Abstract
A screening technique for integrons in members of the family Enterobacteriaceae and nonfermenting gram-negative bacteria by real-time PCR is reported. A total of 226 isolates of gram-negative bacteria obtained from a variety of clinical specimens were screened for class 1 integrons by real-time PCR performed on a LightCycler instrument. This technique used a primer pair specific for a 300-bp conserved region at the 5′ ends of class 1 integrons. The screening assay was evaluated by comparison with results obtained by the conventional, thermal-block PCR (long PCR) by using established conditions and primers for the detection of class 1 integrons, and the real-time PCR technique was thus shown to be both sensitive and specific. DNA from 50 of 226 (22%) isolates screened was identified as containing an integron by the screening PCR, and sequence data were obtained across the integron for 34 of 50 (68%) of these isolates. In an attempt to study the molecular epidemiology of antimicrobial resistance genes carried within integrons, a comparison of the types of gene cassettes carried by isolates from different patients was made. Adenyltransferase genes conferring resistance to streptomycin and spectinomycin were the predominant gene cassettes amplified in the study. Resistance to trimethoprim was also frequently found to be encoded within integrons. Furthermore, multiple bacterial isolates obtained from one patient over a 5-month period were all shown to carry an integron containing the same single adenyltransferase gene cassette, suggesting that these elements were relatively stable in this case.
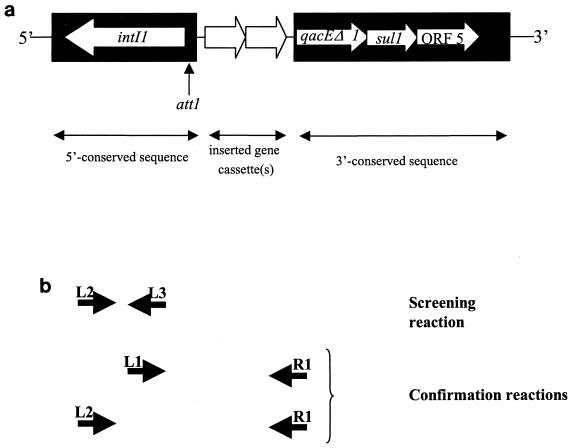
Integrons are potentially mobile genetic elements frequently located on transposons and have been identified as loci at which site-specific incorporation and excision of gene cassettes frequently occur (22). A number of reports have been published detailing the basic genetic structure of these elements, which comprise conserved sequences flanking a variable region that may contain inserted gene cassettes (32). Within the conserved sequence 5′ to any inserted gene cassettes there are located the genes intI1 (encoding DNA integrase) and attI, at which recombination occurs (9). Within the conserved segment 3′ to any inserted gene cassettes there are genes encoding resistance to sulfonamides (sulI) and disinfectants (qacEΔ1) (19) and also a gene of unknown function (ORF5) (32) (Fig. 1a). Almost all of the integrons characterized to date contain gene cassettes coding for resistance to antimicrobial agents, but gene cassettes encoding unknown functions (and provisionally termed “open reading frames”) have also been reported (20). Since promoters within the 5′ conserved region of integrons ensure that integrons act as natural expression vectors for any gene cassettes inserted in the correct orientation (32), the role of integrons in the carriage and dissemination of antimicrobial resistance genes is all too apparent (8, 11, 33).
FIG. 1.
(a) Genetic map of a class 1 integron (adapted from reference 25). The filled areas are the conserved regions at the 5′ and 3′ ends of the integron. The open arrows between the conserved regions represent the inserted gene cassettes (two are illustrated here). The intI1 gene and the bulk of the att1 site used for recombination are located within the 5′ conserved region. The genes qacEΔ1 (a defective quaternary ammonium cation that encodes resistance to disinfectants), sul1 (which encodes sulfonamide resistance), and ORF5 (open reading frame 5) are encoded within the 3′ conserved sequence. (b) Schematic map indicating primer annealing sites for the primers used in the screening reaction (primers L2 and L3) and confirmatory reactions (primers L1, L2, and R1). Primers L1 and R1 anneal at positions 1190 to 1206 and 1342 to 1326, respectively, in the published sequence of integron In0 (GenBank accession number M73819) (4). In the same sequence, L2 anneals at positions 910 to 926 and L3 anneals at positions 1206 to 1190.
With the increasing problem of resistance to a wide spectrum of antimicrobial agents in an increasing number of bacterial isolates (18), both in the hospital and in community settings, the dissemination of antimicrobial resistance genes between organisms and patients is of great concern. Consequently, it is important to recognize the ability of gene cassettes to excise from one integron and insert into another integron within another bacterium of the same or different species or for a given integron within a transposon to transfer between organisms. Such genetic transfer is of particular significance when the new host is a pathogenic organism and the inserted gene confers resistance to a widely used antimicrobial agent.
One of the most rapid PCR machines currently available, the LightCycler instrument, was used in this study to screen for the presence of integrons. The LightCycler instrument uses forced-air heating within the reaction vessel chamber to ensure highly uniform temperatures with rapid temperature transitions. Adapted glass capillary tubes with a high surface area-to-volume ratio are used as reaction vessels, permitting very small reaction volumes (1 to 10 μl) to be tested (36). Together, these features allow 30-cycle reactions to be performed in less than 10 min. Product accumulation may be monitored cycle by cycle by fluorimetric detection of binding of SYBR Green 1, a double-stranded DNA (ds DNA)-specific dye. Following the amplification reaction, the nature of the product is verified by determination of the melting temperature by measuring the decrease in the level of binding of the SYBR Green 1 dye with increasing temperature.
