Abstract
Purpose/Background
Studies for repurposed drugs in severe acute respiratory syndrome coronavirus type 2–infected and coronavirus disease 2019 (COVID-19) patients are ongoing. According to preclinical research, antidepressants (ADs) might be useful in the treatment of COVID-19.
Methods/Procedures
We conducted a scoping review including clinical studies on AD effects on SARS-CoV-2 infection and COVID-19.
Finding/Results
As of January 2, 2022, we found 14 clinical studies, which could be included into this review. Among them, there were 2 randomized, placebo-controlled studies and 2 prospective parallel-group studies about the efficacy/effectiveness and tolerability of fluvoxamine. The remaining studies were mainly retrospective studies considering COVID-19 hospital populations predominantly exposed to fluoxetine (N = 3), other selective serotonin reuptake inhibitors (SSRI), selective norepinephrine reuptake inhibitors (SNRI), and trazodone. The vast majority were hospital studies and assessed COVID-19 severity (morbidity) and mortality as primary endpoints. The only outpatient study (fluvoxamine) investigated the COVID-19–related hospitalization rate, and 1 psychiatric hospital study (SSRI, SNRI, trazodone) focused on the SARS-CoV-2 infection rate.
Implications/Conclusions
At present, the best evidence of an “anti–COVID-19” potential of ADs exists for fluvoxamine and, to a lesser extent, for fluoxetine. Preliminary evidence had found that patients exposed to SSRI or SNRI substance classes might have a reduced mortality risk and that trazodone might reduce SARS-CoV-2 infection rates. Three studies found no relevant influence of ADs on COVID-19 morbidity and mortality, and 1 study described increased mortality. The latter study, however, did not differentiate between psychotropic medication and ADs. Tricyclics and monoamine oxidase inhibitors are still absolute “dark zones” in COVID-19 research. Further controlled studies testing the effectiveness/efficacy and tolerability/safety (as well as the treatment timing and duration) of different AD substance classes in COVID-19 and post/long-COVID patients of various populations are warranted.
Key Words: SARS-CoV-2, clinical studies, antidepressants, COVID-19 morbidity
People with severe mental illness (eg, schizophrenia spectrum disorder, bipolar disorder, unipolar depression) have been repeatedly shown to be at increased risk to experience a severe coronavirus disease 2019 (COVID-19) course and to die in relation therewith.1–4 Per recent findings, however, this risk might be not driven by the psychiatric condition itself. Instead, it appears that the somatic comorbidity burden might play the dominant role in the development of COVID-19 morbidity and mortality, also in this special population of psychiatric patients.5,6 However, what do we know about the contribution of the concomitant psychopharmacotherapy? Do psychotropic medications also impact the COVID-19 outcome significantly? This question is especially interesting because COVID-19 severity is based at least partly on an excessive immune response on the severe acute respiratory syndrome coronavirus type 2 infection and a lot of psychoactive drugs possess the power necessary to modulate and might have an impact on such immune reactions.7–9
A Danish cross-sectional administrative database study1 and an Italian meta-analysis of Vai et al4 have checked a potential influence of psychotropic drugs on COVID-19 severity of psychiatric patients. The Danish large database study found that redemption of psychotropic drugs (lithium, antipsychotics, antidepressants [ADs], psychostimulants) was significantly associated with a poorer COVID-19 outcome (evidence-based medicine [EBM] level 3, Table 1).1 Vai et al4 reported that COVID-19 mortality was significantly associated with prescriptions of antipsychotics and anxiolytics, whereas ADs had no relevant impact on surviving after adjusting for confounding variables (EBM level 2, Table 1). Although evaluating large samples, in both studies, the association of COVID-19 prognosis with psychotropic medications1,4 could have been profoundly biased by the current psychiatric and somatic condition as well as the related lifestyle behavior on health. The same would apply to the results of a smaller Spanish observational study, which, on the contrary, demonstrated a beneficial influence of some ADs on COVID-19–related mortality of psychiatric inpatients.10 To conclude, harmful or even protective effects of psychotropic drugs could have been largely confounded by the psychiatric indication and the overall psychosocial burden independent on COVID-19.
TABLE 1.
