Abstract
Heightened psychological stress during pregnancy has repeatedly been associated with increased risk for offspring development of behavior problems and psychiatric disorders. This review covers a rapidly growing body of research with the potential to advance a mechanistic understanding of these associations grounded in knowledge about maternal-placental-fetal stress biology and fetal brain development. Specifically, we highlight research employing magnetic resonance imaging to examine the infant brain soon after birth in relation to maternal psychological stress during pregnancy to increase capacity to identify specific alterations in brain structure and function and to differentiate between effects of pre- versus postnatal exposures. We then focus on heightened maternal inflammation during pregnancy as a mechanism through which maternal stress influences the developing fetal brain based on extensive preclinical literature and emerging research in humans. We place these findings in the context of recent work identifying psychotherapeutic interventions found to be effective for reducing psychological stress among pregnant individuals, which also show promise for reducing inflammation. We argue that a focus on inflammation, among other mechanistic pathways, has the potential to lead to a productive and necessary integration of research focused on the effects of maternal psychological stress on offspring brain development and prevention and intervention studies aimed at reducing maternal psychological stress during pregnancy. In addition to increasing capacity for common measurements and understanding potential mechanisms of action relevant to maternal mental health and fetal neurodevelopment, this focus can inform and broaden thinking about prevention and intervention strategies.
Keywords: Inflammation, pregnancy, prenatal stress, psychological distress, neurodevelopment, intervention
Introduction
Psychological stress refers to individuals’ “perception and evaluation of the potential harm posed by objective environmental experiences,” with heightened stress occurring when “environmental demands are perceived to exceed their abilities to cope.”(p.6)(1). Psychological stress is multifaceted and can be classified by many characteristics, including intensity, chronicity, and variability. Heightened psychological stress during pregnancy has been repeatedly associated with offspring neurodevelopmental outcomes, including increased risk for psychiatric disorders (1–3). Psychological stress varies in relation to current (4, 5) and past experiences (6, 7), representing a common pathway for diverse environmental and person specific factors to influence the fetus. Recent evidence from studies employing infant neuroimaging has increased capacity to identify alterations in specific aspects of brain structure and functioning associated with elevations in maternal psychological stress and to differentiate effects of pre- versus postnatal influences (8). Stress sensitive aspects of maternal-placental-fetal (MPF) biology, which play obligatory roles in fetal brain development, provide explanatory mechanistic pathways for these observed effects (9). Heightened maternal inflammation represents one such pathway, which has been studied in well-controlled experimental animal models and in translational work with humans. Thus, recent maternal inflammation research provides an opportunity to understand the potential effects of maternal psychological stress on offspring brain development in a manner that is increasingly grounded in multi-level neurobiological evidence. It also provides the groundwork for identifying candidate mechanisms of action of preventive and treatment interventions for reducing prenatal psychological stress and reducing intergenerational transmission of prenatal stress via inflammatory and other MPF pathways.
Several psychotherapeutic interventions are effective in reducing perinatal psychological stress and risk for postpartum psychopathology (10, 11), addressing a critical need given that 10–20% of people experience perinatal depression and 15% perinatal anxiety (12). These interventions also have potential to benefit infant neurodevelopment through multiple mechanisms, including effects on stress-sensitive aspects of MPF biology during pregnancy. Here we highlight the potential for psychotherapeutic intervention during pregnancy to support healthy fetal brain development via reducing maternal psychological stress and associated inflammation. In doing so, we advocate for integrating intervention and prevention research with initiatives that advance understanding of mechanistic pathways through which early life conditions, beginning in the prenatal environment, influence neurodevelopment. We argue that this mechanistic perspective is important from both a scientific and a health systems perspective. Refining understanding of mechanistic pathways most relevant to maternal psychological stress and infant brain development is critical for identifying the most effective psychotherapeutic interventions. Furthermore, understanding relevant mechanistic pathways vis a vis psychotherapeutic intervention will boost confidence in selecting and implementing optimal prenatal health systems changes to support maternal psychological health and infant development.
