Abstract
Although changing patterns in antimicrobial resistance in Streptococcus pneumoniae have prompted several surveillance initiatives in recent years, the frequency with which these studies are needed has not been addressed. To approach this issue, the extent to which resistance patterns change over a 1-year period was examined. In this study we analyzed S. pneumoniae antimicrobial susceptibility results produced in our laboratory with isolates obtained over 2 consecutive years (1997–1998 and 1998–1999) from the same 96 institutions distributed throughout the United States. Comparison of results revealed increases in resistant percentages for all antimicrobial agents studied except vancomycin. For four of the agents tested (penicillin, cefuroxime, trimethoprim-sulfamethoxazole, and levofloxacin), the increases were statistically significant (P < 0.05). Resistance to the fluoroquinolone remained low in both years (0.1 and 0.6%, respectively); in contrast, resistance to macrolides was consistently greater than 20%, and resistance to trimethoprim-sulfamethoxazole increased from 13.3 to 27.3%. Multidrug resistance, concurrent resistance to three or more antimicrobials of different chemical classes, also increased significantly between years, from 5.9 to 11%. The most prevalent phenotype was resistance to penicillin, azithromycin (representative macrolide), and trimethoprim-sulfamethoxazole. Multidrug-resistant phenotypes that included fluoroquinolone resistance were uncommon; however, two phenotypes that included fluoroquinolone resistance not found in 1997–1998 were encountered in 1998–1999. This longitudinal surveillance study of resistance in S. pneumoniae revealed that significant changes do occur in just a single year and supports the need for surveillance at least on an annual basis, if not continuously.
Resistance to β-lactams, macrolides, and trimethoprim-sulfamethoxazole (SXT) continues to increase among clinical isolates of Streptococcus pneumoniae. This trend, coupled with the potential for increasing resistance to fluoroquinolones, has prompted several surveillance studies in recent years (4–6, 10, 17, 22, 23). Although evolving patterns in antimicrobial resistance suggest clearly an exigency for such surveillance initiatives, the frequency with which studies are needed has not been addressed.
The decision to expend resources to perform resistance surveillance must be based on a careful assessment of how frequently the data and information are needed. One approach to making this determination involves establishing the extent to which resistance rates and patterns change over a given time period. Among the surveillance reports published in recent years, there are substantial differences among institutions, geographic regions, and countries represented; the number of institutions involved; and the time periods during which the isolates were obtained (5, 6, 10, 17, 21, 22). These differences make analysis of resistance change over time difficult, and only rarely have studies addressed the extent of changes in resistance on a year-to-year basis (3).
To examine the issue of changing resistance patterns among S. pneumoniae over time, we analyzed antimicrobial susceptibility results produced in our laboratory with isolates obtained over 2 consecutive years from the same 96 institutions distributed throughout the United States. Results were analyzed to determine changes in resistance to individual antimicrobial agents as well as changes in multidrug-resistant (MDR) phenotypes.
(This study was presented in part at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, Calif., 26 to 29 September 1999.)
MATERIALS AND METHODS
Bacterial isolates.
This longitudinal study was conducted using data generated from surveillance studies conducted over 2 consecutive years. In the first year, 4,148 isolates were collected from December 1997 to May 1998 by 163 institutions as previously reported (22). In the second year, 4,296 isolates were collected from September 1998 to March 1999 by 96 of the original 163 institutions that met the criteria of the study and submitted viable isolates. Only data from isolates submitted by the 96 institutions that participated in both years were included in the present analysis; this subgroup included 2,950 isolates from 1997–1998. The 96 institutions were geographically dispersed throughout 40 states.
Of the 2,950 S. pneumoniae isolates collected in 1997–1998 1,700 (57.6%) were taken from respiratory specimens, 842 (28.5%) were taken from blood cultures, and 408 (13.8%) were taken from a variety of other sources. The 4,296 isolates collected during 1998–1999 surveillance comprised 2,427 (56.5%) respiratory isolates, 1,341 (31.2%) isolates from blood cultures, and 528 (12.3%) isolates from other sources. The percentages of isolates tested by patient age were similar for both years of the study. In 1997–1998, 14.5, 2.3, 4.3, 44.3, and 34.6% of isolates were from patients 0 to 4, 5 to 14, 15 to 24, 25 to 64, and ≥65 years of age. In comparison, for 1998–1999, 12.1, 2.8, 5.2, 46.4, and 33.5% of isolates were from patients 0 to 4, 5 to 14, 15 to 24, 25 to 64, and ≥65 years of age. In both years, isolates were submitted to our laboratory on Amies transport swabs (Technical Consultants Ltd., Lancashire, United Kingdom) and were processed identically. All isolates were subcultured to sheep blood agar plates; following incubation, the identification of each isolate was confirmed by the optochin disk test and, if necessary, bile solubility.