Antidepressants for SARS-CoV-2 Infection and Unfavorable COVID-19 Outcomes? Clinical Trials and Observational Studies
| Authors Country |
Population/Sample Size/Context | Study Design | Main Outcome According to ADs | Limitations* | EBM Level† and AD-Related Message |
|---|---|---|---|---|---|
| Randomized clinical trials | |||||
| Reis et al, 202213 TOGETHER trial Brazil/Canada |
High-risk symptomatic Brazilian adults with SARS-CoV-2 (n = 1497; median age, 50 y; 58% females); between June 2, 2020, and September 9, 2021; second and third waves of the pandemic |
Multicenter, adaptive platform RCT; 100 mg fluvoxamine bid vs placebo for 10 d | Serious COVID-19–related emergencies (RR, 0.68; 95% BCI, 0.52–0.88) and deaths (OR, 0.68; 95% CI, 0.36–1.27) were less likely and less frequent in the fluvoxamine group than in the placebo group; both in a clinically relevant fashion. 84 and 64 patients stopped fluvoxamine and placebo treatment owing to issues of tolerability, respectively. No statistically significant difference between the groups in this regard. |
Confounding variables, such as previous and concomitant medications, were not sufficiently controlled; short duration | EBM level 2 Fluvoxamine treatment predicted significantly lower COVID-19–related mortality and fewer emergencies than placebo condition |
| Lenze et al, 202012 United States |
Adult outpatients with severe acute respiratory COVID-19, with symptom onset within 7 d and oxygen saturation of 92% or greater (n = 152); between April 10, 2020, and August 5, 2020, first wave |
Single-center, double-blind, randomized, fully remote (contactless) clinical trial of patients treated with fluvoxamine (100 mg tid, n = 80, mean age: 46 y, 70% females) vs placebo (n = 72, mean age: 45, 74% females) over 15 d |
Significantly fewer clinical deteriorations in the fluvoxamine group (0 of 80 patients) vs the placebo group (6 of 72 patients); absolute difference, 8.7% (95% CI, 1.8%–16.4%) from survival analysis; log-rank, P = 0.009. More serious events in the placebo group (6 vs 1). Fewer serous (1) and other (11) adverse events in the fluvoxamine than in the placebo group (6 and 12) |
Small homogeneous sample, short follow-up duration, vague outcome measures, 20% attrition rate | EBM level 2 Significantly more favorable COVID-19 outcomes among the fluvoxamine-treated patients than in the placebo group |
| Systematic review of prospective and retrospective studies | |||||
| Vai et al, 20214 Italy |
Patients with psychiatric disorders plus COVID-19 (n = 43,938) compared with controls without psychiatric disorders, plus COVID-19 (n = 1,425,793); between January 1, 2020, and March 5, 2021, first and second waves | Epidemiologic systematic review and meta-analysis of COVID-19 outcomes (33 and 23 nonrandomized studies were included, respectively, mainly retrospective cohort studies with EBM level 3 from United States, Israel, South Korea, Brazil, and Western Europe) | COVID-19 mortality was related to prescription of antipsychotics (OR, 3.71 [95% CI, 1·74–7·91]; I2 = 90·31%), anxiolytics (OR, 2.58 [1.22–5.44]; I2 = 96·42%), and ADs (OR, 2·23 [1.06–4.71]; I2 = 95.45%). For psychotic disorders, mood disorders, antipsychotics, and anxiolytics, the relationship remained significant after adjustment for age, sex, and other confounders—but no longer for ADs. Psychiatric disorders were associated with increased risk of hospitalization (OR, 2.24 [1.70–2.94]; I2 = 88.80%). |
Only 9 of the 23 studies included into the meta-analysis considered comorbid somatic conditions, suggesting a considerable underestimation of these conditions. Most of the included evidence relied on electronic medical records that did not allow a precise analysis of clinical variables, eg, separation between unipolar and bipolar mood disorders. Some studies had low quality or examined small samples of patients with psychiatric disorders. |
EBM level 2 In COVID patients with severe mental illness, AD-prescription was not related to COVID-19 mortality |
| Prospective studies | |||||
| Seftel and Boulware, 202115 United States |
SARS-CoV-2–infected adult outpatients (n = 113; mean age, 42 y; 25% females); between November 2020 and December 2020, first wave |
Pragmatic prospective parallel group study, fluvoxamine (n = 64) vs observational group not exposed to fluvoxamine (n = 48); fluvoxamine was prescribed with a 50- to 100-mg loading dose and then 50 mg bid for 14 d |
Hospitalization rate was 0% (0 of 65) with fluvoxamine and 12.5% (6 of 48) with observation alone (P = 0.05). At 14 days, residual symptoms persisted in 0% (0 of 65) with fluvoxamine and 60% (29 of 48) with observation (P < 0.001). No serious adverse event, no premature discontinuation due to an adverse event |
Small sample size; no randomization; confounding variables, such as concomitant medications and comorbidity, were not sufficiently considered; short duration | EBM level 3 Fluvoxamine treatment predicted fewer hospitalizations and residual symptoms of SARS-CoV-2–infected patients |
| Calusic et al, 202114 Croatia |
Adult patients with COVID-19 (n = 102, age stratified, 58% females) admitted to an ICU; between March 2021 and April 2021, second wave | Pragmatic prospective parallel group study; fluvoxamine (100 mg tid) was added to standard therapy of COVID-19 patients admitted to an ICU (n = 41) and was compared with a matched (for age, sex, vaccination) control group of ICU patients with COVID-19 (n = 41); observation period, 15 d; thereafter 7 d fluvoxamine taper | General mortality was significantly lower in the fluvoxamine group (58.8%) than in the control group (76.5%); HR, 0.58; 95% CI (0.36–0.94). In men, no statistically significant differences were found between the groups. In men, coronary artery disease was significantly more frequent than in females. In the fluvoxamine group, CRRT was significantly more frequent. Fluvoxamine was well tolerated; there were no statistically significant differences of adverse effects between the groups with the exception of CRRT. |
EBM level 3 Fluvoxamine treatment significantly predicted lower COVID mortality in females |
|
| Retrospective studies | |||||
| Oskotsky et al, 202116 United States |
