Abstract
The l-nucleoside analog β-l-2′,3′-dideoxy-2′,3′-didehydro-5-fluorocytidine (β-l-Fd4C) was first shown to exhibit potent activity against hepatitis B virus (HBV) in tissue culture and then to significantly inhibit viral spread during acute infection in the duck HBV model (F. Le Guerhier et al., Antimicrob. Agents Chemother. 44:111–122, 2000). We have therefore examined its antiviral activity in a mammalian model of chronic HBV infection, the woodchuck chronically infected with woodchuck hepatitis virus (WHV). Side-by-side comparison of β-l-Fd4C and lamivudine administered intraperitoneally during short-term and long-term protocols demonstrated a more profound inhibition of viremia in β-l-Fd4C-treated groups. Moreover, β-l-Fd4C induced a marked inhibition of intrahepatic viral DNA synthesis compared with that induced by lamivudine. Nevertheless, covalently closed circular (CCC) DNA persistence explained the lack of clearance of infected hepatocytes expressing viral antigens and the relapse of WHV replication after drug withdrawal. Liver histology showed a decrease in the inflammatory activity of chronic hepatitis in woodchucks receiving β-l-Fd4C. An electron microscopy study showed the absence of ultrastructural changes of hepatic mitochondria, biliary canaliculi, and bile ducts. However, a loss of weight was observed in all animals, whatever the treatment, as was a transient skin pigmentation in all woodchucks during β-l-Fd4C treatment. There was no evidence that lamivudine or β-l-Fd4C could prevent the development of hepatocellular carcinoma with the protocols used. These results indicate that β-l-Fd4C exhibits a more potent antiviral effect than lamivudine in the WHV model but was not able to eradicate CCC DNA and infected cells from the liver at the dosage and with the protocol used.
Chronic hepatitis B virus (HBV) infection remains a major public health problem worldwide due to its natural history, which includes the progression to chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma (25). The recent approval of lamivudine [β-l(−)-2′,3′-dideoxy-3′-thiacytidine, also called 3TC or l(−)SddC] has opened new perspectives for the therapy of chronic hepatitis B. In all clinical trials, 3TC was shown to be well tolerated and to be a very potent inhibitor of HBV replication (7, 24). However, due to the long half-life of infected hepatocytes and the persistence of viral covalently closed circular (CCC) DNA in infected hepatocytes, long-term treatment is required to control or eradicate viral infection (29, 34). The spontaneous HBV genome variability gives rise to a progressive selection of drug-resistant variants in 16 to 43% of the patients after 1 year of therapy (7, 24). These mutations located in the conserved B and C domains of the HBV reverse transcriptase confer resistance to 3TC (2, 49).
Therefore, the design and evaluation of new molecules with anti-HBV activity remain major goals. With this aim, new antiviral compounds are usually assessed in experimental models of hepadnavirus replication (30), including in vitro hepatocyte culture (10, 19, 31) and the convenient in vivo model of duck HBV (DHBV) infection (9, 27, 33, 47). The woodchuck model of HBV infection (woodchuck hepatitis virus [WHV] infection) presents many features in common with the natural history of human HBV infection, including the development of chronic hepatitis and hepatocellular carcinoma (40, 41). It is therefore a very useful model for the study of the antiviral activities of new compounds as well as their pharmacokinetics (37) and safety (42). In this model, the potent inhibitory activities of new antivirals such as 3TC (18, 29), emtricitabine (FTC: (−)-β-l-2′,3′-dideoxy-3′-thia-5-fluorocytosine) (6, 23), and entecavir (BMS-200475 or cyclopentyl guanosine) (12) was recently demonstrated. The inability to eradicate WHV CCC DNA, even with the most potent inhibitors of viral replication, was underlined, confirming the requirement of long-term antiviral administration to control viral replication (12, 29). The selection of 3TC-resistant variants harboring mutations in the B domain of the WHV reverse transcriptase was also demonstrated (44). The mitochondrial toxicity of fialuridine [1-(2-deoxy-2-fluoro-β-d-arabinofuranosyl)-5-iodouracil] was analyzed in detail in the woodchuck model, indicating its relevance for the evaluation of new antivirals (42).
Recently, the novel cytidine analog β-l-2′,3′-dideoxy-2′,3′-didehydro-5-fluorocytidine (β-l-Fd4C) was synthesized, and it was shown to inhibit HBV replication in the 2.2.1.5 cell line without inducing significant mitochondrial DNA toxicity in CEM cells (4, 28, 39, 45). Evaluation in the DHBV infection model showed that β-l-Fd4C is a more potent inhibitor of the DHBV reverse transcriptase than 3TC and other cytidine analog triphosphates and inhibits viral DNA synthesis in primary duck hepatocyte cultures. It was also demonstrated that early administration of β-l-Fd4C after experimental infection of ducklings dramatically inhibits viral replication but does not prevent the progression to chronic infection (26). To continue the evaluation of the anti-HBV activity of β-l-Fd4C, we have studied its inhibitory activity in woodchucks chronically infected with WHV in comparison with that of 3TC. The effects of these drugs on viremia, intrahepatic viral DNA synthesis, clearance of infected cells, histologic activity of chronic hepatitis, and development of hepatocellular carcinoma were analyzed. From the studies reported herein, we give evidence that, in the woodchuck model, β-l-Fd4C is a potent inhibitor of WHV replication in vivo.
MATERIALS AND METHODS
Drugs.
β-l-Fd4C was synthesized in the Department of Pharmacology and the Comprehensive Cancer Center, Yale University School of Medicine, New Haven, Conn., as described by Lin et al. (28). 3TC was provided by VION Pharmaceuticals.
Treatment of woodchucks chronically infected with WHV.
Twenty captive-born woodchucks (Marmota monax) (age, 12 months) experimentally infected with WHV were purchased from Northeastern Wildlife (South Plymouth, N.Y.). The woodchucks were chronically infected and were used for in vivo experiments. The treatment protocols were performed 3 months later, from July to December 1998, in accordance with the guidelines for animal care at the facilities of the National Veterinary School of Lyon (Marcy l'Etoile, France). β-l-Fd4C administration was performed by the intraperitoneal route to avoid its degradation at low pH, which may occur if it is given by the oral route. Six animals received β-l-Fd4C for 15 weeks (group 1), four animals received in a first set of experiments β-l-Fd4C for 2 weeks and subsequently received β-l-Fd4C for 9 weeks after a washout period of 8 weeks (group 2), four animals received in a first set of experiments 3TC for 2 weeks and subsequently received 3TC for 9 weeks after a washout period of 8 weeks, and six animals served as a control group. The two nucleoside analogs were administered intraperitoneally to woodchucks by the protocols described in the Results section, followed by a 4-month posttreatment period. Blood samples were collected once a week for analysis of viral markers, drug tolerance, and progression of liver disease. Tolerance of antiviral administration was assessed by monitoring of animal weight and lactic acid levels (Lactate PAP; bioMérieux, Marcy l'Etoile, France) in the serum. Serum markers of liver disease were monitored, including gamma-glutamyltransferase levels (1) with the GGT kit (Boerhinger, Meylan, France) and alpha-fetoprotein levels with the α-FETO RIABEAD Diagnostic kit (Abbott, Rungis, France).
Liver biopsy.
Surgical liver biopsies were performed after laparotomy under general anesthesia with 7.5 mg of tiletamin and 7.5 mg of zolazepam (Zoletil; Virbac, Carros, France) per kg of body weight prior to therapy and also during treatment in all woodchucks except animal 607 because it was feeding its offspring. All the pretreatment biopsies were performed at the same time: 7 weeks before β-l-Fd4C treatment in group 1 and 2 weeks before the first course of β-l-Fd4C treatment (group 2) or 3TC treatment. All the on-treatment biopsies were performed at the same time: 16 weeks after the first biopsy, which corresponded to 9 weeks of β-l-Fd4C treatment in group 1 and to 3 weeks of the second course of β-l-Fd4C treatment in group 2 and the group treated with 3TC. One-third of each sample was snap frozen in liquid nitrogen and subsequently stored at −80°C for viral DNA analysis. Another part of the sample was fixed in formalin and embedded in paraffin for liver histology and immunostaining. The last part of the sample was fixed in 1% osmium tetroxide for the electron microscopy study. A macroscopic liver examination was also performed during biopsy sessions.
Analysis of viremia.
Viremia was assessed by quantitative detection of WHV DNA and WHV endogenous polymerase activity (EPA). WHV DNA from the serum of all woodchucks was detected by a specific dot blot hybridization assay throughout the study. Fifty microliters of serum, previously clarified by centrifugation at 10,600 × g for 6 min at 4°C, was spotted directly onto nitrocellulose filters (Sartorius, Göttingen, Germany) with the Hybri-Dot manifold apparatus (Life Technologies, Cergy Pontoise, France). After denaturation (0.2 M NaOH, 1 M NaCl), neutralization (0.5 M Tris-HCl [pH 8] with 1 M NaCl followed by 2× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]), and fixation (80°C for 2 h), the filters were hybridized with a full-length WHV genomic DNA probe labeled with α-32P (Ready to go; Amersham Pharmacia Biotech, Courtaboeuf, France) (47). The filters were scanned by PhosphoImager scanning software (Amersham Pharmacia Biotech), and spots were quantitated with Image Quant. The limit of detection of viral DNA in serum was 500 pg/ml. WHV-associated DNA polymerase activity was assessed directly in 50-μl serum samples of all woodchucks throughout the study period. The level of specific phosphonoformic acid-inhibitable [3H]dTTP incorporation was calculated by measuring the difference in EPA obtained with and without phosphonoformic acid (16). The limit of detection of this assay was 1,000 cpm/ml.