In this study, 226 clinical isolates of the family Enterobacteriaceae and nonfermenting gram-negative bacteria from a variety of specimens received in this laboratory were screened for the presence of integrons by real-time PCR developed for the LightCycler instrument. When present, integrons including the inserted gene cassette(s) were sequenced, and their molecular epidemiology within a mixed hospital and community population was described. The stability over time of these elements in sequential isolates from one patient is also reported.
MATERIALS AND METHODS
Bacteria.
Three groups of bacteria were isolated from three patient groups. Group A consisted of all 173 gram-negative bacteria isolated during the 8-week period from 19 March 1999 to 15 May 1999 from inpatients at Addenbrooke's Hospital, Cambridge, United Kingdom. All patients were located on wards identified as being highly dependent on antimicrobial chemotherapy: the intensive care unit, the hematological unit, and the hepatic and renal transplant unit.
Group B comprised 33 multiply drug-resistant Escherichia coli isolates obtained from patients at Addenbrooke's Hospital or in the community in the Cambridge area during the week commencing 12 December 1998. Multiply drug-resistant isolates were defined as those reported to be resistant to more than two of the antimicrobial agents routinely included in susceptibility tests in this laboratory. Susceptibility to ampicillin, amoxicillin-clavulanate potassium, azlocillin, cefotaxime, ceftazidime, cephalexin, ciprofloxacin, colistin, gentamicin, meropenem, norfloxacin, and trimethoprim was determined by the breakpoint technique (37) on Iso-Sensitest agar (Oxoid, Basingstoke, United Kingdom). The control strains E. coli NCTC 10418 and Pseudomonas aeruginosa NCTC 10662 were included in each test.
Group C comprised sequential isolates from three patients from whom gram-negative bacteria had been isolated in more than one blood culture. Furthermore, the isolates from each patient were multiply drug resistant, and the resistance pattern altered between sequential isolates. Twenty isolates were obtained from these patients.
The bacterial isolates were identified by standard laboratory procedures (3).
DNA extraction.
Bacteria were grown overnight at 37°C in the absence of antimicrobial agents on cystine–lactose–electrolyte-deficient agar (Oxoid) plates. The bacteria were then treated by the following protocol prior to PCR analysis. A single colony was emulsified in 200 μl of RNase-free water and heated in a boiling water bath for 10 min. The suspension was centrifuged at 11,000 × g for 2 min in a benchtop Eppendorf centrifuge, and the supernatant was stored at −20°C until subsequent PCR analysis. DNA was extracted from the supernatant by the technique of Boom et al. (5) when multiple, nonspecific amplification products were obtained in the PCR.
Initially, a proportion of the isolates was also grown overnight in brain heart infusion broth in the absence of antimicrobial agents, and a comparison was made between the DNA extracted from the broth and agar cultures. Broth cultures were diluted to approximately 106 CFU ml−1 and heated in a boiling water bath for 10 min. DNA was subsequently extracted from 100-μl aliquots of the diluted broth cultures by the protocol of Boom et al. (5). The tubes containing the extracted DNA were stored at −20°C for subsequent PCR analysis. Since there was no apparent advantage in using broth cultures (data not shown), subsequent isolates were grown only on solid medium.
A 100-μl aliquot of diluted broth culture (containing DNA from approximately 105 CFU) or a 50-μl aliquot of supernatant obtained from the isolates grown on agar only (containing DNA from approximately 106 CFU) was used in the extraction done by the technique of Boom et al. (5), with 50 μl of diatomaceous silica (Sigma, Poole, United Kingdom) used as the matrix. The purified DNA was eluted in 100 μl of RNase-free water and stored at −20°C until subsequent analysis by PCR.
Oligonucleotide primers.
The forward and reverse oligonucleotide primers used in the LightCycler (Bio/Gene Ltd., Cambridge, United Kingdom) reaction were L2 (5′-GAC GAT GCG TGG AGA CC-3′) (25) and L3 (5′-CTT GCT GCT TGG ATG CC-3′), respectively. L3 is the reverse complement of the previously published L1 primer (13). The oligonucleotide primers used in the “long PCR” were L1 (5′-GGC ATC CAA GCA GCA AG-3′) (13) or L2 with antisense primer R1 (5′-AAG CAG ACT TGA CCT GA-3′) (13, 25). The oligonucleotides were obtained as a lyophilized purified product from Oswel Research Products, Southampton, United Kingdom, and were diluted to working concentrations with RNase-free water. The positions to which these primers are complementary are shown schematically in Fig. 1b.
Screening PCR with the LightCycler instrument.
The reaction mixture contained 5 μl of 3 mM MgCl2 LC master mix (Bio/Gene Ltd.), 0.5 μl of SYBR Green 1 dye (Bio/Gene Ltd.) diluted according to the manufacturer's instructions, 0.5 μl of TaqStart antibodies (diluted according to the manufacturer's instructions; Sigma), 0.5 μl of RNase-free water, L2 and L3 primers (6.25 pmol of each primer), and 3 μl of bacterial DNA template. The reaction consisted of denaturation at 94°C for 3 min, followed by an amplification protocol of 30 cycles of denaturation at 94°C for 2 s, annealing at 54°C for 2 s, and elongation at 72°C for 8 s. Real-time accumulation of dsDNA was detected by fluorimetry at the end of each elongation step. The nature of the amplification product was determined by melting the PCR product over a temperature range from 70 to 97°C, with a transition rate of 0.1°C s−1, and continuous detection of the fluorescence of the dsDNA. The melting temperatures of the reaction products were compared with that of a positive control. The size of the product was between 295 and 300 bp, and this was verified by agarose gel electrophoresis.
Screening PCR adapted for use in conventional block-based thermal cyclers.
