Abstract
Widespread public health campaigns have reduces the prevalence of tobacco and nicotine exposures during pregnancy in the United States. However, tobacco and nicotine exposures during pregnancy persist as a common modifiable perinatal risk exposure. Furthermore, declines in tobacco use have been accompanied by parallel rises in both the prevalence and incidence of marijuana use in pregnancy. This is worrisome, as the macromolecules which comprise tobacco and marijuana smoke affect placental function. In this chapter we summarize the decades of evidence contributing to our understanding of the placental molecular pathophysiology accompanying these chemical exposures, thereby rendering risk of adverse perinatal outcomes.
Key words: placenta, tobacco, nicotine, marijuana, pregnancy
Introduction
The placenta is a unique organ because while it is only in temporary existence during pregnancy, disruptions in its physiological and molecular function are associated with—and likely causally related to—long-lasting effects on the fetus. In accordance with the Developmental Origins of Health and Disease, we and many others have demonstrated that these long-lasting effects include childhood and adult onset metabolic, cardiovascular, and behavior disease. Indeed, placental biochemical and molecular activities are both responsive to, and are modified by, environmental chemicals. Consequently, the placenta is considered to contain a footprint of the in utero exposures the fetus experiences.1–3
Environmental chemical exposures during pregnancy occur through a combination of primarily inhalation and ingestion, with some nicotine and cannabinoid exposures resulting from adsorption. The first reports on adverse effects of tobacco smoking were documented in the 1950s,4 and have come to include clearly significant independent associations with adverse perinatal outcomes such as placental abruption, premature birth, low birth weight, congenital anomalies, and their accompanying fetal and neonatal deaths.1–6 Since then, numerous studies across geographically and ethnically/racially distinct populations have been published, and show highly consistent findings which have led to strong public health messages regarding the use of nicotine and tobacco products in pregnant patients. Even though these efforts have resulted in a reduction in the use of prenatal tobacco and nicotine (9.2% in 2010 to 6.1% in 2020),5 exposure to other harmful chemicals and macromolecules through opioids, cocaine and marijuana have risen considerably over recent years, with marijuana (THC) being the most used drug during the prenatal period.6
While these public health messages focus on the health of the fetus and neonate with tobacco and marijuana use, the health of the placenta cannot be ignored. Through reductionist and mechanistic-minded studies, which include utilization of trophoblast cell culture, rodent and nonhuman primate models, and robust epidemiological studies, we have an improved understanding of the pathologic and molecular changes which occur in the placenta with these exposures. The focus of this chapter is the reported changes to placental biology in relation to tobacco (both primary and secondary), nicotine and marijuana exposure in the perinatal interval pregnancy. Understanding the functional consequences of these changes is essential to our improved understanding of maternal and fetal health with exposure to these substances.
A Brief History of Placental Biology Studies
The importance of the placenta in human and mammalian development is long known. The placenta is referred to as an “external soul” and “bundle of life” in Hebrew scriptures, was recorded as a “secret helper” an unearthed Egyptian ceremonial slate, and is commonly referred to as the “afterbirth” in almost every modern language. The term “placenta” (meaning “flat cake” in Greek) was first known to be coined in 1559 by Realdus Columbus in De Re Anatomica. 7–9 Interestingly, Leonardo da Vinci, who has generally been well-regarded for the degree of accuracy in his anatomical illustrations, actually failed to include the placenta in his initially early 16th century drawing “fetus in utero,” which was the first detailed depiction of a human baby inside a womb.8 Consequently, it was the absence rather the presence of the placenta that garnished the attention of other illustrators of the time, and the placenta and its distribution of umbilical vessels were accurately depicted in detail by Andreas Vesalius (1555) and Nicolas Hoboken (1669).7 In the late 18th and early 19th centuries, the notion of a lack of vascular continuity between the uterus and placenta was put forward in a first-of-its-kind obstetrical atlas that described the placenta and its membranes in detail.10 In addition to progressive anatomical viewpoints, an appreciation of the functional role of the placenta in maintaining pregnancy and maternal-fetal communication has evolved.11,12 However, the intimacy and importance of the uteroplacental-fetal vascular unit was not firmly established nor largely accepted until the twentieth century.13 Today, the placenta is no longer just seen as an effective maternal-fetal barrier as it once classically was viewed. Instead, a more contemporary, holistic view suggests that the placenta essentially allows for the exchange of all known substances (except macromolecules) by either passive diffusion, facilitated diffusion, active transport, endocytosis, or other mechanisms.7
Tobacco Smoke, Nicotine, and the Placenta
The risks of tobacco use in general and more specifically during pregnancy have been well documented for over 75 years4,14 (Fig. 1). Although studies in the United States have found a gradual decrease in smoking during pregnancy from 25.7% in 1985 to 6.9% in 2017,5 it still remains prevalent and continues to be an important modifiable risk factor to improve pregnancy outcomes (Fig. 2). Surveys show that smoking prevalence varies across geographical locations, race, educational qualifications, and age groups.5,15–17 Various placental functions can be altered because of first and secondhand exposure to tobacco. More than 8400 different chemicals have been characterized from tobacco products and tobacco smoke,18 many of which can readily cross the placenta and affect the fetus adversely. Nicotine is a highly studied, pharmacologically active compound found in tobacco smoke, can easily cross the placenta and reaches a fetal concentration which is 15% higher than in the mother. It is acknowledged that various placental functions are altered because of first and secondhand exposure to combustible tobacco smoke. Several studies, both in vitro and in vivo have reported observable changes in placental morphology following tobacco exposure, including decreases in overall vascularization, vasculosyncytial membrane and cytotrophoblastic proliferation and increases in syncytial knots and syncytiotrophoblast necrosis resulting because of cigarette smoking.19,20 All of these alterations have the potential to contribute to placental insufficiency, which reduces nutrient exchange between maternal and fetal circulation eventually causing various complications.
FIGURE 1.