WHV polymerase gene sequencing.
WHV DNA was amplified by PCR with serum collected either at the end of the treatment period or at the last time point before the animals death, according to a protocol described previously (29), with minor modifications. With this aim, 100 μl of serum was mixed with an equal volume of 10 mM Tris-HCl (pH 7.5)–10 mM EDTA–150 mM NaCl–0.1% sodium dodecyl sulfate and treated overnight with 1 mg of proteinase K (Sigma, Saint-Quentin Fallavier, France) per ml at 45°C. After phenol-chloroform extraction and ethanol precipitation, one-sixth of the DNA was amplified for 35 cycles (94°C for 1 min, 50°C for 1 min, and 72°C for 1 min) with Taq polymerase (Perkin-Elmer, Courtaboeuf, France) and a specific primer pair (5′-AGATTGGTTGGTGCACTTCT-3′ [nucleotides 385 to 403] and 5′-AATTGTCAGTGCCCAACA-3′ [nucleotides 1468 to 1451]), corresponding to the B and C domains of the reverse transcriptase gene, with reference to a previously published sequence (22). Another primer pair was used for a nested PCR with samples that were negative in the first round of amplification: 5′-GGATGTATCTGCGGCGTTT-3′ (nucleotides 510 to 528) and 5′-CCCAAATCAAGAAAAACAGAACA-3′ (nucleotides 953 to 931). Sequencing reactions were performed by PCR amplification in a final volume of 20 μl with 5 pmol of the second primer pair, 100 ng of PCR products, and 8 μl of the BigDye Terminator premix according to the Applied Biosystems (Foster City, Calif.) protocol for 25 cycles (94°C for 30 s, 55°C for 30 s, 60°C for 4 min). Removal of excess of BigDye Terminators was performed with exclusion columns, and the samples, which were dried in a vacuum centrifuge, were dissolved with 2 μl of deionized formamide containing 25 mM EDTA (pH 8). Samples were loaded onto an Applied Biosystems 373A sequencer and run for 12 h on a 4.5% denaturing acrylamide gel. The nucleotide sequence and the derived amino acid sequence of the viral polymerase obtained from each sample were compared to published sequences (22, 29).
WHV surface antigen quantitation.
Due to the genome sequence homology and the cross-reactivity of WHV and HBV surface antigens with polyclonal antibodies (5, 11), WHV surface antigen was detected in the serum of all woodchucks prior to therapy and at the time of the second liver biopsy with the AUSRIA II-125 kit (Abbott) (48). The titer of WHV surface antigen was determined from the last positive dilution.
Analysis of intrahepatic viral DNA synthesis.
Intrahepatic viral DNA was extracted from all liver biopsy specimens by a procedure described in detail elsewhere (20, 21). Liver biopsy samples were snap frozen in liquid nitrogen, stored at −80°C, and then analyzed for viral DNA. After homogenization in 10 mM Tris-HCl (pH 7.5)–10 mM EDTA, the biopsy specimen was divided into two parts: one for isolation of total viral DNA (after proteinase K digestion, phenol-chloroform extraction followed by ethanol precipitation) and the other for isolation of non-protein-bound, CCC viral DNA (after sodium dodecyl sulfate-KCl precipitation of protein-bound DNA, phenol-chloroform extraction of the supernatant followed by ethanol precipitation). In order to normalize the cellular DNA concentration in each sample, the DNA concentration was determined by UV densitometric analysis in comparison with that for previously quantified DNA (High DNA Mass Ladder; Life Technologies) after electrophoresis through an agarose gel. Four hundred nanograms of total DNA or the CCC DNA preparation was then subjected to electrophoresis through 1.5% agarose gels and transferred by blotting to nylon membranes (Hybond N+; Amersham Pharmacia Biotech). Viral DNAs were detected by hybridization with an α-32P-labeled probe representing the complete viral genome and quantitated after PhosphoImager scanning.
Analysis of liver histology prior to and while on therapy.
Three-micrometer-thick, formalin-fixed liver biopsy tissue sections were stained with hematoxylin-eosin-safran (HES) stain and examined under a light microscope. The degree of hepatocyte necrosis (acidophilic bodies), the level of inflammation of the portal tract and the intralobular space, and the fibrosis stage were semiquantitatively assessed by using the Metavir score (3). Liver biopsy sections were also assessed for steatosis, ductular proliferation, hepatocyte dysplasia, and hepatocellular carcinoma.
Immunostaining of liver sections for WHV surface antigen and WHV core antigen.
Five-micrometer-thick deparaffinized liver tissue sections were incubated overnight with rabbit serum containing polyclonal antibody directed against the WHV surface antigen or the WHV core antigen (1/2,000 dilution). This step was followed by incubation with a biotinylated goat anti-rabbit immunoglobulin G (Dako, Trappes, France). The antigen-antibody complex was then revealed with streptavidin-horseradish peroxidase (Dako). The visualization was performed with the 3,3′-diaminobenzidine tetrahydrochloride chromogen substrate according to the manufacturer's instructions (Dako) (43). All specimens were evaluated blind without knowledge of the antiviral protocol.
Examination by electron microscopy.
Liver biopsy tissue was fixed in 1% osmium tetroxide in 150 mM sodium cacodylate HCl (pH 7.4), dehydrated in graded ethanol, and embedded in epoxy resin (Cipec, Paris, France). Seventy-five-nanometer ultrathin sections were obtained on an LKB ultratome V (Leica, Nanterre, France), contrasted with methanolic uranyl acetate and lead citrate, and observed with transmission electron microscope 1200EX (Jeol, Kyoto, Japan).
Examination of skin biopsy sections.
Three-micrometer-thick formalin-fixed skin biopsy sections stained with HES stain and by the Fontana-Masson procedure were examined under a light microscope.
RESULTS
Short-term β-l-Fd4C treatment of WHV-infected woodchucks induced a transient but more potent antiviral activity than 3TC.
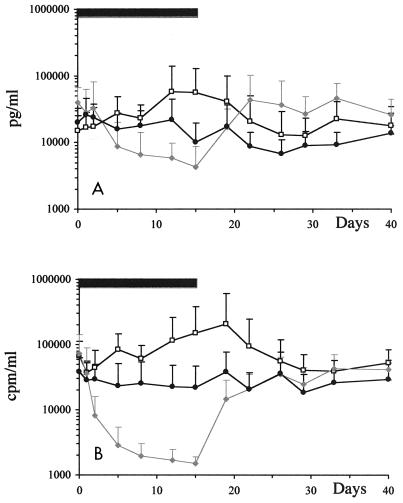
In preliminary dose-finding studies, intraperitoneal administration of β-l-Fd4C at a dosage of 1 mg/kg of body weight per day for 4 consecutive days induced a rapid and profound decrease in serum WHV DNA levels, followed by a rebound of viremia after drug cessation (data not shown). Then, a side-by-side comparison of β-l-Fd4C and 3TC at 1 mg/kg/day for 15 consecutive days was performed with four woodchucks receiving 3TC, four animals treated with β-l-Fd4C, and six control animals receiving no treatment. Analysis of WHV DNA (Fig. 1A) and WHV EPA (Fig. 1B) in serum demonstrated that β-l-Fd4C exhibited a significantly more potent antiviral effect than 3TC at the same dose. In β-l-Fd4C-treated animals, WHV DNA levels decreased by 9.2-fold (range, −51.2- to −3.4-fold), while EPA decreased by 47.8-fold (range, −72.4- to −37.7 fold) and then returned to pretreatment values 1 week after drug withdrawal. The mean serum WHV DNA level and EPA remained stable or increased in 3TC-treated animals and controls, respectively. No variation in body weight or lactic acid levels was observed (data not shown).
FIG. 1.
Short-term β-l-Fd4C administration induces a transient but more potent antiviral effect than 3TC. β-l-Fd4C and 3TC were administered intraperitoneally at 1 mg/kg/day for 15 consecutive days to four woodchucks (per group) chronically infected with WHV, and the results were compared with those for six control animals. Serum WHV DNA levels and WHV EPA were monitored throughout the therapy period and for 25 days after drug withdrawal and are plotted on a logarithmic scale. (A) Mean serum WHV DNA level in each group; (B) mean of WHV EPA. The black bar at the top of each panel indicates the antiviral treatment period. Standard deviations are indicated by error bars. □, controls;  , β-l-Fd4C; •, 3TC.
, β-l-Fd4C; •, 3TC.
Long-term β-l-Fd4C therapy induced a more pronounced inhibition of WHV replication than 3TC.