Maternal Psychological Stress during Pregnancy Influences Infant Brain Development
Heightened psychological stress during pregnancy, including depressive and anxiety symptoms, is associated with elevations in offspring stress responses, emotional dysregulation, and behavioral problems (13–15). A recent meta-analysis identified a 1.5–2 fold increased risk for socioemotional problems among children whose mothers experienced heightened stress during pregnancy (16). Maternal stress during pregnancy is uniquely associated with offspring development after accounting for the presence of objective stressors (17, 18), and effects are not dependent on shared genetics between mother and offspring (19). Stress-sensitive aspects of MPF biology can influence fetal brain development and subsequent offspring neurobehavioral outcomes, including risk for psychiatric disorders (9). Examination of brain outcomes spanning from school-age to early adulthood demonstrate associations between prenatal stress exposure and long-term differences in brain structure and functioning, including alterations in amygdala volume and connectivity, as well as multiple other subcortical and cortical brain regions (see (20) for a review). While these studies indicate potential for enduring effects of prenatal stress, examining the brain at these later time points creates difficulty in disaggregating effects of pre-versus postnatal influences. A growing body of research employing structural, functional, and diffusion magnetic resonance imaging (MRI) with infants has provided evidence for effects of prenatal stress on offspring brain development prior to substantial exposure to co-occurring risk factors in the postnatal environment. MRI provides the necessary spatial resolution to examine specific subcortical brain regions, such as the amygdala and hippocampus, which develop early in gestation (21, 22), are known to be sensitive to stress due to containing high levels of glucocorticoid receptors (23), and are implicated in disrupted emotional processing across psychiatric disorders (24). Although beyond the scope of this review, other noninvasive neuroimaging tools, such as electroencephalogram (EEG) and functional near-infrared spectroscopy (fNIRS), are complementary to MRI due to temporal resolution and portability, and have contributed significantly to understanding prenatal influences on brain development.
In human studies, higher maternal psychological stress during pregnancy is associated with lower left hippocampal volume beginning during the second and third trimesters (25) and continuing into the neonatal period, as well as slower right hippocampal growth from the neonatal period through 6-months-of-age (26). Importantly, null findings have also been reported regarding hippocampal volume (27). To date, few studies have shown alterations to infant amygdala volume in relation to prenatal stress (28, 29), although decreased amygdala volume has been associated with higher levels of stress among male infants. Larger replication studies that examine infant sex as a moderator (1) will be an essential component of future research.
In contrast to overall amygdala volume, alterations in the microstructure (28) functional (30, 31) and structural connectivity (30) of the infant amygdala have been observed in association with prenatal stress. Depressive symptoms during pregnancy have been associated with more pronounced negative functional connectivity between the neonatal amygdala and dorsal medial prefrontal cortex (30) and stronger amygdala functional connectivity with the insula, ventromedial prefrontal cortex, and orbitofrontal cortex (31). This suggests the potential for alterations in brain circuitry integral to emotion processing and regulation and implicated in depression and anxiety later in life. Findings from diffusion tensor imaging (DTI) with infants further indicate potential alterations in the integrity of fiber tracts connecting the amygdala with the prefrontal cortex in association with prenatal stress exposure (30).
Several issues require consideration in interpreting the literature in this area. First, the obligatory role of stress-sensitive aspects of MPF biology in multiple aspects of fetal brain development, including neurogenesis, neuronal migration, synaptogenesis, and myelination (9, 32), indicate that effects of prenatal stress are likely widespread as opposed to restricted to limbic brain regions or connections. Investigation of such effects should harness current understanding of brain organization and functioning, a core feature of which is a limited number of consistently reproducible large-scale brain networks defined by patterns of coordinated functioning between anatomically distant brain regions (33). This network view of brain functioning is highly relevant given increasing evidence for the early emergence of large-scale networks, at least in nascent form already at birth (8). The increasing interest and rapidly advancing technical capacity to better characterize development of large-scale networks during fetal development (34) and early infancy (35) creates an important opportunity to examine how brain organization may be altered in relation to common environmental inputs, such as heightened maternal psychological stress.
Second, well documented challenges with reproducibility in neuroimaging research involving smaller sample sizes (36, 37) indicate a need research in this area with larger samples and testing results across independent datasets as they become available (8). Another limitation is the frequent measurement of maternal psychological stress with a single questionnaire, often at a single time point. Repeated assessment of psychological state in real-time and in ecologically valid contexts, known as ecological momentary assessment (38), has been shown to capture aspects of psychological stress and MPF stress biology during pregnancy in a manner that improves capacity to understand how they relate to one another (39), and to predict birth outcomes (40). Despite these limitations, the literature suggests that: a) maternal stress during pregnancy may impact brain development, and b) this can be quantified soon after birth before exposure to potentially confounding influences of the postnatal environment. Leveraging knowledge regarding potential adverse effects of prenatal stress exposure requires an increased focus on mechanistic pathways. This is critical for generating target mechanisms of action for preventive and treatment interventions.