Antimicrobial susceptibility testing.
For both years, the same antimicrobial agents were tested: penicillin, amoxicillin-clavulanate, cefuroxime, ceftriaxone, azithromycin, clarithromycin, SXT, vancomycin, and levofloxacin. Susceptibility testing was performed by broth microdilution according to the National Committee for Clinical Laboratory Standards (NCCLS) guidelines (14). Colonies taken from overnight growth on sheep blood agar (20 to 24 h at 35°C) were resuspended in broth to match the turbidity of the 0.5 McFarland standard. The resulting bacterial suspension was used to inoculate Sensititre microdilution panels (TREK Diagnostics, Westlake, Ohio) containing cation-adjusted Mueller-Hinton broth supplemented with 2 to 5% lysed horse blood. The inoculated panels were incubated at 35°C for 20 to 24 h in ambient air prior to reading. Throughout the testing period, S. pneumoniae ATCC 49619 was used as a daily control.
PFGE.
Genotypic differentiation of isolates to determine clonal relatedness was assessed by pulsed-field gel electrophoresis (PFGE) as previously described (13). Genomic DNA was digested using SmaI (New England Biolabs, Inc., Beverly, Mass.) prior to PFGE, and gels were interpreted according to the criteria published by Tenover and coworkers (21).
Data analysis.
MIC results were interpreted based on NCCLS susceptible, intermediate, and resistant breakpoints (15). Multidrug resistance excluded intermediate isolates and was defined as resistance (15) to three or more of the following agents: penicillin, ceftriaxone, azithromycin, SXT, and levofloxacin. Although this definition includes two β-lactam agents, our intention in defining multidrug resistance was to use the phenotype as a marker for isolates for which the therapeutic choices would be diminished. Therefore, because ceftriaxone may remain a therapeutic alternative for some penicillin-resistant strains, it was included in the definition criteria. Azithromycin and levofloxacin were chosen as the representative macrolide and fluoroquinolone, respectively, for multidrug resistance analysis.
For statistical analysis, P values were calculated using EpiInfo version 6 STATCALC to perform chi-square analysis for statistical significance. A P value of <0.05 was considered significant; a P value of <0.001 was considered highly significant.
RESULTS
A comparison of results obtained with the antimicrobial agents tested against S. pneumoniae isolates in 1997–1998 and 1998–1999 is shown in Table 1. For each of the nine agents tested except vancomycin, there was an increase in the percentage of resistant isolates between years. The increase was statistically significant for four of the nine agents tested, including penicillin (P = 0.003), cefuroxime (P = 0.007), SXT (P < 0.001), and levofloxacin (P = 0.003). Although penicillin, cefuroxime, and levofloxacin had statistically significant increases in percent resistance in 1998–1999, changes were not seen in their modal MICs and MIC90s (MICs at which 90% of isolates tested are inhibited). Increases in resistance were also noted for azithromycin and clarithromycin, and these were accompanied by increases in MIC90s.
TABLE 1.
Comparison of resistance prevalence and MICs for S. pneumoniae in 1997–1998 (n = 2,950) and 1998–1999 (n = 4,296)
| Antimicrobial | Yr | Modal MIC | MIC90 | % Resistanta |
|---|---|---|---|---|
| Amoxicillin-clavulanate | 1997–1998 | ≤0.015 | 1 | 9.6 |
| 1998–1999 | ≤0.015 | 2 | 10.5 | |
| Penicillin | 1997–1998 | ≤0.03 | 2 | 12.3 |
| 1998–1999 | ≤0.03 | 2 | 14.7b | |
| Ceftriaxone | 1997–1998 | 0.03 | 1 | 2.9 |
| 1998–1999 | ≤0.015 | 1 | 3.4 | |
| Cefuroxime | 1997–1998 | ≤0.12 | 4 | 22.4 |
| 1998–1999 | ≤0.12 | 4 | 25.2b | |
| Azithromycin | 1997–1998 | ≤0.03 | 4 | 20.9 |
| 1998–1999 | 0.06 | >4 | 22.7 | |
| Clarithromycin | 1997–1998 | 0.03 | 4 | 21.9 |
| 1998–1999 | 0.03 | 8 | 23.2 | |
| SXT | 1997–1998 | 0.12 | 4 | 13.8 |
| 1998–1999 | 0.25 | >4 | 27.3c | |
| Levofloxacin | 1997–1998 | 0.5 | 1 | 0.1 |
| 1998–1999 | 0.5 | 1 | 0.6b | |
| Vancomycin | 1997–1998 | 0.25 | 0.5 | 0 |
| 1998–1999 | 0.25 | 0.5 | 0 |
Included resistant isolates only; intermediate isolates were excluded (15).