Since the short-term protocol was not able to induce a sustained inhibition of viral replication, we examined whether a higher dose of β-l-Fd4C could allow spaced dosing for prolonged antiviral therapy. Pilot studies with β-l-Fd4C at 4 mg/kg were performed first with a single injection of drug that induced a rapid drop in viremia levels which was maintained for 4 days (data not shown). Then, β-l-Fd4C was administered at 4 mg/kg/day for 3 consecutive days (induction therapy) and was followed by twice-weekly administration for 2 weeks (maintenance therapy). This regimen with a spacing of the dose every 84 h allowed maintenance of the antiviral effect for the 2 weeks of treatment (data not shown). As expected, a relapse of viremia was observed during the follow-up period.
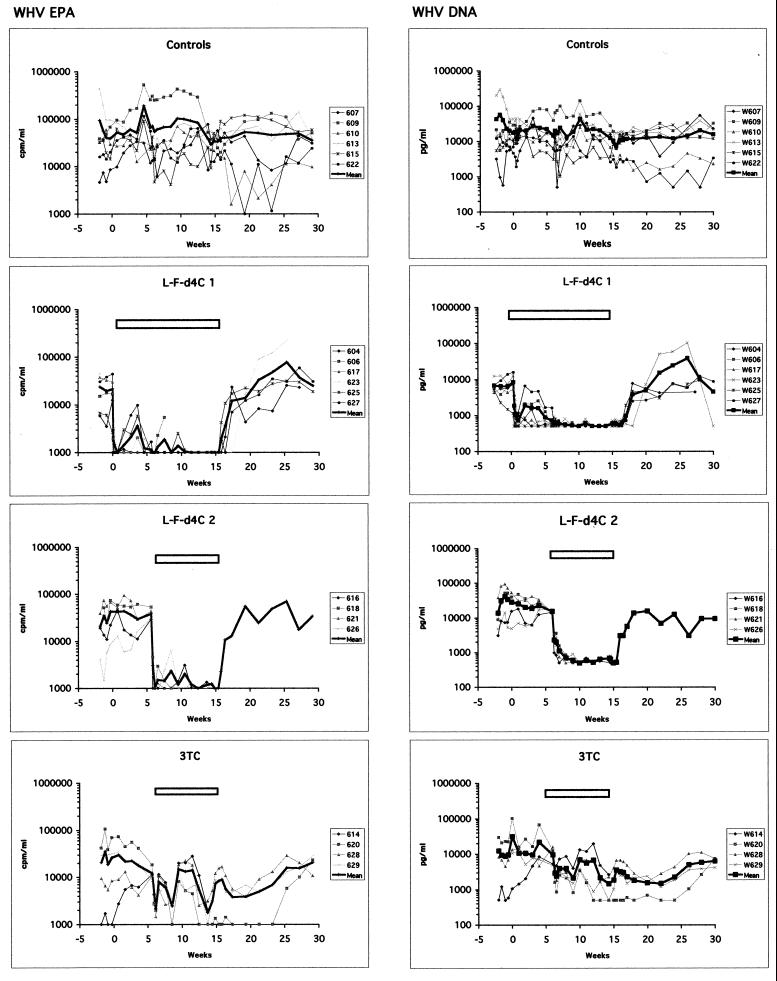
According to these results, a long-term administration of β-l-Fd4C at 4 mg/kg for 15 weeks was initiated in six animals (β-l-Fd4C group 1). The drug was administered for 3 consecutive days as an induction therapy followed by a maintenance therapy (twice weekly for 5 weeks). Results showed first a significant inhibition of viremia (by 21.5-fold) to the limit of detection of the WHV EPA assay or close to the limit of detection of the WHV DNA assay (11.4-fold decrease; range, to 16.6- to −7.1-fold) in all animals, but then three of six woodchucks showed slight increases in viremia levels up to 1,878 pg/ml by the WHV DNA assay or 3,597 cpm/ml by the WHV EPA assay (Fig. 2). Since the evolution of weight and lactic acid levels were similar in treated animals and the animals the control group, the maintenance therapy was modified at week 6 to 4 mg/kg thrice weekly for 9 more weeks. This allowed maintenance of the antiviral effect until the end of the treatment (Fig. 2).
FIG. 2.
Suppression of viral replication during long-term β-l-Fd4C therapy is followed by a relapse of viremia. β-l-Fd4C and 3TC were administered intraperitoneally to woodchucks chronically infected with WHV. The long-term β-l-Fd4C protocol (group 1; six animals) started with an induction treatment at 4 mg/kg/day for 3 consecutive days, followed by a maintenance therapy at the same dosage twice a week, but this was modified at week 6 to a thrice-weekly administration for 9 more weeks. Moreover a side-by-side comparison of the antiviral activities of β-l-Fd4C and 3TC was performed with a schedule consisting of induction therapy for 5 consecutive days followed by maintenance therapy for 8 more weeks with thrice-weekly administration. β-l-Fd4C was administered at 4 mg/kg (group 2; four animals), and 3TC was administered at 10 mg/kg (four animals). The same control group consisting of six woodchucks was used. Viremia was monitored by assays for WHV DNA levels and WHV EPA, as indicated. The level of viremia for each animal and a mean curve for each group were plotted on the graphs (logarithmic scale). The white bars at the top of each panel indicate the antiviral treatment periods. Week 0 indicates the begining of the antiviral administration in β-l-Fd4C group 1.
These results were taken into account to design a second protocol in which β-l-Fd4C was administered at 4 mg/kg to four animals (β-l-Fd4C group 2) for 5 consecutive days as an induction therapy followed by a maintenance therapy thrice weekly for 8 more weeks. The results were compared with those obtained with 3TC, which was administered at a dose of 10 mg/kg by the same schedule used for β-l-Fd4C group 2. In the control group, spontaneous fluctuations of viremia levels were observed throughout the study period (Fig. 2). During the 3TC treatment period, both assays showed a first phase of viremia drop followed by successive phases of a rise and a drop of viremia. In contrast, in β-l-Fd4C group 2 a rapid and significant decrease in the level of viremia to the level of detection of the WHV EPA assay (38.7-fold decrease) or close to the limit of detection of the WHV DNA assay (31-fold decrease) was observed and maintained until the end of the therapy.
At the end of therapy or at the last time point before animal death, polymerase gene sequence analysis was performed with virus from all 20 woodchucks. The results showed the presence of wild-type WHV polymerase sequences, indicating the absence of selection of WHV mutants in the B and C domains of the reverse transcriptase gene (data not shown). After drug withdrawal, viremia levels in all groups of treated animals returned to pretherapy values in 4 to 6 weeks for the β-l-Fd4C groups and in 10 weeks for the 3TC group (Fig. 2).
Long-term β-L-Fd4C therapy strongly inhibited WHV DNA replicative intermediate synthesis but was not sufficient to clear intrahepatic CCC DNA.
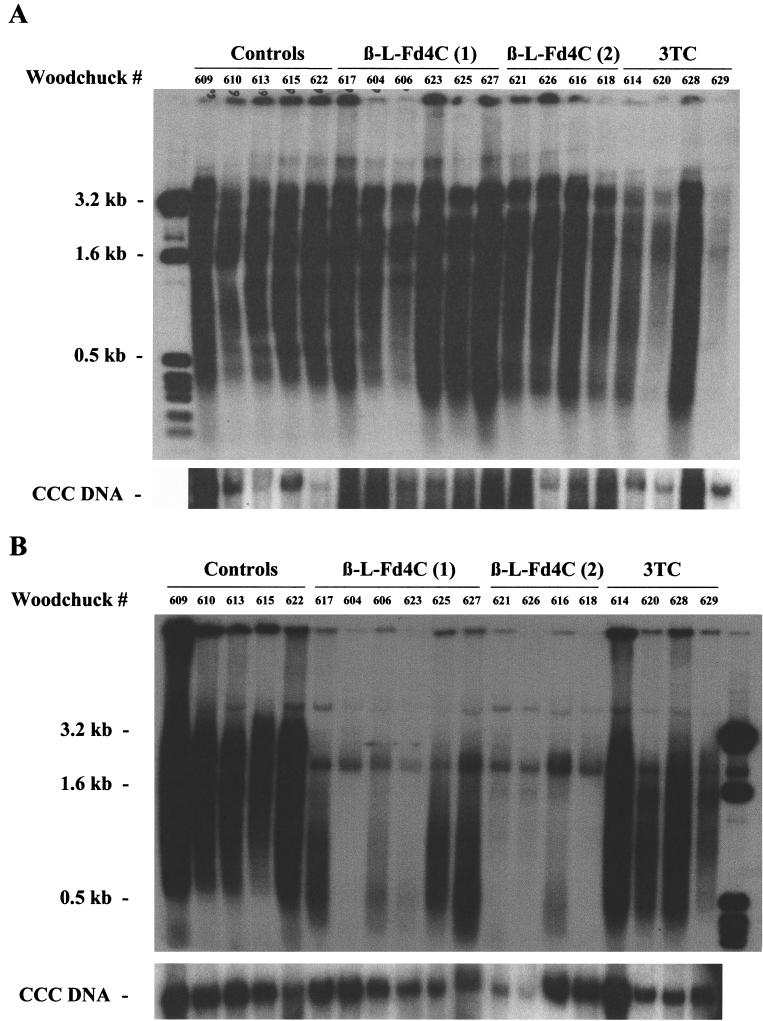
Liver biopsies were performed prior to therapy and after 9 weeks of therapy in β-l-Fd4C group 1 and after 3 weeks of treatment in β-l-Fd4C group 2 or the 3TC group. Southern blot analysis of the intrahepatic viral DNA prior to treatment showed the natural variation of WHV replication from animal to animal in all groups (Fig. 3A). A marked decrease in the level of viral DNA synthesis was observed in both β-l-Fd4C groups, with 5- to 30-fold decreases in the levels of intrahepatic viral DNA intermediates compared to the pretreatment levels (Fig. 3B). In contrast, in the 3TC group, no significant variation in intrahepatic viral replication was observed (range, −3.8- to +2.3-fold variation), which was comparable to that for the control group (range, −3- to +2.2-fold variation). However, the inhibition of WHV replication by β-l-Fd4C administration was not followed by the clearance of intrahepatic viral CCC DNA, as demonstrated by Southern blot analysis (Fig. 3B). Moreover, this viral DNA form persisted whatever the duration of β-l-Fd4C treatment.
