Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disease, manifesting as the progressive development of fluid-filled renal cysts. In approximately half of all patients with ADPKD, end-stage renal disease results in decreased renal function. In this study, we used CRISPR-Cas9 and somatic cell cloning to produce pigs with the unique mutation c.152_153insG (PKD1insG/+). Pathological analysis of founder cloned animals and progeny revealed that PKD1insG/+ pigs developed many pathological conditions similar to those of patients with heterozygous mutations in PKD1. Pathological similarities included the formation of macroscopic renal cysts at the neonatal stage, number and cystogenic dynamics of the renal cysts formed, interstitial fibrosis of the renal tissue, and presence of a premature asymptomatic stage. Our findings demonstrate that PKD1insG/+ pigs recapitulate the characteristic symptoms of ADPKD.
Subject terms: Experimental models of disease, End-stage renal disease
Autosomal dominant polycystic kidney disease (ADPKD) is a genetic disorder characterized by the formation of cysts within the kidneys. The authors generated PKD1 heterozygous knockout (PKD1insG/+) pigs by CRISPR-Cas9 and somatic cell cloning techniques. The founder cloned animals and progeny showed the renal cyst formation from the neonatal stage, interstitial fibrosis of the renal tissue, and the presence of a premature asymptomatic stage. Their findings demonstrate that PKD1insG/+ pigs recapitulate the characteristic symptoms of ADPKD.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) affects one in every 500–1000 people worldwide and is the most common inherited kidney disease1–3. The characteristic pathology of ADPKD is progressive development of fluid-filled cysts that generally arise bilaterally in the kidney tissue. Decreased renal function accompanying renal cyst formation occurs in approximately half of patients succumbing to end-stage renal disease at approximately age 60 years1,4,5. Extrarenal manifestations in patients with ADPKD include hypertension, hepatic and pancreatic cysts, valvular heart disease, and intracranial aneurysm6–8. Although the relatively new drug tolvaptan is available and suppresses the progression of renal cyst formation, a curative therapy for this disease has not been developed9.
The polycystic kidney disease (PKD) genes PKD1 and PKD2 code for polycystin 1 (PC1) and polycystin 2 (PC2), respectively, and are known as the causal genes of ADPKD. Mutations in PKD1 and PKD2 are responsible for approximately 85% and 15%, respectively, of all ADPKD cases10. Although the clinical pathologies accompanying these mutations are similar, PKD1-associated ADPKD exhibits an earlier onset and a more severe disease course than PKD2-associated ADPKD4.
The pathologies and etiologies of ADPKD have been investigated using PKD animal models, such as rats harboring spontaneous mutations in the PKD genes Pkhd1 and Pkdr1 and genetically modified mice with Pkd1 knockout (KO)11,12. Qian et al. proposed a “two-hit” model in which a second somatic mutation, including loss of heterozygosity, is required in addition to intrinsic PKD1 mutation to trigger renal cyst formation13. The gene dosage (haploinsufficiency) model hypothesizes that a reduction in PKD1 expression levels below a critical threshold leads to renal cyst formation14,15. Although these rodent models have provided insights into ADPKD, the specific features of the models limit the ability to extrapolate the research outcomes to human patients. For example, mice carrying heterozygous Pkd1 KO rarely exhibit renal cyst formation, and the manifestation of symptoms is limited in aged mice14,16. Thus, rodent models may not faithfully reproduce the features of human ADPKD symptoms, mainly because of species-specific differences in lifespan, metabolism, and anatomical and physiological characteristics12. Therefore, an animal model displaying ADPKD symptoms that closely resemble those of human patients is needed.
As pigs are considered to exhibit physiological and anatomical characteristics similar to those of humans, they have often been used to develop models for intractable hereditary human diseases17,18. Furthermore, recent progress in genome editing technology has enabled the generation of model pigs for monogenic diseases19,20. Genetically modified cloned pigs with heterozygous mutations in PKD1, including c.642_643insTGCT (PKD1TGCT ins/+) and c642_643insT (PKD1T ins/+), were produced using zinc finger nucleases targeting exon 5 of PKD121. Unlike in the mouse model, this PKD1 KO pig model displayed renal cyst formation at a young age. However, the pig model failed to fully resemble ADPKD in terms of cyst formation onset. Unlike human patients, who begin developing cysts during the fetal period, monoallelic PKD1 KO pigs do not display renal cysts neonatally.
In the current study, we generated heterozygous PKD1 KO cloned pigs harboring the unique mutation c.152_153insG (PKD1insG) in the first exon of PKD1. The resulting PKD1insG/+ cloned pigs displayed characteristics of an ADPKD model, including (i) neonatal renal cyst formation, (ii) progressive cyst development during animal growth, and (iii) sustained fertility after sexual maturation. Symptoms that appeared in the founder generation of the cloned animals were faithfully reproduced in descendants inheriting the mutant gene.
This paper provides a detailed approach for creating heterozygous PKD1 KO (PKD1insG/+) mutant pigs using CRISPR-Cas9 gene-editing technology and somatic cell cloning. Phenotypic features of the mutant pigs, including the founder cloned animals and their progeny, are also discussed.
Materials and methods
Animal care and chemicals
All animal experiments, including genetic modifications performed in this study, were approved by the Institutional Animal Care and Use Committee of Meiji University (IACUC11-0003 and IACUC16-0008). All experiments were performed in accordance with the relevant guidelines and regulations. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise indicated.
Design and preparation of PKD1 targeting guide RNA
Guide RNA (gRNA) was designed using an online CRISPR design tool (http://crispr.mit.edu/) to target the coding region of the first exon of porcine PKD1, which is on chromosome 3. The specificity of the designed gRNA was confirmed by searching for similar porcine sequences in GenBank. The gRNA target sequence (without the protospacer adjacent motif sequence) was as follows: 5′-GCCTGCCGCGTCAACTGCTC−3′. A synthetic DNA template consisting of the gRNA bound to a T7 promoter for in vitro transcription was purchased from Thermo Fisher Scientific (Waltham, MA, USA). The in vitro transcribed gRNA was prepared using the MEGAshortscript T7 transcription Kit (Thermo Fisher Scientific), purified with the MEGAClear kit (Thermo Fisher Scientific), and stored at −80 °C until use.
Isolation of heterozygous PKD1 mutant cells and culture conditions
A primary culture of porcine fetal fibroblast cells (male line) was used as the progenitor line to create PKD1 heterozygous KO cells. The progenitor cells and their derivatives were seeded onto type I collagen-coated dishes or plates (Asahi Glass, Tokyo, Japan) and cultured in α-Minimum Essential Medium (Thermo Fisher Scientific) supplemented with 15% fetal bovine serum (Nichirei Bioscience, Tokyo, Japan), 100 U/mL penicillin, 100 µg/mL streptomycin, and 0.25 µg/mL amphotericin B (antibiotic-antimycotic solution; Thermo Fisher Scientific) in a humidified atmosphere containing 5% CO2 at 37 °C.
