Abstract
Background
Colorectal cancer (CRC) patients have a better prognosis if metastases are resectable. Initially, unresectable liver-only metastases can be converted to resectable with chemotherapy plus a targeted therapy. We assessed which of chemotherapy doublet (2-CTx) or triplet (3-CTx), combined with targeted therapy by RAS status, would be better in this setting.
Methods
PRODIGE 14 was an open-label, multicenter, randomised Phase 2 trial. CRC patients with initially defined unresectable liver-only metastases received either, 2-CTx (FOLFOX or FOLFIRI) or 3-CTx (FOLFIRINOX), plus bevacizumab/cetuximab by RAS status. The primary endpoint was to increase the R0/R1 liver-resection rate from 50 to 70% with the 3-CTx.
Results
Patients (n = 256) were mainly men with an ECOG PS of 0, and a median age of 60 years. In total, 109 patients (42.6%) had RAS-mutated tumours. After a median follow-up of 45.6 months, the R0/R1 liver-resection rate was 56.9% (95% CI: 48–66) with the 3-CTx versus 48.4% (95% CI: 39–57) with the 2-CTx (P = 0.17). Median overall survival was 43.4 months with 3-CTx versus 40 months with 2-CTx.
Conclusion
We failed to increase from 50 to 70% the R0/R1 liver-resection rate with the use of 3-CTx combined with bevacizumab or cetuximab by RAS status in CRC patients with initially unresectable liver metastases.
Subject terms: Cancer therapy, Colorectal cancer
Background
Worldwide, in 2018, colorectal cancer (CRC) was the third most common cancer; with about 1.8 million new cases and second in terms of cancer mortality, with 881,000 deaths [1]. The prognosis of patients with CRC correlates with the tumour stage and most notably with the occurrence of metastases. In metastatic CRC patients with liver-only metastases complete resection of all metastatic sites, if feasible, may be curative. Initially, unresectable liver metastases may become resectable after chemotherapy. Chemotherapy doublets (2-CTx), FOLFOX (fluorouracil, folinic acid and oxaliplatin) or FOLFIRI (fluorouracil, folinic acid and oxaliplatin) are frequently combined with targeted therapies such as bevacizumab [2–5], or cetuximab [3, 5–7] or panitumumab [8] for RAS wild-type (wt) tumours. Studies report a correlation between response rates and secondary resection rate, particularly in patients with liver-limited disease [9–11]. Indeed, chemotherapy triplets (3-CTx), FOLFOXIRI or FOLFIRINOX, (fluorouracil, folinic acid, oxaliplatin and irinotecan) [12–14] alone or with targeted therapies, such as bevacizumab [4, 15–18], cetuximab [19, 20] or panitumumab [21], result in high response rates and more favourable outcomes. When we designed the PRODIGE-14 trial, no prospective study had assessed whether 3-CTx or 2-CTx, combined with targeted therapy (by RAS status), provided a better resection rate.
Methods
Patients
Patients with histologically proven CRC with liver metastases (synchronous or metachronous) considered non-resectable or not optimally resectable, without extrahepatic disease except for a non-symptomatic primary tumour or resectable lung metastases (≤3 lesions, each <2 cm in diameter) were eligible. Liver metastases were considered either technically non-resectable (if any lesion was close to a major vessel or if the predicted post-surgical volume was <30% of the initial volume) or oncologically non-resectable (if they were bilateral with >4 lesions). The non-resectability of the liver lesions was assessed locally by multidisciplinary boards. The eligibility criteria are described in Supplementary Appendix Table 1. Before randomisation, the local molecular platform performed the KRAS exon 2 genotyping of the patient’s tumour. From July 8, 2014, after 172 patients had been enrolled, RAS genotyping was required for the study. Consequently, the required genotyping included KRAS exons 2, 3 and 4; and NRAS exons 2, 3 and 4. The study complied with the Declaration of Helsinki, good clinical practice guidelines, and other local laws. The study documents were approved by a French ethics committee, “Comité de Protection des Personnes Sud Méditerranée IV”. Patients provided written informed consent before enrolment. The PRODIGE 14 was registered in EudraCT (N°2009-012813-22) and ClinicalTrials.gov (NCT01442935).
Intervention and randomisation
The PRODIGE-14 (Partenariat de Recherche en Oncologie DIGEstive) study was an open-label, randomised (1:1) Phase II clinical trial. The trial assessed two chemotherapy regimens, standard 2-CTx or experimental 3-CTx, combined with targeted therapy (bevacizumab or cetuximab by KRAS/RAS status) to treat CRC patients with initially unresectable liver metastases. Patients allocated to 3-CTx received FOLFIRINOX every 2 weeks: oxaliplatin (85 mg/m2) was infused intravenously for 120 min, followed by initially simultaneous intravenous infusions of irinotecan (150 mg/m2), over 90 min, and leucovorin (200 mg/m2), over 120 min. Then, a 400 mg/m2 bolus of fluorouracil was administered, followed by a continuous infusion of fluorouracil (2,400 mg/m2), over 46 h. Patients randomly allocated to 2-CTx received either FOLFOX4 (oxaliplatin [85 mg/m2], folinic acid [400 mg/m2], or l-folinic acid [200 mg/m2], and fluorouracil as a bolus [400 mg/m2] and as a 46-h infusion [2400 mg/m2]) or FOLFIRI (irinotecan [180 mg/m2], folinic acid [400 mg/m2], or l-folinic acid [200 mg/m2], and fluorouracil as a bolus [400 mg/m2] and as a 46-h infusion [2400 mg/m2]) every 2 weeks. Patients with mutated (mt)RAS tumours received bevacizumab (5 mg/kg) and those with wtRAS tumours received cetuximab (500 mg/m2), intravenously, on day 1 of each 2-week chemotherapy cycle. Chemotherapy was administered for at most 12 cycles or until disease progression, unacceptable toxicity or patient withdrawal. Primary prophylaxis with granulocyte colony-stimulating factor was mandatory in the experimental 3-CTx arm.