Adult patients with COVID-19 (n = 90,834), data from the Cerner Real World COVID-19 Database (comprising records of patients diagnosed with COVID-19 or COVID-19 exposure who had an emergency department or urgent care visit or were admitted for observation or hospitalized); between January 2020 and September 2020, first wave |
Retrospective observational clinical register study (n = 3401 exposed to SSRI vs n = 80,183 not exposed to SSRI, cp) | In comparison with matched cp, the RR of mortality was reduced among patients prescribed any SSRI (14.6% vs cp 16.6%, adj P = 0.03); prescribed fluoxetine (9.8% vs cp 13.3%, adj P = 0.03); prescribed fluoxetine or fluvoxamine (10% vs cp 13.3%, adj P = 0.03%); prescribed SSRI that is not fluoxetine or fluvoxamine (15.4% vs cp 17%, adj P = 0.06) | Retrospective design, considerable data heterogeneity, no documentation whether the patients had indeed used their prescription medications, dose uncertainty | EBM level 3 SSRI prescriptions significantly predicted lower COVID-19 mortality, especially the fluoxetine and fluvoxamine prescriptions |
| Barcella et al, 20211 Denmark |
16- to 80-year-old patients with COVID-19 (n = 144,321) |
Cross-sectional study, evaluation of nationwide administrative registries from February 2020 to January 2021, first wave |
Average risk ratio for poor COVID-19 outcome and death was significantly increased for patients with severe mental illness including schizophrenia spectrum disorders (2.43 [95% CI, 1.79–3.07]), bipolar disorder (2.11 [95% CI, 1.25–2.97]), and unipolar depression (1.70 [95% CI, 1.38–2.02]) and for patients who redeemed psychotropic drugs (1.70 [95% CI, 1.48–1.92]). Psychotropic drugs included antipsychotics, lithium, ADs, and psychostimulants. The number of psychotropic drugs was not significantly associated with a higher risk of death and severe COVID-19. |
Considerable data heterogeneity and low specificity. For instance, the increased risk of unfavorable COVID-19 outcomes could result from a delayed diagnosis of COVID-19 owing to treatment nonadherence, which is high among patients with severe psychiatric diseases. | EBM level 3 In COVID patients with severe mental illness, psychopharmaceuticals as a whole (including lithium, antipsychotics, ADs, and psychostimulants) were related with increased COVID-19 severity and mortality, but this finding was not further differentiated for individual substance classes |
| Hoertel et al, 20219 France |
Adult patients at Great Paris University hospitals hospitalized for COVID-19 (n = 7230); of these, n = 345 patients (4.8%) were exposed to an AD at admission. Chart records from the L'AP-HP clinical Data Warehouse initiative (containing various data of all inpatient visits for COVID-19 to any of the 39 AP-HP Greater Paris University hospitals) |
Retrospective multicenter clinical register study, primary endpoint was the time from study baseline to intubation or death, observational period from February 1, 2020, to April 1, 2021, first and second waves |
Significant association between AD use and reduced risk of intubation or death (HR, 0.56; 95% CI, 0.43–0.73; P < 0.001). Exploratory analysis: this association was significant for both SSRI (fluoxetine, paroxetine, escitalopram) and non-SSRI (venlafaxine, mirtazapine). The association between AD use and the endpoint was only observed for patients receiving an AD during the visit and not in those who received it only within the 3 mo before hospital admission. |
Retrospective design; no adjustment for multiple testing; incomplete information about the indication for AD prescriptions, its duration, and adherence as well as the period of COVID-19 preadmission; dose uncertainty | EBM level 3 AD use significantly predicted fewer intubations and mortality rates than non-AD use |
| Hoertel et al, 202119 France |
Adult patients at Great Paris University hospitals hospitalized for COVID-19 (n = 2846, 37.4% females); of these, 277 (9.4%) were exposed to FIASMA medications‡ at admission; AP-P database | Retrospective multicenter clinical register study, primary endpoint was the time from study baseline to intubation or death, observational period from January 24, 2020, to May 1, 2020, first wave |
Significant association between FIASMA medication use and reduced risk of intubation or death. The following ADs were included into the FIASMA medication comprising, eg, additional organic calcium antagonists and neuroleptics: amitriptyline, n = 8; clomipramine, n = 1; paroxetine, n = 16; escitalopram, n = 12; sertraline, n = 7; and duloxetine; n = 5. FIASMA AD medication is associated with significantly lower risk of intubation and death compared with the non-FIASMA medication paracetamol (HR, 0.60 [95% CI, 0.45–0.79]; P < 0.001). No FIASMA medication vs FIASMA medication with low or no affinity to SIR-1s (HR, 0.46 [95% CI, 0.32–0.68]; P < 0.001). |
Retrospective design; no adjustment for multiple testing; incomplete information about the indication for AD prescriptions, its duration, and adherence as well as the period of COVID-19 preadmission; dose un certainty; underestimation of tricyclics (only 9 of 49 charts) | EBM level 3 FISAMA medication use (including ADs, neuroleptics, amlodipine) significantly predicted fewer intubations and mortality rates than non-FIASMA medication use — the same applied to the two subgroups of chart records of patients exposed especially to FIASMA ADs or, more specifically, to low/no SIR-1 receptor FIASMA ADs |
| Diez-Quevedo et al, 20217 Spain |
Adult patients (mean age, 61.3 y; 42.9% females) with COVID-19 (n = 2150), admitted to a tertiary university hospital in Barcelona between January 3, 2020, and November 17, 2020, first wave | Retrospective observational chart record study; 1011 patients (47%) received psychotropic medications: 36% benzodiazepines, 22% ADs, and 21% antipsychotics | Patients with previous year's benzodiazepine and AD treatments (mostly mirtazapine and SSRI) were independently associated with lower mortality risk (hazard ratios: benzodiazepines, 0.47 [95% CI, 0.29–0.78], P = 0.003; and ADs, 0.43 [95% CI, 0.25–0.74], P = 0.002). Antipsychotic treatment history (mostly quetiapine and haloperidol) was not associated with mortality. | Retrospective design, considerable data heterogeneity, no documentation whether the patients had indeed used their prescriptions, dose uncertainty | EBM level 3 AD treatment significantly predicted lower mortality risk |