Isolation of heterozygous PKD1 mutant cells
The fetal fibroblasts were cultured to 70–90% confluence, washed twice with Dulbecco’s phosphate-buffered saline without calcium and magnesium (Thermo Fisher Scientific), and treated with 0.05% trypsin-EDTA (Thermo Fisher Scientific) to detach and collect the cells. Subsequently, 4 × 105 cells were suspended in 40 μL of R buffer (supplied with the Neon Transfection System; Thermo Fisher Scientific). After 4 μL of purified gRNA (200 ng/μL) and 2 μL of Cas9 mRNA (1 µg/µL; Thermo Fisher Scientific) were added, the cells were electroporated under the following conditions: pulse voltage, 1600 V; pulse width, 20 ms; pulse number, 1 (program #4). Following electroporation, the cells were cultured at 37 °C for 24 h in antibiotic-free medium and then for another 48 h in medium with antibiotics. After the second incubation (i.e., 72 h after electroporation), a limiting dilution was performed in five 96-well plates to obtain single cell-derived clones. Fourteen days after the limiting dilution step, cells from wells showing relatively high confluency (>50%) were selected and divided for further culture and mutation analysis, whereas cells from wells with low confluency (~50%) were not used in further experiments.
Analysis of CRISPR-Cas9-induced mutations in nuclear donor cells
The target region of CRISPR-Cas9 was amplified by direct PCR from the cell clones using MightyAmp DNA polymerase (TaKaRa Bio, Shiga, Japan) and the corresponding primers (5′-TGTCGAGCCTGCAGCTGGATGC-3′ and 5′- CAGACAGGCCGCAGCTGCTGC-3′). Nested PCR was performed using PrimeSTAR HS DNA polymerase (TaKaRa Bio) and appropriate primers (5′-CAGACAGGCCGCAGCTGCTGC-3′ and 5′-AGTCCCACCGAGTGAGAAGTC-3′). The PCR fragment including the target region was examined using the sequencing primer 5′-CTTGCGCTGTCCTGACGATG-3′ and BigDye Terminator Cycle Sequencing Kit (Thermo Fisher Scientific) on an ABI PRISM 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
Off-target mutation analysis in nuclear donor cells
All potential off-target sites in the pig genome were predicted by an online CRISPR design tool (http://crispr.mit.edu/). The top 10 potential off-target sites were selected, and regions overlapping the sites were amplified by PCR using appropriate sets of primers (Supplementary Table S1) and cloned into TOPO vector according to the manufacturer’s instructions (Thermo Fisher Scientific). All positive colonies for each potential off-target site were analyzed by DNA sequencing.
Somatic cell nuclear transfer and embryo transfer
Somatic cell nuclear transfer (SCNT) was performed as described previously with slight modifications19,22. Briefly, in vitro-matured oocytes containing the first polar body were enucleated via gentle aspiration of the polar body and adjacent cytoplasm using a beveled pipette in 10 mM HEPES-buffered Tyrode lactose medium containing 0.3% (w/v) polyvinylpyrrolidone, 0.1 μg/mL demecolcine, 5 μg/mL cytochalasin B, and 10% fetal bovine serum. Fibroblasts (cell clone #132) were used as nuclear donors following cell cycle synchronization via serum starvation for 2 days. A single donor cell was inserted into the perivitelline space of each enucleated oocyte. The donor cell-oocyte complexes were placed in a solution of 280 mM mannitol (Nacalai Tesque, Kyoto, Japan) containing 0.15 mM MgSO4, 0.01% (w/v) polyvinyl alcohol, and 0.5 mM HEPES (pH 7.2) and held between two electrode needles. Membrane fusion was induced with a somatic hybridizer (LF201; NEPA GENE, Chiba, Japan) by applying a single direct-current pulse (200 V/mm, 20 μs) and pre- and post-pulse alternating current field of 5 V at 1 MHz for 5 s. The reconstructed embryos were cultured in NCSU23 medium supplemented with 4 mg/mL bovine serum albumin for 1–1.5 h, followed by electrical activation performed as follows: the reconstructed embryos were washed twice in activation solution containing 280 mM mannitol, 0.05 mM CaCl2, 0.1 mM MgSO4, and 0.01% (w/v) polyvinyl alcohol; aligned between two wire electrodes (1.0 mm apart) of a fusion chamber slide filled with activation solution; and subjected to a single direct-current pulse of 150 V/mm for 100 μs using an electrical pulsing machine (Multiporator; Eppendorf, Hamburg, Germany). After activation, the reconstructed embryos were transferred into PZM-5 (porcine zygote medium 5) supplemented with 5 μg/mL cytochalasin B and 500 nM scriptaid for 3 h. The embryos were then transferred into PZM-5 supplemented with scriptaid only and cultured for another 12–14 h. These embryos were transferred to PZM-5 and maintained under a humidified atmosphere of 5% CO2, 5% O2, and 90% N2 at 38.5 °C. Beyond the morula stage, the embryos were cultured in PZM-5 supplemented with 10% fetal bovine serum. Crossbred (Large White/Landrace × Duroc) prepubertal gilts weighing 100–105 kg were used as recipients of the SCNT embryos. The gilts were given a single intramuscular injection of 1000 IU of equine chorionic gonadotropin to induce estrus. Ovulation was induced by intramuscular injection of 1500 IU of human chorionic gonadotropin human chorionic gonadotropin (Kawasaki Pharmaceutical, Kanagawa, Japan) administered at 66 h after equine chorionic gonadotropin injection. SCNT embryos that had been cultured for 5–6 days were surgically transferred into the oviducts of the recipients at approximately 146 h after human chorionic gonadotropin injection.
Genotyping of PKD1 heterozygous KO pigs by PCR-RFLP
The obtained piglets were genotyped using the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method. First, genomic DNA was extracted from the tail biopsies of pigs using a DNeasy Blood and Tissue Kit (QIAGEN, Hilden, Germany) and subjected to nested PCR as described in the subsection “Analysis of CRISPR-Cas9-induced mutations in nuclear donor cells.” PCR products were digested with the restriction enzyme BsrI (New England Biolabs, Ipswich, MA, USA) at 65 °C for 1 h and then subjected to electrophoresis. The band profile was used to determine the zygosity of the sampled animal, as heterozygous PKD1 mutant pigs were expected to display three bands: two from digestion of the mutant allele and one from digestion of the wild-type (WT) allele.
Reverse transcription quantitative PCR
Total RNA was isolated from kidney specimens using RNeasy Plus Mini Kit with RNase-Free DNase Set (QIAGEN). cDNA was synthesized using SuperScript III First-Strand Synthesis Super Mix (Thermo Fisher Scientific). Quantitative PCR was performed using the StepOne Plus Real-Time PCR System (Thermo Fisher Scientific) and Premix Ex Taq (Probe qPCR) (Takara Bio) with the following primers and probes: Porcine PKD1 TaqMan probe, 5′-CCGCGTCACCAGGAGCCTGGATGT-3′; PKD1 forward primer (in exon 6), 5′-ACAGTCCCGCCGTCCAG-3′; PKD1 reverse primer (in exon 7), 5′- CACAGCCGAGAAGCCGATC-3′, porcine ACTB (β-actin) TaqMan probe, 5′-CGGCTTTGCGGGCGACGATGCT-3′; ACTB forward primer, 5′-TGGATGACGATATTGCTGCGC-3′; ACTB reverse primer, 5′-GACACCAGGGCGTGATGG-3′. Both TaqMan probes were obtained from TaKaRa Bio. The ∆∆CT method was used to determine the relative expression normalized to ACTB expression. This experiment was performed three times, and the average results are reported.
