Abstract
The limited therapeutic effect on hypoxic and refractory solid tumors has hindered the practical application of photodynamic therapy. Herein, we report our investigation of an osmium-peroxo complex (Os2), which is inactive in the dark, but can release a peroxo ligand O2•− upon light irradiation even in the absence of oxygen, and is transformed into a cytotoxic osmium complex (Os1). Os1 is cytotoxic in the presence or absence of irradiation in hypoxic tumors, behaving as a chemotherapeutic drug. At the same time, the light-activated Os2 induces photocatalytic oxidation of endogenous 1,4-dihydronicotinamide adenine dinucleotide in living cancer cells, leading to ferroptosis, which is mediated by glutathione degradation, lipid peroxide accumulation and down-regulation of glutathione peroxidase 4. In vivo studies have confirmed that the Os2 can effectively inhibit the growth of solid hypoxic tumors in mice. A promising strategy is proposed for the treatment of hypoxic tumors with metal-based drugs.
Subject terms: Cancer therapy, Photochemistry, Bioinorganic chemistry
Photodynamic therapy has been a promising technique for the treatment of tumours. In this manuscript, the authors report on the photoactivation of the osmium peroxo complex and its potential use for chemotherapy and photodynamic therapy under blue light irradiation against tumours in their hypoxic environment.
Introduction
The hypoxic microenvironment of solid tumors caused by abnormal proliferation and vascularization of cells can greatly compromise the therapeutic effect of traditional photodynamic therapy (PDT)1, which usually requires O2 for the production under light activation of reactive oxygen species (ROS) by a photosensitizer2–9. Many innovative methods have recently been introduced to solve the problem of tumor hypoxia. Some additives such as perfluorocarbons, artificial red blood cells, and covalent organic frameworks10–12, carry O2 directly to the tumor site, and others utilize unique in situ features of the tumor microenvironment to generate O2. These include CaO2 and graphite phase-carbon nitride (gC3N4) carbonization13,14 or high H2O2 concentrations in tumor cells resulting from MnO2 or H2O2 decomposition15,16, or even photocatalytic oxygen production by photosynthetic bacteria17. These nanomaterials drugs however are still not ideal and there are numerous possible adverse side effects18. In addition, in vivo studies have shown that enrichment of O2 promotes proliferation and inhibits apoptosis of malignant cells, results which are not conducive to PDT19. Therefore, improvement of the efficacy of phototherapy for hypoxic tumors is necessary.
It has been reported that some metal complexes show excellent PDT effect toward hypoxic tumors20–26. Most of them do not depend on oxygen through traditional type II mechanism for PDT. For example, Sadler et al. studied that an iridium (III) photoredox catalysis can provide an oxygen-independent mechanism of action to combat hypoxic tumors. The iridium (III) complex photocatalytically oxidized 1,4-dihy-dronicotinamide adenine dinucleotide (NADH), an important coenzyme in living cells, to generate NAD• radicals with a high turnover frequency, and synergistically photoreduced cytochrome C under hypoxia20. Chao et al. designed an iridium (III) complex that upon irradiation, produces free carbon radicals under hypoxia26. In addition, photoactive chemotherapy (PACT) has the potential to overcome the limitations imposed by hypoxia. In PACT, metal complexes can be photoactivated in a controlled manner to produce cytotoxic substances. Toxic gases such as NO27 and CO28, or ligand-centered cytotoxic substances can always be released by photoactivation29–32. The superoxide radical (O2•−) is one of the most toxic ROS and has been identified as a most useful oxidant for cancer treatment and an adjuvant in synergistic chemotherapy33,34. Under the action of intracellular superoxide dismutase, O2•− can form H2O2 and O2. The accumulated H2O2 is further transformed into a hydroxyl radical (OH•) with enhanced toxicity and reactivity, exacerbating oxidative damage of cancer cells and improving the anticancer effect35. The design of a photoactive compound that does not rely on O2 but is photo-controlled to release ROS has significant application prospects in hypoxic tumor treatment.
In this work, we investigate an osmium-peroxo complex (Os2), whose structure is stable in the dark. However, upon photoactivation with 465 nm light, Os2 releases O2•− even in severely hypoxic conditions (1% O2), and is transformed into the cytotoxic Os1 in a Cl− containing PBS solvent (Fig. 1a), thus maintaining useful photoactivation efficacy in hypoxia. Os1 displays toxicity in both light and dark, contributing to a synergistic effect of chemotherapy and PACT of Os2 under light irradiation. We also found that irradiation of Os2 induces distinct ferroptosis mediated by GSH degradation, lipid peroxide accumulation and GPX4 down-regulation. Furthermore, Os2 can photocatalytically oxidize endogenous NADH in living cancer cells, triggering ferroptosis. In vivo studies confirm that Os2 effectively inhibits the growth of solid hypoxic tumors in mice. Thus this work is developing a process to release O2•− for the treatment of hypoxic tumor cells through the mechanism of ferroptosis.
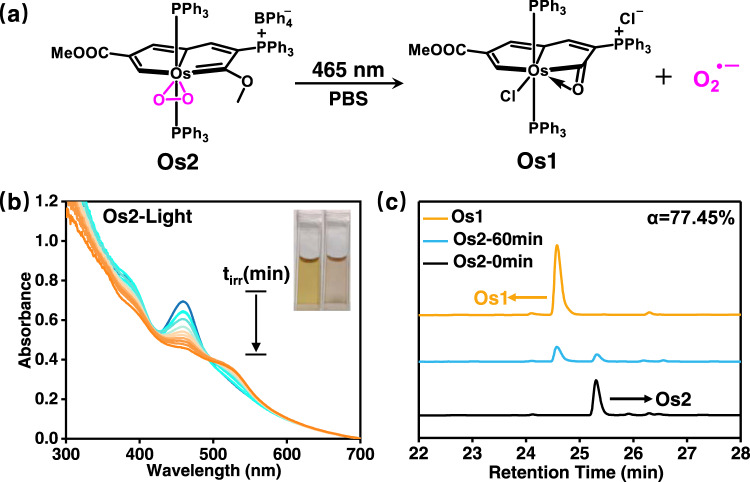
Fig. 1. Photoactivation of Os2.
a Schematic diagram of light-mediated Os2 release of O2•− and Os1. b The UV–Vis absorption spectra of Os2 (100 μM) in PBS solution (pH = 7.4, 1% DMSO) under light illumination (465 nm, 13 mW/cm2) at 298 K. c HPLC analysis of Os2 (10 μM) in PBS solution (1% DMSO) in the dark or after irradiating for 60 min. Mobile phase was CH3CN and water (v/v 1:1).
Results
Photoactivation property
The synthesis of the osmium-peroxo complex Os2 was based on our previously reported method36, and the characterization data can be found in the Supporting Information (SI, experimental section and Supplementary Figs. 1–3). Os2 is highly stable in phosphate-buffered saline (PBS) pH = 7.4 in the dark at room temperature (rt) (Supplementary Fig. 4). However, with 465 nm light irradiation, the solution of Os2 undergoes a gradual color change from brown to light pink (Fig. 1b, insets), which led us to investigate this transformation in detail. High-resolution mass spectrometry (HRMS) measurements were performed to identify the photolytic products, and a prominent peak was observed at m/z = 1175.2351 (Supplementary Fig. 5). The mass and isotope distribution in this ion correspond well to Os1. The formation of Os1 was further evidenced by HPLC analysis. As shown in Fig. 1c, the retention time of the new peak is consistent with that of the independently synthesized Os1 (Supplementary Figs. 6–10, Tables S1–S2. See also SI for the synthesis and characterization of Os1). Moreover, after 60 min of irradiation, the transform efficiency of 77.45% from Os2 to Os1 demonstrated that Os1 is the main photoproduct (Fig. 1c), and the transform rate was determined to be 0.46 μmol L−1 min−1 based on the absorbance changes at 459 nm of Os2 (Fig. 1b and Supplementary Fig. 11).