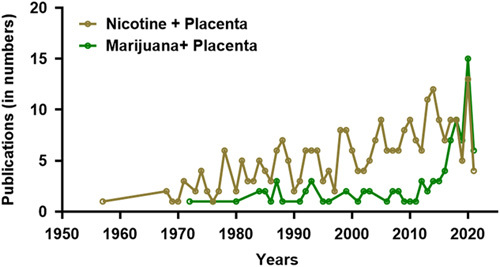
Publication trends as obtained from PubMed search (August 23, 2021) using the “advanced search tool” with field combinations namely, “nicotine + placenta” and “marijuana + placenta,” show a consistent increase in nicotine and placenta studies since 1970s, and a steep rise in marijuana and placenta studies after 2010.
FIGURE 2.
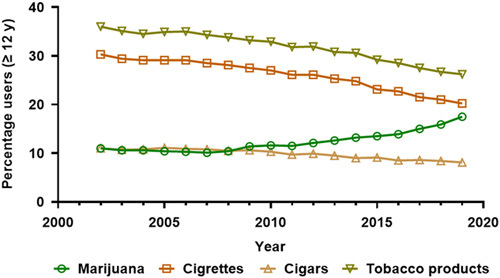
Marijuana and tobacco product use per annum among persons aged 12 or older expressed as percentages of the US population, 2002 to 2019. Data source: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2002 to 2019.
Given the popularity of electronic nicotine delivery systems (ENDS), an understanding of the impact of nicotine alone on placental development (without the other harmful compounds detected in combustible tobacco smoke) is important for improved public health messaging. Only recently, compared with the decades of studies of combustible tobacco smoke exposure, have reports started to document the prevalence of ENDS use as well as the outcomes in pregnancy.21–23 One caveat to large, population-based studies concerning ENDS use, however, is that many users of these devices also smoke combustible tobacco cigarettes. Therefore, studies in cell culture and animal models of nicotine administration have given us insight into changes in the placenta. From our recent comprehensive review of the literature, we have summarized changes in placental development with nicotine exposure.24 Because nicotinic acetyl choline receptors (nAChRs) are expressed in the placenta throughout development, nicotine exposure alters the abundance of these receptors.25,26 Animal model studies and cell culture experiments of nicotine treatment in pregnancy revealed changes in placental histology, gene expression levels, and cellular differentiation.27–29
For a more in-depth description of the effects of tobacco smoke and nicotine on placental development, we refer the reader to our published, publicly available review.24 In this review we summarize relevant studies that use cell culture and rodent models to describe the oxidative damage to the placenta caused by maternal tobacco smoke exposure (MTSE) and the potential for vitamin C supplementation to be utilized to combat these detrimental effects. MTSE is associated with observable changes in epigenetic modifications in the placenta, including alterations to DNA methylation profiles. Furthermore, the placenta expresses nAChRs, which are bound and activated by nicotine. nAChRs are important for cellular proliferation, cell adhesion, and cell migration. Exogenous nicotine from MTSE and ENDS use can activate nAChRs and alter their expression profiles and downstream effects.
Maternal Smoking and Preeclampsia
Preeclampsia (PRE) is one of the most common hypertensive disorders of pregnancy and is thought to have an etiology tied to placental development. A worldwide meta-analysis study of PRE inclusive of data from 30 countries between 1969 and 2019 showed an overall prevalence rate of 6.7%.30 Although smoking increases risks for hypertension in general, as well as adverse obstetrical outcomes like placental abruption and ectopic pregnancy, it is well established that maternal smoking is associated with a reduced risk of pregnancy-induced hypertension. A systematic review and meta-analysis comprising of a total of 13 studies revealed an overall odds ratio of 0.65 for PRE with maternal smoking, however, further subgroup analysis showed that it had protective role only in European and North American women, and not in Asian women.31 Furthermore, dose-dependent protective effects of smoking and PRE risk have been observed.32,33
While the protective effect of smoking on risk for developing PRE has been observed for decades, an understanding of the mechanism behind this decreased risk is lacking. Because the placenta is thought to be central in the pathogenesis of PRE34 an understanding of how maternal tobacco smoking throughout gestation influences placental pathology, physiology, and molecular biology is absolutely essential to fully understand the causes and to uncover potential treatments. On a gross anatomical level, placental pathology at delivery between smokers and nonsmokers with PRE does not reveal any significant differences in placental infarctions, decidual arteriopathy, or abruption.35
Hypotheses for the protective effect of smoking against PRE take into account the molecular mechanisms of trophoblast invasion and the remodeling of the maternal spiral arteries, which are necessary for a successful pregnancy. For example, placental levels of adrenomedulluin expression are higher in placentas from smokers than nonsmokers, and lower in women with PRE.36 Adrenomedullin plays a role in many aspects of a healthy pregnancy including implantation and the regulation of uterine and placental blood flow.37 The elevated levels of placenta growth factor observed in placentas from women who smoke has also been hypothesized to play a role in protection from PRE in smokers. Placenta growth factor has many roles in pregnancy including trophoblast invasion.38
Another potential player in the protective effect of smoking on risk of PE includes increased expression of the Aryl hydrocarbon receptors in the placenta which occurs in association with maternal smoking, prompting increased trophoblast invasion and spiral artery remodeling in the first trimester.39 Aryl hydrocarbon receptors are receptors which recognize and bind to xenobiotic compounds which enter cells. This has been well characterized in studies of polycyclic aromatic hydrocarbons, which are abundant in cigarette smoke.40
Because PRE is prevalent and is accompanied by high maternal and fetal morbidity and mortality, studies aiming to identify biomarkers which predict, diagnose, understand, and even treat PE have uncovered many players in this devastating illness. Exosomes, microRNAs, and placenta-derived molecules have all been noted to be altered in women with PRE.41–43 Studies of how smoking during pregnancy may alter these biomarkers and paradoxically protect against PRE will further aid our understanding of highly morbid disorder of pregnancy.
