Abstract
New inhibitors of peptide deformylase (PDF) which are very potent against the isolated enzyme and show a certain degree of antibacterial activity have recently been synthesized by our group. Several lines of experimental evidence indicate that these inhibitors indeed interfere with the target enzyme in the bacterial cell. (i) The inhibition of Escherichia coli growth could be counteracted by overexpression of PDF from different organisms, including E. coli, Streptococcus pneumoniae, and Haemophilus influenzae. Conversely, reduced expression of PDF in S. pneumoniae resulted in an increased susceptibility to the inhibitors. (ii) Proteome analysis on two-dimensional gels revealed a shift for many proteins towards lower pI in the presence of PDF inhibitors, as would be expected if the proteins still carry their N-formyl-Met terminus. (iii) PDF inhibitors show no antimicrobial activity against E. coli under conditions that make growth independent of formylation and deformylation. The antibacterial activity in E. coli was characterized as bacteriostatic. Furthermore, the development of resistance in E. coli was observed to occur with high frequency (10−7). Resistant mutants show a reduced growth rate, and DNA sequence analysis revealed mutations in their formyl transferase gene. Taking all these aspects into account, we conclude that PDF may not be an optimal target for broad-spectrum antibacterial agents.
Antibiotic resistance is a major health concern, and the existing antibiotics target only a handful of molecules. Therefore, there is an urgent need for antibiotics with novel mechanisms of action. Peptide deformylase (PDF; EC 3.5.1.27) is essential in a variety of pathogenic bacteria but is not required for cytoplasmic protein synthesis in eukaryotes and is therefore an interesting potential target for antibacterial agents. Protein synthesis in eubacteria, under normal conditions, is initiated by formyl-methionyl-tRNA (19). Consequently, all nascent polypeptides are synthesized with N-formyl-methionine at the N terminus. The formyl group is removed by PDF during elongation of the polypeptide chain (1, 7). As methionine aminopeptidase (EC 3.4.11.18) cannot hydrolyze N-blocked polypeptides, deformylation is also a prerequisite for protein maturation (10, 22, 27). Both PDF and MAP, are essential for growth in Escherichia coli (10, 19, 21). pdf gene mutants can only be obtained in E. coli strains lacking the gene for formyltransferase, the enzyme that N-formylates the methionyl-tRNA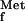 (EC.2.1.2.9) (20).
(EC.2.1.2.9) (20).
In a recent publication, we described the identification, optimization, and biological characterization of novel PDF inhibitors (3). These compounds were potent inhibitors of the isolated enzyme but only moderately active as antibacterials. In the accompanying paper, we describe transcription-translation assays that allowed us to demonstrate that the inhibitors were active as inhibitors of PDF in cell homogenates as well as in intact cells (4a). The experimental evidence presented here demonstrates that (i) antibacterial activity of the compounds results from PDF inhibition, (ii) the inhibitors lead to impaired deformylation of multiple proteins, (iii) the inhibitors are bacteriostatic, and (iv) the development of resistance is relatively rapid. In light of these results and other findings, we discuss the potential of PDF as an antibacterial target.
MATERIALS AND METHODS
Bacterial strains, plasmids, enzymes, and chemicals.
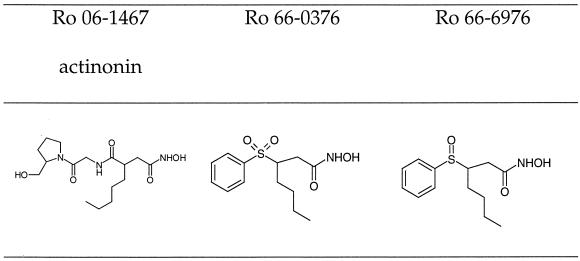
The E. coli strains used in this study were XL2-blue and BL21 (DE3) carrying pLysS (Stratagene, Basel, Switzerland) and DC2 from our own strain collection. The strains were grown in Luria-Bertani medium (Difco Laboratories, Detroit, Mich.) with aeration at 37°C. Streptococcus pneumoniae R6 (6) was routinely grown on sheep blood (3%) agar plates, and liquid cultures were propagated in Todd-Hewitt broth (Difco Laboratories) and incubated with 10% CO2 at 37°C. Haemophilus influenzae ATCC 51907 was grown in minimal medium (8) with a reduced methionine concentration (0.6 μM). The plasmids pET-3a and pET-28a were from Novagen (Abington, United Kingdom). Restriction enzymes were from New England Biolabs (Beverly, Mass.) or Amersham Pharmacia Biotech (Dübendorf, Switzerland) and were used in accordance with the specifications of the manufacturer. All other chemicals, including actinonin (Ro 06-1467), were from Sigma (St. Louis, Mo.). The synthesis of Ro 66-0376 and Ro 66-6976 is described elsewhere (3) (Fig. 1).
FIG. 1.
Chemical structures of PDF inhibitors.
Determination of the MICs.
