Abstract
Background
COVID-19 has been associated with persistent symptoms and functional changes, especially in those surviving severe disease.
Methods
We conducted a prospective multicenter study in patients with severe COVID-19 to determine respiratory sequelae. Patients were stratified into two groups: ward admission (WA) and intensive care unit (ICU) admission. In each follow-up visit, the patients where inquired about cough and dyspnea, and performed spirometry, lung volumes, carbon monoxide diffusion capacity (DLCO), 6-minute walk test (6MWT), and respiratory muscle strength (MIP and MEP). Results of pulmonary function tests at 45 days and 6 months after hospital admission were compared using paired analysis.
Results
211 patients were included, 112 in WA and 99 in ICU. Dyspnea persisted in 64.7% in the WA and 66.7% in the ICU group after 6 months. Lung function measures showed significant improvement between 45 days and 6 months, both in WA and ICU groups in VC, FVC, FEV1, total lung capacity, and 6MW distance measures. The improvement in the proportions of the altered functional parameters was significant in the ICU group for VC (44.2% 45 d; 20.8% 6 m; p = 0,014), FVC (47.6% 45 d; 28% 6 m; p = 0,003), FEV1 (45.1% 45 d; 28% 6 m; p = 0,044), DLCO (33.8% 45 d; 7.7% 6 m; p < 0,0001).
Conclusion
Six months follow-up of patients with the severe forms of COVID-19 showed significant improvement in the lung function measures compared to 45 days post hospital discharge. The difference was more evident in those requiring ICU admission.
Keywords: COVID-19, Lung function, Follow-up, Post-COVID condition
Introduction
COVID-19 has been responsible for millions of deaths worldwide, and is associated with significant morbidity in those who survive the severe form.(1) Fatigue, dyspnea, joint pain, cognitive changes, chest pain and hair loss are frequently observed long after hospital discharge.2 Despite involving multiple organs, respiratory symptoms dominate both the acute phase and long-term sequelae. Dyspnea and fatigue are the most common complaints.1 In a large prospective cohort from Wuhan, China, 76% of 1773 patients reported at least one symptom out of a list of 17, with dyspnea present in 26% at six-month follow-up.3
Studies on respiratory complications after hospital discharge showed interstitial abnormalities on chest tomography (CT) in 55.7% and decreased carbon monoxide diffusing capacity (DLCO) in 34.8%. Changes were more frequent in patients who had undergone mechanical ventilation (MV).4,5 Potential mechanisms to explain the persistence of symptoms would be inflammation and oxidative stress leading to insufficient immune response for complete viral eradication; persistence of viral antigenic remnants causing prolonged inflammatory response, persistent viremia and insufficient antibody generation; or a procoagulant state induced by SARS-CoV-2 infection. Other factors could be the severity of disease, need for intensive care, presence of comorbidities, or the treatment used.1,6,7 This study aimed to describe lung function in patients six months after severe COVID-19 and to compare it with that recorded at 45 days after discharge.8
Methods
This prospective multicenter study evaluated for inclusion patients aged 18 or over, admitted to three public referral hospitals for COVID-19 in Belo Horizonte, Minas Gerais, Brazil, with a confirmed diagnosis of COVID-19 (positive RT-PCR result from nasal or oropharyngeal swabs) and severe acute respiratory syndrome (SARS), between June 16 2020 and January 05 2021. SARS was defined as the presence of fever and cough or sore throat, associated with dyspnea, chest tightness, or SpO2 < 95%.9 Patients with indication for palliative care were considered ineligible. Patients who were too weak to perform the tests, and those who withdrew consent were not included in the analysis.
This study was approved by the national ethics committee (CONEP), protocol number 4.932.048. Approval at the local ethics committee of the three hospitals was also obtained. All participants gave written informed consent.
Patients were stratified into two groups: ward admission (WA) and intensive care unit (ICU). The results of pulmonary function tests at 45 days and six months after hospital admission were compared. Demographics, clinical manifestations, comorbidities, continuous medications, smoking, date of onset of respiratory symptoms, date of hospital admission, length of hospital stay, length of ICU stay, length of mechanical ventilation (MV), and complications during hospitalization were recorded. Laboratory tests and chest imaging at admission were performed at the discretion of the attending clinicians. Arterial blood gases, complete blood workup, C-reactive protein (CRP), LDH, serum albumin, prothrombin time/international normalized ratio (INR), D-dimer, creatinine, ALT, and AST results were recorded when available. Gas exchange was evaluated by the PaO2/FiO2 ratio. The proportion of pulmonary impairment on CT scans was recorded as informed in the reports provided by the hospital radiology specialists.