| Clelland et al, 202117 United States |
Adult patients (n = 165, age and sex stratified) admitted to The Rockland Psychiatric Center, Orangeburg, New York; between June 2, 2020, and July 31, 2020; first wave | Retrospective cohort study of psychiatric in-patients investigating whether ADs can modify the risk of SARS-CoV-2 infection; n = 91 SARS-CoV-2–positive vs n = 74 SARS-CoV-2–negative patients | Patients receiving ADs (SSRI, SNRI, trazodone) experienced significantly fewer COVID-19 infections (fully adj OR, 0.28 [95% CI, 0.09–0.837]; P = 0.023). Exploratory analyses of individual AD use found a significant association between lower infection risk and fluoxetine (P = 0.023), as well as trazodone (P = 0.001) intake. There was small but significant association of chlorpromazine equivalent daily dose with COVID-19 infection rate. |
Retrospective design, small sample size, no documentation of severity outcomes following COVID-19 infection, dose uncertainty | EBM level 3 Fluoxetine and trazodone significantly predicted lower SARS-CoV-2 infection rates |
| Bonnet et al, 202220 Germany |
SARS-CoV-2–infected adult psychiatric inpatients (eligible, n = 107; included, n = 96; mean age, 61.5 y; 54% females); between November 2020 and March 2021, second wave |
Retrospective longitudinal, multicenter psychiatric inpatient study exploring the influence of psychiatric medications on COVID-19 duration and symptom load during the period from diagnosing with SARS-CoV-2 infection via PCR (nasopharyngeal swab) up to the next 21 d | None of the tested medications (AD, neuroleptics, anticonvulsants, benzodiazepines, antihypertensive agents, PPIs, anticoagulants, statins, RAAS inhibitors) was associated with the duration of COVID-19 (primary outcome). According to 95% CIs of regression coefficients, respiratory and neuropsychiatric symptom load was significantly and negatively related to prescription of ADs and anticoagulants, respectively. Fatigue was negatively and positively related to RAAS inhibitors and PPIs, respectively. These significant relationships disappeared with P value adjustment owed to multiple testing. The mean total psychiatric burden was not influenced across the study. | Retrospective design, small sample size, recall errors of the interviewed physicians, no control group with nonpsychiatric patients, loss of follow-up rate of 11.3% (not included were 11 patients who wanted to be directly discharged after diagnosis with SARS-CoV-2 infection — all symptom free at that time) | EBM level 3 ADs (without fluvoxamine and fluoxetine) did not predict COVID-19 duration but significantly negatively predicted respiratory symptom-load (95% CI). The significant relationship disappeared after adjustment for multiple testing. |
| Németh et al, 202118 Hungary |
Adult patients (mean age, 66 y; 45.4% females) with COVID-19–related pneumonia (n = 269), admitted to the Uzsoki Teaching Hospital of the Semmelweis University in Budapest, between March 17, 2021, and April 22, 2021, third wave |
Retrospective case-control study (n = 110 patients with COVID-19 pneumonia receiving as add-on therapy daily 20 mg fluoxetine vs n = 159 patients not receiving fluoxetine) | Patients receiving fluoxetine therapy were 0.33 times (95% CI, 0.16–0.68) less likely to die than those who had not received fluoxetine within 2 to 28 d. This effect was independent on concomitant antiviral therapy. There were 3 adverse events (severe hyponatremia, increase of transaminases, and confusion), which led to fluoxetine treatment stop in each case. |
Retrospective design, small sample size, no documentation of psychiatric comorbidity, significant intergroup differences (fluoxetine group received significantly more frequent antiviral therapy) | EBM level 4 Fluoxetine treatment significantly predicted lower mortality |
| Fei et al, 202121 Italy |
Adult patients (mean age, 70 y; 40.3% females) with COVID-19–related pneumonia (n = 402), admitted to Internal Medicine wards of the Florence University; first wave | Retrospective case-control study (n = 34 AD-treated depressives vs n = 368 non–AD-treated patients) | Mortality rate: no significant difference between the groups; ARDS (P < 0.02) and endotracheal intubation (0.04) significantly lower in the AD-treated group; ADs: SSRI and SNRI | Retrospective design, no documentation whether the patients had indeed used their prescriptions, dose uncertainty, significant intergroup differences (more comorbidity and less concomitant antiviral drugs in the AD group) | EBM level 4 SSRI or SNRI treatment did not influence mortality rate; ARDS and intubation rate was significantly lower in AD-treated patients |
Clinical trials and observational studies are sorted by EBM level and sample size.
*Drugs of abuse (eg, alcohol cannabis, opioids, cocaine, amphetamines) were not sufficiently considered, although these recreational drugs can modulate key components of the immune response machinery.7
†According to the Oxford Centre for Evidence-Based Medicine Levels of Evidence Working Group (Jeremy Howick, Iain Chalmers [James Lind Library], Paul Glasziou, Trish Greenhalgh, Carl Heneghan, Alessandro Liberati, Ivan Moschetti, Bob Phillips, Hazel Thornton, Olive Goddard, and Mary Hodgkinson). “The Oxford Levels of Evidence 2.” The Levels of Evidence, version 2.1. Available at: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence. Accessed February 22, 2022.
‡Definition of FIASMA medication.34
adj indicates adjusted; AP-HP, Assistance Publique—Hôpitaux de Paris; ARDS, acute respiratory distress syndrome; BCI, Bayesian credible interval; bid, twice a day; CI, confidence interval; cp, control patients; CRRT, continuous renal replacement therapy; HR, hazard ratio; ICU, intensive care unit; OR, odds ratio; PCR, polymerase chain reaction; PPI, proton pump inhibitor; RAAS, renin-angiotensin-aldosterone system; RCT, randomized controlled trial; RR, relative risk; tid, 3 times a day; SSRI, selective serotonin reuptake inhibitors; SNRI, selective norepinephrine reuptake inhibitors.