Heightened Inflammation as an Important Pathway for the Influence of Psychological Stress on Offspring Outcome
Increasing evidence identifies heightened inflammation as a key pathway through which elevated maternal psychological stress during pregnancy influences fetal brain development and risk for poor neurodevelopmental outcomes. Maternal inflammation during pregnancy is associated with increased risk for offspring psychiatric disorders, including schizophrenia, autism, and attention deficit hyperactivity disorder (41, 42). Stress induced alterations in immune functioning are thought to occur via interactions between the immune system and hypothalamic-pituitary-adrenal axis (HPA-axis) (43, 44). Cortisol, the end product of the HPA-axis, regulates immune function (43, 44). However, chronic activation of the HPA-axis in response to stress can lead to impaired glucorticoid regulation of immune function, and thus contribute to heightened inflammation (43). A large body of evidence documents effects of stress on immune functioning (45, 46). Different types of stress contribute to heightened inflammation during pregnancy (47) including recent stressful life events (48), and chronic stressors, such as poverty, racial discrimination (49, 50), and childhood adversity (for review see (47)). In addition, mental health conditions with high prevalence during the perinatal period are associated with heightened inflammation (51).
Preclinical research delineates pathways through which maternal inflammation influences the developing fetal brain. Maternal blood elevations in proinflammatory cytokines are accompanied by heightened levels in placental tissues, amniotic fluid, and the fetal brain (52–54), demonstrating that maternal inflammation influences the fetal inflammatory environment. Because cytokines play a role in fetal brain development, including signaling of cell differentiation, axonal growth and synaptogenesis (55, 56) elevations in proinflammatory cytokines alter neurodevelopment. Effects of heightened maternal inflammation during pregnancy on offspring neurodevelopment (57) include decreased total brain volume (58) reduced volume of the prefrontal cortex, hippocampus (59, 60) anterior cingulate cortex, amygdala, striatum, nucleus accumbens and lateral ventricles, and increased volume of the thalamus, ventral mesencephalon, and brain stem (60). Lasting downstream effects on offspring behavior consistent with human psychopathology are also observed (54, 61–64). Effects can be eliminated by antibodies that inactivate certain proinflammatory cytokines (65, 66) highlighting the central role of proinflammatory cytokines in these associations.
Preclinical models also provide evidence that inflammatory factors influence developing fetal neurotransmitter systems critical for behavior regulation, including the serotonergic (67, 68), dopaminergic (69, 70), and glutamatergic systems (71, 72). In addition to directly influencing fetal brain development, in utero exposure to inflammation is postulated to trigger inflammation in the fetal brain (73, 74), which may alter brain development through activation of glial cells (75), increased oxidative stress (76), and aberrant neuronal development among other mechanisms.
In humans, heightened maternal inflammation during pregnancy mediates the association between elevated psychological stress and increased infant negative affect, a transdiagnostic risk factor for psychopathology (77). Recent work further demonstrates associations between heightened maternal inflammation and the structure and function of the infant brain, including greater right amygdala volume, stronger amygdala functional connectivity to cortical and subcortical brain regions in newborns (78), and alterations in frontolimbic structural connectivity through 12-months-of-age (79). These altered neural phenotypes mediate effects of maternal inflammation on children’s development of inhibitory control (78) and cognitive skills (79). Heightened maternal inflammation during pregnancy has also been associated with organization of large scale functional brain systems in the neonatal period, with the salience network implicated in two independent samples (80, 81).
In summary, multiple forms of psychological stress are associated with elevated inflammation during pregnancy, preclinical studies show effects of maternal inflammation on offspring neurobehavioral development, and increasing evidence demonstrates effects of maternal inflammation on neural and behavioral phenotypes relevant to psychiatric disorders in humans. Multiple other aspects of stress-sensitive MPF biology, including the HPA-axis, oxidative stress, serotonin (5-HT) signaling, and epigenetic mechanisms, also effect the developing fetal brain (9, 82). Importantly, inflammation interacts with each of these other aspects of MPF biology through multiple mechanisms. For example, maternal inflammation can reduce 5-HT synthesis and signaling through upregulation of indoleamine-2,3-dioxygenase, which competes for the 5-HT precursor molecule tryptophan (83, 84), and epigenetic alterations to the promoter of the 5-HT transporter gene (85). Thus, inflammation emerges as an important candidate mechanism to better understand and ameliorate potentially detrimental effects of maternal psychological stress during pregnancy, on offspring brain development.
