Abstract
Background
We compared overall survival for metastatic breast cancer (MBC) patients monitored with CE-CT, FDG-PET/CT or a combination of them in an observational setting.
Methods
Patients with biopsy-verified (recurrent or de novo) MBC (n = 300) who were treated at Odense university hospital (Denmark) and response monitored with FDG-PET/CT (n = 83), CE-CT (n = 144), or a combination of these (n = 73) were followed until 2019. Survival was compared between the scan groups, and were adjusted for clinico-histopathological variables representing potential confounders in a Cox proportional-hazard regression model.
Results
The study groups were mostly comparable regarding baseline characteristics, but liver metastases were reported more frequently in CE-CT group (38.9%) than in FDG-PET/CT group (19.3%) and combined group (24.7%). Median survival was 30.0 months for CE-CT group, 44.3 months for FDG-PET/CT group and 54.0 months for Combined group. Five-year survival rates were significantly higher for FDG-PET/CT group (41.9%) and combined group (43.3%), than for CE-CT group (15.8%). Using the CE-CT group as reference, the hazard ratio was 0.44 (95% CI: 0.29–0.68, P = 0.001) for the FDG-PET/CT group after adjusting for baseline characteristics. FDG-PET/CT detected the first progression 4.7 months earlier than CE-CT, leading to earlier treatment change.
Conclusions
In this single-center, observational study, patients with metastatic breast cancer who were response monitored with FDG-PET/CT alone or in combination with CE-CT had longer overall survival than patients monitored with CE-CT alone. Confirmation of these findings by further, preferably randomised clinical trials is warranted.
Subject terms: Breast cancer, Cancer imaging
Introduction
An increasing number of women are living longer with metastatic breast cancer (MBC), making this a chronic disease with a non-negligible prevalence [1]. The prognosis is still poor, however, with 5-year overall survival of only 25% [2–4]. Shorter survival is observed for patients with triple-negative MBC, and longer survival is associated with bone-only metastases [5, 6]. All women with MBC are highly dependent on effective medical treatment along with accurate response assessment. Response monitoring by imaging is widely used to guide treatment decisions, but no specific recommendations for imaging procedures can be found in international MBC guidelines [7–10]. Contrast-enhanced computed tomography (CE-CT) and the corresponding Response Evaluation Criteria in Solid Tumors (RECIST 1.1) have traditionally been used for response monitoring of MBC and are based on changes in the morphological size of metastases [11]. However, CE-CT has low sensitivity for detecting bone metastases [11], low specificity for detection of liver metastases [12], and may also have some limitations for response monitoring in MBC patients [13, 14].
[18F]-fluorodeoxyglucose positron emission tomography (FDG-PET) computed tomography (FDG-PET/CT) has proven highly accurate in detecting MBC and has the potential to provide valid information on tumour metabolic activity by distinguishing active tumour from post-therapeutic changes [15, 16]. Changes in tumour activity potentially occur long before morphological changes appear, and clinical application of FDG-PET/CT for response monitoring may adopt treatment at an earlier timepoint than with conventional imaging [16–18].
Previous studies have shown that disease-specific survival could be better predicted by the response on FDG-PET/CT than on CE-CT [19], but the patient benefit from using FDG-PET/CT for longitudinal response monitoring in patients with MBC is still unknown.
We hypothesised that FDG-PET/CT would improve clinical decision-making and thereby lead to prolonged survival for patients with MBC. Therefore, we aimed to compare the overall survival for patients with metastatic breast cancer response monitored with FDG-PET/CT, CE-CT, or a combination of both modalities in an observational setting. Our objectives were (i) to provide a detailed description of the baseline characteristics to determine if the groups were roughly comparable, (ii) to describe patient management in each group during the response monitoring and (iii) to investigate the survival in each group and in subgroups of triple-negative patients and patients with bone-only metastases.
Material and methods
This was a single-centre, retrospective study based on prospective registration of patients. The study was conducted at the Department of Nuclear Medicine at Odense University Hospital, Denmark, in 2018–2020. The study protocol was approved by the Danish Patient Safety Authority (Ethics permission code: 3-3013-2448/1), including permission to register data from the patients’ electronic medical files until 10.08.2019.
Patient selection and study groups
Women diagnosed with MBC in 2004–2018 were eligible for the study. All patients were treated at the Department of Oncology, and imaging for response assessment was performed at the Departments of Radiology and/or Nuclear Medicine at Odense University Hospital. Inclusion criteria were distant relapsed MBC (biopsy-verified) or de novo breast cancer (biopsy verification of primary tumour and disseminated disease at baseline scan); baseline and at least one follow-up scan for response monitoring; use of either FDG-PET/CT, CE-CT or a combination of the two as the main response monitoring modality; standard response monitoring protocol with imaging intervals of 9–12 weeks [20]; and regular clinical follow-up. As monitoring by CE-CT or FDG-PET/CT was required, patients mainly monitored with magnetic resonance imaging (MRI) were not considered in the population. Exclusion criteria were other known disseminated malignancy, brain metastasis at baseline scan, acute cardiovascular disease or severe dementia at the time of MBC diagnosis, missing clinical data at follow-up, lost to follow-up due to emigration and refusal of treatment.
The modality used for response monitoring was generally decided by the oncologist who met the patient at the initial visit when MBC was diagnosed. No internal algorithm was available to guide the decision about the choice of response monitoring modality, and the choice was thus mainly at the discretion of the oncologist. Patients were allocated arbitrarily to the treating oncologists, with no distinctions due to clinical or performance status. Patients typically saw the same oncologist for each of their follow-up visits, minimising the risk of a change in monitoring modality.
Visual assessment was used for response evaluation without further criteria, i.e. the RECIST were not used regularly for the CE-CT, and the PET Response Criteria in Solid Tumors (PERCIST) were not applied at all. The scan reports typically suggested response (complete/partial response), stable disease or progressive disease. The oncologists based their clinical decisions on the scan report, the patient’s clinical performance status, the patient’s wishes and the potential toxicity of ongoing treatment.
Patients were categorised into three groups based on the imaging modality used for response monitoring: FDG-PET/CT, CE-CT and the combined group. One scan performed on the opposite imaging modality during the treatment period was considered acceptable in the FDG-PET-CT and CE-CT groups, and patients were allocated to the combined group if they were monitored by both scan types more than once. The scans were performed according to the standard guidelines for FDG-PET/CT and CE-CT (Supplementary Material 1) [11, 21].