The screening reaction was prepared with 1 μl of TaqStart antibody, diluted according to the manufacturer's instructions, with 1 U of Taq per reaction mixture. The enzyme-antibody mixture was allowed to bind at room temperature for 10 to 20 min before the addition of the other reactants. The final reaction mixture consisted of 5 μl of DNA template, 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 2 mM MgCl2, each deoxynucleotide triphosphate (Boehringer Mannheim, Lewes, United Kingdom), at a concentration of 50 μM, 25 pmol each of the L2 and L3 oligonucleotide primers, and the Taq-Taqstart antibody mixture. The final volume was made up to 50 μl with RNase-free water. After denaturation at 94°C for 4 min, 40 thermal cycles, each consisting of 96°C for 1 min, 48°C for 1.5 min, and 72°C for 1.5 min, were performed, followed by a final extension at 72°C for 10 min.
Conventional (thermal block) long PCR.
The reaction mixture was prepared by using 2.4 μl of TaqStart antibody, diluted according to manufacturer's instructions, with 2.4 U of Taq per reaction mixture. These were allowed to bind as described above before the remaining reactants were added. The final reaction mixture consisted of 8 μl of the DNA template, 20 mM Tris HCl (pH 8.4), 50 mM KCl, 2.5 mM MgCl2, each deoxynucleotide triphosphate at a concentration of 200 μM, and 20 pmol each of L1 and R1 oligonucleotide primers added to the Taq-TaqStart antibody mixture. The final volume was made up to 40 μl with RNase-free water. After denaturation at 94°C for 5 min, 35 temperature cycles each consisting of 94°C for 1 min, 54°C for 1 min, and 72°C for 5 min with an extension of 5 s/cycle were followed by a final extension at 72°C for 10 min. The reaction mixture for amplification with the L2 and R1 oligonucleotide primers was identical to that described for amplification with the L1 and R1 primers, except that the annealing temperature of the thermal cycling protocol was 58°C. The amplification products were detected by gel electrophoresis of 10 μl of the reaction mixture in agarose gels (1% molecular biology-grade agarose [Oswel Research Products] in 1× TBE [Tris-borate-EDTA]) containing ethidium bromide (4 μg ml−1).
Cloning.
When the sequence data obtained directly from the PCR amplification products were of poor quality, PCR products were cloned prior to sequencing of the DNA. PCR products were concentrated by ethanol precipitation prior to cloning directly into competent E. coli with the TA Original Cloning kit (Invitrogen, Groningen, The Netherlands). A single transformant from each cloning reaction was screened for the presence of the required insert by the PCR described above.
DNA sequencing reactions.
PCR products were purified prior to sequencing by using QIAQuick purification columns (QIAgen Ltd., Crawley, United Kingdom). Purified PCR amplification products were eluted in 10 μl of RNase-free water. Approximately 50 ng of the purified product was sequenced in both directions with the same primers used in the thermal block-based PCR by using either the ABI Prism dye terminator or ABI Prism BigDye terminator cycle sequencing ready reaction kit (Perkin-Elmer Applied Biosystems, Warrington, United Kingdom). The extension products obtained in the sequencing reactions were purified by ethanol precipitation with sodium acetate. All procedures were performed according to the manufacturers' instructions.
Sequence analysis.
Products from the sequencing reactions were sent to the Sequencing Facility at the School of Biological Sciences, University of Durham, Durham, United Kingdom, for separation by polyacrylamide gel electrophoresis and establishment of the primary sequence. Sequence data, obtained in an electronic format, were analyzed with the DNAStar software package (DNAStar Inc., Madison, Wis.). Consensus nucleotide sequences were compared with those available in the GenBank database by using the Basic Local Alignment Search Tool (BLAST2) (1). Consensus sequences were subsequently compared with those in GenBank which were identified as showing significant alignment in the BLAST searches by using the MegAlign program in the DNAStar package. This uses the Clustal method (10) for multiple sequence alignments.
Positive and negative controls.
DNA extracted from Salmonella enterica serovar Typhimurium, which is positive for the presence of two integrons (1.0 and 1.1 kb) (22) and which was a kind gift from E. J. Threlfall, and plasmids pLQ29, pLQ161, pLQ200, pLQ820, and pLQ860, which contain integrons of various lengths (from 100 bp to 3 kb) (12, 15, 24, 27, 38) and which were kind gifts from P. H. Roy, were included as positive controls. RNase-free water was included as a negative control.
RESULTS
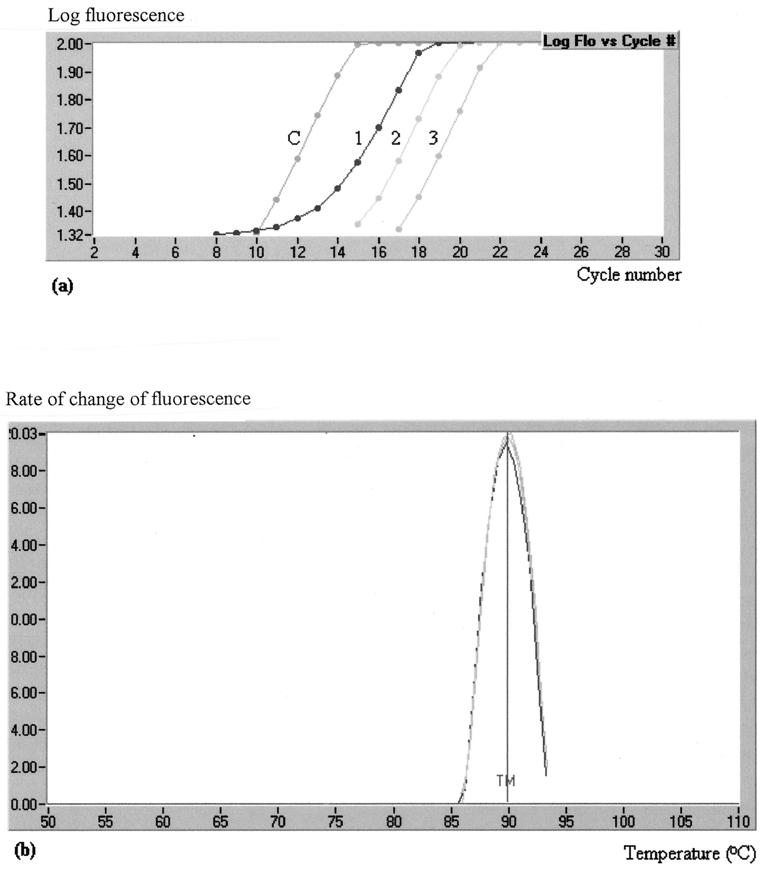
DNA from the 226 bacterial isolates was screened for the presence of integrons by real-time PCR. By using primers to the 5′ conserved region of class 1 integrons, L2 (25) and L3 (Fig. 1b), 50 isolates were identified as being positive for these genetic elements. All products thus obtained were shown to have a melting point of 91.5 ± 1°C, identical to that obtained for the positive control analyzed in each assay run (Fig. 2).