FIG. 3.
β-l-Fd4C treatment inhibits WHV DNA synthesis in the liver but is not sufficient to clear CCC DNA. Total DNA and CCC DNA were extracted from liver biopsy specimens obtained prior to therapy and while on therapy as described in the Materials and Methods section and were then analyzed by Southern blotting followed by specific hybridization. (A) Results prior to the beginning of therapy. (B) Results at week 9 of therapy in β-l-Fd4C group 1 and after 3 weeks of therapy in β-l-Fd4C group 2 or the 3TC group. Data for animal 607 were not included in this analysis, as explained in the Materials and Methods section. The sizes of the molecular weight markers are indicated on the left of each panel.
As viral CCC DNA represents the template for viral gene expression, we analyzed the titers of serum WHV surface antigen prior to and while on therapy, at the time of the liver biopsies. The serum WHV surface antigen titer remained stable (10−4) in the control group. A slight decrease in the surface antigen WHV titer from 1 × 10−4 to 1 × 10−3 was observed in three animals in β-l-Fd4C group 1, while one animal had an increase in titer from 1 × 10−4 to 5 × 10−4 in β-l-Fd4C group 2 and the titers in the other six β-l-Fd4C-treated woodchucks remained stable. Among the animals in the 3TC group, one woodchuck had a decrease in the WHV surface antigen titer (from 5 × 10−4 to 1 × 10−4), while the titers in the other three animals were stable. Overall and consistent with the results obtained by CCC DNA detection, there were no significant changes in the WHV surface antigen titers in the sera of 3TC- and β-l-Fd4C-treated animals.
Absence of clearance of WHV-infected hepatocytes during long-term therapy with β-l-Fd4C.
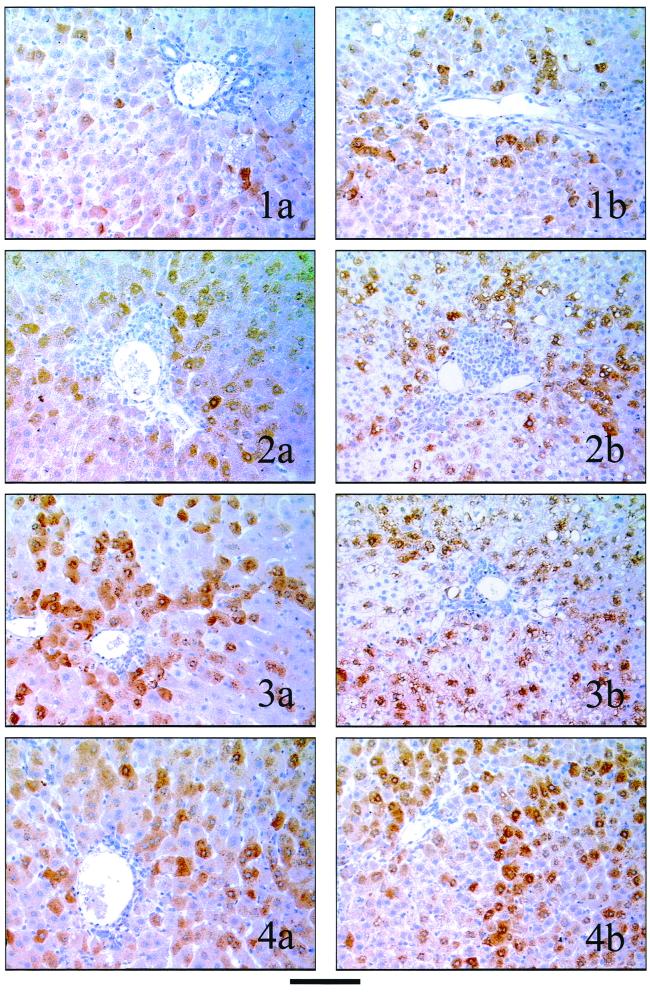
The rate of infected hepatocytes during β-l-Fd4C or 3TC therapy was analyzed by immunostaining with specific antibodies directed against WHV surface or core antigens. Infected hepatocytes expressing viral antigens were observed in the parenchyma and were preferentially clustered in perivascular regions of the hepatic lobule (Fig. 4). The anti-core antigen antibody staining was cytoplasmic and perinuclear (Fig. 4), while the WHV anti-surface antigen antibody staining was mainly cytoplasmic (data not shown). In some animals, the macrovesicular steatosis pushed the cytoplasmic staining to the cellular membrane. Viral envelope and core antigen expression was also noted in some inflammatory cells of the portal tracts, i.e., Kupffer cells, but biliary cells were not stained by anti-WHV antibodies. Whatever the antiviral protocol performed, the number of infected hepatocytes and the level of viral protein expression were not significantly modified in the control group or treated animals (Fig. 4).
FIG. 4.
β-l-Fd4C and 3TC treatments do not decrease significantly the number of infected hepatocytes. The immunostaining studies presented were performed with liver biopsy sections obtained prior to therapy and while on therapy with specific polyclonal anti-WHV core antibodies as described in Materials and Methods. Representative results obtained for each group of animals are depicted. (1a) Liver section of animal 613 (control group) prior to therapy. (1b) Liver section of animal 613 (control group) while on therapy. (2a) Liver section of animal 623 (β-l-Fd4C group 1) prior to therapy. (2b) Liver section of animal 623 (β-l-Fd4C group 1) while on-therapy, (3a) Liver section of animal 621 (β-l-Fd4C group 2) prior to therapy. (3b) Liver section of animal 621 (β-l-Fd4C group 2) while on therapy. (4a) Liver section of animal 620 (3TC group) prior to therapy. (4b) Liver section of animal 620 (3TC group) while on therapy. Bar, 100 μm.
Decrease in chronic hepatitis activity in β-l-Fd4C-treated animals.
Analysis of liver histology was performed with the biopsy specimens obtained prior to and while on therapy (Fig. 5). The Metavir score was used to assess the evolution of the hepatitis activity and liver fibrosis (Table 1). Results showed a decrease in the inflammatory activity of chronic hepatitis and a stability of liver fibrosis in woodchucks treated with β-l-Fd4C for 9 weeks (Fig. 5A and B), while in control animals, hepatitis activity increased and the level of liver fibrosis tended to decrease. After 3 weeks of therapy, the four animals in β-l-Fd4C group 2 showed stable liver histology, while the four woodchucks in the 3TC group showed a stable hepatitis activity and a slight increase in the levels of liver fibrosis.
FIG. 5.
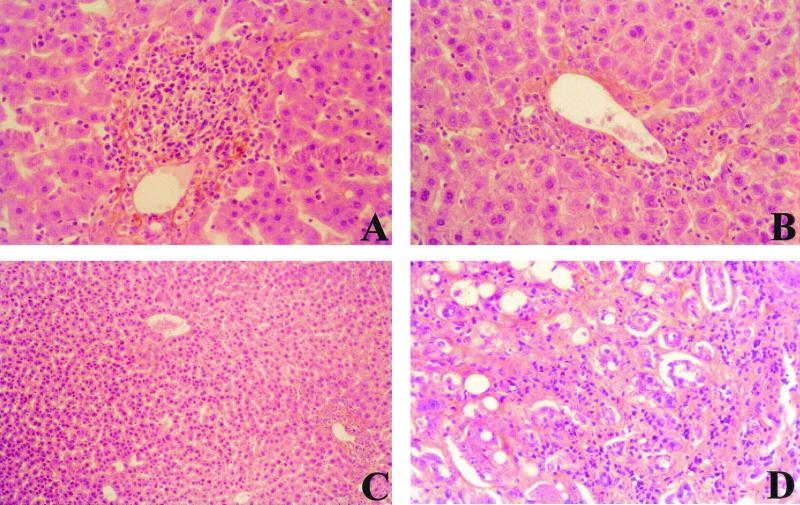
Decrease of hepatitis activity by 9 weeks of β-l-Fd4C therapy but development of hepatocellular carcinoma in all groups of woodchucks. Analysis of liver histology was performed with biopsy specimens obtained prior to and while on therapy. (A) Liver section of animal 625 (β-l-Fd4C group 1) prior to therapy (magnification ×570). (B) Liver section of animal 625 (β-l-Fd4C group 1) while on therapy (magnification, ×570). Note the decrease in the level of the inflammatory infiltrates and the stable liver fibrosis during treatment. (C) Liver section of animal 625 (β-l-Fd4C group 1) while on therapy (magnification, ×228). Note the progression of the liver disease with the presence of a dysplasic nodule (with marked hepatocyte dysplasia). (D) Liver section of animal 623 (β-l-Fd4C group 1) while on therapy (magnification, ×570). Note the progression of the liver disease with the development of hepatocellular carcinoma.
