Abstract
Rationale & Objective
Neutralizing monoclonal antibody treatments have shown promising preliminary results in kidney transplant recipients infected with severe acute respiratory syndrome coronavirus 2. However, their efficacy in kidney transplant recipients infected with the Omicron variant has not been reported yet.
Study Design
Single-center retrospective study.
Setting & Participants
We included all consecutive kidney transplant recipients treated with monoclonal antibodies for severe acute respiratory syndrome coronavirus 2 infections (positive polymerase chain reaction on nasopharyngeal swab) between June 10, 2021, and January 14, 2022. Forty-seven kidney transplant recipients were included. All patients had symptoms evolving for ≤7 days and no oxygen therapy need at monoclonal antibody infusion.
Results
Symptoms at diagnosis were mainly cough (n = 25; 53%) and fever (n = 15; 32%). Eighty-three percent of the cohort (n = 39) had been vaccinated with at least 2 doses before infection, of whom 30 (77%) had demonstrated a vaccine-induced humoral response. They were treated with either casirivimab-imdevimab (n = 16; 34%) or sotrovimab (n = 31; 66%) a median of 2 days (range, 0-6 days) after the onset of symptoms. Except for 1 mild allergic reaction during casirivimab-imdevimab infusion, no side effects were reported. The median viral loads at admission (day 0) and 7 days after monoclonal antibody infusion were 2,110,027 copies/mL (range, 1,000-153,798,962 copies/mL) and 1,000 copies/mL (range, 0-10,000,000 copies/mL), respectively. Genotypes were available for 22 kidney transplant recipients (47%). Omicron, Delta, and Gamma variants were identified in 13 (59%), 8 (36%), and 1 (5%) patients, respectively. In kidney transplant recipients infected with the Omicron variant, the median viral loads at day 0 and day 7 were 752,789 copies/mL (range, 4,000-12,859,300 copies/mL) and 1,353 copies/mL (range, 0-1,211,163 copies/mL), respectively. 2 kidney transplant recipients required hospitalization immediately after sotrovimab perfusion for oxygen therapy that was weaned in 3 days, allowing patients’ discharge. None were admitted to the intensive care unit or died.
Limitations
Small sample size, no control group.
Conclusions
Neutralizing monoclonal antibody therapy is associated with positive outcomes in kidney transplant recipients with mild coronavirus disease 2019, including those infected with the Omicron variant.
Index Words: COVID-19, ESKD, hospitalization, kidney transplant, monoclonal antibody, Omicron, SARS-CoV-2
Plain-Language Summary.
Neutralizing monoclonal antibody treatments have shown promising preliminary results in kidney transplant recipients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, its efficacy in kidney transplant recipients infected with the emerging Omicron variant has not been reported yet. This single-center, retrospective study describes our experience with monoclonal antibody therapy in 47 kidney transplant recipients with mild coronavirus disease 2019 (including 13 with the Omicron variant). Patients were treated with either casirivimab-imdevimab (n = 16) or sotrovimab (n = 31) between June 10, 2021, and January 14, 2022. Monoclonal antibody therapy was associated with a 4% rate of hospitalization for oxygen therapy, without major side effects. The 13 kidney transplant recipients with the Omicron variant of SARS-CoV-2 infection were all treated with sotrovimab, with no need for subsequent hospitalization and a significant drop in the SARS-CoV-2 viral load 7 days after treatment.
As of January 2022, more than 356,000,000 cases and 5,600,000 deaths have been reported by the World Health Organization because of the coronavirus disease 2019 (COVID-19) pandemic, and the numbers continue to increase.1 Solid organ transplant recipients—including kidney transplant recipients—were recognized early as a particularly vulnerable population to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, with increased mortality rates of around 20%-30%.2,3 These excess deaths are most likely triggered by the maintenance immunosuppressive treatments received to prevent graft rejection4 and the comorbid conditions affecting kidney transplant recipients.5,6
Since the onset of the outbreak, an unprecedented, rapid development of therapeutic options occurred, aiming to improve the outcomes of patients with COVID-19. Among them, vaccines have proven to be efficacious in reducing the risks of both severe disease and mortality in the general population.7 Unfortunately, emerging evidence has revealed that kidney transplant recipients actually display a reduction in vaccine-induced humoral responses.8 Moreover, the clinical benefit of vaccines to protect against severe COVID-19 has appeared lower in solid organ transplant recipients compared with the general population.9
Taken together, these data suggest that alternative strategies are required for kidney transplant recipients.10 Among them, neutralizing monoclonal antibody therapy deserves consideration. Indeed, casirivimab-imdevimab11 and sotrovimab12 were recently reported to be safe and effective in reducing a composite end point combining hospitalization and mortality in 2 phase 3 placebo-controlled trials including immunocompetent patients infected with SARS-CoV-2 at risk of progression. Unfortunately, kidney transplant recipients were excluded from the studies. Nevertheless, we and others13, 14, 15, 16, 17 have reported case series suggesting that neutralizing monoclonal antibody therapy may be used safely in kidney transplant recipients with mild forms of COVID-19. However, concerns were recently raised regarding the efficacy of monoclonal antibody therapy in patients infected with the B.1.1.529 (Omicron) variant of SARS-CoV-2. Indeed, in vitro investigations have suggested that some antibodies used in clinics might lose affinity and efficacy against this variant.18,19 Moreover, to the best of our knowledge, the efficacy of monoclonal antibody therapy in kidney transplant recipients infected with the Omicron variant of SARS-CoV-2 has not been investigated yet.