ROS detection in solution
Due to the dissociation of O2 unit from the metal center of Os2 under light irradiation, we sought to verify that the O2 unit is released in the form of a superoxide anion (O2•−). We first tried to detect O2•− by using the non-fluorescent dihydrorhodamine 123 (DHR123) probe24, which can react with O2•− and emit strong green fluorescence around 526 nm. As shown in Fig. 2a, when irradiated at 465 nm (13 mW/cm2), the solution of Os2 showed an increasing emission, suggesting the generation of O2•−. We also simulated the Fenton reaction process37. When Os2 (15 μM) and a small amount of superoxide dismutase (SOD) were added into a PBS solution containing Fe2+, the absorbance of methylene blue (MB) probe (MB, 5 μg/mL) at 665 nm decreased with a degradation rate of 70.26% (Supplementary Fig. 12). On the contrary, in control experiments using only Os2 and Os2 + SOD, respectively, no changes were observed in the characteristic UV–Vis absorption band of MB. On the basis of these findings and published data, we propose the mechanism in Fig. 2f. Under the action of SOD enzyme, O2•− disproportionately generates H2O2 and O2. The OH• is generated from H2O2 under the action of Fe2+, and then reacts with MB, thus decreasing its absorption. These findings confirmed that Os2 can produce O2•− under light irradiation.
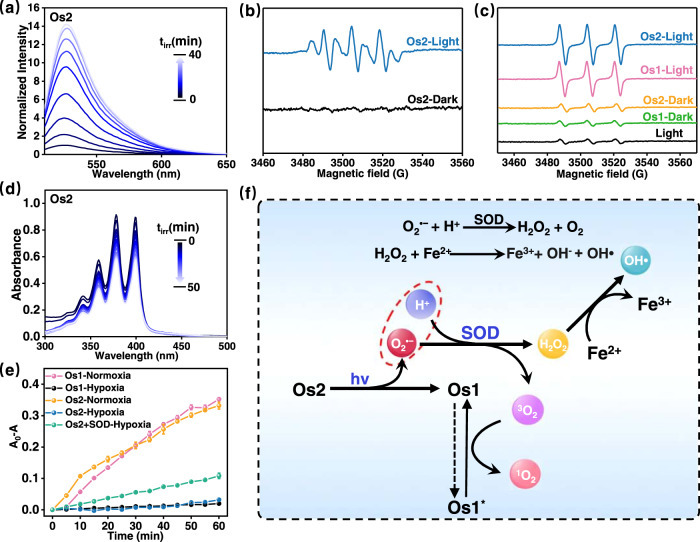
Fig. 2. Detection of ROS in solution.
a The emission spectra for monitoring O2•− generation by Os2 (10 μM) using DHR123 probe (10 μM, λex = 488 nm) in normoxia. b The ESR signal of O2•− trapped by DMPO (18 mM) after light irradiation in normoxia. c The ESR signal of 1O2 trapped by TEMP (8 mM) after light irradiation in normoxia. d The UV–Vis absorption spectra of ABDA (100 μM) for monitoring of 1O2 generated by Os2 (20 μM) under light irradiation in normoxia. e The plot of A0-A of ABDA at 378 nm versus irradiation time in the presence of Os1 or Os2 in hypoxia or normoxia (n = 3 independent experiments). Error bars represent S.D. from the mean. f Schematic diagram of generating O2•−, 1O2 and OH• by light-activating Os2. Light irradiation: 465 nm, 13 mW/cm2; Experiment temperature: 298 K; SOD superoxide dismutase.
To gain more insight into the nature of ROS, we made electron spin resonance (ESR) measurements using 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) and (2,2,6,6-tetramethylpiperidine)oxyl as spin traps. Specifically, DMPO was used to trap O2•− generated by Os2 under 465 nm light irradiation37–39. After the mixture containing DMPO and Os2 was exposed to light irradiation, several signal peaks of DMPO-OOH appeared in the 3480–3540 G (Fig. 2b), which explained the generation of O2•−. In order to study whether Os2 produces 1O2 under light irradiation, we used TEMP to measure 1O2 generation40,41. As shown in Fig. 2c, a three-line signal with the intensity of 1:1:1 between 3480 and 3530 G was observed in the ESR spectrum of Os2 and TEMP mixing solution under light irradiation. A similar signal was observed in the presence of Os1 (the light product of Os2). These ESR results suggest that Os2 can generate both O2•− and 1O2 under light irradiation.
In addition, 9,10-anthracenediyl-bis-(methylene) dimalonic acid (ABDA) was further used to detect the 1O2 quantum yields of Os1 and Os2 under light irradiation. As shown in Fig. 2d and Supplementary Fig. 13, the characteristic absorption peak of ABDA at about 378 nm decreased gradually as the irradiation time increased. The 1O2 quantum yields of Os1 and Os2 were calculated as 0.039 and 0.04 by comparison with Ru(bpy)32+ (Supplementary Fig. 13 and Table S3)42.
The above experiments were all conducted under aerobic condition. Next, we studied the production of ROS in the absence of oxygen (hypoxia, deaeration with nitrogen). As shown in Supplementary Fig. 14, the emission spectra showed enhancing green fluorescence in the presence of Os2 and DHR123 probe. This result proves that Os2 also produces O2•− under hypoxia. We further measured whether 1O2 generation under hypoxia. As shown in Supplementary Fig. 15, the absorption of ABDA in the presence of both Os1 and Os2 failed to change obviously under hypoxia, confirming that no 1O2 generation occurred under hypoxia. However, the absorption of ABDA was reduced in the presence of Os2 + SOD. This is due to Os2 produces O2•− under hypoxia, and that O2•− disproportionately generates H2O2 and O2 under the action of the SOD enzyme, the O2 being available for 1O2 generation by the photoproduct Os1 (Fig. 2f). However, Os1 is unable to produce any ROSs under hypoxia (Supplementary Fig. 15).
Our previous studies showed that Os2 had catalytic activity for alcohol dehydrogenation under relatively harsh conditions (80 °C, K2CO3, pure oxygen atmosphere), and a concerted double-hydrogen transfer mechanism was proposed based on experimental and theoretical results, but the catalytic reaction hardly proceeds at room temperature37. We further verified the stability of Os2 in the cellular environment before the cellular experiment. The results show that it is highly stable in Dulbecco’s Modified Eagle Medium (DMEM) cell culture media or in the PBS solution with different pH values in the dark at 298 K (Supplementary Figs. 16 and 17). Furthermore, treatments of Os2 with reducing agents (NADH, GSH, and Cys) or cell oxidants (such as H2O2, peroxidases, cytochrome p450) in the dark did not result in significant changes in the UV–Vis absorption spectra (Supplementary Fig. 18). Supplementary Figs. 19 and 20 further proved that Os2 does not react with ROS such as 1O2. These results suggest that Os2 is stable in the cellular environment in the dark.