Diagnosis of renal cyst formation
The presence of renal cysts was confirmed by ultrasound examination using an HI VISION Avius (HITACHI, Tokyo, Japan) ultrasound system with an EUP-C175 Convex probe or FC1 (FUJIFILM SonoSite, Bothell, WA, USA) ultrasound device with a convex transducer C60xf (2–5 MHz). Pigs were intramuscularly injected with 1% mafoprazine mesylate (0.5 mg/kg, DS Pharma Animal Health Co., Ltd., Osaka, Japan), followed by intravenous injection of sodium thiopental (Nipro ES Pharma Co., Ltd., Osaka, Japan), and anesthesia was maintained via inhalation of isoflurane (DS Pharma Animal Health Co., Ltd.) while the pigs were examined. After anesthetization, cyst formation in the kidneys of heterozygous PKD1 mutant pigs was examined ultrasonographically. Moreover, their longitudinal and transverse diameters were measured on the longitudinal plane. All ultrasonographic findings were evaluated by two nephrologists.
Histological analysis
After PKD1 heterozygous mutant pigs and age-matched WT pigs were sacrificed under anesthetization, the kidney tissues were dissected, fixed in 4% paraformaldehyde in phosphate-buffered saline without calcium and magnesium (Wako Pure Chemical Industries, Osaka, Japan), embedded in paraffin, sectioned, and subjected to Masson’s trichrome staining. For immunohistochemical analysis, the fixed sections were treated with a mouse anti-PC1 monoclonal (clone: 7e12) antibody (1:200 dilution) overnight at 4 °C. After removing excess antibody, the sections were incubated with Histofine Simple Stain MAX PO (MULTI) (Nichirei Bioscience) and DAB chromogen for 30 min at 25 °C. The slides were counterstained with hematoxylin and visualized under a BIOREVO BZ9000 microscope (Keyence, Osaka, Japan).
Analysis of biochemical blood parameters
Under anesthetization, blood samples were collected in tubes containing heparin to determine the concentrations of blood creatinine (CRE), urea nitrogen (BUN), aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase using a dry-chemistry analyzer (FUJI DRI-CHEM 7000, FUJIFILM Corporation, Tokyo, Japan).
Statistical analysis
Statistical analyses of PKD1 expression in the kidney were performed using SPSS Statistics 20.0 software (SPSS, Inc., Chicago, IL, USA). Student’s t test was used to compare differences between the WT and each PKD1insG/+ pig. A p value < 0.05 was considered to indicate statistically significant results.
Results
Design of gRNA and nuclear donor cell isolation
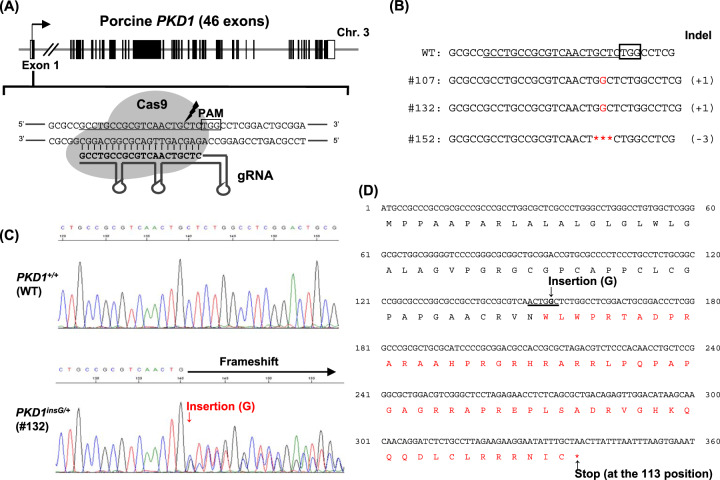
In pigs, as in humans and mice, PKD1 contains 46 exons23. We designed a gRNA targeting exon 1 of PKD1 (Fig. 1A). Heterozygous PKD1 mutant cells were generated by introducing gRNA and Cas9 mRNA into male porcine fetal fibroblasts. Of the 163 cell clones obtained by limiting dilution, mutations were detected in three clones (1.8%, 3/163) (Fig. 1B). Cell clone #132, which showed no morphological aberrations and exhibited high proliferation, was chosen as the nuclear donor cell for SCNT. Cell clone #132 had a guanine nucleotide inserted between bases 152 and 153 of exon 1, generating mutant c.152_153insG (PKD1insG/+) with the resulting frameshift creating a premature stop codon (Fig. 1C–D). The mutated allele was expected to code for a truncated PC1 protein containing 112 amino acids (p.Cys51Trpfs*63). In addition to causing a frameshift, the addition of guanine nucleotide created a BsrI restriction endonuclease site, enabling RFLP genotyping for this mutation (Fig. 1D). DNA sequencing of cell clone #132 revealed no mutations in the top 10 potential off-target sites (Supplementary Table S1).
Fig. 1. Design of CRISPR-Cas9 targeting of porcine PKD1 and isolation of nuclear donor cells.
A Schematic representation of CRISPR-Cas9 targeting site for porcine PKD1 gene. The gRNA targeting sequences are underlined. The black box represents a protospacer adjacent motif (PAM) sequence. B CRISPR-Cas9-induced mutations in the isolated cell clones. The deletion mutation is indicated by asterisks. C Direct DNA sequence analysis of a PKD1insG/+ nuclear donor cell (clone #132). D Deduced amino acid sequence resulting from the induced mutation (PKD1insG) in cell clone #132. The amino acid sequences in red indicate nonsense amino acids. A 1-bp insertion of guanine nucleotide in porcine PKD1 created a BsrI restriction enzyme site (underlined) and stop codon at amino acid residue 113.
Generation of heterozygous PKD1 KO cloned pigs
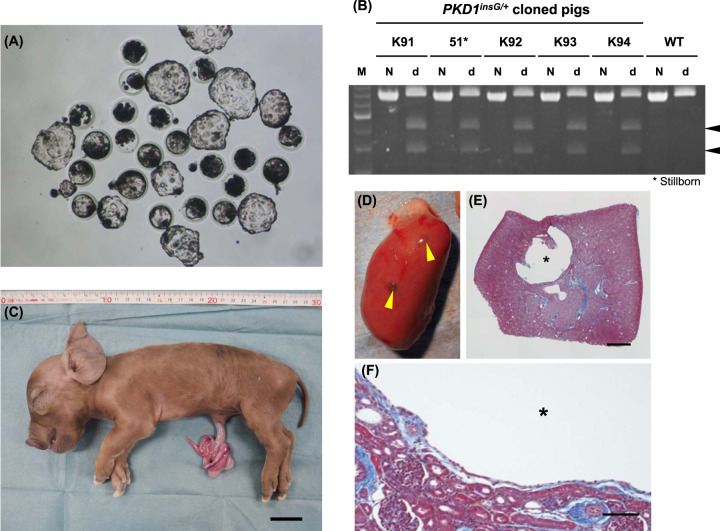
Using cell clone #132 as the nuclear donor, 330 cloned embryos carrying heterozygous PKD1 KO (PKD1insG/+) were generated via SCNT. The in vitro development rate of these embryos was 68.8% (227/330) (Table 1), which is consistent with the results of our previous reports19,24. After transferring the 227 SCNT embryos into two recipient gilts, five heterozygous PKD1 mutant cloned piglets were obtained, including one stillbirth (Table 1 and Fig. 2A–C). The cause of death of the stillborn offspring is unknown but may have occurred because of an accident during labor (Fig. 2C). Genotyping of the cloned pigs using PCR-RFLP analysis confirmed that they harbored the same PKD1 mutation (c.152_153insG; PKD1insG/+) as the nuclear donor cells (Fig. 2B). The production efficiency of the PKD1insG/+ cloned pigs from the two gilts was 2.2% (5/227) (Table 1). These values were similar to those we reported previously19,24–27. Autopsy of one stillborn (No. 51) among the five offspring revealed renal cyst formation during the neonatal stage (Fig. 2C–E). These renal cysts were lined by epithelium (Fig. 2F).