Increase was significant (P < 0.05).
Increase was highly significant (P < 0.001).
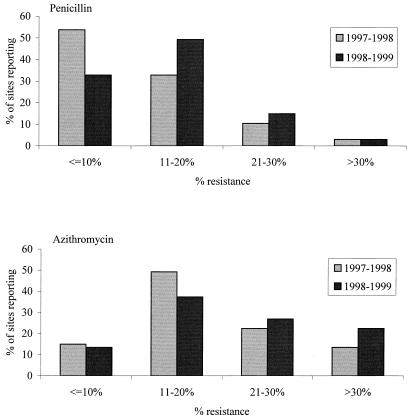
Among penicillin-resistant isolates, the percentage with penicillin MICs of 2 μg/ml increased from 8.9% in 1997–1998 to 10.9% in 1998–1999, while the percentage of isolates with MICs of 4 μg/ml increased from 3.0 to 3.5%. The percentage of isolates with MICs greater than 4 μg/ml stayed relatively stable and low: 0.4% in 1997–1998 and 0.3% in 1998–1999 (data not shown). Among institutions that contributed 20 or more isolates for each year (n = 67), 33 (49.3%) showed increases in penicillin-resistant isolates of more than 5%, 12 (17.9%) showed decreases of more than 5%, and 22 (32.8%) showed changes of ≤5% either way. Resistance to penicillin was ≤10% for 53.7% of the 67 sites in 1997–1998, compared with 32.8% of the same sites in 1998–1999 (Fig. 1). Sites with penicillin resistance rates of between 11 and 20% increased from 32.8% of the 67 sites in 1997–1998 to 49.3% in 1998–1999. In comparison, among the same 67 sites, macrolide (azithromycin) resistance rates of 11 to 20% were identified for 49.3% of sites in 1997–1998 and 37.3% of sites in 1998–1999 (Fig. 1). Sites reporting >30% azithromycin resistance increased from 13.4% of sites in 1997–1998 to 22.4% in 1998–1999.
FIG. 1.
Penicillin and macrolide resistance among 67 sites in the United States submitting 20 or more isolates per year in both 1997–1998 and 1998–1999.
During 1997–1998, four levofloxacin-resistant isolates were encountered out of 2,950 organisms tested (0.1%), all from different institutions. PFGE of the four isolates revealed that they were distinct and clonally unrelated (>3-band difference). Two of the isolates were susceptible to penicillin, and two were intermediate to penicillin. None of the four sites had levofloxacin-resistant isolates in the 1998–1999 study. In 1998–1999, 25 levofloxacin-resistant isolates were identified from the 4,296 tested (0.6%). Nine isolates were resistant only to levofloxacin and susceptible to all other antimicrobial agents tested; five isolates were resistant to both levofloxacin and penicillin; one isolate was resistant to both levofloxacin and SXT; the remaining 10 isolates demonstrated MDR phenotypes and are subsequently described. The 25 levofloxacin-resistant isolates originated from 18 different institutions: 13 institutions contributed one levofloxacin-resistant isolate each, 3 institutions contributed two levofloxacin-resistant isolates each, and 2 institutions contributed three levofloxacin-resistant isolates each. The majority of levofloxacin-resistant isolates submitted by the five institutions submitting multiple isolates were shown to be clonally distinct according to the typing criteria considered (21). The exceptions were two levofloxacin-resistant isolates with identical PFGE patterns contributed by one institution and two closely related but nonidentical isolates (two-band difference) submitted by another institution. In addition, two isolates derived from two geographically distinct hospitals were shown to be related, distinguishable by only a single electrophoretic band difference. When PFGE profiles were compared for the isolates derived from the 1997–1998 and 1998–1999 seasons, clonally identical levofloxacin-resistant S. pneumoniae isolates were not identified. However, two isolates were closely related, having similar antibiograms and only one PFGE band difference.
