Abstract
Clinical and animal studies indicate that with optimal dosing, penicillin may still be effective against penicillin-nonsusceptible pneumococci (PNSP). The present study examined whether the same strains of penicillin-susceptible pneumococci (PSP) and PNSP differed in their pharmacodynamic responses to penicillin by using comparable penicillin dosing regimens in four animal models: peritonitis, pneumonia, and thigh infection in mice and tissue cage infection in rabbits. Two multidrug-resistant isolates of Streptococcus pneumoniae type 6B were used, one for which the penicillin MIC was 0.016 μg/ml and the other for which the penicillin MIC was 1.0 μg/ml. Two additional strains of PNSP were studied in the rabbit. The animals were treated with five different penicillin regimens resulting in different maximum concentrations of drugs in serum (Cmaxs) and times that the concentrations were greater than the MIC (T>MICs). The endpoints were bacterial viability counts after 6 h of treatment in the mice and 24 h of treatment in the rabbits. Similar pharmacodynamic effects were observed in all models. In the mouse models bactericidal activity depended on the T>MIC and to a lesser extent on the Cmax/MIC and the generation time but not on the area under the concentration-time curve (AUC)/MIC. Maximal bactericidal activities were similar for both PSP and PNSP, being the highest in the peritoneum and blood (∼6 log10 CFU/ml), followed by the thigh (∼3 log10 CFU/thigh), and being the lowest in the lung (∼1 log10 CFU/lung). In the rabbit model the maximal effect was ∼6 log10 CFU/ml after 24 h. In the mouse models bactericidal activity became marked when T>MIC was ≥65% of the experimental time and Cmax was ≥15 times the MIC, and in the rabbit model bactericidal activity became marked when T>MIC was ≥35%, Cmax was ≥5 times the MIC, and the AUC at 24 h/MIC exceeded 25. By optimization of the Cmax/MIC ratio and T>MIC, the MIC of penicillin for pneumococci can be used to guide therapy and maximize therapeutic efficacy in nonmeningeal infections caused by PNSP.
Streptococcus pneumoniae remains one of the leading causes of community-acquired bacterial infections, and severe S. pneumoniae infections such as pneumonia and meningitis have significant morbidity and mortality rates (22). Strains of penicillin-nonsusceptible pneumococci were first reported in 1967 (16) and have spread throughout the world and become prevalent in many countries. The high prevalence of penicillin-nonsusceptible pneumococci has created problems in antimicrobial chemotherapy and has resulted in renewed interest in the management of pneumococcal infections (1, 25, 30).
Clinical and animal model studies indicate that by optimizing the dosing of penicillin, it may still be effective against penicillin-nonsusceptible pneumococci (25; V. Magnusson, H. Erlendsdottir, K. G. Kristinsson, and S. Gudmundsson, Program Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A89, p. 17, 1995). Studies in vitro (20; Magnusson et al., 35th ICAAC) and with experimental animals have also demonstrated that the most important pharmacokinetic parameter for establishment of the efficacies of β-lactam antibiotics against both penicillin-susceptible and penicillin-nonsusceptible pneumococci is the time that the antibiotic concentration remains above the MIC (T>MIC) (8, 11, 14, 20, 26, 32; A. Thoroddsen, T. Asgeirsson, H. Erlendsdóttir, and S. Gudmundsson, Program Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-5b, p. 27, 1997). Most studies have examined the correlation of pharmacokinetic and pharmacodynamic parameters with efficacy by using experimental models and several bacterial strains with different antimicrobial susceptibilities (20, 32; Thoroddsen et al., 37th ICAAC). This raises the question of the comparability of infections at different sites. Use of the same bacterial strains in several animal models should eliminate the effect of different strain characteristics and thus provide a better understanding of the effects of pharmacokinetic and pharmacodynamic parameters at the different sites of infection (Thoroddsen et al., 37th ICAAC).
The purpose of the study described here was to examine whether penicillin-susceptible and penicillin-nonsusceptible pneumococci exhibited different pharmacodynamic responses to penicillin at different sites of infection by using the same pneumococcal strains and comparable penicillin dosing regimens in four animal models: peritonitis, pneumonia, and thigh infection in mice and tissue cage infection in rabbits.
(This work was presented in part at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 27 September 1998 [H. Erlendsdóttir, J. D. Knudsen, I. Odenholt, N. Frimodt-Møller, K. Fuursted, F. Espersen, O. Cars, K. G. Kristinsson, and S. Gudmundsson, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-2, p. 1, 1998].)
MATERIALS AND METHODS
Bacteria.
For the mouse models, two clinical isolates belonging to a Spanish-Icelandic clone were used (28). One was an isolate (isolate A-2000) from the middle ear of a child with otitis media, and the other was an isolate (isolate R-2151) from the sputum of a patient with pneumonia. Both strains were multidrug-resistant isolates of serotype 6B with similar susceptibility profiles except for susceptibility to penicillin. They were resistant to tetracycline, trimethoprim-sulfamethoxazole, and erythromycin. Strain A-2000 was susceptible to penicillin (MIC, 0.016 μg/ml), and strain R-2151 was not susceptible to penicillin (MIC, 1.0 μg/ml). For the rabbit model, two clinical penicillin-nonsusceptible isolates were used as well: strain 07126 of serotype 6B (MIC, 0.25 μg/ml) and strain 40932 of serotype 9V (MIC, 2 μg/ml).
MICs.
The MICs were determined by the E test (AB Biodisk, Solna, Sweden), according to the manufacturer's instructions.
Animals.
Female NMRI mice (age, approximately 6 to 8 weeks; weight, 30 ± 2 g) were used for the mouse pneumonia and the mouse thigh infection models. For the mouse peritonitis model, ssc:CF1 female mice (age, approximately 8 weeks; weight, 30 ± 2 g) were used. Female New Zealand White rabbits (age, 3 to 4 months; weight, 2.5 to 3.2 kg) were used in the tissue cage model.
Antibiotic and determination of antibiotic concentrations.
Benzylpenicillin was obtained from Astra AB, Södertälje, Sweden, as a dry powder with known potency. Dilutions were made in distilled water.
