Case Report
A patient with Prader-Willi syndrome (PWS), extreme obesity and hyperglycemia had her body weight increased considerably for 6 months, even with exercise and diet programs. Treatment with metformin and empagliflozin (12.5 mg/day) induced a weight loss of 14 kg (−10.3%) for 6 months and the reduction of glycated hemoglobin A1c.
Keywords: Prader-Willi syndrome; Obesity, Morbid; Exercise; SGLT-2 inhibitors; Case report
Introduction
Characteristics of Prader-Willi syndrome (PWS) include hyperphagia, deficiency of anabolic hormones, small hands and feet, short stature, diminished lean mass and bone mineral density, hypogonadism and mild intellectual disabilities [1, 2]. Empagliflozin, a sodium-glucose cotransporter 2 (SGLT-2) inhibitor, may benefit glycemic control and weight loss [3]. Moreover, regular exercise has been linked to increased maximum oxygen uptake and muscle strength in PWS patients [4].
Special attention needs to be paid regarding lymphedema, which may be masked by the prevailing increase of body fat linked to obesity in PWS patients. Lymphedema is characterized by excessive accumulation of interstitial protein-rich fluid due to an impaired transport or drainage of lymph [1]. Also, the abnormal swelling of tissues is connected with chronic inflammation, infections, and even angiosarcoma in some cases [5]. In this case study, we report a patient with PWS, systemic lymphedema and extreme obesity whose weight and glycemic control were significantly improved following empagliflozin therapy.
Case report
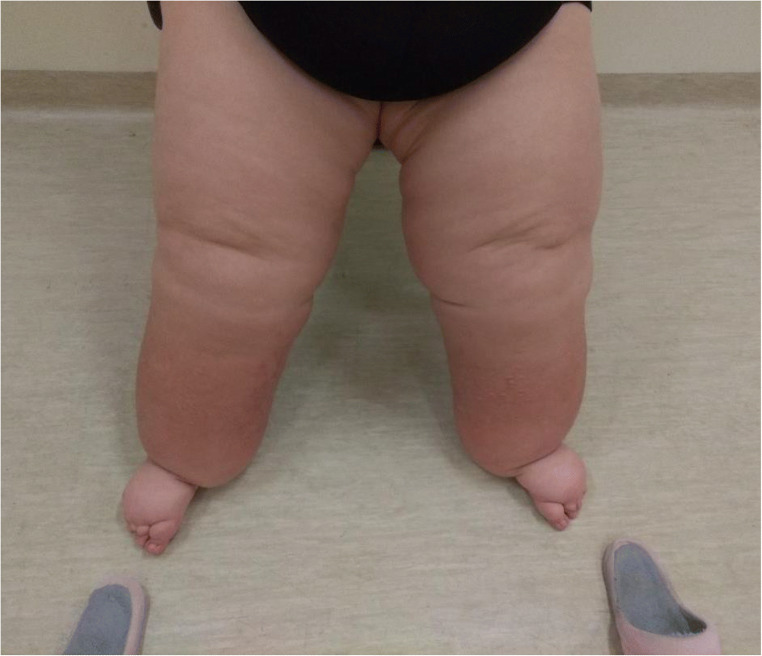
At the age of 23 years and 9 months, a patient was referred by the physician nutrition specialist to a physical exercise–based outpatient service due to progressive weight gain, even in the presence of a controlled diet. Her gradually increasing and persistent feeling of heaviness and swelling in both legs had started approximately 2 years earlier (Fig. 1).
Fig. 1.
Front view of the legs in a patient with Prader-Willi syndrome and lymphedema seated in a chair
The Brazilian patient was delivered by cesarean section associated with acute fetal distress at 32nd week, with a birth weight of 2,140 g. Shortly after birth, hypotonia was observed. She had a global development delay and walked independently at 4 years of age, when PWS was confirmed through the fluorescence in situ hybridization technique. Her further medical history revealed short stature, small hands and feet, mild intellectual disability, severe kyphoscoliosis, skin picking and menarche at age 22. There was no previous history of filariasis, cancer, trauma, leg surgery, underlying systemic diseases or erysipelas. Family history includes cancer and cardiovascular problems, but not skin disease or lymphedema. Manual lymphatic drainage and the use of elastic compression stockings were initiated when patient was 21 years old and did not improve lower limb edema. Last year, due to severe worsening of the edema, the stockings did not fit anymore. Regarding physical activities history, she used to swim during childhood, and during the following years up to 5 years ago she worked out at the gym. In an attempt to reduce edema, the patient reported sleeping with feet up.