SXT resistance nearly doubled, from 13.8 to 27.3%, over the year of the study, with the modal MICs and MIC90s increasing from 0.12 to 0.25 μg/ml and from 4 to >4 μg/ml, respectively (Table 1). This same pattern was noted, but with markedly higher resistance percentages, among penicillin-resistant isolates. In 1997–1998, 46.7% of penicillin-resistant isolates were resistant to SXT; in 1998–1999, 83.8% were resistant to SXT (P < 0.001). In comparison, azithromycin resistance among penicillin-resistant isolates increased from 66.6 to 72.6% over the same time period (data not shown).
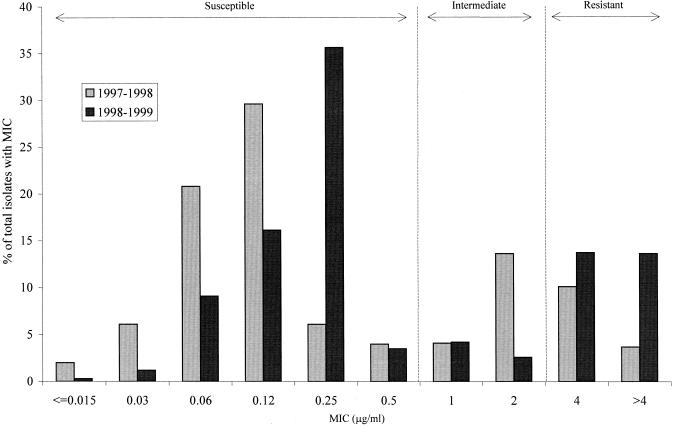
The increase in resistance to SXT was also analyzed by examining changes in MIC distributions between 1997–1998 and 1998–1999 (Fig. 2). At least two shifts in MIC distributions are apparent over the 2 years. First, the percentage of isolates with MICs of 2 μg/ml decreased from 13.6 to 2.6%, while the percentage of isolates with MICs greater than 4 μg/ml increased from 3.7 to 13.6%. This translates into a decrease in the percentage of intermediate isolates and a concomitant increase in resistant isolates (MIC, ≥4 μg/ml) in 1998–1999. A second trend was noted among the MICs within the susceptible range. Among 1998–1999 SXT-susceptible isolates, a substantially higher percentage had MICs equal to 0.25 μg/ml (35.6%) compared with isolates from the previous year (6.1%), of which 20.8% had MICs of 0.06 μg/ml and 29.6% had MICs of 0.12 μg/ml.
FIG. 2.
Comparison of SXT MIC distributions for isolates of S. pneumoniae collected in 1997–1998 (n = 2,950) and 1998–1999 (n = 4,296).
To determine if this increase in SXT resistance was due to certain geographic hot spots or was a geographically dispersed phenomenon, we compared the percent increase on a regional basis. Using regions designated by the U.S. Bureau of the Census, which we have previously applied to pneumococcal surveillance data (22), a statistically significant (P < 0.05) increase in SXT resistance between 1997–1998 and 1998–1999 was observed within every region (Table 2). The extent of this change in each region, however, varied considerably (range, 4.9 to 18.4%).
TABLE 2.
Regional prevalences of SXT-resistant isolates across the United States in 1997–1998 and 1998–1999
| Region | 1997–1998
|
1998–1999
|
P | ||
|---|---|---|---|---|---|
| Total | No. (%) resistant | Total | No. (%) resistant | ||
| East North Central | 408 | 44 (10.8) | 683 | 182 (26.6) | <0.0001 |
| East South Central | 159 | 26 (16.4) | 249 | 70 (28.1) | 0.006 |
| Mid-Atlantic | 383 | 29 (7.6) | 513 | 116 (22.6) | <0.0001 |
| Mountain | 256 | 41 (16.0) | 528 | 150 (28.4) | 0.0002 |
| New England | 269 | 34 (12.6) | 326 | 57 (17.5) | 0.012 |
| Pacific | 254 | 30 (11.8) | 381 | 104 (27.3) | <0.0001 |
| South Atlantic | 484 | 86 (17.8) | 616 | 223 (36.2) | <0.0001 |
| West North Central | 434 | 72 (16.6) | 602 | 151 (25.1) | 0.001 |
| West South Central | 303 | 44 (14.5) | 398 | 119 (29.9) | <0.0001 |
The percentage of S. pneumoniae isolates exhibiting multidrug resistance increased from 5.9% (174 of 2,950 isolates) in 1997–1998 to 11% (472 of 4,296 isolates) in 1998–1999 (P < 0.001). MDR phenotypes (designated A through H) encountered in the 2 years are shown in Table 3. Phenotypes that did not include resistance to penicillin were rare. In the two phenotypes lacking penicillin resistance (E and F), all the isolates except one phenotype F isolate were intermediate to penicillin. For both years, phenotypes A, B, C, and D accounted for more than 96% of isolates with an MDR phenotype. The most common phenotype, A, included resistance to SXT but increased by only 3.2% between the two studies, suggesting that increased SXT resistance in 1998–1999 was not solely responsible for the increased prevalence of multidrug resistance. Resistance to levofloxacin was not included among the four most prominent MDR phenotypes. In 1997–1998 levofloxacin was a part of only 0.6% of MDR phenotypes, while in 1998–1999 it was 2.1%. Phenotype A (resistance to penicillin, azithromycin, and SXT) was the most prevalent in both 1997–1998 (67.8%) and 1998–1999 (71%). The prevalence of phenotype C decreased in 1998–1999, probably as a result of increased SXT resistance, the resistance trait that differentiates phenotype B from C. Macrolide (i.e., azithromycin) resistance was found in all MDR phenotypes except phenotype D.