Penicillin concentrations were determined by the disk plate bioassay method (19). For the mouse models the bioassay organism was Micrococcus luteus ATCC 9341 and the growth medium was Mueller-Hinton agar (Difco Laboratories), and for the rabbit model the bioassay organism was Bacillus stearothermophilus ATCC 3032 and the growth medium was tryptose-glucose agar (23). Penicillin was dissolved in pooled normal mouse serum or in 50% rabbit serum for preparation of the standard curves, and the concentrations were derived from the standard curves. The samples were diluted in either pooled normal mouse serum or 50% rabbit serum, so their concentrations would be within the range of those on the standard curve, and the samples were assayed in duplicate. Results were expressed as micrograms per milliliter of fluid. The correlation coefficients of the standard curves were at least 0.99, and the coefficients of variation were below 5%. The lower limit of detection was 0.1 μg/ml for the experiments with mice and 0.01 μg/ml for the experiments with rabbits. At each dose given, the T>MICs for the different strains were calculated from the serum elimination regression line. The maximum concentration in serum (Cmax) in mice was measured 10 min after injection in all mice, and the Cmax in rabbit serum was measured within 2 h after injection. The serum elimination half-life was estimated by the expression −log 2/β, where β is the slope of the serum elimination regression line (log serum concentration versus time).
Protein binding.
The level of protein binding of penicillin G in human serum, pooled mouse serum, and rabbit serum was determined by the standard ultrafiltration method (7). The concentrations used for human and mouse serum were 100, 50, 25, 12.5, and 6.25 μg/ml, and those used for rabbit serum were 150, 100, and 50 μg/ml. The results obtained with the different concentrations were averaged.
Animal models. (i) Mouse pneumonia and thigh infection model.
Bacterial suspensions were prepared from fresh overnight cultures (made from frozen stock cultures) on 5% blood agar plates. The bacteria were grown in heart infusion broth with 10% horse serum for 6 h at 35°C to an inoculum of ∼108 CFU/ml. The culture was centrifuged (1,600 × g for 20 min) and resuspended in an equal volume of 0.9% saline. The size of the inoculum was determined by viability counting on blood agar plates.
The mice were anesthetized by with pentobarbital (50 mg/kg) by intraperitoneal injection. Pulmonary inoculation was performed by nasal installation of 75 μl (∼7.5 × 106 CFU) of the bacterial suspension, which was dripped onto the nares of each mouse (Magnusson et al., 35th ICAAC). The mice readily aspirated the solution and were suspended on a string by the upper incisors for 10 to 15 min. Ten hours later the same animals were infected in the thigh by injecting 0.1 ml of ∼106 CFU of the same organism in the logarithmic phase per ml of heart infusion broth with 10% horse serum (15). Antibiotic therapy was initiated 12 h after lung inoculation and 2 h after thigh infection and was continued for 6 h thereafter. The penicillin was administered subcutaneously in the neck region in a volume of 0.2 ml per dose (see Tables 2 and 3). Three mice were in each treatment group, and the experiments were done twice. Inoculated untreated control mice were included in all trials.
TABLE 2.
Treatment regimens and dosages with time schedules for the penicillin-susceptible (A-2000) and penicillin-nonsusceptible (R-2151) pneumococci
| Time (h:min) | Dose (mg/kg) administered to the following mice in the indicated regimen no.a:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A-2000
|
R-2151
|
|||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| 0 | 0.15 | 0.075 | 1.5 | 0.75 | 0.3 | 10 | 5 | 100 | 50 | 20 |
| 1 | 0 | 0 | 0 | 0 | 0.3 | 0 | 0 | 0 | 0 | 20 |
| 1:30 | 0 | 0 | 0 | 0.3 | 0 | 0 | 0 | 0 | 20 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0.3 | 0 | 0 | 0 | 0 | 20 |
| 2:30 | 0 | 0.0375 | 0 | 0 | 0 | 0 | 2.5 | 0 | 0 | 0 |
| 3 | 0 | 0 | 1.5 | 0.3 | 0.3 | 0 | 0 | 100 | 20 | 20 |
| 4 | 0 | 0.0375 | 0 | 0 | 0.3 | 0 | 2.5 | 0 | 0 | 20 |
| 4:30 | 0 | 0 | 0 | 0.3 | 0 | 0 | 0 | 0 | 20 | 0 |
| 5 | 0 | 0 | 0 | 0 | 0.3 | 0 | 0 | 0 | 0 | 20 |
The penicillin MIC for strain A-2000 was 0.016 μg/ml; that for strain R-2151 was 1 μg/ml. The end point of treatment was 6 h.
TABLE 3.
Cmax/MIC, T>MIC, and T>MIC f.d. in mouse serum for both pneumococcal strains achieved with all five regimensa used in the mouse models
| Regimen | Cmax/MIC | T>MIC (min [% of treatment period]) | T>MIC f.d. (min) |
|---|---|---|---|
| 1 | 7.4 | 45 (12.5) | 45 |
| 2 | 3.7 | 57 (16) | 29 |
| 3 | 105 | 234 (65) | 117 |
| 4 | 47 | 255 (71) | 81 |
| 5 | 15 | 360 (100) | 60 |
See Table 2.
At the end of the experiment, the animals were killed by cervical dislocation, and the lungs and thighs were removed and homogenized (Omni tissue homogenizer; Omni, Gainesville, Va.) in cold saline with β-lactamase (Penase; 100,000 U/ml) to neutralize residual antibiotics. The total volume of lung homogenate was made to 3 ml, and that of the thigh homogenate was made to 10 ml. The bacterial densities in the lungs and thighs were determined by plating serial 10-fold dilutions on blood agar plates (Difco) containing 5 μg of gentamicin per ml (17) to prevent growth of contaminating bacteria (the MICs of gentamicin for the test strains were 12 to 24 μg/ml). Colony counts were performed after 20 h of incubation at 35°C in 5% CO2-supplemented air. The lowest detection levels for the viability counts in the lungs and thighs were 30 and 100 CFU per lung and thigh, respectively.
(ii) Mouse peritonitis model.
Bacterial suspensions were prepared from fresh overnight cultures (made from frozen stock cultures) on 5% blood agar plates as described above. The inoculum for the mouse peritonitis model was prepared immediately before use by suspending colonies in sterile beef broth medium and was adjusted to an optical density at 540 nm of 0.5, giving a density of approximately 108 CFU/ml. The size of the inoculum was determined by viability counting on 5% blood agar.
Mucin (M-2378; Sigma Chemical Company, St. Louis, Mo.), an enzyme extract of porcine stomach, was used as an adjuvant for inoculation of the peritoneum (12, 20). Immediately before inoculation, a mucin stock solution (10% [wt/vol]) was diluted 1:1 with the pneumococcal suspension in beef broth (final mucin concentration, 5% [wt/vol]), resulting in an inoculum of 5 × 106 to 1 × 107 CFU/ml. The mice were injected intraperitoneally with 0.5 ml of the pneumococcal suspension, resulting in bacteremia within 1 h of inoculation (12). Antibiotic therapy was initiated 1 h after inoculation. Penicillin was administered subcutaneously in the neck region in a volume of 0.1 ml per dose (see Tables 2 and 3). Three mice were in each treatment group. Inoculated untreated control mice were included in all trials.