Generation of cellular ROS under irradiation
We studied the photo-induced ROS generation ability of Os2 in living HeLa cells. Dihydroethidium (DHE), hydroxyphenyl fluorescein (HPF) and singlet oxygen sensor green (SOSG) were used as probes to monitor the production of O2•−, OH• and 1O2, respectively37,38. The detection mechanisms of these probes for various free radicals are shown in Fig. 3a. Confocal microscopy showed that when Os2-incubated HeLa cells were exposed to light under normoxia (20% O2, 465 nm, 13 mW/cm2, 1 h), the fluorescence signal in DHE-stained cells is significantly enhanced (Fig. 3b, c). This confirms the ability of Os2 to release O2•− in cells under light irradiation. Under hypoxia (1% O2), the fluorescence intensity after light irradiation was similar to that obtained under normoxia, indicating that the process of O2•− generation from Os2 is independent of O2. This is different from the mechanism of O2-dependent type I photodynamic mechanism.
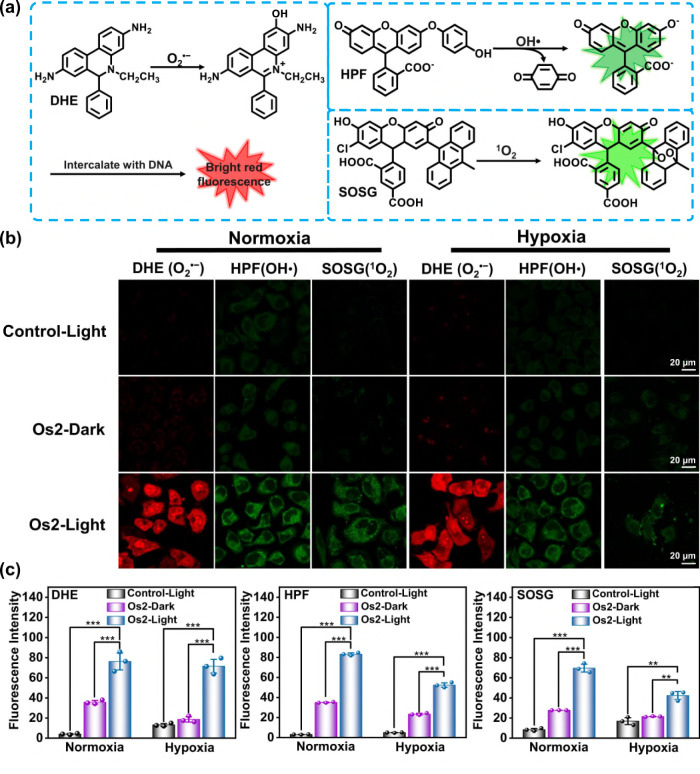
Fig. 3. Cellular ROS mesurement.
a Schematic diagram of detection of O2•−, OH• and 1O2 with DHE, HPF and SOSG probes, respectively. b Confocal microscope images of O2•−, OH• and 1O2 in HeLa cells under normoxia (20% O2) or hypoxia (1% O2) probed by DHE, HPF and SOSG, respectively. c The average fluorescence intensities calculated from the images in b. All experiments were repeated three times independently with similar results. Error bars represent S.D. from the mean. Statistical significance was calculated with two-tailed Student’s t test (*p < 0.05, **p ≤ 0.01 or ***p ≤ 0.001). HeLa cells incubated with Os2 (20 μM) for 8 h, and then treated with DHE (10 μM, 30 min), HPF (10 μM, 1 h) or SOSG (2.5 μM, 30 min). Incubation temperature: 310 K; Light irradiation: 465 nm, 13 mW/cm2, 1 h; DHE: λex = 488 nm, λem = 600 ± 30 nm; HPF: λex = 488 nm, λem = 530 ± 30 nm; SOSG: λex = 488 nm, λem = 525 ± 30 nm. DHE dihydroethidium; HPF hydroxyphenyl fluorescein; SOSG singlet oxygen sensor green.
Because it is known that O2•− can produce OH• in the presence of intracellular SOD enzyme and Fe2+ ions37, we further studied the generation of OH• in the cells by HPF staining. Confocal imaging showed that Os2 can produce OH• in HeLa cells under normoxia or hypoxia (Fig. 3b, c). In addition, we also compared the ability of 1O2 generation of Os2 and the photoproduct Os1 by using a SOSG probe, and found that under hypoxia, Os2 has more advantages in producing 1O2 than Os1. The result showed that the green fluorescence in the Os2 treated cells is strong under both normoxia and hypoxia. However, the fluorescence in the Os1 treated cells was much weaker under hypoxia than that observed under normoxia (Supplementary Fig. 21). These results are consistent with those in Fig. 2f, showing that Os2 firstly induces the release of O2•− and production of Os1, accompanied by the formation of O2 (as an oxygen source for Os1) and OH• by a Fenton reaction. Finally, 1O2 is generated by the photoproduct Os1, but it is difficult to establish whether Os2 can also produce 1O2 due to its conversion to Os1 all the time under 465 nm light irradiaton. These data all show that Os2 may be useful as an effective drug for photoactive treatment of hypoxic tumor cells.
Phototoxicity in vitro
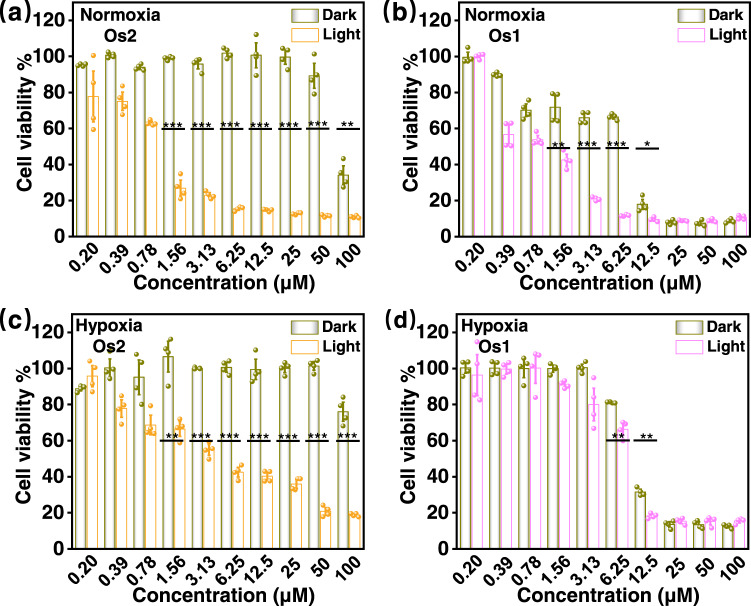
The dark- and photo-cytotoxicities of Os2 and Os1 against HeLa cells were determined with an MTT method40,41. As shown in Fig. 4 and Table 1, Os2 had low dark-cytotoxicity and high photo-cytotoxicity under both normoxia and hypoxia (Fig. 4a, c). The (IC50)dark values, determined with only dark incubation, were 89.2 and >100 μM, respectively (Table 1). The (IC50)Light values of Os2, determined by photoirradiation under normoxia or hypoxia were 1.23 and 5.86 μM, respectively. The photo-cytotoxicity under hypoxia can be explained in terms of the release of O2•− by Os2 and light irradiation, followed by the production of O2, which may be used by the light product Os1 to generate 1O2, and OH• with stronger toxicity via the Fenton reaction. In contrast, the light product (Os1) shows high dark-cytotoxicity and photo-cytotoxicity under both normoxia and hypoxia (Fig. 4b, d). The (IC50)dark values of Os1 under normoxia and hypoxia are 8.12 and 9.95 μM, and the (IC50)Light values under normoxia and hypoxia are 1.31 and 7.51 μM, respectively (Table 1). This result suggests that Os1 is high photo- and dark- cytotoxic, and can be seen as a chemotherapeutic drug. However, with hypoxia, due to the lack of oxygen, Os1 shows no obvious photodynamic effect. The PI value of Os2 (>18) under hypoxia is much higher than that of Os1 (1.3).