TABLE 3.
Comparison of frequencies of MDR phenotypes in S. pneumoniae in 1997–1998 (n = 174) and 1998–1999 (n = 472)
| Phenotype | Pattern of resistancea |
n (%)
|
|
|---|---|---|---|
| 1997–1998 | 1998–1999 | ||
| A | PEN, AZI, SXT | 118 (67.8) | 335 (71.0) |
| B | PEN, CTX, AZI, SXT | 18 (10.3) | 76 (16.1) |
| C | PEN, CTX, AZI | 24 (13.8) | 6 (1.3) |
| D | PEN, CTX, SXT | 11 (6.3) | 39 (8.3) |
| E | CTX, AZI, SXT | 2 (1.1) | 6 (1.3) |
| F | AZI, SXT, LEVO | 1 (0.6) | 5 (1.1) |
| G | PEN, AZI, SXT, LEVO | 0 (0.0) | 4 (0.8) |
| H | PEN, AZI, LEVO | 0 (0.0) | 1 (0.2) |
PEN, penicillin; CTX, ceftriaxone; AZI, azithromycin; SXT, trimethoprim-sulfamethoxazole; LEVO, levofloxacin.
Isolates exhibiting simultaneous resistance to all five antimicrobial agents used to define multidrug resistance in either year were not identified. However, two phenotypes (B and G) demonstrated resistance to four agents. Phenotype B, which included resistance to all agents except levofloxacin (and vancomycin), was the second most prevalent phenotype encountered in both years and showed a substantial increase in prevalence between 1997–1998 (10.3%) and 1998–1999 (16.1%). Phenotype G, which included levofloxacin resistance but not ceftriaxone resistance, was not encountered in 1997–1998 and was much less common than phenotype B. All four phenotype G isolates were intermediate to ceftriaxone. In addition to phenotype G, phenotype H was the other MDR phenotype that was newly encountered in 1998–1999.
DISCUSSION
This longitudinal study was performed to evaluate the extent and nature of changes in antimicrobial resistance profiles that can occur among S. pneumoniae over 2 consecutive years. By analyzing and comparing results obtained with isolates from the same set of 96 institutions distributed throughout the United States, several notable patterns were observed. First, resistance to all classes of antimicrobials except vancomycin was higher in the second year, and for several of these agents, the increase in resistance was statistically significant. Second, the increase in SXT resistance was highly significant and occurred, to various degrees, in all regions represented in the study. Third, multidrug resistance also increased significantly over the study period, and the second most common phenotype included resistance to four antimicrobial agents. Finally, in the second year, new MDR phenotypes not noted in the first year were encountered. These findings not only reinforce the need to perform surveillance of S. pneumoniae but also indicate that the frequency of such surveillance needs to be at least on an annual basis, if not continuous and ongoing.
The findings in Table 1 are in accordance with other recent surveillance studies that indicate that resistance to β-lactams, macrolides, and SXT continues to increase (3, 5–7, 10, 17, 23). However, previous studies have not analyzed the extent to which changes could occur in the same institutions over 1 year. Our finding that increases occurred for every antimicrobial agent studied except vancomycin within a single year is troubling.
