Abstract
Purpose
To provide an updated review of multimodal pain management in arthroscopic surgery by evaluating pain and opioid consumption after shoulder, knee, and hip arthroscopy.
Methods
A comprehensive literature search was performed to identify randomized controlled trials (RCTs) investigating multimodal pain management after shoulder, knee, and hip arthroscopy. Articles were identified from January 2011 through December 2020 using various databases. As the primary outcome variables of this study, differences in postoperative pain and opioid consumption volumes were summarized from all reported postoperative time points.
Results
37 shoulder, 28 knee, and 8 hip arthroscopy RCTs were included in the study. The most frequent bias present in the included RCTs was incomplete outcome data (58%), while group allocation concealment was the least frequent bias (15%). Qualitative analysis of rotator cuff repair (n = 12), anterior cruciate ligament reconstruction (n = 11), meniscectomy (n = 5), femoroacetabular impingement (n = 2), oral medications (n = 8), postoperative interventions (n = 10), and nonpharmacological interventions (n = 6) was performed.
Conclusions
Many multimodal pain management protocols offer improved pain control and decreased opioid consumption after arthroscopic surgery. On the basis of the current literature, the evidence supports an interscalene nerve block with a dexamethasone-dexmedetomidine combination for rotator cuff repair, a proximal continuous adductor canal block for anterior cruciate ligament reconstruction, and local infiltration analgesia (e.g., periacetabular injection with 20 mL of .5% bupivacaine) for hip arthroscopy. When evaluating oral medication, the evidence supports 150 mg Pregabalin for shoulder arthroscopy, 400 mg Celecoxib for knee arthroscopy, and 200 mg Celecoxib for hip arthroscopy, all taken preoperatively. There is promising evidence for the use of various nonpharmacological modalities, specifically preoperative opioid education for rotator cuff repair patients; however, more clinical trials that evaluate nonpharmacological interventions should be performed.
Level of Evidence
Level II, systematic review of Level I and II studies.
Introduction
As pain became the “fifth vital sign” and sustained-release OxyContin (Purdue Pharma, Stamford, CT) was approved for use, opioids were marketed aggressively as an effective treatment for noncancerous pain.1,2 However, excessive opioid usage is associated with increased mortality3,4 and addiction,5 which have been implicated in the current opioid epidemic. In 2020, there were nearly six times more opioid-related overdose deaths than there were in 1999.6 With patient-reported pain remaining unchanged while opioid prescription rates continued to increase,7 research has been increasingly focused on nonopioid pain management techniques.
Optimal management of postoperative pain is associated with decreased morbidity and faster recovery times, as well as improved physical function and quality of life.8 Despite efforts to minimize postoperative pain, 61% of outpatients still experience moderate/extreme pain after discharge.9 Some of the most painful surgeries are orthopedic procedures, with arthroscopic surgeries, such as cruciate ligament reconstruction10 and rotator cuff repair,11 considered among the most painful outpatient orthopedic surgical procedures. Because of the pain associated with these procedures, orthopedic surgeons were the third highest prescribers of opioids based on specialty in the United States, behind only primary care physicians and internists.12 The American Society of Anesthesiologists recommends the use of multimodal pain regimens to minimize opioid use and improve pain control.13 Multimodal pain management uses combinations of opioid prescriptions, nonopioid prescription, regional and local anesthesia, and nonpharmacological therapy. An effective multimodal pain management protocol should limit both postoperative pain and opioid consumption.
Four systematic reviews have evaluated randomized controlled trials (RCTs) within knee,14 hip,15 and shoulder16,17 arthroscopy. Warrender et al.17 suggests that the interscalene nerve block is the most effective analgesic for arthroscopic shoulder surgery, while Hurley et al.16 recommend nerve block adjuncts to improve pain control. In hip arthroscopy, Kunze et al.15 similarly recommend adjunct analgesia, and they suggest that local infiltration analgesia may optimize postoperative pain and opioid consumption. In knee arthroscopy, Secrist et al.14 did not determine an optimal multimodal management protocol for anterior cruciate ligament reconstruction.
In order to optimize postoperative care, treatment plans should minimize both pain and opioid consumption after arthroscopic surgery. However, few reviews have focused on postoperative opioid consumption along with pain management in arthroscopic surgery. The purpose of the study was to provide an updated review of multimodal pain management in arthroscopic surgery by evaluating pain and opioid consumption after shoulder, hip, and knee arthroscopy.
Methods
Study Selection
This systematic review was performed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.18 A comprehensive literature search was performed to identify all RCTs regarding pain management after arthroscopic surgeries of the shoulder, hip, and knee. Articles were identified from a 10-year period ranging from January 1st, 2011 through December 31st, 2020 by using the PubMed, Ovid, and CINAHL databases. The following keywords were used: opioid, pain management, multimodal, sports medicine, shoulder, hip, knee, surgery, surgical, and arthroscopy. Screening of RCTs by title and abstract was performed by two independent researchers, R.W.P. (research fellow) and P.S. (orthopaedic surgery resident), with disagreements settled by K.B.F. (attending orthopaedic surgeon).
Inclusion and Exclusion Criteria
Only RCTs that 1) were related to arthroscopic surgery of the shoulder, hip, and knee, 2) reported both postoperative pain and volume of postoperative opioid consumption, and 3) had a dependent variable focusing on multimodal pain management, were included. Interventions provided preoperatively, intraoperatively, and postoperatively were all included as well. Multimodal pain management was considered any combination of at least two of the following: education, exercise interventions, pharmaceutical medications, regional anesthesia, rehabilitative interventions (exercise, manual therapy, physical modalities), and workplace intervention. Combinations of varying medications were also considered multimodal, as has been done in several other systematic reviews and meta-analyses.19, 20, 21, 22 Studies that 1) were not randomized controlled trials focusing on arthroscopic surgery of the shoulder, hip, and knee, 2) did not report both postoperative pain scores and postoperative opioid consumption, and 3) did not have an intervention regarding multimodal pain management, were excluded.
Assessment of Study Quality
Included studies were evaluated for bias using the Cochrane Risk of Bias tool.23 Six categories of bias assessment were used from the Cochrane Risk of Bias tool: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. Bias in each category was classified as high, low, or unclear.
Data Collection and Abstraction
Surgical category (shoulder, hip, knee arthroscopy), pain management intervention, descriptions of treatment groups, details regarding treatment dosages, and demographic data (age, sex, and BMI) were collected from each included study. As the primary outcome variables of this study, postoperative pain scores and volume of postoperative opioid consumption were collected from all reported postoperative time points. Statistically significant differences in postoperative pain scores and opioid consumption were noted within each included study.
Statistical Analysis
Because of differences in study intervention, patient populations, and surgical procedure, postoperative pain scores and opioid consumption were not pooled. Summary data regarding the postoperative pain scores and opioid consumption from all time points were presented.
Results
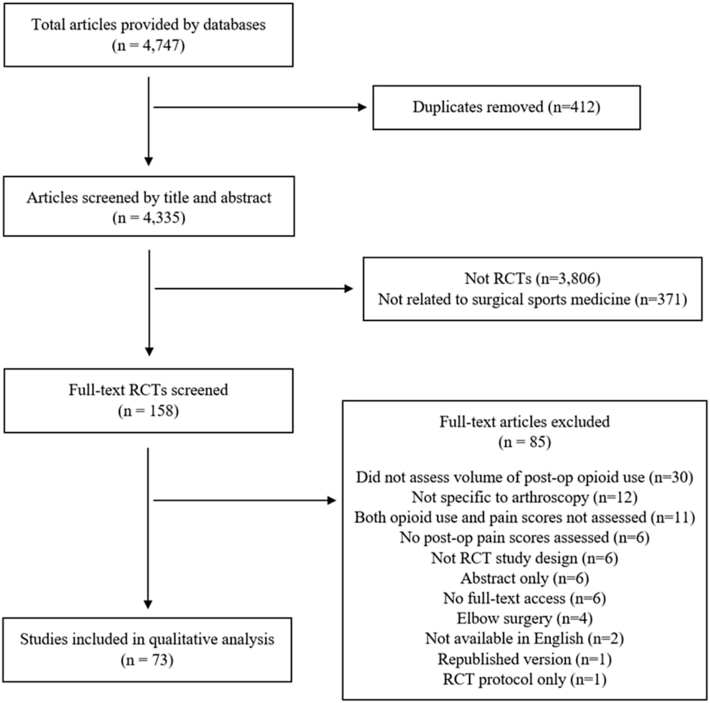
Overall, 4,335 nonduplicate articles were screened by title and abstract for inclusion. After excluding 3,806 studies that were not RCTs and 371 that were not related to arthroscopy, 158 studies were screened by full text. Eighty-five articles were excluded based on full text, with exclusion reasons available in Fig 1. Seventy-three RCTs assessed both postoperative pain and postoperative opioid consumption after arthroscopic procedures and were included in the final qualitative analysis.
Fig 1.
Flowchart of randomized controlled trial (RCT) screening process, with 73 final studies included, and the reasons for excluding 85 other articles are noted.
Study Quality
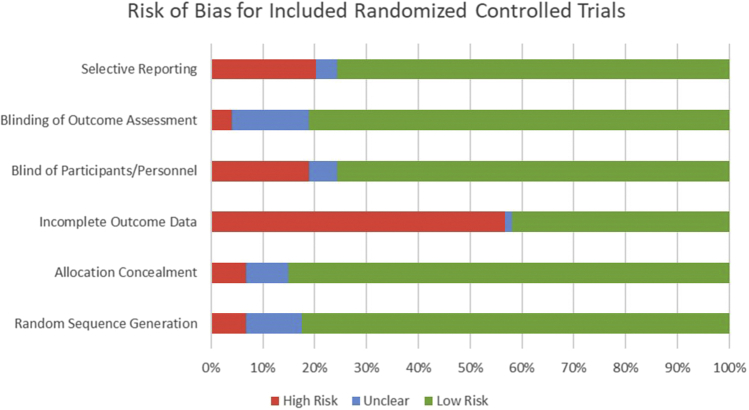
The most frequent bias present in the included RCTs was incomplete outcome data, as 42 out of the 73 included RCTs (58%) either did not provide data for all variables or did not provide adequate statistics, such as standard deviations and exact P values (Table 1, Fig 2). Group allocation concealment was the least frequent bias, as 62 of the 73 included studies (85%) concealed participants’ group allocations, often by using sealed opaque envelopes. The rest of the average Cochrane Risk of Bias tool data is available in Fig 2, with individual studies’ bias scores available in Table 1.
Table 1.
Cochrane Risk of Bias Data for all Included Studies
| Author | Publication Year | Random Sequence Generation | Allocation Concealment | Incomplete Outcome Data | Blind of Participants and Personnel | Blinding of Outcome Assessment | Selective Reporting |
|---|---|---|---|---|---|---|---|
| Abdallah et al.24 | 2016 (knee) | High | Low | High | Low | Low | Low |
| Abdallah et al.24 | 2016 (shoulder) | Low | Low | Low | Low | Low | Low |
| Abdallah et al.25 | 2019 | Low | Low | Low | Low | Low | Low |
| Abdallah et al.26 | 2020 | Unclear | Low | High | Low | Low | Low |
| Ahn et al.27 | 2016 | Low | Low | Low | Low | Low | Low |
| Aksu et al.28 | 2015 | Low | Unclear | Low | High | Low | High |
| Amin et al.29 | 2011 | Low | High | High | Unclear | Unclear | High |
| Arti and Mehdinasab30 | 2011 | High | Low | High | Low | Low | Low |
| Auyong et al.31 | 2018 | Low | Low | Low | Low | Low | Low |
| Baessler et al.32 | 2020 | Low | Low | Low | High | Unclear | Low |
| Bailey et al.33 | 2019 | Low | Unclear | Low | High | Low | Low |
| Behrends et al.34 | 2018 | Low | Low | High | Low | Low | High |
| Bengisun et al.35 | 2014 | Low | Low | High | Low | Low | Low |
| Bjørnholdt et al.36 | 2014 | Unclear | Low | High | Low | Low | Low |
| Cabaton et al.37 | 2019 | Low | Low | High | High | Low | Low |
| Choromanski et al.38 | 2015 | Low | Low | Low | Low | Low | Low |
| Cho et al.39 | 2011 | Low | High | High | High | Unclear | High |
| Cogan et al.40 | 2020 | Low | Low | High | Low | Low | High |
| DeMarco et al.41 | 2011 | Low | Low | High | Low | Low | Low |
| Espelund et al.42 | 2014 | Low | Low | High | Low | Low | Low |
| Espelund et al.43 | 2014 | Low | Low | High | Low | Low | Low |
| Faria-Silva et al.44 | 2016 | Low | Unclear | High | High | Low | Low |
| Glomset et al.45 | 2020 | Low | Unclear | Low | High | Unclear | Low |
| Hanson et al.46 | 2013 | Low | Low | High | Low | Low | Low |
| Hartwell et al.47 | 2020 | High | High | Low | High | High | Low |
| Hsu et al.48 | 2013 | Low | Low | Low | Low | Low | Low |
| Jeske et al.49 | 2011 | Low | Low | High | Low | Low | High |
| Kager et al.50 | 2011 | Low | Low | High | Low | Unclear | Low |
| Kahlenberg et al.51 | 2017 | Unclear | Low | High | Low | Unclear | Low |
| Kahn et al.52 | 2018 | Low | Low | Low | Low | Low | Low |
| Kang et al.53 | 2018 | Low | Low | High | Low | Low | Low |
| Kang et al.54 | 2019 | Low | Low | High | Low | Low | Low |
| Kataria et al.55 | 2019 | Low | Low | High | Low | Low | Low |
| Keller et al.56 | 2019 | Low | Low | Unclear | Low | Low | Unclear |
| Khashan et al.57 | 2016 | Low | Low | Low | Low | Low | Low |
| Kim et al.58 | 2019 | Low | Low | Low | Low | Low | Low |
| Ko et al.59 | 2013 | Low | Low | High | Low | Low | Low |
| Koltka et al.60 | 2011 | Low | Unclear | High | High | Low | High |
| Kraeutler et al.61 | 2015 | Unclear | High | High | High | High | Low |
| Lee et al.62 | 2015 | Low | Low | High | Unclear | Unclear | Low |
| Lee et al.62 | 2012 | Low | Low | High | Low | Low | High |
| Lierz et al.63 | 2012 | Low | Low | High | Low | Low | Low |
| Lu et al.64 | 2017 | Low | Low | Low | Low | Low | Low |
| Lynch et al.65 | 2019 | Low | Low | Low | Low | Low | Low |
| Mahure et al.66 | 2017 | Low | Low | Low | Low | Low | Low |
| Mardani-Kivi et al.67 | 2016 | Low | Low | Low | Low | Low | Low |
| Mardani-Kivi et al.68 | 2013 | Low | Low | Low | Low | Low | Low |
| Marinković et al.69 | 2016 | Unclear | Unclear | High | Unclear | Unclear | High |
| McHardy et al.70 | 2020 | Low | Low | Low | Low | Low | Low |
| Merivirta et al.71 | 2012 | Low | Low | High | Low | Low | Low |
| Merivirta et al.71 | 2013 | Low | Low | High | Low | Unclear | Low |
| Mitra et al.72 | 2011 | Low | Low | Low | Low | Low | High |
| Moyano et al.73 | 2016 | Low | Low | Low | Low | Low | Low |
| Neuts et al.74 | 2018 | Low | Low | High | Low | Low | Low |
| Oh et al.75 | 2018 | Low | Low | High | Low | Low | Low |
| Premkumar et al.76 | 2016 | Low | Low | High | Low | Low | Low |
| Purcell et al.77 | 2019 | High | Low | Low | High | Low | Low |
| Reda et al.78 | 2016 | Low | Low | High | High | Low | Low |
| Sanel et al.79 | 2016 | Unclear | High | Low | Low | Low | Low |
| Saritas et al.80 | 2015 | Low | Low | High | Low | Low | Low |
| Sayin et al.76 | 2015 | Low | Low | Low | Unclear | Unclear | High |
| Schwartzberg et al.81 | 2013 | Low | Low | High | Low | Low | Low |
| Shlaifer et al.82 | 2017 | Low | Low | High | High | Low | Unclear |
| Spence et al.83 | 2011 | Low | Low | Low | Low | Low | Low |
| Syed et al.84 | 2018 | Low | Low | Low | Low | Low | Unclear |
| Thapa et al.66 | 2016 | Unclear | Low | High | Low | Low | High |
| Tompkins et al.85 | 2011 | Low | Low | High | Low | Low | High |
| Westergaard et al.86 | 2014 | Unclear | Low | High | Low | Low | Low |
| Wong et al.87 | 2016 | High | Low | Low | Low | Unclear | Low |
| Xing et al.88 | 2015 | Low | Low | Low | Low | Low | Low |
| Yun et al.89 | 2012 | Low | Low | High | High | High | High |
| Zhang et al.90 | 2014 | Low | Low | High | Low | Low | High |
| Zhou et al.91 | 2017 | Low | Low | Low | Low | Low | Low |
Fig 2.