Study endpoints and assessments
The primary endpoint was the R0/R1 resection rate. Secondary endpoints included 8-week and best response rate, PFS, OS and safety. The response rate was defined as the proportion of complete or partial responses. Tumour response was assessed at baseline and then every 8 weeks until disease progression, by RECIST version (v)1.1, using spiral or conventional computed tomography or magnetic resonance imaging. PFS was the time from randomisation to first reported disease progression or death. OS was the time from randomisation to death. Safety was assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0, except for neurotoxicity assessed by the Levi scale. Efficacy was assessed in the intent-to-treat (ITT) population whereas safety was assessed in patients treated for at least one cycle.
Molecular analyses
During screening, KRAS exon 2 testing, and amended during the study to RAS mutations testing, were performed in regional molecular cancer genetics platforms, labelled and funded by the French National Cancer Institute. A blinded central review of the genotyping was planned and done.
Statistical analysis
This study was designed to evaluate 2 chemotherapy regimens (2-CTx versus 3-CTx) combined with a targeted agent (bevacizumab/cetuximab) according to KRAS/RAS status, to treat CRC patients with initially unresectable liver metastases. We expected a 50% R0/R1 resection rate with induction chemotherapy in the control arm (2-CTx arm). To show a 20% increase in the experimental arm (3-CTx arm) (i.e., R0/R1 resection rate of 70%), using a two-sided α of 5% and a power of 90%, and allowing for a 5% ineligibility rate, the study required 256 patients: 128 in each arm. Patients were randomised by minimisation and stratified by RAS genotype (mtKRAS/mtRAS versus wtKRAS/wtRAS), by primary and metastases presentation (primary not resected and synchronous metastases versus primary resected and synchronous metastases versus primary resected and metachronous metastases), and by non-resectability (technical versus oncological). Qualitative variables were described as percentages and quantitative variables by their medians with the associated ranges. OS and PFS times (with their 95% confidence intervals [CI]) were estimated by the Kaplan–Meier method. After looking at differences between R0/R1 resection rates between treatments, a comparison of liver-resection rates between the two arms will be done using logistic regression adjusted for stratification factors. The homogeneity of the treatment effect will be checked by considering interaction terms in the model. Log-rank and Cox proportional hazards regression model analyses are exploratory. Median follow-up with the 95% CI was estimated by the reverse Kaplan–Meier method. Hazard ratios (HRs) and their 95% CIs were determined using Cox proportional hazards regression models. Analyses were performed using STATA v16.1.
Results
Patients
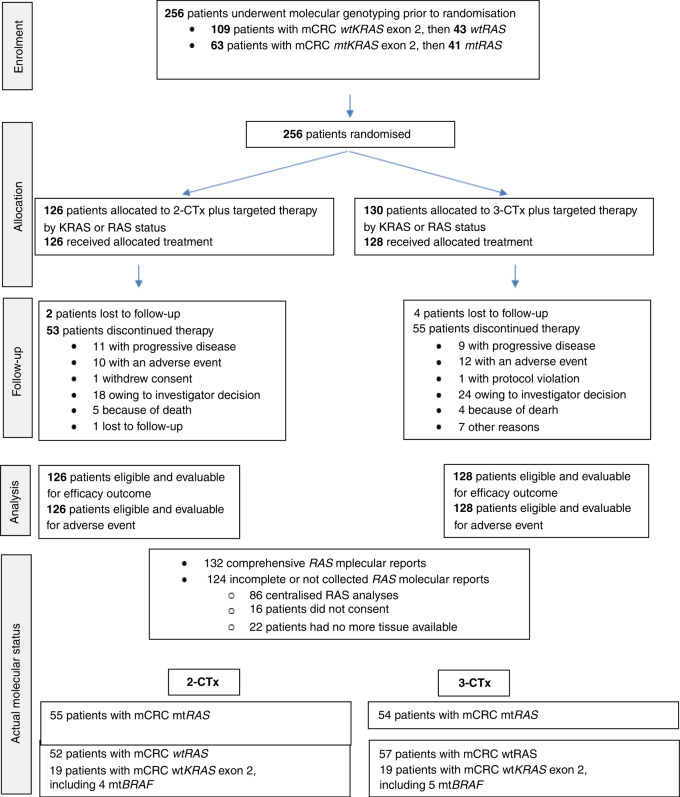
The UNICANCER PRODIGE-14 study enrolled 256 patients between February 9, 2011 and April 30, 2015: 126 in the 2-CTx arm and 130 in the 3-CTx arm (see Fig. 1). Patient baseline characteristics were well balanced between the treatment arms (Table 1). Baseline KRAS exon 2 and actual KRAS and NRAS exon 2, 3, 4 status are reported in Fig. 1.