Table 1.
In vitro development of SCNT embryos and production of cloned pigs.
| In vitro development of reconstructed SCNT embryos | ||
|---|---|---|
| SCNT embryos reconstructed | 330 | |
| Normally cleaved embryos (day 2) | 260 (78.8%) | |
| Blastocysts normally developed | 227 (68.8%) | |
| Production of PKD1insG/+ cloned pigs | ||
| Recipient No. | M174 | M175 |
| No. of embryos transferred | 114 | 113 |
| Cloned piglets obtained | 1 (0.9%) | 4a (3.5%) |
a1 stillborn pig included.
Fig. 2. Generation of PKD1insG/+ cloned pigs by somatic cell nuclear transfer.
A Cloned blastocysts generated using a PKD1insG/+ nuclear donor cell (clone #132), which were then transferred into recipient gilts. B Genotyping of four live PKD1insG/+ cloned pigs (K91, K92, K93, and K94) and one stillborn (No. 51) by PCR-RFLP analysis. One or three bands were obtained after BsrI digestion of the PCR amplicon from WT pigs and PKD1insG/+ pigs, respectively. Arrowheads indicate the digested fragments from the PKD1insG mutant allele with a 1-bp insertion of a guanine nucleotide. M DNA Ladder, N non-digested, d BsrI digested. C Photograph of a stillborn PKD1insG/+ cloned pig (No. 51). The bar represents 3 cm. D Gross morphology of a kidney from the stillborn piglet. Yellow arrowheads indicate renal cysts. E, F Histological section of the kidney (Masson’s trichrome staining). Asterisks indicate a renal cyst. Bars = 1 mm (E) and 100 µm (F).
Phenotypes of PKD1insG/+ mutant cloned pigs
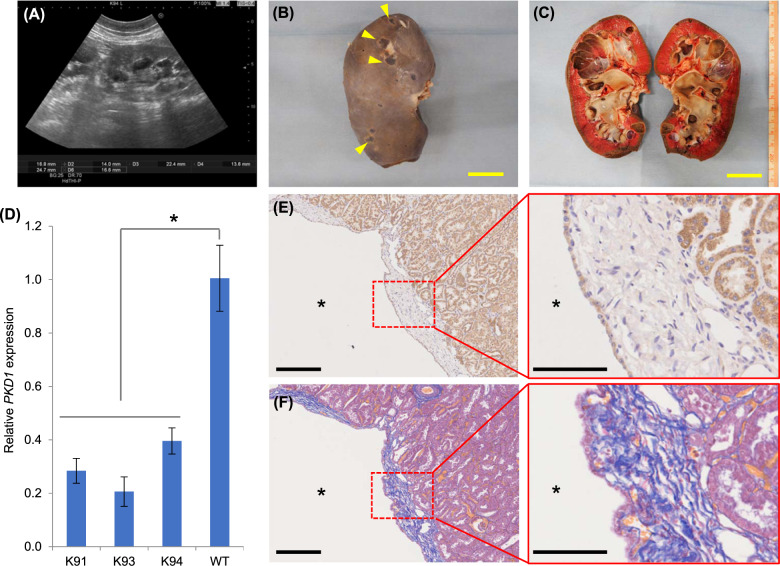
The four surviving PKD1insG/+ cloned pigs (K91–94) grew without any apparent or clinical abnormalities. Ultrasonography performed at 5 months of age confirmed cyst formation in the kidneys of all pigs (Fig. 3A and Supplementary Fig. S1A). Three of the pigs were euthanized and autopsied at 5 months (K93 and K94) or 8 months (K91) of age, and multiple macroscopic cysts were found in both kidneys of each animal (Fig. 3B, C and Supplementary Fig. S1B).
Fig. 3. Phenotypes of PKD1insG/+ founder cloned pigs.
A Ultrasound diagnostic images of the kidney from a PKD1insG/+ cloned pig (K94) at 5 months of age. Gross morphology (B) and coronal section (C) of a kidney from a PKD1insG/+ cloned pig. The yellow bar represents 3 cm. Yellow arrowheads indicate renal cysts. D Relative PKD1 expression in three PKD1insG/+ cloned pigs evaluated using quantitative RT-PCR. The data are presented as the mean ± SD. *p < 0.05. E, F Histological analysis of a PKD1insG/+ founder cloned pig at 5 months of age. Kidney tissue sections with Masson’s trichrome staining (E) and immunostaining using a PKD1-specific antibody (F). Asterisks indicate renal cysts. The black bars represent 250 µm in the left panels and 100 µm in the right panels.
Reverse transcription quantitative PCR analysis of kidney specimens showed that PKD1 expression levels in PKD1insG/+ cloned pigs were reduced by half compared to in WT pigs (Fig. 3D). These data indicate that RNA derived from the mutant allele was selectively degraded by nonsense-mediated decay because of the creation of a premature stop codon associated with the CRISPR-Cas9-induced mutation.
Histochemical analysis of kidney tissue from PKD1insG/+ cloned pigs (K91, K93, and K94) showed that the cyst walls were lined by squamous epithelium. Masson’s trichrome staining revealed fibrosis around the cyst wall (Fig. 3E). Immunostaining using an anti-PKD1 antibody revealed a PKD1-positive signal in the epithelial cells of the cyst wall (Fig. 3F).
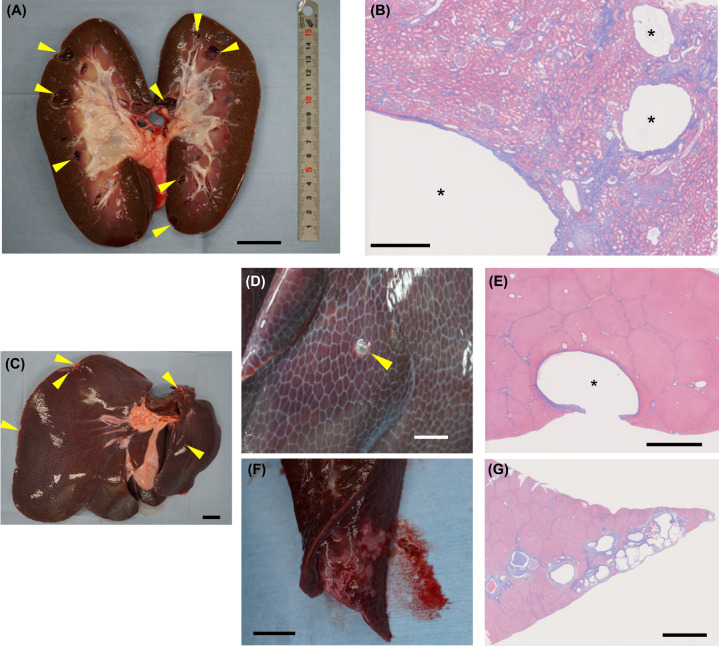
In addition to kidney cysts, multiple cysts were observed in the liver of an autopsied PKD1insG/+ cloned pig (K92) at 36 months of age, and fibrosis was observed around the liver cysts (Fig. 4). Serum CRE and BUN levels, as indicators of renal function, were 2.4 and 15.2 mg/dL, respectively. Aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, as indicators of hepatocellular damage, were 68, 31, and 598 U/L, respectively. These levels were within the range of normal values for WT pigs (CRE, 1.0–2.7 mg/dL; BUN, 10–30 mg/dL; aspartate aminotransferase, 32–84 U/L; alanine aminotransferase, 31–58 U/L; lactate dehydrogenase, 380–630 U/L) 28.