Data collection and variables
From the patients’ medical files, we extracted age, performance status on the World Health Organization scale [22], clinical and histopathological data, referring doctor for the baseline scan, type of treatments received, cause of death and date of death or last clinical visit. All extracted information was located in the routine patient documentation. For patients with more than one primary breast cancer, we used the data for the primary cancer that had most likely led to the metastasis (i.e. had the same molecular profile as the metastasis). In some cases of de novo MBC, where the metastases had not been biopsied, we used information from the initial breast biopsy.
The overall survival time was defined as the time from the metastasis confirmation until death, with end of study period (10 August 2019) as censoring event. Time to the first treatment change was defined as the time between the baseline scan and first progression leading to treatment change. The patients with detected first progression were followed-up until detection of the subsequent progression, leading to a second treatment change in the clinic, and the time in between was considered as the time to detection of the second progression. The follow-up period was defined as the time interval between the baseline scan and the date of the last clinical visit for survivors and the date of death for non-survivors.
Statistical analyses
Continuous data were presented using the median (interquartile range) and mean ± standard deviation. Frequencies and respective percentages were given for categorical variables. The primary endpoint of this study was overall survival. Median 2-, 5- and 10-year survival were evaluated for all study groups with 95% confidence intervals (95% CI). Kaplan–Meier survival curves were used for visualisation [23]. Quantification of the group difference by a hazard ratio (HR) from a Cox-regression model was restricted to the two groups of patients solely monitored by FDG-PET/CT or CE-CT as survival in the combined group was affected by immortality bias, i.e. to experience monitoring by both modalities, patients would have to survive for some time. The HR was adjusted for a wide range of baseline and treatment characteristics to take account of any differences between the groups.
A time-varying HR based on Schoenfeld residuals was used to depict the change in the HR over follow-up time. The significance level was set at 0.05. All statistical analyses were conducted with STATA/IC (version 16.1, StataCorp, College Station, USA).
Results
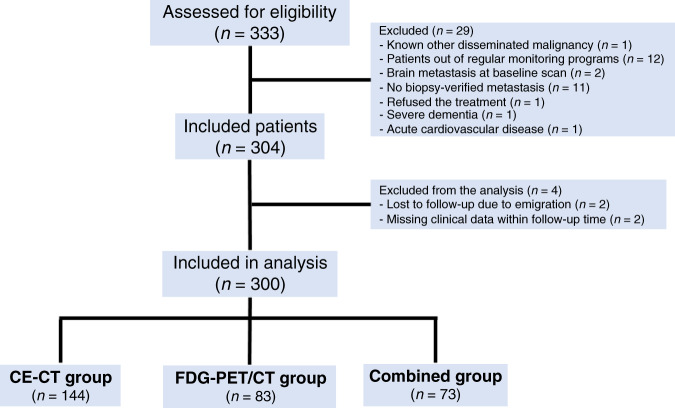
Of 333 eligible patients, 33 were excluded due to the reasons shown in Fig. 1, and the analysis was conducted on 300 patients divided into the CE-CT group (n = 144), the FDG-PET/CT group (n = 83) and the combined group (n = 73). Median follow-up time was 33.0 (3.6–130.6) months. Clinical and histopathological characteristics of the primary and metastatic breast cancer are summarised in Table 1 and Table 2, respectively. The study groups were mostly comparable on baseline characteristics, apart from more biopsy-verified liver metastasis in the CE-CT group and more biopsy-verified lung metastasis in the FDG-PET/CT group.
Fig. 1.
Flowchart of patient’ selection and distribution of included patients to the study groups (CE-CT contrast-enhanced computed tomography, FDG-PET/CT Fluorodeoxyglucose positron emission tomography with integrated computed tomography, MRI Magnetic resonance imaging).
Table 1.
Clinicopathological characteristics of primary breast cancer.
| Characteristicsa | Study groups | |||
|---|---|---|---|---|
| CE-CT (n = 144) | FDG-PET/CT (n = 83) | Combined (n = 73) | p-value | |
| Primary tumour size (mm) | 20 (3–70) | 24 (1–80) | 20 (9–60) | 0.69 |
| Bilateral cancer | 12 (8.3) | 9 (10.8) | 6 (8.2) | 0.81 |
| Histopathology | 0.13 | |||
| Ductal | 111 (77.1) | 57 (68.7) | 50 (68.5) | |
| Lobular | 19 (13.2) | 15 (18.1) | 7 (9.6) | |
| Adenocarcinoma | 6 (4.2) | 2 (2.4) | 6 (8.2) | |
| Unknown | 8 (5.6) | 9 (10.8)b | 10 (13.7) | |
| Surgery of primary tumour | 0.13 | |||
| No surgery | 27 (18.8) | 13 (15.7) | 15 (20.6) | |
| Lumpectomy | 50 (34.7) | 22 (26.5) | 31 (42.5) | |
| Mastectomy | 66 (45.8) | 46 (55.4) | 25 (34.3) | |
| Unknown | 1 (0.7) | 2 (2.4) | 2 (2.7) | |
| Oestrogen receptor status | 0.02 | |||
| Positive | 122 (84.7) | 59 (71.1) | 51 (69.9) | |
| Negative | 16 (11.1) | 12 (14.4) | 11 (15.1) | |
| Unknown | 6 (4.1) | 12 (14.4) | 11 (15.1) | |
| Human epidermal growth factor receptor-2 status | 0.10 | |||
| Positive | 17 (11.8) | 7 (8.4) | 12 (16.4) | |
| Negative | 87 (60.4) | 55 (66.3) | 33 (45.2) | |
| Unknown | 40 (27.8) | 21 (25.3) | 28 (38.4) | |
| Tumour grade | 0.16 | |||
| Grade 1 | 20 (13.9) | 15 (18.1) | 9 (12.3) | |
| Grade 2 | 63 (43.8) | 24 (28.9) | 23 (31.5) | |
| Grade 3 | 30 (20.8) | 24 (28.9) | 16 (21.9) | |
| Unknown | 31 (21.5) | 20 (24.1) | 25 (34.3) | |
| Ki-67 proliferation (%) | 30 (1–100) | 40 (1–95) | 30 (5–90) | 0.54 |
| Lymph node involvement | 0.16 | |||
| None | 30 (20.8) | 23 (27.7) | 18 (24.7) | |
| Single cell / Micro-metastasis | 8 (5.6) | 5 (6.0) | 3 (4.1) | |
| Macro-metastasis | 75 (52.1) | 31 (37.4) | 26 (35.6) | |
| Unknown | 31 (21.5) | 24 (28.9) | 26 (35.6) | |
| Treatment protocol | 0.17 | |||
| Neo-adjuvant treatment | 21 (14.6) | 8 (9.6) | 11 (15.1) | |
| Adjuvant treatmentc | 104 (72.2) | 51 (61.5) | 46 (63.0) | |
| Both of the above | 8 (5.6) | 12 (14.5) | 5 (6.9) | |
| None of protocols | 8 (5.6) | 9 (10.8) | 7 (9.6) | |
| Unknown | 3 (2.1) | 3 (3.6) | 4 (5.5) | |
| Radiotherapy protocol | 0.62 | |||
| None | 66 (45.8) | 40 (48.2) | 34 (46.6) | |
| Breast only | 23 (16.0) | 8 (9.6) | 13 (17.8) | |
| Breast + axilla | 55 (38.2) | 35 (42.2) | 26 (35.6) | |
CE-CT contrast-enhanced computed tomography, FDG-PET/CT Fluorodeoxyglucose positron emission tomography with integrated computed tomography.
aData shown as median (interquartile range) and frequency (%).
bThe histopathology of one patient in FDG-PET/CT group was granular cell tumour.
cAdjuvant treatment included both endocrine and chemotherapy.