The major outcomes studied were lung function (spirometry, lung volumes, DLCO), exercise capacity (6-minute walk distance - 6MWD), and respiratory muscle strength (MIP and MEP) at six months after hospital admission. These data were compared to those registered 45 days post-discharge, in the same cohort, published elsewhere.8 According to the study design, assessment for eligibility took place within 24 hours of admission, and follow-up assessment was scheduled for 45 and 180 days after admission, with a tolerance of ± 15 days.
In each follow-up visit, the patient was inquired about cough and dyspnea (modified Medical Research Council scale).10 Vital data, weight and height were recorded. Lung function tests were performed in the Pulmonary Function Laboratory of the University Hospital of the Federal University of Minas Gerais. A Collins CPL system (Ferraris Respiratory, Louisville, CO, USA) was used for the determination of absolute lung volumes, spirometry parameters, and DLCO in accordance with international criteria.11,12 The helium dilution in a constant volume system was used to measure lung volumes. The following variables were studied: TLC, slow vital capacity (VC), FVC, FEV1, and FEV1/FVC ratio. Measurements were reported as absolute values and %pred for the Brazilian population.13,14
The single breath method was used for the determination of DLCO, considering the values suggested by Guimarães et al.15
The 6MWT was performed in a 30 meters corridor using a portable oximeter (Nonin Medical Inc., Plymouth, MN, USA) in accordance with international standards.16 The following variables were recorded: SpO2, HR, RR, Borg dyspnea scale score at the beginning and end of the test, HR in %pred in relation to the maximum HR in %pred for adults, HR at the end of 6MWT, HR 1 min after recovery time, and 6MWD. Oxygen desaturation ≥ 4% was considered altered result.17,18 The 6MWD was expressed in absolute values and in %pred for the Brazilian population.17
MIP and MEP were measured with an analog manometer (Makil, Londrina, Brazil), as described by Laveneziana et al.19 The maneuver was repeated five to eight times, respecting a 10% reproducibility. The highest measure was recorded. Predicted values were calculated in accordance with Neder et al.20 The lower limit of normal (LLN) for each variable was calculated following prediction equations.12
Diagnosis of COVID-19, lung function measurements, and selection bias were considered possible sources of bias. Diagnosis was defined by the gold-standard RT-PCR and the equipment used for lung function measurements was calibrated according to the recommendations of the manufacturers. Selection bias was minimized by the multicenter design.
Data were collected using the REDCap platform (Vanderbilt University, Nashville, TN, USA) and analyzed with the IBM SPSS Statistics software package, version 28.0 (IBM Corporation, Armonk, NY, USA). Categorical variables are described as frequencies and proportions. Continuous variables with normal distribution are described as means and standard deviations, whereas those with non-normal distribution are described as medians and interquartile ranges. Predicted values and LLN were used as risk to categorize continuous variables. Parametric Student's t-test or nonparametric Mann-Whitney U test with post-hoc analysis were used to verify differences between the groups, pairwise comparisons of continuous variables, and Pearson's chi-square for proportions. Proportions of dependent groups were compared using the McNemar test and continuous variables using paired Student's t-test. Hypothesis testing was two-sided, and the level of significance was set at p < 0.05.
Results
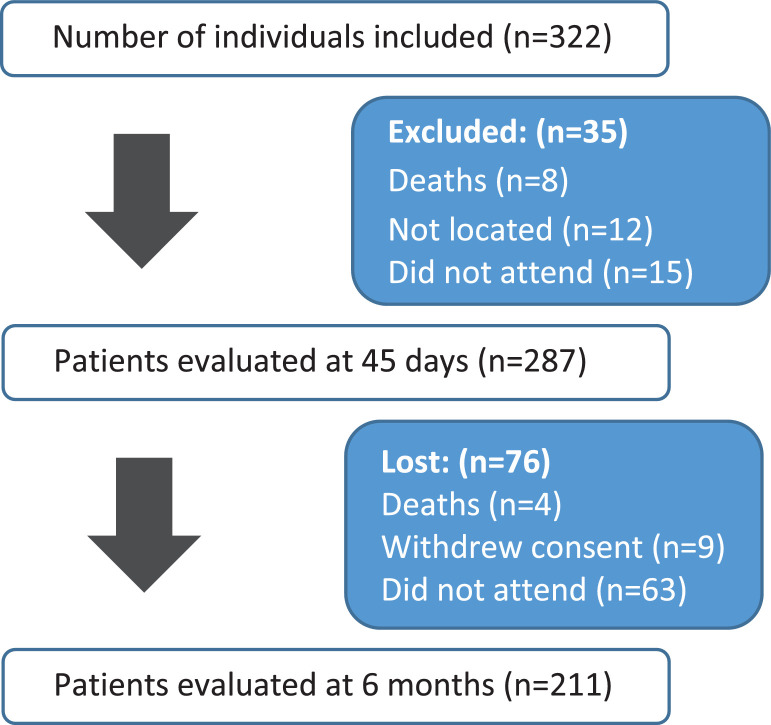
Three hundred and twenty-two patients were considered eligible, 211 were included in the analysis (Fig. 1).