Fig. 4. Renal and hepatic cysts in a PKD1insG/+ founder cloned pig at 36 months of age.
A Coronal section of the kidney from a PKD1insG/+ clone (K92) at 36 months of age. The bar is 3 cm. B Kidney tissue sections with Masson’s trichrome staining. Asterisks indicate renal cysts. The bar is 1 mm. C Gross morphology of the liver from the cloned pig. Hepatic cysts are indicated by yellow arrowheads. The bar is 3 cm. D, F Macroscopic cysts and their histopathological evaluation using Masson’s trichrome staining (E, G). The asterisk indicates a hepatic cyst. The bars represent 2 mm (D and F), 2.5 mm (E), and 5 mm (G).
Reproduction of heterozygous PKD1 mutant pigs and progeny phenotypes
Renal cyst formation was confirmed in all five produced PKD1insG/+ founder cloned pigs. We subsequently checked whether the PKD1insG genotype introduced by CRISPR-Cas9 and its associated phenotype were transmitted to the next generation of animals. A total of 17 F1 offspring were produced by natural mating of a male founder clone pig (K92) and two WT female pigs (PKD1+/+; M237 and W239). Transmission of the mutant PKD1insG was confirmed in 10 of the 17 progeny animals, demonstrating germline transmission of the mutant PKD1 gene (Supplementary Table S2).
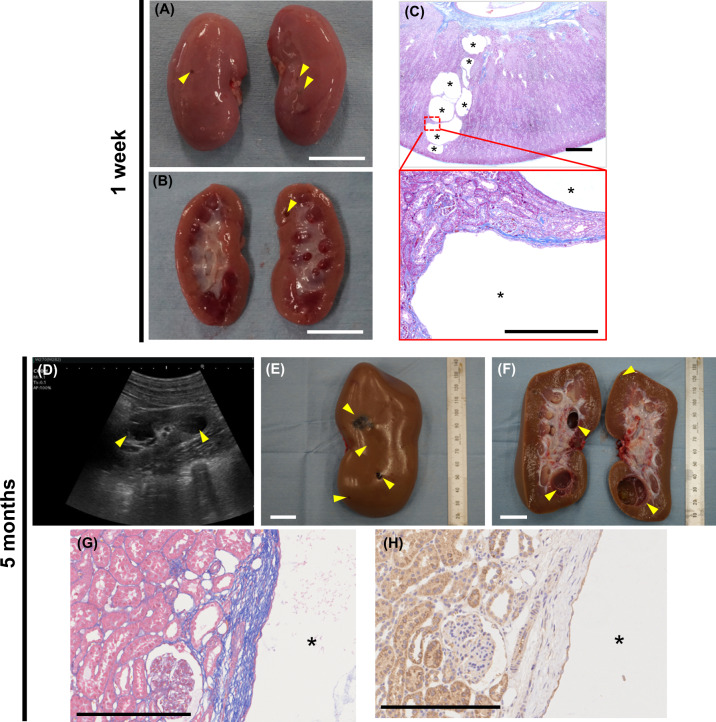
Autopsy of a newborn (1-week-old) PKD1insG/+ F1 animal born from one WT female pig (W239) revealed the presence of macroscopic cysts (Fig. 5A–C). Newborn PKD1insG/+ F1 pigs (n = 5) born from one WT female pig (M237) steadily grew similarly to WT pigs (Supplementary Fig. S2). Ultrasonography performed at 5 months of age revealed renal cyst formation in all five PKD1insG/+ F1 offspring evaluated (Fig. 5D–F, Supplementary Figs. S3 and S4). In addition, ultrasonography evaluation of one PKD1insG/+ F1 offspring (W322) at both 5 and 13 months revealed increased numbers of renal cysts as well as enlargement of the renal cysts from 5 months (1.42 × 1.26 cm) compared to that at 13 months (2.09 × 2.38 cm) (Supplementary Fig. S5A). Increased numbers of renal cysts over time were also observed in other PKD1insG/+ F1 offspring (Supplementary Fig. S5B).
Fig. 5. Phenotypes of PKD1insG/+ F1 progeny presenting early-onset renal cyst formation.
PKD1insG/+ F1 progeny at 1 week of age (A–C) and 5 months of age (D–H). Gross morphology (A) and coronal section of the kidney (B) from PKD1insG/+ F1 progeny at 1 week of age. The arrowheads indicate renal cysts. The white bar represents 2 cm. C Kidney tissue sections with Masson’s trichrome staining of PKD1insG/+ F1 progeny. The black bars represent 1 mm in the upper panels and 250 µm in the lower panels. D Ultrasound diagnostic images of the kidney in PKD1insG/+ F1 progeny. Gross morphology (E) and coronal section (F) of the kidney from PKD1insG/+ F1 progeny (W282) at 5 months of age. The white bar represents 2 cm. Yellow arrowheads indicate renal cysts. G Kidney tissue sections of PKD1insG/+ F1 pigs with Masson’s trichrome staining and immunostaining using a PKD1-specific antibody (H). Asterisks indicate renal cysts. The bar represents 250 µm.
Histochemical analysis of the resected kidneys of PKD1insG/+ mutant F1 progeny showed fibrosis around the cysts, similar to that of PKD1insG/+ mutant clones. Cyst-lining epithelial cells of the cyst walls showed mixed populations of cells with prominent and faint PKD1 signals (Fig. 5G, H).
We confirmed that the mutant allele was stably passed to the next generation (F2) of offspring by natural mating of a male PKD1insG/+ F1 pig (W279) that had reached sexual maturity with a WT female pig (#1730) (Supplementary Fig. S6A). Renal cysts were confirmed in all six PKD1insG/+ F2 newborns (3 days old) (Supplementary Fig. S6B). A very large number of cysts was observed in one of the F2 pigs (#1730-1), and fibrosis was observed around the cysts (Supplementary Fig. S7A–C). Furthermore, PKD1-positive signals were observed in cyst-lining epithelial cells (Supplementary Fig. S7D). These results show that the development of renal cysts in PKD1insG/+ mutant pigs during the neonatal period was not only a feature of the cloned founder but also common to the F1 and F2 progeny.
Serum CRE and serum BUN levels of F1 progeny at 24 months of age were 2.4–2.5 mg/dL (n = 3) and 5.1–13.0 mg/dL (n = 3), respectively. Both the CRE and BUN levels in the F1 progeny were within the normal range of those in the WT pigs. CRE and BUN levels remained in the normal range (2.2 and 17.7 mg/dL, respectively) in an F1 progeny pig that had been raised until 53 months of age. No clinical abnormalities were detected in the renal function of PKD1insG/+ pigs, even after 4 years of age.
Pkd1 homozygous KO mice are known to exhibit embryonic or prenatal lethality16. Mating of two F1 PKD1insG/+ boars (W279 and W280) with two gilts (W273 and W322) with the same mutation gave rise to a total of 19 F2 offspring including only PKD1insG/+ and PKD1+/+ pigs, with no homozygous PKD1-KO pigs (PKD1insG/insG) born (Supplementary Table S3). In addition, genotyping of 39-day-old fetuses (n = 6) produced by mating F1 PKD1insG/+ animals did not reveal homozygous PKD1-KO fetuses (Supplementary Table S3). These results indicate early embryonic lethality of the homozygous PKD1-KO in the pig.