Statistically significant p < 0.05 values are in bold.
Table 2.
Clinicopathological characteristics of metastatic diseasea.
| Characteristics | Study groups | |||
|---|---|---|---|---|
| CE-CT (n = 144) | FDG-PET/CT (n = 83) | Combined (n = 73) | p-value | |
| Year of diagnosis | 2013 (2007–2017) | 2015 (2009–2018) | 2013 (2004–2017) | <0.001 |
| Age at diagnosis (years) | 66.1 (28.4–84.5) | 64.7 (28.2–86.7) | 60.4 (31.0–95.3) | 0.003 |
| Performance status | 0.08 | |||
| 0 | 71 (49.3) | 40 (48.2) | 35 (48.0) | |
| 1 | 40 (28.8) | 26 (31.3) | 18 (24.7) | |
| 2 | 11 (7.6) | 6 (7.2) | 1 (1.37) | |
| ≥3 | 5 (3.5) | 3 (3.6) | 0 (0) | |
| Unknown | 17 (11.8) | 8 (9.6) | 19 (26.0) | |
| Time until relapseb (months) | 69.8 (0–271.4) | 59.3 (0–313.2) | 78 (0–364.0) | 0.94 |
| De novo metastatic cancer | 31 (21.5) | 17 (20.5) | 20 (27.4) | 0.56 |
| Histopathology | 0.69 | |||
| Ductal | 36 (25.0) | 14 (16.9) | 14 (19.2) | |
| Lobular | 16 (11.1) | 9 (10.8) | 5 (6.9) | |
| Adenocarcinoma | 58 (40.3) | 36 (43.4) | 35 (48.0) | |
| Unknown | 34 (23.6) | 24 (28.9) | 19 (26.0) | |
| Oestrogen receptor status | 0.24 | |||
| Positive | 118 (81.9) | 69 (83.1) | 55 (75.3) | |
| Negative | 17 (11.8) | 13 (15.7) | 14 (19.2) | |
| Unknown | 9 (6.6) | 1 (1.2) | 4 (5.5) | |
| Human epidermal growth factor receptor-2 status | 0.02 | |||
| Positive | 13 (9.0) | 8 (9.6) | 18 (24.7) | |
| Negative | 103 (71.5) | 64 (77.1) | 44 (60.3) | |
| Unknown | 28 (19.4) | 11 (13.3) | 11 (15.1) | |
| Ki-67 proliferation (%) | 32.5 (1–95) | 30 (5–95) | 40 (5–80) | 0.83 |
| Origin of biopsy | 0.03 | |||
| Bone | 41 (28.5) | 24 (28.9) | 19 (26.0) | |
| Liver | 27 (18.8) | 4 (4.8) | 7 (9.6) | |
| Lung | 16 (11.1)c | 13 (15.7) | 9 (12.3) | |
| Pleural fluid | 7 (4.9) | 13 (15.7) | 4 (5.5) | |
| Lymph nodes | 18 (12.5) | 12 (14.5) | 13 (17.8) | |
| Breast | 21 (14.6) | 11 (13.3) | 11 (15.1) | |
| Skin | 8 (5.6) | 3 (3.6) | 5 (6.9) | |
| Others | 6 (4.2) | 3 (3.6) | 5 (6.9) | |
| Triple negatived | 12 (8.3) | 8 (9.6) | 5 (6.9) | 0.78 |
| Detected metastatic lesions at baseline scan | ||||
| Oligometastatic cancere | 18 (12.5) | 8 (9.6) | 15 (20.6) | 0.14 |
| Bone | 95 (66.0) | 59 (71.1) | 45 (61.6) | 0.47 |
| Liver | 56 (38.9) | 16 (19.3) | 18 (24.7) | 0.005 |
| Lung / pleural effusion | 53 (36.8) | 37 (44.6) | 21 (28.8) | 0.12 |
| Lymph nodes (locoregional/distant) | 80 (55.6) | 60 (72.3) | 48 (65.8) | 0.04 |
| Breast / local recurrence | 31 (21.5) | 17 (20.5) | 14 (19.2) | 0.95 |
| Soft tissue | 9 (6.3) | 8 (9.6) | 3 (4.1) | 0.39 |
| Bone-only metastasis | 19 (13.2) | 7 (8.4) | 11 (15.1) | 0.41 |
| Bone and/or soft tissue dominant | 31 (21.5) | 15 (18.1) | 20 (27.4) | 0.38 |
| Organ-specific metastasis (according to baseline scan) | ||||
| Single organ metastasis | 42 (29.2) | 13 (15.7) | 22 (30.1) | 0.06 |
| Two–four organs metastases | 101 (70.1) | 68 (81.9) | 49 (67.1) | |
| Five-organs metastases | 1 (0.7) | 2 (2.4) | 2 (2.7) | |
CE-CT contrast-enhanced computed tomography, FDG-PET/CT Fluorodeoxyglucose positron emission tomography with integrated computed tomography.
aData shown as median (interquartile range) and frequency (%).
bTime until relapse for patients with primary disseminated disease was considered zero.
cOne patient was diagnosed with metastatic breast cancer and primary lung cancer at the same time.
dNegative for excess HER2 protein, oestrogen and progesterone receptors.
eOligometastatic cancer refers to patients with fewer than five metastatic lesions in a single organ.
Statistically significant p < 0.05 values are in bold.
Information on treatment and performed scans is shown in Table 3 for the three groups. Comparing the FDG-PET/CT and CE-CT groups, the main difference between them was that fewer patients in the FDG-PET/CT group received chemotherapy at least once. The number of scans was significantly higher in the combined group (P < 0.001), but after adjusting for the number of performed scans per follow-up time (Number of scans / 3 months of response monitoring), there was no statistically significant difference between the study groups (P = 0.40).