Just taking a closer look on ADs, it appears that increasing more-in-depth investigations7,9,10 point to a predominantly useful effect of these drugs against SARS-CoV-2 infections and the development of severe COVID-19 cases. This encouraged us to conduct the following scoping review11 including clinical studies on this possibly promising drug repurposing approach for COVID-19.
METHODS/PROCEDURES
A scoping systematic review11 was performed using the search term combinations “antidepressants” and “study” and “COVID” OR SARS-COV” in https://pubmed.ncbi.nlm.nih.gov/ up to January 2, 2022. Furthermore, we evaluated the secondary literature of the resulting 117 or 49 hits with respect to our inclusion criteria: case reports, or clinical or epidemiological studies about the influence of ADs on COVID-19 outcomes or on patients' infection with SARS-CoV-2. Exclusion criteria comprised all animal, nonclinical, and nonepidemiological studies.
RESULTS/FINDINGS
We could include 14 studies (Fig. 1), which were summarized in Table 1. In short, 2 placebo-controlled randomized studies,12,13 both EBM level 2, demonstrated a beneficial role of fluvoxamine (100 mg twice a day or 3 times a day over 14 days) as add-on in the treatment of COVID-19: in these studies,12,13 fluvoxamine significantly reduced serious COVID-19 outcomes. This and the good tolerability and safety of fluvoxamine were supported by a small pragmatic prospective Croatian study (EBM level 3).14 Another small pragmatic prospective study tested a fluvoxamine loading dose protocol (50 to 100 mg, followed by 50 mg twice a day) alongside 14 days in SARS-CoV-2–positive adult outpatients, who were not necessarily affected by COVID-19. In this 14-day study, fluvoxamine treatment predicted fewer hospitalizations and residual symptoms of SARS-CoV-2–infected patients (EBM level 3).15 The second best evidence of an “anti-COVID” potential of ADs exists for fluoxetine; at present, it is shown only in retrospective studies: 1 larger study (EBM level 3)16 and the other 2 with smaller sample sizes (EBM level 317; EBM level 4).18 The smallest study in this context (EBM level 4)18 tested an add-on treatment of 20 mg fluoxetine up to 28 days in patients receiving an antiviral treatment of COVID-19 pneumonia, whereas the other 2 studies16,17 had dose uncertainties. Other selective serotonin reuptake inhibitors (SSRI), mirtazapine, venlafaxine, and trazodone were associated with reduced SARS-CoV-2 infection rates as well as reduced COVID-19 morbidity and mortality in 4 retrospective studies, 1 with psychiatric inpatients (EBM level 3)17 and the other 3 evaluating larger general hospital samples affected by COVID-19, all EBM level 3.7,9,19 Three further retrospective studies described no relevant influence of ADs on COVID-19 severity and mortality, 2 of them using small sample sizes (EBM level 320 and EBM level 4),21 and the remaining study with a large heterogeneous COVID-19 population (EBM level 2).4 The least specific study was cross-sectional and described that psychotropic drugs (including ADs) were associated with increased COVID-19 severity and mortality in the Danish general population (EBM level 3).1
FIGURE 1.
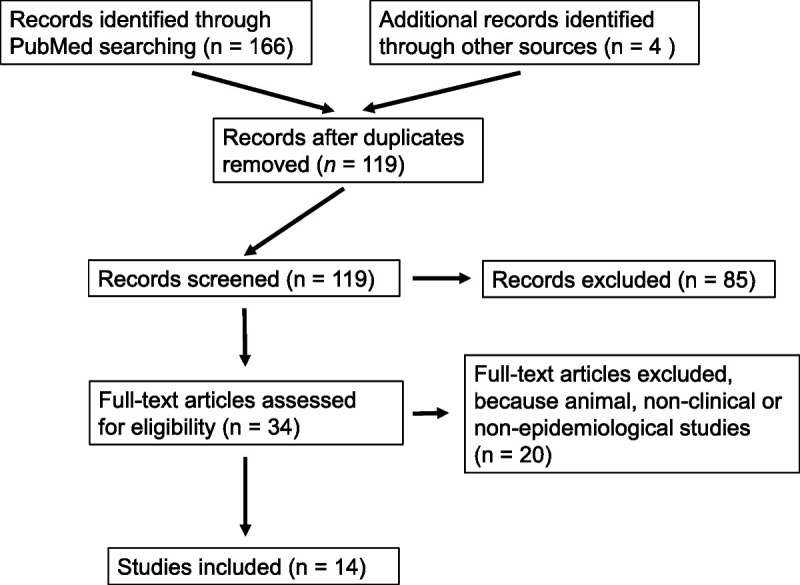
Flow chart of the search strategy used during the scoping review of epidemiological and clinical studies about influence of ADs on SARS-CoV-2 infection and COVID-19 outcomes.