Maternal Inflammation during Pregnancy as a Target for Psychotherapeutic Intervention
A robust literature supports the effect of psychotherapeutic intervention on immune functioning. Engagement in psychotherapeutic interventions affects immune function in individuals with multiple medical conditions known to increase inflammation, such as cancer, rheumatoid arthritis, and HIV (for reviews, see (86–88)). A recent meta-analysis examined 56 RCTs for psychosocial interventions across a variety of adult populations, including individuals with medical disorders, mental health disorders, and psychological distress (86). They found a small, pooled effect of improved immune function for psychosocial intervention participants (as compared to controls), with an associated 15% improvement in immune system functioning (defined by levels of anti-inflammatory cytokines, antibodies, immune cell counts, and natural killer cell activity) and 18% decrease in harmful immune function (defined by levels of proinflammatory cytokines and viral load). Significant findings were most consistently characterized by decreases in proinflammatory cytokines and increases in immune cells, and were present up to at least 6 months post-treatment (86).
Non-pharmacological interventions (e.g., psychotherapy) for individuals with heightened psychological stress are associated with increased immune function, most consistently decreased levels of proinflammatory markers C-reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) (for reviews, see (86–89)). Cognitive behavior therapy (CBT) is a specific psychotherapeutic intervention for psychological stress (e.g., depression, anxiety) with some of the strongest evidence for treatment-related reductions in inflammation over time in a variety of medical populations, psychiatric populations, and individuals with elevated mental health symptoms or psychological distress (86, 88, 89). In a recent meta-analysis of CBT RCTs, CBT was associated with a 15% improvement in immune function and a 34% reduction in harmful immune function. Associations did not differ by age, intervention duration, or comorbid medical presentation, and changes persisted for at least six months after the intervention ended (86). While there are no direct studies of CBT and inflammation in perinatal populations, CBT treatment and lower depressive symptoms were correlated with lower IL-6 levels in women experiencing their first depressive episode (90).
Similar to CBT, mindfulness-based interventions (MBIs), such as mindfulness-based cognitive therapy (MBCT) (91) and mindfulness-based stress reduction (MBSR) (92), are efficacious in reducing psychological stress, improving well-being, and improving clinical and functional outcomes in adults with medical and psychiatric disorders (93–98). Unlike CBT, MBIs are also conducted with healthy populations and therefore hold promise as more universal interventions to target inflammatory pathways. Initial evidence suggests that MBIs may reduce proinflammatory immune response in healthy populations, including reductions in levels of TNF-α after completing MBSR (99) and reductions in levels of CRP after completing MBSR and MBCT (100, 101). Completion of an intensive MBI retreat by stressed healthy controls was associated with decreases in the IL-6 (98). Across meta-analyses of RCTs and clinical research of MBIs with heterogeneous populations of medical, psychiatric, and healthy populations, MBIs are consistently associated with reduced CRP levels (87, 102, 103)(except see (104)). Evidence for the impact of MBIs on IL-6 and TNF-α has been inconclusive (87, 102, 103). Therefore, while MBIs hold potential to affect proinflammatory immune functioning, larger studies are needed to replicate findings and establish potential target mechanisms.
Changes in immune functioning during pregnancy (105, 106) raise the possibility of increased plasticity and thus potential responsiveness to intervention. The potential to reduce inflammation during pregnancy is highly relevant both for the developing fetal brain and maternal mental health due to the proposed role of inflammation in the emergence and maintenance of perinatal depression (107). Addressing perinatal mental health and well-being is increasingly recognized as a public health imperative, evidenced by guidelines recently published by the US Preventive Services Task Force (108). Traditional care approaches have focused on pharmacological interventions (109), which reduce risk of depression relapse during pregnancy (110), and have potential to reduce inflammation (111, 112); however, many pregnant people have reservations about pharmacological strategies (109, 113). Non-pharmacological interventions, including CBT, MBIs, and interpersonal therapy (IPT), are safe and acceptable during pregnancy (10, 114–116). There are robust effects of CBT and IPT on reducing depressive symptoms and preventing perinatal depression (10, 11, 117), with CBT also showing efficacy in reducing perinatal anxiety and psychological stress (118, 119).
MBIs are also feasible and acceptable during the perinatal period (10, 116, 120, 121) and are associated with reductions in anxiety and stress (116, 120, 121). MBCT has a strong evidence base for reducing psychological stress during pregnancy and reducing risk for perinatal depression (10, 122, 123). However, results on the effectiveness of MBIs more generally at reducing perinatal depressive symptoms are mixed, likely reflecting methodological differences, limited RCTs, and variability in adherence to treatment protocols (116, 121).