Marijuana Use and Placental Development
Marijuana (also referred to by its predominant cannabanoid, THC) is derived from the whole plant (dried leaves, flowers, stems, and seeds) of Cannabis sativa, otherwise known as the hemp plant. Cannabis plants contain over 500 chemicals, of which 104 cannabinoids have been presently identified.44 These cannabinoids can have both physical and mental effects when consumed45 and two cannabinoids in particular have been the subject of many scientific investigations for their pharmacological properties: Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD).44 Over the past 2 decades, the average THC content of cannabis, also known as cannabis potency, has increased from ∼4% to 12%,45 but levels as high as 30% have been detected in legal cannabis grown for recreational use.46
Although cannabis is commonly referred to as the most used “illicit” drug in United States, its medical use is currently legalized in 36 states and 4 territories. In addition, 18 states, 2 territories, and the District of Columbia have all enacted legislation to regulate cannabis for nonmedical, recreational use. Subsequently, there has been a significant increase in marijuana use in all age groups (Fig. 2).47 For women who are pregnant, marijuana is the most used recreational drug with more than 112,000 users in 2019, of whom 36,000 reported daily or almost daily use.47 There has been an overall increase in usage amongst gravidae from 3.4% in 2002-2003 to 7.0% in 2016-2017.48 While the components and the dose-related adverse perinatal outcomes of tobacco and alcohol are known, there is insufficient data regarding the varied properties of different cannabis components and products. As marijuana has several routes of application and use—through inhalation, oral and gastrointestinal tract absorption, skin absorption, or administration through rectal or vaginal suppositories— it is challenging to study and exceedingly difficult to identify causal risks.
Marijuana Use During Pregnancy
Marijuana use continues to rise across the United States, with the Centers for Disease Control and Prevention reporting an estimated 22.2 million users each month.47 This rise is associated with the increased legalization of marijuana as well as a rise in its potency over the years. An estimated 20% of female adolescents and young adults consume cannabis, according to the National Survey on Drug Use and Health.47 Marijuana has been identified as the most commonly utilized recreational drug during pregnancy,6 and the CDC reports that ∼1 in 20 women continue or begin using marijuana at some point during gestation.47 Epidemiological studies have identified certain groups of women who have an increased risk for gestational cannabis use. Within the 18 to 25 age group, marijuana use climbs to as high as 7.5% during gestation, differing by up to 3% from the general population of women who are pregnant, all based on data collected between 2002 and 2014.49 ACOG estimates that cannabis use during pregnancy increases up to 15% to 28% in young, urban, socioeconomically disadvantaged women.6 This finding especially raises concerns for women in minority populations.
Other investigators have attempted to elucidate the reasons why women may elect to utilize marijuana while pregnant, given the recent increase in consumption (Fig. 2). Nausea appears to be a common symptom inciting use, and is a common symptom of early pregnancy. A survey of Canadian women revealed that up to 77% of cannabis consumption during pregnancy was related to nausea.46 Further, data obtained from the Pregnancy Risk Assessment Monitoring System in Hawai’i showed that women who are experiencing significant gestational nausea report higher rates of use.50 Other pregnancy-related symptoms leading women to use marijuana include insomnia, lack of appetite, general pain, anxiety, depression, and fatigue.46
Although nausea is consistently the most common symptom prompting cannabis use, marijuana’s efficacy in treating or alleviating gestational nausea has yet to be validated, and current evidence suggests nonefficacy as an antiemetic. Preliminary data warrants further research into the relatively rare but increasingly documented cannabinoid hyperemesis syndrome (CHS). CHS has generally been characterized by cyclic nausea, vomiting, and abdominal pain in long-term marijuana users that temporarily improves with hot showers or baths.51 Characterization of this condition is undoubtedly more complicated in pregnant marijuana users but nonetheless, CHS is a condition provider should be familiar with as marijuana rates of usage continue to increase.
Despite an abundance of epidemiological data, most studies focusing upon the effects of marijuana during pregnancy are subject to many confounding factors, most notably polysubstance use.52 In addition, investigators rely on patient’s self-reported marijuana use, with no methods currently in existence to precisely validate the amount consumed through biological sampling, especially over a longitudinal time frame.52 To further complicate these efforts, marijuana use during pregnancy continues to evolve with the rise of new psychoactive substances, including synthetic cannabinoids, often with a higher-reported potency than natural cannabis.53
The Endocannabinoid System
The identification of THC in the early 1960s and the search for its mechanism of action lead to the discovery of the endocannabinoid system (ECS).44 The ECS is a complex cell-cell signaling system involving cannabinoid receptors, enzymes, and endogenous cannabinoids—endogenous molecules or endocannabinoids (eCBs) made by the body with similar properties to the cannabinoids derived from cannabis plants. Within this ECS system, two important cannabinoid receptors 1 and 2, respectively known as CB1 and CB2 and 2 eCBs—anandamide (AEA) and 2-arachidonoylglycerol (2-AG)—have been identified and studied.54
eCBs are inherent to the functioning of the ECS. They are primarily esters, amides or ethers synthesized from membrane phospholipids, and each eCB has its own distinct metabolic pathways.54 Derived from membrane phospholipids, AEA is synthesized through the action of 2 enzymes, N-acyltransferase (NAT) and N-acylphosphatidylethanolamine-phospholipaseD (NAPE-PLD) sequentially, with degradation catalyzed by fatty acid amide hydrolase (FAAH). 2-AG is produced from phospholipaseC (PLC) and diacylglycerol lipases (DAGL) respectively and degraded through the action of monoacylglycerol lipase (MAGL).54 Once produced, eCBs are released, which bind to cannabinoid receptors present on adjacent cell membranes. CB1 and CB2 are the 2 major types of G-coupled protein cannabinoid receptors, each binding both AEA and 2-AG. These cellular interactions ultimately lead to the modulation of the cAMP/PKA pathway. An understanding of this pathway is important because over the years, ECS signaling has been found to be ubiquitously expressed throughout the human body. It has even been shown to regulate a diverse array of physiological and pathophysiological events of the reproductive system including but not limited to oogenesis, spermatogenesis, implantation, decidualization, and labor.54–56 For these reasons, understanding the role of the ECS and its effects on placental development and function is of significant interest and likely importance.