Fig. 4. Cytotoxicity against HeLa cells.
The viabilities of HeLa cells treated with different concentrations of Os2 or Os1 under normoxia (20% O2, a, b) or hypoxia (1% O2, c, d) in the dark or upon light irradiation. All cell viability data was performed as duplicates of quadruplicate (n = 4 biologically independent samples). Error bars represent S.D. from the mean. Statistical significance was calculated with two-tailed Student’s t test (*p < 0.05, **p ≤ 0.01 or ***p ≤ 0.001). Incubation temperature: 310 K. Light irradiation: 465 nm, 13 mW/cm2, 1 h.
Table 1.
The dark- and photo-IC50 values of the compounds against HeLa cells under normoxia and hypoxia.
| Compounds | Condition | Dark [μM] | Light[a] [μM] | PI[b] |
|---|---|---|---|---|
| Os2 | Normoxia (20% O2) | 89.2 ± 2.6 | 1.23 ± 0.09 | 72.5 |
| Os2 | Hypoxia (1% O2) | >100 | 5.86 ± 0.19 | >18 |
| Os1 | Normoxia (20% O2) | 8.12 ± 0.26 | 1.31 ± 0.05 | 6.2 |
| Os1 | Hypoxia (1% O2) | 9.95 ± 0.14 | 7.51 ± 0.35 | 1.3 |
| 5-ALA | Normoxia (20% O2) | >100 | 68.62 ± 1.99 | >1.45 |
| Cisplatin | Normoxia (20% O2) | 33.22 ± 0.77 | 47.75 ± 1.5 | – |
5-ALA is 5-aminolevulinic acid.
[a]Photoirradiation was imposed (465 nm, 13 mW/cm2, 1 h) after 8 h of the complexes incubation and then 40 h recovery.
[b]Photo-cytotoxicity index, the ratio of (IC50)dark/(IC50)Light.
Cisplatin and 5-aminolevulinic acid (5-ALA) were used as controls (Table 1). Very low dark- and photo- toxicities (both (IC50)dark and (IC50)light > 50 μM) were showed for HeLa cells after 8 h 5-ALA drug exposure and 40 h recovery (the same conditions as Os2). Cisplatin has a certain dark toxicity ((IC50)dark = 33.22 μM) after 8 hours of administration, and its phototoxicity is not obvious compared with dark toxicity. We further measured that the cellular uptake of Os2 or cisplatin towards HeLa cancer cells after 2, 4 and 8 h incubation (Supplementary Fig. 22). The results showed that the accumulation of Os2 in cells was less than that of cisplatin. This cause lower dark toxicity of Os2 than cisplatin, thus Os2 is a feasible drug that can reduce toxic and side effects compared with cisplatin in the dark. However, the phototoxicity of Os2 is much higher than that of cisplatin under irradiation (Table 1).
Staining with calcein acetoxymethyl ester (Calcein-AM) or propidium iodide (PI) were also used to distinguish living cells (green) from dead cells (red). As shown in Supplementary Fig. 23, the dark group of Os2 in normoxia and hypoxia shows strong green fluorescence (living cells) but no red fluorescence (dead cells), and the control group in the dark or under light irradiation also shows no dead cells. In contrast, the light group of Os2 shows weak green fluorescence and strong red fluorescence, indicating that Os2 leads to a large number of dead cells under light irradiation.
Ferroptosis mechanism
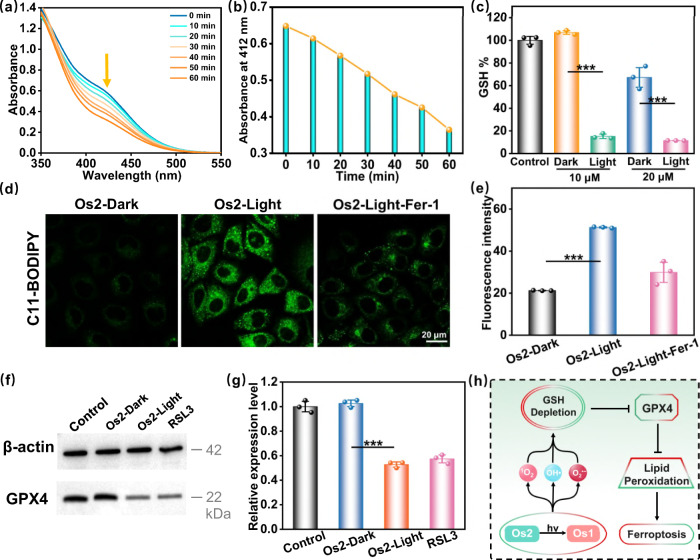
It has been reported that the photo-chemical process of ROS generation could cause ferroptosis in cancer cells6,43,44. This led us to consider if Os2 can induce ferroptosis. GSH is closely related to ferroptosis45,46, and we first detected the ability of Os2 to consume GSH. As shown in Fig. 5a, b, with an increase of light irradiation time, the absorption at 412 nm decreased, indicating that Os2 when irradiated, could consume GSH. We determined the GSH levels in the cells after treatment with Os2, and found that the GSH level in the irradiated group was significantly lower than that in the non-irradiated group (Fig. 5c). Therefore, we concluded that Os2 can consume cellular GSH under irradiation conditions.
Fig. 5. Ferroptosis induction by Os2 under light irradiation.
a Irradiation time-dependent GSH (200 μM) depletion by Os2 (20 μM) upon blue light irradiation at 298 K and b the absorption at 412 nm was decreased by increasing the irradiation time. c The GSH levels in cells after different treatments. All the experiments were performed as duplicates of triplicates (n = 3 biologically independent samples, p values (p): 10 μM-0.000025, 20 μM-0.00065). d The fluorescence images of lipid peroxides in the treated cells detected by C11-BODIPY probe (30 μM, 310 K, 0.5 h). C11-BODIPY: λex = 488 nm, λem = 570 ± 50 nm. e The average fluorescence intensities calculated from the images in d. The experiment was repeated three times independently with similar results, p = 0.000024. f Western blot analysis of GPX4 in HeLa cells after treatment with Os2 (20 μM, 310 K, 8 h) with or without light treatment. RSL3 is the positive control group. g The relative expression levels of GPX4 calculated from f All the experiments were performed as duplicates of triplicates (n = 3 biologically independent samples, p = 0.000025). Error bars represent SD from the mean. Statistical significance was calculated with two-tailed Student’s t test (*p < 0.05, **p ≤ 0.01 or ***p ≤ 0.001). h The process of ferroptosis in this photoactive antitumor therapy. Light irradiation: 465 nm, 13 mW/cm2. GSH glutathione, Fer-1 ferrostatin-1, GPX4 glutathione peroxidase 4.
Ferroptosis is a type of iron-dependent cell death caused by excessive lipid peroxidation. The main feature of ferroptosis is that after the inactivation of cell antioxidant capacity, phospholipids containing polyunsaturated fatty acids are peroxidated on the cell membrane, destroying the cell membrane and leading to ferroptosis47–49. The antioxidant glutathione peroxidase 4 (GPX4) specifically catalyzes loss of oxidative activity in the lipid peroxides in a glutathione-dependent manner50,51, and subsequent inhibition of GPX4 induces ferroptosis. GSH consumption can indirectly inhibit the expression of GPX4. We speculated that Os2 could further inhibit the expression of GPX4 and we verified this hypothesis by western blot analysis of GPX4. As shown in Fig. 5f–g, Os2 fails to reduce the expression of GPX4 in the dark. However, upon light irradiation, Os2 significantly reduces the expression of GPX4, and the GSH consumption caused by ROS and the inhibition of GPX4 will further lead to the accumulation of lipid peroxides and induce ferroptosis. We used C11-BODIPY as a lipid peroxide probe with which to monitor intracellular accumulation of lipid peroxides (Fig. 5d, e). Confocal microscopy showed that the fluorescence of HeLa cells treated with Os2 was significantly enhanced after exposure to light, indicating a significant accumulation of lipid peroxides, which could be effectively inhibited by Ferrostatin-1 (Fer-1, a ferroptosis inhibitor). All the above results confirm that Os2 induces ferroptosis as shown in Fig. 5h.