The effects of the various treatment regimens were determined after 6 h of treatment by evaluation of bacterial counts in the blood and peritoneal fluid. Blood samples were obtained by periorbital cuts after anesthetization of the mice with CO2. After the mice were killed, peritoneal washes were performed by injecting 2 ml of sterile saline intraperitoneally, followed by massage of the abdomen and then opening of the peritoneum to collect the fluid. Blood and peritoneal fluids were immediately diluted 10-fold in saline, from which 20 μl was plated onto 5% blood agar plates in spots, with subsequent counting of colonies after incubation overnight at 35°C in ambient air. The lowest detection levels for bacterial counts in blood and peritoneal fluid were 50 and 250 CFU/ml, respectively.
(iii) Rabbit tissue cage model.
The animals were anesthetized by intramuscular injection of 0.5 ml of fentanyl-fluanisone (Hyponorm), followed by disinfection of the back of the rabbits with 70% alcohol and the administration of local anesthesia (lidocaine at 40 mg/ml). A 5-cm incision was then made in the midline, and four well-separated pouches were bluntly dissected in the subcutaneous layer. An autoclaved cylindrical steel-net cage with a volume of 4 ml was implanted in each pouch (23). The incision was closed with sutures. To reverse the anesthesia, the rabbits were given 0.3 to 0.4 ml of naloxone hydrochloride (0.4 mg/ml; Narcante) through an intravenous needle in the ear vein. Three to 4 weeks after the implantation, the tissue cages had sealed with a thin layer of connective tissue and filled with clear, yellowish tissue cage fluid (TCF). Earlier studies have shown that the TCF albumin/serum albumin ratio corresponds very well to the skin albumin/plasma albumin ratio of 0.45 found in humans (3, 23).
The bacterial suspensions used in the rabbit tissue cage model were grown in Todd-Hewitt broth for 6 h at 37°C in 5% CO2-supplemented air, resulting in an inoculum of approximate 5 × 108 CFU/ml. The four test strains, for which the MICs were different, then in the logarithmic phase of growth, were diluted 10−2 in phosphate-buffered saline (PBS), and thereafter, 0.4 ml of the dilutions was injected into the four different cages, one strain in each cage, giving a starting inoculum in the cages of approximately 5 × 105 CFU/ml.
Since the Cmax of penicillin in the tissue cages is reached approximately 1 h after injection, antibiotic therapy was initiated 1 h before inoculation (23). The penicillin was administered by intravenous injection in the ear vein of one dose of 4, 7.5, 15, 75, or 150 mg of penicillin per kg of body weight. Samples from the cages were obtained by percutaneous aspiration every 2nd h up to 12 h and, in addition, after 24 h. Two rabbits were used for each dosing regimen. To avoid antibiotic carryover, the samples were treated with penicillinase (Penase; 100,000 U/ml) and, if necessary, were diluted in PBS before seeding onto blood agar plates. Only plates with 20 to 200 colonies were counted. The lower limit of detection was 20 CFU/ml.
Pharmacokinetic studies with mice.
Pharmacokinetic studies were done with NMRI mice. Concentrations in sera were determined after the administration of a single dose of 1, 10, 50, or 100 mg of penicillin per kg. The drug was administered by subcutaneous injection in the neck region in a volume of 0.2 ml per dose. At 10, 20, 40, 60, 90, 120, and 180 min following penicillin administration, blood samples were obtained from the mice in groups of three by periorbital cuts during ether anesthesia. Three to four groups of mice with three mice per group were used, and two to three samples were obtained from each mouse. After collection, the blood was centrifuged and the serum was stored at −80°C until it was analyzed.
Pharmacokinetic studies with rabbits.
Penicillin concentrations in TCF were determined after the administration of single doses of 4, 7.5, 15, 75, or 150 mg of penicillin per kg. The drug was administered by intravenous injection in the ear vein. Samples from the cages were obtained by percutanous aspiration every 2nd h up to 14 h. TCF pooled from all four cages was used. Two rabbits were used for each dosing regimen.
Treatment regimens.
The designs of the treatment regimens for the mouse models were based upon the results from the pharmacokinetic studies (see Results) (Table 1). Dosages not included in Table 1 were derived by interpolation. The mice were treated and monitored for 6 h. The treatment regimens and dosages for the animals infected with penicillin-susceptible and penicillin-nonsusceptible organisms are shown in Table 2. The regimens were selected to provide a range of Cmaxs and T>MICs. The range of Cmaxs in serum was ∼4 to 100 times the MIC, and the range of T>MICs was 45 to 360 min; i.e., the T>MIC lasted for 12.5 to 100% of the 6-h experiment (see Table 3).
TABLE 1.
Half-lives, Cmaxs, and T>MICs for the two strains used in the mouse models achieved after administration of a single subcutaneous dose of 1, 10, 50, or 100 mg of penicillin per kg
| Dose (mg/kg) | Half-life (min) | Cmax (μg/ml) |
T>MIC (min)
|
|
|---|---|---|---|---|
| MIC, 0.016 μg/ml | MIC, 1.0 μg/ml | |||
| 100 | 16.1 | 105 | 220 | 117 |
| 50 | 13.6 | 47 | 167 | 81 |
| 10 | 12.3 | 7.4 | 123 | 45 |
| 1 | 12.1 | 0.6 | 102 | 0 |
The rabbits were treated with one dose of either 4, 7.5, 15, 75, or 150 mg/kg to provide different T>MICs (0 to 100% of the time during the 24 h after the administration of each dose) for the four different strains used (see the Results and Table 4).
TABLE 4.
Half-lives and T>MIC in the TCF for the four strains used in the rabbit model achieved after the administration of a single intravenous dose of 4, 7.5, 15, 75, or 150 mg of penicillin per kg
| Dose (mg/kg) | Half-life (h) |
T>MIC (h [% of 24 h])
|
|||
|---|---|---|---|---|---|
| MIC, 0.016 μg/ml | MIC, 0.25 μg/ml | MIC, 1.0 μg/ml | MIC, 2.0 μg/ml | ||
| 150 | 3.9 | 44 (100) | 28 (100) | 20 (83) | 16 (67) |
| 75 | 2.9 | 32 (100) | 20 (83) | 14 (58) | 12 (50) |
| 15 | 4.8 | 32 (100) | 13 (54) | 3.2 (13) | 0 |
| 7.5 | 3.5 | 22 (92) | 8.3 (35) | 1.4 (5.8) | 0 |
| 4 | 4.4 | 21 (88) | 3.3 (14) | 0 | 0 |
Data analysis and presentation.