Despite advancement in the field of prenatal intervention, there is limited understanding of how prenatal psychotherapeutic intervention affects immune function. Evidence for treatment effects on downstream neonatal and infant outcomes (124) suggests potential involvement of stress-sensitive aspects of MPF biology, such as immune function. Ongoing RCTs are attempting to identify direct intervention effects on infant neurodevelopment and potential mechanistic pathways, including inflammation (for review, see (125)).
Discussion
Inflammation as a common factor
As understanding of potentially efficacious interventions to prevent and reduce psychological stress during pregnancy advances, a parallel line of research provides increasing understanding of the mechanisms through which stress can influence the developing fetal brain and risk for psychiatric disorders. We propose focusing on inflammation as a promising avenue for joining these two lines of research. Given the likely role of inflammation in the effects of prenatal psychological stress on fetal brain development and in the emergence of more severe forms of perinatal psychological stress (e.g., major depressive disorder), the proposed focus on inflammation has implications for improving maternal health and fetal development.
Intervention implementation- proposing a unified and universal approach
Focusing on pregnancy inflammation as a mechanistic pathway for effects of psychotherapeutic intervention on the developing fetal brain may broaden our conceptualization of intervention for pregnant people. First, multiple other aspects of the prenatal environment, including nutrition, metabolic health, and substance use, impact the maternal inflammatory milieu during pregnancy (see Box 1). By anchoring intervention efforts to inflammation as a key mechanism, intervention evaluation remains grounded in biology and has a common measurable outcome. Second, the perinatal period represents a time of potentially unique plasticity and vulnerability in immune function for all pregnant people. Further, predicting who is at greatest psychological risk during pregnancy and postpartum remains challenging, with prenatal anxiety or depressive symptoms being the best predictors of postpartum psychopathology (126).
BOX 1: Factors commonly co-occurring with heightened maternal psychological stress with potential to influence inflammation and offspring neurodevelopment.
In the current manuscript, we focus on maternal psychological stress during pregnancy as one important factor relevant to infant neurodevelopment. This focus, however, is not intended to imply that this is the only relevant prenatal input, nor that the effects of prenatal stress occur in isolation. Rather, there are several factors that have been shown to commonly co-occur with heightened psychological stress and that may exert independent, interactive, or bidirectional effects on child brain and behavior. Among these factors are poor nutrition, obesity, environmental toxicant exposure, and substance use during pregnancy (137–140). Interestingly, each of these factors have been associated with greater systemic or intra-uterine inflammation (141–145), suggesting a possible overlapping biological mechanism through which these factors jointly influence child brain and behavioral development. While a comprehensive review of each of these areas of research is outside of the scope of the current manuscript, we highlight two exemplar factors herein, to illustrate our point.
First, heightened psychological stress during pregnancy has been shown to be related to poor nutrition, such that pregnant individuals who are stressed are more likely to consume calorically dense diets that are higher in fats and carbohydrates, and to consume lower levels of some micronutrients that are important for fetal development, including folate (146). Psychological stress is purported to be related to dietary intake via alterations in food choices, in hormones that regulate hunger and satiety, and in digestive processes (see 142 for a more thorough discussion of these points). There have been several excellent reviews on this topic that highlight the various ways in which stress and nutrition may interact to influence offspring neurodevelopment (e.g., 138, 147). Of relevance to the biological mechanisms described herein, poor nutrition can contribute to systemic inflammation, such that some nutrients can amplify (e.g., saturated fats) or dampen (e.g., omega-3 fatty acids) symptoms of psychological stress and its associated inflammatory response.
A second example comes from the literatures examining substance use during pregnancy. Previous research suggests that individuals who misuse alcohol, tobacco, and prescription or illicit drugs during pregnancy often have a psychiatric history and endorse increased psychological stress during pregnancy (148, 149). Similar to the other examples described herein, alcohol, tobacco, and drug use have each been associated with increased systemic inflammation (though most studies in this area have investigated these associations in non-pregnant populations) (144, 145, 150) and has been linked with compromised neurodevelopmental outcomes in offspring (140), though few studies have examined their relative influence on child outcomes. Future research that simultaneously considers the effects of stress and other factors such as these is clearly needed, both to better contextualize stress effects and to disentangle the multiple influences on maternal gestational inflammation and child neurodevelopment, as a means to finding the most powerful target for intervention.