TABLE 1.
Analysis of liver histology after surgical biopsy of woodchucks, prior to therapy, and after 3 weeks of treatment with β-l-Fd4C (group 2) or 3TC and 9 weeks of therapy in β-l-Fd4C (group 1)a
| Woodchuck group and animal no. | Pretreatment biopsy
|
On-treatment biopsy
|
γGT activity | Necropsy after week 0
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| A | F | Observation | A | F | Observation | Wk | Observation | ||
| Controls | |||||||||
| 607 | ND-1 | ND-1 | 37 | HCC, 7 cm; euthanatized | |||||
| 609 | 1 | 2 | 0 | 1 | HCC; dysplasic nodule | γ | 57 | HCC, multiple (1 to 5 cm) | |
| 610 | 0 | 1 | 2 | 1 | HCC; no dysplasia | 33b | HCC, 1.5 cm | ||
| 613 | 1 | 1 | 2 | 1 | HCC; no dysplasia | 64 | HCC, 3.5 cm | ||
| 615 | 1 | 3 | ND-2 | γ | 30b | No apparent HCC, but liver with necrosis | |||
| 622 | 2 | 2 | 3 | 1 | Two dysplasic foci | 39 | HCC, multiple (1 cm) | ||
| β-l-Fd4C group 1 | |||||||||
| 604 | 1 | 0 | HCC | 0 | 0 | HCC; no dysplasia | γ | 27b | HCC, multiple (1 to 5.5 cm); euthanatized |
| 606 | 2 | 1 | 1 | 1 | HCC | γ | 9b | Laparotomy; HCC | |
| 617 | 2 | 1 | 0 | 1 | HCC | γ | 6b | HCC, multiple (1 cm) | |
| 623 | 1 | 1 | 1 | 1 | HCC | γ | 26b | HCC, multiple (1 to 7 cm) | |
| 625 | 2 | 1 | 1 | 1 | Dysplasic nodule | 69 | HCC, multiple (5 cm) euthanatized | ||
| 627 | 0 | 2 | 1 | 1 | 53 | HCC, multiple | |||
| β-l-Fd4C group 2 | |||||||||
| 616 | 1 | 2 | 0 | 1 | HCC; tumoral nodule | 15b | HCC, multiple (1 cm) | ||
| 618 | 0 | 0 | 0 | 1 | HCC | 9b | Laparotomy; HCC (1 cm) | ||
| 621 | 0 | 1 | HCC | 1 | 1 | HCC; no dysplasia | 9b | Laparotomy; HCC (1 to 4.5 cm) | |
| 626 | 0 | 0 | HCC; dysplastic nodule | 0 | 0 | HCC; no dysplasia | γ | 65 | HCC global |
| 3TC | |||||||||
| 614 | 2 | 2 | HCC | 2 | 2 | HCC; tumoral nodule | γ | 14b | HCC, multiple (1 to 8 cm); euthanatized |
| 620 | 1 | 1 | 1 | 2 | 77 | HCC, multiple (2 cm) | |||
| 628 | 1 | 0 | HCC | 1 | 1 | HCC | γ | 47 | HCC, multiple (6 to 10 cm) |
| 629 | 2 | 2 | 2 | 2 | 39 | No apparent HCC, but marked dysplasia of hepatocytes | |||
Observations were performed in reference to the beginning of the treatment in β-l-Fd4C group 1 (week 0), which started 4 months after the arrival of the 12-month-old animals. Woodchucks were also studied for 4 months after the cessation of drug treatment. Micro- or macroscopic examinations were performed at the time of the two liver biopsies and at the time of animal death. Further necropsy observations performed after the end of the study are also indicated. The Metavir score was used to assess the severity of the disease. Abbreviations: A, activity of chronic hepatitis (a score of 0 to 3 indicates the degree of hepatitis activity); F, fibrosis of the liver (a score of 0 to 4 indicates the degree of liver fibrosis); ND-1, Liver biopsy not performed for that woodchuck because it was feeding its two offspring; ND-2, granulomatous hepatitis due to bacterial infection; impossibility of scoring for viral hepatitis activity; HCC, hepatocellular carcinoma; γ, elevated gamma-glutamyltransferase (γ GT) levels (>40 IU/ml) during the study period.
The woodchuck died naturally or was euthanatized or did not survive to the time of surgical biopsy during the study.
Although β-l-Fd4C and 3TC administration inhibited viral replication to different extents and acted on liver disease, six animals died of hepatocellular carcinoma during the study period (4 of 10 in the β-l-Fd4C groups, 1 of 4 in the 3TC group, and 1 of 6 in the control group), 3 other animals died during the second biopsy session but already presented with hepatocellular carcinoma, and one animal died with a liver with signs of necrosis (Table 1). All the remaining animals died naturally or were euthanatized during the 10-month period following the end of the study and presented with hepatocellular carcinoma (9 of 10 animals) or marked liver dysplasia (1 of 10 animals). Diagnosis of hepatocellular carcinoma was made by both macroscopic and microscopic examination of the liver. Interestingly, liver histology analysis showed in most animals the sequential occurrence of dysplasic nodules and progression to hepatocellular carcinoma during antiviral treatment (Fig. 5C and D). Follow-up of alpha-fetoprotein levels did not reveal significant variations over time and was not associated with the evolution toward hepatocellular carcinoma (data not shown).
Analysis of animal tolerance to antiviral therapy.
Lactic acid levels and animal weight were assessed throughout the study. A loss of weight was observed in the animals in the β-l-Fd4C groups (from 4 ± 0.6 to 2.5 ± 0.3 kg) as well as in those in the 3TC group (from 4.3 ± 0.8 to 3.2 ± 0.1 kg) and in the control group (from 3.7 ± 0.7 to 2.9 ± 0.4 kg), although lactic acid levels were normal for all animals (data not shown). Gamma-glutamyltransferase quantitation revealed at the time of the second liver biopsy the presence of elevated gamma-glutamyltransferase levels in nine animals distributed among all groups tested (Table 1). However, although the gamma-glutamyltransferase level elevation was specifically associated with the occurrence of hepatocellular carcinoma (observed in eight animals), the association was not sensitive enough since in five animals in which hepatocellular carcinoma was observed macroscopically gamma-glutamyltransferase levels were within the normal range. Study of liver sections from all treated animals after 3 or 9 weeks of therapy by electron microscopy showed the absence of ultrastructural modification of hepatocyte mitochondria (Fig. 6A), biliary canaliculi (Fig. 6B), and bile duct epithelial cells (Fig. 6C) in β-l-Fd4C- and 3TC-treated animals as well as in the control group (Fig. 6D). Surprisingly, homogeneous skin hyperpigmentation slowly appeared in all β-l-Fd4C-treated animals, after 4 weeks of treatment in group 2 and 10 weeks of treatment in group 1, corresponding to the switch from biweekly to thrice-weekly administration. Skin histology performed at the end of therapy and 8 weeks after drug cessation revealed an accumulation of melanin pigment throughout the entire epidermal layer that was progressively eliminated to the upper level of the epidermis by natural cell turnover after drug withdrawal (Fig. 7).
FIG. 6.