The pharmacokinetic parameters Cmax, half-lives, area under the concentration-time curve (AUC)/MIC, T>MIC, and the time that the antibiotic concentration after administration of the first dose (f.d.) of antibiotic remains above the MIC (T>MIC f.d.) were determined from the concentration curves. Total drug concentrations were used in all calculations.
The bactericidal efficacies of the treatment regimens in the mouse models were calculated by subtracting the results for each treated mouse from the mean results for control mice at the end of therapy (6 h). In the rabbit model the bactericidal efficacy was determined as the difference between the starting inoculum and the colony count at the end of the experiment at 24 h for all strains. Correlation between pharamacokinetic variables and bactericidal activity was examined by linear regression (Pearson's).
The generation time was defined as the time for the viability counts for the untreated organisms in the different mouse models to double.
A P value of <0.05 was considered significant. All statistical comparisons were two-tailed.
RESULTS
Pharmacokinetic studies with mice.
Table 1 shows the different half-lives and Cmaxs achieved after the administration of various doses of penicillin and the times that the serum drug levels exceed the MICs for the two strains used in the mouse models. Table 3 demonstrates the Cmax/MICs and T>MICs for both pneumococcal strains achieved with all five regimens. By univariate analysis, the Cmaxs and T>MICs correlated by a coefficient (r2) of 0.14% (P was not significant).
Pharmacokinetic studies with rabbits.
Tables 4 and 5 similarly show the different half-lives, T>MICs, and Cmaxs achieved after the administration of five different single-dose regimens. For all the regimens the Cmax was reached within 2 h.
TABLE 5.
Cmax and Cmax/MIC in TCF for the four pneumococcal strains achieved with all five regimens in the rabbit model
| Dose (mg/kg) | Cmax (μg/ml) |
Cmax/MIC
|
|||
|---|---|---|---|---|---|
| MIC, 0.016 μg/ml | MIC, 0.25 μg/ml | MIC, 1.0 μg/ml | MIC, 2.0 μg/ml | ||
| 150 | 32.5 | 2,031 | 130 | 33 | 16 |
| 75 | 32.5 | 2,031 | 130 | 33 | 16 |
| 15 | 1.58 | 99 | 6.3 | 1.6 | 0.8 |
| 7.5 | 1.32 | 83 | 5.3 | 1.3 | 0.7 |
| 4 | 0.42 | 26 | 1.7 | 0.4 | 0.2 |
Protein binding.
The mean protein binding of penicillin G in human serum was 29.5% (range, 25 to 46%), that in mouse serum was 8.5% (range, 0 to 17%), and that in rabbit serum was 24% (range, 13 to 36%).
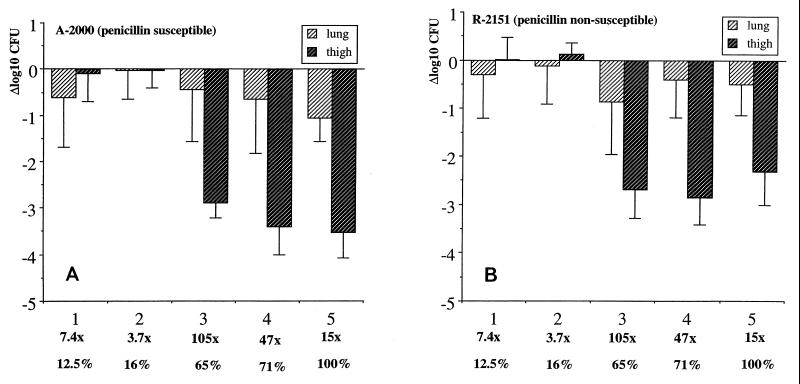
Bactericidal activity in mouse lungs and thighs.
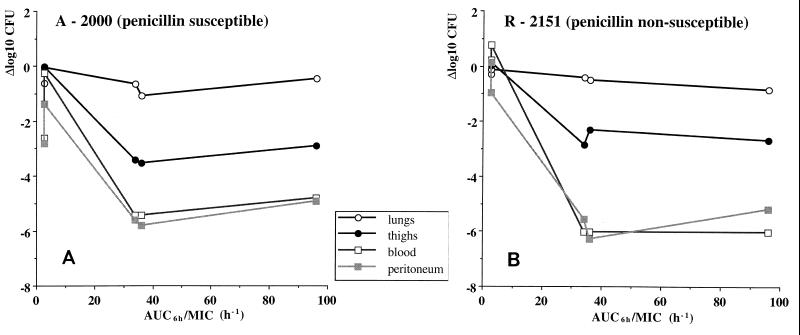
The bactericidal activities of the different treatment regimens in the lungs and thighs are shown in Fig. 1A and 1B. The pharmacodynamic parameters were similar for both the susceptible (Fig. 1A) and the nonsusceptible (Fig. 1B) strains, with maximal bactericidal activities in the thighs of 3.5 and 2.9 log10 CFU, respectively, and maximal bactericidal activities in the lungs of 1.1 and 0.9 log10 CFU, respectively. In the thigh model, bactericidal activity became pronounced only when T>MIC was ≥65% of the experimental time or Cmax was at least 15 times the MIC, or both. Similarly, even though the bactericidal activity was very limited in the lungs, it was observed only when T>MIC was at least 65% of the experimental time. The correlation of Cmax, T>MIC, T>MIC f.d., and AUC/MIC with bactericial activity by univariate analysis is shown in Table 6, and the association with AUC/MIC is demonstrated further in Fig. 2.
FIG. 1.
Bactericidal activities of five treatment regimens in mouse lungs and thighs. (A) Susceptible strain A-2000; (B) nonsusceptible strain R-2151. Results are given for each regimen as log10 CFU reduction between control mice and treated mice at 6 h. The bars show the means and standard deviations for six mice. Below the x axis the multiples of the MIC (Cmax/MIC) and the T>MIC as a percentage of the 6-h experiment for each regimen are provided.
TABLE 6.