Fig. 1.
CONSORT flow chart.
Table 1.
Baseline demographic and clinical characteristics.
| Characteristic | Patients, no. (%) | |
|---|---|---|
| Chemotherapy doublet plus targeted therapy by RAS* status (n = 126) | Chemotherapy triplet plus targeted therapy by RAS* status (n = 130) | |
| Age, median (range) | 61 (29–75) | 60 (27–78) |
| Sex | ||
| Male | 82 (65.1) | 81 (62.3) |
| Female | 44 (34.9) | 49 (37.7) |
| ECOG performance status | ||
| 0 | 84 (66.7) | 82 (64.1) |
| 1 | 42 (33.3) | 46 (35.9) |
| Primary tumour location | ||
| Right colon | 32 (25.4) | 28 (21.7) |
| Left colon | 58 (46) | 63 (48.8) |
| Rectum | 33 (26.2) | 34 (26.4) |
| Multiple | 3 (2.4) | 4 (3.1) |
| Unknown | 0 | 1 |
| Surgery of the primary tumour | 40 (31.7) | 43 (33.1) |
| Synchronous metastases | 111 (88.1) | 117 (90.0) |
| Lung metastases | 16 (12.7) | 14 (11.2) |
| Non-resectability reason | ||
| Technical | 90 (71.4) | 92 (72.4) |
| Oncological | 23 (18.3) | 27 (21.3) |
| Both | 13 (10.3) | 8 (6.3) |
| Unknown | 0 | 3 |
| Median CEA (ng/mL) | 96 | 58.7 |
| Median leucocyte count (109/L) | 9.1 | 6.8 |
ECOG Eastern Cooperative Oncology Group, CEA carcinoembryonic antigen.
RAS* is KRAS/NRAS exon 2, exon 3, exon 4 genotyping.
We centrally reviewed molecular analyses in 218/256 cases. This quality control was impossible in 38 cases due to the absence of consent or tumour tissue samples. The review identified nine patients with mtRAS tumours initially classified as wtKRAS exon 2 and treated with cetuximab. Furthermore, nine patients with mtBRAF tumours were identified, four in the 2-CTx arm and five in the 3-CTx arm. In 86/218 molecular analyses, genotyping was inconclusive, so we performed a centralised post hoc pyrosequencing of KRAS exons 3 and 4 and NRAS exons 2, 3 and 4.
Treatment
In both the 2-CTx and 3-CTx arms, the median number of treatment cycles administered was 12 (range: 1–12). Granulocyte colony-stimulating factor was administered to 26% of patients in the 2-CTx arm and to 89% in the 3-CTx arm. For patients who had liver surgery, the median number of cycles administered before liver surgery was 9 (range: 1–12) with 2-CTx and 9 (range: 1–12) with 3-CTx. Before liver surgery, the median relative dose intensities were 93.3% (range: 29.8–105.5) vs 84.4% (range: 21.5–120.0) for irinotecan, 86.9% (range: 0–130.5) vs 82.9% (range: 0–115.3) for oxaliplatin, and 91.8% (range: 0–129.0) vs 86.2% (range: 0–115.3) for fluorouracil in the 2-CTx arm vs 3-CTx arm, respectively.
Efficacy
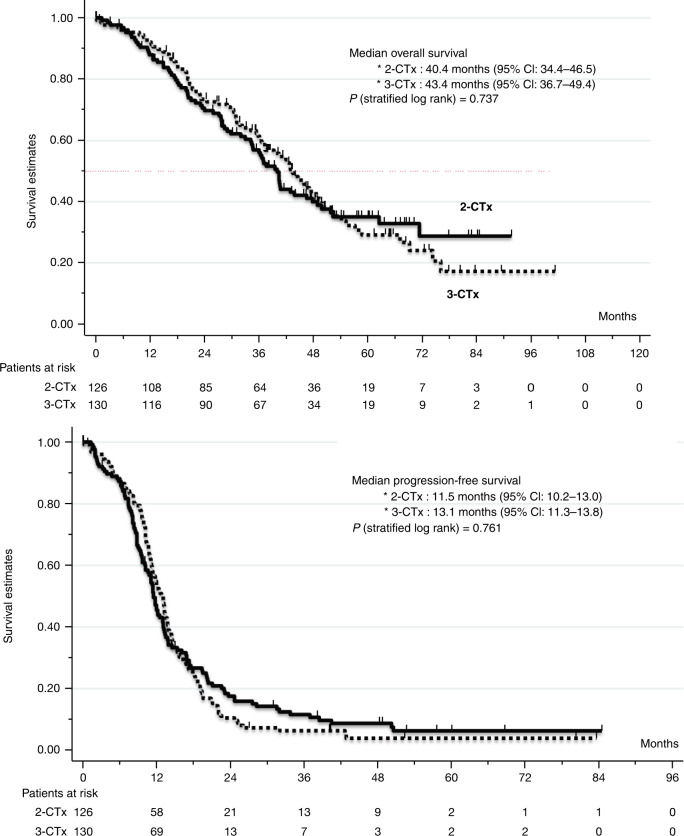
In the 2-CTx arm, 64/126 patients (50.8%) underwent liver surgery and in the 3-CTx arm, 77/130 patients (59.2%). After a median follow-up of 57.4 months, the median time to resection was 5.1 months in the 2-CTx arm and 5.5 months in the 3-CTx arm. The efficacy results (ITT population) are summarised in Table 2. The R0/R1 resection rate, the primary endpoint, was 48.4% (R0/R1 in 61/126 patients) in the 2-CTx arm and 56.9% (R0/R1 in 74/130 patients) in the 3-CTx arm (P = 0.17). An analysis using a logistic regression model, adjusted for stratification factors, gave odds of 1.7 (95% CI: 1.1–2.7; P = 0.016) for R0/R1 