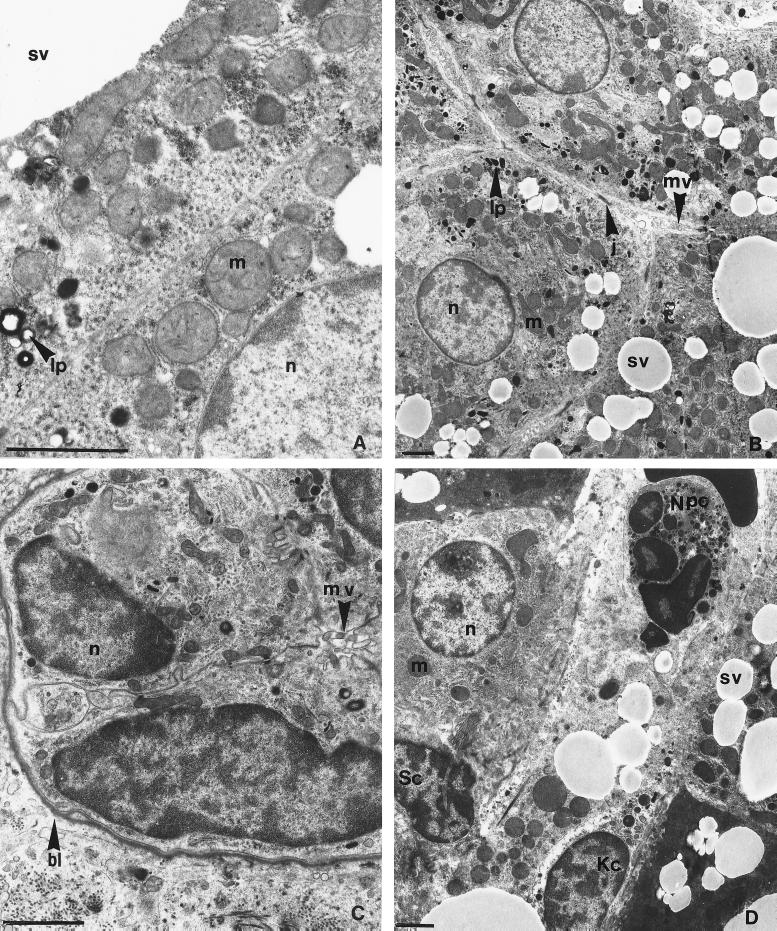
Absence of cytotoxicity-related ultrastructural change of hepatocyte mitochondria, biliary canaliculi, and bile ducts during therapy. The ultrastructure of the mitochondria, biliary canaliculi, and bile ducts were analyzed by electron microscopy of liver biopsy specimens after 3 or 9 weeks of therapy, as described in Materials and Methods. (A) Hepatic trabecula of animal 623 (β-l-Fd4C group 1). Mitochondria showed a normal aspect, with tight intermembrane spaces and regularly distributed cristae into the homogeneously electron-dense matrix. (B) Hepatocytic biliary poles of animal 620 (3TC group). The bile canaliculi were regularly distributed between hepatocytes, with well-preserved limiting-membrane junctional complexes and well-developed intracanalicular hepatocytic microvilli. A physiological amount of lipopigments was deposited in the pericanalicular hepatocytic cytoplasm. Note the normal mitochondrial population. (C) Bile ductule of animal 620 (3TC group). Note the normal aspect of the periductal basal lamina, the polarized distribution of epithelial cell organelles, interepithelial cell contact, and ductal lumen with epithelial cell microvilli. (D) Hepatocytic trabecula with sinusoid of animal 609 (control group). Major steatosis was observed, with micro- and macrovesicular accumulations of triglycerides. The sinusoidal vessel was congested with hepatocytic cytoplasmic fragments and inflammatory cells (neutrophil polymorphonuclear cell, hypertrophic Kupffer cell). A stellate cell was present in the Disse space. Note the normal aspect of mitochondrias. m, mitochondria; n, nucleus; sv, steatosis vesicle; j, membrane junctional complexes; mv, microvilli; lp, lipopigment; bl, basal lamina; Kc, Kupffer cell; Sc, stellate cell; Npc, neutrophilic polymorphonuclear cell. Bars, 2 μm.
FIG. 7.
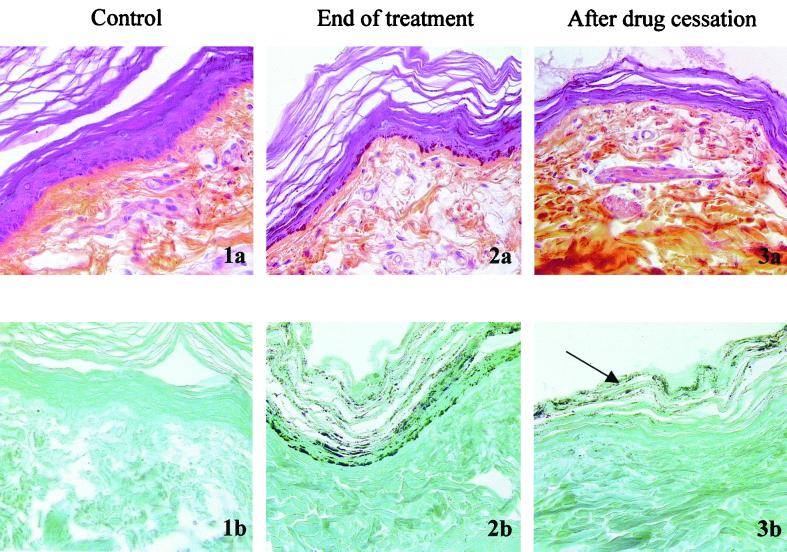
Transient skin pigmentation during β-l-Fd4C therapy of WHV-infected woodchucks. Skin biopsies were performed at the end of β-l-Fd4C therapy and 8 weeks after drug withdrawal. (1a) Skin section of animal 613 (control group) analyzed by staining with HES stain at the end of therapy. (1b) Skin section of animal 613 (control group) stained with Fontana-Masson stain at the end of therapy. Note the normal epidermis and upper dermis in the control with the absence of melanin pigment on Fontana-Masson staining. (2a) Skin section of animal 623 (β-l-Fd4C group 1) analyzed by staining with HES stain at the end of therapy. (2b) Skin section of animal 623 (β-l-Fd4C group 1) stained with Fontana-Masson stain at the end of therapy. Note the overload of all the epidermal layers including the stratum corneum by melanic pigmentation, as visualized by Fontana-Masson staining. (3a) Skin section of animal 623 (β-l-Fd4C group 1) analyzed by staining with HES stain posttherapy. (3b) Skin section of animal 623 (β-l-Fd4C group 1) stained with Fontana-Masson stain posttherapy. Note the transient effect of β-l-Fd4C on the skin pigmentation after drug cessation. The hyperpigmentation is retained in the upper part of the epidermis (horny layer [arrow]), while the basal layer and spinosum stratum are free of pigment. Magnifications, ×225.
DISCUSSION
In the work described here the antiviral activity of β-l-Fd4C was analyzed in vivo in the mammalian model of WHV infection and was compared with that of 3TC, another l-deoxycytidine analog. Both short-term and long-term treatments with β-l-Fd4C at a dose of 1 or 4 mg/kg administered intraperitoneally allowed inhibition of WHV replication. However, this was followed by a relapse of viral replication after drug withdrawal, as already described in this model with other nucleoside analogs (6, 12, 18, 42), but with a longer delay for the case of the 3TC group in our experiment. The inhibition of viremia assessed by WHV DNA detection and determination of EPA was, however, less pronounced than that observed with a recently developed compound, cyclopentyl guanosine (entecavir; BMS-200475), which allowed 107- to 108-fold decreases in viremia titers (12). By comparison, 3TC was only moderately active against WHV replication, as already observed with a dosage of 5 mg/kg administered orally (12). In a recently published study, 3TC had to be administered at an oral dose of 200 mg/kg to obtain a significant antiviral effect in woodchucks (29). These results are consistent with the more potent inhibitory effect of β-l-Fd4C on the DHBV reverse transcriptase in vitro and in vivo in comparison with that of 3TC in the duck model of HBV infection (26). Therefore, it would be interesting to gain comparative information on the in vivo metabolisms of 3TC and β-l-Fd4C in both the duck and woodchuck models by comparison with those in humans.
Prolonged β-l-Fd4C treatment for 15 weeks was not able to achieve clearance of intrahepatic viral CCC DNA, explaining the relapse of viral replication after the cessation of therapy. This is consistent with the results obtained with the duck model of HBV infection with antiviral therapy based on 2′-carbodeoxyguanosine or FTC (9), as well as in the woodchuck model with 3TC (29), FTC (6), and cyclopentyl guanosine (12). This was associated with the absence of a decline in the number of infected hepatocytes and the WHV surface antigen titer in serum, suggesting that the residual viral CCC DNA is an active template for viral genome expression, allowing the reinitiation of viral replication when therapy is stopped. Genome sequence analysis of the B and C domains of the polymerase gene of the dominant virus in the sera of the animals demonstrated the wild-type sequence, suggesting the absence of selection of drug-resistant mutants in β-l-Fd4C- or 3TC-treated animals with this duration of treatment. However, selection of drug-resistant mutants has previously been observed after longer durations (>9 to 12 months) of 3TC treatment in the woodchuck model (44). As the rise in the viremia titer associated with the selection of drug-resistant mutants was shown to depend on the clearance of hepatocytes infected with wild-type virus (44), in our experiments we cannot rule out that this process was ongoing when therapy was stopped. As direct sequencing of the PCR product may allow detection of minor variants representing 10 to 20% of the circulating viral quasispecies, clonal analysis may already have revealed the presence of mutants as minor species.
It was recently shown in the woodchuck model of hepadnavirus infection that the recruitment of CD4+ and CD8+ T cells and the production of gamma interferon and tumor necrosis factor alpha in the infected liver, accompanied by a significant increase in apoptosis and regeneration of heptocytes, are critical events involved in viral clearance (15). In light of these observations, the evaluation of combination treatment strategies based on the use of reverse transcriptase inhibitors and immune modulators such as the DNA vaccine approach (38, 46) to enhance the clearance of infected cells is warranted.
Interestingly, we observed a decrease in liver histology activity in β-l-Fd4C-treated animals. Since the number of animals was limited in this study, further investigations are warranted to determine whether prolonged antiviral treatment may control the progression of the liver disease, as suggested in clinical trials with 3TC (7, 24). Since expression of WHV antigen in the liver induces an antiviral immune response that leads to chronic disease, the decrease of hepatitis activity during β-l-Fd4C therapy could also be explained by an immunomodulatory action of this antiviral since the number of infected cells and the level of viral protein expression were apparently not significantly modified by our protocol. However, in our woodchuck cohort, which was obtained at 12 months of age and treated 4 months later in the long-term β-l-Fd4C group 1 protocol, this beneficial effect on liver histology was not sufficient to prevent or delay the occurrence of liver cell dysplasia and subsequently the development of hepatocellular carcinoma. Accordingly, in another study, long-term 3TC therapy of chronically infected woodchucks did not prevent the occurrence of hepatocellular carcinoma (29). WHV-induced liver cancer involves a cascade of complex events including early viral genome integration in the N-myc and c-myc proto-oncogenes as well as hepatocyte turnover (8, 32). As the rate of hepatocellular carcinoma reaches 25% of the infected animals each year (13, 36), our findings suggest that early antiviral intervention should be evaluated as prophylaxis for hepatocellular carcinoma.