Table 3.
Treatment types and imaging information in response monitoring.
| Characteristicsa | Study groups | |||
|---|---|---|---|---|
| CE-CT (n = 144) | FDG-PET/CT (n = 83) | Combined (n = 73) | p-value | |
| Exposure of patients to treatment categories | ||||
| Endocrine therapy | 107 (74.3) | 61 (73.5) | 53 (72.6) | 0.97 |
| Chemotherapy | 99 (68.8) | 45 (54.2) | 57 (78.1) | 0.006 |
| Palliative radiotherapy | 32 (22.2) | 9 (10.9) | 12 (16.4) | 0.09 |
| Anti-HER2 therapyb | 13 (9.0) | 7 (8.4) | 17 (23.3) | 0.009 |
| CDK4/6 inhibitors | 19 (13.2) | 19 (22.9) | 12 (16.4) | 0.18 |
| Bone-target therapy | 105 (72.9) | 60 (72.3) | 52 (71.2) | 0.98 |
| No treatment / Unknown | 0 (0) | 1 (1.2) | 0 (0) | 0.52 |
| Protocolled experimental treatments | 15 (10.4) | 0 (0) | 13 (17.8) | <0.001 |
| Imaging information during follow-up period | ||||
| Total number of scans | 11 (3–36) | 11 (3–36) | 18 (5–51) | <0.001 |
| Number of scans per 3 months | 1.2 (0.4–3.6) | 1.1 (0.3–5.8) | 1.2 (0.2–2.7) | 0.40 |
| Patients experienced switch to opposite modality (once) | 37 (25.7) | 39 (47.0) | – | 0.001 |
| Performed MRI scans | 1 (0–8) | 1 (0–10) | 1 (0–9) | 0.26 |
CE-CT contrast-enhanced computed tomography, FDG-PET/CT Fluorodeoxyglucose positron emission tomography with integrated computed tomography, MRI magnetic resonance imaging, HER2 human epidermal growth factor receptor-2.
aData shown as frequency (%) at the patient level and as median (interquartile range) for performed scans.
bAnti-HER2 therapies consisted of Trastuzumab, Pertuzumab and T-DM1.
Statistically significant p < 0.05 values are in bold.
Information on mortality and median survival is presented in Table 4. Of the 215 patients who died within the follow-up period, the cause of death was MBC in 209 (97.2%) patients and unknown in six (2.8%). Median survival time was longer in the FDG-PET/CT and combined groups than in the CE-CT group. Survival curves are shown in the Kaplan–Meier plot in Fig. 2. An extended Kaplan–Meier survival curve including pointwise 95% CI can be found in Supplementary Material 2. Two-year survival probabilities differed only slightly between the groups, but 5-year survival for the FDG-PET/CT and combined groups were considerably higher (advantage of 26.1% and 27.5%, respectively) than for the CE-CT group.
Table 4.
Survival among study groups according to Kaplan–Meier estimates.
| Characteristics | Study groups | ||
|---|---|---|---|
| CE-CT (n = 144) | FDG-PET/CT (n = 83) | Combined (n = 73) | |
| Total number of deathsa | 123 (85.4) | 45 (54.2) | 47 (64.4) |
| Survival probabilitiesb (%) | |||
| 2-year survival | 63.9 (55.4–71.1) | 69.3 (58.0–78.1) | 87.7 (77.6–93.4) |
| 5-year survival | 15.8 (9.8–23) | 41.9 (29.0–54.2) | 43.3 (30.6–55.4) |
| 10-year survival | – | 20.9 (7.4–39.1) | 23.0 (12.3–35.7) |
| Survival timeb (months) | |||
| All patients | 30.0 (25.4–36.0) | 44.3 (29.7–80.2) | 54.0 (44.3–80.1) |
| Triple-negative patientsc | 12.4 (8.4–31.6) | 12.2 (4.2–20.5) | 44.3 (7.4–∞) |
| Patients with bone-only metastasis | 33.9 (17.5–52.9) | 82.6 (15.5–∞) | 58.0 (31.2–80.1) |
| Patients with oligometastatic cancer | 40.3 (29.1–54.4) | 94.0 (46.5–∞) | 87.1 (44.3–∞) |
| Patients received experimental treatments | 31.8 (12.0–48) | – | 85.0 (33.9–90.9) |
CE-CT Contrast-enhanced computed tomography, FDG-PET/CT Fluorodeoxyglucose positron emission tomography with integrated computed tomography.
aData shown as frequency (%).
bData shown as median (95% confidence interval).
cNegative for oestrogen receptors, progesterone receptors and excess HER2 protein.
Fig. 2.
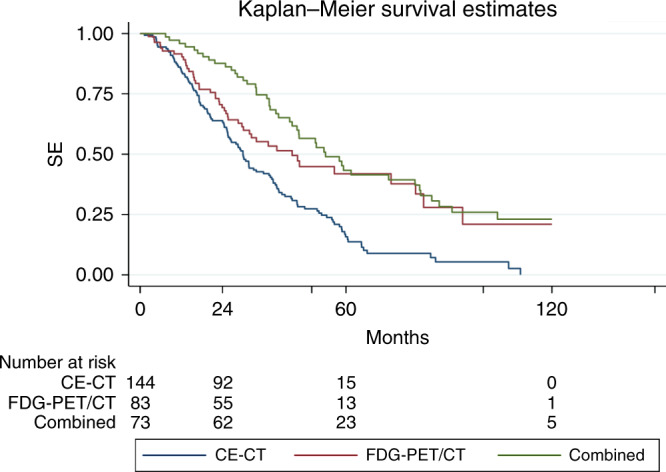
Kaplan–Meier plot and risk table showing survival of metastatic breast cancer patients according to response monitoring method (CE-CT contrast-enhanced computed tomography, FDG-PET/CT Fluorodeoxyglucose positron emission tomography with integrated computed tomography).
The FDG-PET/CT and combined groups had statistically significantly longer survival with HR of 0.56 (95% CI: 0.40–0.80, P = 0.001) and 0.41 (95% CI: 0.29–0.59, P < 0.001), respectively, when using the CE-CT group as reference in univariate survival analyses. When considering only the FDG-PET/CT and CE-CT groups and adjusting for baseline characteristics (selected variables from Table 1 and Table 2), there was a statistically significantly longer survival for the FDG-PET/CT group with HR of 0.44 (P = 0.001, Table 5). The results of the multivariable analysis also showed a negative prognostic value of short time until relapse, negative oestrogen and HER2 receptors in the metastasis, and bone and liver metastases at the baseline scan. A visual display of HR over time corroborated the increasing difference between groups over time as already visible in the survival curve (Supplementary Material 3). Adding a time x group interaction term to the model supported this visual finding (P < 0.001).