resection in favour of the 3-CTx arm over the 2-CTx arm. The 8-week response rate was 50.4% (95% CI: 41.1–59.7%) and the overall response rate was 65.9% (95% CI: 56.9–74.1%) with 2-CTx. Similarly, the 8-week response rate was 57.4% (95% CI: 48.1–66.3%) and the response rate was 75.0% (95% CI: 66.6%-82.2%) with 3-CTx. The median PFS time was 11.5 months (95% CI: 10.2–13.0) in the 2-CTx arm and 13.1 months (95% CI: 11.3–13.8) in the 3-CTx arm (HR for disease progression, 1.04; 95% CI: 0.79–1.38; P = 0.762) (Fig. 2). Median OS was 40.0 months (95% CI: 34.4–46.5) in the 2-CTx and 43.4 months (95% CI: 36.7–49.4) in the 3-CTx arm (HR for death, 0.94; 95% CI: 0.67–1.32; P = 0.737) (Fig. 2). In the multivariate analysis (per-protocol population), the non-resectability (technical/oncological), sex, age, primary tumour resected, primary tumour sidedness (left/right colon), baseline CEA and alkaline phosphatase level, treatment arm and post hoc RAS assessment) were assessed as predictive of R0/R1 resection. Among these parameters: age (<60 years old), left colon primary tumours, primary tumour resected before randomisation, and baseline CEA < 80 IU/ml were found to independently predict R0/R1 resection (P < 0.01) (see Table 3). The median survival was 54.2 months (95% CI: 48–74) in patients with R0/R1 liver resection in either treatment arm and was 28.4 months (95% CI: 21–37) in those with R2 resections or non-resected. We performed an exploratory analysis of the nine patients with mtBRAF tumours: four in the 2-CTx arm and five in the 3-CTx arm (Appendix Table 2). Only 2/9 patients, both in the 3-CTx arm, had a R0/R1 liver resection. In the 2-CTx arm the median PFS was 1.8 months and median OS was 6.6 months. Similarly, in the 3-CTx, median PFS was 6.1 months and median OS was 21.3 months (Supplementary Appendix Table 2).
Table 2.
Efficacy outcomes.
| Chemotherapy doublet plus targeted therapy by RAS status (n = 126) | Chemotherapy triplet plus targeted therapy by RAS status (n = 130) | |
|---|---|---|
| Variable | ||
| R0/R1 resection, n (%, 95% CI) | 61 (48.4, 39–57) | 74 (56.9, 48–66) |
| 8-week overall response, n (%, 95% CI) | 60 (50.4, 41.1–659.7) | 69 (57.4, 48.1–66.3) |
| Complete response, n (%) | 1 (0.8) | 2 (1.7) |
| Partial response, n (%) | 59 (49.6) | 68 (56.2) |
| Stable disease, n (%) | 52 (43.7) | 48 (39.7) |
| Progressive disease, n (%) | 7 (5.9) | 3 (2.5) |
| Unknown, n | 7 | 7 |
| Best overall response, n (%, 95% CI) | 83 (65.9, 56.9–74.1) | 96 (75.0, 66.6–82.2) |
| Complete response, n (%) | 20 (16.8) | 25 (20.7) |
| Partial response, n (%) | 63 (52.9) | 71 (58.7) |
| Stable disease, n (%) | 31 (26.1) | 22 (18.2) |
| Progressive disease, n (%) | 5 (4.2) | 3 (2.5) |
| Unknown, n | 7 | 7 |
| Progression-free survival time | ||
| Median (95% CI), months | 11.5 (10.2–13.0) | 13.1 (11.3–13.8) |
| Overall survival time | ||
| Median (95% CI), months | 40.4 (34.4–46.5) | 43.4 (36.7–49.4) |
Fig. 2. Survival times in the intent-to-treat populations.
Kaplan–Meier estimates of overall survival (up) and of progression-free survival (below).
Table 3.
Predictive factors for R0/R1 resection after multivariate analysis.