Fig. 1.
Flow Chart: patients evaluated between May 23rd 2020 and January 5th 2021.
One hundred twelve patients (53.1%) were in WA and 99 (46.9%) in ICU groups. Groups were homogeneous regarding age (60.8 ± 13.9 years), sex (male 51.7%), education, family income, self-reported skin color, marital status, and pre-existing medical conditions. The majority (88.2%) of participants had at least one comorbidity. Hypertension was reported in 74.1% patients, obesity in 39.4%, diabetes mellitus in 33%. Other cardiovascular diseases were described in 29 (15.9%) patients. The use of immunosuppressants was reported in 4.5%, and 2.2% had undergone bone marrow or solid organ transplantation. Asthma and chronic obstructive pulmonary disease (COPD) were diagnosed in 10.3 and 7.7%, respectively. Eight (4.4%) patients had chronic renal disease and 59 (28.8%) patients reported current or former smoking (Table 1).
Table 1.
Sociodemographic baseline characteristics and pre-existing conditions.
| Variable | Total n = 211 |
Ward n = 112 |
ICU n = 99 |
p-value | |
|---|---|---|---|---|---|
| Age (mean ± SD) | 60.8 (13.9) | 62.4 (13.9) | 59.1 (13.3) | 0.083 | |
| Male, n (%) | 109 (51.7) | 53 (47.3) | 56 (56.6) | 0.180 | |
| Variable | Category | n (%) | |||
| Educationa | Higher education/post-graduation | 16 (11.2) | 8 (10.4) | 8 (12.1) | 0.546 |
| Elementary to high school | 59 (41.3) | 35 (45.5) | 24 (36.4) | ||
| No education or incomplete elementary school (< 8 years) | 68 (47.6) | 34 (44.2) | 34 (51.5) | ||
| Incomea | > 3 MW | 21 (15.2) | 11 (15.1) | 10 (15.4) | 0.837 |
| Up to 3 MW | 112 (81.2) | 60 (82.2) | 52 (80.0) | ||
| No income | 5 (3.6) | 2 (2.7) | 3 (4.6) | ||
| Self-reported skin colora | White | 37 (24.2) | 23 (28.0) | 14 (19.7) | 0.484 |
| Brown | 85 (55.6) | 43 (52.4) | 42 (59.2) | ||
| Black | 31 (20.3) | 16 (19.5) | 15 (21.1) | ||
| Marital Statusa | Not Married | 71 (48.3) | 38 (47.5) | 34 (49.3) | 0.832 |
| Married | 76 (51.7) | 42 (52.5) | 34 (50.7) | ||
| Pre-existing conditions | |||||
| Presence of comorbidities | 186 (88.2) | 100 (89.3) | 86 (86.9) | 0.588 | |
| High blood pressureb | 137 (74.1) | 69 (69.7) | 68 (79.1) | 0.147 | |
| Obesityb | 71 (39.4) | 33 (33.3) | 38 (46.9) | 0.064 | |
| Diabetes mellitusb | 61 (33.0) | 30 (30.0) | 31 (36.5) | 0.351 | |
| Other cardiovascular diseaseb | 29 (15.9) | 16 (16.2) | 13 (15.7) | 0.927 | |
| Asthmab | 19 (10.3) | 12 (12.1) | 7 (8.2) | 0.388 | |
| COPDb | 14 (7.7) | 7 (7.1) | 7 (8.4) | 0.731 | |
| Chronic kidney diseaseb | 8 (4.4) | 3 (3.0) | 5 (6.0) | 0.335 | |
| Other comorbiditiesb | 88 (47.6) | 52 (52.0) | 36 (42.4) | 0.190 | |
| Smokinga | 59 (28.8) | 32 (29.6) | 27 (27.8) | 0.777 | |
| Use of immunosuppressive medicationcb | 8 (4.5) | 5 (5.3) | 3 (3.6) | 0.574 | |
| Solid organ or bone marrow transplantationb | 3 (2.2) | 1 (1.3) | 2 (3.2) | 0.435 | |
Missing data (≤ 10%)
Missing data (10-20%);
ICU: intensive care unit; SD: standard deviation; MW: minimum wage (3 MW = $613.50); COPD: chronic obstructive pulmonary disease;
Prednisone > 20 mg/day for more than two weeks, cyclosporine, cyclophosphamide, mycophenolate, rituximab, azathioprine or chemotherapy within the past 30 days.
