doi: 10.1038/nrdp.2018.8. [DOI] [PubMed] [Google Scholar]
- 15.Bartova L, Dold M, Kautzky A, Fabbri C, Spies M, Serretti A, et al. Results of the European Group for the Study of Resistant Depression (GSRD) - basis for further research and clinical practice. World J Biol Psychiatry. 2019;20:427–48. doi: 10.1080/15622975.2019.1635270. [DOI] [PubMed] [Google Scholar]
- 16.Schosser A, Serretti A, Souery D, Mendlewicz J, Zohar J, Montgomery S, et al. European Group for the Study of Resistant Depression (GSRD)--where have we gone so far: review of clinical and genetic findings. Eur Neuropsychopharmacol. 2012;22:453–68. doi: 10.1016/j.euroneuro.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Clark SL, Hattab MW, Chan RF, Shabalin AA, Han LK, Zhao M, et al. A methylation study of long-term depression risk. Mol Psychiatry. 2020;25:1334–43. doi: 10.1038/s41380-019-0516-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang C, Wardenaar KJ, Bosker FJ, Li J, Schoevers RA. Inflammatory markers and treatment outcome in treatment resistant depression: a systematic review. J Affect Disord. 2019;257:640–9. doi: 10.1016/j.jad.2019.07.045. [DOI] [PubMed] [Google Scholar]
- 19.Carvalho LA, Torre JP, Papadopoulos AS, Poon L, Juruena MF, Markopoulou K, et al. Lack of clinical therapeutic benefit of antidepressants is associated overall activation of the inflammatory system. J Affect Disord. 2013;148:136–40. doi: 10.1016/j.jad.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 20.Fabbri C, Kasper S, Zohar J, Souery D, Montgomery S, Albani D, et al. Drug repositioning for treatment-resistant depression: hypotheses from a pharmacogenomic study. Prog Neuropsychopharmacol Biol Psychiatry. 2021;104:110050. doi: 10.1016/j.pnpbp.2020.110050. [DOI] [PubMed] [Google Scholar]
- 21.Du J, Machado-Vieira R, Maeng S, Martinowich K, Manji HK, Zarate CA., Jr Enhancing AMPA to NMDA throughput as a convergent mechanism for antidepressant action. Drug Discov Today Ther Strateg. 2006;3:519–26. doi: 10.1016/j.ddstr.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadriu B, Musazzi L, Henter ID, Graves M, Popoli M, Zarate CA., Jr Glutamatergic neurotransmission: pathway to developing novel rapid-acting antidepressant treatments. Int J Neuropsychopharmacol. 2019;22:119–35. doi: 10.1093/ijnp/pyy094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadriu B, Farmer CA, Yuan P, Park LT, Deng ZD, Moaddel R, et al. The kynurenine pathway and bipolar disorder: intersection of the monoaminergic and glutamatergic systems and immune response. Mol Psychiatry. 2019 Nov 15 doi: 10.1038/s41380-019-0589-8. doi: . Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Downar J, Geraci J, Salomons TV, Dunlop K, Wheeler S, McAndrews MP, et al. Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry. 2014;76:176–85. doi: 10.1016/j.biopsych.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 25.Riva-Posse P, Choi KS, Holtzheimer PE, McIntyre CC, Gross RE, Chaturvedi A, et al. Defining critical white matter pathways mediating successful subcallosal cingulate deep brain stimulation for treatment-resistant depression. Biol Psychiatry. 2014;76:963–9. doi: 10.1016/j.biopsych.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nierenberg AA, Ostacher MJ, Calabrese JR, Ketter TA, Marangell LB, Miklowitz DJ, et al. Treatment-resistant bipolar depression: a STEP-BD equipoise randomized effectiveness trial of antidepressant augmentation with lamotrigine, inositol, or risperidone. Am J Psychiatry. 2006;163:210–6. doi: 10.1176/appi.ajp.163.2.210. [DOI] [PubMed] [Google Scholar]
- 27.Ishibashi T, Horisawa T, Tokuda K, Ishiyama T, Ogasa M, Tagashira R, et al. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther. 2010;334:171–81. doi: 10.1124/jpet.110.167346. [DOI] [PubMed] [Google Scholar]
- 28.Loebel A, Cucchiaro J, Silva R, Kroger H, Sarma K, Xu J, et al. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171:169–77. doi: 10.1176/appi.ajp.2013.13070985. [DOI] [PubMed] [Google Scholar]