Indeed, a study performed with younger animals showed that long-term 3TC treatment may delay the development of hepatocellular carcinoma in this model (S. F. Peek, I. A. Toshkov, H. N. Erb, R. F. Shinazi, B. E. Korba, P. J. Cote, J. L. Gerin, and B. C. Tennant, Abstr. Am. Assoc. Study Liver Dis., abstr. 957, 1997).
As fialuridine was responsible for major mitochondrial toxicity in humans as confirmed with the woodchuck model (42), the potential toxicity of β-l-Fd4C was another very important issue that was addressed with the woodchuck model. Whether the loss of weight that was observed during β-l-Fd4C therapy could be related to the occurrence of hepatocellular carcinoma, the physiology of the woodchuck during hibernation, or side effects of β-l-Fd4C needs to be determined by further toxicological studies. Indeed, careful electron microscopy analysis of liver samples during antiviral treatment did not reveal any significant ultrastructural modifications to hepatocyte mitochondria, biliary canaliculi, or bile ducts in β-l-Fd4C- and 3TC-treated animals, suggesting an absence of liver cytotoxicity of these antivirals under our experimental conditions, as already observed with 3TC treatment in humans (17). However, skin hyperpigmentation related to an accumulation of melanin pigment was observed during β-l-Fd4C treatment and slowly disappeared after drug withdrawal. This skin modification may be related to the particular physiology of the woodchuck, especially since therapy was performed in part during the hibernation period, when the general metabolism of these animals is greatly modified. A similar hyperpigmentation phenomenon has been described in patients treated with zidovudine and was reproduced in mice experimentally receiving zidovudine (14, 35). The molecular mechanisms responsible for this increase in melanocyte activity as a result of nucleoside analog administration and their relation to hibernation remain to be elucidated.
In conclusion, the results of our study suggest that although β-l-Fd4C exhibits a more potent antiviral effect than 3TC in a mammalian model of HBV infection, clearance of viral CCC DNA and infected cells from the liver is difficult to achieve with monotherapy with a potent inhibitor of viral replication. Furthermore, effective prevention of hepatocellular carcinoma in this animal model may require an early therapeutic intervention, before the integration of viral genome sequences in the host genome. Future approaches that combine antivirals and immune modulators for the eradication of chronic hepadnavirus infection should be evaluated.
ACKNOWLEDGMENTS
This work was supported by grants from INSERM, the French Association for Research against Cancer, and the French League against Cancer and grants AI38204 and CA63477. F. Le Guerhier was the recipient of a fellowship from the French League against Cancer.
REFERENCES
- 1.Abe K, Kurata T, Shikata T, Tennant B C. Enzyme-altered liver cell foci in woodchucks infected with woodchuck hepatitis virus. Jpn J Cancer Res. 1988;79:466–472. doi: 10.1111/j.1349-7006.1988.tb01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen M I, Deslauriers M, Andrews C W, Tipples G A, Walters K A, Tyrell D L J, Brown N, Condreay L D. Identification and characterization of mutations in hepatitis B virus resistant to lamivudine. Hepatology. 1998;27:1670–1677. doi: 10.1002/hep.510270628. [DOI] [PubMed] [Google Scholar]
- 3.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 4.Chen S H, Lin S, King I, Spinka T, Dutschman G E, Gullen E A, Cheng Y C, Doyle T W. Synthesis and comparative evaluation of two antiviral agents: beta-l-Fd4C and beta-D-Fd4C. Bioorg Med Chem Lett. 1998;8:3245–3250. doi: 10.1016/s0960-894x(98)00599-x. [DOI] [PubMed] [Google Scholar]
- 5.Cote P J, Engle R E, Langer C A, Ponzetto A, Gerin J L. Antigenic analysis of woodchuck hepatitis virus surface antigen with site-specific radioimmunoassays. J Virol. 1984;49:701–708. doi: 10.1128/jvi.49.3.701-708.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cullen J M, Smith S L, Davis M G, Dunn S E, Botteron C, Cecchi A, Linsey D, Linzey D, Frick L, Paff M T, Goulding A, Biron K. In vivo antiviral activity and pharmacokinetics of (−)-cis-5-fluoro-1-(2-(hydroxymethyl)-1,3-oxathiolan-5-yl)cytosine cytosine in woodchuck hepatitis virus-infected woodchucks. Antimicrob Agents Chemother. 1997;41:2076–2082. doi: 10.1128/aac.41.10.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dienstag J L, Schiff E R, Wright T L, Perrillo R P, Hann H W, Goodman Z, Crowther L, Condready L D, Woessner M, Rubin M, Brown N A. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256–1263. doi: 10.1056/NEJM199910213411702. [DOI] [PubMed] [Google Scholar]
- 8.Fourel G, Trépo C, Bougueleret L, Henglein B, Ponzetto A, Tiollais P, Buendia M A. Frequent activation of N-myc genes by hepadnavirus insertion in woodchuck liver tumors. Nature. 1990;347:294–298. doi: 10.1038/347294a0. [DOI] [PubMed] [Google Scholar]
- 9.Fourel I, Cullen J M, Saputelli J, Aldrich C E, Schaffer P, Averett D R, Pugh J, Mason W S. Evidence that hepatocyte turnover is required for rapid clearance of duck hepatitis B virus during antiviral therapy of chronically infected ducks. J Virol. 1994;68:8321–8330. doi: 10.1128/jvi.68.12.8321-8330.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fourel I, Saputelli J, Schaffer P, Mason W S. The carbocyclic analog of 2′-deoxyguanosine induces a prolonged inhibition of duck hepatitis B virus DNA synthesis in primary hepatocyte cultures and in the liver. J Virol. 1994;68:1059–1065. doi: 10.1128/jvi.68.2.1059-1065.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galibert F, Chen T N, Mandart E. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: comparison with the hepatitis B virus sequence. J Virol. 1982;41:51–65. doi: 10.1128/jvi.41.1.51-65.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genovesi E V, Lamb L, Medina I, Taylor D, Seifer M, Innaimo S, Colonno R J, Standring D N, Clark J M. Efficacy of the carbocyclic 2′-deoxyguanosine nucleoside BMS-200475 in the woodchuck model of hepatitis B virus infection. Antimicrob Agents Chemother. 1998;42:3209–3217. doi: 10.1128/aac.42.12.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gouillat C, Manganas D, Zoulim F, Vitrey D, Saguier G, Guillaud M, Ain J F, Duque-Campos R, Jamard C, Praves M, Trépo C. Woodchuck hepatitis virus-induced carcinoma as a relevant natural model for therapy of human hepatoma. J Hepatol. 1997;26:1324–1330. doi: 10.1016/s0168-8278(97)80468-0. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg R G, Berger T G. Nail and mucocutaneous hyperpigmentation with azidothymidine therapy. J Am Acad Dermatol. 1990;22:327–330. doi: 10.1016/0190-9622(90)70039-k. [DOI] [PubMed] [Google Scholar]
- 15.Guo J T, Zhou H, Liu C, Aldrich C, Saputelli J, Whitaker T, Barrasa M I, Mason W S, Seeger C. Apoptosis and regeneration of hepatocytes during recovery from transient hepadnavirus infections. J Virol. 2000;74:1495–1505. doi: 10.1128/jvi.74.3.1495-1505.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hantz O, Ooka T, Vitvitski L, Pichoud C, Trépo C. Comparison of properties of woodchuck hepatitis virus and human hepatitis B virus endogenous DNA polymerases. Antimicrob Agents Chemother. 1984;25:242–246. doi: 10.1128/aac.25.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honkoop P, de Man R A, Scholte H R, Zondervan P E, Van Den Berg J W O, Rademekers L H P M, Schalm S W. Effect of lamivudine on morphology and function of mitochondria in patients with chronic hepatitis B. Hepatology. 1997;26:211–215. doi: 10.1002/hep.510260128. [DOI] [PubMed] [Google Scholar]