The MICs of the test compounds were determined by broth microdilution. The MIC of a compound was defined as the lowest concentration that prevented visible growth of bacteria after incubation at 37°C for 24 h, or 72 h for slow-growing strains. Iso-Sensitest broth (Oxoid, Basingstoke, United Kingdom) was used as the test medium.
Time-kill assay.
For time-kill studies, glass tubes containing 7 ml of Iso-Sensites broth were inoculated with approximately 5 × 107 CFU of an exponentially growing culture of E. coli DC2/ml. The concentration of the antibiotics was 32 μg/ml, i.e., approximately eight times the MIC. The cultures were incubated at 37°C in a shaking water bath, and viability counts were performed at different time points by plating appropriate dilutions on Trypticase soy agar (Difco). Colony counts were recorded after incubation at 37°C for 24 h.
General DNA techniques and transformation.
Chromosomal DNA preparation was performed using the Qiagen (Hilden, Germany) genomic DNA purification system. Preparation of plasmid DNA was performed using the Promega (Madison, Wis.) Wizard mini- or maxipurification system. Plasmids, PCR products, and chromosomal DNA were cleaved with the appropriate restriction enzymes, ligated, and transformed into E. coli XL2 blue cells (5, 24). Transformants were selected on Luria-Bertani agar plates containing ampicillin (100 μg/ml) for pET-3a and pDS56Δcat, kanamycin (20 μg/ml) for pET-28a, or erythromycin (500 μg/ml) for pJDC9 and its derivatives. Transformation of S. pneumoniae was performed as described by Havarstein et al. (14) with modifications. Briefly, frozen aliquots of competent cells were thawed, diluted 10-fold with prewarmed medium (16), and incubated for 20 min at 37°C in an atmosphere of 10% CO2. One microliter of plasmid DNA (1 μg/μl) was added to 500 μl of the mixture, and incubation continued for an additional 3 h. Transformants were selected on sheep blood (3%) agar plates containing erythromycin (0.5 μg/ml). Competent cells were obtained by growing R6 in Todd-Hewitt medium, supplemented with 5% calf serum, to an optical density at 660 nm (OD660) of 0.3 to 0.5. The culture was diluted (1/10), glycerol was added to a final concentration of 10%, and aliquots were flash frozen at −80°C.
PCR and sequencing.
PCR was performed with the Expand High-Fidelity DNA system (Roche Diagnostics, Rotkreuz, Switzerland) according to the manufacturer's recommendations in a Perkin-Elmer (Foster City, Calif.) thermocycler. Sequencing was performed using the dideoxy chain termination method (26) with a modified DNA sequencing kit (dye terminator cycle sequencing; PE Applied Biosystems, Foster City, Calif.) and an automated DNA sequencing system (ABI Prism 310 genetic analyzer; PE Applied Biosystems). Nucleotide and amino acid sequences were analyzed with the University of Wisconsin Genetics Computer Group sequence analysis package (13) and with the Lasergene program (DNASTAR, Madison, Wis.).
Construction of expression plasmids for PDF.
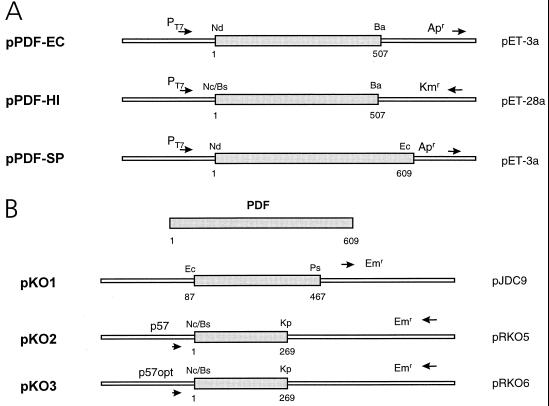
The pdf genes from E. coli (EMBL accession no. ecdeff), H. influenzae (EMBL accession no., hi32745), and S. pneumoniae (EMBL accession no., spn278785) were expressed in an IPTG (ispropyl-β-d-thiogalactopyranoside)-inducible system (Fig. 2A). In all cases, full-length genes were PCR amplified using the appropriate forward and reverse primers. The selected forward primer introduced a BsaI site (5′-GGTCTCTCATG-3′) for H. influenzae or an NdeI site (5′-GCCATATG-3′) for E. coli and S. pneumoniae overlapping with the start codon. The reverse primer introduced a BamHI site for E. coli and H. influenzae and an EcoRI site for S. pneumoniae downstream from the stop codon. The PCR products were digested with the corresponding restriction enzymes, isolated from an agarose gel, and cloned into pET-3a for the E. coli and S. pneumoniae genes and into pET-28a for H. influenzae, resulting in pPDF-EC, pPDF-SP, and pPDF-HI (Fig. 2A). The correctness of the PCR inserts was verified by DNA sequencing. Both pET-3a and pET-28a carry T7 promoters and are designed to allow protein expression via activation of T7 polymerase synthesis by IPTG in an appropriate E. coli strain, such as BL21, which carries the T7 polymerase gene under the control of the lacUV5 promoter (29). The T7 promoter carried by pET-28a contains an additional binding site for the lac repressor.