- 29.Schaffer CB, Schaffer LC, Nordahl TE, Stark NM, Gohring CE. An open trial of lurasidone as an acute and maintenance adjunctive treatment for outpatients with treatment-resistant bipolar disorder. J Clin Psychopharmacol. 2016;36:522–3. doi: 10.1097/JCP.0000000000000567. [DOI] [PubMed] [Google Scholar]
- 30.Cuomo A, Crescenzi BB, Goracci A, Bolognesi S, Giordano N, Rossi R, et al. Drug safety evaluation of aripiprazole in bipolar disorder. Expert Opin Drug Saf. 2019;18:455–63. doi: 10.1080/14740338.2019.1617847. [DOI] [PubMed] [Google Scholar]
- 31.Sugawara H, Sakamoto K, Harada T, Shimizu S, Ishigooka J. A retrospective study of predictive factors for effective aripiprazole augmentation of antidepressant therapy in treatment-resistant depression. Neuropsychiatr Dis Treat. 2016;12:1151–6. doi: 10.2147/NDT.S104115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bahji A, Ermacora D, Stephenson C, Hawken ER, Vazquez G. Comparative efficacy and tolerability of pharmacological treatments for the treatment of acute bipolar depression: a systematic review and network meta-analysis. J Affect Disord. 2020;269:154–84. doi: 10.1016/j.jad.2020.03.030. [DOI] [PubMed] [Google Scholar]
- 33.Zhuo C, Ji F, Tian H, Wang L, Jia F, Jiang D, et al. Transient effects of multi-infusion ketamine augmentation on treatment-resistant depressive symptoms in patients with treatment-resistant bipolar depression - an open-label three-week pilot study. Brain Behav. 2020;10:e01674. doi: 10.1002/brb3.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen MH, Cheng CM, Gueorguieva R, Lin WC, Li CT, Hong CJ, et al. Maintenance of antidepressant and antisuicidal effects by D-cycloserine among patients with treatment-resistant depression who responded to low-dose ketamine infusion: a double-blind randomized placebo-control study. Neuropsychopharmacology. 2019;44:2112–8. doi: 10.1038/s41386-019-0480-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savitz JB, Teague TK, Misaki M, Macaluso M, Wurfel BE, Meyer M, et al. Treatment of bipolar depression with minocycline and/or aspirin: an adaptive, 2x2 double-blind, randomized, placebo-controlled, phase IIA clinical trial. Transl Psychiatry. 2018;8:27. doi: 10.1038/s41398-017-0073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Consoli A, Formoso G. Do thiazolidinediones still have a role in treatment of type 2 diabetes mellitus? Diabetes Obes Metab. 2013;15:967–77. doi: 10.1111/dom.12101. [DOI] [PubMed] [Google Scholar]
- 38.Aftab A, Kemp DE, Ganocy SJ, Schinagle M, Conroy C, Brownrigg B, et al. Double-blind, placebo-controlled trial of pioglitazone for bipolar depression. J Affect Disord. 2019;245:957–64. doi: 10.1016/j.jad.2018.11.090. [DOI] [PubMed] [Google Scholar]
- 39.Shahripour RB, Harrigan MR, Alexandrov AV. N-acetylcysteine (NAC) in neurological disorders: mechanisms of action and therapeutic opportunities. Brain Behav. 2014;4:108–22. doi: 10.1002/brb3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibson KR, Neilson IL, Barrett F, Winterburn TJ, Sharma S, MacRury SM, et al. Evaluation of the antioxidant properties of N-acetylcysteine in human platelets: prerequisite for bioconversion to glutathione for antioxidant and antiplatelet activity. J Cardiovasc Pharmacol. 2009;54:319–26. doi: 10.1097/FJC.0b013e3181b6e77b. [DOI] [PubMed] [Google Scholar]
- 41.Al-Samhari MM, Al-Rasheed NM, Al-Rejaie S, Al-Rasheed NM, Hasan IH, Mahmoud AM, et al. Possible involvement of the JAK/STAT signaling pathway in N-acetylcysteine-mediated antidepressant-like effects. Exp Biol Med (Maywood) 2016;241:509–18. doi: 10.1177/1535370215619707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandes BS, Dean OM, Dodd S, Malhi GS, Berk M. N-Acetylcysteine in depressive symptoms and functionality: a systematic review and meta-analysis. J Clin Psychiatry. 2016;77:e457–66. doi: 10.4088/JCP.15r09984. [DOI] [PubMed] [Google Scholar]