With regard to penicillin resistance, the 14.7% resistance found in 1998–1999 not only was a marked increase from the 12.3% resistance that we found among 1997–1998 isolates (Table 1), but also was a marked increase from the 12.1% resistance reported by Doern et al. (5) for isolates from 1997 and 1998. The overall increase in penicillin resistance in our longitudinal study was nearly 3% in 1 year and was accompanied, as expected, by increased resistance to other β-lactams (cefuroxime, amoxicillin-clavulanate, and ceftriaxone) and macrolides. The prevalence of penicillin resistance in other countries, for example, 36.5% in Spain (2) and 19.6% in Hong Kong (12), clearly indicates that the United States may be some distance from a peak in the prevalence of penicillin resistance (8, 9, 18, 20). It is also worth noting that nearly half (49.3%) of the sites that contributed ≥20 isolates to the study demonstrated increases in penicillin resistance of >5%, with 65.7% (44 of 67) of participating institutions experiencing at least a marginal increase in penicillin resistance.
Resistance to SXT nearly doubled over the year studied, from 13.8 to 27.3% for all isolates and from 46.7 to 83.8% for penicillin-resistant isolates. The higher level of resistance among penicillin-resistant isolates has been documented in previous studies (5–7, 22), but again, in this longitudinal study the substantial increase that occurred over 1 year is noteworthy. In contrast, select global surveillance studies have not always shown a similar correlation between SXT and penicillin resistance among S. pneumoniae (24). Interestingly, analysis of the findings on a geographic basis indicated that this trend was widely dispersed and therefore did not result from extraordinary increases in resistance among a few institutions (Table 2). In addition, the MIC distribution data in Fig. 2 demonstrate that even among susceptible populations, SXT MICs tended to be higher in 1998–1999 (for most isolates, ≥0.12 μg/ml) than in 1997–1998 (for most isolates, <0.12 μg/ml). It is also important to note that S. pneumoniae resistance to SXT is increasing despite not having a primary indication for use in the treatment of upper and lower respiratory tract infections. However, SXT is a broad-spectrum antimicrobial with a variety of indications for infections attributable to both gram-positive and gram-negative pathogens and is widely used. Although SXT resistance in S. pneumoniae is likely due to mutations, and perhaps heterologous recombination, in the genes encoding dihydropteroate synthase and dihydrofolate reductase, our understanding of these mechanisms and their genetic dissemination is incomplete (1, 16). Further knowledge in this area is needed to better understand how SXT resistance could become so pervasive and how it could increase so dramatically over 1 year. Certainly, the increase in resistance in every geographic region over such a short period of time makes the clonal dissemination of resistant strains an unlikely explanation.
Although fluoroquinolone resistance (as determined using levofloxacin as a marker agent) increased from 0.1 to 0.6% between 1997–1998 and 1998–1999, more than 99% of the isolates remained susceptible. In contrast, resistance to all other agents except vancomycin and ceftriaxone exceeded 10%, and in the case of macrolides, resistance was greater than 20%. While it is difficult to estimate the extent to which fluoroquinolone resistance will increase, the potential of pneumococci to acquire resistance to fluoroquinolones (4, 11, 25) dictates that timely surveillance be continued. Currently, however, fluoroquinolone resistance in the United States does not appear to be due to clonal distribution of strains and is not associated with the most prevalent MDR phenotypes (Table 3).
Whereas monitoring resistance trends among S. pneumoniae against individual antimicrobial agents is a useful component of surveillance, tracking the prevalence of MDR pneumococci is also important, as the therapeutic choices for such organisms can become quite limited. Although multidrug resistance is a concern, this aspect has been examined in only a few previous surveillance studies (3, 5, 6). The near doubling in the percentage of isolates exhibiting multidrug resistance between 1997–1998 (5.9%) and 1998–1999 (11%) found in our study is similar to the findings reported by Butler et al. (3), in which the percentage of MDR isolates increased from 6.5 to 12.1% between 1992 and 1993. Different definitions of multidrug resistance likely underlie the 12.1% reported by Butler et al. in 1993 compared with the 11% presented in the present study for 1998–1999. Our criteria included only isolates resistant by NCCLS standards (15), while the definition used by Butler and coworkers included isolates at or above the intermediate breakpoint.
Doern et al. (6) also reported an increase in the prevalence of MDR isolates from 9.1% in 1994–1995 to 16% in 1997–1998 (5). The higher percentages reported previously compared with the findings presented here, again, are likely due to differences in definition of multidrug resistance, as the former criteria included both penicillin-intermediate and penicillin-resistant isolates. Also, in the study involving S. pneumoniae from 1997 to 1998, isolates showing resistance to only two agents, penicillin and SXT, were considered multidrug-resistant (5).