FIG. 2.
Plasmids used in this study. (A) Plasmids used for controlled expression of pdf genes from E. coli, H. influenzae, and S. pneumoniae. (B) Plasmids for generating controlled pdf gene disruptions in S. pneumoniae. A small internal fragment of the gene was cloned into pJDC9, resulting in pKO1. An amino-terminal fragment of the pdf gene was cloned into the insertion vectors pRKO5 and pRKO6, resulting in plasmids pKO2 and pKO3, which allow tetracycline-regulated pdf expression in the transformants. For details, see Material and Methods. The numbers indicate the nucleotides, starting with 1 at the putative initiation codon of each gene. Abbreviations for restriction enzymes: Ba, BamHI; Bs, BsaI; Ec, EcoRI; Kp, KpnI; Nc, NcoI; Nd, NdeI; Ps, PstI.
Construction of plasmids for generating PDF gene disruptions.
A small (381-bp) fragment internal to the pdf gene from S. pneumoniae (nucleotides 87 to 467) was amplified by PCR for use in disrupting the pdf gene and terminating expression of the downstream genes in the operon. The forward primer used introduced an EcoRI site, and the reverse primer introduced a PstI site. The PCR product was cloned into pJDC9 (EcoRI-PstI) (12), resulting in pKO1 (Fig. 2B). As a positive control for gene disruption experiments, pJDC9 carrying a DNA fragment internal to the nonessential amiC gene was used (2). In order to introduce two different tetracycline-regulatable promoters into the promoter region of the pdf operon, an N-terminal fragment of the pdf gene (nucleotides 1 to 269) was amplified by PCR. The forward primer introduced a BsaI site (5′-GGTCTCTCATG-3′) upstream from the start codon, and the reverse primer introduced a KpnI site. The digested PCR product was cloned into vectors pRKO5 and pRKO6 (NcoI-KpnI), resulting in plasmids pKO2 and pKO3 (Fig. 2B). The vectors pRKO5 and pRKO6 are derivatives of pRKO1 and -2, respectively. The last plasmids were designed for insertional gene inactivation and introduce a Tet operator upstream from the start codon of the targeted gene (4, 28). The plasmids pRKO5 and -6 are improved derivatives of these vectors that allow the use of the authentic start codons for expression studies (M. Stieger, unpublished data). Recombinants were propagated in E. coli XL2. Mutants were generated in S. pneumoniae R6 by transformation using the protocol described above. The authenticity of each clone was confirmed by sequencing.
2D polyacrylamide gel electrophoresis (PAGE).
H. influenzae cultures were grown under aeration (200 rpm) to an OD600 of 0.4. The compounds Ro 66-0376 and actinonin were added to final concentrations of 32 and 16 μg/ml, respectively. After incubation for 30 and 90 min, the cultures were pulse-labeled with 20 μCi of l-[35S]methionine (20 fmol), rapidly chilled on ice, and harvested by centrifugation at 15,000 × g. In parallel, an untreated control culture was labeled correspondingly. The bacterial pellets were washed once in a solution containing 10 mM Tris-HCl (pH 8.0) 1 mM EDTA, and 7% (wt/vol) glucose and resuspended in lysis solution containing 8 M urea, 4% (wt/vol) 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), 40 mM Tris base, 65 mM 1,4-dithioerythritol, and 2% (vol/vol) ampholytes Resolyte, pH 6 to 9.5 (BDH, Poole, United Kingdom). Soluble extracts were prepared by centrifugation of the lysate at 100,000 × g for 1 h at 10°C. The incorporated radioactivity was determined by scintillation counting. An aliquot of the protein solution (4 × 106 cpm) was loaded onto Immobiline 3-10 nonlinear pH gradient (IPG) strips (Pharmacia, Uppsala, Sweden) at the basic end and resolved according to the manufacturer's recommendations. After isoelectric focusing, the strips were equilibrated as described previously (25), and electrophoresis was carried out on 1-mm-thick 20- by 25-cm 12% polyacrylamide slab gels. The gels were dried, and the images were acquired on a PhosphorImager device (Molecular Dynamics, Sunnyvale, Calif.). The protein identities and pI values were tentatively assigned by comparison with an H. influenzae reference two-dimensional (2D) map (17). The pI estimations were based on the IPG strip manufacturer's specifications and on the theoretical pI values of reference proteins.
RESULTS
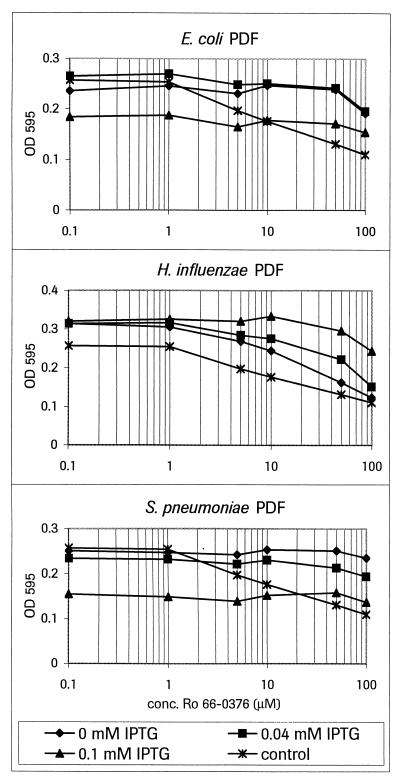
Overexpression of PDF counteracts the growth inhibition in E. coli caused by PDF inhibitor Ro 66-0376.