Time elapsed from symptom onset to hospitalization was similar between groups, 9.2 ± 8.6 days. The most commonly reported symptom on admission was dyspnea (82.4%), more frequent in the ICU. Cough (dry or productive), fever, myalgia, sore throat, rhinorrhea and abdominal pain were similar in both groups. Changes in taste and smell, as well as diarrhea were more frequent in WA group. Complications during hospitalization were more frequent in ICU group: acute renal failure in 14 (14.4%) patients and and vascular thrombosis in 20 (20. 6%). Antibiotics were used by 194 (92.8%) patients, with no difference in the two groups (Supplement Table).
Some laboratory changes and severity scores on admission showed significant differences between groups. Increase in inflammation and acute phase markers – CRP, LDH, albumin, AST, ALT – were more pronounced in the ICU group. Total leukocyte and neutrophil counts, creatinine, and INR were also more significantly increased in ICU group. Average PaO2/FiO2 was significantly lower in ICU group. Similarly, the Sequential Sepsis-related Organ Failure Assessment (SOFA) scores in the first 24 hours were significantly higher in ICU group. One hundred and two patients had CT during hospital stay. Thirty-five (34.3%) had lung damage ≥ 50%, 22 (62.9%) in ICU group (p = 0.004).
The length of hospital stay was longer in ICU group (WA: 8 days (5-10), ICU: 16 days (10.5-24); p < 0.001). The first post-discharge functional pulmonary evaluation took place at 49.5 ± 34.7 days and the second at 180.7 ± 34.9 days after hospital admission. Average time between the first and second evaluations was 131.9 ± 31.2 days (Supplement Table).
Paired comparisons of lung function measurements assessed after 45 and 180 days showed significant improvement of several parameters VC, FVC, FEV1, TLC, DLCO, 6MWD, and 6MWD%pred which was more pronounced in ICU group (Table 2).
Table 2.
Paired analysis of pulmonary function tests measurements at 45 and 180 days.
| Variable | n | Ward, mean (±SD) |
p | n | ICU, mean (±SD) |
p-value | ||
|---|---|---|---|---|---|---|---|---|
| D45 | D180 | D45 | D180 | |||||
| BMI | 109 | 31.0 (7.1) | 31.2 (7.2) | 0.294 | 98 | 31.2 (7.0) | 31.9 (6.9) | < 0.0001 |
| VC, liter a | 99 | 3.1 (0.9) | 3.2 (0.9) | < 0.0001 | 84 | 3.0 (0.8) | 3.3 (0.9) | < 0.0001 |
| VC, % of preda | 99 | 88.1 (13.7) | 92.9 (15.1) | < 0.0001 | 84 | 81.3 (17.0) | 89.4 (16.5) | < 0.0001 |
| FVC, litersa | 105 | 2.9 (0.9) | 3.1 (0.9) | < 0.0001 | 94 | 2.9 (0.8) | 3.2 (0.9) | < 0.0001 |
| FVC, % of preda | 105 | 83.7 (13.8) | 88.7 (15.4) | < 0.0001 | 94 | 78.0 (16.0) | 87.1 (19.7) | < 0.0001 |
| FEV1, litersa | 105 | 2.2 (0.7) | 2.3 (0.7) | < 0.0001 | 94 | 2.3 (0.6) | 2.5 (0.7) | < 0.0001 |
| FEV 1,% of preda | 105 | 80.1 (16.5) | 84.6 (18.0) | < 0.0001 | 94 | 77.2 (15.6) | 84.7 (16.5) | < 0.0001 |
| FEV 1/FVC, %a | 105 | 76.3 (10.5) | 75.9 (10.8) | 0.394 | 93 | 79.9 (6.7) | 78.9 (8.5) | 0.212 |
| TLC, litersa | 92 | 4.9 (1.1) | 5.1 (1.2) | 0.001 | 79 | 4.8 (1.3) | 5.2 (1.2) | < 0.0001 |
| TLC, % of preda | 92 | 92.9 (13.3) | 98.4 (16.0) | < 0.0001 | 78 | 84.7 (16.2) | 94.4 (19.6) | < 0.0001 |
| RV, litersa | 92 | 1.8 (0.5) | 1.9 (0.5) | 0.161 | 79 | 1.7 (0.6) | 1.8 (0.5) | 0.089 |
| RV, % preda | 92 | 93.9 (26.5) | 101.5 (44.8) | 0.148 | 78 | 88.6 (27.7) | 94.2 (24.9) | 0.151 |
| RV/TLC, % preda | 92 | 111.0 (32.3) | 111.4 (24.6) | 0.922 | 77 | 110.4 (27.4) | 111.0 (26.2) | 0.849 |
| DLCO, ml, min−1, mmHg−1a | 88 | 23.1 (6.4) | 24.3 (6.7) | 0.028 | 74 | 20.8 (8.2) | 22.9 (6.0) | 0.001 |
| DLCO, % predb | 88 | 110.7 (21.9) | 116.0 (18.2) | 0.004 | 73 | 91.6 (26.0) | 103.9 (21.4) | < 0.0001 |
| MIP, cmH2Ob | 91 | 75.9 (31.3) | 76.8 (29.2) | 0.655 | 88 | 77.2 (26.0) | 76.1 (27.8) | 0.595 |