NADH photocatalytic oxidation
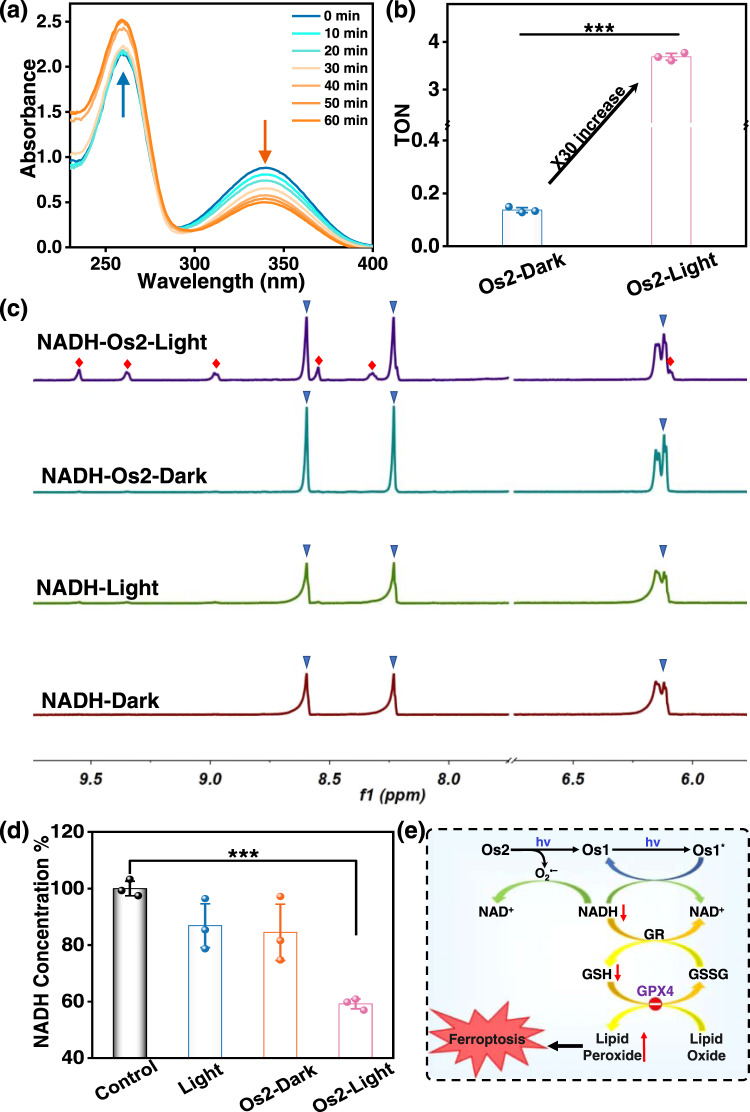
As a cofactor, 1,4-dihydro-nicotinamide adenine dinucleotide (NADH) regulates the redox balance of cellular mitochondria, and plays an important role in regulating energy production. If NADH is oxidized to NAD+, it can destroy the whole respiratory chain and kill cells19,52,53. We studied whether NADH can be oxidized by Os2 under light irradiation, which could provide a photocatalytic oxidation pathway to kill cancer cells. The photocatalytic efficiency of Os2 (20 μM) towards NADH (175 μM) was first determined by UV–Vis absorption spectroscopy. As shown in Fig. 6a, the absorbance at 339 nm decreases and the absorbance at 259 nm increases gradually with increase of the irradiation time. In contrast, the absorption of the non-illuminated Os2 group exhibits no obvious changes (Supplementary Fig. 24). This indicates that Os2 can reduce the enzyme activity of NADH under light irradiation. We also calculated the NADH oxidation turnover number (TON) of Os2 at 339 nm to evaluate its photocatalytic efficiency. The TON value of NADH oxidation by Os2 under light irradiation is 3.888, which is 30-times higher than that of the non-illuminated group (TONdark = 0.137) (Fig. 6b). Similarly, Os1 also shows a similar photocatalytic oxidation effect on NADH (Supplementary Fig. 25).
Fig. 6. Photocatalytic oxidation of NADH by Os2 under light irradiation.
a Reaction of Os2 (20 μM) and NADH (175 μM) in PBS solution under blue light irradiation monitored by UV–Vis absorption spectra at 298 K. b TON of Os2 under dark or irradiation conditions (n = 3 independent experiments, p = 0.0000016). c Photocatalytic oxidation of NADH (3.5 mM) by Os2 (0.25 mM) under dark or irradiation conditions monitored by 1H NMR spectroscopy at 298 K. Peaks associated with blue triangles represent NADH, those with red squares represent NAD+. d NADH concentrations in the treated HeLa cells. Os2: 20 μM, 310 K, 8 h. All the experiments were performed as duplicates of triplicates (n = 3 biologically independent samples, p = 0.000039). Error bars represent S.D. from the mean. Statistical significance was calculated with two-tailed Student’s t test (*p < 0.05, **p ≤ 0.01 or ***p ≤ 0.001). e Schematic diagram of the photocatalytic oxidation of NADH by Os2 and followed by induced ferroptosis. Light irradiation: 465 nm, 13 mW/cm2. TON turnover number, NADH 1,4-dihydro-nicotinamide adenine dinucleotide, GSH glutathione; GSSG oxidized glutathione, GPX4 glutathione peroxidase 4, GR glutathione reductase.
The photocatalytic oxidation of NADH was also monitored by 1H NMR in D2O/CD3OD (1/3, v/v) at 298 K. As shown in Fig. 6c, In the Os2 and NADH illuminated group, new peaks from hydrogens on the nicotinamide ring of NAD+ are observed at 6.13, 8.31, 8.55, 8.99, 9.36 and 9.58, but no new NAD+ peak is observed in either the non-illuminated group or the non-Os2 group. Intuitively, this shows that Os2 could oxidize NADH under light irradiation, transforming it into NAD+. Subsequently, we measured the NADH photocatalytic oxidation ratio at the cellular level using a NAD/NADH-GloTM method, which is a bioluminescence method for the detection of NAD+ and NADH. As shown in Fig. 6d, the chemical luminescence intensity of Os2-light group proved to be lower than that of other groups. These results show that Os2 can effectively oxidize NADH at the cellular level by light irradiation, thereby killing cancer cells. As can be seen in Fig. 6e, NADH can not only be converted to NAD+ by interacting with Os1* (the excited state of Os1), but can also be oxidized to NAD+ by O2•− released from Os2. The downregulation of NADH indirectly aids the reduction of oxidized glutathione (GSSG) to GSH by glutathione reductase54, resulting in the accumulation of lipid peroxides, and ultimately achieving the synergistic induction of ferroptosis.