This single-center, retrospective study describes our experience with neutralizing monoclonal antibody therapy in kidney transplant recipients infected with SARS-CoV-2, including 13 patients with the Omicron variant. Of note, preliminary results of this study have previously been published.13
Methods
Patient Selection and Study Design
Soon after the onset of the outbreak, all kidney transplant recipients were informed to contact our center, Cliniques universitaires Saint-Luc, in case of possible SARS-CoV-2 infection (because of symptoms or high-risk contact). Since the availability of monoclonal antibody therapy in Belgium (June 2021), all kidney transplant recipients with a possible infection (or confirmed infection based on an antigen or polymerase chain reaction [PCR] test performed outside of our clinic) have been clinically evaluated by a physicians (AD, NK, JCY, LB, JDG) from our team and systematically tested (or retested) for SARS-CoV-2 (PCR on nasopharyngeal swab on day 0). Neutralizing monoclonal antibodies are then locally administrated in all kidney transplant recipients who meet the following criteria: have a laboratory-confirmed SARS-CoV-2 infection (positive PCR on nasopharyngeal swab) <5 days, had symptoms evolving for ≤10 days, and can maintain oxygen saturation ≥93% when breathing room air, according to the Belgian guidelines.20
Baseline characteristics at monoclonal antibody infusion (including data regarding vaccination and clinical symptoms), immunosuppression management data, and outcomes were retrospectively recorded from the numeric files of patients. Data were collected until 30 days after monoclonal antibody infusion (to collect potential COVID-19 linked complications) or death.
This study was conducted with the approval of the Cliniques universitaires Saint-Luc institutional ethics review board (2021/12NOV/470; B403/). The need for informed consent was waived because of the use of deidentified information.
Management of Patients
Patients were given monoclonal antibodies intravenously in our COVID-19 unit before being discharged after 2 hours of surveillance. The study was conducted from June 10, 2021, to January 14, 2022. Two different monoclonal antibodies were used during this period, depending on their availability in Belgium: casirivimab at 1,200 mg and imdevimab at 1,200 mg (Regeneron) from June 10, 2021, to November 11, 2021, and then sotrovimab (GlaxoSmithKline and Vir Biotechnology) at 500 mg onwards. The last patient included in the present study was treated on January 14, 2022. We repeated viral load measurements 7-8 days after monoclonal antibody infusion, according to previously published studies reporting on monoclonal antibody therapy.21,22
In COVID-19 cases not requiring oxygen therapy, our protocol regarding immunosuppression management consists of a withdrawal of the antimetabolite drug for 10 days after the onset of symptoms in all patients or a 50% dose decrease in high-risk patients (first year of transplant, recent history of acute rejection). The doses of calcineurin inhibitors and steroids remain unchanged. In patients with mild COVID-19 (no need for oxygen) transplanted within the month, the immunosuppression regimen is not modified.
Microbiology
The vaccine-induced humoral response was measured using immunoassay detecting antibodies against the spike protein receptor-binding domain (Elecsys anti-SARS-CoV-2, Roche Diagnostics GmbH; positive threshold, >0.8 binding antibody units [BAU] per mL).
The detection of SARS-CoV-2 on nasopharyngeal swab was performed using the Alinity m SARS-CoV-2 assay (Abbott Molecular Inc). This real-time reverse transcription PCR is a dual target assay for the RdRp and N genes. To detect and identify SARS-CoV-2 variants of concern, RNA extracted from nasopharyngeal swabs stored in a deep freezer were subjected to the Allplex SARS-CoV-2 Variants I and II assays (Seegene) using the CFX96 real-time PCR detection system (Bio-Rad). These assays enable the simultaneous amplification and detection of target nucleic acids of spike protein mutations (N501Y, E484K, W152C, K417N, K417T, L452R mutations, and HV69/70 deletion), as well as the RdRp gene and an endogenous internal control. The data analysis and results interpretation were achieved with the Seegene Viewer Software.