Cochrane Risk of Bias tool categorical scores of all included randomized controlled trials (RCTs). Red denotes high risk. Blue denotes unclear. Green denotes low risk.
Shoulder Arthroscopy
There were 37 RCTs that assessed pain and opioid consumption after shoulder arthroscopy (Table 2); 18 of the RCTs assessed nerve blocks, while 3 evaluated localized injections, 3 assessed oral medications, and 3 evaluated nonpharmacological interventions. Also, 12 RCTs isolated patients that underwent rotator cuff repair, 2 RCTs isolated patients that underwent subacromial decompression, and 1 RCT isolated patients that underwent Bankart repair. Finally, 20 studies showed significant differences in postoperative pain, and 21 studies found significant differences in opioid consumption.
Table 2.
Treatment Provided, Patient Population, Postoperative Pain, and Postoperative Opioid Consumption Summarized from all Included RCTs Regarding Shoulder Arthroscopy
| Author, Publication Year | Level of Evidence | Surgical Procedure | Intervention | Treatment Groups | Dosage | Patients (n) | Age (years) | Post-Op Pain Differences | Post-Op Opioid Consumption Differences |
|---|---|---|---|---|---|---|---|---|---|
| Cabaton et al., 201937 | 1 | Rotator cuff repair | Nerve block | SCB: Supraclavicular nerve block | 100 mg levobupivacaine with clonidine | 52 | 57 | NRS scale: SCB = ISB, from 0 to 48 hours | Total morphine consumed: SCB < ISB, from 0 to 48 hours |
| ISB: Ultrasound-guided interscalene nerve block | 100 mg levobupivacaine with clonidine | 51 | 58 | ||||||
| Wong et al., 201687 | 2 | Rotator cuff repair | Nerve Block | .1%: Phrenic nerve block, .1% ropivacaine | Ultrasound-guided interscalene block with 20 mL of .1% ropivacaine | 18 | 48.3 | DVPRS: .1% = .2%, at 30 min and 1 hour | PACU fentanyl consumption, and codeine equivalents at 72 hours: .1% = .2%, in PACU. .1% > .2%, at 72 hours post-block |
| .2%: Phrenic nerve block, .2% ropivacaine | Ultrasound-guided interscalene block with 20 mL of .2% ropivacaine | 19 | 40.5 | ||||||
| Faria-Silva et al., 201644 | 1 | Rotator cuff repair | Nerve block | LA + CL: Brachial plexus block with ropivacaine and clonidine | 30 mL of .33% ropivacaine and .15 mg clonidine | 26 | 52 ± 11 | NRS: LA+CL=LA, from 6-24 hours | Doses of rescue analgesic: LA+CL=LA, total consumption |
| LA (local anesthetics): Brachial plexus block with ropivacaine | 30 mL of .33% ropivacaine | 24 | 54 ± 10 | ||||||
| Auyong et al., 201831 | 1 | Rotator cuff repair (90%) or Bankart repair (10%) | Nerve block | ISB: Interscalene nerve block | 15 mL of .5% ropivacaine | 63 | 54 ± 13 | NRS: ISB = SCB = SSB, in PACU and at 1 hour | Fentanyl consumption: ISB = SCB = SSB, in PACU and at 1 hour |
| SCB: Supraclavicular nerve block | 15 mL of .5% ropivacaine | 63 | 53 ± 14 | ||||||
| SSB: Suprascapular nerve block | 15 mL of .5% ropivacaine | 63 | 55 ± 14 | ||||||
| Kang 201853 | 2 | 85% rotator cuff repair, 11% Bankart repair, 4% other | Nerve block | Control | 50 mL of .9% normal saline | 18 | 47.8 ± 14.4 | VAS: DEX 2.0 < control only, at 12 hour. All groups are equal at 6 hours and 24 hours | Opioid Consumption: ∗DEX 2.0 < DEX 1.0, DEX .5, and control, at 24 hour. All groups are equal at 6 hour and 12 hour |
| DEX .5: Dexmedetomidine (DEX), .5 μg/kg | IV DEX .5 μg/kg added to 50 mL of .9% normal saline | 18 | 53.7 ± 13.6 | ||||||
| DEX 1.0: DEX- 1.0 μg/kg | IV DEX 1.0 μg/kg added to 50 mL of .9% normal saline | 18 | 49.7 ± 12.5 | ||||||
| DEX 2.0: DEX- 2.0 μg/kg | IV DEX 2.0 μg/kg added to 50 mL of .9% normal saline | 18 | 52.9 ± 10.5 | ||||||
| Kataria 201955 | 1 | 73% Bankart repair, 27% rotator cuff repair | Nerve block | A: Ultrasound-guided interscalene block (ISB) with dexmedetomidine (DXM) | 20 mL .5% ropivacaine + 2 mL saline containing DXM .5 mcg/kg | 30 | 30.1 ± 10.9 | VAS: DXA < DXM, at 24 hours DXM = DXA at 30 minutes, 1 hour, 2 hours, 3 hours, 6 hours, and 12 hours | Analgesic consumption: DXA < DXM, total consumption |
| A: Ultrasound-guided interscalene block (ISB) with dexamethasone (DXA) | 20 mL .5% ropivacaine + 2 mL saline containing DXA .5 mcg/kg | 30 | 30.2 ± 11.7 | ||||||
| Neuts 201874 | 1 | Rotator cuff repair + decompression (38%) and subacromial decompression (31%), other (31%) | Nerve block | Interscalene brachial plexus nerve block (ISBPNB) | 20 mL .75% ropivacaine | 50 | 54 ± 10 | NRS: ISBPNB < SSB/AX, from 0-8 hours ISBPNB = SSB/AX from 8-24 hours | Oxycodone Equivalents: ISBPNB < SSB/AX, from 0-8 hours ISBPNB = SBB/AX from 8-24 hours |
| Suprascapular + axillary nerve bock (SSB/AX) | 10 mL .75% ropivacaine | 48 | 51 ± 10 | ||||||
| Kim 201958 | 1 | Rotator cuff repair (48%), other (52%) | Nerve Block | Superior trunk block (STB) | 15 mL .5% bupivicaine | 62 | 51.5 | NRS: STB = ISBPNB, from 1-48 hours | Morphine Equivalents: STB = ISBPNB, from 0-48 hours |
| Interscalene brachial plexus nerve block (ISBPNB) | 15 mL .5% bupivicaine | 63 | 50 | ||||||
| Choromanski 201538 | 2 | Bankart repair (17%), superior labrum anterior and posterior repair (30%), arthroscopic rotator cuff repair (30%), other (23%) | Continuous Nerve Block | Continuous interscalene nerve block catheter with .125% bupivacaine | 400 mL .125% bupivacaine @ 6 mL/h | 14 | 54 ± 18.8 | VAS: Ropivacaine = bupivacaine, on postop day 1 | Oxycodone Equivalents: Ropivacaine = Bupivacaine, from 0 to 24 hours |
| Continuous interscalene brachial plexus nerve block catheter with .2% ropivacaine | 400 mL .2% ropivacaine @ 6 mL/h | 16 | 48.2 ± 17.7 | ||||||
| Abdallah et al., 202026 | 1 | Acromioplasty (29%), rotator cuff repair (22%), biceps tenodesis (11%), other (37%) | Nerve block | Interscalene block (ISB) | 15 mL .5% ropivacaine with epinephrine 1:200,000 | 69 | 40 ± 15 | VAS: ISB = SASB, from 0 to 24 hours | Morphine Equivalent Consumption: ISB < SASB, in PACU. ISB = SASB, until 24 hours |
| Subomohyoid anterior suprascapular block (SASB) | 15 mL .5% ropivacaine with epinephrine 1:200,000 | 67 | 46 ± 15 | ||||||
| Baessler et al., 202032 | 1 | Rotator cuff repair, with frequent concomitant biceps tenodesis (46%) and biceps tenotomy (46%) | Nerve block | LBD group: Liposomal bupivacaine (LB) + dexamethasone + conventional bupivacaine | 15 mL .5% bupivacaine, 10 mL (133 mg) LB, .4 mL (4 mg) dexamethasone, and 5 mL saline solution | 26 | 57.5 ± 8.8 | VAS: LBD<LB, day 3. All groups were similar at all other time points, days 1-4 | Oral Morphine Milligram Equivalents: LB = LBD, days 1-4. ∗LB < Control, day 2. LB < Control, day 3. LB < Control, day 2. ∗LB < Control, day 3 |
| LB group: Liposomal bupivacaine + conventional bupivacaine | 15 mL .5% bupivacaine, 10 mL (133 mg) LB, and 5.4 mL saline | 24 | 56.9 ± 9.6 | ||||||
| Control group: Conventional bupivacaine + dexamethasone | 30 mL .5% bupivacaine and .4 mL (4 mg) of preservative-free dexamethasone | 26 | 59.1 ± 9.0 | ||||||
| DeMarco 201141 | 1 | 83% bursectomy, 79% subacromial decompression, 32% rotator cuff repair, and other concomitant procedures | Nerve block | ISB: Preoperative interscalene nerve block | 30 mL of .5% ropivacaine | 28 | VAS: ∗ISB < Placebo, at 6 hr. ISB = Placebo, from 12-80 hours | Narcotic Pills Used: ISB = Placebo, from 6-80 hours | |
| Placebo | 100 mL saline solution | 25 | |||||||
| Ko 201359 | 2 | Acromioplasty | Nerve block | UG SSB: Ultrasound-guided suprascapular nerve block | 10 mL of .375% ropivacaine | 15 | 42.8 ± 14.3 | VAS: UG SSB < EG SSB + Blind SSB, at 4 hours. UG SSB = EG SSB = Blind SSB, from 24-72 hours | Morphine Consumption: EG SSB + UG SSB < Blind SSB, from 0 to 72 hours |
| EG SSB: Electrophysiology-guided suprascapular nerve block | 10 mL of .375% ropivacaine | 18 | 39.3 ± 15.3 | ||||||
| Blind SSB: Suprascapular nerve block using anatomic landmarks | 10 mL of .375% ropivacaine | 19 | 40.8 ± 15.8 | ||||||
| Bengisun et al., 201435 | 1 | Subacromial decompression | Nerve block | LE: Levobupivacaine + epinephrine | Interscalene block with 20 mL of 100 mg levobupivacaine (.5%) + 50 μg epinephrine | 25 | 50.4 ± 12.9 | VAS: ∗LED < LE, from 2 to 24 hours | Lornoxicam consumption: ∗LED < LE, at 24 hours |
| LED: Levobupivacaine + epinephrine + dexmedetomidine | Interscalene block with 20 mL of 100 mg levobupivacaine (.5%) + 50 μg epinephrine + 10 μg dexmedetomidine | 23 | 55.9 ± 8.5 | ||||||
| Jeske et al., 201149 | 2 | Subacromial decompression | Nerve Block vs. Subacromial injection | SSN: Suprascapular nerve block | 10 mL of 1% ropivacaine | 15 | 59.1 ± 6.1 | VAS: SSN < SAI + Placebo, at 6 hours. ∗SSN < SAI, from 24 to 48 hours. SSN = Placebo, from 24 to 48 hours. Placebo = SAI, at 6 hours. ∗Placebo < SAI, from 24 to 48 hours | Total analgesic consumption: SSN < SAI + Placebo, from 0 to 24 hours. SSN < Placebo, from 0 to 48 hours. SSN = Placebo, from 0 to 48 hours |
| SAI: Subacromial infiltration | 20 mL of 1% ropivacaine, soon after end of surgery | 15 | 62.9 ± 6.9 | ||||||
| Placebo | 10 mL of .9% saline solution | 15 | 63.6 ± 9.0 | ||||||
| McHardy et al., 202070 | 1 | Subacromial decompression or rotator cuff repair | Perineural vs intravenous nerve block | PN: Interscalene nerve block with perineural (PN) dexamethasone | Injectate mixture, 3 mL 1% ropivacaine, 1 mL .4% dexamethasone, 2 mL .9% saline | 90 | 51.6 | NRS: PN = IV, at 12 hours, 24 hours, and 7 days | Oral Morphine Equivalents: PN = IV, at 12 hours, 24 hours, and 7 days |
| IV: Interscalene nerve block with intravenous (IV) dexamethasone | IV infusion, 50 mL .9% saline (infusion bag), 1 mL .4% dexamethasone | 89 | 52.8 | ||||||
| Abdallah et al., 201624 | 1 | Rotator cuff repair + acromioplasty (35%), acromioplasty (24%), Bankart repair (23%), other (18%) | IV vs Perineural vs Placebo nerve block | PN: Perineural dexmedetomidine with single-injection interscalene nerve block | .5 μg/kg dexmedetomidine + 15 mL ropivacaine .5% | 33 | 42 | VAS: PN < IV < Placebo, at 30 min. PN = IV from 60 min to 14 days | Morphine Equivalents: PN and IV < Placebo, at 8 hours, and for cumulative 24-hour consumption. PN and IV = Placebo from 24 hours to 14 days. PN = V, at all time points |
| IV: Intravenous Dexmedetomidine with single-injection interscalene nerve block | .5 μg/kg dexmedetomidine IV | 34 | 36.1 | ||||||
| Placebo: Saline with single-injection interscalene nerve block | Saline | 32 | 38 | ||||||
| Kahn et al., 201852 | 1 | Shoulder Arthroscopy | IV vs. Perineural nerve block | IV: Dexamethasone with interscalene nerve block | 1 mg IV dexamethasone | 62 | 47 ± 15 | NRS: IV=PN for ISBPNB, in PACU and on days 2 and 3 | Morphine Equivalents: IV=PN for ISBPNB, on days 0, 2, and 3 |
| PN: Perineural dexamethasone with interscalene nerve block | 30 mL bupivacaine .5% and 2 mL (2 mg) dexamethasone | 63 | 50 ± 14 | ||||||
| Aksu et al., 201528 | 1 | Rotator cuff repair and acromioplasty (67%), Bankart repair (20%), other (13%) | Nerve block vs. local analgesic injection | IBSP: Interscalene brachial plexus nerve block | 20 mL of .25% bupivacaine | 20 | 45.1 ± 15.5 | VAS: IBSP < Ia < Control, from 0 to 6 hours. All groups are equal from 12 to 24 hours | Morphine Equivalents: IBSP < Ia < Control, from 0 to 24 hour |
| Ia: Intra-articular injection | 20 mL of .25% bupivacaine, at the end of surgery | 20 | 44.2 ± 15.9 | ||||||
| Control: No block or intra-articular injection | 20 | 43.4 ± 13.5 | |||||||
| Merivirta et al., 201371 | 1 | 54% acromioplasty, 46% rotator cuff repair | Subacromial Catheter | Bupivacaine | Continuous infusion of 5 mg/mL bupivacaine, at 2 mL/hr | 39 | 53 ± 9 | NRS: Bupivacaine = Saline, from 0 to 12 hours. Bupivacaine < Saline, at 18 hours. Bupivacaine = Saline, on days 1 and 3 | Opioid and codeine consumption: Bupivacaine < Saline, on days 0 and 1. ∗Bupivacaine < Saline, on day 2. Bupivacaine = Saline, on day 3 |
| Saline | Continuous infusion of 9 mg/mL saline, at 2 mL/hr | 43 | 55 ± 6 | ||||||