The Endocannabinoid System in Normal Placenta
While the complex and multifaceted role of the ECS in placental biology remains to be completely uncovered, ECS macromolecules are expressed and present in the human placenta. CB1 and CB2 receptors are transcribed in human placental villous tissue during both the first and third trimester.57 In addition, the concentrations of both CB2 receptor mRNA and protein have been shown to increase in the third trimester.57 The placenta expresses alternative eCB targets as well, which adds to the complexity of ECS involvement in placental biology.54 DAGL and MAGL, the enzymes that synthesize and degrade 2-AG respectively, are similarly ubiquitously present across all trimesters of pregnancy, and expressed by both human cytotrophoblasts and syncytiotrophoblasts, and physiological levels of these enzymes are consistent across gestation and throughout trophoblast cellular differentiations.58 NAPE-PLD and FAAH, the enzymes that synthesize and degrade AEA respectively, are also present in the human placenta. Immunohistochemical analysis reveals that NAPE-PLD is primarily localized to the syncytial layer, whereas FAAH is expressed more prominently in the connective tissue.59 Just as ECS receptors and enzymes are found in the human placenta, it has been expected and validated that both of the primary eCBs, AEA59 and 2-AG,60 are transcribed and demonstrate function as well.
Trophoblasts, the characteristic epithelial cells of the placenta, undergo coordinated cellular events to achieve the normal growth, development, and function of the placenta. Much of what we know about the role of the ECS in placental biology comes from studies in which the levels of endogenous cannabinoids were manipulated. Utilizing an in vitro model (BeWo cells), it has been shown that AEA reduces trophoblast proliferation while inducing apoptosis through the action of caspases 3/7 and poly (ADP-ribose)polymerase 1 (PARP-1), coupled to a loss of mitochondrial membrane potential and the formation of reactive oxygen species (ROS).61,62 Both AEA and 2-AG appear to decrease proliferation of BeWo cells through a pathway mediated by cannabinoid receptors.56,58,63 Syncytiotrophoblast differentiation from cytotrophoblast cells is critical for the creation of the maternal-fetal interface. In human term cytotrophoblasts (38 to 40 wk of gestation) analyzed in vitro, 2-AG impedes differentiation through interaction with cannabinoid receptors.58 AEA does not appear to have the same effect.61 Trophoblast migration and invasion, also essential processes for the creation and maintenance of the maternal-fetal interface, appear to be modulated through intervention of the ECS. In a CB1-knockout murine model, invasion of trophoblast stem cells was hindered as compared with the controls.64 Interestingly, eCBs have been shown to hinder invasion in tumor cells, with similar migratory capacities to the aforementioned trophoblasts, partially because of lowered levels of matrix metalloproteinases.64,65
The placenta plays a critical role in maternal-fetal transport, with the ECS appearing to have an influence on the transport of important nutrients and resources. Folic acid transport, imperative for the development of the nervous system in utero, was decreased in BeWo cells in response to acute exposure to AEA, while transport increased in response to chronic treatment with AEA. Further, the baseline constriction of chorionic plate arteries and veins have been shown to increase with exposure to AEA, although this finding appears to be dependent on oxygen concentration.66,67
In vivo animal models have also helped to elucidate the role of the ECS in normal placental development. ECS components are expressed in both the murine and rat placenta.54 In the absence of CB1 receptors in mice, trophoblast proliferation, differentiation, and invasion are all impaired.64 Modulation of CB1 signaling can also limit trophoblast attachment, migration, and spreading in mice at the genetic level.68 In addition to rodent models, baboons have also been used to better understand the function of the ECS in placental development. One study utilizing a nonhuman primate model suggested that the placenta may be a major source of 2-AG during pregnancy, as evidenced by 10-fold higher levels of 2-AG in the placenta as compared with maternal blood.69 Thus, the levels of eCBs and their associated receptors and enzymatic machinery must be carefully regulated for the placenta to develop and function appropriately and for pregnancy to be maintained.
THC Metabolism in the Body
Upon exposure to marijuana, THC—the primary psychoactive component of cannabis—is extensively metabolized. With over 80 potential metabolites, <0.05% of THC consumed is ultimately excreted without modification.70 While the liver is the primary site of THC metabolism (so-called first pass metabolism), other sites including the gut, lungs, and heart are thought to contribute as well.71 The action of these alternative sites is ultimately dependent on the route of marijuana exposure, with methods of use including smoking, oral absorption, skin absorption, and vaginal and rectal suppositories.52 After smoking cannabis, the highest concentrations of THC are observed within minutes, whereas with oral intake, the peak concentration takes ∼1 to 3 hours to manifest.72 Smoking also leads to greater average peak concentrations overall, as compared with oral intake.73 When administered intravenously, the greatest psychological “high” is reported to occur within 15 minutes.74 Rates of systemic clearance exist along a range of ∼12 to 36 L/h.70 Ultimately, there is variability in the concentrations of THC found in the body post exposure, along with variable rates of clearance among individuals.