| MEP, % predb | 91 | 85.6 (31.7) | 87.2 (31.5) | 0.533 | 87 | 84.3 (30.8) | 84.5 (32.8) | 0.937 |
| MEP, cmH2Ob | 91 | 91.0 (37.5) | 86.4 (35.8) | 0.139 | 87 | 91.5 (31.2) | 87.6 (30.3) | 0.186 |
| MEP, % pred | 91 | 53.2 (19.1) | 50.8 (18.3) | 0.181 | 86 | 52.8 (18.6) | 50.8 (16.9) | 0.259 |
| 6MWD,ma | 102 | 445.1 (100.6) | 462.0 (111.6) | 0.048 | 92 | 440.3 (99.8) | 466.6 (83.6) | 0.001 |
| 6MWD % preda | 101 | 86.2 (16.7) | 90.3 (18.8) | 0.025 | 92 | 84.5 (20.0) | 90.0 (16.3) | 0.001 |
| HRR1,bpma | 102 | 96.5 (15.7) | 94.0 (16.5) | 0.074 | 90 | 94.8 (17.5) | 89.2 (16.1) | 0.002 |
| ΔHRFinalHRR1, bpma | 102 | 15.8 (13.8) | 20.4 (15.7) | 0.015 | 90 | 17.7 (16.6) | 21.1 (10.9) | 0.109 |
| % HRmaxa | 100 | 70.8 (12.6) | 72.3 (13.2) | 0.298 | 92 | 69.9 (11.7) | 68.1 (10.4) | 0.109 |
Missing data (≤ 10%);
Missing data (11-12%);
ICU: intensive care unit; SD: standard deviation; BMI: body mass index; VC: vital capacity; FVC: forced vital capacity; FEV1: forced expiratory volume in the first second; TLC: total lung capacity; RV: residual volume; DLCO: carbon monoxide diffusion; MIP: maximal inspiratory pressure; MEP: maximal expiratory pressure; 6MWD,m: six minute walk distance, meters; HRR1: recovery heart rate in the first minute; bpm: beats per minute; Δ: variation; HR: heart rate; % HRmax: percentage of maximum heart rate achieved.
The frequency of altered functional parameters after 45 and 180 days decreased more markedly in ICU than WA groups for VC (44.2% vs 20.8%), FVC (47.6% vs 28.0%), FEV1 (45.1% vs 28.0%), and DLCO (33.8% vs 7.7%). The frequency of stress desaturation ≥ 4% increased at six months (83.6%) compared to 45 days (60.3%) in ICU group, whereas Final Borg Scale ≥ 4 decreased only in WA group (50% vs 25%) (Table 3).
Table 3.
Paired analysis of the categorical variables of pulmonary function tests results at 45 and 180 days.
| Variable | Total pairs | Ward |
P** | Total pairs | ICU |
P-value** | ||
|---|---|---|---|---|---|---|---|---|
| Proportion changed D45 n (%) |
Proportion changed 180 n (%) |
Proportion changed D45 n (%) |
Proportion changed 180 n (%) |
|||||
| Dyspnea | 85 | 41 (48.2) | 38 (44.7) | 0.711 | 73 | 41 (56.2) | 30 (41.1) | 0.071 |
| Dyspnea (mMRC) >=2 | 17 | 10 (58.8) | 11 (64.7) | 1.000 | 15 | 10 (66.7) | 10 (66.7) | 1.000 |
| Cough | 83 | 20 (24.1) | 21 (25.3) | 1.000 | 73 | 22 (30.1) | 13 (17.8) | 0.064 |
| VC< LLN, (%) a | 85 | 16 (18.8) | 17 (20.0) | 1.000 | 77 | 34 (44.2) | 16 (20.8) | 0.014 |
| FVC< LLN, (%) a | 93 | 25 (26.9) | 22 (23.7) | 0.728 | 82 | 39 (47.6) | 23 (28.0) | 0.003 |
| FEV1< LLN, (%) a | 93 | 31 (33.3) | 22 (23.7) | 0.200 | 82 | 37 (45.1) | 23 (28.0) | 0.044 |
| FEV1/FVC< LLN, (%) a | 93 | 37 (39.8) | 30 (32.3) | 0.371 | 82 | 27 (32.9) | 33 (40.2) | 0.430 |
| TLC< LLN, (%) a | 82 | 78 (95.1) | 79 (96.3) | 1.000 | 69 | 67 (97.1) | 68 (98.6) | 1.000 |
| DLCO < LLN, (%) b | 78 | 6 (7.7) | 8 (10.3) | 0.791 | 65 | 22 (33.8) | 5 (7.7) | < 0.0001 |
| MIP < LLN, (%) b | 80 | 32 (40.0) | 36 (45.0) | 0.644 | 74 | 27 (36.5) | 27 (36.5) | 1.000 |
| MEP < LLN, (%) b | 80 | 76 (95.0) | 75 (93.8) | 1.000 | 73 | 68 (93.2) | 72 (98.6) | 0.219 |
| Exercise oxygen desaturation (Δ SpO2 ≥ 4%) a | 88 | 62 (70.5) | 61 (69.3) | 1.000 | 73 | 44 (60.3) | 61 (83.6) | 0.003 |
| BorgFinal ≥ 4 a | 72 | 36 (50.0) | 18 (25.0) | 0.005 | 61 | 23 (37.7) | 18 (29.5) | 0.473 |
Missing data (≤ 10%);
Missing data (11-12%);
ICU: intensive care unit; VC: vital capacity; FVC: forced vital capacity; LLN: lower limit of normality; FEV1: forced expiratory volume in the first second; mMRC: modified Medical Research Council; TLC: total lung capacity; RV: residual volume; DLCO: carbon monoxide diffusion; MIP: maximal inspiratory pressure; MEP: maximal expiratory pressure; SpO2: pulse oxygen saturation; Δ:variation.** McNemar's chi-square.