| Schwartzberg and Reuss 201381 | 1 | Rotator cuff repair | Subacromial Catheter | Catheter with bupivacaine | Postoperative infusion catheter with 200 mL of .5% bupivacaine without epinephrine | 32 | 56 | VAS: No catheter < Catheter with saline solution, immediately after surgery. No catheter = Catheter with bupivacaine, and Catheter with bupivacaine = No catheter, immediately after surgery. All groups are equal, from 1 to 12 hour | Oxycodone consumption: All groups are equal, days 0-4 |
| Catheter with saline solution | Postoperative infusion catheter with 200 mL of sterile saline solution | 29 | 56 | ||||||
| No catheter | 27 | 58 | |||||||
| Kang et al., 201954 | 1 | 73% rotator cuff repair, 20% Bankart repair, 8% other | IV Injection | Control: saline | Intravenous .9% saline injection with interscalene nerve block, prior to surgery | 22 | 46.3 ± 16.6 | VAS: Control = D1 = D2, at 6 hours. D1 + D2 < Control, at 12 hours. D2 < Control + D1, from 18 to 24 hours | Morphine Equivalents: D1 and D2 < Control, from 12-24 hours. D2 < D1 from 18 to 24 hours. Control = D1 and D2, at 6 hours |
| D1: Dexamethasone | Intravenous dexamethasone .11 mg/kg with interscalene nerve block, prior to surgery | 22 | 46.1 ± 17.0 | ||||||
| D2: Dexamethasone + dexmedetomidine | Coadministered intravenous dexamethasone .11 mg/kg + intravenous dexmedetomidine, with interscalene nerve block, prior to surgery | 22 | 47.4 ± 13.5 | ||||||
| Bjørnholdt et al., 201436 | 2 | Subacromial decompression and/or acromioclavicular joint resection | Intravenous medication | D40: 40 mg Dexamethasone | 40 mg dexamethasone intravenously, preoperatively | 25 | 53 ± 10 | NRS: D40 = D8 = Placebo, from surgery until day 3 | Total analgesic consumption: D40 = D8 = Placebo, from surgery until day 3 |
| D8: 8 mg dexamethasone | 8 mg dexamethasone intravenously, preoperatively | 26 | 55 ± 11 | ||||||
| Placebo | Placebo infused, preoperatively | 22 | 49 ± 11 | ||||||
| Oh et al., 201875 | 1 | Shoulder Arthroscopy | Intravenous Patient-Controlled Analgesia (PCA) | Nefopam | PCA provided once awake, of 120 mg nefopam, 20 μg/kg fentanyl, and 16 mg ondansetron | 46 | 53.3 ± 12.8 | VAS and NRS: Nepofam = Ketorolac, from 10 min to 48 hours | Total PCA: Nepofam = Ketorolac, from 10 min to 24 hours |
| Ketorolac | PCA provided once awake, of 2 mg/kg ketorolac, 20 μg/kg fentanyl, and 16 mg ondansetron | 46 | 51.9 ± 11.5 | ||||||
| Yun et al., 201289 | 1 | Rotator cuff repair, with SLAP lesion in 62% and biceps tear in 17% | IV PCA vs. Subacromial PCA | SA-PCA: Subacromial patient-controlled analgesia | 150 mL of .5% ropivacaine, infused at 2 mL/hour, for hours 0-48 postoperatively | 30 | 54.1 ± 11.6 | VAS: ∗SA-PCA < IV-PCA, at 1 hour. SA-PCA = IV-PCA, from 4 to 48 hours | Rescue boluses received: SA-PCA = IV-PCA, from 1 to 48 hours |
| IV-PCA: Intravenous patient-controlled analgesia | Fentanyl (.3-.5 μg/kg/mL), keterolac (.03-.05 mg/kg/mL), and ondansetron (.08 mg/mL), infused at 1 mL/hr | 30 | 51.5 ± 17.4 | ||||||
| Merivirta et al., 201371 | 1 | 50% acriomioplasty, 50% rotator cuff repair | Patch vs infusion | Fentanyl | 12 μg/hour fentanyl patch for 72 hours, with 4 mL/hr saline infusion in a subacromial manner, for 72 hours | 30 | 52 ± 9 | NRS: Fentanyl = Bupivacaine, from immediately after surgery to day 90 | Rescue Analgesics Used: Fentanyl = Bupivacaine, from recovery room to day 3 |
| Bupivacaine | 2.5 mg/mL bupivacaine infusion in a subacromial manner, with placebo patch, for 72 hours | 30 | 54 ± 9 | ||||||
| Khashan et al., 201657 | 2 | Rotator cuff repair | Intra-articular injection | M: Morphine | 20 mg/10 mL morphine, 20 minutes before surgery | 15 | 50.7 ± 2.4 | NRS: M < KM + S, on ward. M + S < KM, in PACU. M < KM < S. from 1 to 2 weeks. All groups are equal at 3 months | Morphine Equivalents until Discharge, Number of Paracetamol and Oxycodone Capsules Consumed for Weeks 1 and 2: M = KM = S, from 0 to 2 weeks |
| KM: Ketamine + morphine | 50 mg ketamine + 10 mg/10 mL morphine, 20 minutes before surgery | 15 | 57.7 ± 2.4 | ||||||
| S: Saline | .9% 10 mL saline, 20 minutes before surgery | 15 | 54.1 ± 2.6 | ||||||
| Saritas et al., 201580 | 1 | Rotator cuff repair | Intra-articular injection | Magnesium | 1,000 mg magnesium sulfate (100 mg/mL) intra-articularly in 10 mL saline, at end of surgery | 30 | 39.8 ± 9.2 | VAS: Magnesium < Control, from 1 to 12 hours. Magnesium = Control, from 18 to 24 hours | Total PCA Morphine: ∗Magnesium < Control, total consumption |
| Control | 10 mL IV saline, at end of surgery | 30 | 41.6 ± 10.4 | ||||||
| Lee et al., 201562 | 1 | Rotator cuff repair | Local analgesic injection | GJ: Glenohumeral joint injection | 20 mL bupivacaine + 10 mL lidocaine, postoperatively | 40 | 57.2 | VAS: GJ = SS = GJ + SS, from 20 min to 24 hours | Boluses of Rescue Analgesic: GJ = SS = GJ + SS, from 1 to 24 hours |
| SS: Subacromial space injection | 20 mL bupivacaine + 10 mL lidocaine, postoperatively | 42 | 58.1 | ||||||
| GJ+SS: Glenohumeral joint + subacromial space injection | 10 mL bupivacaine + 5 mL lidocaine in each of the two injection sites, postoperatively | 39 | 58.6 | ||||||
| Lu et al., 201764 | 1 | Shoulder arthroscopy | Infusion | SD: Sufentanil + dexmedetomidine | .04 μg/kg/h Sufenanil + .06 μg/kg/h Dexmedetomidine, postoperatively | 75 | 65.5 ± 5.3 | VAS: SD < S from 6 to 48 hours | Amount of Rescue Analgesia, and Analgesic Liquid Pump Volume: SD < S, from 24 to 48 hours. SD = S, from 1 to 3 hours |
| S: Sufentanil only | .04 μg/kg/h Sufenanil, postoperatively | 76 | 65 ± 5.8 | ||||||
| Spence et al., 201183 | 1 | Shoulder arthroscopy | Oral medication | Gabapentin | 300 mg Gabapentin 1 hour before surgery, then twice a day for 2 days after surgery. Interscalene nerve block also used. | 26 military patients | 31.8 ± 10.48 | NRS: Gabapentin = Control, on days 1 and 2 | Morphine Equivalents: Gabapentin = Control, on days 1 and 2 |
| Control | Placebo 1 hour before surgery, then twice a day for 2 days after surgery. Interscalene nerve block also used. | 31 military patients | 31.51 ± 8.9 | ||||||
| Ahn et al., 201627 | 1 | Bankart repair (25%) and rotator cuff repair (75%) | Oral medication | Pregabalin | 1 150-mg Pregabalin capsule, 1 hour before anesthesia induction | 30 | 55 ± 9 | NRS: Pregabalin < Control, from 6 to 48 hours. Pregabalin = Control, in PACU | Fentanyl Consumption: Pregabalin < Control, 0-6 hours and 0-48 hours. ∗Pregabalin < Control, 24-48 hours. Pregabalin = Control, total consumption and 6-24 hours |
| Control | Placebo capsule, 1 hour before anesthesia induction | 30 | 51 ± 12 | ||||||
| Mardani-Kivi et al., 201667 | 1 | Bankart repair | Oral medication | Gabapentin | 1 600-mg Gabapentin capsule, 2 hours before surgery | 38 | 30.2 ± 5.0 | VAS: Gabapentin = Placebo | Pethidine Consumption: Gabapentin < Placebo, from 6 to 24 hours |
| Placebo | Identical placebo capsule, 2 hours before surgery | 38 | 28.3 ± 4.4 | ||||||
| Cho et al., 201139 | 1 | Rotator cuff repair | Multimodal protocol | Multimodal pain control | Preoperative written and oral education + pre-op prophylactic oral medication + intra-op 50 mL cocktail of local analgesics | 40 | 57.6 ± 8.2 | VAS: Multimodal = IV PCA, days 1 and 2. Multimodal < IV PCA, immediately after surgery. ∗Multimodal < IV PCA, days 3-5 | Analgesic Consumption: Multimodal < IV PCA, days 0-5 |
| Intravenous patient-controlled analgesia (IV PCA) | Individualized doses of fentanyl, ketorolac, and ondansteron HCl | 30 | 55.1 ± 7.5 | ||||||
| Kraeutler et al., 201561 | 1 | All had rotator cuff repair and/or subacromial decompression, with distal clavicle excision (41%) and biceps tenodesis (37%) as most common concomitant procedures | Nonpharmacological intervention | CC: Postoperative compressive cryotherapy | Used cryotherapy device every other hour for days 0-2 postsurgery, then 2-3 times per day for an hour on days 3-7 postsurgery | 25 | 55.4 | VAS: CC = IW, from 4 to 6 hours and on days 1-7 | Morphine Equivalents: CC = IW, from days 1-7 |
| IW: Postoperative standard ice wrap | Used standard ice wrap every other hour for days 0-2 postsurgery, then 2-3 times per day for an hour on days 3-7 postsurgery | 21 | 55.8 | ||||||
| Mahure et al., 201766 | 2 | Rotator Cuff Repair | Non-pharmacological intervention | Active transcutaneous electrical nerve stimulation (TENS) | Continuous frequency of 150 pps with pulse duration of 150 microseconds, active for 30 seconds then ramp down for 15 seconds. Use TENS unit 4 sessions/day, 45 minutes/session, through first postoperative week | 21 | 60.5 ± 11.1 | VAS: TENS < Placebo, from 12 to 48 hours, and from days 3 to 7 | Percocet pills used: TENS < Placebo, on days 2 and 7 |
| Placebo TENS | 16 | 56.4 ± 12.2 | |||||||
| Syed et al., 201884 | 1 | Rotator cuff repair | Nonpharmacological intervention | No pre-op opioid education | No video or handout | 66 | 58.0 ± 9.4 | VAS: ∗Pre-op Education < No Pre-op Education from 2 to 6 weeks. Pre-op Education = No Pre-op Education, at 3 months | Percocet pills used: Pre-op education < No Pre-op Education, from 6 weeks to 3 months. Pre-op Education = No Pre-op Education, at 2 weeks |
| Pre-op opioid education | 2-minute narrated video with handout detailing the risks of narcotic overuse and abuse | 68 | 59.2 ± 9.2 |
Findings with a P value <.01 are marked with an asterisk (∗) and findings with a P value <.001 are in bold.
VAS, visual analog scale; NRS, numeric pain rating scale, DVPRS, defense and veterans pain rating scale, PACU, post-anesthesia care unit.
Rotator Cuff Repair
Four studies evaluated nerve blocks for rotator cuff repair specifically.32, 37, 44, 87 Cabaton et al.37 found that a supraclavicular nerve block with 100 mg levobupivacaine and clonidine provided similar pain management but much less opioid consumption from 0 to 48 hours postoperatively compared to an ultrasound-guided interscalene nerve block with the same dosages. Wong et al.87 shows that an interscalene block with 20 mL of .2% ropivacaine decreases opioid consumption at 72 hours postoperatively compared to the same block with only .1% ropivacaine. Faria-Silva et al.44 found that adding .15 mg clonidine to a brachial plexus block with .33% ropivacaine does not affect pain or opioid consumption within the first day postop. Lastly, Baessler et al.32 found that the addition of liposomal bupivacaine in an interscalene nerve block leads to less opioid consumption for several days after surgery. Baessler et al.32 also showed that providing dexamethasone with liposomal bupivacaine and conventional bupivacaine may decrease postoperative pain, while providing comparable opioid consumption. However, this difference in pain was only observed at postoperative day 3, and not on days 1, 2, or 4.
Three studies evaluated injections for rotator cuff repair.57, 62, 80 Khashan et al.57 found that adding 50 mg ketamine to a preoperative intra-articular injection of morphine provided worse pain relief than morphine alone from 0-2 weeks postoperatively, while morphine also provides better pain relief than a saline control from 0 to 2 weeks. However, all three groups had comparable pain relief at 3 months, and no differences in opioid consumption. Saritas et al.80 found that an intra-articular injection with 1,000 mg of magnesium sulfate decreases pain from postoperative hours 1-12, while also significantly decreasing opioid consumption. Lastly, Lee et al.62 showed that local analgesic injections of bupivacaine and lidocaine used in the glenohumeral joint, in the subacromial space, and in both spaces all provide similar pain relief and opioid consumption.
Knee Arthroscopy
Twenty-eight RCTs assessed pain and opioid consumption after knee arthroscopy (Table 3). Eleven studies evaluated various types of nerve blocks, while 8 compared local injections and 2 assessed nonpharmacological intervention. Eleven RCTs isolated patients that underwent anterior cruciate ligament (ACL) reconstruction, and 5 isolated patients that underwent meniscectomy. Of these studies, 19 studies showed significant differences in postoperative pain, while 19 studies also found significant differences in opioid consumption.