Genotype assessment was not conducted between September 18, 2021, and December 10, 2021 (because of staffing issues and because the B.1.617.2 [Delta] variant was detected in more than 98% of SARS-CoV-2 infections in Belgium during this period).23
Results
Baseline Characteristics and Vaccination Status
Forty-seven kidney transplant recipients infected with SARS-CoV-2 met the criteria to receive neutralizing monoclonal antibody treatment in our center.
As summarized in Table 1, the median age at diagnosis was 50 years (range, 19-78 years), and 26 patients (55%) were men. The median time from transplant was 61 months (range, 1-348 months). Hypertension and diabetes (66% [n = 31] and 34% [n = 16], respectively) were the 2 most prevalent comorbid conditions among infected kidney transplant recipients.
Table 1.
Baseline Characteristics
| Characteristics | Casirivimab/Imdevimab, n = 16 (34%) | Sotrovimab, n = 31 (66%) | Total Cohort, N = 47 |
|---|---|---|---|
| Age, y, median (range) | 48.5 (21-78) | 50 (19-70) | 50 (19-78) |
| Male, n (%) | 8 (50) | 18 (58) | 26 (55) |
| Time from KT, mo, median (range) | 57.5 (1-348) | 70 (1-279) | 61 (1-348) |
| Comorbid condition | |||
| Hypertension, n (%) | 9 (56) | 22 (71) | 31 (66) |
| Diabetes, n (%) | 8 (50) | 8 (26) | 16 (34) |
| Cardiovascular history, n (%) | 1 (6) | 4 (13) | 5 (11) |
| Obesity, n (%) | 1 (6) | 4 (13) | 5 (11) |
| Baseline eGFR, mL/min/1.73 m2, median (range) | 51.5 (10-105) | 56 (11-121) | 56 (10-121) |
| Maintenance immunosuppression | |||
| Tac-MMF-steroids, n (%) | 11 (69) | 23 (74) | 34 (72) |
| Prior anti-SARS-CoV-2 vaccination | |||
| At least 2 doses,a n (%) | 13 (81) | 26 (84) | 39 (83) |
| Three doses, n (%) | 1 (6) | 16 (52) | 17 (36) |
| Time from the last dose, d, median (range) | 117 (39-211) | 103.5 (29-274) | 113 (29-274) |
| Documented humoral response,b n (%) | 10 (77) | 20 (77) | 30 (77) |
| Anti-RBD antibody titer, BAU/mL, median (range) | 199.7 (1-257) | 257 (1-257) | 257 (1-257) |
Abbreviations: BAU, binding antibody units; eGFR, estimated glomerular filtration rate; KT, kidney transplant; MMF, mycophenolate mofetil; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; Tac, tacrolimus.
One patient received 1 dose of the Ad26.COV2.S vaccine (Janssen/Johnson & Johnson).
Percentages were calculated among vaccinated patients (at least 2 doses; n = 39). Positivity, anti-RBD antibody titer >0.8 BAU/mL.
Thirty-nine patients (83%) had received at least 2 doses of an anti-SARS-CoV-2 vaccine and 17 (43%) of them had received a third dose. The last doses were administered at medians of 113 days (range, 29-274 days), 171 days (range, 39-274 days), and 82 days (range, 29-115 days) before infection in all patients, in kidney transplant recipients who were only vaccinated twice, and in those who had received a booster dose, respectively. The BNT162b2 (Pfizer-BioNTech) vaccine was administered in all but 3 kidney transplant recipients (ChAdOx1 nCoV-19/AZD1222 vaccine [AstraZeneca] in 2 and Ad26.COV2.S vaccine [Janssen/Johnson & Johnson] in 1).
Among vaccinated patients (n = 39), 30 (77%) were seropositive (antireceptor-binding domain antibody titer >0.8 BAU/mL). The 2 kidney transplant recipients who required hospitalization were seronegative. The median anti-SARS-CoV-2 antibody titer among responders was 257 BAU/mL (range, 1-257 BAU/mL) at medians of 54 days (range, 0-243 days) after the last dose and 8.5 days (range, 0-126 days) before infection.
Presentation at Diagnosis
The most common symptoms were cough and fever (n = 25 [53%] and n = 15 [32%], respectively); dyspnea was uncommon (n = 3; 6%; Table 2).