The PDF proteins from different species (E. coli, H. influenzae, and S. pneumoniae) were overexpressed in E. coli. The level of expression was regulated by the level of inducer added (0, 0.04, and 0.1 mM IPTG) (Fig. 3). For the E. coli and S. pneumoniae enzymes, we could show an induction level-dependent decrease of susceptibility to the PDF inhibitor (Ro 66-0376) treatment. For the H. influenzae PDF, this effect was less pronounced. Induction of either the S. pneumoniae or the E. coli enzyme with an IPTG concentration of 1 mM was lethal for the host bacteria.
FIG. 3.
Counteracting effect of overexpressing PDF from different species on growth inhibition by Ro 66-0376. E. coli BL21 transformed with the indicated plasmids was grown to an OD578 of 0.05 and distributed into 96-well plates. Increasing amounts of IPTG (as indicated) were used to induce the expression of the PDFs, and increasing amounts of the inhibitor Ro 66-0376 were added. The OD595 was measured after 5 h of incubation. The E. coli strain harboring vector pET-3a is shown as the control (noninduced). Conc., concentration.
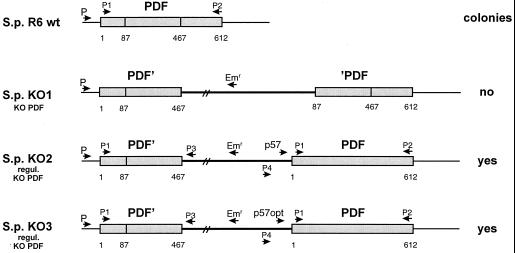
Construction and analysis of regulatable pdf gene disruptions in S. pneumoniae.
Knockout mutants were constructed in S. pneumoniae by site-directed insertional mutagenesis (23) to investigate the function of the pdf gene. A small internal gene fragment was cloned into the plasmid pJDC9, which does not replicate in pneumococci. The construct (pKO1 [Fig. 2B]) was transformed into S. pneumoniae R6 and plasmid-encoded erythromycin resistance (Emr) was used to select for single homologous recombination events. No Emr transformants were obtained in several repeated experiments, suggesting that pdf is essential in S. pneumoniae.
To further study the role of the pdf gene in bacterial growth, we used a regulatable knockout system that allows conditional gene expression (Fig. 4). Regulation of gene expression by using the TetR and TetO regulatory elements had been shown to be extremely efficient (18). Therefore, the potential promoter region upstream from the streptococcal pdf gene was replaced by two different tetracycline-regulatable promoters: (i) p57 in pKO2 (weak promoter; relatively tight) and (ii) p57opt in pKO3 (p57optimal; stronger promoter and higher basal expression level then p57) (28). Transforming S. pneumoniae R6 with these plasmids resulted in erythromycin-resistant transformants in the presence and absence of tetracycline. Contrary to our expectations, we could not observe any difference between the growth rates of cultures grown in the presence and in the absence of tetracycline (data not shown; several independent transformants were tested), indicating that the basal transcription level from these promoters, even p57, was sufficient to allow normal growth of the transformants.
FIG. 4.
Schematic representation of the chromosomal pdf mutants of S. pneumoniae R6 with integrational plasmids. The numbers indicate the nucleotides, starting with 1 at the initiation codon of each gene. P1 to P4 indicate the primers used in the PCR to prove the correct integration of the plasmids. regul., regulatable.
A decrease in pdf expression in S. pneumoniae enhances the growth inhibition caused by PDF inhibitor Ro 66-0376.
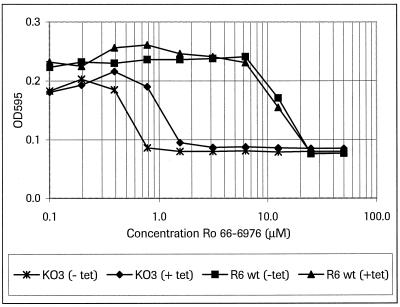
The degrees of inhibition of bacterial growth by Ro 66-0376 in S. pneumoniae R6 and the conditional knockout mutant S. pneumoniae KO3 (Fig. 4) were compared. For strain R6, as expected, no difference in growth rate was detected between tetracycline-induced cultures and control cultures: the 50% inhibitory concentration was around 13 μM (Fig. 5). Strain KO3 exhibited a higher susceptibility to the PDF inhibitor in the absence of tetracycline. Growth inhibition (the half-maximal growth rate) could already be detected at a concentration of 0.5 to 0.6 μM. The addition of tetracycline (20 ng/ml) shifted the 50% inhibitory concentration to a slightly higher concentration (1.2 μM).