Table 3.
Treatment Provided, Patient Population, Postoperative Pain, and Postoperative Opioid Consumption Summarized from all Included RCTs Regarding Knee Arthroscopy
| Author, Publication Year | Level of Evidence | Surgical Procedure | Intervention | Treatment Groups | Dosage | Patients (n) | Age (years) | Post-Op Pain Differences | Post-Op Opioid Consumption Differences |
|---|---|---|---|---|---|---|---|---|---|
| Abdallah et al., 201624 | 1 | Unilateral ACL Reconstruction | Nerve block | A: Adductor canal block (ACB) | 20 mL .5% ropivacaine (with epinephrine) | 52 | 31.6 | VAS: ACB = FNB, from 30 min to 24 hours | Oral Morphine Equivalents: ACB = FNB, at 24 hours |
| B: Femoral nerve block (FNB) | 20 mL .5% ropivacaine (with epinephrine) | 48 | 33.3 | ||||||
| Abdallah et al., 2019 | 1 | ACL reconstruction | Nerve block | Proximal adductor canal block | 20 mL of 1:1 ropivacaine .5% and Lidocaine 2% with epi 1:200,000 | 34 | 30 | VAS: ∗Proximal Adductor Canal Block < Mid < Distal, from PACU-6 hr. All groups are equal from 12 to 24 hours | Total Morphine and Morphine Equivalents Consumed: Proximal Adductor Canal Block < Mid + Distal, until discharge and from 1 to 24 hours |
| Mid adductor canal block | 20 mL of 1:1 ropivacaine .5% and Lidocaine 2% with epi | 38 | 31 | ||||||
| Distal Canal Block | 20 mL of 1:1 ropivacaine .5% and Lidocaine 2% with epi | 36 | 29 | ||||||
| Thapa et al., 201692 | 1 | ACL reconstruction | Nerve block | Continuous Adductor Canal Block (ACB) | .5% ropivacaine @ 2.5 mL/hr | 25 | 25.2 ± 6.4 | VAS: Intermittent ACB < Continuous ACP, from 4 to 12 hours. Intermittent ACB = Continuous ACB, at 2 and 24 hours | Cumulative Oral Morphine Equivalents: Intermittent ACB < Continuous ACB, total consumption |
| Intermittent ACB | .5% ropivacaine, every 6 hours | 25 | 27.7 ± 7.2 | ||||||
| Lynch et al., 201965 | 1 | ACL Reconstruction, with concomitant partial meniscectomy (35%) or meniscal repair (10%) | Nerve block | Adductor canal block (ACB) | 20 mL .5% ropivacaine | 30 | 21.2 ± 4.2 | VAS: ACB=FNB, from 0 hours to 3 days | Morphine Equivalents: ACB < FNB, from 0 to 4 hours. ACB = FNB, 4 hours to 3 days |
| Femoral nerve block (FNB) | 30 mL .5% ropivacaine | 30 | 21.5 ± 5.4 | ||||||
| Bailey et al., 201933 | 1 | ACLR with patellar tendon autograft, concomitant meniscal repair in 78% | Nerve block | Femoral nerve blockade (FNB) | 30 mL of 0.2% ropivacaine with 100 mcg clonidine | 38 | 24.4 ± 8.8 | NRS: FNB = ACB, until discharge | Morphine Equivalents: FNB = ACB, until discharge |
| Adductor canal nerve blockade (ACB) | 15 mL of 0.2% ropivacaine with 100 mcg clonidine | 40 | 21.0 ± 7.3 | ||||||
| Espelund et al., 201442 | 1 | Minor arthroscopic knee surgery | Nerve block | Ropivacaine | 30 mL of 7.5 mg/mL ropivacaine | 36 | 46 ± 14 | VAS: Ropivacaine=control, from 0 to 24 hours | Total opioid consumption: Ropivacaine < control, from 0 to 2 hours. Ropivacaine = control, from 2 to 24 hours. |
| Control | 30 mL of isotonic saline | 35 | 43 ± 14 | ||||||
| Espelund et al., 201443 | 1 | 50% ACL reconstruction, 50% other major arthroscopic knee surgery | Nerve block | Ropivacaine | 30 mL of 7.5 mg/mL ropivacaine, 30 mL isotonic saline 45 minutes later | 25 | 38 ± 12 | VAS: Ropivacaine < control, from 15-45 min. Ropivacaine = control, from 60-90 min | Sufentanil consumption: Ropivacaine = control, from 0 to 90 min |
| Control | 30 mL of isotonic saline, 30 mL of 7.5 mg/mL ropivacaine 45 minutes later | 25 | 34 ± 14 | ||||||
| Hanson et al., 201346 | 1 | Medial meniscectomy | Nerve block | ACB: Ultrasound-guided adductor canal block | 15 mL of .5% ropivacaine with 1:400,000 epinephrine | 24 | 54 ± 11 | NRS: ACB < sham, in PACU, at discharge, and from 12 to 24 hr. ACB = sham, at 6 hours | Oral Morphine Equivalents: ACB < Sham, over 24 hours |
| Sham: Ultrasound-guided sham injection | 2 mL normal saline | 24 | 51 ± 11 | ||||||
| Hsu et al., 201348 | 1 | 87% soft-tissue (meniscectomy, meniscal repair), 26% single osseous, 24% multiple osseous procedures | Nerve block | INF: Block of infrapatellar branch of saphenous nerve | 10 mL of .25% bupivacaine | 33 | 51.7 ± 12.1 | NRS: INF < Placebo, immediately postoperatively, at 1 hour, and on arrival at home. INF = Placebo, from 2 to 4 hours postoperatively, and 0-24 hours after arriving home | IV Ketorolac, Hydrocodone, and Fentanyl, oral Hydrocodone, and Total Oral Morphine Equivalents: INF = Placebo, 0-48 hours |
| Placebo | Saline solution | 32 | 49.6 ± 14.1 | ||||||
| Westergaard et al., 201486 | 1 | 61% synovectomy, 53% meniscectomy, 37% chondrosectomy, with other concomitant procedures | Nerve block | Ropivacaine | 20 mL of .75% ropivacaine prepared – 7.5 mL around the saphenous nerve and 7.5 mL around the posterior branch of obturator nerve | 29 | 31 | NRS: ropivacaine = Saline, from 0 to 24 hours | Morphine consumed: ropivacaine = Saline, from 0 to 24 hours |
| Saline | 20 mL of isotonic saline prepared – 7.5 mL around the saphenous nerve and 7.5 mL around the posterior branch of obturator nerve | 30 | 42 | ||||||
| Marinković et al., 201669 | 1 | Knee arthroscopy | Peripheral nerve block | GA: General anesthesia | ‒‒ | 30 children | 13.5 ± 3.2 | Wong Baker Faces Scale: PNB + GA < GA, from 2 to 12 hours | Morphine Equivalents: PNB + GA < GA, until discharge |
| PNB+GA: Peripheral nerve block + general anesthesia/sedation | 1 mL/kg of .25% or.33% levobupivacaine administered with ultrasound guidance. 43% femoral, obturator, ischiatic block; 33% femoral, obturator block; 23% femoral, ischiatic block | 30 children | 15.2 ± 1.6 | ||||||
| Keller et al., 201956 | 1 | ACL reconstruction | Nerve block vs nerve block + local injection | Femoral Nerve Block (FNB) | 20 mL .5% Bupivacaine without epi | 21 | 35.1 | VAS: ∗FNB + PCI < FNB, at until discharge and at 1 hour. FNB + PCI = FNB, at 20 min and days 1-4 | Number of Vicodin Pills Used: FNB + PCI < FNB, on day 4 |
| FNB + Posterior capsule injection (PCI) | FNB + 20 mL .5% Bupivacaine without epi in posterior capsule, injected before drilling femoral tunnel | 21 | 32.5 | ||||||
| Moyano et al., 201673 | 1 | 35% ACL repair, 28% multiple procedures, 24% meniscectomy, 13% other | IV injection | DM: Dexamethasone | 2 mL of a 5 mg/mL dexamethasone phosphate solution, during anesthetic induction | 37 | 39.9 | VAS: DM > S, at 4 hours. DM = S, in PACU, and at 8 and 12 hours | Number of Codeine Tablets Taken: DM = S, from 0 to 48 hours |
| S: Saline | 2 mL of .9% normal saline, during anesthetic induction | 41 | 44.3 | ||||||
| Amin et al., 201129 | 2 | ACL reconstruction | Intraarticular patient-controlled analgesia (PCA) | RMX: Morphine + ropivacaine + xefocam mixture | PCA of .25% ropivacaine, .2 mg/mL morphine, 1 mg/mL xefocam (Lornoxicam) | 15 | 32 ± 3 | VAS: RMX = RM = C, at 4 hr. RMX < RM + C, at 8-16 hours. RMX < RM < C, at 24 hours | Rescue IV Morphine: RMX < RM, at 24 hours. ∗RM < C, at 24 hours |
| RM: Ropivacaine + morphine mixture | PCA of .25% ropivacaine, .2 mg/mL morphine | 15 | 27 ± 3 | ||||||
| Control: No drug | ‒‒ | 15 | 35 ± 3 | ||||||
| Sanel et al., 201679 | 1 | Isolated partial meniscectomy | Intra-articular injection | TEN: tenoxicam with bupivacaine | 22 mL of .5% bupivacaine 100 mg + tenoxicam 20 mg, after the surgery and before tourniquet deflation | 120 | 36 | VAS and NRS: TEN < MOR, at 12 hours. TEN = MOR, at 1, 2, 4, 6, and 24 hours | Total Analgesic: TEN < MOR, at 24 hours |
| MOR: morphine with bupivacaine | 22 mL of .5% bupivacaine 100 mg + morphine 2 mg, after the surgery and before tourniquet deflation | 120 | 40 | ||||||
| Arti and Mehdinasab, 201130 | 1 | ACL reconstruction | Intra-articular injection | Morphine | At end of procedure: 9.5 mL bupivacaine + 5 mg morphine | 30 | 31.5 ± 5.9 | VAS: Morphine < all other groups, 0-12 hours after surgery. All other groups < Placebo, 0-12 hours after surgery | Morphine Equivalents: Morphine + Methadone < Pethidine + Tramadol < Placebo, 0-12 hours after surgery |
| Methadone | At end of procedure: 9.5 mL bupivacaine + 5 mg methadone | 30 | 28.9 ± 7.6 | ||||||
| Pethidine | At end of procedure: 9.5 mL bupivacaine + 37.5 mg pethidine | 30 | 26.8 ± 7.8 | ||||||
| Tramadol | At end of procedure: 9.5 mL bupivacaine + 100 mg tramadol | 30 | 27.5 ± 7.4 | ||||||
| Placebo | At end of procedure: 9.5 mL bupivacaine + .5 mL normal saline | 30 | 28.6 ± 5.3 | ||||||
| Mitra et al., 201172 | 1 | 70% ACL repair, 20% diagnostic arthroscopy, 10% other | Intra-articular injection | Tramadol | 30 mL .25% bupivacaine + 1 mL (50 mg) tramadol, at end of surgery | 20 | 31.65 ± 12.86 | VAS: Fentanyl < Tramadol + Saline, from 0 to 8 hr. ∗Tramadol < Saline, from 0 to 8 hours | Total Analgesic: Fentanyl + Tramadol < Saline, from 0 to 8 hours |
| Fentanyl | 30 mL .25% bupivacaine + 1 ML (50 μg) fentanyl, at end of surgery | 20 | 26.55 ± 8.02 | ||||||
| Saline | 30 mL .5% bupivacaine + 1 mL normal saline, at end of surgery | 20 | 28.05 ± 10.76 | ||||||
| Kager et al., 201150 | 2 | 75% meniscus resection and cartilage smoothing, 19% cartilage smoothing only, and 6% cruciate ligament repair | Intra-articular injection | 5 mg labetalol | 20 mL intra-articularly with 5 mg labetalol, at end of surgery | 21 | 48.0 ± 3.5 | VAS and VRS: 5 mg = 2.5 mg = Placebo, from 30 min to 24 hours | Morphine Consumption: Placebo + 5 mg < 2.5 mg, from 30 min to 24 hours. Placebo < 5 mg, from 30 min to 1 hour, and from 4 to 24 hours. Placebo = 5 mg, from 2 to 3 hours |
| 2.5 mg labetalol | 20 mL intra-articularly with 2.5 mg labetalol, at end of surgery | 18 | 41.4 ± 3.9 | ||||||
| Placebo | 20 mL intra-articularly with normal saline, at end of surgery | 24 | 49.0 ± 2.5 | ||||||
| Koltka et al., 201160 | 1 | Meniscectomy | Intra-articular injection | Magnesium | 500 mg magnesium sulfate intra-articularly in 20 mL saline, before tourniquet deflation | 30 | 48.4 ± 11 | NRS: All groups are equal at 1 hour ∗Lornoxicam < Placebo, at 2 hours. Levobupivacaine < Placebo, at 2 hours. Magnesium = Placebo, at 2 hours. Magnesium + Levobupivacaine + Lornoxicam < Placebo, at 4 hours. All groups are equal from 12 to 48 hours | Tramadol Consumption and Number of Pills Consumed: ∗Lornoxicam < Placebo, from 0 to 4 hr. Magnesium + Levobupivacaine < Placebo, from 0 to 4 hr. ∗Lornoxicam < Placebo, from 0 to 48 hr. Magnesium = Levobupivacaine = Placebo, from 0 to 48 hours. Lornoxicam < Magnesium, from 0 to 24 hours |
| Levobupivacaine | 100 mg levobupivacaine (.5%) of 20 mL local anesthetic, before tourniquet deflation | 30 | 50.6 ± 12 | ||||||
| Lornoxicam | 8 mg lornoxicam intra-articularly in 20 mL saline, before tourniquet deflation | 30 | 42.5 ± 9.7 | ||||||
| Placebo | 20 mL saline intra-articularly, before tourniquet deflation | 30 | 46 ± 15.6 | ||||||
| Lee et al., 201293 | 2 | Partial meniscectomy | Intrathecal injection | 10 HM: 10 μg hydromorphone | Spinal anesthesia with: 10 μg hydromorphone + 1.2 mL (6 mg) of .5% hyperbaric bupivacaine, in .05 mL isotonic saline, prior to surgery | 15 | 36.3 ± 12.3 | VAS: All groups are equal, at 30 min and 2 hours 2.5 HM, 5 HM, and 10 HM < Control, from 4-6 hours. 5 HM and 10 HM < Control, at 12 hours. 2.5 HM = Control, at 12 hours. All groups are equal, at 24 hours | Number of required analgesic injections: 5 HM and 10 HM < Control and 2.5 HM, 0-24 hours |
| 5 HM: 5 μg hydromorphone | Spinal anesthesia with: 5 μg hydromorphone + 1.2 mL (6 mg) of .5% hyperbaric bupivacaine, in .05 mL isotonic saline, prior to surgery | 15 | 36.5 ± 15.1 | ||||||
| 2.5 HM: 2.5 μg hydromorphone | Spinal anesthesia with: 2.5 μg hydromorphone + 1.2 mL (6 mg) of .5% hyperbaric bupivacaine, in .05 mL isotonic saline, prior to surgery | 15 | 38.9 ± 12.4 | ||||||
| Control | Spinal anesthesia with: 6 mg hyperbaric bupivacaine in .05 mL isotonic saline, prior to surgery | 15 | 39.9 ± 13.7 | ||||||
| Sayin et al., 201576 | 1 | Meniscopathic knee surgery | Local anesthesia | C: Control | No description | 20 | 30.2 ± 6.8 | VAS: ∗L+T and L + F < C and L, from 1 to 24 hours. Also ∗L + T < L + F, from 2 to 4 hours | Number of times needing post-op Analgesia: L + T and L + F < C and L, total doses |
| L: Levobupivacaine | 20 mL of Levobupivacaine 2.5 mg/mL, 7 mL before surgery and 13 mL at the end of surgery | 20 | 32.6 ± 7.0 | ||||||
| L+T: Levobupivacaine + Tramadol | 20 mL of Levobupivacaine 2.5 mg/mL + 50 mg Tramadol, 7 mL before surgery and 13 mL at the end of surgery | 20 | 36.2 ± 8.8 | ||||||