Table 2.
Characteristics at Admission for Monoclonal Antibody Treatment
| Characteristics | Total Cohort, N = 47 |
|---|---|
| Symptoms | |
| Fever, n (%) | 15 (32) |
| Cough, n (%) | 25 (53) |
| Shortness of breath, n (%) | 3 (6) |
| Diarrhea, n (%) | 7 (15) |
| SARS-CoV-2 viral loada | |
| Day 0,b copies/mL, median (range) | 2,110,027 (1,000-153,798,962) |
| Day 7,c copies/mL, median (range) | 1,000 (0-10,000,000) |
| Monoclonal antibody treatment | |
| Casirivimab-imdevimab, n (%) | 16 (34) |
| Sotrovimab, n (%) | 31 (66) |
| SARS-CoV-2 variant | |
| Delta, n (%) | 8 (17) |
| Omicron, n (%) | 13 (28) |
| Gamma, n (%) | 1 (2) |
| Not available, n (%) | 25 (53) |
Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Polymerase chain reaction.
Day of monoclonal antibody treatment.
7-8 days after treatment.
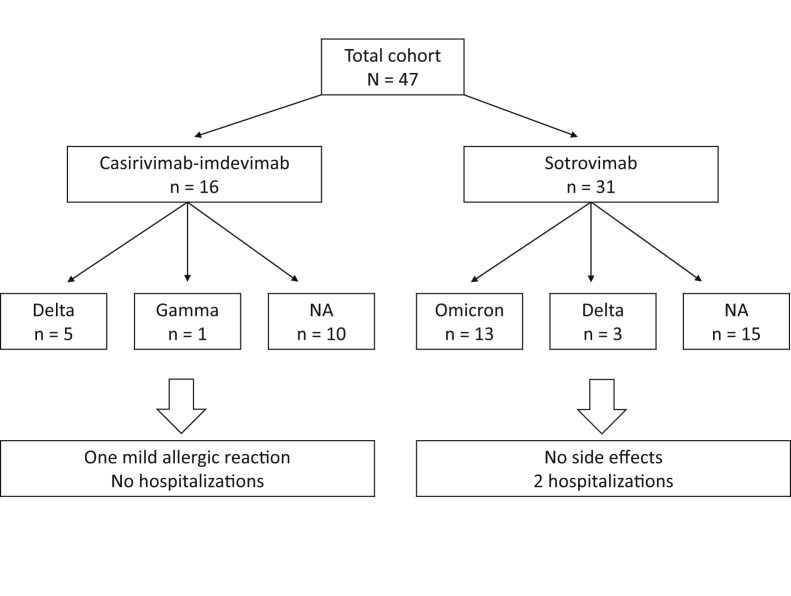
Among the 22 available SARS-CoV-2 genotypes, the Omicron variant was the most prevalent (n = 13; 59%) followed by the Delta (n = 8; 36%) and P.1 (Gamma) variants (n = 1; 5%). The last Delta variant was identified on December 17, 2021, and Omicron has been detected since then (Table 2; Fig 1).
Figure 1.
Description of the cohort. Abbreviation: NA, not available.
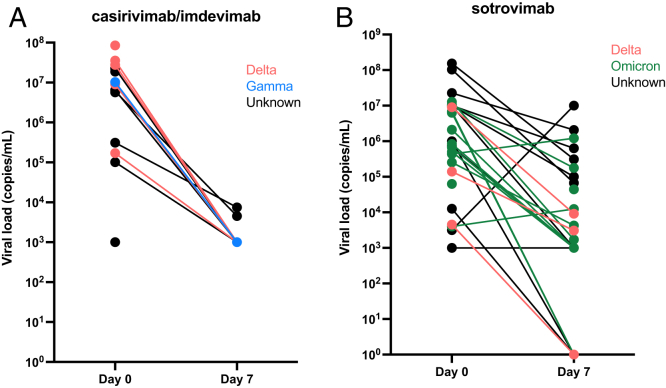
The median viral loads at diagnosis were 2,110,027 copies/mL (range, 1,000-153,798,962 copies/mL) and 752,789 copies/mL (range, 4,000-12,859,300 copies/mL) for the entire cohort and the Omicron-variant cohort, respectively (Table 2; Fig 2).
Figure 2.
Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load between the day of monoclonal antibody perfusion (day 0) and 7 days after (day 7) in patients treated with (A) casirivimab-imdevimab and (B) sotrovimab.
Management and Outcomes
On day 0, the antimetabolite drug was interrupted or decreased by 50% in 34 (83%) and 6 (15%) patients, respectively. The dose was unchanged in 1 patient who had a transplant 22 days before infection.