The FEV1/FVC ratio below the LLN, translating obstructive ventilatory disorder, was observed in 30 (32.3%) patients of WA and 33 (40.2%) of ICU group at six months (Table 3). Among the 76 individuals classified as having obstruction at six months, 27 (35.5%) were smokers, 8 (10.5%) had asthma, and 12 (15.8%) had COPD.
MIP and MEP below the LLN was 36 (45.0%) and 27 (36.5) at six months, in WA and ICU respectively, with no significant difference from the 45-day assessment (Table 3).
Dyspnea ≥ 2 was observed in 11 (64.7%) of WA and 10 (66.7%) of ICU group at six monyhs (Table 3).
Discussion
To the best of our knowledge, this is the first study from South America to prospectively compare clinical and functional data of survivors of severe COVID-19, 45 days and six months after hospitalization. The main results of this study are that time heals almost all wounds, including those in patients who required ICU admission. At six months, residual abnormalities in lung function were still present in most of the cohort, but with significant improvement compared to the 45-day assessment.8 Restrictive ventilatory disorder was the most prevalent abnormality seen at six months and was more frequent in ICU group (98%), but with mild severity (mean CPT% 94.4 ± 19.6). The ICU group had a greater reduction in the frequency of lung function abnormalities such as FVC, FEV1 and DLCO. Our results agree with studies that included patients with moderate and severe COVID-19 in long-term follow-up.3 Lung involvement > 50% was present in 34.3% of those with CT on admission, and this rate was higher in ICU group. Post-COVID-19 fibrotic changes may account for the restriction. Pulmonary fibrosis was described in 10% of patients with persistent symptoms after three months. Need for mechanical ventilation during hospitalization and persistence of dyspnea at follow-up were independent risk factors for post-COVID-19 fibrosis.21
Decreased DLCO is the most frequently described change in long-term follow-up after COVID-19, whether in mild or severe forms.3,22 However, in these studies, as in our cohort, a significant improvement in DLCO was observed after six months.3,23,24 Zhang et al. reported a 32% reduction in DLCO after severe COVID-19 after eight months. In their cohort, 30% of patients had interstitial lung abnormalities, with ground glass being the most frequent (50%), followed by irregular lines.24 Risk factors for developing fibrotic changes after six months were age greater than 50 years, extensive lung involvement on admission CT, and acute respiratory distress syndrome.25 Wu et al. also reported altered DLCO in 33% of patients at 12 months.22 However, in their cohort, they did not include patients who required ICU admission or with comorbidities. In contrast, our cohort also included critically ill patients, and we observed a persistent 6-month DLCO reduction in only 8% of WA and 7.7% of ICU group. A possible explanation would be a differentiated recovery due to the already incorporated use of corticosteroid for treatment during the inclusion of our patients.27
Obstructive ventilatory disorder was observed in 32.3% in WA and 40.2% in ICU group at six months. These results cannot be fully explained by the reported frequencies of asthma (10.3%) and COPD (7.7%). Smokers accounted for 28.8% of our cohort. An important epidemiological study conducted in six Latin American cities, the Platino study, showed that COPD was underdiagnosed in up to 70%.26 It is possible that the high frequency of obstructive disorder found in our cohort, higher than in most post-COVID-19 lung function studies, is related to those who could have previous undiagnosed smoking COPD. Obstruction may also be associated with emphysematous changes related to direct parenchymal destruction by viral infection or ventilator-induced lung injury.27
From a list of 17 symptoms evaluated after six months, muscle weakness and fatigue were the most common, seen in 63% of a cohort of 1,733 patients.3 The impairment of inspiratory and expiratory muscle strength was similar and remained unchanged at six months regardless of the group, and may be attributed to physical deconditioning. Deconditioning was identified as a predominant factor causing dyspnea/fatigue symptoms in three studies that evaluated persistent symptoms after COVID-19 using cardiopulmonary exercise testing. Deconditioning is the main mechanism of impaired cardiopulmonary exercise capacity three months after COVID-19 hospitalization.28,29 Other authors have linked respiratory muscle weakness to the occurrence of interstitial lung disease after COVID-19.30