| Variables | Comparison | Odds ratio | 95% CI | P value |
|---|---|---|---|---|
| Age | <60 vs ≥60 | 1 vs 0.39 | 0.22–0.78 | 0.003 |
| Tumour location | Left vs right side | 1 vs 0.30 | 0.15–0.63 | 0.001 |
| Primary resected | No vs yes | 1 vs 10.78 | 5.60–20.75 | <0.001 |
| Baseline CEA | <80 vs ≥80 | 1 vs 0.28 | 0.15–053 | <0.001 |
| Arm | 2-CTx vs 3-CTx | 1 vs 1.16 | 0.56–2.39 | 0.693 |
| Sex | Male vs female | 1 vs 2.10 | 0.96–4.61 | 0.063 |
| PAL | <128 vs ≥128 | 1 vs 0.97 | 0.45–2.08 | 0.936 |
| KRAS | Mutated vs WT | 1 vs 0.76 | 0.37–1.60 | 0.477 |
| Non-resectable | Technically vs oncologically | 1 vs 1.50 | 0.57–3.96 | 0.413 |
Tolerability
Serious adverse events (AEs) were reported in 86/126 patients (68.3%) in the 2-CTx arm and in 104/128 patients (81.3%) in the 3-CTx arm. The most common grade 3–4 AEs reported in >3% of the patients were neutropenia, diarrhoea, thrombosis, peripheral neuropathy, fatigue, and hypertension. The incidence of these AEs was similar in the study arms except for fatigue that occurred more frequently in the 3-CTx arm (24/128 patients, 18.8%) compared to in the 2-CTx arm (8/126, 6.3%; P = 0.003). Grade 5 toxicities (leading to death) in the 2-CTx arm was gastrointestinal bleeding, and, in the 3-CTx arm a multi-organ failure, a sepsis and sudden death in the 1st cycle.
Discussion
Any fit CRC patient with initially unresectable liver-only metastases origin may eventually benefit from surgery. However, no criteria exist to distinguish those patients needing palliative treatment and those that may benefit from conversion therapy and then surgery. Indeed, survival is only slightly shorter for patients who undergo conversion therapy and then surgery compared to those with initially resectable metastatic disease. In contrast, survival is much shorter in patients with non-resected metastases [22]. Possible conversion therapies include, 2-CTx like FOLFOX or FOLFIRI combined with targeted therapies (bevacizumab or an anti-epithelial growth factor receptor monoclonal antibody) or the 3-CTx FOLFOXIRI alone or with bevacizumab [22, 23]. In our trial, we compared conversion therapy with either FOLFOX/FOLFIRI or FOLFIRINOX, combined with bevacizumab or cetuximab, by RAS status, in CRC patients with initially unresectable liver metastases.
We observed a not significantly higher rate of curative-intent liver surgery, 56.9% with 3-CTx (FOLFIRINOX) plus cetuximab/bevacizumab compared to 48.4% with 2-CTx (FOLFIRI or FOLFOX) plus cetuximab/bevacizumab. The 56.9% R0/R1 resection rate observed with FOLFIRINOX plus cetuximab/bevacizumab is similar to the 61%, reported by Gruenberger et al. [4], with FOLFOXIRI-bevacizumab (41 patients). The TRIBE study reported only a 15% R0/R1 resection rate in the FOLFOXIRI-bevacizumab group (252 patients). However, accrual in the TRIBE study was not restricted to patients with liver-only metastases [16]. The benefit of 3-CTx plus targeted therapy on the R0/R1 resection rate was confirmed using a logistic regression model adjusted for the stratification factors. There were higher odds for R0/R1 resection in the 3-CTx arm than in the 2-CTx arm (odds ratio, 1.8; 95% CI: 1.1–2.7; P < 0.02). However, the 8.5% improvement in R0/R1 resection rate in the 3-CTx arm is below the hypothesised 20% considered as clinically meaningful. In addition, the 3-CTx treatment arm was not identified as an independent predictor of R0/R1 resection, as opposed to younger age, prior resection of the primary tumour, low baseline CEA level, and left-sidedness of the primary tumour. At baseline, most patients (70%) had an unresected primary tumour and technically unresectable liver metastases. These characteristics may have lowered the R0/R1 resection rate observed. Also at study design, we may have overestimated the extent of the benefit with 3-CTx.
The 10% increase in response rate with 3-CTx plus targeted therapy is similar to that report by Loupakis [16] comparing FOLFOXIRI-bevacizumab to FOLFIRI-bevacizumab. However, in contrast, we did not observe a significant benefit in OS in the 3-CTx arm compared to the 2-CTx arm. However, our results confirm that patients with curative surgery of their liver metastases after conversion therapy live longer than those without liver-metastases resection.
PRODIGE has substantial experience in using FOLFIRINOX in the metastatic and adjuvant setting in CRC and pancreatic cancers [12, 24–26]. Therefore, we preferred FOLFIRINOX, as 3-CTx, rather than FOLFOXIRI which is used by the GONO group [16]. These regimes differ with respect to their fluorouracil dosing. With FOLFIRINOX, fluorouracil is administered as a continuous infusion (2400 mg/m²) combined with a bolus (400 mg/m²). In contrast, with FOLFOXIRI, fluorouracil is only administered as a continuous infusion (3200 mg/m²), but at a higher concentration. Interestingly, in our study, with FOLFIRINOX the fluorouracil dose intensity was 90% for the continuous infusion and 71% for the bolus. While, in the TRIBE study [16], with FOLFOXIRI, the fluorouracil dose intensity was 83% for the continuous infusion.
In the nine patients with mtBRAF tumours, those treated with 3-CTx had numerically more R0/R1 resections, extended median PFS and OS than those treated with 2-CTx. Our data with FOLFIRINOX plus cetuximab are consistent with those reported, by Cremolini [15], in 16 patients with mtBRAF tumours treated with FOLFOXIRI-bevacizumab.