Monoclonal antibodies were given immediately after diagnosis, a median time of 2 days (range, 0-6 days) after symptoms onset.
Casirivimab-imdevimab was administered to the first 16 patients (34%). One patient reported a mild allergic reaction at infusion, characterized by skin flush, pruritus, and fever, which rapidly resolved after antihistamine and steroid administration, allowing the completion of the monoclonal antibody infusion. Of the 6 available genotypes, the Delta and Gamma variants of SARS-CoV-2 were detected in 5 and 1 patients, respectively (Fig 1). Sotrovimab was used in the following 31 patients (66%). No side effects were reported. Of the 16 available genotypes, the Omicron and Delta variants of SARS-CoV-2 were identified in 13 (81%) and 3 (19%) patients, respectively (Fig 1).
The median viral loads at day 7 after monoclonal antibody infusion were 1,000 copies/mL (range, 0-10,000,000 copies/mL) and 1,353 copies/mL (range, 0-1,211,163 copies/mL) in the entire cohort and in the Omicron-variant cohort, respectively (Table 2; Fig 2). Of note, 10 patients (21%) did not perform a day-7 PCR: 1 with the Omicron variant, 1 with the Delta variant, and 8 without an available genotype at diagnosis. Six patients (13%) displayed a negative PCR at day 7 (including 2 patients with the Omicron variant).
Immediately after monoclonal antibody perfusion, 2 patients required hospitalization for oxygen therapy that was weaned in 3 days, allowing patients’ discharge. Both patients had received 2 doses of messenger RNA vaccine before infection but did not exhibit a humoral response. The genotypes were not available for them, but the infections occurred during the Delta-prevalent SARS-CoV-2 wave in Belgium and they were both treated with sotrovimab.
One patient was infected in the first month (22 days) after kidney transplant. He was a 24-year-old man who had received 2 doses of the BNT162b2 (Pfizer-BioNTech) vaccine, the second dose was 171 days before infection. He mounted a serological response, with an anti-SARS-CoV-2 antibody titer of 257 BAU/mL measured 52 days before infection. He did not require hospitalization and there was no adjustment in his immunosuppression regimen.
No patient required further hospital readmission. None were admitted to the intensive care unit or died during the study period.
Discussion
We herein report reassuring data regarding both the efficacy and safety of neutralizing monoclonal antibody treatment use in kidney transplant recipients with mild to moderate COVID-19 (with no need for oxygen therapy). Indeed, out of the included 47 patients, only 2 (4%) required a short hospitalization for oxygen supply after monoclonal antibody treatment, and none died or required intensive care unit admission within a 30-day follow-up time. These results are in line with previous observations.13, 14, 15, 16, 17 Moreover, the 30-day hospitalization rate after monoclonal antibody infusion in our cohort seems lower than those reported in 2 recent case series (8.7%24 and 15%25).
No major side effects were observed. Interestingly, the use of sotrovimab in infected kidney transplant recipients has been poorly reported so far. In this study, we provide reassuring data, as 66% of our cohort was treated with sotrovimab with no safety concerns. However, the only 2 patients who needed hospitalization were treated with sotrovimab. They became hypoxic soon after monoclonal antibody infusion. Although a direct side effect of monoclonal antibody therapy cannot be totally excluded, oxygen therapy was weaned after 3 days, which seems like a long period for allergy. In our opinion, these 2 seronegative patients had infections that were already too advanced for the monoclonal antibody therapy to prevent hospitalization, but their infusion most likely participated in the rapid recovery.26 Both patients were quickly discharged and did not require rehospitalization.
Another important original aspect of the present study is the reassuring information about monoclonal antibody (sotrovimab only) use in cases of the Omicron variant. Indeed, all patients displaying the Omicron variant of SARS-CoV-2 were treated with sotrovimab and were discharged after monoclonal antibody infusion without any complications. In addition, a significant drop in the viral load 7 days after treatment was observed. Recent in vitro investigations have suggested that antibodies used in clinics might lose affinity and efficacy against this variant.18,19 VanBlargan et al18 tested several monoclonal antibodies in clinical practice for their ability to neutralize infectious Omicron isolates. They found that the neutralizing activity of sotrovimab against this variant was less effective compared with that of other antibodies. Although this requires validation in a larger-scale study, our data seem to confirm this finding in real-life practice. Consequently, sotrovimab might currently be the monoclonal antibody therapy of choice given the massive prevalence of the Omicron SARS-CoV-2 variant worldwide today.