DISCUSSION
At present, especially fluvoxamine (100 mg twice a day or 3 times a day) seems to exert protective effects on COVID-19 morbidity and mortality (supported by 2 randomized placebo-controlled clinical trials [both EBM level 2, Table 1]12,13 and 1 pragmatic prospective study [EBM level 3, Table 1]).14 The only outpatient study included into this review used lower fluvoxamine doses (50 mg twice a day) in an outpatient everyday setting and found significantly reduced COVID-19–related hospitalization (EBM level 3, Table 1).15
In this context, it should be outlined that fluvoxamine, like other ADs, is no “panacea,” given its special pharmacokinetics as a powerful inhibitor of the CYP11A2 and CYP2C19 systems as well as an average inhibitor of CYP3A4, all together catabolic pathways with a relevant genetic variability in the general population.22 Against this background, problematic drug-drug interactions have been reported with fluvoxamine, such as considerable increases of the blood levels of, for example, diazepam, bromazepam, alprazolam, clozapine, tricyclics, mirtazapine, and many other somatic medications as well as adverse effects during cross-over titration from fluvoxamine (itself mainly metabolized by CYP2D6 and CYP1A2) to other serotonergic drugs.22,23 Citing the last Cochrane analysis, fluvoxamine was neither superior nor inferior to any other ADs in terms of efficacy and tolerability in the acute phase treatment of depression;24 especially nausea/vomiting was more frequently reported with fluvoxamine than with tricyclics.24 According to the studies elaborated here, fluvoxamine was well tolerated by COVID-19 patients (Table 1). More data are necessary about dosing, treatment timing and duration, tolerability/safety in various populations, and effectiveness/efficacy against post/long COVID and different SARS-CoV-2 variants. We read that 4 larger trials are underway in United States and Canada.25
Known Fluvoxamine and Fluoxetine Anti–COVID-19 Mechanisms
The idea to test the anti–COVID-19 potential of fluvoxamine, an old and inexpensive SSRI, however less frequently prescribed in these days, was based upon preclinical research, where fluvoxamine was shown to augment a cellular key anti-inflammatory system by stimulating the sigma-1 receptor (SIR-1), an endoplasmic reticulum (ER) chaperone membrane protein involved, for example, in the control of the ER stress response.12 For instance, SIR-1 was identified as an essential inhibitor of cytokine production in a murine model of septic shock .26 Another SSRI, that is, fluoxetine, was demonstrated to inhibit SARS-CoV-2 entry into epithelial cells as well as SARS-CoV-2 replication.27–30 Because fluoxetine and fluvoxamine share a potent SIR-1 agonism, it is possible that both SSRIs can prevent severe COVID-19 by abating hyperinflammatory events, such as the cytokine storm after ER stress due to SARS-CoV-2 replication.27,31 Moreover, decreased immunoglobulin E–mediated mast-cell degranulation and interference with endolysosomal viral trafficking were discussed as mechanisms to limit this hyperinflammatory immune response.31,32 A recent large retrospective clinical register study corroborated that both fluoxetine and fluvoxamine prescriptions negatively predicted COVID-19 mortality (EBM level 3, Table 1).16 Moreover, an anti–COVID-19 potential of fluoxetine was supported by 2 further, although smaller, retrospective studies.17,18
Further Broader Possible Anti–COVID-19 Mechanisms of ADs
The SIR-1 agonist effect as a central explanation of the effect of fluvoxamine remains to date uncertain in the absence of any preclinical or clinical data specific to COVID-19 supporting this effect and seems unable to explain associations observed between non–SIR-1 or very-low SIR-1 ADs (such as paroxetine, mirtazapine, and venlafaxine) and reduced risk of intubation or death (Table 1)19 as well as anti-inflammatory effects observed with a broad range of ADs (and not only the SIR-1 ADs fluvoxamine and fluoxetine) in individuals with major depression.8 Contrariwise, several preclinical (in vitro and ex vivo) and observational studies suggest that inhibition of acid sphingomyelinase (ASM)/ceramide system plays a potentially important role and may explain both potential antiviral and anti-inflammatory effects of certain ADs in COVID-19.32,33 Particularly, among SSRIs, the magnitude of the in vitro inhibition of ASM, which varies across molecules (eg, fluoxetine > paroxetine > fluvoxamine > other SSRIs),34,35 appears to correlate with the magnitude of the in vitro antiviral effect against SARS-CoV-2.28,36
In this context, most ADs belong to a large group of special lipophilic, lysosomotropic, amine, and weak bases (comprising also many tricyclic ADs, some antipsychotics like chlorpromazine and promethazine, and nonpsychotropic drugs, such as organic calcium antagonists and ambroxol), which can inhibit the lysosomal and cell-surface ASM.33–38 This enzyme converts sphingomyelin to phosphorylcholine and ceramide, and high ceramide concentrations in the cell membrane are assumed to weaken the membrane integrity, thereby possibly supporting virus entry.2 Three studies found that plasma ceramide levels strongly correlate with clinical and inflammation severity among patients with COVID-19.39–41 Intriguingly, SARS-CoV-2 infection was demonstrated to stimulate even this ASM/ceramide system.2 Thus, an inhibition of sphingomyelin break down by the aforementioned special weakly basic drugs, so-called functional inhibitors of acid sphingomyelinase (FIASMAs), might by a promising approach to prevent SARS-CoV-2 entry into cells.19,27,33–38 Indeed, the latter phenomenon was demonstrated with tricyclics (amitriptyline, imipramine, maprotiline) as well as SSRI (sertraline, fluoxetine, escitalopram); all of which prevented infection of nasal epithelial cells with SARS-CoV-2.27 Furthermore, volunteers treated with a low dose of amitriptyline developed no infection of freshly isolated nasal epithelial cells with pseudoviral particles presenting SARS-CoV-2 spike protein, a model preferentially used for mimicking SARS-CoV-2 infection.27 In addition, the non-SSRI trazodone was found to be associated with a protective effect on SARS-CoV-2 infection rates.17 In this context, a German retrospective clinical study pointed also to protective influences of the whole substance class of ADs on respiratory COVID-outcomes; however, the statistical significance of this effect faded after adjustment to multiple testing (EBM level 3, Table 1).20 Remarkably, this study had a preferential use of venlafaxine, duloxetine, mirtazapine, agomelatine, sertraline, and citalopram but, in fact, had examined zero cases with fluoxetine and fluvoxamine treatment (unreported information).20 Nevertheless, it cannot be excluded that the accidental absence of fluoxetine and fluvoxamine treatments had negatively modulated the results of this explorative clinical study.20
Beyond SIR-1 and FIASMA potential mechanisms, the antiplatelet activity of SSRIs might be beneficial to COVID-19 prognosis.9,19,36 Finally, it is still possible that the whole substance class of ADs can be characterized by an intrinsic property of filtering overactive destructive immune responses by reducing pro-inflammatory cytokine activities and increasing anti-inflammatory cytokine activities,8,20 thereby curbing also a consecutive post-COVID autoimmunity. Toward this end, beyond or overlapping with their FIASMA activity, ADs might control destructive immune activity by increasing the resilience of vulnerable cells, in general, for example, by activating non–SIR-1-inositol-requiring enzyme pathways,32,36 balancing a disturbed tryptophan metabolism,42,43 limiting oxidative stress, promoting mild intracellular acidification (assumed to be cyto/neuroprotective),44,45 and/or increasing the net vagal influence.46
CONCLUSIONS
At present, the best evidence of an anti–COVID-19 potential of ADs exists for fluvoxamine and, to a lesser extent, for fluoxetine. Preliminary evidence was found for other SSRI, selective norepinephrine reuptake inhibitors, and trazodone exposures. Tricyclics and MAO inhibitors are still “dark zones” in COVID-19 research. Further well-controlled studies testing the effectiveness/efficacy and tolerability/safety (and treatment timing/duration) of different AD substance classes in COVID-19 patients of various populations are warranted.