- 43.Vian J, Pereira C, Chavarria V, Kohler C, Stubbs B, Quevedo J, et al. The renin-angiotensin system: a possible new target for depression. BMC Med. 2017;15:144. doi: 10.1186/s12916-017-0916-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams LJ, Pasco JA, Kessing LV, Quirk SE, Fernandes BS, Berk M. Angiotensin converting enzyme inhibitors and risk of mood disorders. Psychother Psychosom. 2016;85:250–2. doi: 10.1159/000444646. [DOI] [PubMed] [Google Scholar]
- 45.Halaris A, Cantos A, Johnson K, Hakimi M, Sinacore J. Modulation of the inflammatory response benefits treatment-resistant bipolar depression: a randomized clinical trial. J Affect Disord. 2020;261:145–52. doi: 10.1016/j.jad.2019.10.021. [DOI] [PubMed] [Google Scholar]
- 46.Fekadu A, Wooderson S, Donaldson C, Markopoulou K, Masterson B, Poon L, et al. A multidimensional tool to quantify treatment resistance in depression: the Maudsley staging method. J Clin Psychiatry. 2009;70:177–84. doi: 10.4088/jcp.08m04309. [DOI] [PubMed] [Google Scholar]
- 47.Dodd S, Fernandes BS, Dean OM. Future directions for pharmacotherapies for treatment-resistant bipolar disorder. Curr Neuropharmacol. 2015;13:656–62. doi: 10.2174/1570159X13666150630175841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kurczewska E, Ferensztajn-Rochowiak E, Jasinska-Mikolajczyk A, Chlopocka-Wozniak M, Rybakowski JK. Augmentation of pharmacotherapy by sleep deprivation with sleep phase advance in treatment-resistant depression. Pharmacopsychiatry. 2019;52:186–92. doi: 10.1055/a-0695-9138. [DOI] [PubMed] [Google Scholar]
- 49.Sikkens D, Riemersma-Van der Lek RF, Meesters Y, Schoevers RA, Haarman BC. Combined sleep deprivation and light therapy: clinical treatment outcomes in patients with complex unipolar and bipolar depression. J Affect Disord. 2019;246:727–30. doi: 10.1016/j.jad.2018.12.117. [DOI] [PubMed] [Google Scholar]
- 50.Tang VM, Blumberger DM, Dimitrova J, Throop A, McClintock SM, Voineskos D, et al. Magnetic seizure therapy is efficacious and well tolerated for treatment-resistant bipolar depression: an open-label clinical trial. J Psychiatry Neurosci. 2020;45:313–21. doi: 10.1503/jpn.190098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bulteau S, Beynel L, Marendaz C, Dall'Igna G, Pere M, Harquel S, et al. Twice-daily neuronavigated intermittent theta burst stimulation for bipolar depression: a randomized sham-controlled pilot study. Neurophysiol Clin. 2019;49:371–5. doi: 10.1016/j.neucli.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 52.Tavares DF, Myczkowski ML, Alberto RL, Valiengo L, Rios RM, Gordon P, et al. Treatment of bipolar depression with deep tms: results from a double-blind, randomized, parallel group, sham-controlled clinical trial. Neuropsychopharmacology. 2017;42:2593–601. doi: 10.1038/npp.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rapinesi C, Bersani FS, Kotzalidis GD, Imperatori C, Del Casale A, Di Pietro S, et al. Maintenance deep transcranial magnetic stimulation sessions are associated with reduced depressive relapses in patients with unipolar or bipolar depression. Front Neurol. 2015;6:16. doi: 10.3389/fneur.2015.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nierenberg AA, Alpert JE, Gardner-Schuster EE, Seay S, Mischoulon D. Vagus nerve stimulation: 2-year outcomes for bipolar versus unipolar treatment-resistant depression. Biol Psychiatry. 2008;64:455–60. doi: 10.1016/j.biopsych.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 55.Aaronson ST, Sears P, Ruvuna F, Bunker M, Conway CR, Dougherty DD, et al. A 5-year observational study of patients with treatment-resistant depression treated with vagus nerve stimulation or treatment as usual: comparison of response, remission, and suicidality. Am J Psychiatry. 2017;174:640–8. doi: 10.1176/appi.ajp.2017.16010034. [DOI] [PubMed] [Google Scholar]