The rate of vaccination in our cohort was high (83% of the cohort had received at least 2 doses of vaccine before infection), possibly contributing to the good outcomes observed in our patients treated with neutralizing monoclonal antibody therapy. However, the impact of vaccination on kidney transplant recipients in protecting against severe forms of COVID-19 is a matter of debate. Indeed, a recent, large, multicenter study showed that compared with 101 million fully vaccinated adults in the United States, fully vaccinated solid organ transplant recipients (n = 18,215) had an 82-fold higher risk of breakthrough infection and a 485-fold higher risk of breakthrough infection with associated hospitalization and death.27 Aslam et al28 reported in a single-center, US, retrospective study including 2,151 solid organ transplant recipients that vaccinated patients had a significantly decreased risk of developing symptomatic COVID-19. In comparison, a recent analysis from the UK registry including 43,481 solid organ transplant recipients showed that vaccination was associated with much lower protection against severe disease compared with that achieved in the general population.9 Recently, Yetmar et al17 published a case series of 32 solid organ transplant recipients diagnosed with COVID-19 at least 14 days after completing SARS-CoV-2 vaccination, demonstrating that COVID-19 breakthrough occurs in immunocompromised populations even after full vaccination. However, 28 patients with mild to moderate infections received casirivimab-imdevimab, with only 1 requiring further hospitalization, and no intensive care unit admission or death was reported. Similar to our findings, these results suggest that monoclonal antibody therapy might have an additional impact to protect even vaccinated SARS-CoV-2-infected transplant patients against the development of severe COVID-19. However, as in our study, the lack of a control group does not allow for drawing firm conclusions. Nevertheless, the combination of both strategies (vaccine and early use of monoclonal antibody therapy in case of infection) is likely the most effective strategy for this particularly vulnerable population.
An interesting perspective is the use of monoclonal antibodies as a prophylactic strategy in kidney transplant recipients. Indeed, its use in immunocompetent patients after a household contact has been shown to reduce the risks of asymptomatic and symptomatic infections, as well as reduce the duration of symptoms.29 Some countries (such as France) have authorized the use of monoclonal antibodies for prophylaxis in immunocompromised patients (including kidney transplant recipients) with no antibody response after 3 doses of anti-SARS-CoV-2 vaccine administration. Ducloux and Courivaud30 have recently published their experience of casirivimab-imdevimab as pre-exposition prophylaxis in 91 patients who did not show an antibody response after 3 doses of messenger RNA vaccine. After 3 months, no patient experienced COVID-19 infection, compared with a 16% rate of infection in kidney transplant recipients without a vaccine-induced response who had refused the monoclonal antibody prophylaxis. If confirmed on a larger scale and with a longer follow-up, this strategy might become a more efficient preventive measure against COVID-19 in kidney transplant recipients.
Our study has limitations. First, its retrospective design makes it subject to collection bias. Second, the sample size is small. Third, the rate of unavailable genotypes is high (53%). However, systematic genotype assessments were no longer performed in our center during a period of time when the Delta variant of SARS-CoV-2 was highly prevalent in our country and were resumed when the Omicron variant emerged in Belgium. Consequently, it is highly plausible that a vast majority of unavailable genotypes in our study were actually Delta variants and that we did not miss any Omicron variant. Fourth, we have used casirivimab-imdevimab and sotrovimab as monoclonal antibody therapies. The first has shown no efficacy on the Omicron variant and the second may be less active against the BA-2 Omicron subvariant. Nevertheless, it is highly likely that no patient with the Omicron variant was actually treated with casirivimab-imdevimab, as sotrovimab had been exclusively used before the emergence of the Omicron variant. However, it is possible that some patients with the BA-2 Omicron subvariant were treated with sotrovimab, as not all genotypes were available. Fifth, as we included only kidney transplant recipients with mild to moderate COVID-19, our observations cannot be extrapolated to more severe clinical situations (eg, oxygen supplementation needed at admission). Of note, a recent study showed no clinical benefit of sotrovimab in immunocompetent adults hospitalized for severe COVID-19.31 Also, as we did not include patients with severe disease, the clinical severity of the Omicron variant of SARS-CoV-2 in kidney transplant recipients could not be evaluated. In the general population, the Omicron variant might be associated with less severe outcomes compared to previous variants, especially in previously immunized populations.32,33 However, the clinical impact of this variant in kidney transplant recipients has not been reported yet. Finally, the lack of a control group does not allow us to clearly assess the clinical impacts of neutralizing monoclonal antibody therapy. Two studies so far have compared the outcomes between solid organ transplant recipients who received monoclonal antibodies and those who did not, and found contrasting results. Sarrell et al24 compared the outcomes of 93 solid organ transplants treated with monoclonal antibody therapy for mild to moderate COVID-19 (between November 22, 2020, and February 2, 2021) versus 72 solid organ transplants that did not. After adjustment for age, the 30-day hospitalization rates were not statistically different between the 2 groups (odds ratio, 0.49; P = 0.16). Klein et al25 reported on 95 kidney transplant recipients (including 20 who received monoclonal antibody therapy) with COVID-19 between March 1, 2020, and April 30, 2021. After adjustment for potential confounders, monoclonal antibody administration was associated with a significant decrease in hospitalization rates (adjusted hazard ratio, 0.216; P = 0.04). Interestingly, both study periods occurred before the Omicron wave’s onset.24,25
In conclusion, our study provides reassuring data about the efficacy and safety of neutralizing monoclonal antibody treatment in kidney transplant recipients with mild COVID-19, including those infected with the Omicron variant of SARS-CoV-2. Larger controlled studies are required to confirm our observations.