Undergoing first pass maternal metabolism, THC is largely processed by cytochrome P450 enzymes (CYP). Two of the major THC metabolites, including 11-hydroxy-tetrahydrocannabinol (11-OH-THC) and 11-nor-9-carboxy-tetrahydrocannabinol (THC-COOH), are processed in a sequential metabolic pathway.75 CYP2C9 and CYP2C19 catalyze the hydroxylation of THC to produce 11-OH-THC, and CYP2C9 and CYP3A4 oxidize 11-OH-THC thereafter to produce 11-nor-THC-COOH.75 These metabolites can then undergo glucuronidation through the action of UDP-glucuronosyltransferase (UGT) enzymes.76 UGT1A9 and UGT1A10 act upon 11-OH-THC, and UGT1A1 and UGT1A3 act on THC-COOH.76 THC and its metabolites are subsequently hematogenously transported and exert actions on target tissues, or undergo storage or excretion. 11-OH-THC has been noted to have even more pharmacological and psychoactive effects than unmodified THC.77 Inactive THC-COOH is often detected in traditional drug tests, considering its very low rate of clearance. THC and its metabolites can be stored in fatty tissue, because of their highly lipophilic nature, or can be excreted through the urine.75 In addition, THC and its metabolites can exit the body through the fecal route, either through biliary secretion of metabolites or THC-processing post oral intake.78
In addition to this traditional maternal first pass metabolic pathway, THC can also reach the placenta and fetal bloodstream in women who are pregnant. The metabolites 11-OH-THC and THC-COOH can cross the placenta as well, although with less efficiency than THC.52 The concentration of THC in fetal circulation generally falls between one-10th to one-third of maternal concentrations. The placenta controls fetal exposure to the components of marijuana, with variability in rates of placental transport and permeability existing between individuals. In addition, differing routes of THC intake, as well as differences in the length and timing of intake, add additional complexities to studying fetal marijuana exposure in humans.55
THC is an Antioxidant but Causes Oxidative Damage to the Placenta
Interestingly, THC is characterized as an antioxidant, yet induces oxidative stress in the placenta. Studies utilizing in vitro models have demonstrated THC’s role in the production of ROS. Cannabinoids, including THC, activate mitochondrial and endoplasmic reticulum stress pathways.49 This can occur independently of cannabinoid receptors,79 ultimately resulting in the production of ROS and decreased trophoblast viability.80 THC exposure can increase ROS production by up to 2-fold in BeWo.49 This increase in ROS is accompanied by elevated expression of the heat shock proteins 60 and 70 (HSP60 and HSP70) as well as superoxide dismutase 1 and 2 (SOD1 and SOD2), the cytosolic and mitochondrial isoforms, respectively.81 Considering that SOD1 and SOD2 are responsible for regulating the production of ROS, this elevation in enzyme expression may be a compensatory mechanism used by the placenta in response to THC-induced oxidative stress. Researchers also observed elevated expression of DRP1, a mitochondrial fission effector, in response to THC treatment, leading to trophoblast dysfunction. This may ultimately be the result of increased oxidative stress.81–83
Although THC ubiquitously induces cellular stress, this effect appears to be concentration dependent. Low THC concentrations (1 to 25 μmol/l) led to lowered oxidative and nitrative stress, along with increased production of oxidized glutathione, increasing syncytiotrophoblast viability.79 Low THC concentrations also led to increased mitochondrial activity in syncytiotrophoblasts, as appreciated through an MTT assay. Since cytotrophoblast and syncytiotrophoblast remodeling and turnover are critical for the proper growth and development of the placenta, this antioxidant effect may ultimately inhibit placental function, despite some studies suggesting a protective effect.84 At higher concentrations (75 μmol/l), THC led to increased oxidative and nitrative stress, prompting the proposal of THC’s dual effect, independent of cannabinoid receptors.53 Overall, THC appears to have a dual effect particularly on syncytiotrophoblasts, adding to the complexity of marijuana’s effects on placental biology.
Conclusion
We have detailed herein the biochemistry and functional pathophysiology of tobacco, nicotine, and marijuana metabolism during pregnancy, focusing on the role of the placenta as both effector and mediator of these processes. While the first reports on adverse effects of tobacco smoking were documented in the 1950s,4 hundreds of studies in the decades hence have documented and explained the underlying placental molecular mechanisms driving these adverse associations. In the recent 2 decades, similar studies have elucidated how potential harm with cannabinoids may similarly occur during pregnancy. Even though these efforts have resulted in a reduction in the use of prenatal tobacco and nicotine (9.2% in 2010 to 6.1% in 2020),5 exposure to other harmful chemicals and macromolecules through opioids, cocaine and marijuana have risen considerably over recent years, with marijuana (THC) being the most used drug during the prenatal period.6
Culturally, we are at a crossroads. On the one hand, decriminalization and legalization of marijuana has allowed for some nonpregnant persons to receive potential (or presumed) health benefits. However, in pregnancy, we appropriately set a high bar for determination of both safety and efficacy of exposures in pregnancy. This is for both maternal and fetal safety and wellbeing. Until well-conducted, rigorously controlled preclinical and clinical studies can be completed, it is most appropriate and scientifically justified to assume that no amount of tobacco, nicotine, nor marijuana is known to be unequivocally safe for use in pregnancy.
Acknowledgments
The authors acknowledge funding from the NIH for their partial support this work (R01HD091731, R21ES029462, P42ES027725 to KMA). The authors would like to thank Dr Alexa Sassin for her input during the revision of this chapter.
Footnotes
The authors declare that they have nothing to disclose.
Contributor Information
SOHINI BANERJEE, Email: sohini.banerjee@bcm.edu.
ALYSSA DEACON, Email: ahadeacon@gmail.com.
MELISSA A. SUTER, Email: masuter@bcm.edu.
KJERSTI M. AAGAARD, Email: aagaardt@bcm.edu.