| L+F: Levobupivacaine + Fentanyl | 20 mL of Levobupivacaine 2.5 mg/mL + 50 mcg Fentanyl, 7 mL before surgery and 13 mL at the end of surgery | 20 | 34.7 ± 10.2 | ||||||
| Premkumar et al., 201698 | 2 | ACL reconstruction with quadriceps autograft | Surgical site injection | Local liposomal bupivacaine (LLB) | 40 mL suspension of 20 mL liposomal bupivacaine + 20 mL Saline, 30 mL injected into graft harvest site and 10 mL into superficial skin | 14 | NRS: LLB = LB, from PACU until day 6 | PACI IV Hydromorphone, PACE Fentanyl, and PACE Oxycodone Equivalents Consumed: LLB = LB, from PACU until day 6 | |
| Local bupivacaine (LB) | 40 mL suspension of 20 mL bupivacaine + 20 mL Saline, 30 mL injected into graft harvest site and 10 mL into superficial skin | 15 | |||||||
| Zhou et al., 201791 | 1 | Partial Meniscectomy | Oral medication | Celecoxib 4 hours post-op | 400 mg Celecoxib | 60 | 35.9 ± 6.6 | VAS: Very Early + Early < Post op Celecoxib, from 4 to 36 hours | Rescue analgesic consumed: Very Early + Early almost < Post-op Celecoxib (P = .06), from 24 to 48 hours |
| Celecoxib 1 hour Pre-op (early) | 400 mg Celecoxib | 62 | 36.0 ± 6.1 | ||||||
| Celecoxib 1 Day Pre-op (very early) | 400 mg Celecoxib | 60 | 34.7 ± 7.1 | ||||||
| Lierz et al., 201263 | 1 | Therapeutic knee arthroscopy | Oral medication | Etoricoxib | One tablet of 120 mg etoricoxib, 1 hour before anesthesia induction | 33 | 54 ± 10 | VAS: Etoricoxib < Placebo, at 0 hour and from 4 to 24 hours. Etoricoxib = Placebo, at 2 hours | Total morphine consumption: Etoricoxib < Placebo, from 2 to 24 hours |
| Placebo | One look-alike placebo tablet, 1 hour before anesthesia induction | 33 | 56 ± 14 | ||||||
| Mardani-Kivi et al., 201368 | 1 | 52% isolated ACL reconstruction (ACLR), 48% isolated partial meniscectomy | Oral medication | Celecoxib | 400 mg celecoxib, 2 hours prior to operation | 57 | ACL: 25.8 ± 7.7. Meniscectomy: 32.7 ± 8 | VAS: Celecoxib < Placebo, from 6 to 24 hours, for both ACLR and meniscectomy | Opioid (Pethidine) Consumption: Celecoxib < Placebo, from 6-24 hours, for meniscectomy only. ∗Celecoxib < Placebo, at 6 hours, for ACLR. Celecoxib < Placebo, at 24 hours |
| Placebo | Identical placebo, 2 hours prior to operation | 60 | ACL: 26.7 ± 4.9. Meniscectomy: 32.2 ± 9.8 | ||||||
| Tompkins et al., 201185 | 2 | ACL reconstruction | Oral medication | Postoperative Zolpidem (sleep-aid) with ibuprofen | 7 zolpidem tartrate tablets (10 mg), taken once a day for 7 days after surgery. Also 800 mg ibuprofen, taken every 8 hours as needed. | 6 | 36.9 | VAS: All groups are equal, days 0-7 | Number of Vicodin tablets: Zolpidem groups < Placebo groups, days 0-7. Ibuprofen did not affect opioid consumption. |
| Postoperative Zolpidem without ibuprofen | 7 zolpidem tartrate tablets (10 mg), taken once a day for 7 days after surgery. | 7 | |||||||
| Postoperative Placebo with ibuprofen | 7 gelatin pills, taken once a day for 7 days after surgery. Also 800 mg ibuprofen, taken every 8 hours, as needed. | 7 | 35.6 | ||||||
| Postoperative Placebo without ibuprofen | 7 gelatin pills, taken once a day for 7 days after surgery. | 9 | |||||||
| Reda et al., 201678 | 1 | ACL reconstruction with anatomical single-bundle technique | Nonpharmacological intervention | A: Tourniquet | Inflated to 350 mm mercury (64 ± 8.7 min) | 29 | 25.5 ± 4.0 | VAS: ∗No Tourniquet < Tourniquet, from 4 to 10 hours. Tourniquet = No Tourniquet, from 16 to 22 hours | Morphine Equivalent Consumption: ∗No Tourniquet < Tourniquet, until discharge |
| B: No tourniquet | Tourniquet not inflated; received intra-articular injection of 60 cc (250 cc saline + 10 mg morphine + 1 mg Adrenaline) | 29 | 25.0 ± 4.6 | ||||||
| Hartwell et al., 202094 | 1 | Knee arthroscopy with partial meniscal debridement | Nonpharmacological | Electronic prescription: automatically provided opioids with multimodal pain medications from pharmacist | 20 tablets of 5-mg oxycodone, automatically provided after surgery | 48 | 45.0 ± 12.3 | VAS: Electronic prescription = paper prescription, at 2, 24, and 48 hours, as well as at 7 and 21 days | Number of Pills Taken: Electronic prescription = paper subscription, total number of pills taken |
| Paper prescription: optional opioids, only if absolutely necessary for pain control | 20 tablets of 5-mg oxycodone, available if needed after surgery | 47 | 43.6 ± 12.8 |
Findings with a P value <.01 are marked with an asterisk (∗) and findings with a P value <.001 are in bold. NRS, numeric pain rating scale; PACU, postanesthesia care unit; VAS, visual analog scale.
ACL Reconstruction
Five studies assessed the adductor canal block for ACL reconstruction.24, 25, 33, 65, 92 Three studies compared the adductor canal block to the femoral nerve block,24,65,33 with all three studies finding no difference in pain relief, and only Lynch et al.65 finding a difference in opioid consumption, with the adductor canal block allowing for less opioid consumption from 0 to 4 hours postoperatively. Meanwhile, Abdallah et al.25 used an adductor canal block with 20 mL of 1:1 ropivacaine and Lidocaine at three different locations: proximal, middle, and distal. They found that using the proximal block significantly decreases pain from 0 to 6 hours postoperatively, while dramatically decreasing opioid consumption from 0 to 24 hours as well. Lastly, Thapa et al.92 showed that an intermittent adductor canal block with .5% ropivacaine provides similar pain relief, but dramatically decreased opioid consumption, relative to a continuous adductor canal block with the same dosage.
Meniscectomy
Three studies evaluated injections for meniscectomy.60, 79, 93 Sanel et al.79 found that adding 20 mg of tenoxicam to an intra-articular injection with .5% bupivacaine dramatically decreases both pain at 12 hours postoperatively and opioid consumption at 24 hours postoperatively, as opposed to adding 2 mg of morphine with the bupivacaine. However, pain was similar between the groups at 1, 2, 4, 6, and 24 hours postoperatively, so the dramatic difference in pain at 12 hours is not clear. Koltka et al.60 also evaluated intra-articular injections, but instead compared 500 mg magnesium, 100 mg of .5% levobupivacaine, 8 mg lornoxicam, and a saline placebo. All three intervention groups helped with pain relief and opioid consumption, with the only clear difference between intervention groups being that Lornoxicam decreased opioid consumption relative to magnesium from 0 to 24 hours postoperatively. Lastly, Lee et al.93 compared a control group with 2.5-, 5-, and 10-μg dosages of hydromorphone added to bupivacaine intrathecal injections, finding that all the intervention groups provided better outcomes than the control group, but no significant differences between the hydromorphone dosage groups.
Hip Arthroscopy
Eight RCTs assessed pain and opioid consumption after hip arthroscopy (Table 4): 3 studies evaluated various types of nerve blocks, while 1 compared nerve block to intra-articular injection, 2 assessed localized injections, and 2 evaluated oral medication. Four RCTs isolated patients that underwent femoroacetabular impingement surgery. Four studies showed significant differences in postoperative pain, and 2 studies found significant differences in opioid consumption.
Table 4.
Treatment Provided, Patient Population, Postoperative Pain, and Postoperative Opioid Consumption Summarized from All Included RCTs Regarding Hip Arthroscopy
| Author, Publication Year | Level of Evidence | Surgical Procedure | Intervention | Treatment Groups | Dosage | Patients (n) | Age (years) | Post-Op Pain Differences | Post-Op Opioid Consumption Differences |
|---|---|---|---|---|---|---|---|---|---|
| Behrends et al., 201834 | 1 | Femoroacetabular impingement | Nerve block | Fascia iliaca block (FI) | 40 mL .2% ropivacaine | 38 | 35 ± 11 | NRS: FI=Saline, from 0 to 24 hours | Morphine and Morphine Equivalent Consumption: FI = Saline, from 1 to 24 hours |
| Saline | Saline | 37 | 32 ± 9 | ||||||
| Xing et al., 201588 | 1 | Femoroacetabular impingement | Nerve block | Femoral nerve block (FNB) | 20 mL .5% bupivacaine | 27 | 32 ± 11 | VAS: FNB < Saline, at 6 hours. ∗FNB < Saline, from 30 min to 1 hour and from 2 to 4 hours. FNB < Saline, at 48 hours and day 7. FNB = Saline, at 90 minutes and 24 hours | Total morphine consumed: ∗FNB < Saline, from 24 to 48 hours. FNB = Saline, from 1-24 hours and from days 2-7 |
| Saline | Saline | 23 | 31 ± 8 | ||||||
| Purcell et al., 201977 | 1 | 96% labral repair, 93% pincer resection, 87% cam osteoplasty, 83% capsular repair | Nerve block | PB: Plain bupivacaine | 40 mL of .25% plain bupivacaine (100 mg) | 37 military veterans | 30.2 | DVPRS: PB = LB + PB, from the PACU until 2 weeks | Oxycodone Consumed: PB = LB + PB, from days 1-14 |
| LB + PB: Liposomal bupivacaine + plain bupivacaine | 20 mL of .5% plain bupivacaine (100 mg) + 20 mL of liposomal bupivacaine (266 mg) | 33 military veterans | 32.8 | ||||||
| Glomset et al., 202045 | 1 | Labral repair with both acetabuloplasty and femoroplasty (76%), labral repair with acetabuloplasty or femoroplasty (14%), other (10%) | Nerve block vs. intra-articular injection | Ultrasound-guided fascia iliaca block (FIB) | Up to 60 mL .35% ropivacaine at 3 mg/kg, with 100-mg clonidine (per 60 mL) and epinephrine 1:400,000 | 41 | 40.6 ± 12.4 | VAS: FIB = IA, in PACU, and at 2 weeks, 6 weeks, and 3 months | Morphine Equivalents: FIB = IA, in PACU |
| Intra-articular (IA) injection, at completion of surgery | 20 mL .5% ropivacaine, at the end of surgery | 43 | 36.8 ± 12.1 | ||||||
| Cogan et al., 202040 | 1 | Hip arthroscopy, labral repair, and acetabuloplasty | Intra-articular injection | M + C: Morphine + clonidine | 11 mL of 10 mg morphine, and 100 mcg clonidine, in .9% NaCl solution, at the conclusion of arthroscopy | 33 | 40 | VAS: M + C = control, in PACU, and at 7, 18, 24, and 48 hours, as well as at 7 days | Morphine Equivalents: M + C = control, in PACU, and at 7, 18, 24, and 48 hours, as well as at 7 days |
| Control: Normal saline | 11 mL of .9% NaCl solution, at conclusion of arthroscopy | 36 | |||||||
| Shlaifer et al., 201782 | 1 | Femoroacetabular impingement | Surgical Site Injection | Periacetabular Injection | 20 mL .5% bupivacaine with epi (1:200,000), before surgery | 21 | 39.6 ± 16.1 | VAS: Periacetabular < Intra-articular, 30 min. ∗Periacetabular < Intra-articular, at 18 hours Periacetabular = Intra-articular, from 1 to 12 hours, and from days 1 to 14 | Morphine Equivalents: Periacetabular = Intra-articular, until discharge and days 1-7 |
| Intra-articular Hip Injection | 20 mL .5% bupivacaine with epi (1:200,000), before surgery | 21 | 36 ± 15.6 | ||||||
| Kahlenberg et al., 201751 | 1 | 61% labral repair, 24% labral repair with acetabular osteoplasty, 11% other | Oral medication | Celecoxib | 1 hour pre-op: 2 pills, 200 mg celecoxib each | 50 | 34.2 | VAS: Celecoxib < control at 1 hour, and Celecoxib almost < control at 2 hours (P = .06). Celecoxib = control, until discharge | Morphine Equivalents: Celecoxib = control, in PACU |
| Placebo | 1 hour pre-op: 2 lactose-based placebo pills | 48 | 35.8 | ||||||
| Zhang et al., 201490 | 1 | Femoroacetabular impingement with labral tears | Oral medication | Celecoxib | 200 mg celecoxib 1 hour before surgery | 27 | 41.0 ± 4.9 | VAS: Celecoxib = Placebo, in recovery room. Celecoxib < Placebo, from 12 to 24 hours | Number of Narcotic Pills Used: Celecoxib < Placebo, in recovery room |
| Placebo | 200 mg placebo 1 hour before surgery | 26 | 43.5 ± 5.1 |
Findings with a P value <.01 are marked with an asterisk (∗), and findings with a P value <.001 are in bold.
DVPRS, defense and veterans pain rating scale; VAS, visual analog scale; NRS, numeric pain rating scale, PACU, postanesthesia care unit.
Femoroacetabular Impingement
Two studies evaluated nerve blocks for femoroacetabular impingement surgery.34,88 Behrends et al.34 found that a preoperative fascia iliaca block with 40 mL of .2% bupivacaine provides similar pain relief and opioid consumption as a saline placebo. Xing et al.88 found more encouraging pain management results, showing that a preoperative femoral nerve block with 20 mL of .5% bupivacaine significantly decreases pain at several time points throughout the first postoperative week, and that opioid consumption also decreases from 1 to 2 days postoperatively, relative to a saline placebo. Unfortunately, the femoral nerve block also increased the rate of postoperative falls.