Article Information
Authors’ Full Names and Academic Degrees
Guillaume Fernandes, MD, Arnaud Devresse, MD, PhD, Anais Scohy, PharmD, Julien De Greef, MD, Jean Cyr Yombi, MD, Leila Belkhir, MD, PhD, Tom Darius, MD, PhD, Michel Mourad, MD, PhD, Antoine Buemi, MD, Benoit Kabamba, PharmD, PhD, Eric Goffin, MD, and Nada Kanaan, MD.
Authors’ Contributions
Data acquisition: GF, AD, AS; data analysis: GF, AD, NK; polymerase chain reaction analysis: AS, BK; patient care: GF, AD, JDG, JCY, LB, TD, AB, MM, EG, NK. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Peer Review
Received February 18, 2022. Evaluated by 3 external peer reviewers, with direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form April 1, 2022.
Footnotes
Complete author and article information provided before references.
References
- 1.WHO coronavirus (COVID-19) dashboard. World Health Organization. https://covid19.who.int
- 2.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jager K.J., Kramer A., Chesnaye N.C., et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98(6):1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goffin E., Candellier A., Vart P., et al. COVID-19-related mortality in kidney transplant and haemodialysis patients: a comparative, prospective registry-based study. Nephrol Dial Transplant. 2021;36(11):2094–2105. doi: 10.1093/ndt/gfab200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadi Y.B., Naqvi S.F.Z., Kupec J.T., Sofka S., Sarwari A. Outcomes of COVID-19 in solid organ transplant recipients: a propensity-matched analysis of a large research network. Transplantation. 2021;105(6):1365–1371. doi: 10.1097/TP.0000000000003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devresse A., De Greef J., Yombi J.C., Belkhir L., Goffin E., Kanaan N. Immunosuppression and SARS-CoV-2 infection in kidney transplant recipients. Transplant Direct. 2022;8(3) doi: 10.1097/TXD.0000000000001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald I., Murray S.M., Reynolds C., Altmann D.M., Boyton R.J. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. NPJ Vaccines. 2021;6(1):74. doi: 10.1038/s41541-021-00336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caillard S., Thaunat O. COVID-19 vaccination in kidney transplant recipients. Nat Rev Nephrol. 2021;17(12):785–787. doi: 10.1038/s41581-021-00491-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callaghan C.J., Mumford L., Curtis R.M.K., et al. Real-world effectiveness of the Pfizer-BioNTech BNT162b2 and Oxford-AstraZeneca ChAdOx1-S vaccines against SARS-CoV-2 in solid organ and islet transplant recipients. Transplantation. 2022;106(3):436–446. doi: 10.1097/TP.0000000000004059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ducloux D., Bamoulid J., Chabannes M., et al. Current vaccine strategies against SARS_CoV-2 only poorly protect kidney transplant recipients. J Infect. 2022;84(3):e34–e35. doi: 10.1016/j.jinf.2022.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weinreich D.M., Sivapalasingam S., Norton T., et al. REGEN-COV antibody combination and outcomes in outpatients with COVID-19. N Engl J Med. 2021;385(23):e81. doi: 10.1056/NEJMoa2108163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta A., Gonzalez-Rojas Y., Juarez E., et al. Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385(21):1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 13.Fernandes G., Devresse A., Scohy A., et al. Monoclonal antibody therapy for SARS-CoV-2 infection in kidney transplant recipients: a case series from Belgium. Transplantation. 2022;106(1):e107–e108. doi: 10.1097/TP.0000000000003974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catalano C., Servais S., Bonvoisin C., et al. Preemptive antibody therapy for vaccine breakthrough SARS-CoV-2 infection in immunocompromised patients. Transplantation. 2021;105(12):e282. doi: 10.1097/TP.0000000000003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Del Bello A., Marion O., Vellas C., Faguer S., Izopet J., Kamar N. Anti-SARS-CoV-2 monoclonal antibodies in solid-organ transplant patients. Transplantation. 2021;105(10):e146–e147. doi: 10.1097/TP.0000000000003883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angarone M., Kumar R.N., Stosor V. Organ transplant patients, COVID-19, and neutralizing monoclonal antibodies: the glass is half full. Transpl Infect Dis. 2021;23(5) doi: 10.1111/tid.13724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yetmar Z.A., Bhaimia E., Bierle D.M., Ganesh R., Razonable R.R. Breakthrough COVID-19 after SARS-CoV-2 vaccination in solid organ transplant recipients: an analysis of symptomatic cases and monoclonal antibody therapy. Transpl Infect Dis. 2022;24(2) doi: 10.1111/tid.13779. [DOI] [PubMed] [Google Scholar]