The patient presented an extreme obesity status (BMI: 62 kg/m2) with lymphedema (nonpitting) mainly of the lower limbs (extensive soft tissue induration), but also of belly and arms. The patient was taking the following drugs: metformin 1,000 mg/day, levothyroxine sodium 50 mcg/day, cholecalciferol 14,000 IU/week, paroxetine 20 mg/day.
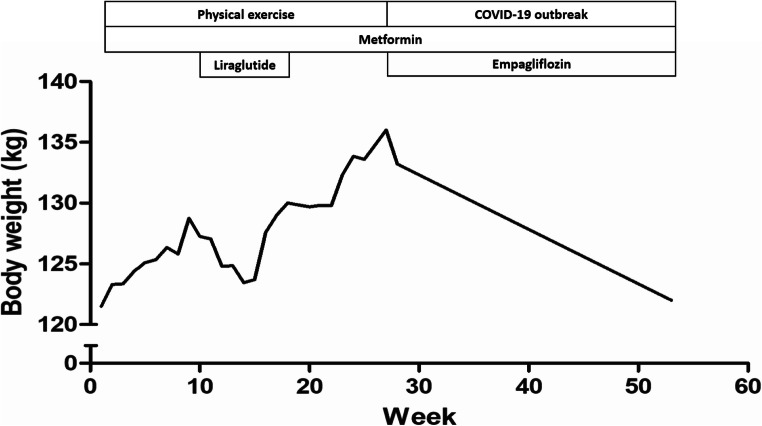
An individual and supervised exercise program was performed, twice a week. The program consisted of free-weight and exercises with elastic bands, up and down stairs, and exercises with balls of different sizes. Each exercise session lasted 45–50 min, and heart rate and oxygen saturation were measured before and after each exercise session. No adverse events were reported; the patient presented high adherence and was excited with the exercises program. Two months after starting exercises, liraglutide (1.2 mg/day), a glucagon-like peptide-1 analog (GLP-1), was prescribed. This drug caused a transient and relatively small weight reduction (Fig. 2), but was discontinued after 2 months due to the relatively high cost and little efficacy on glucose levels and weight loss in this patient.
Fig. 2.
Course of treatment graph showing the corresponding effects on body weight. The duration of interventions/conditions and pharmacological agents are represented by the length of each box relative to the time period on the x-axis
Exercise program was interrupted with 6 months of duration due to elective hospitalization for a hospital-based supervised intervention to reduce weight gain, control glycemic levels and evaluate for obstructive sleep apnea syndrome (OSAS). So, at the age of 24 years and 3 months, she was hospitalized. Upon admission, empagliflozin (12.5 mg/day) was initiated. She received a restricted diet of 1,000 kcal/day + 140 g protein and lost a total of 2.8 kg during 5 days of hospitalization. She was discharged due to the outbreak of coronavirus disease 2019. At hospital discharge, a hypocaloric and hyperproteic diet (1,300 kcal + 113 g protein/day) was prescribed.
When administered only with metformin (1,000 mg/day) for glycemic control, empagliflozin (12.5 mg/day) induced a weight loss of 14 kg (−10.3%) for 6 months (Fig. 2). Moreover, glycated hemoglobin A1c (HbA1c) levels changed from 7.9 to 6.5% some months after utilizing empagliflozin. Polysomnography (PSG) was performed and confirmed severe OSAS. A second PSG for continuous positive airway pressure titration was performed and CPAP therapy was prescribed, although she is not yet using the device.
Discussion
The progression of obesity is associated with impaired cutaneous lymphatic collecting vessel pumping rate, lymphatic leakiness and macromolecule clearance. The prevalence of lymphedema ranges from 49 to 63% in PWS [6, 7]. Recently, liraglutide treatment (0.9 mg/day) did not reduce body weight [3],3 while this GLP-1 analog (0.6→1.2 mg/day) stimulated weight reduction in another PWS patient [8]. In our study, liraglutide (1.2 mg/day) provided a transient weight reduction. However, when added to metformin treatment (up to 1,750 mg/day), empagliflozin (10 mg/day) caused a weight loss of approximately 5.5 kg (−7.4%) for 5 months (average monthly loss of 1.1 kg) in a recent study [3]. In our case report, when administered only with metformin (1,000 mg/day), empagliflozin (12.5 mg/day) induced a weight loss of 14 kg (−10.3%) for 6 months (average monthly loss of 2.3 kg). Noteworthy, the absolute body weight of our patient before empagliflozin therapy was 136 kg, much greater than those observed in similar case reports with PWS patients [3, 9]. In this regard, empagliflozin presents protector role against cardiovascular and renal events [10].