FIG. 2.
(a) Accumulation of products in the rapid real-time screening reaction with the L2-L3 primer pair, as monitored by fluorimetry for three specimens (1, 2, and 3) and one control strain (C). The cycle number at which product accumulation is initially detected is dependent on the amount of template in the reaction mixture. (b) The melting temperature (TM; 91.5°C) is shown for the products from these three specimens and the positive control and is plotted as the rate of change of fluoresence (y axis; arbitrary units) against temperature (x axis). The resulting curves for all four samples are virtually identical. Flo, fluorescence.
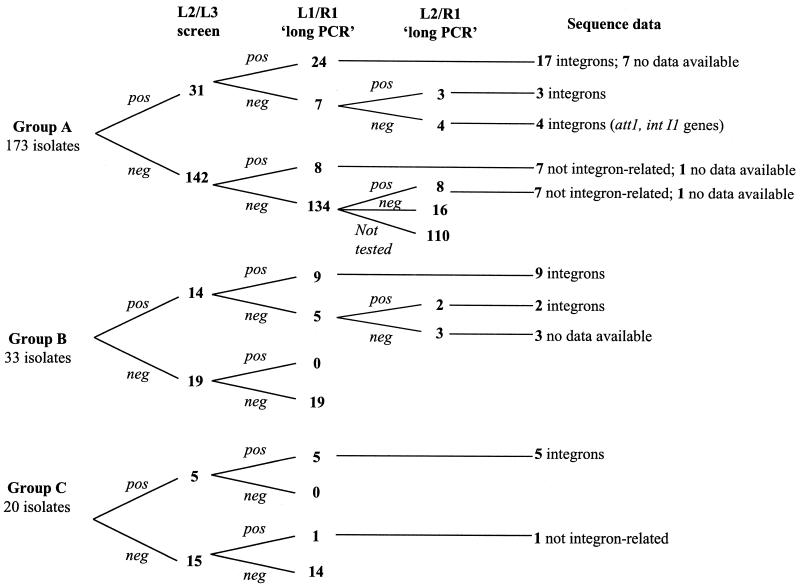
Confirmation of the presence of integrons was sought by conventional thermal block-based long PCR technology. DNA from all 226 bacterial isolates screened in the LightCycler instrument was examined. PCR with the L1-R1 primer pair (Fig. 1b) produced detectable amplification products in 47 of 226 reactions, of which 38 had previously been positive by the LightCycler reaction (Fig. 3). DNA from a selection of isolates, including those 12 isolates which were positive for integrons by the screening reaction but which were negative by the long PCR were subjected to a second long PCR with the L2-R1 (25) primer combination (Fig. 1b). This reaction gave a positive result for 5 of 12 isolates which were positive by the screening reaction and also for 8 isolates which were negative by both the screening reaction and the first long PCR with the L1-R1 primer pair (Fig. 3).
FIG. 3.
Relationship between results obtained in the screening reaction with the L2-L3 primer pair and in each of the two long PCRs (with the L1-R1 and L2-R1 primer pairs). For some isolates no sequence data were available for analysis because either the data obtained were of inadequate quality or insufficient PCR product was available for sequencing. PCR products from the screening reactions were obtained from the seven screening reaction-positive, long PCR-negative isolates. Adequate sequence data were obtained for four of these isolates, and analysis identified the amplified region as being the same as that of the att1 and intI1 genes within the 5′ conserved sequence of class 1 integrons. pos, positive result; neg, negative result.
The nature of all amplification products obtained in the reactions with either the L1-R1 or L2-R1 primer pair was determined by DNA sequencing. DNA from 15 of 17 isolates that yielded a positive reaction by the long PCR (with the L1-R1 or L2-R1 primer pair) but that scored negative by the screening reaction yielded sequences which failed to show any homology with that of other integron sequence data available in GenBank. Sequence data of sufficiently high quality for analysis were unobtainable for the remaining two amplification products.
Integrons were detected by the screening reaction with L2-L3 primer pair in DNA from seven isolates with which long PCR failed to detect any integrons. DNA from these isolates was subjected to amplification with the screening primers (primers L2 and L3) in an analogous reaction adapted for use on a conventional thermal cycler. Sufficient product for sequencing was obtained from four isolates, and analysis of the sequence data indicated a high degree of identity (>99%) with the corresponding region of a class 1 integron.