Table 5.
Multivariable COX-regression analyses of baseline clinical and histopathological characteristics on patient survivala.
| Variable | HR | 95% CI | p-value |
|---|---|---|---|
| Arm | |||
| CE-CT (Reference) | |||
| FDG-PET/CT | 0.44 | 0.29–0.68 | 0.001 |
| Primary breast cancer (clinical and surgical parameters) | |||
| Tumour size | 0.99 | 0.999–1.002 | 0.19 |
| Tumour histopathology (invasive ductal carcinoma as reference) | |||
| Invasive lobular carcinoma | 1.54 | 0.77–3.06 | 0.22 |
| Adenocarcinoma | 1.65 | 0.61–4.48 | 0.33 |
| Unknown | 1.004 | 0.36–2.78 | 0.99 |
| Oestrogen receptor status (positive as reference) | |||
| Negative | 1.58 | 0.72–3.50 | 0.26 |
| Unknown | 0.73 | 0.29–1.83 | 0.50 |
| HER2 receptor status (positive as reference) | |||
| Negative | 0.82 | 0.36–1.84 | 0.63 |
| Unknown | 1.40 | 0.55–3.52 | 0.48 |
| Tumour grade (grade 1 as reference) | |||
| Grade 2 | 0.88 | 0.51–1.51 | 0.65 |
| Grade 3 | 1.10 | 0.60–2.01 | 0.76 |
| Unknown | 0.72 | 0.36–1.45 | 0.36 |
| Lumpectomy / mastectomy | 1.30 | 0.57–3.00 | 0.53 |
| Lymph node involvement (no involvement as reference) | |||
| Single cell / micro-metastasis | 0.69 | 0.31–1.51 | 0.35 |
| Macro-metastasis | 1.09 | 0.71–1.69 | 0.68 |
| Unknown | 1.13 | 0.51–2.52 | 0.76 |
| Receiving adjuvant therapy | 1.26 | 0.70–2.26 | 0.44 |
| Receiving radiotherapy | 0.91 | 0.60–1.38 | 0.66 |
| Time until relapse (months) | 0.992 | 0.988–0.996 | <0.001 |
| Metastatic cancer (clinico-histopathological parameters) | |||
| Year of metastasis diagnosis | 1.15 | 1.06–1.26 | 0.001 |
| Age at metastasis (years) | 0.994 | 0.979–1.010 | 0.48 |
| Performance status ≥ 2 | 1.32 | 0.77–2.27 | 0.31 |
| Primary disseminated disease | 0.71 | 0.33–1.51 | 0.37 |
| Tumour histopathology (invasive ductal carcinoma as reference) | |||
| Invasive lobular carcinoma | 0.61 | 0.26–1.43 | 0.26 |
| Adenocarcinoma | 0.63 | 0.39–1.04 | 0.07 |
| Unknown | 0.51 | 0.29–0.90 | 0.02 |
| Oestrogen receptor status (positive as reference) | |||
| Negative | 3.02 | 1.38–6.62 | 0.006 |
| Unknown | 1.07 | 0.44–2.61 | 0.89 |
| HER2 receptor status (positive as reference) | |||
| Negative | 3.62 | 1.44–9.08 | 0.006 |
| Unknown | 8.21 | 2.99–22.60 | <0.001 |
| Referral oncologist (oncologist A as reference) | |||
| Oncologist B | 1.34 | 0.79–2.28 | 0.28 |
| Oncologist C | 0.65 | 0.36–1.18 | 0.15 |
| Oncologist D | 1.88 | 0.94–3.74 | 0.07 |
| Other oncologists | 0.91 | 0.54–1.53 | 0.71 |
| Tumour characteristics and region of metastasis at baseline scan | |||
| Oligometastases | 0.47 | 0.24–0.89 | 0.02 |
| Bone | 1.71 | 1.09–2.69 | 0.02 |
| Liver | 2.73 | 1.82–4.10 | <0.001 |
| Lung / Pleural | 0.97 | 0.66–1.41 | 0.86 |
| Regional / Distant lymph node | 0.97 | 0.65–1.42 | 0.86 |
HR hazard ratio, CI confidence interval, CE-CT contrast-enhanced computed tomography, FDG-PET/CT Fluorodeoxyglucose positron emission tomography with integrated computed tomography, HER2 human epidermal growth factor receptor-2.
aVariables considered in this model included baseline characteristics that are assumed to have potentially a prognostic value and in particular those which showed some imbalance between the groups.
Statistically significant p < 0.05 values are in bold.
Information on the duration of treatment during follow-up is summarised in Table 6 for the three groups. Patients in the FDG-PET/CT group received fewer treatment lines, experienced longer duration of treatment courses, and had shorter time on chemotherapy than patients in the CE-CT group. The first progression leading to treatment change occurred on average 4.7 months earlier in the FDG-PET/CT group than in the CE-CT group, while the second progression was detected on average 4.0 months later in the FDG-PET/CT group compared with the CE-CT group (12.1 vs. 8.1 months, P = 0.0001). Sensitivity analyses showing the effect of excluding patients who received one scan from the other scan modality and who received protocolled experimental treatments are presented in Supplementary Material 4 and Supplementary Material 5, respectively. Furthermore, sensitivity analysis of replacing region of metastases by the number of metastatic organs is presented in Supplementary Material 6.
Table 6.
Received treatment according to study groups.