Correlation (by univariate analysis) of bactericidal activity with Cmax, T>MIC, T>MIC f.d., and AUC/MIC in the mouse infection models for two type 6B pneumococcal isolates, A-2000 and R-2151a
| Mouse model | Parameter | r2 for A-2000 | P for A-2000 | r2 for R-2151 | P for R-2151 |
|---|---|---|---|---|---|
| Thigh | Cmax | 0.295 | 0.345 | 0.487 | 0.190 |
| T>MIC | 0.925 | 0.009 | 0.781 | 0.047 | |
| T>MIC f.d. | 0.494 | 0.186 | 0.684 | 0.084 | |
| AUC/MIC | 0.661 | 0.225 | 0.763 | 0.134 | |
| Lung | Cmax | 0.002 | 0.947 | 0.793 | 0.043 |
| T>MIC | 0.543 | 0.155 | 0.368 | 0.278 | |
| T>MIC f.d. | 0.041 | 0.743 | 0.878 | 0.019 | |
| AUC/MIC | 0.141 | 0.821 | 0.967 | 0.007 | |
| Peritoneum | Cmax | 0.242 | 0.400 | 0.286 | 0.353 |
| T>MIC | 0.861 | 0.023 | 0.939 | 0.006 | |
| T>MIC f.d. | 0.686 | 0.201 | 0.453 | 0.213 | |
| AUC/MIC | 0.604 | 0.280 | 0.673 | 0.213 | |
| Blood | Cmax | 0.278 | 0.362 | 0.421 | 0.237 |
| T>MIC | 0.766 | 0.052 | 0.870 | 0.021 | |
| T>MIC f.d. | 0.521 | 0.169 | 0.629 | 0.109 | |
| AUC/MIC | 0.618 | 0.267 | 0.752 | 0.143 |
The penicillin MIC for strain A-2000 was 0.016 μg/ml; that for strain R-2151 was 1.0 μg/ml. Statistically significant values are boldfaced.
FIG. 2.
Association of bactericidal activity of five different regimens with AUC/MIC at four different infection sites in mice. (A) Results for susceptible strain A-2000. (B) Results for nonsusceptible strain R-2151.
Bactericidal activity in mouse blood and peritoneum.
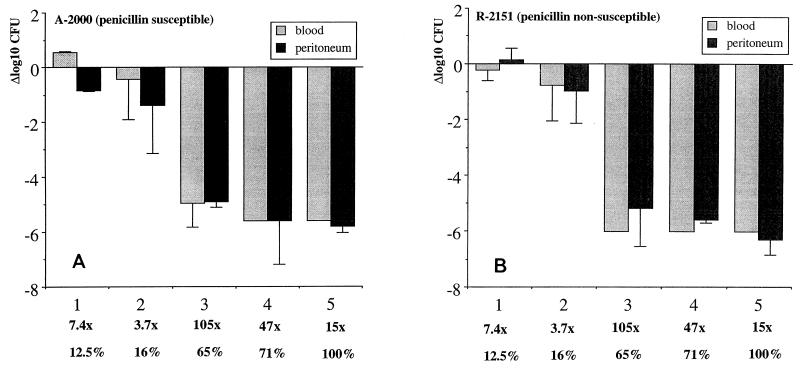
The bactericidal activity of penicillin in blood and peritoneum is shown in Fig. 3A and B. Pronounced bactericidal efficacy was seen against both blood and peritoneal infections. The pharmacodynamic parameters were similar for both the susceptible (Fig. 3A) and the nonsusceptible (Fig. 3B) strains, with maximal activities in blood of 5.4 and 6.0 log10 CFU/ml, respectively, and maximal activities in the peritoneum of 5.8 and 6.3 log10 CFU/ml, respectively. In these infections, bactericidal activity became significant only when T>MIC was ≥65% of the experimental time or Cmax was at least 15 times the MIC, or both. The correlation of Cmax, T>MIC, T>MIC f.d., and AUC/MIC with bactericial activity by univariate analysis is shown in Table 6, and the association with AUC/MIC is demonstrated further in Fig. 2.
FIG. 3.
Bactericidal activities of five different treatment regimens in mouse peritoneum and blood. (A) Results for susceptible strain A-2000. (B) Results for nonsusceptible strain R-2151. Results are given for each regimen as log10 CFU reduction between control mice and treated mice at 6 h. The bars show the means and standard deviations for three mice. Below the x axis the multiples of the MIC (Cmax/MIC) and the T>MIC as a percentage of the 6-h experiment for each regimen are provided.
Bactericidal activity in rabbit TCF.
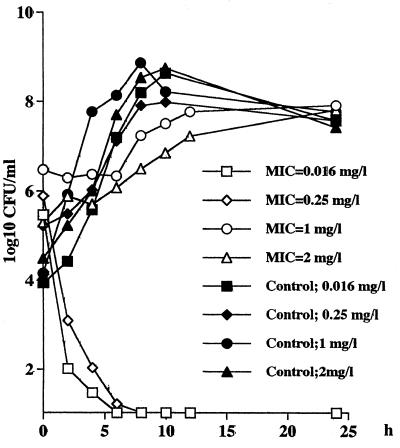
As shown in Table 4, the concentration of penicillin in the cages after the administration of a dose of 15 mg/kg never exceeds the MICs for strain 40932 (MIC, 2.0 μg/ml). As an example, Fig. 4 shows the different bactericidal activity curves for the four test strains following the administration of a penicillin dose of 15 mg/kg. For the strain for which the MIC was 1.0 μg/ml, the T>MIC after this dose was 3.2 h (13% of the 24-h experiment), but the Cmax/MIC was only 1.6 and there was no bactericidal activity against the two nonsusceptible strains. For the intermediate (MIC, 0.25 μg/ml), the 15-mg/kg dose provided a T>MIC of 13 h (54%) and the Cmax/MIC was 6.3. For the sensitive strain, the T>MIC was 32 h (100%) and the Cmax/MIC was 99. Significant bactericidal activity was achieved only against these two strains (MICs, 0.016 and 0.25 μg/ml, respectively).
FIG. 4.
Killing and growth curves for the four pneumococcal strains (MICs, 0.016, 0.25, 1, and 2 μg/ml, respectively) for 24 h in the rabbit tissue cages following the intravenous administration of 15 mg of penicillin per kg. The curves show the mean number of CFU per milliliter for two rabbits at each time point.