These considerations signal the need to reshape how and who we are reaching during pregnancy. We suggest that a universal approach to intervention may be warranted. A recent meta-analysis of 10 studies including universal mental health preventive prenatal interventions found a moderate effect of universal interventions on stress (d = .52) (127). This indicates that shifting our attention away from narrow populations and instead focusing on well-integrated, preventive mental health screening and care may result in a greater ability to support healthy perinatal processes and infant brain development. Several models exist that might guide a unified, universal screening and psychotherapeutic intervention approach to prenatal care (128, 129). Presented in this manner, universal prenatal psychotherapeutic interventions could become similar to the prenatal vitamin – a normalized approach that is commonly used for health promotion and disease prevention, benefitting both the pregnant person and the developing fetus. A paradigm shift such as this also holds promise to decrease barriers, such as stigma, known to hinder access to mental health care.
Universal intervention also reframes responsibility for reducing psychological stress during pregnancy – shifting responsibility away from pregnant people and towards our healthcare systems, systems of support, and payers to provide increased access, availability, and coverage of preventive services. Effective and sustainable solutions will require mental health experts to work across disciplines, including collaboration with maternity care experts and implementation scientists, as shifting responsibility solely to maternity care providers will be an ineffective strategy (130).
We therefore suggest that future research on mental health and wellness interventions during pregnancy be designed to consider the potential for broad implementation within the prenatal practice setting, where most pregnant people receive care. To accomplish this, it will be essential to consider implementation concerns, such as acceptability, feasibility, delivery preferences, and satisfaction among pregnant people and community-based prenatal care providers. Ideally, these newly defined or modified interventions will: a) retain key components of interventions with known efficacy (e.g., learning and employing CBT skills), b) measure psychological stress and symptoms every two to four weeks to allow for obtaining point prevalence estimates and trajectories (131), c) be tested against one another, and d) examine the effects of intervention timing (e.g., early versus later pregnancy intervention). Pre- and post-intervention measurement of inflammation may be accomplished by blood draws in clinic settings, or through the use of innovative methods for remote biological sample collection during pregnancy, which facilitate sampling at home (132). Finally, given the challenges in predicting who will develop perinatal depression, accomplishing depression screening in many prenatal care settings, realizing timely and effective mental health care when pregnant people screen positive for perinatal mood disorders, and the many maternal/child benefits of preventing rather than treating perinatal mood disorders, we recommend that future research examine models of care with the capacity to reach all pregnant people and the flexibility to identify and respond appropriately to non-responders. Specifically, we recommend a stepped approach with a shorter intervention focused on mood disorder screening and mental wellness skill building that is offered to all pregnant people followed by additional intervention sessions for those with heightened symptoms, increasing symptoms, or a psychiatric disorder history.
Reaching all pregnant people
Importantly, a universal approach to intervention does not mean one size fits all. Contextualizing factors, particularly factors reflecting chronic stress experiences of diverse populations (e.g., racism, discrimination, health disparities) must be the focus of future research and can be advanced with a focus on inflammation and other aspects of MPF stress biology. Heightened proinflammatory cytokines have frequently been observed in association with chronic stress (49, 133), and studies in which the immune system has been stimulated via a temporal stressor (134) or ex vivo stimulation (135) show increased levels of IL-6 in pregnant Black women compared to pregnant white women. Further, in one study pregnant Black women had reduced ability to regulate IL-6 response compared to pregnant white women (135). Maternal trauma history has also been associated with heightened inflammation during pregnancy, and effects on the developing fetal brain (47). Additional research in this area to advance understanding of how contextualizing factors contribute to inflammation during pregnancy and how they can inform interventions aimed at reducing psychological stress during pregnancy must be a top priority. Consideration must be given to known differences in psychotherapeutic intervention perceptions, preferences, beliefs, and needs among pregnant people (136) Psychotherapeutic intervention efficacy and implementation must be more assertively examined outside of white, urban populations using appropriate scientific methods and applying a human-centered design framework.
Conclusion
Evidence documenting the effects of maternal psychological stress on offspring brain development has become increasingly grounded in understanding mechanistic pathways involving stress-sensitive aspects of MPF biology, particularly inflammation. In parallel, evidence-based interventions for reducing psychological stress among pregnant people have proliferated, and the need to address perinatal psychological stress has been firmly stated as a policy imperative (10, 108). The current state of the science suggests that we are ready to more widely consider inflammation as both a key mechanism and clinical translation of effective interventions. There is a pressing need to make pregnancy interventions that effectively improve mental health more widely available, more widely appealing, functional, and sustainable within the standard prenatal care setting. We are poised to simultaneously and collaboratively advance both missions. This will be particularly important as we seek to better address the contextualizing factors that perpetuate health inequity across generations by including minoritized populations in this research aimed at identifying mechanistic pathways for and improving prevention of the influence of stress on the developing brain.