FIG. 5.
Effect of reduced PDF expression on growth inhibition by Ro 66-6976 in S. pneumoniae. Strains R6 and KO3 were grown to an OD620 of 0.05 and distributed into 96-well plates. Increasing amounts of the inhibitor Ro 66-6976 with or without 20 ng of tetracycline (tet)/ml were added. The OD595 was measured after 16 h of incubation. One representative experiment is shown. wt, wild type.
Analysis of changes in protein pattern caused by treatment of H. influenzae Rd KW 20 with PDF inhibitors.
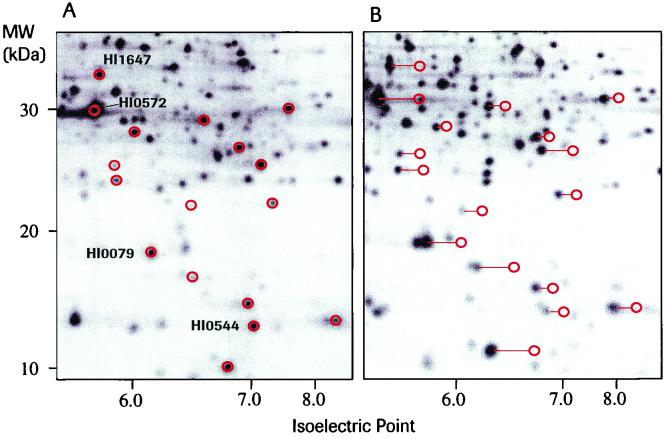
Mid-log-phase cultures of H. influenzae Rd KW 20 were treated with subinhibitory concentrations of the PDF inhibitor actinonin (Ro 06-1467) for 90 min and pulse-labeled for 5 min with l-[35S]methionine. Extracts were prepared, and the proteins were resolved by 2D PAGE. The protein patterns detected were compared to those obtained from an untreated pulse-labeled control culture. An overlay of the two images revealed that numerous spot shifts, directed towards the anode, were caused by treatment with the inhibitor. This indicates a decrease in the isoelectric points of the shifted proteins (Fig. 6). Little change was observed for the high-molecular-weight proteins, while the pIs of the majority of the low-molecular-weight proteins were changed. As a protein map of H. influenzae Rd KW 20 was available (17), the differences in pI with and without PDF inhibitor treatment could be estimated for some proteins and compared to the calculated values. In all cases examined, the pI difference was in good agreement with the assumption that the shifts are due to the formyl groups that are not removed and that block the N-terminal amino group (Table 1). Experiments carried out with compound Ro 66-0376 yielded results identical to those obtained with actinonin. Studies using S. pneumoniae as a gram-positive model organism also revealed spot shifts towards a more acidic pI (data not shown). Of note, 2D PAGE analysis of unlabeled protein extracts from long-term-treated bacterial cultures followed by Coomassie staining did not detect spot shifts (data not shown), which indicates that the residual PDF activity remaining after treatment with subinhibitory concentrations of the inhibitors suffices for the near-complete deformylation of the newly synthesized proteins. The effect of the inhibitors visualized with pulse-labeled cultures, therefore, corresponds to a delay rather than to a complete block of deformylation.
FIG. 6.
Isoelectric point shifts in proteins triggered by treatment with inhibitors of PDF. in H. influenzae as visualized by 2D PAGE of pulse-labeled protein extracts. Only sections of the 2D gels are shown. (A) Control culture without of addition of inhibitor. The red circles indicate spots that were shifted after the addition of deformylase inhibitors. The spots for which a detailed analysis of the amplitude of the shifts was undertaken are labeled. (B) Culture treated with 16 μg of Ro 06-1467/ml for 90 min. The red circles indicate the positions of spots without treatment. The lines illustrate the spot shifts.
TABLE 1.
Spot shifts observed on 2D gels after treatment with PDF inhibitors
| Proteina | pI
|
|||
|---|---|---|---|---|
| Observed
|
Theoretical
|
|||
| Control | With inhibitor | Control | Formylated | |
| RL9_HAEIN | 7.0 | 6.8 | 7.0 | 6.5 |
| CYPB_HAEIN | 6.1 | 5.8 | 6.1 | 5.7 |
| Y572_HAEIN | 5.8 | 5.5 | 5.7 | 5.5 |
| YG47_HAEIN | 5.8 | 5.6 | 5.8 | 5.6 |
SWISS-PROT identifier.
Antibacterial activity of inhibitors of PDF is reversed by trimethoprim in rich medium.
The antibiotic trimethoprim inhibits dihydrofolate reductase and thereby promotes the depletion of the tetrahydrofolate pool. Mazel et al. showed that E. coli with an inactivated pdf gene can grow slowly in a rich medium containing trimethoprim and thymidine, supposedly using unformylated Met-tRNA, which results in slow growth independent of the action of deformylase (20). This artificial condition mimics a mutation in the fmt gene. Under such conditions, a specific PDF inhibitor should not inhibit bacterial growth. This was indeed observed with Ro 66-0376 and Ro 66-6976 in medium supplied with trimethoprim and thymidine (Table 2).