References
- 1. Suter MA, Aagaard K. What changes in DNA methylation take place in individuals exposed to maternal smoking in utero? Editorial. Epigenomics. 2012;4:115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Suter MA, Aagaard-Tillery KM. Environmental influences on epigenetic profiles. Research Support, N.I.H., Extramural Review. Semin Reprod Med. 2009;27:380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suter MA, Anders AM, Aagaard KM. Maternal smoking as a model for environmental epigenetic changes affecting birthweight and fetal programming. Research Support, N.I.H., Extramural Review. Mol Hum Reprod. 2013;19:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simpson WJ. A preliminary report on cigarette smoking and the incidence of prematurity. Am J Obstet Gynecol. 1957;73:808–815. [PubMed] [Google Scholar]
- 5. Azagba S, Manzione L, Shan L, et al. Trends in smoking during pregnancy by socioeconomic characteristics in the United States, 2010–2017. BMC Preg Childbirth. 2020;20:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ayonrinde OT, Ayonrinde OA, Van Rooyen D, et al. Association between gestational cannabis exposure and maternal, perinatal, placental, and childhood outcomes. J Develop Origins Health Dis. 2020;12:694–703. [DOI] [PubMed] [Google Scholar]
- 7. Longo LD, Reynolds LP. Some historical aspects of understanding placental development, structure and function. Int J Develop Biol. 2009;54:237–255. [DOI] [PubMed] [Google Scholar]
- 8. Loke YW. Life’s Vital Link: The Astonishing Role of the Placenta. New York, NY: Oxford University Press; 2013. [Google Scholar]
- 9. Colombo R. De re anatomica libri XV. Ex typographia Nicolai Bevilacquae, Venetiis. 1559.
- 10. Wagoner N. Anatomia Uteri Humani Gravidi Tabulis Illustrata [The Anatomy of the Human Gravid Uterus Exhibited in Figures], (1774) by William Hunter. Embryo Project Encyclopedia. 2017.
- 11. Keibel F, Mall FP. Manual of Human Embryology. Philadelphia, PA: J.B. Lippincott Company; 1910. [Google Scholar]
- 12. Mossman HW. Classics revisited: comparative morphogenesis of the fetal membranes and accessory uterine structures. Placenta (Eastbourne). 1991;12:1–5. [DOI] [PubMed] [Google Scholar]
- 13. Weber EH. Handbuch der Anatomie des Menschen. Braunschweig: Verlag der Schulbuchhandlung; 1832. [Google Scholar]
- 14. Bayne-Jones S. Smoking and Health: Report of the Advisory Committee to the Surgeon General of the Public Health Service. Washington, DC: US Department of Health, Education, and Welfare, Public Health Service; 1964. [Google Scholar]
- 15. Nighbor TD, Doogan NJ, Roberts ME, et al. Smoking prevalence and trends among a US national sample of women of reproductive age in rural versus urban settings. PLoS One. 2018;13:e0207818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tong VT, Dietz PM, Morrow B, et al. Trends in smoking before, during, and after pregnancy—Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000–2010. Morbid Mortal Wkly Rep. 2013;62:1–19. [PubMed] [Google Scholar]
- 17. Azagba S, Shan L, Latham K, et al. Trends in cigarette smoking among American Indians and Alaska natives in the USA: 1992–2015. Cancer Causes Control. 2020;31:73–82. [DOI] [PubMed] [Google Scholar]
- 18. Rodgman A, Perfetti TA. The Chemical Components of Tobacco and Tobacco Smoke. Boca Raton, FL: CRC Press; 2008. [Google Scholar]
- 19. Sbrana E, Suter MA, Abramovici AR, et al. Maternal tobacco use is associated with increased markers of oxidative stress in the placenta. Am J Obstet Gynecol. 2011;205:246.e1–246.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pintican D, Poienar AA, Strilciuc S, et al. Effects of maternal smoking on human placental vascularization: a systematic review. Taiwan J Obstet Gynecol. 2019;58:454–459. [DOI] [PubMed] [Google Scholar]
- 21. Hawkins SS, Wylie BJ, Hacker MR. Use of ENDS and cigarettes during pregnancy. Am J Prev Med. 2020;58:122–128. [DOI] [PubMed] [Google Scholar]
- 22. Cardenas VM, Fischbach LA, Chowdhury P. The use of electronic nicotine delivery systems during pregnancy and the reproductive outcomes: a systematic review of the literature. Tob Induc Dis. 2019;17:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cardenas VM, Cen R, Clemens MM, et al. Use of Electronic Nicotine Delivery Systems (ENDS) by pregnant women I: risk of small-for-gestational-age birth. Tob Induc Dis. 2019;17:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Suter MA, Aagaard KM. The impact of tobacco chemicals and nicotine on placental development. Prenat Diagn. 2020;40:1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holloway AC, Salomon A, Soares MJ, et al. Characterization of the adverse effects of nicotine on placental development: in vivo and in vitro studies. Am J Physiol Endocrinol Metab. 2014;306:E443–E456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Machaalani R, Ghazavi E, Hinton T, et al. Cigarette smoking during pregnancy regulates the expression of specific nicotinic acetylcholine receptor (nAChR) subunits in the human placenta. Toxicol Appl Pharmacol. 2014;276:204–212. [DOI] [PubMed] [Google Scholar]
- 27. Correia-Branco A, Keating E, Martel F. Placentation-related processes in a human first-trimester extravillous trophoblast cell line (HTR-8/SVneo cells) are affected by several xenobiotics. Drug Chem Toxicol. 2019;42:541–545. [DOI] [PubMed] [Google Scholar]
- 28. Zhou J, Liu F, Yu L, et al. nAChRs-ERK1/2-Egr-1 signaling participates in the developmental toxicity of nicotine by epigenetically down-regulating placental 11β-HSD2. Toxicol Appl Pharmacol. 2018;344:1–12. [DOI] [PubMed] [Google Scholar]