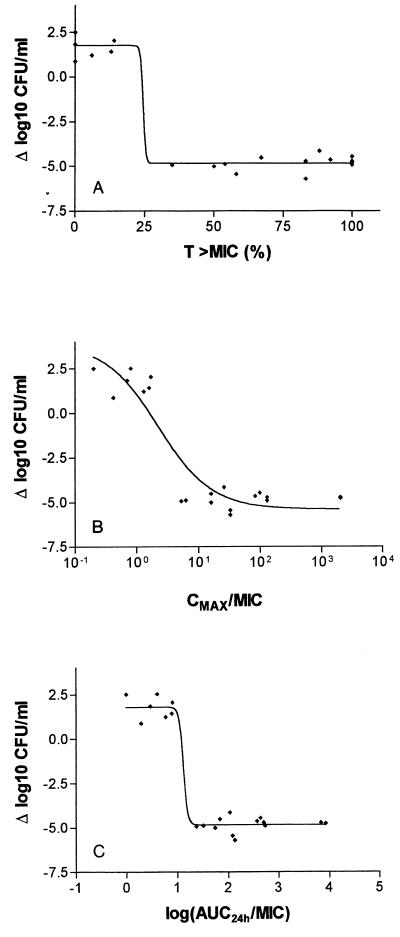
The bactericidal activities of all five regimens against the four test strains are shown in Fig. 5 as a correlate of the respective T>MICs, Cmax/MICs, and AUC/MICs 24 h after dosing. Maximal bactericidal activity was obtained when the T>MIC was at least 35% of the experimental time, Cmax exceeded 5 times the MIC, and AUC/MIC exceeded 25.
FIG. 5.
Bactericidal activities of all five regimens (4, 7.5, 15, 75, and 100 mg/kg) against the four test strains in the rabbit tissue cages as a function of the respective T>MICs (A) Cmax/MIC (B), and AUC/MIC (C) 24 h after dosing.
Generation times.
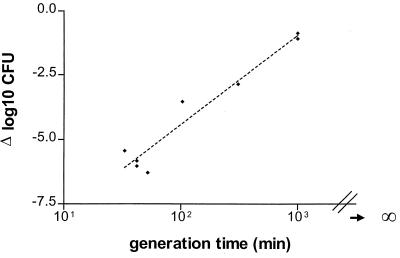
Maximum bactericidal activity was associated with the generation times of the untreated control organisms in the different mouse models, as demonstrated in Fig. 6 (r2 = 0.94; P < 0.01). The generation time in the lungs was long, approaching infinity, and strain R-2151 (MIC, 1.0 μg/ml) had a 1.3- to 3-fold longer generation time than strain A-2000 (MIC, 0.016 μg/ml).
FIG. 6.
Correlation between generation time of the untreated control organisms in the different mouse models and maximum bactericidal activity. The generation time in the lungs approached infinity, but the duration was arbitrarily chosen as 1,000 min.
DISCUSSION
The major aim of the present study was to evaluate the bactericidal activity of penicillin against penicillin-susceptible and penicillin-nonsusceptible pneumococcal strains in four different animal models. Previous studies with experimental animals (2, 13, 29; Magnusson et al., 35th ICAAC) and limited studies with humans (25) have shown that penicillin-nonsusceptible pneumococci can be treated with penicillin. The experimental studies were performed with single animal models. The current study is the first that has examined these issues concurrently in different animal models at different sites of infection but with the same organisms and the same antimicrobial agent at comparable dosages. Furthermore, the end points in this study were not survival but bactericidal activity as determined by the viable counts in all models. Since the half-life in the rabbit model was 2.9 to 4.4 h but was only 12.1 to 16.1 min in mice, the end point of therapy in the tissue cages was chosen to be 24 h after dosing of the drug, but in the mouse models the end point was chosen to be 6 h after the initiation of therapy.
The main findings of this study are as follows. (i) Similar pharmacodynamic patterns were observed in all models, although bactericidal activity was lowest in the lung. (ii) The bactericidal effect of penicillin in all models against both the penicillin-susceptible and penicillin-nonsusceptible pneumococci was dependent on both T>MIC and, to a lesser extent, Cmax, but it was not dependent on AUC/MIC. (iii) The bactericidal activity in the mouse models correlated with the in vivo generation time.
The virulence of pneumococci in mice varies with capsular type. Penicillin-nonsusceptible pneumococci are dominated by capsular types with low levels of virulence for mice, i.e., types 6B, 14, 19, and 23 (4). In the present study pneumococcal strains of serotype 6B were used in all models, and in addition, one strain of serotype 9V was used in the tissue cage model. The serotype 6B strains were multidrug resistant and exhibited similar levels of resistance to most antibiotics tested except penicillin, which had MICs from 0.016 to 1.0 μg/ml. The studies were done with immunocompetent mice. Previous data (21; H. Erlendsdóttir, S. Ómarsdóttir, V. Magnússon, and S. Gudmundsson, Program Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-5c, p. 27; 1997) have indicated that pharmacokinetic parameters in infected and uninfected mice are comparable.
It has previously been shown with experimental animal models that the most important pharmacokinetic parameter for prediction of the effects of β-lactam antibiotics in vivo against penicillin-susceptible pneumococci is T>MIC (8, 11, 20, 32). An earlier study examined the efficacy of penicillin in 100 different penicillin dosing regimens against a penicillin-sensitive pneumococcus (MIC, 0.008 μg/ml) in the mouse thigh model (32). Therapeutic efficacy was achieved when levels in serum were constantly maintained above the MIC. In the present study, the pharmacokinetic datum points did not allow the complete separation of the interdependence between T>MIC and Cmax, but it was not the primary goal. Nevertheless, the correlation between the two parameters was poor by univariate analysis (r2 = 14%). Furthermore, the correlations of the bactericidal activity with T>MIC were higher (r2 = 54 to 94%) in all mouse models for both isolates than the correlations with Cmax (r2= 0.2 to 49%) and AUC/MIC (r2 = 14 to 75%) except for those for the nonsusceptible isolate in the lung (for T>MIC, r2 = 37%; for Cmax, r2 = 79%; for AUC/MIC, r2 = 97%). Thus, the results in the present study achieved with the same isolates at several different infection sites are consistent with those of previous single-infection-site studies (8, 11, 20, 31). In the present study the T>MIC after administration of the first dose of antibiotic did not correlate with the bactericidal activity at the end of treatment except for that against the nonsusceptible isolate in the lung (P = 0.019). It should be noted, however, that a certain maximum-effect threshold for Cmax, T>MIC, and T>MIC f.d. can be deducted from the results from studies with mice (Fig. 1 and 3).