Gene cassette sequence data were obtained for 34 of the isolates for which both screening and long PCR gave positive results. Integron amplification products ranged in size from 850 bp (containing a single gene cassette) to 3,000 bp (in which multiple gene cassettes were inserted). All the integrons for which adequate sequencing data were obtained contained at least one gene cassette. A single gene cassette was present in integrons from 17 isolates, and more than one gene cassette was identified in integrons from 15 isolates (Table 1).
TABLE 1.
Summary of gene cassettes present in identified integrons
| Cassettec | No. of isolates containing an integrona
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Group A
|
Group B, E. colib | Group C
|
Total | |||||
| E. coli | Citrobacter freundii | Enterobacter spp. | Klebsiella spp. | E. coli | Agrobacterium radiobacter | |||
| aadA or aadA1(a) | 10 | 3 (H) | 13 | |||||
| aadA6 | 1 | 1 | 1 | 3 | ||||
| dhfrVII | 1 (G) | 1 | ||||||
| dhfrI-aadA1(a) | 3 (G) | 4 | 7 | |||||
| dhfrI-aadA3 | 1 | 1 | ||||||
| dhfrA17-aadA5 | 2 | 2 (G) | 4 | |||||
| Gene-aadA | 1 (G) | 1 | ||||||
| aadB-gene-aadA | 1 | 1 | ||||||
| aadB-gene-cmlA | 1 | 1 | ||||||
| Unidentified peptide | 1 | 1 (G) | 2 | |||||
| Data not available | 4 | 1 | 2 | 2 | 3 (G) | 12 | ||
| intI1 and promoters | 3 | 1 | 4 | |||||
The total numbers of isolates for groups A, B, and C were 31, 14, and 5, respectively.
G, the isolates were from general-practice patients; H, the isolates were from hospitalized patients.
“Gene” indicates the presence of a gene for which sequence data of insufficient quality for analysis were obtained.
Several sequences indicative of known resistance genes were identified. Aminoglycoside adenyltransferase genes (aadA), which confer resistance to streptomycin and spectinomycin, were the most frequently found gene cassettes and were detected in 30 of 34 of the isolates for which integron sequence data were obtained. The carriage of an aadA gene as a single gene cassette within an integron occurred in 16 of these isolates.
Gene cassettes within integrons from 13 strains encoded trimethoprim resistance (dhfr) genes. Of these, the integrons from 12 isolates contained the dhfr gene together with an aadA gene cassette, and the integron from only 1 isolate contained a dhfr gene as a single gene insert. In those integrons that contained dhfr and aadA resistance genes, the dhfr gene was, without exception, 5′ to the aadA gene.
Amplification of integrons from 12 isolates produced insufficient product for DNA sequencing, and inadequate sequence data were obtained for the 5′ end of the integron from another isolate. The sequences obtained for gene cassettes amplified from a further two isolates showed no appreciable identity with genes held in the GenBank database and thus have been labeled “unidentified gene cassettes” flanked by confirmed integron sequences.
Of the 39 integrons found in the hospital isolates (of which 31 were from group A, 3 were from the 14 identified in group B, and 5 were from group C), no sequencing data were available for 9 integrons. An unidentified gene cassette was found in one integron, and sequence data for only the intI1 gene were obtained for four integrons. Of the 25 integrons characterized, 7 contained a dhfr gene cassette, 24 contained an aadA gene cassette, 2 contained an aadB gene cassette, and 1 contained a cmlA gene cassette that encoded resistance to chloramphenicol.
Of the 33 isolates in group B identified as containing an integron, 30 were obtained from general-practice patients, and of these, 11 were found to harbor an integron. Of these, five contained a dhfr gene cassette upstream of an aadA gene cassette, one contained a single gene cassette (dhfr), and one contained an unidentified polypeptide. Sequence data of inadequate quality for analysis were obtained for the remaining three isolates.
E. coli was isolated over a 5-month period (from 8 December 1998 to 5 May 1999) from three wound swabs and seven urine specimens from one patient (patient X), a long-term patient in the transplant unit. During this period the patient had received various antimicrobial treatment regimens including the following agents: meropenem, vancomycin, ciprofloxacin, gentamicin, erythromycin, and co-trimoxazole. All isolates from this patient contained an integron of 1,000 bp, and sequence analysis identified a single aadA gene in each. There were between 97.5 and 100% identities between the aadA sequences of the isolates (data not shown).
From 11 sets of blood for culture taken over a 2-month period from patient Y, a long-term patient in the hematology unit, there were 11 isolates of Stenotrophomonas maltophilia, 1 isolate each of Burkholderia cepacia, Acinetobacter lwoffii, and Agrobacterium radiobacter, and 2 unidentified nonfermenting gram-negative bacilli. Only the A. radiobacter isolate carried a class 1 integron, and the inserted gene showed the greatest identity (ca. 64%) with adenyltransferase resistance gene aadA6 (16).
DISCUSSION
Recently, there have been a number of reports of the use of PCR techniques to determine the presence of integrons and the nature of any inserted gene (12, 22, 25, 29). However, the unpredictable nature of the amplification products, both in length and in DNA sequence, renders optimization of the PCR conditions difficult. At best, the conditions are optimized for a specific product, and other amplification products may be produced under suboptimal conditions. In the study described here we used an optimized, rapid, real-time PCR for the detection of class 1 integrons in members of the family Enterobacteriaceae and nonfermenting gram-negative bacilli.