Limitations
During the study, we needed to extend KRAS-genotyping to RAS genotyping. Unfortunately, we could only centrally review the molecular analyses of 218/256 cases. When the molecular analysis was inconclusive (86/218 reports), we centrally reassessed KRAS exons 3 and 4 and NRAS exons 2, 3 and 4. Eventually, we identified nine tumours initially genotyped as wtKRAS that had other RAS mutations in the newly assessed genes and the corresponding patients treated (retrospectively inadequately) with cetuximab plus 2-CTx or 3-CTx. Finally, the fact that there was no central assessment of technical irresectability might have induced some heterogeneity between centres.
Conclusion
In our study, the use of FOLFIRINOX instead of 2-CTx, combined with bevacizumab or cetuximab by RAS status, did not increase from 50 to 70% the tumour resection rates in CRC patients with initially unresectable liver-only metastases.
Supplementary information
Acknowledgements
We thank the patients and their families for participating in the study. Also, we would like to thank Trevor Stanbury (UNICANCER) for medical writing support. We are also indebted to all the participating centers and to The Ligue Nationale Contre le Cancer. This trial was made possible through an unrestricted grant from Merck-Serono France.
Author contributions
Dr. MY had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: MY, RA, OB and MR. Acquisition, analysis or interpretation of the data: MY, AA and ST. Drafting of the manuscript: AA, EL-C, ST and MY. Critical revision of the manuscript for important intellectual content: all the authors. Statistical analysis: Thezenas. Administrative, technical or material support: MY, MR, RG, FG, EL-C, AM-B, LM, EF, FK, MC, RK, MF, PH, TA, M-PG, FA, EA, CJ, AA and OB; supervision: MY.
Funding
This trial was made possible through an unrestricted grant from Merck-Serono France. Investigators list: INVESTIGATOR NAME: SITE, CITY. Pr. Marc YCHOU: Institut Régional du Cancer de Montpellier/Val d’Aurelle (ICM), Montpellier; Dr. Laurent MINEUR: Institut Ste Catherine, Avignon; Pr. Olivier BOUCHE: CHU de Reims, Reims; Dr. Pascale MARIANI: Institut Curie, Paris; Dr. Kartine BOUHIER-LEPORIER: CHU Côte de Nacre, Caen; Dr. Marianne FONCK: Institut Bergonié, Bordeaux; Dr. Roger FAROUX: CHD Vendée, La Roche sur Yon; Dr. Alice GAGNAIRE: CHU Dijon - Hôpital Du Bocage, Dijon; Dr. Eric FRANCOIS: Centre Antoine Lacassagne, Nice; Dr. Julien FORESTIER: Hôpital Edouard Herriot, Lyon; Pr François GHIRINGHELLI: Centre G. F. Leclerc, Dijon; Dr. Driffa MOUSSATA: Centre hospitalier Lyon Sud, Pierre-Benite; Dr. Gaël DEPLANQUE: Centre Hospitalier Saint-Joseph, Paris; Dr. Jean-Louis LEGOUX: CHR d’Orléans - La Source, Orleans; Dr. Cédric LECAILLE: Polyclinique de Bordeaux Nord Aquitaine, Bordeaux; Pr. Nicole TUBIANA-MATHIEU: CHU Dupuytren, Limoges; Mr. Michel RIVOIRE: Centre Léon Bérard, Lyon; Dr. Marie-Pierre GALAIS: Centre François Baclesse, Caen; Pr. Rosine GUIMBAUD: Centre hospitalier Rangueil, Toulouse; Dr. Francine FEIN: CHU Jean Minjoz, Besancon; Dr. Philippe HOUYAU: Clinique Claude Bernard, Albi; Pr. Thomas APARICIO: Hôpital Avicenne, Bobigny; Pr. Jean-François SEITZ: CHU La Timone, Marseille; Dr. Stephen ELLIS: Centre Catalan d’oncologie, Perpignan; Dr. Stéphane REMY: Centre d’oncologie et de radiothérapie du Pays basque, Bayonne; Dr. Faiza KHEMISSA - AKOUZ: Hôpital Saint Jean, Perpignan; Dr. Mohammed RAMDANI: Centre Hospitalier de Béziers, Béziers; Dr. Anne MERCIER - BLAS: CHP Saint Grégoire, Saint Gregoire; Pr. Eric ASSENAT: CHU Saint Eloi - CHRU de Montpellier, Montpellier; Pr. Julien TAIEB: Hôpital Européen Georges Pompidou, Paris; Pr. René ADAM: Hôpital Paul Brousse, Villejuif; Dr. Hervé PERRIER: Hôpital St Joseph, Marseille; Dr. Agnès PELAQUIER: Centre hospitalier Montelimar, Montelimar; Pr. Emmanuel MITRY: Hôpital Huguenin - Institut Curie, Saint-Cloud; Dr. Serge FRATTE: CHBM - Site du Mittan, Montbeliard; Dr. Franck AUDEMAR: Centre hospitalier Côte Basque, Bayonne; Dr. Jean-Luc LABOUREY: Centre hospitalier de Carcassonne, Carcassone; Dr. Mathilde MARTINEZ: Clinique Pasteur, Toulouse; Dr. Christian BOREL: Centre Paul Strauss, Strasbourg; Dr. Etienne SUC: Clinique St Jean du Languedoc, Toulouse; Dr. Christine LE FOLL: Centre hospitalier Marne la Vallée, Jossigny; Dr. Etienne MAURIAC: Polyclinique Côte Basque Sud, Saint Jean de Luz; Dr. Denis PÈRE-VERGĒ: Centre Hospitalier Saint-Joseph-Saint-Luc, Lyon.