TABLE 2.
Trimethoprim antagonism to PDF inhibitors
| Inhibitora | Growth of E. colib
|
|||
|---|---|---|---|---|
| IST | IST + TMP + thymidine | IST + thymidine | IST + TMP | |
| None | + | + | + | − |
| Ro 66-0376 | − | + | − | − |
| Ro 66-6976 | − | + | − | − |
30 μg/ml.
IST, Iso-Sensitest broth. The concentrations of compounds added were as follows: trimethoprim (TMP), 50 μg/ml; thymidine, 10 μg/ml. Growth of E. coli DC2 was recorded after 48 h and classified as growth (+) or no growth (−).
Antibacterial activity is bacteriostatic.
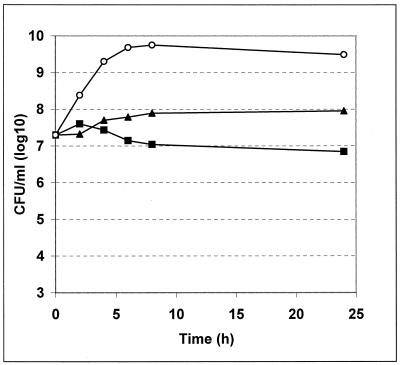
PDF inhibitors inhibited the growth of E. coli DC2 in a bacteriostatic manner and are not bactericidal. This is comparable to the action of erythromycin (Fig. 7).
FIG. 7.
Time-kill kinetics with E. coli DC2. The effect of the addition of Ro 66-0376 (32 μg/ml; solid squares) compared to that of erythromycin (32 μg/ml; solid triangles) on CFU is shown. Open circles, untreated control.
Development of in vitro resistance to Ro 66-6976 and analysis of resistant mutants.
Mutants resistant to Ro 66-6976 were selected by plating E. coli XL2-blue on agar containing 64 μg of Ro 66-6976/ml. Resistant colonies appeared after 3 days at a frequency of 10−7. All isolated mutants exhibited lower growth rates than the wild-type strain, i.e., doubling times of 1.2 to 2 h compared to 0.4 h in the wild type (data not shown). The two selected mutants were completely resistant to both inhibitors, Ro 66-0376 and Ro 66-6796, whereas their susceptibilities to unrelated antibiotics were not significantly altered (Table 3). The fmt-pdf operons of both mutants were sequenced, and a Tn10 insertion in the fmt gene was found in both cases.
TABLE 3.
Susceptibilities of spontaneous Ro 66-6976-resistant mutants compared to that of the E. coli wild-type strain for two PDF inhibitors and two unrelated antibiotics
| Strain | MIC (μg/ml)b
|
|||
|---|---|---|---|---|
| Ro 66-6976 | Ro 66-0376 | Erythromycin | Trimethoprim | |
| XL2 (wt)a | 8 | 32 | 32 | 0.5 |
| XL2-66-6976-1 | >128 | >128 | 16 | 0.5 |
| XL2-66-6976-2 | >128 | >128 | 16 | 1 |
wt, wild type.
Determined after 24 (wild-type strain) and 72 (mutants) h.
DISCUSSION
In the accompanying paper, we demonstrated that the PDF inhibitors recently synthesized by our group show activity in E. coli cell homogenates and intact cells. In this report, the following questions are addressed: (i) do the compounds exert their antibacterial action by targeting PDF, (ii) do they lead to impaired deformylation of multiple intracellular proteins in species other than E. coli, and (iii) at what rate does the development of resistance occur?
Regulation of the level of expression of the target enzyme is one approach to demonstrate that the antibacterial activity of the PDF inhibitors is really related to inhibition of deformylase activity and is not caused by the inhibition of an unrelated target. Using this approach, Chen et al. have successfully demonstrated that actinonin, a naturally occurring antibacterial agent, acts by inhibiting PDF in E. coli (11). Using similar methodology, we cloned the pdf genes from several bacterial strains (E. coli, S. pneumoniae, and H. influenzae) into vectors that allow the regulation of their expression by varying the IPTG concentration. For the E. coli and S. pneumoniae enzymes, it could be clearly shown that the susceptibility to PDF inhibitors decreases with increasing levels of expression of pdf. Overexpression of the H. influenzae enzyme also had this effect, but to a lesser degree. Conversely, decreasing levels of pdf expression in S. pneumoniae resulted in an increased susceptibility to the inhibitors. Taken together, these experiments strongly suggest that the compounds tested inhibit bacterial-cell growth by targeting PDF.
We analyzed the protein extracts from pulse-labeled PDF inhibitor-treated and untreated bacterial cultures by 2D PAGE. In gram-positive and gram-negative model organisms it was shown that treatment with PDF inhibitors leads to multiple spot shifts that are most likely due to the blocking of PDF activity, which again argues strongly in favor of the proposed mode of action. This effect was seen in a multitude of spots. Finally, the observations that impaired methionyl-tRNAMet formylation triggered by trimethoprim treatment antagonizes the action of the PDF inhibitors and that resistant mutants carry a mutation in the fmt gene are further pieces of evidence indicating that PDF inhibition is responsible for the antibacterial action of the hydroxamic acid derivatives tested.