The screening reaction, which amplifies a short, conserved sequence at the 5′ ends of integrons, was shown to be highly specific. The sensitivity for the detection of integrons was higher than that achieved by conventional long PCR methods. The L2 (25) sense primer anneals to a site within the intI1 gene, and antisense primer L3, complementary to the previously published sequence of L1 (13), anneals to the integrase promoter. The 295- to 300-bp products obtained from this reaction are highly conserved between class 1 integrons, and consequently, this reaction is easy to optimize. In the present study, 226 clinical isolates of the family Enterobacteriaceae and nonfermenting gram-negative bacilli were screened by this technique, and 50 (22%) gave positive results. The results obtained by the screening assay were compared with those obtained by the conventional long PCR assay. When the long PCR was performed with these isolates with primers to the 5′ (primer L1 or L2) and 3′ (primer R1) conserved regions of integrons, 60 of 226 (27%) yielded a positive reaction.
Discrepancies between the rapid and conventional reactions were investigated by sequencing of the PCR products from the conventional reaction in order to determine whether the products were indeed from integrons. Of the 60 isolates found to be positive by the long PCR, 43 (72%) were also positive by the screening reaction, and DNA sequencing data from these long products were consistent with the presence of antimicrobial resistance genes encoded within an integron for 34 isolates. DNA from the remaining nine isolates gave data of insufficient quality, precluding sequence analysis. The amplification products obtained by the long PCR for the 17 isolates that gave a negative result by the screening assay were also subjected to DNA sequencing. Sequence data of a quality adequate for evaluation were obtained for 14 of these isolates, and when the sequences were compared with those available in the GenBank database, none of these showed any identity with integron sequences. It is therefore concluded that the long PCR is prone to nonspecific amplifications, probably as a result of the large amounts of bacterial template DNA present at the start of the reaction, which may facilitate nonspecific annealing of the primers, and as a result of suboptimal PCR conditions that allow nonspecific amplification.
The LightCycler screening reaction yielded positive results for seven isolates that were negative by the long PCR (with both the L1-R1 and L2-R1 primer pairs). The DNA from these isolates was thus amplified with the L2-L3 primer pair by the PCR adapted for use in conventional block-based thermal cyclers. The PCR products obtained in this way were subjected to DNA sequencing. Data adequate for sequence analysis were obtained from four isolates. On alignment of the sequences thus obtained with GenBank sequences for class 1 integrons, a high degree of identity (>99%) was seen, and these isolates were deemed to contain an integron. It is possible that they failed to give a positive result by the long PCRs as a result of point mutations at the R1 (antisense) primer annealing site.
From the sequence data obtained, the prevalence and nature of class 1 integrons containing antibiotic resistance genes in bacteria from selected hospitalized patients and from patients in the community were compared. The predominance of gene cassettes that encode resistance to streptomycin and spectinomycin (aadA-type genes) is unexpected, as these drugs have not been in widespread clinical use in the United Kingdom for over 20 years. However, this finding is in agreement with reports from other laboratories, where streptomycin resistance genes have frequently been observed in both integrons and other genetic elements (6, 14). Streptomycin-resistant bacteria can be isolated from animals (22, 26), probably as a result of the use of streptomycin and spectinomycin in animal husbandry (31). Humans may become colonized with streptomycin-resistant bacteria by contact with animals or via the food chain (7, 35). The high rates of carriage of trimethoprim resistance gene cassettes may be due to the fact that this antimicrobial agent is commonly used as a first-line treatment for urinary tract infections in community medicine.
The frequent occurrence of integrons containing both aadA and dhfr genes may reflect a common origin for all these integrons, and the presence of one of these genes, e.g., aadA, inserted into an integron may increase the probability of integration of specific gene cassettes, such as dhfr, into the same integron. This may also account for the consistent order of these two genes within the integrons studied.
The potential for integrons to undergo site-specific integration of gene cassettes would suggest that there may be some instability in these elements, especially in the absence of any selective pressure from antimicrobial agents. Indeed, several previous studies of integrons have suggested this (21, 30). It was therefore of interest to compare integrons of sequential isolates obtained from the same patient. The high degree of identity (97.5 to 100%) between the 10 integron sequences from patient X suggests a high degree of stability of these genetic elements during the 5-month period over which these isolates were obtained. The identification of only 1 bacterial isolate containing an integron among 16 bacterial isolates from 11 specimens from long-term patient Y suggested that integron-containing and integron-free gram-negative bacteria can coexist in a host without movement of the integrons (or gene cassettes) between strains.
The albeit limited data presented here suggest that these genetic elements were infrequently lost or gained by bacteria and that they did not readily take up other gene cassettes, even under selective pressure from antimicrobial therapy. Furthermore, when more than one bacterial species was isolated from the same patient, the presence of an integron in more than one species was not observed, suggesting that integron transfer between bacteria may not be as common as was previously thought. These findings are similar to those of Martinez-Freijo et al. (14). In the current work, support for transfer of the complete element containing integrons (14) was derived from the fact that a single isolate of E. coli from a patient in the community harbored an integron that carried a dhfrVII gene with 99% identity with that previously identified in Shigella flexneri (17), although the gene that encoded resistance to trimethoprim (dhfrVII) was originally identified in E. coli (2). The integron identified in the present work probably originated from the same source as that reported for S. flexneri, and it is possible that it moved between species as a whole element, for example, as part of a transposon, rather than by transfer of gene cassettes. Since an organism which carries an integron will probably retain the integron and its gene cassettes, screening for and identification of these genetic elements by probing or sequencing may be useful in the monitoring of cross-infections.
The occurrence of gene cassettes that encode unidentified polypeptides, as determined by sequencing of integrons, has been observed by other groups (9, 23, 34), who reported that integrons, although commonly found to carry antimicrobial resistance genes, occasionally carry cassettes which are open reading frames that do not encode a specific gene, or at least not a gene identified to date.