Photoactive antitumor therapy in vivo
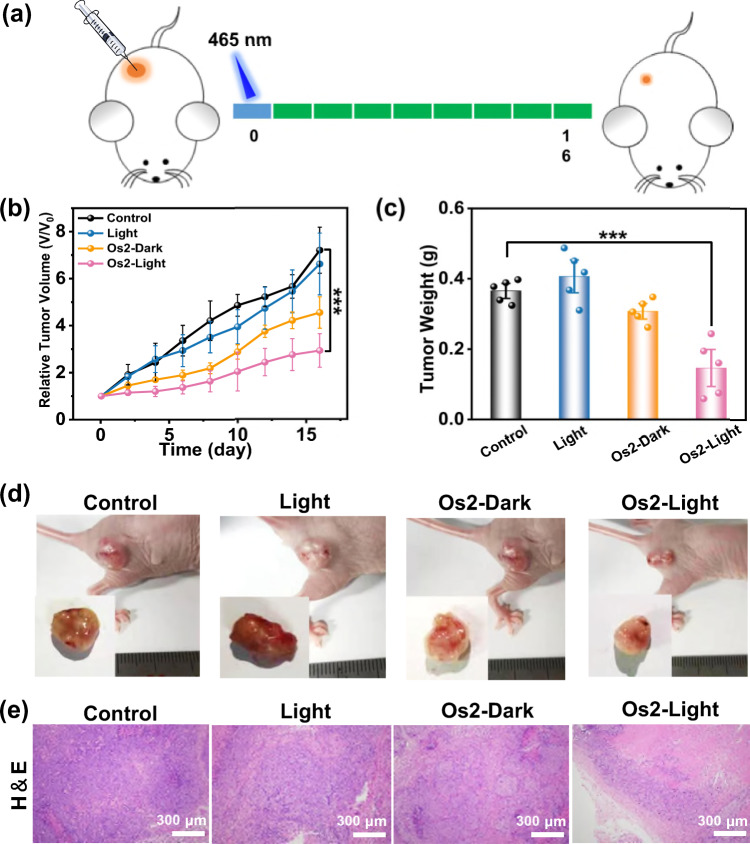
We studied the feasibility of photoactive therapy in vivo by Os2. Since Os2 is a small molecule and there is no specific targeted group, it can be administered intratumorally (Fig. 7a). As shown in Fig. 7b, compared with the other three groups, the tumor growth in mice treated with Os2-light group was inhibited. The tumor size of the Os2-light group was the smallest in the four groups (Fig. 7d), and the average tumor weight of the Os2-light group was significantly lower than that of the other groups (Fig. 7c). The tumor tissues after final treatment were collected for histological assessment. The hematoxylin and eosin (H&E) staining showed obvious destruction of tumor tissues in the Os2-light group, while tumor tissues in the other three groups were not affected (Fig. 7e).
Fig. 7. In vivo photoactive chemotherapy.
a Schematic of the in vivo (HeLa tumor-bearing Balb/c mice) therapeutic protocol. Mice were irradiated by light (465 nm, 13 mW/cm2) for 60 min after i.t. injection with 25 μL of PBS containing 500 μM Os2. b Tumor growth curves after treatment. Error bars were standard errors (±SD) based on five mice in each group. Statistical significance was calculated with two-tailed Student’s t test, p = 0.0004 (*p < 0.05, **p ≤ 0.01 or ***p ≤ 0.001). c Tumor weights of mice at day 16 after various treatments. Error bars were standard errors (±SD) based on five mice in each group. Statistical significance was calculated with two-tailed Student’s t test, p = 0.00042 (*p < 0.05, **p ≤ 0.01 or ***p ≤ 0.001). d The digital photos of representative mice after various treatments. e H&E staining images of HeLa tumor tissues in mice after various treatments. The experiment was repeated three times independently with similar results.
In order to evaluate the biological safety of Os2, we first analyzed the H&E staining slices of the main organs of healthy mice i.v. injected with three times of the therapeutic dose (2.69 mg kg−1). The results showed no obvious tissue damage in these slices (Supplementary Fig. 26). We also used zebrafish to test the biological safety of Os2 (Supplementary Fig. 27) with the green fluorescent protein (GFP) commonly used as a biomarker to visualize the physiological processes. After 5 days of incubation with Os2, the blood vessels of the zebrafish were apparently not damaged, showing good biocompatibility in vivo.
Discussion
In summary, we have demonstrated that an osmium-peroxo complex (Os2) can release O2•− under light irradiation in the absence of O2, and at the same time is transformed into another active osmium complex (Os1), which exhibits both chemotherapeutic and photodynamic properties, thus maintaining good phototoxicity in hypoxic tumors. The osmium-peroxo complex Os2 can induce ferroptosis, which is characterized by GSH degradation, GPX4 down-regulation and lipid peroxide accumulation. In addition, under light irradiation, the same osmium-peroxo complex oxidizes NADH into NAD+, further helping induction of ferroptosis. At the in vivo level, the osmium-peroxo complex achieves highly effective photoactive therapy of solid hypoxic tumors. This study reports an interesting example of a metal-peroxo complex for O2-independent photoactive therapy and provides a promising strategy for combating hypoxic tumors.
Methods
ROS detection in solution
O2•− detection
Dihydrorhodamine 123 (DHR123) was used as the superoxide anion radical indicator, which can be converted to Rhodamine 123 in the presence of O2•−. Os2 (10 μM) and DHR123 (10 μM) were mixed. Then the cuvette was exposed to 465 nm monochromatic light for different time, and the fluorescence spectra were observed immediately after each irradiation. The O2•− generation was studied in nornoxia (20% O2) and hypoxia (deaeration with nitrogen, <1% O2), respectively.
1O2 detection
20 μM Os1 or Os2 and 100 μM ABDA mixing solution was measured using UV–Vis spectrophotometer after different light (465 nm, 13 mW/cm2) irradiation durations. The absorbance changes of ABDA at 378 nm were recorded to quantify the quantum yields (Ф) of 1O2. The 1O2 generation was studied in normoxia (20% O2) and hypoxia (deaeration with nitrogen, <1% O2), respetively.
OH• detection
15 μM the osmium complex and 5 μg mL−1 MB mixing solution (pH = 4.5 containing Fe2+ (0.2 mM)) was measured using UV–vis spectrophotometer after different light (465 nm, 13 mW/cm2) irradiation durations.
Intracellular ROS measurement
Intracellular O2•− measurement
HeLa cells were incubated with 20 μM Os2 for 8 h followed by incubation with 10 μM DHE for another 30 min under hypoxia (1% O2) or normoxia (20% O2). After that, cells were irradiated with 465 nm blue light for 1 h at a power density of 13 mW/cm2. The red fluorescence was immediately observed using CLSM with the excitation wavelength of 488 nm, and emission collection wavelength from 570 to 630 nm.
Intracellular OH• measurement
HeLa were incubated with 20 μM Os2 for 8 h followed by incubation with 10 μM HPF for 1 h under hypoxia (1 % O2) or normoxia (20% O2). After that, cells were washed with PBS and then irradiated with 465 nm blue light for 1 h at a power density of 13 mW/cm2. The green fluorescence was immediately observed using CLSM with the excitation wavelength of 488 nm, and emission collection wavelength from 500 to 560 nm.
Intracellular 1O2 measurement
HeLa cells were incubated with 20 μM Os2 or Os1 for 8 h followed by incubation with 2.5 μM SOSG for 30 min under hypoxia (1% O2) or normoxia (20% O2). After that, cells were washed with PBS and then irradiated with 465 nm blue light for 1 h at a power density of 13 mW/cm2. The green fluorescence was immediately observed using CLSM with the excitation wavelength of 488 nm, and emission collection wavelength from 495 to 555 nm.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
We appreciate the financial support of the National Natural Science Foundation of China (22077085 for P.Z., 22007104 and 21931002 for H.X.), and the Science and Technology Foundation of Shenzhen (JCYJ20210324095200002 and JCYJ20190808153209537 for P.Z., JCYJ20200109140812302 for H.X.). We appreciate the Instrumental Analysis Center of Shenzhen University for the assistance with confocal microscopy analysis.