- 29. Kwon J-Y, Bai S-W, Kwon Y-G, et al. The effect of nicotine on the production of soluble fms-like tyrosine kinase-1 and soluble endoglin in human umbilical vein endothelial cells and trophoblasts. Acta Obstet Gynecol Scand. 2010;89:565–571. [DOI] [PubMed] [Google Scholar]
- 30. Macedo TC, Montagna E, Trevisan CM, et al. Prevalence of preeclampsia and eclampsia in adolescent pregnancy: a systematic review and meta-analysis of 291,247 adolescents worldwide since 1969. Eur J Obstet Gynecol Reprod Biol. 2020;248:177–186. [DOI] [PubMed] [Google Scholar]
- 31. Wang J, Yang W, Xiao W, et al. The association between smoking during pregnancy and hypertensive disorders of pregnancy: a systematic review and meta-analysis. Int J Gynecol Obstet. 2021. doi: 10.1002/ijgo.13709. [DOI] [PubMed] [Google Scholar]
- 32. Hammoud AO, Bujold E, Sorokin Y, et al. Smoking in pregnancy revisited: findings from a large population-based study. Am J Obstet Gynecol. 2005;192:1856–1862. [DOI] [PubMed] [Google Scholar]
- 33. Wei J, Liu C-X, Gong T-T, et al. Cigarette smoking during pregnancy and preeclampsia risk: a systematic review and meta-analysis of prospective studies. Oncotarget. 2015;6:43667–43678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rana S, Lemoine E, Granger JP, et al. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124:1094–1112. [DOI] [PubMed] [Google Scholar]
- 35. Vinnars MT, Falkare S, Papadogiannakis N, et al. Placental pathology in smoking and non-smoking preeclamptic women. J Matern Fetal Neonat Med. 2016;29:733–736. [DOI] [PubMed] [Google Scholar]
- 36. Kraus DM, Feng L, Heine RP, et al. Cigarette smoke-induced placental adrenomedullin expression and trophoblast cell invasion. Reprod Sci. 2014;21:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Di Iorio R, Marinoni E, Letizia C, et al. Adrenomedullin in perinatal medicine. Regul Pept. 2003;112:103–113. [DOI] [PubMed] [Google Scholar]
- 38. Kawashima A, Koide K, Hasegawa J, et al. Maternal smoking history enhances the expression of placental growth factor in invasive trophoblasts at early gestation despite cessation of smoking. PLoS One. 2015;10:e0134181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang K, Zhou Q, He Q, et al. The possible role of AhR in the protective effects of cigarette smoke on preeclampsia. Med Hypotheses. 2011;77:872–874. [DOI] [PubMed] [Google Scholar]
- 40. Klingbeil E, Hew K, Nygaard UC, et al. Polycyclic aromatic hydrocarbons, tobacco smoke, and epigenetic remodeling in asthma. Immunol Res. 2014;58:369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pillay P, Moodley K, Moodley J, et al. Placenta-derived exosomes: potential biomarkers of preeclampsia. Int J Nanomed. 2017;12:8009–8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lv Y, Lu C, Ji X, et al. Roles of microRNAs in preeclampsia. J Cell Physiol. 2019;234:1052–1061. [DOI] [PubMed] [Google Scholar]
- 43. Ahmed A, Rezai H, Broadway-Stringer S. Evidence-based revised view of the pathophysiology of preeclampsia. Hypertension. 2016;956:355–374. [DOI] [PubMed] [Google Scholar]
- 44. Lafaye G, Karila L, Blecha L, et al. Cannabis, cannabinoids, and health. Dialogues Clin Neurosci. 2017;19:309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. ElSohly MA, Mehmedic Z, Foster S, et al. Changes in cannabis potency over the last 2 decades (1995-2014): analysis of current data in the United States. Biol Psychiatry. 2016;79:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grant KS, Petroff R, Isoherranen N, et al. Cannabis use during pregnancy: pharmacokinetics and effects on child development. Pharmacol Ther. 2018;182:133–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. SAMHSA. Key Substance Use and Mental Health Indicators in the United States: Results From the 2019 National Survey on Drug Use and Health (HHS Publication No PEP20-07-01-001, NSDUH Series H-55). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2020. Available at: https://www.samhsa.gov/data/ Accessed July, 2021. [Google Scholar]
- 48. Volkow ND, Han B, Compton WM, et al. Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA. 2019;322:167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Walker OLS, Ragos R, Gurm H, et al. Delta-9-tetrahydrocannabinol disrupts mitochondrial function and attenuates syncytialization in human placental BeWo cells. Physiologic Rep. 2020;8:e14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ryan SA, Ammerman SD, O’Connor ME. Marijuana use during pregnancy and breastfeeding: implications for neonatal and childhood outcomes. Pediatrics. 2018;142:3. [DOI] [PubMed] [Google Scholar]
- 51. Sorensen CJ, DeSanto K, Borgelt L, et al. Cannabinoid hyperemesis syndrome: diagnosis, pathophysiology, and treatment—a systematic review. J Med Toxicol. 2017;13:71–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Thompson R, DeJong K, Lo J. Marijuana use in pregnancy: a review. Obstet Gynecologic Surv. 2019;74:415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Carlier J, Huestis MA, Zaami S, et al. Monitoring perinatal exposure to cannabis and synthetic cannabinoids. Ther Drug Monit. 2020;42:194–204. [DOI] [PubMed] [Google Scholar]
- 54. Costa M. The endocannabinoid system: a novel player in human placentation. Reprod Toxicol. 2016;61:58–67. [DOI] [PubMed] [Google Scholar]
- 55. Dubovis M, Muneyyirci-Delale O. Effects of marijuana on human reproduction. Reprod Toxicol. 2020;94:22–30. [DOI] [PubMed] [Google Scholar]
- 56. Habayeb OM, Taylor AH, Bell SC, et al. Expression of the endocannabinoid system in human first trimester placenta and its role in trophoblast proliferation. Endocrinology. 2008;149:5052–5060. [DOI] [PubMed] [Google Scholar]
- 57. Chang X, Bian Y, He Q, et al. Suppression of STAT3 signaling by Δ9-tetrahydrocannabinol (THC) induces trophoblast dysfunction. Cell Physiol Biochem. 2017;42:537–550. [DOI] [PubMed] [Google Scholar]