In conclusion, the rapid screening assay for the presence of integrons is more specific and sensitive for the detection of integrons than the long PCR protocols reported previously. For the screening of large numbers of isolates by this technique, the long PCR may be reserved for the characterization of integrons carried by isolates identified as harboring these genetic elements in the screening reaction. By using this screening method, cross-infection with integron-carrying bacteria within clinical units was not observed during the period of the present study, as isolates obtained from several patients within the same ward at the same time did not contain the same integrons, nor, indeed, was there more than one patient from whom an integron was isolated on any ward at the same time. Integrons from sequential isolates of the family Enterobacteriaceae and nonfermenting gram-negative bacilli have not been examined previously. Studies have concentrated on, for example, blood culture isolates within a given hospital (28), all isolates of a given species of bacteria (29), or several bacteria from widely different geographical locations (14).
ACKNOWLEDGMENTS
We thank E. J. Threlfall, Central Public Health Laboratory, London, United Kingdom, for the donation of a positive control strain of S. enterica serovar Typhimurium, and P. H. Roy, Centre de Recherche du CHUL, Quebec, Quebec, Canada, for the donation of positive control plasmids pLQ29, pLQ161, pLQ200, pLQ820, and pLQ860.
The work was supported by a Public Health Laboratory Service training grant.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, W. M E, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Amyes S G B, Towner K J, Carter G I, Thomason C J, Young H-K. The type VII dihydrofolate reductase: a novel plasmid-encoded trimethoprim-resistant enzyme from gram-negative bacteria isolated in Britain. J Antimicrob Chemother. 1989;24:111–119. doi: 10.1093/jac/24.2.111. [DOI] [PubMed] [Google Scholar]
- 3.Barrow G, Feltham R, editors. Cowan and Steel's manual for the identification of medical bacteria. 3rd ed. Cambridge, United Kingdom: Cambridge University Press; 1993. [Google Scholar]
- 4.Bissonnette L, Roy P H. Characterization of In0 of Pseudomonas aeruginosa plasmid pVS1, an ancestor of integrons of multiresistance plasmids and transposons of gram-negative bacteria. J Bacteriol. 1992;174:1248–1257. doi: 10.1128/jb.174.4.1248-1257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boom R, Sol C, Salismans M, Jansen C, Wertheim-van-Dillen P, van der Noordaa J. Rapid and simple method for the purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiew Y F, Yeo S F, Hall L M, Livermore D M. Can susceptibility to an antimicrobial be restored by halting its use? The case of streptomycin versus Enterobacteriaceae. J Antimicrob Chemother. 1998;41:247–251. doi: 10.1093/jac/41.2.247. [DOI] [PubMed] [Google Scholar]
- 7.Collignon P. Vancomycin-resistant enterococci and use of avoparcin in animal feed: is there a link? Med J Aust. 1999;171:144–146. doi: 10.5694/j.1326-5377.1999.tb123568.x. [DOI] [PubMed] [Google Scholar]
- 8.Hall R M. Mobile gene cassettes and integrons: moving antibiotic resistance genes in gram-negative bacteria. Ciba Found Symp. 1997;207:192–202. doi: 10.1002/9780470515358.ch12. [DOI] [PubMed] [Google Scholar]
- 9.Hall R M, Brookes D E, Stokes H W. Site-specific insertion of genes into integrons: role of the 59-base element and determination of the recombination cross-over point. Mol Microbiol. 1991;5:1941–1959. doi: 10.1111/j.1365-2958.1991.tb00817.x. [DOI] [PubMed] [Google Scholar]
- 10.Higgins D, Bleasby A, Fuchs R. Clustal V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992;8:189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- 11.Jones M, Peters E, Weersink A, Fluit A, Verhoef J. Widespread occurrence of integrons causing multiple antibiotic resistance in bacteria. Lancet. 1997;349:1742–1743. doi: 10.1016/S0140-6736(05)62954-6. [DOI] [PubMed] [Google Scholar]
- 12.Levesque C, Piche L, Larose C, Roy P H. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob Agents Chemother. 1995;39:185–191. doi: 10.1128/aac.39.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levesque C, Roy P. PCR analysis of integrons. In: Persing D H, Smith T F, Tenover F C, White T J, editors. Diagnostic molecular microbiology: principles and applications. Washington, D.C.: American Society for Microbiology; 1993. pp. 590–594. [Google Scholar]
- 14.Martinez-Freijo P, Fluit A C, Schmitz F J, Verhoef J, Jones M E. Many class 1 integrons comprise distinct stable structures occurring in different species of Enterobacteriaceae isolated from widespread geographic regions in Europe. Antimicrob Agents Chemother. 1999;43:686–689. doi: 10.1128/aac.43.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer J, Nies B, Weidemann B. Amikacin resistance mediated by multiresistance transposon Tn2424. J Bacteriol. 1983;155:755–760. doi: 10.1128/jb.155.2.755-760.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naas T, Sougakoff W, Casetta A, Nordmann P. Molecular characterization of OXA-20, a novel class D β-lactamase, and its integron from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:2074–2083. doi: 10.1128/aac.42.8.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navia M M, Capitano L, Ruiz J, Vargas M, Urassa H, Schellemberg D, Gascon J, Vila J. Typing and characterization of resistance of Shigella spp. isolated from feces of children under 5 years of age from Ifakara, Tanzania. J Clin Microbiol. 