While direct comparisons between this study and previous studies describing the prevalence of multidrug resistance are difficult, our findings for two contiguous years clearly indicate that multidrug resistance is increasing. Furthermore, the second most common phenotype (phenotype B [Table 3]) encountered in this longitudinal study included resistance to all antimicrobial classes commonly used against pneumococci except vancomycin and fluoroquinolones (as represented by levofloxacin). Also, MDR phenotypes that included resistance to fluoroquinolones were found among 1998–1999 isolates that were not encountered in 1997–1998. These findings and trends support the need to include monitoring and tracking of MDR phenotypes as a key component of pneumococcal surveillance initiatives.
In summary, longitudinal surveillance of S. pneumoniae resistance to several antimicrobial agents over 2 consecutive years and involving the same set of participating institutions revealed that significant changes do and will occur in just a single year. These changes include increasing resistance to individual agents, with changes for some agents (such as SXT) being highly significant; increasing prevalence of isolates exhibiting multidrug resistance; and the emergence of new MDR phenotypes. These findings support the need for surveillance at least on an annual basis; however, a strong case could be made for performing continuous surveillance throughout the year. Because of advances in information technology, this type of surveillance is now feasible (19).
ACKNOWLEDGMENTS
Ortho-McNeil Pharmaceutical, Inc. (Raritan, N.J.) supported this work.
We thank David Diakun of MRL Information Systems for providing technical assistance in preparation of the manuscript. We acknowledge all of the clinical testing institutions that participated in both surveillance studies and that contributed valuable data to this study.
REFERENCES
- 1.Adrian P V, Klugman K P. Mutations in the dihydrofolate reductase gene of trimethoprim-resistant isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:2406–2413. doi: 10.1128/aac.41.11.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baquero F, Garcia-Rodriguez J A, Garcia de Lomas J, Aguilar L The Spanish Surveillance Group for Respiratory Pathogens. Antimicrobial resistance of 1,113 Streptococcus pneumoniae isolates from patients with respiratory tract infections in Spain: results of a 1-year (1996–1997) multicenter surveillance study. Antimicrob Agents Chemother. 1999;43:357–359. doi: 10.1128/aac.43.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler J C, Hofmann J, Cetron M S, Elliott J A, Facklam R R, Breiman R F the Pneumococcal Sentinel Surveillance Working Group. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention's Pneumococcal Sentinel Surveillance System. J Infect Dis. 1996;174:986–993. doi: 10.1093/infdis/174.5.986. [DOI] [PubMed] [Google Scholar]
- 4.Chen D K, McGeer A, De Azavedo J C, Low D E for the Canadian Bacterial Surveillance Network. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. N Engl J Med. 1999;341:233–239. doi: 10.1056/NEJM199907223410403. [DOI] [PubMed] [Google Scholar]
- 5.Doern G V, Brueggemann A B, Huynh H, Wingert E, Rhomberg P. Antimicrobial resistance with Streptococcus pneumoniae in the United States, 1997–1998. Emerg Infect Dis. 1999;5:757–765. doi: 10.3201/eid0506.990603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doern G V, Brueggemann A B, Holley H P, Jr, Rauch A M. Antimicrobial resistance of Streptococcus pneumoniae recovered from outpatients in the United States during the winter months of 1994 to 1995: results of a 30-center national surveillance study. Antimicrob Agents Chemother. 1996;40:1208–12013. doi: 10.1128/aac.40.5.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doern G V, Pfaller M A, Kugler K, Freeman J, Jones R N. Prevalence of antimicrobial resistance among respiratory tract isolates of Streptococcus pneumoniae in North America: 1997 results from the Sentry Antimicrobial Surveillance Program. Clin Infect Dis. 1998;27:764–770. doi: 10.1086/514953. [DOI] [PubMed] [Google Scholar]