If Coomassie staining was used for protein visualization in the 2D PAGE experiments, no spot shifts could be seen even after prolonged incubation with the inhibitors. Together with the observation that the low level of pdf expression in the regulatable S. pneumoniae knockout strains did not impair their viability, this indicates that a low level of PDF activity in the cells suffices to sustain growth. However, the observation that no mutants with a totally inactivated pdf gene could be obtained in S. pneumoniae strongly suggests that the enzyme is essential in this organism, although the results presented cannot exclude polar effects.
The compounds tested were bacteriostatic, and rapid development of resistance occurred in E. coli. Although bacteriostatic action has also been described for actinonin (11), this may not be a general characteristic of PDF inhibitors, as a series of peptide thiols described recently (15) show bactericidal activity. However, this was only convincingly demonstrated in Bacillus subtilis, which may be particularly susceptible to this class of compounds. The same authors also did not observe any development of resistance in B. subtilis, suggesting that rapid development of resistance may not be a general property of PDF inhibitors.
Sequence alignments from public data banks show that the pdf gene is ubiquitous in the bacterial domain, and the essential residues are highly conserved. In some bacteria, such as S. pneumoniae, Streptococcus pyogenes, Staphylococcus aureus, B. subtilis, Enterococcus faecalis, and Vibrio cholerae, two pdf-like sequences can be detected by sequence similarity searching. The roles of the two individual genes are as yet unclear. However, their presence raises the possibility that resistance in these organisms may arise through a bypass mechanism. Moreover, a partial sequence with similarity to an internal part of pdf was identified in a proprietary data bank of human DNA sequences. So far, our attempts to express functionally active human protein have been unsuccessful. It can be speculated that the protein encoded by this gene is active in the mitochondria, as mitochondrial protein synthesis is initiated by methionyl-tRNA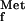 (9) and no pdf gene has been detected in this organelle. This may be an argument against the concept that PDF activity is not required in eukaryotes.
(9) and no pdf gene has been detected in this organelle. This may be an argument against the concept that PDF activity is not required in eukaryotes.
The existence of multiple pdf-like genes in some bacterial species and the possibility of the existence of a human homologue shed some doubt on the value of PDF as a target for broad-spectrum antibacterial drugs. Furthermore, the questions of the development of resistance and bactericidal or bacteriostatic action in different bacterial species and by different chemical classes of inhibitors need to be more broadly addressed to fully validate PDF as an antibacterial target.
ACKNOWLEDGMENTS
We acknowledge the expert technical assistance of Karin Di Padova, Christian Lacoste, Olivier Partouche, Bernadette Prud'hon, and Bernard Rutten.
REFERENCES
- 1.Adams J M. On the release of the formyl group from nascent protein. J Mol Biol. 1968;33:571–589. doi: 10.1016/0022-2836(68)90307-0. [DOI] [PubMed] [Google Scholar]
- 2.Alloing G, de Philip P, Claverys J P. Three highly homologous membrane-bound lipoproteins participate in oligopeptide transport by the Ami system of the gram-positive Streptococcus pneumoniae. J Mol Biol. 1994;241:44–58. doi: 10.1006/jmbi.1994.1472. [DOI] [PubMed] [Google Scholar]
- 3.Apfel C, Banner D W, Bur D, Dietz M, Hirata T, Hubschwerlen C, Locher H, Page M G, Pirson W, Rosse G, Specklin J L. Hydroxamic acid derivatives as potent peptide deformylase inhibitors and antibacterial agents. J Med Chem. 2000;43:2324–2331. doi: 10.1021/jm000018k. [DOI] [PubMed] [Google Scholar]
- 4.Apfel C M, Takacs B, Fountoulakis M, Stieger M, Keck W. Use of genomics to identify bacterial undecaprenyl pyrosphosphate synthetase: cloning, expression, and characterization of the essential uppS gene. J Bacteriol. 1999;181:483–492. doi: 10.1128/jb.181.2.483-492.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4a.Apfel C M, Evers S, Hubschwerlen C, Pirson W, Page M G P, Keck W. Peptide deformylase as an antibacterial drug target: assays for detection of its inhibition in Escherichia coli cell homogenates and intact cells. Antimicrob Agents Chemother. 2001;45:1053–1057. doi: 10.1128/AAC.45.4.1053-1057.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: Wiley Interscience; 1997. [Google Scholar]