1999;37:3113–3117. doi: 10.1128/jcm.37.10.3113-3117.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neu H C. The crisis in antibiotic resistance. Science. 1992;257:1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 19.Paulsen I T, Littlejohn T G, Radstrom P, Sundstrom L, Skold O, Swedberg G, Skurray R A. The 3′ conserved segment of integrons contains a gene associated with multidrug resistance to antiseptics and disinfectants. Antimicrob Agents Chemother. 1993;37:761–768. doi: 10.1128/aac.37.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Recchia G D, Hall R M. Plasmid evolution by acquisition of mobile gene cassettes: plasmid pIE723 contains the aadB gene cassette precisely inserted at a secondary site in the incQ plasmid RSF1010. Mol Microbiol. 1995;15:179–187. doi: 10.1111/j.1365-2958.1995.tb02232.x. [DOI] [PubMed] [Google Scholar]
- 21.Recchia G D, Stokes H W, Hall R M. Characterisation of specific and secondary recombination sites recognised by the integron DNA integrase. Nucleic Acids Res. 1994;22:2071–2078. doi: 10.1093/nar/22.11.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ridley A, Threlfall E J. Molecular epidemiology of antibiotic resistance genes in multiresistant epidemic Salmonella typhimurium DT 104. Microb Drug Resist. 1998;4:113–118. doi: 10.1089/mdr.1998.4.113. [DOI] [PubMed] [Google Scholar]
- 23.Rosser S, Young H-K. Identification and characterization of class 1 integrons in bacteria from an aquatic environment. J Antimicrob Chemother. 1999;44:11–18. doi: 10.1093/jac/44.1.11. [DOI] [PubMed] [Google Scholar]
- 24.Rubens C, McNeill W, Farrar W. Transposable plasmid deoxyribonucleic acid sequence in Pseudomonas aeruginosa which mediates resistance to gentamicin and four other antimicrobial agents. J Bacteriol. 1979;139:877–882. doi: 10.1128/jb.139.3.877-882.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sallen B, Rajoharison A, Desvarenne S, Mabilat C. Molecular epidemiology of integron-associated antibiotic resistance genes in clinical isolates of Enterobacteriaceae. Microb Drug Resist. 1995;1:195–202. doi: 10.1089/mdr.1995.1.195. [DOI] [PubMed] [Google Scholar]
- 26.Sandvang D, Aarestrup F M, Jensen L B. Characterisation of integrons and antibiotic resistance genes in Danish multiresistant Salmonella enterica Typhimurium DT104. FEMS Microbiol Lett. 1998;160:37–41. doi: 10.1111/j.1574-6968.1998.tb12887.x. [DOI] [PubMed] [Google Scholar]
- 27.Scavizzi M. Nouveaux groupes d'incompatibilite des plasmides. Interet dans les epidemies de creche a Eschericia coli O11:B4. Ann Microbiol (Institut Pasteur) 1973;124B:153–167. [Google Scholar]
- 28.Schmitz F-J, Martinez-Freijo P, Theis S, Fluit A C, Verhoef J, Heinz A-P, Jones M E. Class 1 integrons: prevalence and impact on antibiotic susceptibility in 278 consecutive unrelated gram-negative blood isolates. Clin Microbiol Infect. 1999;5:496–498. doi: 10.1111/j.1469-0691.1999.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 29.Seward R J, Lambert T, Towner K J. Molecular epidemiology of aminoglycoside resistance in Acinetobacter spp. J Med Microbiol. 1998;47:455–462. doi: 10.1099/00222615-47-5-455. [DOI] [PubMed] [Google Scholar]
- 30.Seward R J, Towner K J. Detection of integrons in worldwide nosocomial isolates of Acinetobacter spp. Clin Microbiol Infect. 1999;5:308–318. doi: 10.1111/j.1469-0691.1999.tb00149.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith H, Tucker F. The effect of antibiotic therapy on the faceal excretion of Salmonella typhimurium by experimentally infected chickens. J Hyg. 1975;57:275–292. doi: 10.1017/s0022172400047306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stokes H, Hall R. A novel family of potentially mobile DNA elements encoding site-specific gene-integration functions: integrons. Mol Microbiol. 1989;3:1669–1683. doi: 10.1111/j.1365-2958.1989.tb00153.x. [DOI] [PubMed] [Google Scholar]
- 33.Sundstrom L. The potential of integrons and connected programmed rearrangements for mediating horizontal gene transfer. APMIS Suppl. 1998;84:37–42. doi: 10.1111/j.1600-0463.1998.tb05646.x. [DOI] [PubMed] [Google Scholar]
- 34.Sundstrom L, Skold O. The dhfrI trimethoprim resistance gene of Tn7 can be found at specific sites in other genetic surroundings. Antimicrob Agents Chemother. 1990;34:642–650. doi: 10.1128/aac.34.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wegener H, Aarestrup F, Jensen L, Hammerum A, Bager F. Use of antimicrobial growth promoters in food animals and Enterococcus faecium resistance to therapeutic antimicrobial drugs in Europe. Emerg Infect Dis. 1999;5:329–335. doi: 10.3201/eid0503.990303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wittwer C, Ririe K, Andrew R, David D, Gundry R, Balis U. The LightCyclerTM: a microvolume multisample fluorimeter with rapid temperature control. BioTechniques. 1997;22:176–181. doi: 10.2144/97221pf02. [DOI] [PubMed] [Google Scholar]
- 37.Working Party, British Society for Antimicrobial Chemotherapy. A guide to sensitivity testing. Report of the Working Party on Antibiotic Sensitivity Testing of the British Society for Antimicrobial Chemother. J Antimicrob Chemother. 1991;27(Suppl. D):1–50. [PubMed] [Google Scholar]
- 38.Yamamoto T, Tanaka M, Baba R, Yamagishi S. Physical and functional mapping of Tn2603, a transposon encoding ampicillin, streptomycin, sulphonamide and mercury resistance. Mol Gen Genet. 1981;181:464–469. doi: 10.1007/BF00428737. [DOI] [PubMed] [Google Scholar]