Figure 1.
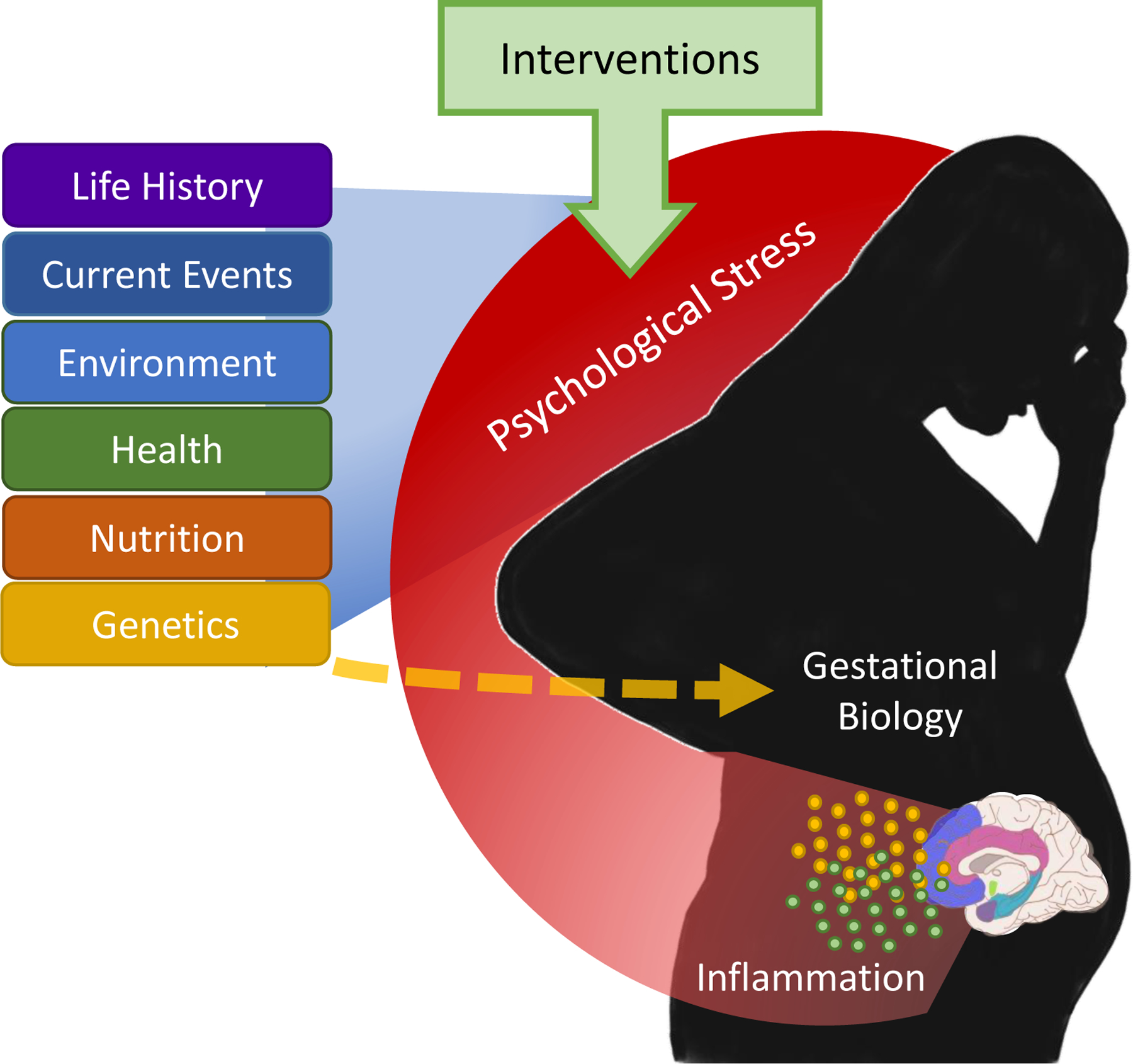
Psychological stress during pregnancy is hypothesized to be a common pathway through which life history, current events, environmental conditions, physical and mental health, and other factors influence stress-sensitive aspects of gestational biology, including immune functioning. Psychological stress during pregnancy has potential to both mediate and moderate these other influences on gestational biology (e.g. impacts of psychological stress on metabolism would lead to consideration of stress as a moderator of the effects of birth parent nutrition on gestational biology). Direct pathways also exist from these other factors to gestational biology, but we highlight the role of psychological stress here as a potentially modifiable factor. Evidence-based psychotherapeutic interventions are hypothesized to reduce inflammation via reduction in psychological stress during pregnancy.