| Characteristics | Study groups | |||
|---|---|---|---|---|
| CE-CT (n = 144) | FDG-PET/CT(n = 83) | Combined (n = 73) | p-value | |
| Number of treatment lines | <0.001 | |||
| Mean ± standard deviation | 3.1 ± 1.6 | 2.5 ± 1.5 | 3.6 ± 2.0 | |
| Median (interquartile range) | 3 (1–8) | 2 (0–8) | 3 (1–9) | |
| Duration of treatment courses (months) | 0.01 | |||
| Mean ± standard deviation | 10.3 ± 9.5 | 13.9 ± 14.5 | 15.9 ± 15.9 | |
| Median (interquartile range) | 6.8 (0.50–49.3) | 7.7 (0.50–76.7) | 9.3 (1.4–105) | |
| Time to first treatment changea (months) | 0.03 | |||
| Mean ± standard deviation | 17.6 ± 13.5 | 12.9 ± 11.6 | 16.0 ± 15.3 | |
| Median (interquartile range) | 13.6 (2.4–61.7) | 10.1 (1.8–61.3) | 11.4 (1.5–77.1) | |
| Time to detect second progressionb (months) | <0.001 | |||
| Mean ± standard deviation | 8.1 ± 7.9 | 12.1 ± 8.1 | 14.7 ± 15.8 | |
| Median (interquartile range) | 5.6 (1–42.7) | 10.4 (2.8–33.5) | 11 (1.6–106.2) | |
| Time period receiving treatment (months) | <0.001 | |||
| Mean ± standard deviation | 26.7 ± 20.1 | 27.0 ± 23.8 | 43.5 ± 31.2 | |
| Median (interquartile range) | 22.2 (0.9–84.8) | 19.3 (0–112.6) | 33.5 (2.8–135.3) | |
| Time period receiving chemotherapy (months) | 0.005 | |||
| Mean ± standard deviation | 12.6 ± 11.6 | 8.9 ± 7.2 | 19.2 ± 20.7 | |
| Median (interquartile range) | 8.6 (0.1–61.9) | 7.2 (0.2–26.9) | 12.5 (0.1–123.9) | |
CE-CT contrast-enhanced computed tomography, FDG-PET/CT Fluorodeoxyglucose positron emission tomography with integrated computed tomography.
aTime period between baseline scan and first detected progression leading treatment change.
bTime period between first and second detected progressions leading treatment change.
Statistically significant p < 0.05 values are in bold.
Discussion
This study revealed a survival benefit of 14–24 months for patients with metastatic breast cancer who were response monitored with FDG-PET/CT alone or in combination with CE-CT compared with patients monitored with CE-CT alone. Increased survival was confirmed for patients response monitored by FDG-PET/CT (HR: 0.44) in a multivariable Cox-regression analysis using the CE-CT group as a reference controlled for relevant baseline factors. The 5-year survival rate was considerably higher in the FDG-PET/CT group and the combined group than in the CE-CT group, and the difference in survival increased over time. FDG-PET/CT detected the first progression dictating treatment change ~5 months earlier than CE-CT, which may have had an impact on the effect and tolerability of subsequent treatment lines. Earlier detection of progression leading to treatment change could be a potential reason for the achievement of a more efficacious next treatment line in the FDG-PET/CT group, as we observed a longer time to experience the second progression in this group. Overall, patients in the FDG-PET/CT group received a lower number of treatment lines over a longer time than patients in the CE-CT group. We could consider the overall survival equal to disease-specific survival in this study as 97% of the mortality was due to metastatic breast cancer.
The results of this study suggest that FDG-PET/CT-based response monitoring may improve the clinical management of MBC patients through earlier termination of ineffective treatment, which result in longer survival. A reduction of false positive decisions implying stop of efficient treatment when using CT may also contribute to this effect although this could not be analysed in this observational study. Deducing intervention effects from observational data should only be interpreted cautiously. However, the present study had some favourable circumstances in that the choice of response modality probably reflected a preference by the treating oncologist, and major prognostic patient characteristics seem to be rather balanced or do not systematically favour one group.
We found that liver metastasis at the baseline scan was predictive of poor prognosis, which is in line with the results of a Danish registry-based cohort study [24]. Actually, the incidence of liver metastasis at baseline scan was higher in the CE-CT group than in the FDG-PET/CT group (38% vs. 19%). We conjecture that this reflects the lower specificity of CE-CT for diagnosing liver metastasis compared to FDG-PET/CT [12], and not a true difference between the two groups. However, the overall distribution of biopsy-verified liver/lung metastases, known as negative prognostic factors [25], was almost the same between CE-CT (34.8%) and FDG-PET/CT (36.1%) groups. In any case, we adjusted the multivariable analysis for the region of metastasis at baseline scan to take the potential differences between the groups into account.
It has already been demonstrated that MBC patients with bone-only metastasis have better long-term survival than patients with metastases in other regions [5]. In the subgroup of patients with bone-only metastasis (n = 27), however, we still observed longer survival in the FDG-PET/CT group compared with the CE-CT group (82.6 vs. 33.9 months). In contrast, patients with triple-negative MBC have a poor prognosis with a reported median survival of ~12 months [26, 27]; this was the same in our study regardless of whether monitoring with CE-CT or FDG-PET/CT. Patients with such aggressive tumour types may not benefit from response monitoring to guide treatment decisions.
Previous reviews and smaller studies have suggested that an FDG-PET/CT approach for monitoring distant metastases in MBC could improve treatment decisions by detecting non-response earlier than conventional methods and preventing patients from receiving ineffective, potentially toxic treatments [15, 16, 20]. In one larger study, FDG-PET/CT could identify non-responders earlier than CE-CT [17]. These results are supported by our findings indicating that FDG-PET/CT might prolong the survival of MBC patients through earlier detection of the first progression and hence sparing non-responding patients for ineffective treatment. However, effective medical treatment and clinicopathological features have the main role in improved long-term survival of MBC patients [25], and response monitoring could only guide clinical management of patients through improved prognostic stratification [28].
A few studies have proposed that FDG-PET/CT could play a role in predicting overall survival or progression-free survival when evaluating response to chemotherapy or endocrine treatment [29, 30]. In a study on 65 MBC patients who underwent FDG-PET/CT and CE-CT as their response evaluation after initial treatment line, it was concluded that responders on PERCIST (FDG-PET/CT) had significantly better progression-free and disease-specific survival than responders on RECIST (CE-CT) [19]. This corresponds well with the results of the current study that favour FDG-PET/CT for guiding treatment decisions.
Novel treatments such as CDK4/6 inhibitors have improved survival in MBC patients [31–34]. Evaluation of the effects of costly new treatments might be improved by adding FDG-PET/CT for response monitoring, or even by using more dedicated PET tracers that are currently under development in pre-clinical trials [35, 36]. Our data did not reflect the effects of this new generation of treatments, which were only sparsely administered in the current retrospective setting.
The overall economic burden of managing MBC patients will increase as the number of women with MBC increases [37–41]. Most of the cost is due to treatment costs as expenses related to imaging and diagnostic tests are approximately one-sixth of the treatment costs [39]. It is thus important to select the most accurate method for treatment response monitoring to improve long-term survival of MBC patients and potentially decrease costs to the healthcare system by reducing treatment intensity.