Oral Medications
Several studies show encouraging results for oral medications across shoulder, knee, and hip arthroscopy. For shoulder arthroscopy, Ahn et al.27 provided either a 150 mg Pregabalin capsule or a placebo 1 hour before anesthesia induction (25% Bankart repair, 75% rotator cuff repair), and found that Pregabalin dramatically decreased pain from 6 to 48 hours postoperatively and opioid consumption from 0 to 48 hours postoperatively. Mardani-Kivi et al.67 instead provided either 600 mg Gabapentin or a placebo to Bankart repair patients 2 hours before surgery, finding similar pain relief but significantly decreased opioid consumption from 6 to 24 hours postoperatively in the Gabapentin group. However, Spence et al.83 did not see a difference due to Gabapentin when provided to military patients preoperatively and postoperatively from shoulder arthroscopy, clouding the overall efficacy of Gabapentin. Across ACL reconstruction, meniscectomy, and knee arthroscopy, in general, providing either 120 mg Etoricoxib and 400 mg Celecoxib preoperatively leads to significant improvements in both pain and opioid consumption.63,68 Furthermore, Zhou et al.91 provided 400 mg Celecoxib at various time points (1 day preoperatively, 1 hour preoperatively, and 4 hours postoperatively) to partial meniscectomy patients, finding that both the 1 day and 1 hour preoperative groups have better pain relief and slightly less opioid consumption relative to the 4 hours postoperative group. Lastly, in hip arthroscopy, 200 mg Celecoxib given 1 hour before surgery may provide some pain relief and slightly decrease opioid consumption after femoroacetabular impingement surgery or labral repair; however, the effects are not strong and differ across studies.51,90
Postoperative Interventions for Pain Management
Ten studies evaluated interventions for postoperative pain management protocols, six in shoulder arthroscopy,61, 66, 71, 75, 83 four in knee arthroscopy,29, 85, 91, 94 and none in hip arthroscopy. Notable in shoulder arthroscopy, Yun et al.89 found that subacromial patient-controlled analgesia (PCA) provided postoperatively for 48 hours to rotator cuff repair patients provided better pain relief at 1 hour postoperatively compared to IV PCA, while opioid consumption was similar. Merivirta et al.71 and Oh et al.75 found that a 72-hour postoperative fentanyl batch provided similar outcomes to a bupivacaine infusion and that Nefopam IV PCA provided similar outcomes to a Ketorolac IV PCA, respectively. For knee arthroscopy, Tompkins et al.85 found that providing the sleep aid Zolpidem (10 mg) to ACL reconstruction patients for days 0-7 postoperatively decreased opioid consumption, while not affecting pain relief. Amin et al.29 compared three groups of intra-articular PCA for ACL reconstruction patients: an RM group (.25% ropivacaine + .2 mg/mL morphine), an RMX group (ropivacaine + morphine + 1 mg/mL xefocam [i.e. Lornoxicam]), and a control group. They found that the RMX group had the best pain relief from 8 to 24 hours postoperatively and dramatically less opioid consumption than the RM group at 24 hours.
Nonpharmacological Interventions
Six studies evaluated nonpharmacological interventions for pain management, four for shoulder arthroscopy,39,61,66,84 two for knee arthroscopy,78,94 and none for hip arthroscopy. Both Cho et al.39 (orally and written) and Syed et al.84 (2-minute video) provided preoperative opioid education to rotator cuff repair patients, and both studies found opioid education to decrease postoperative pain and opioid consumption throughout short-term (<1 week) and mid-term (up to 3 months) recovery. Mahure et al.66 also evaluated rotator cuff repair patients, finding that using a transcutaneous electrical nerve stimulation (TENS) unit throughout the first postoperative week can decrease pain and opioid consumption throughout that week. However, Kraeutler et al.61 found no difference in outcomes between shoulder arthroscopy patients who used compressive cryotherapy versus a standard ice wrap postoperatively. Reda et al.78 showed that for ACL reconstruction patients, the use of a tourniquet negatively affects outcomes, with tourniquet use showing increased pain from 4 to 10 hours postoperatively and increased opioid consumption until discharge. Also, Hartwell et al.94 evaluated whether the mode of prescription affects postoperative outcomes by providing one group of patients with optional paper prescriptions for 20 tablets of 5 mg oxycodone and the other group with the same prescriptions automatically provided (not optional) from the pharmacist. However, there were no differences in pain or opioid consumption at any time point up to 21 days postoperatively.
Discussion
On the basis of current evidence, we recommend interscalene nerve blocks with a dexamethasone-dexmedetomidine combination for rotator cuff repair, a proximal continuous adductor canal block for ACL reconstruction, and local infiltration analgesia (e.g., periacetabular injection with 20 mL of .5% bupivacaine) for hip arthroscopy. Several oral medications appear to be optimal as well, such as 150 mg Pregabalin for shoulder arthroscopy, 400 mg Celecoxib for knee arthroscopy, and 200 mg Celecoxib for hip arthroscopy. There is promising evidence for the use of various nonpharmacological modalities, specifically preoperative opioid education for rotator cuff repair patients; however more clinical trials evaluating nonpharmacological interventions should be performed.
Shoulder Arthroscopy
A number of different nerve block locations and formulations were examined following shoulder arthroscopy. While bupivacaine alone was shown to reduce postoperative opioid consumption, the addition of dexamethasone to the interscalene block resulted in even lower postoperative pain for rotator cuff repair patients.32 However, Kang et al.54 compared IV dexamethasone to IV dexamethasone-dexmedetomidine and showed that a combination of dexamethasone and dexmedetomidine (IV dexamethasone .11 mg/kg + IV dexmedetomidine 1.0 μg/kg) decreased postoperative pain and opioid consumption in a cohort of 73% rotator cuff repair patients. These findings were further supported by Bengisun et al.,35 who reported similarly superior outcomes with the addition of dexmedetomidine to an interscalene levobupivacaine and epinephrine block; however, this study involved a cohort of subacromial decompression patients. While other studies conflicted on the effects of block location, the overall trend was that interscalene brachial plexus blocks performed equal to or greater than supraclavicular and suprascapular blocks for both pain and opioid control.26, 31, 74 Cabaton et al.37 had results opposing the superiority of the interscalene brachial plexus block for rotator cuff repair patients, as they reported less opioid consumption after injection at a supraclavicular site; however, these authors used a levobupivacaine and clonidine block rather than a ropivacaine formulation.
Nerve blocks appear to be the optimal pain management modality for shoulder arthroscopy, as the bupivacaine interscalene brachial plexus block was superior to bupivacaine intra-articular injections for both pain control and opioid consumption in a cohort of mostly rotator cuff repair (67%) and Bankart repair (20%) patients.28 However, intra-articular injections for shoulder arthroscopy may be optimized by using morphine (20 mg morphine/10 mL) or magnesium (1 g magnesium sulfate in 10 mL saline).57,80 Before a clear clinical recommendation can be provided, further high-quality studies comparing morphine, magnesium, bupivacaine, and any other viable intra-articular injections formulations should be conducted. Lastly, preemptively providing the oral medication Pregabalin can help patients after shoulder arthroscopy. Mardani-Kivi et al.67 found that 600 mg Gabapentin significantly decreased postoperative opioid consumption in Bankart repair patients; however, Spence et al.83 had conflicting results in a military patient population undergoing unspecified shoulder arthroscopy. Meanwhile, Ahn et al.27 showed that 150 mg Pregabalin decreased both postoperative pain and opioid consumption compared to placebo in a cohort of 75% rotator cuff repair patients, ultimately providing pain relief, while Gabapentin did not significantly decrease pain in either aforementioned study.
Knee Arthroscopy
Postoperative pain following knee arthroscopy was reported as equivalent, and opioid consumption appears similar, when comparing ropivacaine as an adductor canal block (ACB) to a femoral nerve block (FNB) in ACL reconstruction patients.24,33,65 This is supported by a network meta-analysis performed by Davey et al.95 who found that nerve blocks are efficacious for ACL reconstruction, but that no specific nerve block proved superior. However, proximal ACBs were found to significantly reduce pain within the first 6 hours after ACL reconstruction compared to middle and distal ACBs.25 In addition, continuous nerve blocks were shown to reduce pain between hours 4 and 12 after ACL reconstruction compared to intermittent ACBs.92 Proximal and continuous ACBs were also superior to comparison groups for the minimization of postoperative opioid consumption, suggesting that the proximal and continuous ACB may be the most effective modality for pain management for ACL reconstruction.25,92 Regarding intra-articular injections, Davey et al.95 also found that intra-articular injections with bupivacaine decrease pain for up to 12 hours postoperatively, while also decreasing postoperative opioid consumption. Unfortunately, the optimal pain management for meniscectomy is less clear, as several injections provided better outcomes than placebo, but no studies have compared these interventions to determine superiority.60, 79, 93 Providing either 120 mg Etoricoxib and 400 mg Celecoxib preoperatively leads to significant improvements in both pain and opioid consumption for various knee arthroscopy procedures.63, 68, 91 An RCT comparing preoperative Etoricoxib and Celecoxib would help clarify whether one oral medication is superior to the other for knee arthroscopy.
Hip Arthroscopy
Following hip arthroscopy, Xing et al.88 found a decrease in postoperative pain relief and opioid consumption with the use of a bupivacaine femoral nerve block compared to saline controls. However, the nerve block was also associated with a significant increase in the risk of falls within the first 24 hours following the procedure, which ultimately led to the discontinuation of bupivacaine femoral nerve blocks for outpatient hip arthroscopy procedures at the respective institution.88 The fascia iliaca block also may be an inferior form of pain management, as the fascia iliaca block provides equal pain relief compared to saline placebo.34,45,77 No RCTs included in this study evaluated the lumbar plexus nerve block; however, it is possible that the lumbar plexus nerve block may provide the best clinical outcomes of the common hip arthroscopy nerve blocks.96,97 For example, YaDeau et al.97 described in a brief report of an RCT that the addition of a lumbar plexus nerve block with a combined spinal epidural leads to significantly decreased postoperative pain compared to only receiving the spinal epidural. Also, in a retrospective cohort study, Wolff et al.96 found that a lumbar plexus nerve block with general anesthesia leads to much less postoperative pain than a fascia iliaca nerve block with general anesthesia. However, until several high-quality RCTs compare the lumbar plexus nerve block to other viable nerve blocks, the lumbar plexus nerve block should not be considered the gold standard of nerve blocks for hip arthroscopy.
Interestingly, the network meta-analyses performed by Kunze et al.15 suggest that local infiltration anesthesia is more effective than nerve block for limiting both postoperative pain and opioid consumption for hip arthroscopy. Specifically regarding local infiltration anesthesia within our included studies, Shlaifer et al.82 reported a decrease in postoperative pain if 20 mL of .5% bupivacaine was used as periacetabular injection as opposed to an intra-articular injection. Interestingly, the use of morphine and clonidine as an intra-articular injection provided equivalent pain relief and opioid consumption compared to placebo.40 Lastly in regard to oral medication, while the use of oral celecoxib resulted in decreased postoperative pain compared to controls, only Zhang et al.90 reported a decrease in postoperative opioid consumption.51,90
Nonpharmacological Interventions
Several nonpharmacological interventions appear to provide clinical benefit for arthroscopic surgery patients. Preoperative patient opioid education of any form appears to provide clear benefits regarding both postoperative pain and opioid consumption for rotator cuff repair patients.39,84 Syed et al.84 also found that preoperative education patients were more than 2 times more likely to stop their narcotic use by 3 months postoperatively. Considering how easily preoperative opioid education was provided by Syed et al.,84 providing patients with an educational 2-minute video and a handout, preoperative opioid education can be a realistic and beneficial intervention for managing postoperative pain and opioid consumption. The use of a TENS unit throughout the first postoperative week may also help rotator cuff repair patients; however, the feasibility depends on the finances of each institution and adherence of the patient.66 For ACL reconstruction, abandoning the tourniquet appears advantageous.78 Surgeons that feel comfortable abandoning tourniquet use may be able to decrease postoperative pain and opioid consumption for ACL reconstruction patients.78 Unfortunately, several nonpharmacological interventions do not appear to provide additional pain relief or minimize opioid consumption, such as cryotherapy for shoulder arthroscopy patients or optional paper prescriptions for knee arthroscopy patients.61,94
Postoperative Interventions for Pain Management
Similarly, several postoperative interventions may prove beneficial for certain arthroscopic surgery populations. For example, rotator cuff patients have decreased postoperative pain when given subacromial PCA instead of IV PCA for 48 hours postoperatively.89 Similarly, postoperative pain and opioid consumption can be decreased for ACL reconstruction patients by including ropivacaine, morphine, and xefocam (Lornoxicam) for intra-articular PCA.29 ACL reconstruction patients may also benefit from the use of a sleep aid such as Zolpidem 10 mg.85 While taking Zolpidem postoperatively days 0-7 did not decrease pain, opioid consumption significantly decreased.85
Clinical Recommendations
Development of an optimal analgesic strategy based on the articles examined is difficult due to the paucity of direct comparisons between various treatment modalities. However, each anatomic location demonstrated similar trends in regard to maximizing pain relief and minimizing opioid consumption. First, a distinct nerve block may be superior at each surgical location. Interscalene brachial plexus blocks with ropivacaine appear superior for rotator cuff repair,17,32, 37, 87, 44 while proximal continuous adductor canal blocks were superior for ACL reconstruction.24, 25, 33, 65, 92, 95 Second, certain oral medications taken preoperatively may limit both postoperative pain and opioid consumption, with 150 mg Pregabalin being optimal for shoulder arthroscopy,27, 67, 83 400 mg Celecoxib for knee arthroscopy,63,68 and 200 mg Celecoxib for hip arthroscopy.51,90 Lastly, several nonpharmacological interventions have the potential to improve pain management with minimal risk to the patient. For example, preoperative patient opioid education, minimization of tourniquet use, and postoperative TENS unit usage can decrease pain and opioid consumption, while replacing electronic prescriptions with paper prescriptions may minimize the amount of unused opioid tablets available to the public.39,66,84,94
Limitations
This review is not without limitations. The included studies were separated on the basis of anatomic location. However, many studies included a number of different surgical procedures at the anatomic site. In addition, studies used different drug formulations for pain control and different morphine equivalents for opioid consumption. As a result, directly comparing outcomes between studies was not feasible. Also, the heterogeneity of data prevented pooling of results, which weakens the strength of the study conclusions. Plus, complications and patient-reported outcomes were not evaluated in this systematic review. Finally, minimal clinically important differences (MCIDs) were not evaluated in this study, only statistical significance.