Data availability
Unicancer will share de-identified individual data that underlie the results reported under the following conditions: the data shared will be limited to that required for independent mandated verification of the published results, the reviewer will need authorisation from Unicancer for personal access, and data will only be transferred after signing of a data access agreement. A decision concerning the sharing of other study documents, including protocol and statistical analysis plan, will be examined upon request. Unicancer will consider access to study data upon written detailed request sent to l-monard@unicancer.fr, from 6 months until 5 years after the publication of this article.
Ethics approval and consent to participate
The study complied with the Declaration of Helsinki, good clinical practice guidelines, and other local laws. The study documents were approved by a French ethics committee, “Comité de Protection des Personnes Sud Méditerranée IV”. Patients provided written informed consent before enrolment.
Consent to publish
Not applicable.
Competing interests
MY reported receiving honoraria from Amgen, Bayer, Merck, Roche, and Servier. MR has nothing to disclose. ST has nothing to disclose. RG reported personal fees from AAA, Amgen, Astra-Zeneca, BMS, P. Fabre, Novartis, Roche, Servier, outside the submitted work. FG served on external advisory boards for Roche; research funding from Roche, Genentech, Amgen, Enterome, Servier; received funding for a clinical trial from Astra-Zeneca, received fee for communication by Amgen, Astra-Zeneca, BMS, Sanofi, Merck-Serono, Servier and received fee for travel by Roche and Servier. AM-B has nothing to disclose. LM has nothing to disclose. EF reported personal fees from ROCHE, personal fees from SERVIER, personal fees from NOVARTIS, personal fees from MSD, outside the submitted work. FK reported personal feed from Sanofi, other fees from Roche (congress support), other fees from Ipsen (congress support), outside the submitted work. MC has nothing to disclose. RK has nothing to disclose. MF has nothing to disclose. PH has nothing to disclose. TA reported personal fees from Roche, personal fees from Servier, personal fees from Amgen, personal fees from Ipsen, personal fees from Sanofi, non-financial support from Bayer, outside the submitted work. M-PG reported other relevant financial interest from Roche (travel), other relevant financial interest from Amgen (travel, board), outside the submitted work. FA reported personal fees from Sanofi, personal fees from Merck, personal fees from Amgen, personal fees from Servier, personal fees from Roche, outside the submitted work. EA reported other fees (advisory board) from Roche, from Astrazeneca, from Ipsen, from Bayer, from Sanofi, from AMGEN, from AAA, outside the submitted work. EL-C has nothing to disclose, CJ has nothing to disclose. AA reported receiving honoraria from Bayer, Bristol-Myers Squibb, Merck-Sharp Dohme, Sanofi, and Servier. RA reported personal fees from Merck (congress presentation), personal fees from Sanofi (congress presentation), outside the submitted work. OB reported personal fees from ROCHE (self honoraria, advisory/consultancy), personal fees from AMGEN (self honoraria, speaker bureau/expert testimony), personal fees from MERCK KGaA (self honoraria, advisory/consultancy), personal fees from SERVIER (self honoraria, speaker bureau/expert testimony), personal fees from BAYER (self honoraria, advisory/consultancy), personal fees from PIERRE FABRE (self honoraria, speaker bureau/expert testimony), personal fees from Astra-Zeneca (self honoraria, advisory/consultancy), personal fees from Grunenthal (self honoraria, advisory/consultancy), personal fees from MSD (Self honoraria, advisory/consultancy), non-financial support from Roche (travel/accommodation/expenses) and non-financial support from Servier (travel/accommodation/expenses) outside the submitted work.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-021-01644-y.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Wong R, Cunningham D, Barbachano Y, Saffery C, Valle J, Hickish T, et al. A multicentre study of capecitabine, oxaliplatin plus bevacizumab as perioperative treatment of patients with poor-risk colorectal liver-only metastases not selected for upfront resection. Ann Oncol. 2011;22:2042–8. doi: 10.1093/annonc/mdq714. [DOI] [PubMed] [Google Scholar]
- 3.Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065–75. doi: 10.1016/S1470-2045(14)70330-4. [DOI] [PubMed] [Google Scholar]
- 4.Gruenberger T, Bridgewater J, Chau I, Garcia Alfonso P, Rivoire M, Mudan S, et al. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26:702–8. doi: 10.1093/annonc/mdu580. [DOI] [PubMed] [Google Scholar]
- 5.Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. J Am Med Assoc. 2017;317:2392–2401. doi: 10.1001/jama.2017.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai SY, et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol. 2013;31:1931–8. doi: 10.1200/JCO.2012.44.8308. [DOI] [PubMed] [Google Scholar]