SGLT-2 inhibitors reduce glycemia and body weight by inhibiting glucose absorption and transportation in the kidney [3]. We believe that the diuretic effects of this drug could, at least in part, have helped in weight reduction by lymphedema improvement. Although liraglutide induced a transient weight reduction, we concluded that the later observed weight loss was specifically due to empagliflozin administration. Taken together, these findings indicate empagliflozin drug as a suitable approach is an add-on drug to metformin for treating diabetic patients with PWS, although the results of our single case report should be extrapolated with caution.
Acknowledgments
The authors greatly thank the Clínica Vissom® for the technical support and research incentive.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Ethics Committee (Permit number 40430520.6.0000.5317).
Consent to participate and publication
The volunteer and her guardian provided written informed consent about study participation and publication.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Juliano Boufleur Farinha, Email: juliano.farinha@ebserh.gov.br.
Letícia Schwerz Weinert, Email: leticiasweinert@yahoo.com.br.
Lidiane Pozza Costa, Email: lidiane.pozza@ebserh.gov.br.
Marcelo Zanusso Costa, Email: marcelo.zanusso@ebserh.gov.br.
Patrícia Peres de Peres, Email: patricia.peres@ebserh.gov.br.
Cláudia Fernandes Lorea, Email: claudialorea@hotmail.com.
References
- 1.Heitink MV, Sinnema M, van Steensel MAM, Schrander-Stumpel CTRM, Frank J, Curfs LMG. Lymphedema in Prader-Willi syndrome. Int J Dermatol. 2008;47(1):42–44. doi: 10.1111/j.1365-4632.2008.03959.x. [DOI] [PubMed] [Google Scholar]
- 2.Manzardo AM, Heinemann J, McManus B, Loker C, Loker J, Butler MG. Venous Thromboembolism in Prader-Willi Syndrome: a questionnaire survey. Genes (Basel). 2019;10(7). 10.3390/genes10070550. [DOI] [PMC free article] [PubMed]
- 3.Sano H, Kudo E, Yamazaki T, Ito T, Hatakeyama K, Kawamura N. Efficacy of sodium-glucose cotransporter 2 inhibitor with glucagon-like peptide-1 receptor agonist for the glycemic control of a patient with Prader-Willi syndrome: a case report. Clin Pediatr Endocrinol. 2020;29(2):81–84. doi: 10.1297/cpe.29.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morales JS, Valenzuela PL, Pareja-Galeano H, Rincón-Castanedo C, Rubin DA, Lucia A. Physical exercise and Prader-Willi syndrome: a systematic review. Clin Endocrinol (Oxf). 2019;90(5):649–661. doi: 10.1111/cen.13953. [DOI] [PubMed] [Google Scholar]
- 5.Grada AA, Phillips TJ. Lymphedema: pathophysiology and clinical manifestations. J Am Acad Dermatol. 2017;77(6):1009–1020. doi: 10.1016/j.jaad.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Laurier V, Lapeyrade A, Copet P, Demeer G, Silvie M, Bieth E, Coupaye M, Poitou C, Lorenzini F, Labrousse F, Molinas C, Tauber M, Thuilleaux D, Jauregi J. Medical, psychological and social features in a large cohort of adults with Prader-Willi syndrome: experience from a dedicated centre in France. J Intellect Disabil Res. 2015;59(5):411–421. doi: 10.1111/jir.12140. [DOI] [PubMed] [Google Scholar]
- 7.Partsch CJ, Lämmer C, Gillessen-Kaesbach G, Pankau R. Adult patients with Prader-Willi syndrome: clinical characteristics, life circumstances and growth hormone secretion. Growth Horm IGF Res. 2000;10(B):S81–S85. doi: 10.1016/s1096-6374(00)80015-5. [DOI] [PubMed] [Google Scholar]
- 8.Kim YM, Lee YJ, Kim SY, Cheon CK, Lim HH. Successful rapid weight reduction and the use of liraglutide for morbid obesity in adolescent Prader-Willi syndrome. Ann Pediatr Endocrinol Metab. 2020;25(1):52–56. doi: 10.6065/apem.2020.25.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horikawa Y, Enya M, Komagata M, Hashimoto KI, Kagami M, Fukami M. Effectiveness of sodium-glucose cotransporter-2 inhibitor as an add-on drug to GLP-1 receptor agonists for glycemic control of a patient with Prader-Willi syndrome: a case report. Diabetes Ther. 2018;9(1):421–426. doi: 10.1007/s13300-018-0369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neeland IJ, Eliasson B, Kasai T, Marx M, Zinman B, Inzucchi SE, et al. The impact of empagliflozin on obstructive sleep apnea, cardiovascular, and renal outcomes: an exploratory analysis of the EMPA-REG OUTCOME trial. Diabetes Care. 2020;43:3007–3015. doi: 10.2337/dc20-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.