A limitation of the current study was the single-centre, observational retrospective design, meaning that patients were not randomly allocated to the study groups. A multivariable Cox-regression model was used to adjust for a variety of clinico-histopathological variables representing potential measured confounders (e.g. a higher rate of liver metastases in the CT group), but we cannot exclude the existence of further unmeasured confounders. Further, FDG-PET/CT is a newer modality, and patients diagnosed more recently had a higher chance to be followed by this modality and to have the advantage of new anti-cancer therapy. However, year of diagnosis was included as a potential confounder in the multivariable analysis, and hence this difference was taken into account. The RECIST criteria were only applied sporadically for CE-CT, and no standardised evaluation was used for FDG-PET/CT. We allowed one of the opposite scan types to be performed in each of the CE-CT and the FDG-PET/CT groups, which may have affected our results to some degree. However, in a sensitivity analysis of ‘pure’ FDG-PET/CT and CE-CT groups (where patients having one of the opposite scan types were excluded), the superiority of FDG-PET/CT was unchanged (Supplementary Material 4). We have to mention that participation of patients in an experimental protocol during the follow-up period, influenced group membership, as Food and Drug Administration and European Medicines Agency required response evaluation based on RECIST. In total, 28 patients participated at some timepoint during follow-up in such a protocol, and in 13 of them, such participation implied a change from the FDG-PET/CT group to the combined group, which resulted in an excellent survival (median survival of 85 months) among them.
Strengths of the current study were that: it was conducted in a relatively large patient population representative of daily clinical practice and with a long follow-up time (median of 33 months) that allowed us to analyze long-term survival probabilities. All included patients had biopsy-verified metastatic disease and were treated and monitored using the same equipment in a single university hospital. The data quality was high due to regular and careful registration of patients’ electronic files. As Danish residents, the patients had equal access to the healthcare services, and all were covered by the same national insurance system [42].
Our results require confirmation in prospective multicenter studies with extended follow-up times to allow further insights into the long-term consequences of using these modalities. These may be randomised studies or studies monitoring a systematic change from one modality to another with routine application of RECIST and PERCIST.
Conclusion
In this single-center, observational study, we found improved patient management and prolonged overall survival for patients with metastatic breast cancer when FDG-PET/CT was used alone or in combination with CE-CT for response monitoring as compared with using CE-CT alone. Using FDG-PET/CT for response monitoring provided earlier detection of the first progression, leading to change or termination of ineffective treatment, and longer effective next treatment line. The advantage of using FDG-PET/CT increased clearly over time, as we expect from an effective response strategy. Our results indicate that using FDG-PET/CT for response monitoring in patients with metastatic breast cancer may improve clinical decision-making and patient survival. This potential advantage requires confirmation in prospective trials, preferable using a randomised design.
Supplementary information
Acknowledgements
We thank Claire Gudex, MD, PhD, University of Southern Denmark, for language editing the manuscript.
Author contributions
Study concept and design: MN-B, MV, KK, ARK, WW, MGH. Data collection: MN-B, MV, RMV, MMBO, HO. Statistical analyses: MN-B, OG, WV. Interpretation of data: MN-B, MV, P-EB, JTA, OG, WV, ARK, MGH. Critical revision of the manuscript: All of the authors. Approval of final draft: All of the authors.
Funding
This study was supported by the Centre for Personalized Response Monitoring in Oncology (Odense University Hospital, Denmark) and the University of Southern Denmark.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study protocol was approved by the Danish Patient Safety Authority (Ethics permission code: 3-3013-2448/1), including permission to register data from the patients’ electronic medical files until 10.08.2019.
Consent for publication
Not applicable.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01654-w.
References
- 1.Mariotto AB, Etzioni R, Hurlbert M, Penberthy L, Mayer M. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol Biomark Prev. 2017;26:809–15.. doi: 10.1158/1055-9965.EPI-16-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim B, Hortobagyi GN. Current challenges of metastatic breast cancer. Cancer Metastasis Rev. 2016;35:495–514. doi: 10.1007/s10555-016-9636-y. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone A, Krapcho M, Garshell J, Miller D, Altekruse S, et al. SEER cancer statistics review, 1975-2012. Bethesda: National Cancer Institute; 2015.
- 4.Alteri R, Bertaut T, Brooks D, Chambers W, Chang E, DeSantis C, et al. Cancer facts & figures 2015. Atlanta: American Cancer Society; 2015. pp. 58–72.
- 5.Kono M, Fujii T, Matsuda N, Harano K, Chen H, Wathoo C, et al. Somatic mutations, clinicopathologic characteristics, and survival in patients with untreated breast cancer with bone-only and non-bone sites of first metastasis. J Cancer. 2018;9:3640–6. doi: 10.7150/jca.26825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 7.Khatcheressian JL, Hurley P, Bantug E, Esserman LJ, Grunfeld E, Halberg F, et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:961–5. doi: 10.1200/JCO.2012.45.9859. [DOI] [PubMed] [Google Scholar]
- 8.Ramakrishna N, Temin S, Chandarlapaty S, Crews JR, Davidson NE, Esteva FJ, et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2018;36:2804–7. doi: 10.1200/JCO.2018.79.2713. [DOI] [PubMed] [Google Scholar]
- 9.Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, et al. Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol. 2016;34:3069–103. doi: 10.1200/JCO.2016.67.1487. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31:1623–49. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Chua SC, Groves AM, Kayani I, Menezes L, Gacinovic S, Du Y, et al. The impact of 18F-FDG PET/CT in patients with liver metastases. Eur J Nucl Med Mol Imaging. 2007;34:1906–14. doi: 10.1007/s00259-007-0518-y. [DOI] [PubMed] [Google Scholar]
- 13.Hildebrandt MG, Gerke O, Baun C, Falch K, Hansen JA, Farahani ZA, et al. [18F]Fluorodeoxyglucose (FDG)-positron emission tomography (PET)/computed tomography (CT) in suspected recurrent breast cancer: a prospective comparative study of dual-time-point FDG-PET/CT, contrast-enhanced CT, and bone scintigraphy. J Clin Oncol. 2016;34:1889–97. doi: 10.1200/JCO.2015.63.5185. [DOI] [PubMed] [Google Scholar]