- 7.Kohne CH, Poston G, Folprecht G, Ciardiello F, Ronga P, Beier F, et al. FOLFIRI plus cetuximab in patients with liver-limited or non-liver-limited RAS wild-type metastatic colorectal cancer: a retrospective subgroup analysis of the CRYSTAL study. Eur J Surg Oncol. 2016;42:1540–7. doi: 10.1016/j.ejso.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 8.Leone F, Artale S, Marino D, Cagnazzo C, Cascinu S, Pinto C, et al. Panitumumab in combination with infusional oxaliplatin and oral capecitabine for conversion therapy in patients with colon cancer and advanced liver metastases. The MetaPan study. Cancer. 2013;119:3429–35. doi: 10.1002/cncr.28223. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Sun Y, Zhao B, Zhang H, Yu Q, Yuan X. Chemotherapy plus targeted drugs in conversion therapy for potentially resectable colorectal liver metastases: a meta-analysis. Oncotarget. 2016;7:55732–40. doi: 10.18632/oncotarget.9675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folprecht G, Grothey A, Alberts S, Raab HR, Kohne CH. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann Oncol. 2005;16:1311–9. doi: 10.1093/annonc/mdi246. [DOI] [PubMed] [Google Scholar]
- 11.Okuno M, Hatano E, Nishino H, Seo S, Taura K, Uemoto S. Does response rate of chemotherapy with molecular target agents correlate with the conversion rate and survival in patients with unresectable colorectal liver metastases?: a systematic review. Eur J Surg Oncol. 2017;43:1003–1012. doi: 10.1016/j.ejso.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Ychou M, Rivoire M, Thezenas S, Quenet F, Delpero JR, Rebischung C, et al. A randomized phase II trial of three intensified chemotherapy regimens in first-line treatment of colorectal cancer patients with initially unresectable or not optimally resectable liver metastases. The METHEP trial. Ann Surg Oncol. 2013;20:4289–97. doi: 10.1245/s10434-013-3217-x. [DOI] [PubMed] [Google Scholar]
- 13.Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670–6. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 14.Masi G, Vasile E, Loupakis F, Cupini S, Fornaro L, Baldi G, et al. Randomized trial of two induction chemotherapy regimens in metastatic colorectal cancer: an updated analysis. J Natl Cancer Inst. 2011;103:21–30. doi: 10.1093/jnci/djq456. [DOI] [PubMed] [Google Scholar]
- 15.Masi G, Loupakis F, Salvatore L, Fornaro L, Cremolini C, Cupini S, et al. Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:845–52. doi: 10.1016/S1470-2045(10)70175-3. [DOI] [PubMed] [Google Scholar]
- 16.Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N. Engl J Med. 2014;371:1609–17. doi: 10.1056/NEJMoa1403108. [DOI] [PubMed] [Google Scholar]
- 17.Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306–15. doi: 10.1016/S1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 18.Cremolini C, Casagrande M, Loupakis F, Aprile G, Bergamo F, Masi G, et al. Efficacy of FOLFOXIRI plus bevacizumab in liver-limited metastatic colorectal cancer: a pooled analysis of clinical studies by Gruppo Oncologico del Nord Ovest. Eur J Cancer. 2017;73:74–84. doi: 10.1016/j.ejca.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 19.Assenat E, Desseigne F, Thezenas S, Viret F, Mineur L, Kramar A, et al. Cetuximab plus FOLFIRINOX (ERBIRINOX) as first-line treatment for unresectable metastatic colorectal cancer: a phase II trial. Oncologist. 2011;16:1557–64. doi: 10.1634/theoncologist.2011-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cremolini C, Antoniotti C, Lonardi S, Aprile G, Bergamo F, Masi G, et al. Activity and safety of cetuximab plus modified FOLFOXIRI followed by maintenance with cetuximab or bevacizumab for RAS and BRAF wild-type metastatic colorectal cancer: a randomized phase 2 clinical trial. JAMA Oncol. 2018;4:529–536. doi: 10.1001/jamaoncol.2017.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geissler M, Riera-Knorrenschild J, Tannapfel A, Greeve J, Florschütz A, Wessendorf S, et al. mFOLFOXIRI + panitumumab versus FOLFOXIRI as first-line treatment in patients with RAS wild-type metastatic colorectal cancer m(CRC): a randomized phase II VOLFI trial of the AIO (AIO- KRK0109) J Clin Oncol. 2018;36:3509–3509. doi: 10.1200/JCO.2018.36.15_suppl.3509. [DOI] [Google Scholar]
- 22.Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 23.Phelip JM, Tougeron D, Léonard D, Benhaim L, Desolneux G, Dupré A, et al. Metastatic colorectal cancer (mCRC): French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR) Dig Liver Dis. 2019;51:1357–1363. doi: 10.1016/j.dld.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 24.Ychou M, Viret F, Kramar A, Desseigne F, Mitry E, Guimbaud R, et al. Tritherapy with fluorouracil/leucovorin, irinotecan and oxaliplatin (FOLFIRINOX): a phase II study in colorectal cancer patients with non-resectable liver metastases. Cancer Chemother Pharm. 2008;62:195–201. doi: 10.1007/s00280-007-0588-3. [DOI] [PubMed] [Google Scholar]
- 25.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 26.Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wie AC, Raoul JL, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N. Engl J Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Unicancer will share de-identified individual data that underlie the results reported under the following conditions: the data shared will be limited to that required for independent mandated verification of the published results, the reviewer will need authorisation from Unicancer for personal access, and data will only be transferred after signing of a data access agreement. A decision concerning the sharing of other study documents, including protocol and statistical analysis plan, will be examined upon request. Unicancer will consider access to study data upon written detailed request sent to l-monard@unicancer.fr, from 6 months until 5 years after the publication of this article.