Discussion
In heterozygous Pkd1-KO mice, renal cyst formation is very rare, even at an old age29,30. This may reflect the limitations of rodent models for studying this disease, as rodents have a short lifespan and markedly different anatomy and physiology compared to humans. In contrast, the PKD1insG/+ pigs produced in this study (Supplementary Table S4) with a mutation introduced into exon 1 of PKD1 (c.152_153insG) by CRISPR-Cas9 exhibited a similar phenotype as human patients with ADPKD, including high penetrance of renal cyst formation. We confirmed that the mutant allele was transmitted following Mendelian inheritance to progeny offspring. Furthermore, the progeny pigs faithfully reproduced the renal cystic phenotype, which is the major symptom of ADPKD.
Patients with ADPKD generally show slow progression of renal cyst formation, and most patients are clinically asymptomatic until their 4th to 5th decade of life1,4,5. However, renal cyst formation in patients with ADPKD occurs starting during the fetal period, with cysts progressively increasing in number and size with age31–33. In the heterozygous PKD1-KO minipigs reported previously21, no macroscopic or microscopic cysts were observed in the neonatal stage. In contrast, PKD1insG/+ pigs produced in the current study exhibited macroscopic cyst formation during the neonatal stage, similar to in human patients with ADPKD. These human-like cystogenic dynamics, combined with progressive renal cyst formation in conjunction with age, may be a hallmark of PKD1insG/+ pigs.
The difference in the timing of renal cyst formation between the current study and that reported by He et al.21 may be related to the differences in the type of mutation of the causative gene. Various types of PKD1 mutations have been identified in patients with ADPKD, including missense, nonsense, in-frame deletion/insertion, and aberrant splicing mutations34,35. It has been reported that over 70% of detected mutations are unique36. Together, our findings suggest that diverse causal mutations result in ADPKD symptoms in pigs.
In contrast, differences in pathological conditions within a family of patients with the same mutation and among siblings have been reported37. The genetic background of PKD1insG/+ cloned pigs (K91–K94) obtained in this study is identical. The degree of cyst formation exhibited by these individuals varied. Although gene mutation is among the major determinants of disease severity, various factors are involved in cyst formation in a complicated manner in ADPKD. Our PKD1insG/+ cloned pig data suggest the presence of modifiers that differ from the genetic background of the individual. Regarding the difference in the pathophysiology of K94 and K92 in PKD1insG/+ cloned pigs, we have not yet identified the cause of individual differences. Differences in genetic background, nongenetic factors including epigenetic factors, and microRNAs are thought to have caused this discrepancy38–41. The development of ADPKD model pigs with various mutation types will provide diverse cystogenic dynamics to give a detailed understanding of the pathophysiology of ADPKD and improve the development of treatment methods.
A two-hit theory has been proposed as a mechanism of cyst formation in patients with ADPKD13. According to this concept, the etiology of ADPKD involves a heterozygously inherited mutation and somatic mutation in the other normal PKD1 allele, resulting in the complete loss of function of PKD1, ultimately leading to renal cyst formation. In this theory, the proliferation of cystic epithelial cells is explained by the monoclonal proliferation of constituent cells in the nephron harboring the two genetic hits13. However, Nishio et al.42 described that renal cyst formation cannot be ascribed only to monoclonal proliferation of cystic epithelial cells.
In our PKD1insG/+ pigs, PKD1-positive signals were observed in many epithelial cells forming the renal cyst walls. Similarly, cysts in many patients with ADPKD have been reported to contain PKD1-positive cells43,44. This finding is consistent with the low frequency of cells displaying complete loss of PKD1 function in individual renal cysts of patients with ADPKD. The two-hit theory can explain the slow onset of ADPKD and focal cyst formation but fails to adequately explain the full extent of the pathological manifestation of ADPKD45,46. Further study is required, such as investigating the mechanism of haploinsufficiency proposed in recent years15,47.
In addition to renal cyst formation during the neonatal stage, PKD1insG/+ pigs exhibited several characteristics similar to those of human ADPKD. First, the number of cysts that developed in the kidneys was similar to that in human patients with ADPKD. The diagnostic criteria for human ADPKD are met if a patient under 30 years of age has more than two or three unilateral or bilateral kidney cysts48,49. All young adult to adult PKD1insG/+ pigs evaluated presented with an adequate number of cysts to meet the diagnostic criteria of ADPKD in human patients.
The second similarity was interstitial fibrosis. Various degrees of interstitial fibrosis are observed around expanding renal cysts in patients with ADPKD50. Fibrosis was also observed around the renal cysts of PKD1insG/+ pigs, demonstrating a pathological presentation similar to that observed in humans.
Similarities or differences between PKD1insG/+ and human patients with ADPKD in the mechanisms of renal cyst development require further investigation. In human ADPKD, renal cysts derived from the collecting duct are thought to develop consistently, whereas renal cysts can originally arise from all segments of the nephron51–53. The origin of cyst formation in PKD1insG/+ pigs should be identified in further studies.
In many cases of ADPKD, renal function remains undiminished until midlife. It has been reported that in patients with progressive renal enlargement, their nephrons undergo compensatory hyperfiltration to maintain the glomerular filtration rate for decades, which continues until more than half of the functioning parenchyma is destroyed. Furthermore, CRE levels are maintained within the normal range54. The timing of clinical symptom onset in PKD1insG/+ pigs was also similar to that of human patients with ADPKD. No clinical renal hypofunction was detected in either the 36-month-old PKD1insG/+ founder clone or 53-month-old PKD1insG/+ F1 pig analyzed in the current study. The age of these pigs, considering the average lifespan of livestock pigs as approximately 20 years55, would correspond to a premature age in humans. Accordingly, we considered that PKD1insG/+ pigs also had an asymptomatic period during the first half of life, similar to that in humans with ADPKD. Further long-term studies are needed to clarify disease progression and disease latency in PKD1insG/+ pigs.
Recently, ADPKD models with mutations in PKD1 of cynomolgus monkeys were created by zygote injection of CRISPR-Cas956. The resulting PKD1-mutated monkeys exhibit a phenotype similar to our heterozygous PKD1-KO pigs. In fact, heterozygous PKD1 monkeys show few renal cysts perinatally, and many live monkeys with PKD1 mutations present only mild cyst formation. It has also been reported that there are no abnormalities in CRE or urinary urea nitrogen levels at 6 or 12 months. Therefore, PKD1-mutated monkeys are also thought to exhibit asymptomatic stages, similar to in our current PKD1insG/+ pigs.
Co-development of liver cysts, in addition to renal cyst formation, is known to be a common phenotype of patients with ADPKD57. The onset dynamics of liver cysts in PKD1insG/+ pigs is not clear, although we observed liver cyst formation in one of the founder clones at 36 months of age. Autosomal recessive polycystic kidney disease (ARPKD) due to PKHD1 mutation is characterized by severe hepatic fibrosis with bile duct dysplasia and intrahepatic periportal fibrosis. In addition, congenital hepatic fibrosis is a complication associated with ARPKD58. The pathophysiology of the ADPKD model introduced with the PKD1 mutation in this study differs from that of the fibrocystic liver phenotype in ARPKD. Other extrarenal manifestations of ADPKD include cyst formation in the pancreas and testes, hypertension, valvular heart disease, cerebral aneurysm, and diverticulosis5. Extrarenal manifestations in PKD1insG/+ pigs have not yet been determined. Pigs show greater fecundity than monkeys, and large animals have relatively early sexual maturation (pigs, 6–8 months; monkeys, 3–4 years). The normal reproductive ability of PKD1insG/+ pigs will allow for further research.