AUTHOR DISCLOSURE INFORMATION
The authors declare no conflicts of interest.
REFERENCES
- 1.Barcella CA Polcwiartek C Mohr GH, et al. Severe mental illness is associated with increased mortality and severe course of COVID-19. Acta Psychiatr Scand. 2021;144:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen S Fernandez-Egea E Jones PB, et al. Longer-term mortality following SARS-CoV-2 infection in people with severe mental illness: retrospective case-matched study. BJPsych Open. 2021;7:e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toubasi AA AbuAnzeh RB Tawileh HBA, et al. A meta-analysis: the mortality and severity of COVID-19 among patients with mental disorders. Psychiatry Res. 2021;299:113856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vai B Mazza MG Delli Colli C, et al. Mental disorders and risk of COVID-19-related mortality, hospitalisation, and intensive care unit admission: a systematic review and meta-analysis. Lancet Psychiatry. 2021;8:797–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoertel N Sánchez-Rico M Herrera-Morueco JJ, et al. Comorbid medical conditions are a key factor to understand the relationship between psychiatric disorders and COVID-19-related mortality: results from 49,089 COVID-19 inpatients. Mol Psychiatry. 2021;1–3. doi: 10.1038/s41380-021-01393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sánchez-Rico M, Limosin F, Hoertel N. Is a diagnosis of schizophrenia spectrum disorder associated with increased mortality in patients with COVID-19? Am J Psychiatry. 2022;179:71–73. [DOI] [PubMed] [Google Scholar]
- 7.Friedman H, Pross S, Klein TW. Addictive drugs and their relationship with infectious diseases. FEMS Immunol Med Microbiol. 2006;47:330–342. [DOI] [PubMed] [Google Scholar]
- 8.Köhler CA Freitas TH Stubbs B, et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol. 2018;55:4195–4206. doi: 10.1007/s12035-017-0632-1. [DOI] [PubMed] [Google Scholar]
- 9.Hoertel N Sánchez-Rico M Vernet R, et al. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study. Mol Psychiatry. 2021;26:5199–5212. [DOI] [PubMed] [Google Scholar]
- 10.Diez-Quevedo C Iglesias-González M Giralt-López M, et al. Mental disorders, psychopharmacological treatments, and mortality in 2150 COVID-19 Spanish inpatients. Acta Psychiatr Scand. 2021;143:526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters MD Godfrey CM Khalil H, et al. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc. 2015;13:141–146. [DOI] [PubMed] [Google Scholar]
- 12.Lenze EJ Mattar C Zorumski CF, et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. JAMA. 2020;324:2292–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reis G Dos Santos Moreira-Silva EA Silva DCM, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Glob Health. 2022;10:e42–e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calusic M Marcec R Luksa L, et al. Safety and efficacy of fluvoxamine in COVID-19 ICU patients: an open label, prospective cohort trial with matched controls. Br J Clin Pharmacol. 2021. doi: 10.1111/bcp.15126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seftel D, Boulware DR. Prospective cohort of fluvoxamine for early treatment of coronavirus disease 19. Open forum. Infect Dis. 2021;8:ofab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oskotsky T Maric I Tang A, et al. Mortality risk among patients with COVID-19 prescribed selective serotonin reuptake inhibitor antidepressants. JAMA Netw Open. 2021;4:e2133090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clelland CL Ramiah K Steinberg L, et al. Analysis of the impact of antidepressants and other medications on COVID-19 infection risk in a chronic psychiatric in-patient cohort. BJPsych Open. 2021;8:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Németh ZK Szûcs A Vitrai J, et al. Fluoxetine use is associated with improved survival of patients with COVID-19 pneumonia: a retrospective case-control study. Ideggyogy Sz. 2021;74:389–396. [DOI] [PubMed] [Google Scholar]
- 19.Hoertel N Sánchez-Rico M Gulbins E, et al. Association between FIASMAs and reduced risk of intubation or death in individuals hospitalized for severe COVID-19: an observational multicenter study. Clin Pharmacol Ther. 2021;110:1498–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonnet U Claus B Schaefer M, et al. Impact of psychiatric and related somatic medications on the duration and severity of COVID-19: a retrospective explorative multi-center study from the German metropolitan Ruhr-area. Pharmacopsychiatry. 2022;55:30–39. [DOI] [PubMed] [Google Scholar]
- 21.Fei L Santarelli G D'Anna G, et al. Can SSRI/SNRI antidepressants decrease the 'cytokine storm' in the course of COVID-19 pneumonia? Panminerva Med. 2021. doi: 10.23736/S0031-0808.21.04436-0. [DOI] [PubMed] [Google Scholar]
- 22.Sproule BA Naranjo CA Brenmer KE, et al. Selective serotonin reuptake inhibitors and CNS drug interactions. A critical review of the evidence. Clin Pharmacokinet. 1997;33:454–471. [DOI] [PubMed] [Google Scholar]