Conclusions
Many multimodal pain management protocols offer improved pain control and decreased opioid consumption after arthroscopic surgery. On the basis of the current evidence, we recommend an interscalene nerve block with a dexamethasone-dexmedetomidine combination for rotator cuff repair, a proximal continuous adductor canal block for ACL reconstruction, and local infiltration analgesia (e.g., periacetabular injection with 20 mL of .5% bupivacaine) for hip arthroscopy. When evaluating oral medication: the evidence supports 150 mg Pregabalin for shoulder arthroscopy, 400 mg Celecoxib for knee arthroscopy, and 200 mg Celecoxib for hip arthroscopy, all taken preoperatively. There is promising evidence for the use of various nonpharmacological modalities, specifically preoperative opioid education for rotator cuff repair patients; however, more clinical trials evaluating nonpharmacological interventions should be performed.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: K.B. F. is a board/committee member of AOSSM. He is a paid consultant for DePuy and Vericel. He has received payment for development of educational presentations from Liberty Surgical. F.P.T. is a board or committee member of AOSSM, AAOS, and ABOS. He owns stock options in Franklin/Keystone Biosciences and Trice Medical. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
References
- 1.Chisholm-Burns M.A., Spivey C.A., Sherwin E., Wheeler J., Hohmeier K. The opioid crisis: Origins, trends, policies, and the roles of pharmacists. Am J Health-Syst Pharm. 2019;76:424–435. doi: 10.1093/ajhp/zxy089. [DOI] [PubMed] [Google Scholar]
- 2.Skolnick P. The opioid epidemic: Crisis and solutions. Annu Rev Pharmacol Toxicol. 2018;58:143–159. doi: 10.1146/annurev-pharmtox-010617-052534. [DOI] [PubMed] [Google Scholar]
- 3.Chen L.H. Drug-poisoning deaths involving opioid analgesics: United States, 1999–2011. NCHS Data Brief. 2014;(166):1–8. [PubMed] [Google Scholar]
- 4.Dart R.C., Surratt H.L., Cicero T.J., et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- 5.Vital Signs: Overdoses of Prescription Opioid Pain Relievers—United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60:1487–1492. [PubMed] [Google Scholar]
- 6.Opioid data analysis and resources. https://www.cdc.gov/opioids/data/analysis-resources.html
- 7.Daubresse M., Chang H.-Y., Yu Y., et al. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000-2010. Med Care. 2013;51:870–878. doi: 10.1097/MLR.0b013e3182a95d86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gan T.J. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res. 2017;10:2287–2298. doi: 10.2147/JPR.S144066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gan T.J., Habib A.S., Miller T.E., White W., Apfelbaum J.L. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30:149–160. doi: 10.1185/03007995.2013.860019. [DOI] [PubMed] [Google Scholar]
- 10.Meissner W., Mescha S., Rothaug J., et al. Quality improvement in postoperative pain management. Dtsch Ärztebl Int. 2008;105:865–870. doi: 10.3238/arztebl.2008.0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calvo E., Torres M.D., Morcillo D., Leal V. Rotator cuff repair is more painful than other arthroscopic shoulder procedures. Arch Orthop Trauma Surg. 2019;139:669–674. doi: 10.1007/s00402-018-3100-0. [DOI] [PubMed] [Google Scholar]
- 12.Volkow N.D., McLellan T.A., Cotto J.H., Karithanom M., Weiss S.R.B. Characteristics of opioid prescriptions in 2009. JAMA. 2011;305:1299–1301. doi: 10.1001/jama.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Practice guidelines for acute pain management in the perioperative setting: An updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–273. doi: 10.1097/ALN.0b013e31823c1030. [DOI] [PubMed] [Google Scholar]
- 14.Secrist E.S., Freedman K.B., Ciccotti M.G., Mazur D.W., Hammoud S. Pain management after outpatient anterior cruciate ligament reconstruction: A systematic review of randomized controlled trials. Am J Sports Med. 2016;44:2435–2447. doi: 10.1177/0363546515617737. [DOI] [PubMed] [Google Scholar]
- 15.Kunze K.N., Polce E.M., Lilly D.T., et al. Adjunct analgesia reduces pain and opioid consumption after hip arthroscopy: A systematic review of randomized controlled trials. Am J Sports Med. 2020;48:3638–3651. doi: 10.1177/0363546520905884. [DOI] [PubMed] [Google Scholar]
- 16.Hurley E.T., Maye A.B., Thompson K., et al. Pain control after shoulder arthroscopy: A systematic review of randomized controlled trials with a network meta-analysis. Am J Sports Med. 2021;49:2262–2271. doi: 10.1177/0363546520971757. [DOI] [PubMed] [Google Scholar]
- 17.Warrender W.J., Syed U.A.M., Hammoud S., et al. Pain management after outpatient shoulder arthroscopy: A systematic review of randomized controlled trials. Am J Sports Med. 2017;45:1676–1686. doi: 10.1177/0363546516667906. [DOI] [PubMed] [Google Scholar]
- 18.Moher D., Shamseer L., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abou-Setta A.M., Beaupre L.A., Rashiq S., et al. Comparative effectiveness of pain management interventions for hip fracture: A systematic review. Ann Intern Med. 2011;155:234–245. doi: 10.7326/0003-4819-155-4-201108160-00346. [DOI] [PubMed] [Google Scholar]
- 20.Brubaker L., Kendall L., Reina E. Multimodal analgesia: A systematic review of local NSAIDs for non-ophthalmologic postoperative pain management. Int J Surg. 2016;32:158–166. doi: 10.1016/j.ijsu.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Lee Y., Yu J., Doumouras A.G., et al. Intravenous acetaminophen versus placebo in post-bariatric surgery multimodal pain management: A meta-analysis of randomized controlled trials. Obes Surg. 2019;29:1420–1428. doi: 10.1007/s11695-019-03732-8. [DOI] [PubMed] [Google Scholar]
- 22.Tong Y., Fernandez L., Bendo J.A., Spivak J.M. Enhanced recovery after surgery trends in adult spine surgery: A systematic review. Int J Spine Surg. 2020;14:623–640. doi: 10.14444/7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins J.P.T., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdallah F.W., Whelan D.B., Chan V.W., et al. Adductor canal block provides noninferior analgesia and superior quadriceps strength compared with femoral nerve block in anterior cruciate ligament reconstruction. Anesthesiology. 2016;124:1053–1064. doi: 10.1097/ALN.0000000000001045. [DOI] [PubMed] [Google Scholar]
- 25.Abdallah F.W., Mejia J., Prasad G.A., et al. Opioid- and motor-sparing with proximal, mid-, and distal locations for adductor canal block in anterior cruciate ligament reconstruction: A randomized clinical trial. Anesthesiology. 2019;131:619–629. doi: 10.1097/ALN.0000000000002817. [DOI] [PubMed] [Google Scholar]
- 26.Abdallah F.W., Wijeysundera D.N., Laupacis A., et al. Subomohyoid anterior suprascapular block versus interscalene block for arthroscopic shoulder surgery: A multicenter randomized trial. Anesthesiology. 2020;132:839–853. doi: 10.1097/ALN.0000000000003132. [DOI] [PubMed] [Google Scholar]
- 27.Ahn S., Byun S.H., Park K., Ha J.L., Kwon B., Kim J.C. Analgesic efficacy of preemptive pregabalin administration in arthroscopic shoulder surgery: A randomized controlled trial. Can J Anaesth J Can Anesth. 2016;63:283–289. doi: 10.1007/s12630-015-0510-0. [DOI] [PubMed] [Google Scholar]
- 28.Aksu R., Biçer C., Ülgey A., et al. Comparison of interscalene brachial plexus block and intra-articular local anesthetic administration on postoperative pain management in arthroscopic shoulder surgery. Braz J Anesthesiol. 2015;65:222–229. doi: 10.1016/j.bjane.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Amin S., Yosry M., El D.I. The effect of the addition of lornoxicam (xefocam) intrarticularly on the WOMAC Scale in patients undergoing arthroscopic anterior cruciate ligament reconstruction. Middle East J Anaesthesiol. 2011;21:15–21. [PubMed] [Google Scholar]
- 30.Arti H., Mehdinasab S.A. The comparison effects of intra-articular injection of different opioids on postoperative pain relieve after arthroscopic anterior cruciate ligament reconstruction: A randomized clinical trial study. J Res Med Sci. 2011;16:1176–1182. [PMC free article] [PubMed] [Google Scholar]
- 31.Auyong D.B., Hanson N.A., Joseph R.S., Schmidt B.E., Slee A.E., Yuan S.C. Comparison of anterior suprascapular, supraclavicular, and interscalene nerve block approaches for major outpatient arthroscopic shoulder surgery: A randomized, double-blind, noninferiority trial. Anesthesiology. 2018;129:47–57. doi: 10.1097/ALN.0000000000002208. [DOI] [PubMed] [Google Scholar]
- 32.Baessler A.M., Moor M., Conrad D.J., Creighton J., Badman B.L. Single-shot liposomal bupivacaine reduces postoperative narcotic use following outpatient rotator cuff repair: A prospective, double-blinded, randomized controlled trial. J Bone Joint Surg Am. 2020;102:1985–1992. doi: 10.2106/JBJS.20.00225. [DOI] [PubMed] [Google Scholar]
- 33.Bailey L., Griffin J., Elliott M., et al. Adductor canal nerve versus femoral nerve blockade for pain control and quadriceps function following anterior cruciate ligament reconstruction with patellar tendon autograft: A prospective randomized trial. Arthroscopy. 2019;35:921–929. doi: 10.1016/j.arthro.2018.10.149. [DOI] [PubMed] [Google Scholar]
- 34.Behrends M., Yap E.N., Zhang A.L., et al. Preoperative fascia iliaca block does not improve analgesia after arthroscopic hip surgery, but causes quadriceps muscles weakness: A randomized, double-blind trial. Anesthesiology. 2018;129:536–543. doi: 10.1097/ALN.0000000000002321. [DOI] [PubMed] [Google Scholar]
- 35.Bengisun Z.K., Ekmekçi P., Akan B., Köroğlu A., Tüzüner F. The effect of adding dexmedetomidine to levobupivacaine for interscalene block for postoperative pain management after arthroscopic shoulder surgery. Clin J Pain. 2014;30:1057–1061. doi: 10.1097/AJP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 36.Bjørnholdt K.T., Mønsted P.N., Søballe K., Nikolajsen L. Dexamethasone for pain after outpatient shoulder surgery: a randomised, double-blind, placebo-controlled trial. Acta Anaesthesiol Scand. 2014;58:751–758. doi: 10.1111/aas.12333. [DOI] [PubMed] [Google Scholar]
- 37.Cabaton J., Nové-Josserand L., Mercadal L., Vaudelin T. Analgesic efficacy of ultrasound-guided interscalene block vs. supraclavicular block for ambulatory arthroscopic rotator cuff repair: A randomised noninferiority study. Eur J Anaesthesiol. 2019;36:778–786. doi: 10.1097/EJA.0000000000001065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choromanski D.W., Patel P.S., Frederick J.M., Lemos S.E., Chidiac E.J. The effect of continuous interscalene brachial plexus block with 0.125% bupivacaine vs 0.2% ropivacaine on pain relief, diaphragmatic motility, and ventilatory function. J Clin Anesth. 2015;27:619–626. doi: 10.1016/j.jclinane.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Cho C.-H., Song K.-S., Min B.-W., et al. Multimodal approach to postoperative pain control in patients undergoing rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2011;19:1744–1748. doi: 10.1007/s00167-010-1294-y. [DOI] [PubMed] [Google Scholar]
- 40.Cogan C.J., Tjong V.K., Dunne K.F., Sahota S., Tuttle J., Terry M.A. Intra-articular morphine and clonidine injection after hip arthroscopy: A randomized, triple-blind controlled trial. Ambul Surg. 2020;26:5–9. [Google Scholar]
- 41.DeMarco J.R., Componovo R., Barfield W.R., Liles L., Nietert P. Efficacy of augmenting a subacromial continuous-infusion pump with a preoperative interscalene block in outpatient arthroscopic shoulder surgery: a prospective, randomized, blinded, and placebo-controlled study. Arthroscopy. 2011;27:603–610. doi: 10.1016/j.arthro.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Espelund M., Fomsgaard J.S., Haraszuk J., Dahl J.B., Mathiesen O. The efficacy of adductor canal blockade after minor arthroscopic knee surgery--a randomised controlled trial. Acta Anaesthesiol Scand. 2014;58:273–280. doi: 10.1111/aas.12224. [DOI] [PubMed] [Google Scholar]
- 43.Espelund M., Grevstad U., Jaeger P., et al. Adductor canal blockade for moderate to severe pain after arthroscopic knee surgery: a randomized controlled trial. Acta Anaesthesiol Scand. 2014;58:1220–1227. doi: 10.1111/aas.12407. [DOI] [PubMed] [Google Scholar]
- 44.Faria-Silva R., de Rezende D.C., Ribeiro J.M., et al. Association of clonidine and ropivacaine in brachial plexus block for shoulder arthroscopy. Braz J Anesthesiol Elsevier. 2016;66:335–340. doi: 10.1016/j.bjane.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 45.Glomset J.L., Kim E.J., Tokish J.M., et al. Reduction of hip arthroscopy post-operative pain using ultrasound-guided fascia-iliaca block: A prospective randomized control trial. Orthop J Sports Med. 2020;48:682–688. doi: 10.1177/0363546519898205. [DOI] [PubMed] [Google Scholar]
- 46.Hanson N.A., Derby R.E., Auyong D.B., et al. Ultrasound-guided adductor canal block for arthroscopic medial meniscectomy: a randomized, double-blind trial. Can J Anaesth. 2013;60:874–880. doi: 10.1007/s12630-013-9992-9. [DOI] [PubMed] [Google Scholar]
- 47.Hartwell M.J., Selley R.S., Terry M.A., Tjong V.K. Can We Eliminate Opioid Medications for Postoperative Pain Control? A Prospective, Surgeon-Blinded, Randomized Controlled Trial in Knee Arthroscopic Surgery. Am J Sports Med. 2020;48:2711–2717. doi: 10.1177/0363546520941861. [DOI] [PubMed] [Google Scholar]
- 48.Hsu L.P., Oh S., Nuber G.W., et al. Nerve block of the infrapatellar branch of the saphenous nerve in knee arthroscopy: a prospective, double-blinded, randomized, placebo-controlled trial. J Bone Joint Surg Am. 2013;95:1465–1472. doi: 10.2106/JBJS.L.01534. [DOI] [PubMed] [Google Scholar]
- 49.Jeske H.-C., Kralinger F., Wambacher M., et al. A randomized study of the effectiveness of suprascapular nerve block in patient satisfaction and outcome after arthroscopic subacromial decompression. Arthroscopy. 2011;27:1323–1328. doi: 10.1016/j.arthro.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 50.Kager I., Mousa S.A., Sieper J., Stein C., Pipam W., Likar R. Blockade of intra-articular adrenergic receptors increases analgesic demands for pain relief after knee surgery. Rheumatol Int. 2011;31:1299–1306. doi: 10.1007/s00296-010-1489-z. [DOI] [PubMed] [Google Scholar]
- 51.Kahlenberg C.A., Patel R.M., Knesek M., Tjong V.K., Sonn K., Terry M.A. Efficacy of celecoxib for early postoperative pain management in hip arthroscopy: A prospective randomized placebo-controlled study. Arthroscopy. 2017;33:1180–1185. doi: 10.1016/j.arthro.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 52.Kahn R.L., Cheng J., Gadulov Y., Fields K.G., YaDeau J.T., Gulotta L.V. Perineural low-dose dexamethasone Prolongs interscalene block analgesia with bupivacaine compared with systemic dexamethasone: A randomized trial. Reg Anesth Pain Med. 2018;43:572–579. doi: 10.1097/AAP.0000000000000817. [DOI] [PubMed] [Google Scholar]