Only a few studies have investigated whether the same pharmacodynamic parameters apply to penicillin-susceptible and penicillin-nonsusceptible pneumococci, but in the present studies there have been indications that penicillin at the appropriate dosages can be used to treat infections caused by penicillin-nonsusceptible pneumococci. Azoulay-Dupuis et al. (2) showed in a leukopenic mouse pneumonia model that the dose of amoxicillin (50 mg/kg) required to protect mice and to eradicate penicillin-nonsusceptible strains was 10 times higher than the dose which was effective against penicillin-susceptible strains. Tateda et al. (29) showed that the ratio of the penicillin MICs for the two pneumococcal strains tested (1.0/0.015 μg/ml) was the same as the ratio of the two penicillin dosages (40/0.6 mg/kg) required to clear the pneumonia from immunocompetent mice. The doses were given six times at 1-h intervals. These results are consistent with those of Knudsen et al. (20), who showed a highly significant correlation between the log MIC and the log 50% effective dose in a mouse peritonitis model. Moreover, earlier Magnusson et al. (35th ICAAC) showed that high penicillin dosages of up to 200 mg/kg/24 h, with doses given every 1 or every 3 h, were efficacious in the treatment of pneumonia due to penicillin-nonsusceptible pneumococci in mice. That study also indicated that a certain threshold penicillin Cmax of approximately 6 μg/ml was necessary for in vivo efficacy. However, beyond this, an increased dosage did not improve the bactericidal activity further. This was seen with both the penicillin-susceptible and the penicillin-nonsusceptible strains tested and also in the in vitro experiments with the same strains.
Few studies with humans have obtained results consistent with these findings. Pallares et al. (25) reported that penicillin resistance is not associated with an increased rate of mortality in patients with bacteremic pneumococcal infections that were treated with penicillin. Friedland (10) showed that penicillin nonsusceptibility was not associated with increased mortality in a series of 208 children with pneumococcal bacteremia without infection in the central nervous system. Einarsson et al. (9) found in a recent case control study that patients with pneumonia due to penicillin-nonsusceptible strains presented with a milder illness than patients infected with susceptible strains but had prolonged hospital stays and required more expensive antibiotics. These results might be explained by the fact that penicillin resistance is more common among serotypes with lower levels of virulence (18).
In the present study the bactericidal activity in lungs was limited, the reason for which is not entirely clear, but the long generation time in the lungs is probably the most important factor. Several investigators have previously reported a significant association between the generation time in untreated organisms and the bactericidal activities of β-lactams, for example, for Escherichia coli (31) and group A beta-hemolytic streptococci (24). In a previous study (Thoroddsen et al., 37th ICAAC) of pneumococcal serotype 6B infection in the lungs and thighs in healthy and neutropenic mice, the bactericidal activity of penicillin was monitored at regular intervals for 24 h after the administration of one 100-mg/kg dose of penicillin. The maximal bactericidal effect was reached 4 to 12 hours later in the lungs than in the thighs. The antibiotic concentration at the infection site does not explain this difference, because values for the most important parameter for establishment of the effect of β-lactams, T>MIC, were similar at both sites (Erlendsdóttir et al., 37th ICAAC). Thus, the bactericidal activity in the lungs in the present study may have been more pronounced if it had been monitored for a longer period, but the studies were done with immunocompetent mice, which limited the experimental time.
The antibiotic concentration at the infection site is also important. In rabbits it has been shown that the concentrations of β-lactams in muscle interstitial fluid closely approximate those observed in serum, with only a short lag time (5, 27). Similarly, comparison of the pharmacokinetics of penicillin in mouse serum and lungs (Erlendsdóttir et al., 37th ICAAC) has shown good penetration of penicillin into murine lung tissue. However, Cmax was much higher in serum than in lungs, with a ratio of 2/1 to 3/1. In contrast, the half-lives were considerably longer in lungs than in serum (20%), resulting in comparable T>MICs. However, in the tissue cage, due to a large volume-to-surface area ratio, an even antibiotic concentration at the infection site is achieved during the induction phase (23). The β-lactams accumulated slowly in TCF, and the Cmax in TCF was lower than that in serum, but the half-life was much longer. For instance, a single intravenous injection of ampicillin (20 mg/kg) to rabbits resulted in a half-life of 28 min in serum and one of approximately 2.5 h in TCF (6). In the present study the half-life of penicillin in TCF ranged from 2.9 to 4.9 h. Therefore, levels in serum were used as surrogate markers for the concentration at the infection sites in the mouse models, while the concentrations in TCF were used as surrogate markers in the rabbit model. The question arises as to whether infection alters the pharmacokinetic parameters. It has been shown that the pharmacokinetic parameters for penicillin 18 to 22 h after infection were the same in both serum and lungs in infected and uninfected mice (Erlendsdóttir et al., 37th ICAAC). By the same token, peritonitis does not alter the parameters at the start of the infection (21).
In summary, the present study showed that the pharmacokinetic parameters associated with the efficacy of penicillin against pneumococci with different susceptibilities were remarkably similar in the four animal models and nearly identical at the different sites of infection in the mice. Thus, in conclusion, the MIC of penicillin for pneumococci can be used to adjust the dosage required to achieve an optimum outcome with penicillin treatment regimens when one is treating nonmeningeal infections.
ACKNOWLEDGMENT
This work was supported by a Scandinavian Society of Chemotherapy research grant for research in antimicrobial chemotherapy.