Significant improvement in walking distance was observed in both groups at six months in our cohort. There was no change in the frequency of stress desaturation in WA group between the two assessments, but ICU group showed a significant increase in this finding at six months. This worsening may be due to the higher metabolic-energy expenditure during the test, which is compatible with the deconditioning expected in the more severe patients. Similar results were reported in a cohort that included 72 patients undergoing MV, assessed six months after hospital discharge.31 Wu et al. found higher mean 6MWD values at six months (585 m); however, patients who required MV and had comorbidities were not included.22
There is information on persistent respiratory symptoms in the follow-up of survivors of severe COVID-19.3,22,24 We observed dyspnea grade > 1 in 64.7% (WA) and 66.7% (ICU) of the patients after six months. Our findings differed from those of Huang et al.3 In their cohort of 1,773 patients only 26% had dyspnea grade >1, six months after discharge. The risk was higher in the groups requiring high-flow oxygen and MV during hospitalization.3
The strengths of this study design are the multicenter and prospective nature; the inclusion of patients at different levels of severity; and the assessment of different aspects of pulmonary functional capacity.
This study has limitations. One, the absence of pre-hospitalization information on lung function, especially in smokers. Two, chest imaging examinations at follow-up were not evaluated, limiting the correlation of ventilatory disturbances with structural changes. Finally, appropriate investigation of respiratory muscle weakness as a cause of reduced MIP and MEP should include non-voluntary techniques such as diaphragm ultrasound and transdiaphragmatic pressure.
In conclusion, six months follow-up of patients with severe COVID-19 showed overall improvement in lung function, more expressive among those who required ICU admission.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgment
This work was funded by the Pro Reitoria de Pesquisa of The Federal University of Minas Gerais (UFMG). The authors thank the Federal University of Ouro Preto for their support.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.bjid.2022.102352.
Appendix. Supplementary materials
References
- 1.Nalbandian A, Sehgal K, Gupta A, Madhavan M, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Razak F, Katz GM, Cheung AM, Herridge MS, Slutsky AS, Allen U, et al. Understanding the post COVID-19 condition (long COVID) and the expected burden for Ontario. Science briefs of the Ontario COVID-19 science advisory table. 2021;2(44).
- 3.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet Respir Med. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.So M, Kabata H, Fukunaga K, Takagi H, Kuno T. Radiological and functional lung sequelae of COVID-19: a systematic review and meta-analysis. BMC Pulm Med. 2021;21:97. doi: 10.1186/s12890-021-01463-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qin W, Chen S, Zhang Y, Dong F, Zhang Z, Hu B, et al. Diffusion capacity abnormalities for carbon monoxide in patients with COVID-19 at 3-month follow-up. Eur Respir J. 2021;58 doi: 10.1183/13993003.03677-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naeije R, Caravita S. Phenotyping long COVID. Eur Respir J. 2021;58 doi: 10.1183/13993003.01763-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akbarialiabad H, Taghrir MH, Abdollahi A, Ghahramani N, Kumar M, Paydar S, et al. Long COVID, a comprehensive systematic scoping review. Infection. 2021;49:1163–1186. doi: 10.1007/s15010-021-01666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mancuzo EV, Marinho CC, Luiz G, Machado-Coelho L, Batista AP, Oliveira JF, et al. Lung function of patients hospitalized with COVID-19 at 45 days after hospital discharge: first report of a prospective multicenter study in Brazil. J Bras Pneumol. 2021;2021(47) doi: 10.36416/1806-3756/e20210162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brasil. Ministério da Saúde. [homepage on the Internet]. Brasília: Ministério da Saúde; c2020 [cited 2021 Mar 1]. Protocolo de Tratamento do Novo Coronavírus (2019-nCoV). [Adobe Acrobat document, 32p.]. Available from: https://portalarquivos2.saude.gov.br/images/pdf/2020/fevereiro/05/Protocolo-de-manejo-clinico-para-o-novo-coronavirus-2019-ncov.pdf
- 10.Lareau C, Meek PM, Roos PJ. Development and testing of the modified version of the Pulmonary Functional Status and Dyspnea Questionnaire (PFSDQ-M) Heart Lung. 1998;27:159–168. doi: 10.1016/s0147-9563(98)90003-6. [DOI] [PubMed] [Google Scholar]