- 18.VanBlargan L.A., Errico J.M., Halfmann P.J., et al. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med. 2022;28(3):490–495. doi: 10.1038/s41591-021-01678-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCallum M., Czudnochowski N., Rosen L.E., et al. Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagement. Science. 2022;375(6583):864–868. doi: 10.1126/science.abn8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Interim clinical guidance for adults with suspected or confirmed COVID-19 in Belgium. Sciensano. https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_InterimGuidelines_Treatment_ENG.pdf
- 21.Chen P., Nirula A., Heller B., et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19. N Engl J Med. 2021;384(3):229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta A., Gonzalez-Rojas Y., Juarez E., et al. Effect of sotrovimab on hospitalization or death among high-risk patients with mild to moderate COVID-19: a randomized clinical trial. JAMA. 2022;327(13):1236–1246. doi: 10.1001/jama.2022.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.COVID-19 weekly report. Sciensano. https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_Weekly_report_FR.pdf
- 24.Sarrell B.A., Bloch K., El Chediak A., et al. Monoclonal antibody treatment for COVID-19 in solid organ transplant recipients. Transpl Infect Dis. 2022;24(1) doi: 10.1111/tid.13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein E.J., Hardesty A., Vieira K., Farmakiotis D. Use of anti-spike monoclonal antibodies in kidney transplant recipients with COVID-19: efficacy, ethnic and racial disparities. Am J Transplant. 2022;22(2):640–645. doi: 10.1111/ajt.16843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.RECOVERY Collaborative Group Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399(10325):665–676. doi: 10.1016/S0140-6736(22)00163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin C.X., Moore L.W., Anjan S., et al. Risk of breakthrough SARS-CoV-2 infections in adult transplant recipients. Transplantation. 2021;105(11):e265–e266. doi: 10.1097/TP.0000000000003907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aslam S., Adler E., Mekeel K., Little S.J. Clinical effectiveness of COVID-19 vaccination in solid organ transplant recipients. Transpl Infect Dis. 2021;23(5) doi: 10.1111/tid.13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Brien M.P., Forleo-Neto E., Musser B.J., et al. Subcutaneous REGEN-COV antibody combination to prevent COVID-19. N Engl J Med. 2021;385(13):1184–1195. doi: 10.1056/NEJMoa2109682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ducloux D., Courivaud C. REGEN-Cov antibody combination to prevent COVID-19 infection in kidney transplant recipient without detectable antibody response to optimal vaccine scheme. Kidney Int. 2022;101(3):645–646. doi: 10.1016/j.kint.2021.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ACTIV-3/Therapeutics for Inpatients with COVID-19 (TICO) Study Group Efficacy and safety of two neutralising monoclonal antibody therapies, sotrovimab and BRII-196 plus BRII-198, for adults hospitalised with COVID-19 (TICO): a randomised controlled trial. Lancet Infect Dis. 2022;22(5):622–635. doi: 10.1016/S1473-3099(21)00751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolter N., Jassat W., Walaza S., et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399(10323):437–446. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danza P., Koo T.H., Haddix M., et al. SARS-CoV-2 infection and hospitalization among adults aged ≥18 years, by vaccination status, before and during SARS-CoV-2 B.1.1.529 (Omicron) variant predominance - Los Angeles County, California, November 7, 2021-January 8, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:177–181. doi: 10.15585/mmwr.mm7105e1. [DOI] [PMC free article] [PubMed] [Google Scholar]