Source data
Author contributions
N.L., J.G., H.X., and P.Z. designed the study. Z.D. synthesized and characterized the complexes. N.L. and C.L. performed the experiments in vitro and in vivo. N.L., Z.D., J.G., H.X., and P.Z. analyzed the data and wrote the paper. All authors contributed to the general discussion.
Peer review
Peer review information
Nature Communications thanks the other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The authors declare that all data needed to evaluate the conclusion of this work are presented in the paper, the supplementary information or source data file. The source data have been deposited in the Figshare database under accession code 10.6084/m9.figshare.19333802 [https://figshare.com/articles/figure/Daet_of_An_osmium-peroxo_complex_for_photoactive_therapy_of_hypoxic_tumors_/19333802]. The crystal structure of Os1 was deposited in the Cambridge Crystallographic Data Centre (CCDC 1913382), and the data can be obtained free of charge via www.ccdc.cam.ac.uk/structures.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nong Lu, Zhihong Deng.
Contributor Information
Haiping Xia, Email: hpxia@xmu.edu.cn.
Pingyu Zhang, Email: p.zhang6@szu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-29969-z.
References
- 1.Zhao X, Liu J, Fan J, Chao H, Peng X. Recent progress in photosensitizers for overcoming the challenges of photodynamic therapy: from molecular design to application. Chem. Soc. Rev. 2021;50:4185–4219. doi: 10.1039/D0CS00173B. [DOI] [PubMed] [Google Scholar]
- 2.Imberti C, Zhang P, Huang H, Sadler PJ. New designs for phototherapeutic transition metal complexes. Angew. Chem. Int. Ed. 2020;59:61–73. doi: 10.1002/anie.201905171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monro S, et al. Transition metal complexes and photodynamic therapy from a tumor-centered approach: challenges, opportunities, and highlights from the development of TLD1433. Chem. Rev. 2019;119:797–828. doi: 10.1021/acs.chemrev.8b00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karges J, et al. Rationally designed ruthenium complexes for 1- and 2-photon photodynamic therapy. Nat. Commun. 2020;11:3262. doi: 10.1038/s41467-020-16993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karges J, Basu U, Blacque O, Chao H, Gasser G. Polymeric encapsulation of novel homoleptic bis(dipyrrinato) zinc(II) complexes with long lifetimes for applications as photodynamic therapy photosensitisers. Angew. Chem. Int. Ed. 2019;58:14334–14340. doi: 10.1002/anie.201907856. [DOI] [PubMed] [Google Scholar]
- 6.Yuan H, et al. Ferroptosis photoinduced by new cyclometalated iridium(III) complexes and its synergism with apoptosis in tumor cell inhibition. Angew. Chem. Int. Ed. 2021;60:8174–8181. doi: 10.1002/anie.202014959. [DOI] [PubMed] [Google Scholar]
- 7.Lifshits LM, et al. Near-infrared absorbing Ru(ii) complexes act as immunoprotective photodynamic therapy (PDT) agents against aggressive melanoma. Chem. Sci. 2020;11:11740–11762. doi: 10.1039/D0SC03875J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smithen DA, et al. Bis[pyrrolyl Ru(ii)] triads: a new class of photosensitizers for metal-organic photodynamic therapy. Chem. Sci. 2020;11:12047–12069. doi: 10.1039/D0SC04500D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan RT, et al. Bis-tridentate N-heterocyclic carbene Ru(II) complexes are promising new agents for photodynamic therapy. Inorg. Chem. 2020;59:8882–8892. doi: 10.1021/acs.inorgchem.0c00686. [DOI] [PubMed] [Google Scholar]
- 10.Wan G, et al. Nanoscaled red blood cells facilitate breast cancer treatment by combining photothermal/photodynamic therapy and chemotherapy. Biomaterials. 2018;155:25–40. doi: 10.1016/j.biomaterials.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Tang X, et al. Overcome the limitation of hypoxia against photodynamic therapy to treat cancer cells by using perfluorocarbon nanodroplet for photosensitizer delivery. Biochem. Biophys. Res. Commun. 2017;487:483–487. doi: 10.1016/j.bbrc.2017.03.142. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, et al. Covalent organic frameworks as favorable constructs for photodynamic therapy. Angew. Chem. Int. Ed. 2019;58:14213–14218. doi: 10.1002/anie.201909020. [DOI] [PubMed] [Google Scholar]
- 13.Huang CC, et al. An implantable depot that can generate oxygen in situ for overcoming hypoxia-induced resistance to anticancer drugs in chemotherapy. J. Am. Chem. Soc. 2016;138:5222–5225. doi: 10.1021/jacs.6b01784. [DOI] [PubMed] [Google Scholar]
- 14.Zheng DW, et al. Carbon-Dot-Decorated Carbon Nitride Nanoparticles for Enhanced Photodynamic Therapy against Hypoxic Tumor via Water Splitting. ACS Nano. 2016;10:8715–8722. doi: 10.1021/acsnano.6b04156. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Tian J, He W, Guo Z. H2O2-activatable and O2-evolving nanoparticles for highly efficient and selective photodynamic therapy against hypoxic tumor cells. J. Am. Chem. Soc. 2015;137:1539–1547. doi: 10.1021/ja511420n. [DOI] [PubMed] [Google Scholar]
- 16.Zhu W, et al. Modulation of hypoxia in solid tumor microenvironment with MnO2 nanoparticles to enhance photodynamic therapy. Adv. Funct. Mater. 2016;26:5490–5498. doi: 10.1002/adfm.201600676. [DOI] [Google Scholar]
- 17.Huo M, et al. Photosynthetic tumor oxygenation by photosensitizer-containing cyanobacteria for enhanced photodynamic therapy. Angew. Chem. Int. Ed. 2020;59:1906–1913. doi: 10.1002/anie.201912824. [DOI] [PubMed] [Google Scholar]
- 18.Liu CP, et al. Self-Supplying O2 through the catalase-like activity of gold nanoclusters for photodynamic therapy against hypoxic cancer cells. Small. 2017;13:1700278. doi: 10.1002/smll.201700278. [DOI] [PubMed] [Google Scholar]
- 19.Wang YG, et al. Hyperbaric oxygen promotes malignant glioma cell growth and inhibits cell apoptosis. Oncol. Lett. 2015;10:189–195. doi: 10.3892/ol.2015.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang H, et al. Targeted photoredox catalysis in cancer cells. Nat. Chem. 2019;11:1041–1048. doi: 10.1038/s41557-019-0328-4. [DOI] [PubMed] [Google Scholar]
- 21.Lv W, et al. A mitochondria-targeted photosensitizer showing improved photodynamic therapy effects under hypoxia. Angew. Chem. Int. Ed. 2016;55:9947–9951. doi: 10.1002/anie.201604130. [DOI] [PubMed] [Google Scholar]
- 22.Guo S, et al. Mitochondria-localized iridium(III) complexes with anthraquinone groups as effective photosensitizers for photodynamic therapy under hypoxia. Sci. China Chem. 2019;62:1639–1648. doi: 10.1007/s11426-019-9583-4. [DOI] [Google Scholar]
- 23.Novohradsky V, et al. Towards novel photodynamic anticancer agents generating superoxide anion radicals: a cyclometalated Ir(III) complex conjugated to a far-red emitting coumarin. Angew. Chem. Int. Ed. 2019;58:6311–6315. doi: 10.1002/anie.201901268. [DOI] [PubMed] [Google Scholar]