- 11.Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of spirometry 2019 update. An official American thoracic society and European respiratory society technical statement. Am J Respir Crit Care Med. 2019;27:159–168. doi: 10.1164/rccm.201908-1590ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 13.Pereira CA, Sato T, Rodrigues SC. New reference values for forced spirometry in Brazilian adults of white race. J Bras Pneumol. 2007;33:397–406. doi: 10.1590/s1806-37132007000400008. [DOI] [PubMed] [Google Scholar]
- 14.Lessa T, Pereira CAC, Soares MR. Reference equations for plethysmographic lung volumes in white adults in Brazil as derived by linear regression. J Bras Pneumol. 2021;47 doi: 10.36416/1806-3756/e20200359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guimarães VP, de Miranda DM, Reis MAS, Andrade TL, Matos RL, Soares MR, et al. Reference values for the carbon monoxide diffusion (Transfer factor) in a Brazilian sample of white race. J Bras Pneumol. 2019;45 doi: 10.1590/1806-3713/e20180262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, et al. An official European respiratory society/American thoracic society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 17.Soares MR, Pereira CA. Six-minute walk test: reference values for healthy adults in Brazil. J Bras Pneumol. 2011;37:576–583. doi: 10.1590/s1806-37132011000500003. [DOI] [PubMed] [Google Scholar]
- 18.Singh SJ, Puhan MA, Andrianopoulos V, Hernandes NA, Mitchell KE, Hill CJ, et al. An official systematic review of the European Respiratory Society/American thoracic society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1447–1478. doi: 10.1183/09031936.00150414. [DOI] [PubMed] [Google Scholar]
- 19.Laveneziana P, Albuquerque A, Aliverti A, Babb T, Barreiro E, Dres M, et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J. 2019;53 doi: 10.1183/13993003.01214-2018. [DOI] [PubMed] [Google Scholar]
- 20.Neder JA, Andreoni S, Lerario MC, Nery LE. Reference values for lung function tests. II. Maximal respiratory pressures and voluntary ventilation. Braz J Med Biol Res. 1999;32:719–727. doi: 10.1590/s0100-879x1999000600007. [DOI] [PubMed] [Google Scholar]
- 21.Aul DR, Gates DJ, Draper DA, Dunleavy DA, Ruickbie DS, Meredith DH, et al. Complications after discharge with COVID-19 infection and risk factors associated with development of post-COVID pulmonary fibrosis. Respir Med. 2021;188 doi: 10.1016/j.rmed.2021.106602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu X, Liu X, Zhou Y, Yu H, Li R, Zhan Q, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9:747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darcis G, Bouquegneau A, Maes N, Thys M, Henket M, Labye F, et al. Long-term clinical follow-up of patients suffering from moderate-to-severe COVID-19 infection: a monocentric prospective observational cohort study. Int J Infect Dis. 2021;109:209–216. doi: 10.1016/j.ijid.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S, Bai W, Yue J, Qin L, Zhang C, Xu S, et al. Eight months follow-up study on pulmonary function, lung radiographic, and related physiological characteristics in COVID-19 survivors. Sci Rep. 2021;11:13854. doi: 10.1038/s41598-021-93191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia manuscript type: original research. Radiology. 2021;299:E177–E186. doi: 10.1148/radiol.2021203153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreira GL, Manzano BM, Gazzotti MR, Nascimento OA, Perez-Padilla R, Menezes AM, et al. PLATINO, a nine-year follow-up study of COPD in the city of São Paulo, Brazil: the problem of underdiagnosis. J Bras Pneumol. 2014;40:30–37. doi: 10.1590/S1806-37132014000100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rinaldo RF, Mondoni M, Parazzini EM, Pitari F, Brambilla E, Luraschi S, et al. Deconditioning as main mechanism of impaired exercise response in COVID-19 survivors. Eur Respir J. 2021;58 doi: 10.1183/13993003.00870-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skjørten I, Ankerstjerne OAW, Trebinjac D, Brønstad E, Rasch-Halvorsen Ø, Einvik G, et al. Cardiopulmonary exercise capacity and limitations 3 months after COVID-19 hospitalisation. Eur Respir J. 2021;58 doi: 10.1183/13993003.00996-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spagnolo P, Balestro E, Aliberti S, Cocconcelli E, Biondini D, Casa GD, et al. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med. 2020;8:750–752. doi: 10.1016/S2213-2600(20)30222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cabo-Gambin R, Benítez ID, Carmona P, Santiesteve S, Mínguez O, Vaca R, et al. Three to six months evolution of pulmonary function and radiological features in critical COVID-19 patients: a prospective cohort. Arch Bronconeumol. 2021 Jul 27 doi: 10.1016/j.arbres.2021.07.005. S0300-2896(21)00208-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.