- 8.Fenoll A, Jado I, Vicioso D, Perez A, Casal J. Evolution of Streptococcus pneumoniae serotypes and antibiotic resistance in Spain: update (1990 to 1996) J Clin Microbiol. 1998;36:3447–3454. doi: 10.1128/jcm.36.12.3447-3454.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho P L, Que T, Tsang D N-C, Ng T-K, Chow K-H, Seto W H. Emergence of fluoroquinolone resistance among multiply resistant strains of Streptococcus pneumoniae in Hong Kong. Antimicrob Agents Chemother. 1999;43:1310–1313. doi: 10.1128/aac.43.5.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs M R, Bajaksouzian S, Zilles A, Lin G, Pankuch G A, Appelbaum P C. Susceptibilities of Streptococcus pneumoniae and Haemophilus influenzae to 10 oral antimicrobial agents based on pharmacodynamic parameters: 1997 U.S. surveillance study. Antimicrob Agents Chemother. 1999;43:1901–1908. doi: 10.1128/aac.43.8.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones M E, Sahm D F, Martin N, Scheuring S, Heisig P, Thornsberry C, Köhrer K, Schmitz F-J. A prevalence study of gyrA, gyrB, parC, and parE mutations in clinical isolates of Streptococcus pneumoniae with decreased susceptibilities to different fluoroquinolones and originating from worldwide surveillance studies during the 1997–1998 respiratory season. Antimicrob Agents Chemother. 2000;44:462–466. doi: 10.1128/aac.44.2.462-466.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kam K M, Luey K Y, Fung S M, Yiu P P, Harden T J, Cheung M M. Emergence of multiple-antibiotic-resistant Streptococcus pneumoniae in Hong Kong. Antimicrob Agents Chemother. 1995;39:2667–2670. doi: 10.1128/aac.39.12.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEllistrem M C, Stout J E, Harrison L H. Simplified protocol for pulsed-field gel electrophoresis analysis of Streptococcus pneumoniae. J Clin Microbiol. 2000;38:351–353. doi: 10.1128/jcm.38.1.351-353.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 4th ed. Vol. 17. 1997. , no. 2. Approved standard M7–A4. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Performance standard for antimicrobial susceptibility testing; ninth informational supplement, M100–S9. Vol. 18 1999. , no. 1. National Committee for Clinical Laboratory Standards. Wayne, Pa. [Google Scholar]
- 16.Padayachee T, Klugman K P. Novel expansions of the gene encoding dihydropteroate synthase in trimethoprim-sulfamethoxazole-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother. 1999;43:2225–2230. doi: 10.1128/aac.43.9.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfaller M A, Jones R N, Doern G V, Kugler K the SENTRY Participants Group. Bacterial pathogens isolated from patients with bloodstream infection: frequencies of occurrence and antimicrobial susceptibility patterns from the SENTRY antimicrobial surveillance program (United States and Canada, 1997) Antimicrob Agents Chemother. 1998;42:1762–1770. doi: 10.1128/aac.42.7.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahm D F, Jones M E, Hickey M L, Diakun D R, Mani S, Thornsberry C. Resistance surveillance of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis isolated in Asia and Europe, 1997–1998. J Antimicrob Chemother. 2000;45:457–466. doi: 10.1093/jac/45.4.457. [DOI] [PubMed] [Google Scholar]
- 19.Sahm D F. Information technology: a means for enhancing surveillance of antimicrobial resistance. Clin Microbiol Newsl. 1999;21:169–172. [Google Scholar]
- 20.Song J-H, Lee N Y, Ichiyama S, Yoshida R, Hirakata Y, et al. Spread of drug-resistant Streptococcus pneumoniae in Asian countries: Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Clin Infect Dis. 1999;28:1206–1211. doi: 10.1086/514783. [DOI] [PubMed] [Google Scholar]
- 21.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thornsberry C, Jones M E, Hickey M L, Mauriz Y, Kahn J, Sahm D F. Resistance surveillance of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis isolated in the United States, 1997–1998. J Antimicrob Chemother. 1999;44:749–759. doi: 10.1093/jac/44.6.749. [DOI] [PubMed] [Google Scholar]
- 23.Thornsberry C, Oglivie P T, Kahn J, Mauriz Y the Laboratory Investigator Group. Surveillance of antimicrobial resistance in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis in the United States in the 1996–1997 respiratory season. Diagn Microbiol Infect Dis. 1997;29:249–257. doi: 10.1016/s0732-8893(97)00195-8. [DOI] [PubMed] [Google Scholar]
- 24.Thornsberry C, Sahm D F. Antimicrobial resistance in respiratory tract pathogens: results of an international surveillance study. Chemother. 1999;46(Suppl 1):15–23. doi: 10.1159/000048488. [DOI] [PubMed] [Google Scholar]
- 25.Waites K, Rand K, Jenkins S, Yangco B, Brookings E, Gaskins D, Lewis J, Halkias K. Multicenter in vitro comparative study of fluoroquinolones after four years of widespread clinical use. Diagn Microbiol Infect Dis. 1994;3:181–189. doi: 10.1016/0732-8893(94)90089-2. [DOI] [PubMed] [Google Scholar]