- 6.Avery O T, MacLeod C M, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a desoxyribonucleic acid fraction isolated from Pneumococcus type III. J Exp Med. 1944;79:137–158. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ball L A, Kaesberg P. Cleavage of the N-terminal formylmethionine residue from a bacteriophage coat protein in vitro. J Mol Biol. 1973;79:531–537. doi: 10.1016/0022-2836(73)90404-x. [DOI] [PubMed] [Google Scholar]
- 8.Barcak G J, Chandler M S, Redfield R J, Tomb J F. Genetic systems in Haemophilus influenzae. Methods Enzymol. 1991;204:321–342. doi: 10.1016/0076-6879(91)04016-h. [DOI] [PubMed] [Google Scholar]
- 9.Bianchetti R, Lucchini G, Crosti P, Tortora P. Dependence of mitochondrial protein synthesis initiation on formylation of the initiator methionyl-tRNAf. J Biol Chem. 1977;252:2519–2523. [PubMed] [Google Scholar]
- 10.Chang S Y, McGary E C, Chang S. Methionine aminopeptidase gene of Escherichia coli is essential for cell growth. J Bacteriol. 1989;171:4071–4072. doi: 10.1128/jb.171.7.4071-4072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen D Z, Patel D V, Hackbarth C J, Wang W, Dreyer G, Young D C, Margolis P S, Wu C, Ni Z J, Trias J, White R J, Yuan Z. Actinonin, a naturally occurring antibacterial agent, is a potent deformylase inhibitor. Biochemistry. 2000;39:1256–1262. doi: 10.1021/bi992245y. [DOI] [PubMed] [Google Scholar]
- 12.Chen J D, Morrison D A. Construction and properties of a new insertion vector, pJDC9, that is protected by transcriptional terminators and useful for cloning of DNA from Streptococcus pneumoniae. Gene. 1988;64:155–164. doi: 10.1016/0378-1119(88)90489-1. [DOI] [PubMed] [Google Scholar]
- 13.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Havarstein L S, Coomaraswamy G, Morrison D A. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc Natl Acad Sci USA. 1995;92:11140–11144. doi: 10.1073/pnas.92.24.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huntington K M, Yi T, Wei Y, Pei D. Synthesis and antibacterial activity of peptide deformylase inhibitors. Biochemistry. 2000;39:4543–4551. doi: 10.1021/bi992452y. [DOI] [PubMed] [Google Scholar]
- 16.Lacks S. Integration efficiency and genetic recombination in pneumococcal transformation. Genetics. 1966;53:207–235. doi: 10.1093/genetics/53.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Langen H, Takacs B, Evers S, Berndt P, Lahm H W, Wipf B, Gray C, Fountoulakis M. Two-dimensional map of the proteome of Haemophilus influenzae. Electrophoresis. 2000;21:411–429. doi: 10.1002/(SICI)1522-2683(20000101)21:2<411::AID-ELPS411>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Lutz R, Bujard H. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1–I2 regulatory elements. Nucleic Acids Res. 1997;25:1203–1210. doi: 10.1093/nar/25.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcker K, Sanger F. N-Formyl-methionyl-tRNA. J Mol Biol. 1963;8:835–840. doi: 10.1016/s0022-2836(64)80164-9. [DOI] [PubMed] [Google Scholar]
- 20.Mazel D, Pochet S, Marliere P. Genetic characterization of polypeptide deformylase, a distinctive enzyme of eubacterial translation. EMBO J. 1994;13:914–923. doi: 10.1002/j.1460-2075.1994.tb06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meinnel T, Blanquet S. Characterization of the Thermus thermophilus locus encoding peptide deformylase and methionyl-tRNA(fMet) formyltransferase. J Bacteriol. 1994;176:7387–7390. doi: 10.1128/jb.176.23.7387-7390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meinnel T, Mechulam Y, Blanquet S. Methionine as translation start signal: a review of the enzymes of the pathway in Escherichia coli. Biochimie. 1993;75:1061–1075. doi: 10.1016/0300-9084(93)90005-d. [DOI] [PubMed] [Google Scholar]
- 23.Pozzi G, Oggioni M R, Manganelli R, Plevani R. Genetic manipulation of streptococci by chromosomal integration of recombinant DNA. In: Dunny G M, Cleary P P, McKay L L, editors. Genetics and molecular biology of streptococci, lactococci, and enterococci. Washington, D.C.: American Society for Microbiology; 1991. pp. 59–61. [Google Scholar]
- 24.Sambrook J, Fritsch E, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Sanchez J, Appel R, Golaz O, Pasquali C, Ravier F, Bairoch A, Hochstrasser D. Inside SWISS 2DPAGE database. Electrophoresis. 1995;16:1131–1151. doi: 10.1002/elps.11501601190. [DOI] [PubMed] [Google Scholar]
- 26.Sanger F, Nicklen S, Coulson A. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solbiati J, Chapman-Smith A, Miller J L, Miller C G, Cronan J E., Jr Processing of the N termini of nascent polypeptide chains requires deformylation prior to methionine removal. J Mol Biol. 1999;290:607–614. doi: 10.1006/jmbi.1999.2913. [DOI] [PubMed] [Google Scholar]
- 28.Stieger M, Wohlgensinger B, Kamber M, Rolf L, Keck W. Integrational plasmids for the tetracycline-regulated expression of genes in Streptococcus pneumoniae. Gene. 1999;226:243–251. doi: 10.1016/s0378-1119(98)00561-7. [DOI] [PubMed] [Google Scholar]
- 29.Studier F W, Moffatt B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]