- 53.Kang R., Jeong J.S., Yoo J.C., et al. Effective dose of intravenous dexmedetomidine to prolong the analgesic duration of interscalene brachial plexus block: A single-center, prospective, double-blind, randomized controlled trial. Reg Anesth Pain Med. 2018;43:488–495. doi: 10.1097/AAP.0000000000000773. [DOI] [PubMed] [Google Scholar]
- 54.Kang R.A., Jeong J.S., Yoo J.C., et al. Improvement in postoperative pain control by combined use of intravenous dexamethasone with intravenous dexmedetomidine after interscalene brachial plexus block for arthroscopic shoulder surgery: A randomised controlled trial. Eur J Anaesthesiol. 2019;36:360–368. doi: 10.1097/EJA.0000000000000977. [DOI] [PubMed] [Google Scholar]
- 55.Kataria S., Mitra S., Saroa R., Jindal S., Gupta R. A randomized double blinded trial comparing dexmedetomidine with dexamethasone as an adjunct to ropivacaine in ultrasound guided interscalene block for arthroscopic shoulder surgery. Asian J Anesthesiol. 2019;57:10–18. doi: 10.6859/aja.201903_57(1).0003. [DOI] [PubMed] [Google Scholar]
- 56.Keller R.A., Birns M.E., Cady A.C., Limpisvasti O., Banffy M.B. Posterior capsule injection of local anesthetic for post-operative pain control after ACL reconstruction: a prospective, randomized trial. Knee Surg Sports Traumatol Arthrosc. 2019;27:822–826. doi: 10.1007/s00167-018-5111-3. [DOI] [PubMed] [Google Scholar]
- 57.Khashan M., Dolkart O., Amar E., et al. Effect of preemptive intra-articular morphine and ketamine on pain after arthroscopic rotator cuff repair: a prospective, double-blind, randomized controlled study. Arch Orthop Trauma Surg. 2016;136:233–239. doi: 10.1007/s00402-015-2346-z. [DOI] [PubMed] [Google Scholar]
- 58.Kim D.H., Lin Y., Beathe J.C., et al. Superior trunk block: A phrenic-sparing alternative to the interscalene block: A randomized controlled trial. Anesthesiology. 2019;131:521–533. doi: 10.1097/ALN.0000000000002841. [DOI] [PubMed] [Google Scholar]
- 59.Ko S.H., Kang B.S., Hwang C.H. Ultrasonography- or electrophysiology-guided suprascapular nerve block in arthroscopic acromioplasty: a prospective, double-blind, parallel-group, randomized controlled study of efficacy. Arthroscopy. 2013;29(5):794–801. doi: 10.1016/j.arthro.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 60.Koltka K., Koknel-Talu G., Asik M., Ozyalcin S. Comparison of efficacy of intraarticular application of magnesium, levobupivacaine and lornoxicam with placebo in arthroscopic surgery. Knee Surg Sports Traumatol Arthrosc. 2011;19:1884–1889. doi: 10.1007/s00167-011-1497-x. [DOI] [PubMed] [Google Scholar]
- 61.Kraeutler M.J., Reynolds K.A., Long C., McCarty E.C. Compressive cryotherapy versus ice—A prospective, randomized study on postoperative pain in patients undergoing arthroscopic rotator cuff repair or subacromial decompression. J Shoulder Elbow Surg. 2015;24:854–859. doi: 10.1016/j.jse.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 62.Lee H.-J., Kim Y.-S., Park I., Ha D.-H., Lee J.-H. Administration of analgesics after rotator cuff repair: A prospective clinical trial comparing glenohumeral, subacromial, and a combination of glenohumeral and subacromial injections. J Shoulder Elbow Surg. 2015;24:663–668. doi: 10.1016/j.jse.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 63.Lierz P., Losch H., Felleiter P. Evaluation of a single preoperative dose of etoricoxib for postoperative pain relief in therapeutic knee arthroscopy: A randomized trial. Acta Orthop. 2012;83:642–647. doi: 10.3109/17453674.2012.747053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu J., Chen G., Zhou H., Zhou Q., Zhu Z., Wu C. Effect of parecoxib sodium pretreatment combined with dexmedetomidine on early postoperative cognitive dysfunction in elderly patients after shoulder arthroscopy: A randomized double blinded controlled trial. J Clin Anesth. 2017;41:30–34. doi: 10.1016/j.jclinane.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lynch J.R., Okoroha K.R., Lizzio V., Yu C.C., Jildeh T.R., Moutzouros V. Adductor canal block versus femoral nerve block for pain control after anterior cruciate ligament reconstruction: A prospective randomized trial. Am J Sports Med. 2019;47:355–363. doi: 10.1177/0363546518815874. [DOI] [PubMed] [Google Scholar]
- 66.Mahure S.A., Rokito A.S., Kwon Y.W. Transcutaneous electrical nerve stimulation for postoperative pain relief after arthroscopic rotator cuff repair: A prospective double-blinded randomized trial. J Shoulder Elbow Surg. 2017;26:1508–1513. doi: 10.1016/j.jse.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 67.Mardani-Kivi M., Karimi Mobarakeh M., Keyhani S., Haghighi M., Hashemi-Motlagh K., Saheb-Ekhtiari K. Arthroscopic bankart surgery: Does gabapentin reduce postoperative pain and opioid consumption? A triple-blinded randomized clinical trial. Orthop Traumatol Surg Res. 2016;102:549–553. doi: 10.1016/j.otsr.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 68.Mardani-Kivi M., Karimi Mobarakeh M., Haghighi M., et al. Celecoxib as a pre-emptive analgesia after arthroscopic knee surgery: A triple-blinded randomized controlled trial. Arch Orthop Trauma Surg. 2013;133:1561–1566. doi: 10.1007/s00402-013-1852-0. [DOI] [PubMed] [Google Scholar]
- 69.Marinković D., Simin J.M., Drasković B., Kvrgić I.M., Pandurov M. Efficiency of ultrasound guided lower limb peripheral nerve blocks in perioperative pain management for knee arthroscopy in children. A randomized study. Med Pregl. 2016;69:5–10. doi: 10.2298/mpns1602005m. [DOI] [PubMed] [Google Scholar]
- 70.McHardy P.G., Singer O., Awad I.T., et al. Comparison of the effects of perineural or intravenous dexamethasone on low volume interscalene brachial plexus block: a randomised equivalence trial. Br J Anaesth. 2020;124:84–91. doi: 10.1016/j.bja.2019.08.025. [DOI] [PubMed] [Google Scholar]
- 71.Merivirta R., Äärimaa V., Aantaa R., et al. Postoperative fentanyl patch versus subacromial bupivacaine infusion in arthroscopic shoulder surgery. Arthroscopy. 2013;29:1129–1134. doi: 10.1016/j.arthro.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 72.Mitra S., Kaushal H., Gupta R.K. Evaluation of analgesic efficacy of intra-articular bupivacaine, bupivacaine plus fentanyl, and bupivacaine plus tramadol after arthroscopic knee surgery. Arthroscopy. 2011;27(12):1637–1643. doi: 10.1016/j.arthro.2011.08.295. [DOI] [PubMed] [Google Scholar]
- 73.Moyano J., García M., Caicedo M. Analgesic Effect of Dexamethasone after Arthroscopic Knee Surgery: A Randomized Controlled Trial. Pain Res Manag. 2016;2016:4216469. doi: 10.1155/2016/4216469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neuts A., Stessel B., Wouters P.F., et al. Selective suprascapular and axillary nerve block versus interscalene plexus block for pain control after arthroscopic shoulder surgery: A noninferiority randomized parallel-controlled clinical trial. Reg Anesth Pain Med. 2018;43:738–744. doi: 10.1097/AAP.0000000000000777. [DOI] [PubMed] [Google Scholar]
- 75.Oh Y.N., Kim K.N., Jeong M.A., Kim D.W., Kim J.Y., Ki H.S. Effects of nefopam with fentanyl in intravenous patient-controlled analgesia after arthroscopic orthopedic surgery: A prospective double-blind randomized trial. Turk J Med Sci. 2018;48:142–149. doi: 10.3906/sag-1707-113. [DOI] [PubMed] [Google Scholar]
- 76.Sayın P., Dobrucalı H., Türk H.Ş., Totoz T., Işıl C.T., Hancı A. Effects of intra-articular levobupivacaine, fentanyl-levobupivacaine and tramadol-levobupivacaine for postoperative pain in arthroscopic knee surgery. Acta Orthop Traumatol Turc. 2015;49(3):267–273. doi: 10.3944/AOTT.2015.14.0107. [DOI] [PubMed] [Google Scholar]
- 77.Purcell R.L., Brooks D.I., Steelman T.J., et al. Fascia iliaca blockade with the addition of liposomal bupivacaine versus plain bupivacaine for perioperative pain management during hip arthroscopy: A double-blinded prospective randomized control trial. Arthroscopy. 2019;35:2608–2616. doi: 10.1016/j.arthro.2019.03.056. [DOI] [PubMed] [Google Scholar]
- 78.Reda W., ElGuindy A.M.F., Zahry G., Faggal M.S., Karim M.A. Anterior cruciate ligament reconstruction: Is a tourniquet necessary? A randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2016;24:2948–2952. doi: 10.1007/s00167-015-3582-z. [DOI] [PubMed] [Google Scholar]
- 79.Sanel S., Arpaz O., Unay K., Turkmen I., Simsek S., Ugutmen E. Comparison of intra-articular bupivacaine-morphine with bupivacaine-tenoxicam combinations on post-operative analgesia in patients with arthroscopic meniscectomy: A prospective, randomised study. Int Orthop. 2016;40:601–605. doi: 10.1007/s00264-015-2990-5. [DOI] [PubMed] [Google Scholar]
- 80.Saritas T.B., Borazan H., Okesli S., Yel M., Otelcioglu Ş. Is intra-articular magnesium effective for postoperative analgesia in arthroscopic shoulder surgery? Pain Res Manag. 2015;20:35–38. doi: 10.1155/2015/648063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schwartzberg R.S., Reuss B.L., Rust R. Efficacy of continuous subacromial bupivacaine infusion for pain control after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. 2013;22(10):1320–1324. doi: 10.1016/j.jse.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 82.Shlaifer A., Sharfman Z.T., Martin H.D., et al. Preemptive analgesia in hip arthroscopy: A randomized controlled trial of preemptive periacetabular or intra-articular bupivacaine in addition to postoperative intra-articular bupivacaine. Arthroscopy. 2017;33:118–124. doi: 10.1016/j.arthro.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 83.Spence D., Goff J., Mohan E., Bowen K., Osborne L., Maye J. Perioperative administration of gabapentin for shoulder arthroscopy: A prospective, randomized, double-blind, placebo-controlled study. AANA J. 2011;79(4 Suppl):S43–S50. [PubMed] [Google Scholar]
- 84.Syed U.A.M., Aleem A.W., Wowkanech C., et al. Neer Award 2018: The effect of preoperative education on opioid consumption in patients undergoing arthroscopic rotator cuff repair: A prospective, randomized clinical trial. J Shoulder Elbow Surg. 2018;27:962–967. doi: 10.1016/j.jse.2018.02.039. [DOI] [PubMed] [Google Scholar]
- 85.Tompkins M., Plante M., Monchik K., Fleming B., Fadale P. The use of a non-benzodiazepine hypnotic sleep-aid (Zolpidem) in patients undergoing ACL reconstruction: A randomized controlled clinical trial. Knee Surg Sports Traumatol Arthrosc. 2011;19:787–791. doi: 10.1007/s00167-010-1368-x. [DOI] [PubMed] [Google Scholar]
- 86.Westergaard B., Jensen K., Lenz K., et al. A randomised controlled trial of ultrasound-guided blockade of the saphenous nerve and the posterior branch of the obturator nerve for postoperative analgesia after day-case knee arthroscopy. Anaesthesia. 2014;69:1337–1344. doi: 10.1111/anae.12794. [DOI] [PubMed] [Google Scholar]
- 87.Wong A.K., Keeney L.G., Chen L., Williams R., Liu J., Elkassabany N.M. Effect of local anesthetic concentration (0.2% vs 0.1% ropivacaine) on pulmonary function, and analgesia after ultrasound-guided interscalene brachial plexus block: A randomized controlled study. Pain Med Malden Mass. 2016;17:2397–2403. doi: 10.1093/pm/pnw057. [DOI] [PubMed] [Google Scholar]
- 88.Xing J.G., Abdallah F.W., Brull R., et al. Preoperative femoral nerve block for hip arthroscopy: A randomized, triple-masked controlled trial. Am J Sports Med. 2015;43:2680–2687. doi: 10.1177/0363546515602468. [DOI] [PubMed] [Google Scholar]
- 89.Yun M.J., Oh J.H., Yoon J.P., Park S.H., Hwang J.W., Kil H.Y. Subacromial patient-controlled analgesia with ropivacaine provides effective pain control after arthroscopic rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2012;20:1971–1977. doi: 10.1007/s00167-011-1841-1. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Z., Zhu W., Zhu L., Du Y. Efficacy of celecoxib for pain management after arthroscopic surgery of hip: A prospective randomized placebo-controlled study. Eur J Orthop Surg Traumatol Orthop Traumatol. 2014;24:919–923. doi: 10.1007/s00590-013-1359-y. [DOI] [PubMed] [Google Scholar]
- 91.Zhou F., Du Y., Huang W., Shan J., Xu G. The efficacy and safety of early initiation of preoperative analgesia with celecoxib in patients underwent arthroscopic knee surgery: A randomized, controlled study. Medicine (Baltimore) 2017;96 doi: 10.1097/MD.0000000000008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thapa D., Ahuja V., Verma P., Gombar S., Gupta R., Dhiman D. Post-operative analgesia using intermittent vs. continuous adductor canal block technique: A randomized controlled trial. Acta Anaesthesiol Scand. 2016;60:1379–1385. doi: 10.1111/aas.12787. [DOI] [PubMed] [Google Scholar]
- 93.Lee Y.-S., Park Y.-C., Kim J.-H., et al. Intrathecal hydromorphone added to hyperbaric bupivacaine for postoperative pain relief after knee arthroscopic surgery: A prospective, randomised, controlled trial. Eur J Anaesthesiol. 2012;29:17–21. doi: 10.1097/EJA.0b013e3283476055. [DOI] [PubMed] [Google Scholar]
- 94.Hartwell M.J., Selley R.S., Terry M.A., Tjong V.K. Can we eliminate opioid medications for postoperative pain control? A prospective, surgeon-blinded, randomized controlled trial in knee arthroscopic surgery. Am J Sports Med. 2020;48:2711–2717. doi: 10.1177/0363546520941861. [DOI] [PubMed] [Google Scholar]
- 95.Davey M.S., Hurley E.T., Anil U., et al. Pain management strategies after anterior cruciate ligament reconstruction: A systematic review with network meta-analysis. Arthroscopy. 2021;37:1290–1300.e6. doi: 10.1016/j.arthro.2021.01.023. [DOI] [PubMed] [Google Scholar]
- 96.Wolff A.B., Hogan G.W., Capon J.M., Napoli A.M., Smith H.J., Gaspar P.S. Pre-operative lumbar plexus block provides superior post-operative analgesia when compared with fascia iliaca block or general anesthesia alone in hip arthroscopy. J Hip Preserv Surg. 2016;3:338–345. doi: 10.1093/jhps/hnw021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.YaDeau J.T., Tedore T., Goytizolo E.A., et al. Lumbar plexus blockade reduces pain after hip arthroscopy: A prospective randomized controlled trial. Anesth Analg. 2012;115:968–972. doi: 10.1213/ANE.0b013e318265bacd. [DOI] [PubMed] [Google Scholar]
- 98.Premkumar A., Samady H., Slone H., Hash R., Karas S., Xerogeanes J. Liposomal bupivacaine for pain control after anterior cruciate ligament reconstruction: A prospective, double-blinded, randomized, positive-controlled trial. Am J Sports Med. 2016;44:1680–1686. doi: 10.1177/0363546516640772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.