- 58. Costa M, Keating E, Fonseca B, et al. 2-Arachidonoylglycerol impairs human cytotrophoblast cells syncytialization: influence of endocannabinoid signalling in placental development. Mol Cell Endocrinol. 2015;399:386–394. [DOI] [PubMed] [Google Scholar]
- 59. Maia J, Midão L, Cunha S, et al. Effects of cannabis tetrahydrocannabinol on endocannabinoid homeostasis in human placenta. Arch Toxicol. 2019;93:649–658. [DOI] [PubMed] [Google Scholar]
- 60. Maia J, Fonseca BM, Cunha SC, et al. Impact of tetrahydrocannabinol on the endocannabinoid 2-arachidonoylglycerol metabolism: ABHD6 and ABHD12 as novel players in human placenta. Biochim Biophy Acta. 2020;1865:158807. [DOI] [PubMed] [Google Scholar]
- 61. Costa M, Fonseca B, Keating E, et al. Transient receptor potential vanilloid 1 is expressed in human cytotrophoblasts: induction of cell apoptosis and impairment of syncytialization. Int J Biochem Cell Biol. 2014;57:177–185. [DOI] [PubMed] [Google Scholar]
- 62. Costa M, Fonseca B, Teixeira N, et al. The endocannabinoid anandamide induces apoptosis in cytotrophoblast cells: involvement of both mitochondrial and death receptor pathways. Placenta. 2015;36:69–76. [DOI] [PubMed] [Google Scholar]
- 63. Costa M, Fonseca B, Keating E, et al. 2-arachidonoylglycerol effects in cytotrophoblasts: metabolic enzymes expression and apoptosis in BeWo cells. Reproduction. 2014;147:301–311. [DOI] [PubMed] [Google Scholar]
- 64. Sun X, Xie H, Yang J, et al. Endocannabinoid signaling directs differentiation of trophoblast cell lineages and placentation. Proc Natl Acad Sci. 2010;107:16887–16892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Freimuth N, Ramer R, Hinz B. Antitumorigenic effects of cannabinoids beyond apoptosis. J Pharmacol Exp Ther. 2010;332:336–344. [DOI] [PubMed] [Google Scholar]
- 66. Wareing M, Bai X, Seghier F, et al. Expression and function of potassium channels in the human placental vasculature. Am J Physiol. 2006;291:R437–R446. [DOI] [PubMed] [Google Scholar]
- 67. Araújo JR, Goncalves P, Martel F. Effect of cannabinoids upon the uptake of folic acid by BeWo cells. Pharmacology. 2009;83:170–176. [DOI] [PubMed] [Google Scholar]
- 68. Xie H, Sun X, Piao Y, et al. Silencing or amplification of endocannabinoid signaling in blastocysts via CB1 compromises trophoblast cell migration. J Biol Chem. 2012;287:32288–32297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Brocato B, Zoerner AA, Janjetovic Z, et al. Endocannabinoid crosstalk between placenta and maternal fat in a baboon model (Papio spp.) of obesity. Placenta. 2013;34:983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hunt CA, Jones RT. Tolerance and disposition of tetrahydrocannabinol in man. J Pharmacol Exp Ther. 1980;215:35–44. [PubMed] [Google Scholar]
- 71. Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42:327–360. [DOI] [PubMed] [Google Scholar]
- 72. Ahmed AIA, Elsen GAHvd, Colbers A, et al. Safety, pharmacodynamics, and pharmacokinetics of multiple oral doses of delta-9-tetrahydrocannabinol in older persons with dementia. Psychopharmacology (Berl). 2015;232:2587–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kauert GF, Ramaekers JG, Schneider E, et al. Pharmacokinetic properties of 9-tetrahydrocannabinol in serum and oral fluid. J Anal Toxicol. 2007;31:288–293. [DOI] [PubMed] [Google Scholar]
- 74. Ohlsson A, Lindgren JE, Wahlen A, et al. Plasma delta-9-tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol Ther. 1980;28:409–416. [DOI] [PubMed] [Google Scholar]
- 75. Neradugomma NK, Drafton K, Mor GG, et al. Marijuana-derived cannabinoids inhibit uterine endometrial stromal cell decidualization and compromise trophoblast-endometrium cross-talk. Reprod Toxicol. 2019;87:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mazur A, Lichti CF, Prather PL, et al. Characterization of human hepatic and extrahepatic UDP-glucuronosyltransferase enzymes involved in the metabolism of classic cannabinoids. Drug Metab Dispos. 2009;37:1496–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Christensen HD, Freudenthal RI, Gidley JT, et al. Activity of delta8- and delta9-tetrahydrocannabinol and related compounds in the mouse. Science. 1971;172:165–167. [DOI] [PubMed] [Google Scholar]
- 78. Lemberger L, Axelrod J, Kopin IJ. Metabolism and disposition of tetrahydrocannabinols in näive subjects and chronic marijuana users. Ann N Y Acad Sci. 1971;191:142–154. [Google Scholar]
- 79. Costa MA, Fonseca BM, Marques F, et al. The psychoactive compound of Cannabis sativa, Δ(9)-tetrahydrocannabinol (THC) inhibits the human trophoblast cell turnover. Toxicology. 2015;334:94. [DOI] [PubMed] [Google Scholar]
- 80. Lojpur T, Easton Z, Raez-Villanueva S, et al. Δ9-Tetrahydrocannabinol leads to endoplasmic reticulum stress and mitochondrial dysfunction in human BeWo trophoblasts. Reprod Toxicol. 2019;87:21–31. [DOI] [PubMed] [Google Scholar]
- 81. Guo X, Sesaki H, Qi X. Drp1 stabilizes p53 on the mitochondria to trigger necrosis under oxidative stress conditions in vitro and in vivo. Biochem J. 2014;461:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang Y, Walsh SW. Placental mitochondria as a source of oxidative stress in pre-eclampsia. Placenta. 1998;19:581–586. [DOI] [PubMed] [Google Scholar]
- 83. Gan X, Huang S, Wu L, et al. Inhibition of ERK-DLP1 signaling and mitochondrial division alleviates mitochondrial dysfunction in Alzheimer’s disease cybrid cell. Bioch Biophys Acta Mol Basis Dis. 2014;1842:220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Neradugomma NK, Drafton K, O’Day DR, et al. Marijuana use differentially affects cannabinoid receptor expression in early gestational human endometrium and placenta. Placenta. 2018;66:36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]