In conclusion, PKD1insG/+ pigs showing 100% penetrance of a cystic kidney phenotype have many symptomatic similarities to patients with ADPKD caused by heterozygous mutation of PKD1. Similarities include the formation of macroscopic renal cysts during the neonatal stage, number and cystogenic dynamics of renal cysts formed, pathological features such as interstitial fibrosis, and presence of an asymptomatic prematuration stage. ADPKD is treated clinically as an adult-onset disease; however, intervention starting at an earlier stage is needed59. PKD1insG/+ pigs can be used to study early intervention in pediatric patients with ADPKD, verify the effects of prophylactic treatment, and test long-term treatments that cannot be performed using rodent models.
Supplementary information
Acknowledgements
We thank Dr. Yoshikazu Arai for technical help and Hiroshi Kadoi for help with maintenance of pigs.
Author contributions
H.Nagashima conceived and designed this study. M.W. and H.Nagashima wrote the manuscript with contributions from H.Nakauchi, N.M., and T.Y. M.W., K.U., K.N., T.F., K.H, K.O., S.Tajiri, and S.Y. performed data acquisition, data analysis. K.M. and S.Tajiri. performed ultrasound examination. M.W. and S.Takayanagi performed isolation of gene knockout cells and genetic and biochemical experiments. H.Nagashima, H.M, K.N., A.U., and K.O. performed the experiments for generation of cloned pigs. All authors reviewed and approved the final manuscript.
Funding
This work was supported by Grants-in-Aid form the Meiji University International Institute for Bio-Resource Research (MUIIBR) and the Japan Agency for Medical Research and Development (AMED-LEAP, Generation of Functional Organs using developmental niches, JP19gm0010002).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
H.Nagashima is a founder and shareholder of PorMedTec Co., Ltd. These associations do not alter the authors’ adherence to the journal’s policies on sharing data and materials. The other authors declare no competing interests.
Ethics approval
All animal experiments, including genetic modifications performed in this study, were approved by Meiji University Institutional Animal Care and Use Committee (IACUC11-0003 and IACUC16-0008). All experiments were performed in accordance with the relevant guidelines and regulations.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41374-021-00717-z.
References
- 1.Gabow PA. Autosomal dominant polycystic kidney disease. N. Engl. J. Med. 1993;329:332–342. doi: 10.1056/NEJM199307293290508. [DOI] [PubMed] [Google Scholar]
- 2.Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287–1301. doi: 10.1016/S0140-6736(07)60601-1. [DOI] [PubMed] [Google Scholar]
- 3.Harris PC, Torres VE. Polycystic kidney disease. Annu. Rev. Med. 2009;60:321–337. doi: 10.1146/annurev.med.60.101707.125712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hateboer N, et al. Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet. 1999;353:103–107. doi: 10.1016/S0140-6736(98)03495-3. [DOI] [PubMed] [Google Scholar]
- 5.Spithoven EM, et al. Analysis of data from the ERA-EDTA Registry indicates that conventional treatments for chronic kidney disease do not reduce the need for renal replacement therapy in autosomal dominant polycystic kidney disease. Kidney Int. 2014;86:1244–1252. doi: 10.1038/ki.2014.120. [DOI] [PubMed] [Google Scholar]
- 6.Perrone RD. Extrarenal manifestations of ADPKD. Kidney Int. 1997;51:2022–2036. doi: 10.1038/ki.1997.276. [DOI] [PubMed] [Google Scholar]
- 7.Rossetti S, et al. Association of mutation position in polycystic kidney disease 1 (PKD1) gene and development of a vascular phenotype. Lancet. 2003;361:2196–2201. doi: 10.1016/S0140-6736(03)13773-7. [DOI] [PubMed] [Google Scholar]
- 8.Ecder T, Schrier RW. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat. Rev. Nephrol. 2009;5:221–228. doi: 10.1038/nrneph.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torres VE, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris PC, Rossetti S. Molecular diagnostics for autosomal dominant polycystic kidney disease. Nat. Rev. Nephrol. 2010;6:197–206. doi: 10.1038/nrneph.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagao S, Kugita M, Yoshihara D, Yamaguchi T. Animal models for human polycystic kidney disease. Exp. Anim. 2012;61:477–488. doi: 10.1538/expanim.61.477. [DOI] [PubMed] [Google Scholar]
- 12.Happe H, Peters DJ. Translational research in ADPKD: lessons from animal models. Nat. Rev. Nephrol. 2014;10:58. doi: 10.1038/nrneph.2014.137. [DOI] [PubMed] [Google Scholar]
- 13.Qian F, Watnick TJ, Onuchic LF, Germino GG. The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell. 1996;87:979–987. doi: 10.1016/S0092-8674(00)81793-6. [DOI] [PubMed] [Google Scholar]
- 14.Lantinga-van Leeuwen IS, et al. Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum. Mol. Genet. 2004;13:3069–3077. doi: 10.1093/hmg/ddh336. [DOI] [PubMed] [Google Scholar]
- 15.Hopp K, et al. Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J. Clin. Invest. 2012;122:4257–4273. doi: 10.1172/JCI64313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu W, et al. Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation. Nat. Genet. 1997;17:179–181. doi: 10.1038/ng1097-179. [DOI] [PubMed] [Google Scholar]
- 17.Matsunari H, Nagashima H. Application of genetically modified and cloned pigs in translational research. J. Reprod. Dev. 2009;55:225–230. doi: 10.1262/jrd.20164. [DOI] [PubMed] [Google Scholar]
- 18.Aigner B, et al. Transgenic pigs as models for translational biomedical research. J. Mol. Med. 2010;88:653–664. doi: 10.1007/s00109-010-0610-9. [DOI] [PubMed] [Google Scholar]
- 19.Umeyama K, et al. Generation of heterozygous fibrillin-1 mutant cloned pigs from genome-edited foetal fibroblasts. Sci. Rep. 2016;6:24413. doi: 10.1038/srep24413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe M, Nagashima H. Genome editing of pig. Methods Mol. Biol. 1630;121-139:2017. doi: 10.1007/978-1-4939-7128-2_11. [DOI] [PubMed] [Google Scholar]
- 21.He J, et al. PKD1 mono-allelic knockout is sufficient to trigger renal cystogenesis in a mini-pig model. Int. J. Biol. Sci. 2015;11:361–369. doi: 10.7150/ijbs.10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurome M, Kessler B, Wuensch A, Nagashima H, Wolf E. Nuclear transfer and transgenesis in the pig. Methods Mol. Biol. 2015;1222:37–59. doi: 10.1007/978-1-4939-1594-1_4. [DOI] [PubMed] [Google Scholar]
- 23.He J, Wang Q, Ye J, Hu X, Li N. Identification of porcine polycystic kidney disease 1 (PKD1) gene: molecular cloning, expression profile, and implication in disease model. Gene. 2011;490:37–46. doi: 10.1016/j.gene.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe M, et al. Generation of interleukin-2 receptor gamma gene knockout pigs from somatic cells genetically modified by zinc finger nuclease-encoding mRNA. PLoS One. 2013;8:e76478. doi: 10.1371/journal.pone.0076478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsunari H, et al. Transgenic-cloned pigs systemically expressing red fluorescent protein, Kusabira-Orange. Cloning Stem Cells. 2008;10:313–323. doi: 10.1089/clo.2008.0024. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe M, et al. Production of transgenic cloned pigs expressing the far-red fluorescent protein monomeric Plum. J. Reprod. Dev. 2015;61:169–177. doi: 10.1262/jrd.2014-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyagawa S, et al. Generation of alpha1,3-galactosyltransferase and cytidine monophospho-N-acetylneuraminic acid hydroxylase gene double-knockout pigs. J. Reprod. Dev. 2015;61:449–457. doi: 10.1262/jrd.2015-058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson, P., Cockcroft, P. Handbook of pig medicine, (Saunders, 2007).