- 23.Hori H Yoshimura R Ueda N, et al. A case with occurring adverse effects when cross-over titration from fluvoxamine to paroxetine associated with increasing the plasma fluvoxamine level in major depressive disorder. World J Biol Psychiatry. 2009;10(Pt 2):620–622. [DOI] [PubMed] [Google Scholar]
- 24.Omori IM Watanabe N Nakagawa A, et al. Fluvoxamine versus other anti-depressive agents for depression. Cochrane Database Syst Rev. 2010;17:CD006114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Facente SN Reiersen AM Lenze EJ, et al. Fluvoxamine for the early treatment of SARS-CoV-2 infection: a review of current evidence. Drugs. 2021;81:2081–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen DA Seki SM Fernández-Castañeda A, et al. Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci Transl Med. 2019;11:eaau5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpinteiro A Edwards MJ Hoffmann M, et al. Pharmacological inhibition of acid sphingomyelinase prevents uptake of SARS-CoV-2 by epithelial cells. Cell Rep Med. 2020;1:100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schloer S Brunotte L Goretzko J, et al. Targeting the endolysosomal host-SARS-CoV-2 interface by clinically licensed functional inhibitors of acid sphingomyelinase (FIASMA) including the antidepressant fluoxetine. Emerg Microbes Infect. 2020;9:2245–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brunotte L Zheng S Mecate-Zambrano A, et al. Combination therapy with fluoxetine and the nucleoside analog GS-441524 exerts synergistic antiviral effects against different SARS-CoV-2 variants in vitro. Pharmaceutics. 2021;13:1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimniak M Kirschner L Hilpert H, et al. The serotonin reuptake inhibitor fluoxetine inhibits SARS-CoV-2 in human lung tissue. Sci Rep. 2021;11:5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hashimoto Y, Suzuki T, Hashimoto K. Old drug fluvoxamine, new hope for COVID-19. Eur Arch Psychiatry Clin Neurosci. 2022;272:161–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoertel N Sánchez-Rico M Cougoule C, et al. Repurposing antidepressants inhibiting the sphingomyelinase acid/ceramide system against COVID-19: current evidence and potential mechanisms. Mol Psychiatry. 2021;26:7098–7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carpinteiro A Gripp B Hoffmann M, et al. Inhibition of acid sphingomyelinase by ambroxol prevents SARS-CoV-2 entry into epithelial cells. J Biol Chem. 2021;296:100701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kornhuber J Tripal P Reichel M, et al. Functional inhibitors of acid sphingomyelinase (FIASMAs): a novel pharmacological group of drugs with broad clinical applications. Cell Physiol Biochem. 2010;26:9–20. [DOI] [PubMed] [Google Scholar]
- 35.Kornhuber J, Hoertel N, Gulbins E. The acid sphingomyelinase/ceramide system in COVID-19. Mol Psychiatry. 2021;1–8. doi: 10.1038/s41380-021-01309-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoertel N. Do the selective serotonin reuptake inhibitor antidepressants fluoxetine and fluvoxamine reduce mortality among patients with COVID-19? JAMA Netw Open. 2021;4:e2136510. [DOI] [PubMed] [Google Scholar]
- 37.Le Corre P, Loas G. Repurposing functional inhibitors of acid sphingomyelinase (FIASMAs): an opportunity against SARS-CoV-2 infection? J Clin Pharm Ther. 2021;46:1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kornhuber J Muehlbacher M Trapp S, et al. Identification of novel functional inhibitors of acid sphingomyelinase. PLoS One. 2011;6:e23852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khodadoust MM. Inferring a causal relationship between ceramide levels and COVID-19 respiratory distress. Sci Rep. 2021;11:20866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torretta E Garziano M Poliseno M, et al. Severity of COVID-19 patients predicted by serum sphingolipids signature. Int J Mol Sci. 2021;22:10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marín-Corral J Rodríguez-Morató J Gomez-Gomez A, et al. Metabolic signatures associated with severity in hospitalized COVID-19 patients. Int J Mol Sci. 2021;22:4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lionetto L Ulivieri M Capi M, et al. Increased kynurenine-to-tryptophan ratio in the serum of patients infected with SARS-CoV2: an observational cohort study. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shader RI. COVID-19, interferons, and depression: a commentary. Psychiatry Res. 2020;291:113198. [DOI] [PubMed] [Google Scholar]
- 44.Bonnet U Bingmann D Wiltfang J, et al. Modulatory effects of neuropsychopharmaca on intracellular pH of hippocampal neurones in vitro. Br J Pharmacol. 2010;159:474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonnet U. The sour side of vitamin C might mediate neuroprotective, anticonvulsive and antidepressant-like effects. Med Hypotheses. 2019;131:109320. [DOI] [PubMed] [Google Scholar]
- 46.Ondicova K Tillinger A Pecenak J, et al. The vagus nerve role in antidepressants action: efferent vagal pathways participate in peripheral anti-inflammatory effect of fluoxetine. Neurochem Int. 2019;125:47–56. [DOI] [PubMed] [Google Scholar]