- 18.Hurwitz S J, Tennant B C, Korba B E, Gerin J L, Schinazi R F. Pharmacodynamics of (−)-β-2′,3′-dideoxy-3′-thiacytidine in chronically virus-infected woodchucks compared to its pharmacodynamics in humans. Antimicrob Agents Chemother. 1998;42:2804–2809. doi: 10.1128/aac.42.11.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Innaimo S F, Seifer M, Bisacchi G S, Standring D N, Zahler R, Colonno R J. Identification of BMS-200475 as a potent and selective inhibitor of hepatitis B virus. Antimicrob Agents Chemother. 1997;41:1444–1448. doi: 10.1128/aac.41.7.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jilbert A R, Wu T T, England J M, De La P, Hall M, Carp N Z, O'Connell A P, Mason W S. Rapid resolution of duck hepatitis B virus infections occurs after massive hepatocellular involvement. J Virol. 1992;66:1377–1388. doi: 10.1128/jvi.66.3.1377-1388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kajino K, Jilbert A, Saputelli J, Aldrich C, Cullen J, Mason W. Woodchuck hepatitis virus infections: very rapid recovery after a prolonged viremia and infection of virtually every hepatocyte. J Virol. 1994;68:5792–5803. doi: 10.1128/jvi.68.9.5792-5803.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kodama K, Ogasawara N, Yoshikawa H, Murakami S. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: evolutional relationship between hepadnaviruses. J Virol. 1985;56:978–986. doi: 10.1128/jvi.56.3.978-986.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korba B E, Schinazi R F, Cote P, Tennant B C, Gerin J L. Effect of oral administration of emtricitabine on woodchuck hepatitis virus replication in chronically infected woodchucks. Antimicrob Agents Chemother. 2000;44:1757–1760. doi: 10.1128/aac.44.6.1757-1760.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai C L, Chine R W, Leung N W Y, Chang T T, Guan R, Tai D I, Ng K Y, Wu P C, Dent J C, Barber J, Stephenson S L, Gray F. A one year trial of lamivudine for chronic hepatitis B. N Engl J Med. 1998;339:61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 25.Lee W M. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–1745. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 26.Le Guerhier F, Pichoud C, Guerret S, Chevallier M, Jamard C, Hantz O, Li X Y, Chen S H, King I, Trépo C, Cheng Y C, Zoulim F. Characterization of the antiviral effect of 2′, 3′-dideoxy-2′, 3′-didehydro-β-l-5-fluorocytidine in the duck hepatitis B virus infection model. Antimicrob Agents Chemother. 2000;44:111–122. doi: 10.1128/aac.44.1.111-122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin E, Luscombe C, Wang Y, Shaw T, Locarnini S. The guanine nucleoside analog penciclovir is active against chronic duck hepatitis B virus infection in vivo. Antimicrob Agents Chemother. 1996;40:413–418. doi: 10.1128/aac.40.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin T-S, Luo M-Z, Liu M-C, Zhu Y-L, Gullen E, Dutschmann G E, Cheng Y-C. Design and synthesis of 2′,3′-dideoxy-2′,3′-didehydro-β-l-cytidine (β-l-d4C) and 2′,3′-dideoxy-2′,3′-didehydro-β-l-5-fluorocytidine (β-l-Fd4C), two exceptionally potent inhibitors of human immunodeficiency virus (HIV) in vitro. J Med Chem. 1996;39:1757–1759. doi: 10.1021/jm950836q. [DOI] [PubMed] [Google Scholar]
- 29.Mason W S, Cullen J, Moraleda G, Saputelli J, Aldrich C E, Miller D S, Tennant B, Frick L, Averett D, Condreay L D, Jilbert A R. Lamivudine therapy of WHV-infected woodchucks. Virology. 1998;245:18–32. doi: 10.1006/viro.1998.9150. [DOI] [PubMed] [Google Scholar]
- 30.Mason W S, Taylor J M. Experimental systems for the study of hepadnavirus and hepatitis delta virus infections. Hepatology. 1989;9:635–645. doi: 10.1002/hep.1840090420. [DOI] [PubMed] [Google Scholar]
- 31.Moraleda G, Saputelli J, Aldrich C E, Averett D, Condreay L, Mason W S. Lack of effect of antiviral therapy in nondividing hepatocyte cultures on the closed circular DNA of woodchuck hepatitis virus. J Virol. 1997;71:9392–9399. doi: 10.1128/jvi.71.12.9392-9399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moroy T, Marchio A, Etiemble J, Trépo C, Tiollais P, Buendia M. Rearrangement and enhanced expression of c-myc in hepatocellular carcinoma of hepatitis virus infected woodchucks. Nature. 1986;324:276–279. doi: 10.1038/324276a0. [DOI] [PubMed] [Google Scholar]
- 33.Nicoll A J, Colledge D L, Toole J J, Angus P W, Smallwood R A, Locarnini S A. Inhibition of duck hepatitis B virus replication by 9-(2-phosphonylmethoxyethyl) adenine, an acyclic phosphonate nucleoside analogue. Antimicrob Agents Chemother. 1998;42:3130–3135. doi: 10.1128/aac.42.12.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nowak M, Bonhoeffer S, Hill A, Boehme R, Thomas H, McDade H. Viral dynamics in hepatitis B virus infection. Proc Natl Acad Sci USA. 1996;93:4398–4402. doi: 10.1073/pnas.93.9.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obuch M L, Baker G, Roth R I, Yen T S, Levin J, Berger T G. Selective cutaneous hyperpigmentation in mice following zidovudine administration. Arch Dermatol. 1992;128:508–513. [PubMed] [Google Scholar]
- 36.Popper H, Roth L, Purcell R, Tennant B C, Gerin J L. Hepatocarcinogenicity of the woodchuck hepatitis virus. Proc Natl Acad Sci USA. 1987;84:866–870. doi: 10.1073/pnas.84.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajagopalan P, Boudinot F, Chu C, Tennant B, Baldwin B, Schinazi R. Pharmacokinetics of (−)-2′,3′-dideoxy-3′-thiacytidine in woodchucks. Antimicrob Agents Chemother. 1996;40:642–645. doi: 10.1128/aac.40.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rollier C, Sunyach C, Barraud L, Madani N, Jamard C, Trépo C, Cova L. Protective and therapeutic effect of DNA-based immunization against hepadnavirus large envelope protein. Gastroenterology. 1999;116:658–665. doi: 10.1016/s0016-5085(99)70188-5. [DOI] [PubMed] [Google Scholar]
- 39.Shi J, McAtee J J, Schlueter Wirtz S, Tharnish P, Juodawlkis A, Liotta D C, Schinazi R F. Synthesis and biological evaluation of 2′,3′-didehydro-2′,3′-dideoxy-5-fluorocytidine (D4FC) analogues: discovery of carbocyclic nucleoside triphosphates with potent inhibitory activity against HIV-1 reverse transcriptase. J Med Chem. 1999;42:859–867. doi: 10.1021/jm980510s. [DOI] [PubMed] [Google Scholar]
- 40.Summers J. Three recently described animal virus models for human hepatitis B virus. Hepatology. 1981;1:179–183. doi: 10.1002/hep.1840010215. [DOI] [PubMed] [Google Scholar]
- 41.Summers J, Smolec J M, Snyder R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc Natl Acad Sci USA. 1978;75:4533–4537. doi: 10.1073/pnas.75.9.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tennant B C, Baldwin B H, Graham L A, Ascenzi M A, Hornbuckle W E, Rowland P H, Tochkov I A, Yeager A E, Erb H N, Colacino J M, Lopez C, Engelhardt J A, R. B R, Richarson F C, Lewis W, Cote P J, Korba B E, Gerin J L. Antiviral activity and toxicity of fialuridine in the woodchuck model of hepattis B virus infection. Hepatology. 1998;28:179–191. doi: 10.1002/hep.510280124. [DOI] [PubMed] [Google Scholar]
- 43.Trigueiro de Araujo M S, Guerret S, Gérard F, Chossegros P, Chevallier M, Grimaud J A. Quantitative studies on liver fibrosis and alpha-smooth muscle actin expression in heroin abusers. Cell Mol Biol. 1997;43:589–596. [PubMed] [Google Scholar]
- 44.Zhou T, Saputelli J, Aldrich C E, Deslauriers M, Condreay L D, Mason W S. Emergence of drug-resistant populations of woodchuck hepatitis virus in woodchucks treated with the antiviral nucleoside lamivudine. Antimicrob Agents Chemother. 1999;43:1947–1954. doi: 10.1128/aac.43.8.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu Y-L, Dutschman G E, Liu S-H, Bridges E G, Cheng Y-C. Anti-hepatitis B virus activity and metabolism of 2′,3′-dideoxy-2′,3′-didehydro-β-l-(−)-5-fluorocytidine. Antimicrob Agents Chemother. 1998;42:1805–1810. doi: 10.1128/aac.42.7.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zoulim F. Therapy of chronic hepatitis B virus infection: inhibition of the viral polymerase and other antiviral strategies. Antivir Res. 1999;44:1–30. doi: 10.1016/s0166-3542(99)00056-x. [DOI] [PubMed] [Google Scholar]
- 47.Zoulim F, Dannaoui E, Borel C, Hantz O, Lin T-S, Liu S-H, Trépo C, Cheng Y-C. 2′,3′-Dideoxy-β-l-5-fluorocytidine inhibits duck hepatitis B virus reverse transcription and suppresses viral DNA synthesis in hepatocytes, both in vitro and in vivo. Antimicrob Agents Chemother. 1996;40:448–453. doi: 10.1128/aac.40.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zoulim F, Saputelli J, Seeger C. Woodchuck hepatitis virus X protein is required for viral replication in vivo. J Virol. 1994;68:2026–2030. doi: 10.1128/jvi.68.3.2026-2030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zoulim F, Trépo C. Drug therapy for chronic hepatitis B: antiviral efficacy and influence of hepatitis B virus polymerase mutations on the outcome of therapy. J Hepatol. 1998;29:151–168. doi: 10.1016/s0168-8278(98)80191-8. [DOI] [PubMed] [Google Scholar]