REFERENCES
- 1.Appelbaum P C. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Azoulay-Dupuis E, Moine P, Bedos J P, Rieux V, Vallee E. Amoxicillin dose-effect relationship with Streptococcus pneumoniae in a mouse pneumonia model and roles of in vitro penicillin susceptibilities, autolysis, and tolerance properties of the strains. Antimicrob Agents Chemother. 1996;40:941–946. doi: 10.1128/aac.40.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergholm A M, Henning C, Holm S E. Static and dynamic properties of tissue cage fluids in rabbits. Eur J Clin Microbiol. 1984;3:126–130. doi: 10.1007/BF02014329. [DOI] [PubMed] [Google Scholar]
- 4.Briles D E, Crain M J, Gray B M, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:111–116. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cars O. Tissue distribution of ampicillin: assay in muscle tissue and subcutaneous tissue cage fluid from normal and nephrectomized rabbits. Scand J Infect Dis. 1981;13:283–289. doi: 10.3109/inf.1981.13.issue-4.09. [DOI] [PubMed] [Google Scholar]
- 6.Cars O, Henning C, Holm S E. Penetration of ampicillin and dicloxacillin into tissue cage fluid in rabbits: relation to serum and tissue protein binding. Scand J Infect Dis. 1981;13:69–74. doi: 10.1080/00365548.1981.11690370. [DOI] [PubMed] [Google Scholar]
- 7.Craig W A, Suh B. Protein binding and antimicrobial effects: methods for determination of protein binding. In: Lorian V, editor. Antibiotics in labaratory medicine. 3rd ed. Baltimore, Md: The Williams & Wilkins Co.; 1991. pp. 367–393. [Google Scholar]
- 8.Drusano G L, Goldstein F W. Relevance of the Alexander Project: pharmacodynamic considerations. J Antimicrob Chemother. 1996;38(Suppl. A):141–154. doi: 10.1093/jac/38.suppl_a.141. [DOI] [PubMed] [Google Scholar]
- 9.Einarsson S, Kristjansson M, Kristinsson K G, Kjartansson G, Jonsson S. Pneumonia caused by penicillin non-susceptible and penicillin susceptible pneumococci in adults: a case-control study. Scand J Infect Dis. 1998;30:253–256. doi: 10.1080/00365549850160882. [DOI] [PubMed] [Google Scholar]
- 10.Friedland I R. Comparison of the response to antimicrobial therapy of penicillin-resistant and penicillin-susceptible pneumococcal disease. Pediatr Infect Dis J. 1995;14:885–890. doi: 10.1097/00006454-199510000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Frimodt-Møller N, Bentzon M W, Thomsen V F. Experimental pneumococcus infection in mice: comparative in vitro activity and pharmacokinetic parameters with in vivo effect for 14 cephalosporins. J Infect Dis. 1986;154:511–517. doi: 10.1093/infdis/154.3.511. [DOI] [PubMed] [Google Scholar]
- 12.Frimodt-Møller N, Knudsen J D, Espersen F. The mouse peritonitis/sepsis model. In: Zak O, et al., editors. Handbook of animal models of infection. New York, N.Y: Academic Press, Inc.; 1999. pp. 127–136. [Google Scholar]
- 13.Gavalda J, Capdevila J A, Almirante B, Otero J, Ruiz I, Laguarda M, Allende H, Crespo E, Pigrau C, Pahissa A. Treatment of experimental pneumonia due to penicillin-resistant Streptococcus pneumoniae in immunocompetent rats. Antimicrob Agents Chemother. 1997;41:795–801. doi: 10.1128/aac.41.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerber A U. Impact of the antibiotic dosages schedule on efficacy in experimental soft tissue infections. Scand J Infect Dis Suppl. 1990;74:147–154. [PubMed] [Google Scholar]
- 15.Gudmundsson S, Erlendsdottir H. Murine thigh infection model. In: Zak O, et al., editors. Handbook of animal models of infection. New York, N.Y: Academic Press, Inc.; 1999. pp. 137–144. [Google Scholar]
- 16.Hansman D, Bullen M M. A resistant pneumococcus. Lancet. 1967;ii:264–265. doi: 10.1016/s0140-6736(75)91547-0. [DOI] [PubMed] [Google Scholar]
- 17.Hendley J O. Spread of Streptococcus pneumoniae in families. I. Carriage rates and distribution of types. J Infect Dis. 1975;132:55–61. doi: 10.1093/infdis/132.1.55. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan S L, Mason E O. Management of infections due to antibiotic-resistant Streptococcus pneumoniae. Clin Microbiol Rev. 1998;11:628–644. doi: 10.1128/cmr.11.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klassen M, Edberg S C. Measurement of antibiotics in human body fluids: techniques and significance. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: The Williams & Wilkins Co.; 1996. pp. 233–243. [Google Scholar]
- 20.Knudsen J D, Frimodt-Møller N, Espersen F. Experimental Streptococcus pneumoniae infection in mice for studying correlation of in vitro and in vivo activities of penicillin against pneumococci with various susceptibilities to penicillin. Antimicrob Agents Chemother. 1995;39:1253–1258. doi: 10.1128/aac.39.6.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knudsen J D, Frimodt-Møller N, Espersen F. Pharmacodynamics of penicillin are unaffected by bacterial growth phases of Streptococcus pneumoniae in the mouse peritonitis model. J Antimicrob Chemother. 1998;41:451–459. doi: 10.1093/jac/41.4.451. [DOI] [PubMed] [Google Scholar]
- 22.Mufson M A. Streptococcus pneumoniae. In: Mandell G L, Douglas R G, Bennet J E, editors. Principles and practice of infectious diseases. 3rd ed. New York, N.Y: Churchill Livingstone Inc.; 1990. pp. 1539–1551. [Google Scholar]
- 23.Odenholt I, Holm S E, Cars O. An in vivo model for evaluation of the postantibiotic effect. Scand J Infect Dis. 1988;20:97–103. doi: 10.3109/00365548809117224. [DOI] [PubMed] [Google Scholar]
- 24.Odenholt I. Pharmacodynamics of β-lactam antibiotics, p. 29. Ph.D. thesis. Uppsala, Sweden: Akademitryk; 1989. [Google Scholar]
- 25.Pallares R, Linares J, Vadillo M, Cabellos C, Manresa F, Viladrich P F, Martin R, Gudiol F. Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N Engl J Med. 1995;333:474–480. doi: 10.1056/NEJM199508243330802. [DOI] [PubMed] [Google Scholar]
- 26.Roosendaal R, Bakker-Woudenberg I A J M. Impact of the antibiotic dosage schedule on efficacy in experimental lung infections. Scand J Infect Dis Suppl. 1990;74:147–154. [PubMed] [Google Scholar]
- 27.Ryan M, Cars O. Antibiotic assays in muscle: are conventional tissue levels misleading as indicator of the antibacterial activity? Scand J Infect Dis. 1980;12:307–309. doi: 10.3109/inf.1980.12.issue-4.12. [DOI] [PubMed] [Google Scholar]
- 28.Soares S, Kristinsson K G, Muser J M, Tomasz A. Evidence for introduction of a multiresistant clone of serotype 6B Streptococcus pneumoniae from Spain to Iceland in the late 1980's. J Infect Dis. 1992;168:158–163. doi: 10.1093/infdis/168.1.158. [DOI] [PubMed] [Google Scholar]
- 29.Tateda K, Takashima K, Miyazaki H, Matsumoto T, Hatori T, Yamaguchi K. Noncompromised penicillin-resistant pneumococcal pneumonia CBA/J mouse model and comparative efficacies of antibiotics in this model. Antimicrob Agents Chemother. 1996;40:1520–1525. doi: 10.1128/aac.40.6.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomasz A. Pneumococcus at the gates. N Engl J Med. 1995;333:514. doi: 10.1056/NEJM199508243330810. [DOI] [PubMed] [Google Scholar]
- 31.Tuomanen E, Cozens R, Tosch W, Zak O, Tomasz A. The rate of killing of Escherichia coli by beta-lactam antibiotics is strictly proportional to the rate of bacterial growth. J Gen Microbiol. 1986;132:1297–1304. doi: 10.1099/00221287-132-5-1297. [DOI] [PubMed] [Google Scholar]
- 32.Vogelman B, Gudmundsson S, Leggett J, Turnidge J, Ebert S, Craig W A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988;158:831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]