- 56.Clair AH, Haynes W, Mallet L. Recent advances in deep brain stimulation in psychiatric disorders. F1000Res. 2018;7 doi: 10.12688/f1000research.14187.1. F1000 Faculty Rev-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fenoy AJ, Schulz PE, Selvaraj S, Burrows CL, Zunta-Soares G, Durkin K, et al. A longitudinal study on deep brain stimulation of the medial forebrain bundle for treatment-resistant depression. Transl Psychiatry. 2018;8:111. doi: 10.1038/s41398-018-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haber SN, Yendiki A, Jbabdi S. Four deep brain stimulation targets for obsessive-compulsive disorder: are they different? Biol Psychiatry. 2020 Jul 25:S0006-3223(20)31773-X. doi: 10.1016/j.biopsych.2020.06.031. doi: . Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martinez GV, Justicia A, Salgado P, Gines JM, Guardiola R, Cedron C, et al. A randomized trial of deep brain stimulation to the subcallosal cingulate and nucleus accumbens in patients with treatment-refractory, chronic, and severe anorexia nervosa: initial results at 6 months of follow up. J Clin Med. 2020;9:1946. doi: 10.3390/jcm9061946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holtzheimer PE, Kelley ME, Gross RE, Filkowski MM, Garlow SJ, Barrocas A, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Arch Gen Psychiatry. 2012;69:150–8. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crowell AL, Riva-Posse P, Holtzheimer PE, Garlow SJ, Kelley ME, Gross RE, et al. Long-term outcomes of subcallosal cingulate deep brain stimulation for treatment-resistant depression. Am J Psychiatry. 2019;176:949–56. doi: 10.1176/appi.ajp.2019.18121427. [DOI] [PubMed] [Google Scholar]
- 62.Ramasubbu R, Clark DL, Golding S, Dobson KS, Mackie A, Haffenden A, et al. Long versus short pulse width subcallosal cingulate stimulation for treatment-resistant depression: a randomised, double-blind, crossover trial. Lancet Psychiatry. 2020;7:29–40. doi: 10.1016/S2215-0366(19)30415-8. [DOI] [PubMed] [Google Scholar]
- 63.Gippert SM, Switala C, Bewernick BH, Kayser S, Brauer A, Coenen VA, et al. Deep brain stimulation for bipolar disorder-review and outlook. CNS Spectr. 2017;22:254–7. doi: 10.1017/S1092852915000577. [DOI] [PubMed] [Google Scholar]
- 64.Fernandes BS, Williams LM, Steiner J, Leboyer M, Carvalho AF, Berk M. The new field of ‘precision psychiatry'. BMC Med. 2017;15:80. doi: 10.1186/s12916-017-0849-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Passos IC, Ballester PL, Barros RC, Librenza-Garcia D, Mwangi B, Birmaher B, et al. Machine learning and big data analytics in bipolar disorder: a position paper from the International Society for Bipolar Disorders Big Data Task Force. Bipolar Disord. 2019;21:582–94. doi: 10.1111/bdi.12828. [DOI] [PubMed] [Google Scholar]
- 66.Rajpurkar P, Yang J, Dass N, Vale V, Keller AS, Irvin J, et al. Evaluation of a machine learning model based on pretreatment symptoms and electroencephalographic features to predict outcomes of antidepressant treatment in adults with depression: a prespecified secondary analysis of a randomized clinical trial. JAMA Netw Open. 2020;3:e206653. doi: 10.1001/jamanetworkopen.2020.6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173:373–84. doi: 10.1176/appi.ajp.2015.14091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hippius H, Jungkunz G. Some general remarks to the problem of therapy resistant depression. In: Pichot P, Berner P, Wolf R, Than K, editors. Psychiatry: the state of the art. 3rd ed. New York/London:: Plennum; 1983. [Google Scholar]
- 70.Freyhan RA. Treatment-resistant or untreatable? Compr Psychiatry. 1978;19:97–101. doi: 10.1016/0010-440x(78)90052-4. [DOI] [PubMed] [Google Scholar]
- 71.Deisseroth K. Optogenetics and psychiatry: applications, challenges, and opportunities. Biol Psychiatry. 2012;71:1030–2. doi: 10.1016/j.biopsych.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 72.Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry. 2016;3:472–80. doi: 10.1016/S2215-0366(15)00579-9. [DOI] [PMC free article] [PubMed] [Google Scholar]