- 24.Nam JS, et al. Endoplasmic reticulum-localized iridium(III) complexes as efficient photodynamic therapy agents via protein modifications. J. Am. Chem. Soc. 2016;138:10968–10977. doi: 10.1021/jacs.6b05302. [DOI] [PubMed] [Google Scholar]
- 25.Bevernaegie R, et al. Exploring the phototoxicity of hypoxic active iridium(III)-based sensitizers in 3D tumor spheroids. J. Am. Chem. Soc. 2019;141:18486–18491. doi: 10.1021/jacs.9b07723. [DOI] [PubMed] [Google Scholar]
- 26.Kuang S, et al. A mitochondrion-localized two-photon photosensitizer generating carbon radicals against hypoxic tumors. Angew. Chem. Int. Ed. 2020;59:20697–20703. doi: 10.1002/anie.202009888. [DOI] [PubMed] [Google Scholar]
- 27.Carpenter AW, Schoenfisch MH. Nitric oxide release: part II. Therapeutic applications. Chem. Soc. Rev. 2012;41:3742–3752. doi: 10.1039/c2cs15273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Askes SHC, Reddy GU, Wyrwa R, Bonnet S, Schiller A. Red light-triggered CO release from Mn2(CO)10 using triplet sensitization in polymer nonwoven fabrics. J. Am. Chem. Soc. 2017;139:15292–15295. doi: 10.1021/jacs.7b07427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tso KK, Leung KK, Liu HW, Lo KK. Photoactivatable cytotoxic agents derived from mitochondria-targeting luminescent iridium(III) poly(ethylene glycol) complexes modified with a nitrobenzyl linkage. Chem. Commun. 2016;52:4557–4560. doi: 10.1039/C6CC00918B. [DOI] [PubMed] [Google Scholar]
- 30.Presa A, et al. Photoactivation of the cytotoxic properties of platinum(II) complexes through ligand photoswitching. Inorg. Chem. 2018;57:4009–4022. doi: 10.1021/acs.inorgchem.8b00146. [DOI] [PubMed] [Google Scholar]
- 31.Joshi T, et al. A bis(dipyridophenazine)(2-(2-pyridyl)pyrimidine-4-carboxylic acid)ruthenium(II) complex with anticancer action upon photodeprotection. Angew. Chem. Int. Ed. 2014;53:2960–2963. doi: 10.1002/anie.201309576. [DOI] [PubMed] [Google Scholar]
- 32.Leonidova A, et al. Photo-induced uncaging of a specific Re(i) organometallic complex in living cells. Chem. Sci. 2014;5:4044–4056. doi: 10.1039/C3SC53550A. [DOI] [Google Scholar]
- 33.Dai Y, et al. Toxic reactive oxygen species enhanced synergistic combination therapy by self-assembled metal-phenolic network nanoparticles. Adv. Mater. 2018;30:1704877. doi: 10.1002/adma.201704877. [DOI] [PubMed] [Google Scholar]
- 34.Dai Y, et al. Hypochlorous acid promoted platinum drug chemotherapy by myeloperoxidase-encapsulated therapeutic metal phenolic nanoparticles. ACS Nano. 2018;12:455–463. doi: 10.1021/acsnano.7b06852. [DOI] [PubMed] [Google Scholar]
- 35.Shen Z, et al. Emerging strategies of cancer therapy based on ferroptosis. Adv. Mater. 2018;30:1704007. doi: 10.1002/adma.201704007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng Z, et al. Dioxygen activation by internally aromatic metallacycle: crystallographic structure and mechanistic investigations. iScience. 2020;23:101379. doi: 10.1016/j.isci.2020.101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M, et al. Near-infrared light-initiated molecular superoxide radical generator: rejuvenating photodynamic therapy against hypoxic tumors. J. Am. Chem. Soc. 2018;140:14851–14859. doi: 10.1021/jacs.8b08658. [DOI] [PubMed] [Google Scholar]
- 38.Sun J, et al. GSH and H2O2 Co-activatable mitochondria-targeted photodynamic therapy under normoxia and hypoxia. Angew. Chem. Int. Ed. 2020;59:12122–12128. doi: 10.1002/anie.202003895. [DOI] [PubMed] [Google Scholar]
- 39.Zhang W, et al. Selective aerobic oxidation reactions using a combination of photocatalytic water oxidation and enzymatic oxyfunctionalisations. Nat. Catal. 2018;1:55–62. doi: 10.1038/s41929-017-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang C, et al. A highly potent ruthenium(II)-sonosensitizer and sonocatalyst for in vivo sonotherapy. Nat. Commun. 2021;12:5001. doi: 10.1038/s41467-021-25303-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie J, et al. Water-soluble iridic-porphyrin complex for non-invasive sonodynamic and sono-oxidation therapy of deep tumors. ACS Appl. Mater. Interfaces. 2021;13:27934–27944. doi: 10.1021/acsami.1c06381. [DOI] [PubMed] [Google Scholar]
- 42.Abdel-Shafi AA, Worrall DR, Ershov AY. Photosensitized generation of singlet oxygen from ruthenium(ii) and osmium(ii) bipyridyl complexes. Dalton Trans. 2004;1:30–36. doi: 10.1039/b310238f. [DOI] [PubMed] [Google Scholar]
- 43.Li H, et al. Ferroptosis accompanied by (*)OH generation and cytoplasmic viscosity increase revealed via dual-functional fluorescence probe. J. Am. Chem. Soc. 2019;141:18301–18307. doi: 10.1021/jacs.9b09722. [DOI] [PubMed] [Google Scholar]
- 44.Meng X, et al. Triggered all-active metal organic framework: ferroptosis machinery contributes to the apoptotic photodynamic antitumor therapy. Nano Lett. 2019;19:7866–7876. doi: 10.1021/acs.nanolett.9b02904. [DOI] [PubMed] [Google Scholar]
- 45.Dixon SJ, Stockwell BR. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014;10:9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 46.Liu H, Schreiber SL, Stockwell BR. Targeting dependency on the GPX4 lipid peroxide repair pathway for cancer therapy. Biochemistry. 2018;57:2059–2060. doi: 10.1021/acs.biochem.8b00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dixon SJ, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stockwell BR, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang L, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520:57–62. doi: 10.1038/nature14344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang WS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell. Biol. 2018;20:1181–1192. doi: 10.1038/s41556-018-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Z, et al. Reduction of quinones by NADH catalyzed by organoiridium complexes. Angew. Chem. Int. Ed. 2013;52:4194–4197. doi: 10.1002/anie.201300747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rajagopalan S, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Investig. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guo X, et al. Electron-accepting micelles deplete reduced nicotinamide adenine dinucleotide phosphate and impair two antioxidant cascades for ferroptosis-induced tumor eradication. ACS Nano. 2020;14:14715–14730. doi: 10.1021/acsnano.0c00764. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all data needed to evaluate the conclusion of this work are presented in the paper, the supplementary information or source data file. The source data have been deposited in the Figshare database under accession code 10.6084/m9.figshare.19333802 [https://figshare.com/articles/figure/Daet_of_An_osmium-peroxo_complex_for_photoactive_therapy_of_hypoxic_tumors_/19333802]. The crystal structure of Os1 was deposited in the Cambridge Crystallographic Data Centre (CCDC 1913382), and the data can be obtained free of charge via www.ccdc.cam.ac.uk/structures.