- 29.Lu W, et al. Late onset of renal and hepatic cysts in Pkd1-targeted heterozygotes. Nat. Genet. 1999;21:160–161. doi: 10.1038/5944. [DOI] [PubMed] [Google Scholar]
- 30.Wu G, et al. Trans-heterozygous Pkd1 and Pkd2 mutations modify expression of polycystic kidney disease. Hum. Mol. Genet. 2002;11:1845–1854. doi: 10.1093/hmg/11.16.1845. [DOI] [PubMed] [Google Scholar]
- 31.MacDermot KD, Saggar-Malik AK, Economides DL, Jeffery S. Prenatal diagnosis of autosomal dominant polycystic kidney disease (PKD1) presenting in utero and prognosis for very early onset disease. J. Med. Genet. 1998;35:13–16. doi: 10.1136/jmg.35.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyer O, et al. Prognosis of autosomal dominant polycystic kidney disease diagnosed in utero or at birth. Pediatr. Nephrol. 2007;22:380–388. doi: 10.1007/s00467-006-0327-8. [DOI] [PubMed] [Google Scholar]
- 33.Reed B, et al. Renal ultrasonographic evaluation in children at risk of autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 2010;56:50–56. doi: 10.1053/j.ajkd.2010.02.349. [DOI] [PubMed] [Google Scholar]
- 34.Autosomal dominant polycystic kidney disease mutation database: PKDB, (PKD Foundation, Missouri,1982). https://pkdb.mayo.edu.
- 35.Rossetti S, et al. A complete mutation screen of the ADPKD genes by DHPLC. Kidney Int. 2002;61:1588–1599. doi: 10.1046/j.1523-1755.2002.00326.x. [DOI] [PubMed] [Google Scholar]
- 36.Cornec-Le Gall E, Audrezet MP, Le Meur Y, Chen JM, Ferec C. Genetics and pathogenesis of autosomal dominant polycystic kidney disease: 20 years on. Hum. Mutat. 2014;35:1393–1406. doi: 10.1002/humu.22708. [DOI] [PubMed] [Google Scholar]
- 37.Persu A, et al. Comparison between siblings and twins supports a role for modifier genes in ADPKD. Kidney Int. 2004;66:2132–2136. doi: 10.1111/j.1523-1755.2004.66003.x. [DOI] [PubMed] [Google Scholar]
- 38.Rossetti S, Harris PC. Genotype-phenotype correlations in autosomal dominant and autosomal recessive polycystic kidney disease. J. Am. Soc. Nephrol. 2007;18:1374–1380. doi: 10.1681/ASN.2007010125. [DOI] [PubMed] [Google Scholar]
- 39.Chapman AB. The fetal environment: a critical phase that determines future renal outcomes in autosomal dominant polycystic kidney disease. Kidney Int. 2012;81:814–815. doi: 10.1038/ki.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X. Epigenetics and autosomal dominant polycystic kidney disease. Biochim. Biophys. Acta. 2011;1812:1213–1218. doi: 10.1016/j.bbadis.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan YC, Blumenfeld J, Rennert H. Autosomal dominant polycystic kidney disease: genetics, mutations and microRNAs. Biochim. Biophys. Acta. 2011;1812:1202–1212. doi: 10.1016/j.bbadis.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Nishio S, et al. Pkd1 regulates immortalized proliferation of renal tubular epithelial cells through p53 induction and JNK activation. J. Clin. Invest. 2005;115:910–918. doi: 10.1172/JCI22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward CJ, et al. Polycystin, the polycystic kidney disease 1 protein, is expressed by epithelial cells in fetal, adult, and polycystic kidney. Proc. Natl. Acad. Sci. USA. 1996;93:1524–1528. doi: 10.1073/pnas.93.4.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geng L, et al. Identification and localization of polycystin, the PKD1 gene product. J. Clin. Invest. 1996;98:2674–2682. doi: 10.1172/JCI119090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brasier JL, Henske EP. Loss of the polycystic kidney disease (PKD1) region of chromosome 16p13 in renal cyst cells supports a loss-of-function model for cyst pathogenesis. J. Clin. Invest. 1997;99:194–199. doi: 10.1172/JCI119147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ong AC, Harris PC. Molecular basis of renal cyst formation–one hit or two? Lancet. 1997;349:1039–1040. doi: 10.1016/S0140-6736(05)62286-6. [DOI] [PubMed] [Google Scholar]
- 47.Rossetti S, et al. Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney Int. 2009;75:848–855. doi: 10.1038/ki.2008.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ravine D, et al. Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet. 1994;343:824–827. doi: 10.1016/S0140-6736(94)92026-5. [DOI] [PubMed] [Google Scholar]
- 49.Pei Y, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J. Am. Soc. Nephrol. 2009;20:205–212. doi: 10.1681/ASN.2008050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norman J. Fibrosis and progression of autosomal dominant polycystic kidney disease (ADPKD) Biochim. Biophys. Acta. 2011;1812:1327–1336. doi: 10.1016/j.bbadis.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baert L. Hereditary polycystic kidney disease (adult form): a microdissection study of two cases at an early stage of the disease. Kidney Int. 1978;13:519–525. doi: 10.1038/ki.1978.75. [DOI] [PubMed] [Google Scholar]
- 52.Grantham JJ, Geiser JL, Evan AP. Cyst formation and growth in autosomal dominant polycystic kidney disease. Kidney Int. 1987;31:1145–1152. doi: 10.1038/ki.1987.121. [DOI] [PubMed] [Google Scholar]
- 53.Verani RR, Silva FG. Histogenesis of the renal cysts in adult (autosomal dominant) polycystic kidney disease: a histochemical study. Mod. Pathol. 1988;1:457–463. [PubMed] [Google Scholar]
- 54.Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N. Engl. J. Med. 2008;359:1477–1485. doi: 10.1056/NEJMcp0804458. [DOI] [PubMed] [Google Scholar]
- 55.Swindle, M. M. Swine in the laboratory (CRC Press, Florida, 2007).
- 56.Tsukiyama T, et al. Monkeys mutant for PKD1 recapitulate human autosomal dominant polycystic kidney disease. Nat. Commun. 2019;10:5517. doi: 10.1038/s41467-019-13398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Everson GT. Hepatic cysts in autosomal dominant polycystic kidney disease. Am. J. kidney Dis. 1993;22:520–525. doi: 10.1016/S0272-6386(12)80923-1. [DOI] [PubMed] [Google Scholar]
- 58.Ward CJ, et al. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat. Genet. 2002;30:259–269. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- 59.Cadnapaphornchai MA. Autosomal dominant polycystic kidney disease in children. Curr. Opin. Pediatr. 2015;27:193–200. doi: 10.1097/MOP.0000000000000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.