- 14.Yang HL, Liu T, Wang XM, Xu Y, Deng SM. Diagnosis of bone metastases: a meta-analysis comparing (1)(8)FDG PET, CT, MRI and bone scintigraphy. Eur Radiol. 2011;21:2604–17.. doi: 10.1007/s00330-011-2221-4. [DOI] [PubMed] [Google Scholar]
- 15.Dose Schwarz J, Bader M, Jenicke L, Hemminger G, Jänicke F, Avril N. Early prediction of response to chemotherapy in metastatic breast cancer using sequential 18F-FDG PET. J Nucl Med. 2005;46:1144–50. [PubMed] [Google Scholar]
- 16.Avril S, Muzic RF, Jr, Plecha D, Traughber BJ, Vinayak S, Avril N. (18)F-FDG PET/CT for monitoring of treatment response in breast cancer. J Nucl Med. 2016;57:34s–9s. doi: 10.2967/jnumed.115.157875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin NU, Guo H, Yap JT, Mayer IA, Falkson CI, Hobday TJ, et al. Phase II study of lapatinib in combination with trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: clinical outcomes and predictive value of early [18F]fluorodeoxyglucose positron emission tomography imaging (TBCRC 003) J Clin Oncol. 2015;33:2623–31. doi: 10.1200/JCO.2014.60.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naghavi-Behzad M, Oltmann HR, Alamdari TA, Bülow JL, Ljungstrøm L, Braad PE, et al. Clinical impact of FDG-PET/CT compared with CE-CT in response monitoring of metastatic breast cancer. Cancers (Basel) 2021;13:4080. doi: 10.3390/cancers13164080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riedl CC, Pinker K, Ulaner GA, Ong LT, Baltzer P, Jochelson MS, et al. Comparison of FDG-PET/CT and contrast-enhanced CT for monitoring therapy response in patients with metastatic breast cancer. Eur J Nucl Med Mol Imaging. 2017;44:1428–37. doi: 10.1007/s00259-017-3703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graham LJ, Shupe MP, Schneble EJ, Flynt FL, Clemenshaw MN, Kirkpatrick AD, et al. Current approaches and challenges in monitoring treatment responses in breast cancer. J Cancer. 2014;5:58–68. doi: 10.7150/jca.7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sok M, Zavrl M, Greif B, Srpčič M. Objective assessment of WHO/ECOG performance status. Support Care Cancer. 2019;27:3793–8. doi: 10.1007/s00520-018-4597-z. [DOI] [PubMed] [Google Scholar]
- 23.Jager KJ, van Dijk PC, Zoccali C, Dekker FW. The analysis of survival data: the Kaplan-Meier method. Kidney Int. 2008;74:560–5. doi: 10.1038/ki.2008.217. [DOI] [PubMed] [Google Scholar]
- 24.Ording AG, Heide-Jørgensen U, Christiansen CF, Nørgaard M, Acquavella J, Sørensen HT. Site of metastasis and breast cancer mortality: a Danish nationwide registry-based cohort study. Clin Exp Metastasis. 2017;34:93–101. doi: 10.1007/s10585-016-9824-8. [DOI] [PubMed] [Google Scholar]
- 25.Wang R, Zhu Y, Liu X, Liao X, He J, Niu L. The Clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer. 2019;19:1091. doi: 10.1186/s12885-019-6311-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pogoda K, Niwińska A, Murawska M, Pieńkowski T. Analysis of pattern, time and risk factors influencing recurrence in triple-negative breast cancer patients. Med Oncol. 2013;30:388. doi: 10.1007/s12032-012-0388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kassam F, Enright K, Dent R, Dranitsaris G, Myers J, Flynn C, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer. 2009;9:29–33. doi: 10.3816/CBC.2009.n.005. [DOI] [PubMed] [Google Scholar]
- 28.Cochet A, David S, Moodie K, Drummond E, Dutu G, MacManus M, et al. The utility of 18 F-FDG PET/CT for suspected recurrent breast cancer: impact and prognostic stratification. Cancer Imaging. 2014;14:13. doi: 10.1186/1470-7330-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mortazavi-Jehanno N, Giraudet AL, Champion L, Lerebours F, Le Stanc E, Edeline V, et al. Assessment of response to endocrine therapy using FDG PET/CT in metastatic breast cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2012;39:450–60. doi: 10.1007/s00259-011-1981-z. [DOI] [PubMed] [Google Scholar]
- 30.Zhang FC, Xu HY, Liu JJ, Xu YF, Chen B, Yang YJ, et al. (18)F-FDG PET/CT for the early prediction of the response rate and survival of patients with recurrent or metastatic breast cancer. Oncol Lett. 2018;16:4151–8. doi: 10.3892/ol.2018.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926–36. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 32.Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–48. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 33.Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–46. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 34.Martin M, Garcia-Saenz JA, Manso L, Llombart A, Cassinello A, Atienza M, et al. Abemaciclib, a CDK4 and CDK6 inhibitor for the treatment of metastatic breast cancer. Future Oncol. 2020;16:2763–78. doi: 10.2217/fon-2020-0604. [DOI] [PubMed] [Google Scholar]
- 35.Elmi A, Makvandi M, Weng C-C, Hou C, Clark AS, Mach RH, et al. Cell-proliferation imaging for monitoring response to CDK4/6 inhibition combined with endocrine-therapy in breast cancer: comparison of [18 F]FLT and [18 F]ISO-1 PET/CT. Clin Cancer Res. 2019;25:3063–73. doi: 10.1158/1078-0432.CCR-18-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramos N, Baquero-Buitrago J, Ben Youss Gironda Z, Wadghiri YZ, Reiner T, Boada FE, et al. Noninvasive PET imaging of CDK4/6 activation in breast cancer. J Nucl Med. 2020;61:437–42. doi: 10.2967/jnumed.119.232603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen MT, Sun HF, Zhao Y, Fu WY, Yang LP, Gao SP, et al. Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: a SEER population-based analysis. Sci Rep. 2017;7:9254. doi: 10.1038/s41598-017-10166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foster TS, Miller JD, Boye ME, Blieden MB, Gidwani R, Russell MW. The economic burden of metastatic breast cancer: a systematic review of literature from developed countries. Cancer Treat Rev. 2011;37:405–15. doi: 10.1016/j.ctrv.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Sorensen SV, Goh JW, Pan F, Chen C, Yardley D, Martin M, et al. Incidence-based cost-of-illness model for metastatic breast cancer in the United States. Int J Technol Assess Health Care. 2012;28:12–21. doi: 10.1017/S026646231100064X. [DOI] [PubMed] [Google Scholar]
- 40.Sorenson S, Benedict A, Yardley DA, Martin M, Knopf KB, Pan F, et al. Burden of illness estimates of metastatic breast cancer (MBC) in the United States. J Clin Oncol. 2010;28:6009-. doi: 10.1200/jco.2010.28.15_suppl.6009. [DOI] [Google Scholar]
- 41.Vondeling GT, Menezes GL, Dvortsin EP, Jansman FGA, Konings IR, Postma MJ, et al. Burden of early, advanced and metastatic breast cancer in The Netherlands. BMC Cancer. 2018;18:262. doi: 10.1186/s12885-018-4158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olejaz M, Juul Nielsen A, Rudkjøbing A, Okkels Birk H, Krasnik A, Hernández-Quevedo C. Denmark health system review. Health Syst Transit. 2012;14:i–xxii. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.