Acknowledgments
Dr. Graham (R00-MH111805, PI Graham), Dr. Sullivan (R01-MH117177, PI Sullivan; R01-MH124824, PI Sullivan), Dr. Gustafsson (K01-MH120507, PI Gustafsson), and Dr. Marr (F30-MH118762) were supported by funding from the NIHM. Dr. Graham also received funding from NIH/NIDA (R34-DA050291, PI Graham), as did Dr. Mackiewicz Seghete and Ms. Doyle (P50-DA048756, MPIs Fisher and Leve, OHSU Site PI Mackiewicz Seghete). Dr. Mackiewicz Seghete additionally received funding from NIH/NCCIH (R21-AT010292, PI Mackiewicz Seghete). Dr. Tilden was supported by funding from NCATS (UL1TR002369).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Dr. Graham reported no biomedical financial interests or potential conflicts of interest.
Ms. Doyle reported no biomedical financial interests or potential conflicts of interest.
Dr. Tilden reported no biomedical financial interests or potential conflicts of interest.
Dr. Sullivan reported no biomedical financial interests or potential conflicts of interest.
Dr. Gustafsson reported no biomedical financial interests or potential conflicts of interest.
Dr. Marr reported no biomedical financial interests or potential conflicts of interest.
Dr. Mackiewicz Seghete reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Lautarescu A, Craig MC, Glover V. Prenatal stress: Effects on fetal and child brain development. Int Rev Neurobiol 2020;150:17–40. [DOI] [PubMed] [Google Scholar]
- 2.O’Donnell KJ, Glover V, Barker ED, O’Connor TG. The persisting effect of maternal mood in pregnancy on childhood psychopathology. Dev Psychopathol 2014;26(2):393–403. [DOI] [PubMed] [Google Scholar]
- 3.Kinney DK, Miller AM, Crowley DJ, Huang E, Gerber E. Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. J Autism Dev Disord 2008;38(3):481–8. [DOI] [PubMed] [Google Scholar]
- 4.Oni O, Harville E, Xiong X, Buekens P. Relationships among stress coping styles and pregnancy complications among women exposed to Hurricane Katrina. J Obstet Gynecol Neonatal Nurs 2015;44(2):256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Da Costa D, Larouche J, Dritsa M, Brender W. Variations in stress levels over the course of pregnancy: factors associated with elevated hassles, state anxiety and pregnancy-specific stress. J Psychosom Res 1999;47(6):609–21. [DOI] [PubMed] [Google Scholar]
- 6.Seng JS, Sperlich M, Low LK. Mental health, demographic, and risk behavior profiles of pregnant survivors of childhood and adult abuse. J Midwifery Womens Health 2008;53(6):511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang AJ, Rodgers CS, Lebeck MM. Associations between maternal childhood maltreatment and psychopathology and aggression during pregnancy and postpartum. Child Abuse Negl 2006;30(1):17–25. [DOI] [PubMed] [Google Scholar]
- 8.Graham AM, Marr M, Buss C, Sullivan EL, Fair DA. Understanding Vulnerability and Adaptation in Early Brain Development using Network Neuroscience. Trends Neurosci 2021;44(4):276–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Entringer S, Buss C, Wadhwa PD. Prenatal stress, development, health and disease risk: A psychobiological perspective-2015 Curt Richter Award Paper. Psychoneuroendocrinology 2015;62:366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Connor E, Senger CA, Henninger ML, Coppola E, Gaynes BN. Interventions to Prevent Perinatal Depression: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2019;321(6):588–601. [DOI] [PubMed] [Google Scholar]
- 11.Huang L, Zhao Y, Qiang C, Fan B. Is cognitive behavioral therapy a better choice for women with postnatal depression? A systematic review and meta-analysis. PLoS One 2018;13(10):e0205243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br J Psychiatry 2017;210(5):315–23. [DOI] [PubMed] [Google Scholar]
- 13.Glynn LM, Howland MA, Sandman CA, Davis EP, Phelan M, Baram TZ, et al. Prenatal maternal mood patterns predict child temperament and adolescent mental health. J Affect Disord 2018;228:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graignic-Philippe R, Dayan J, Chokron S, Jacquet AY, Tordjman S. Effects of prenatal stress on fetal and child development: a critical literature review. Neurosci Biobehav Rev 2014;43:137–62. [DOI] [PubMed] [Google Scholar]
- 15.Lahti M, Savolainen K, Tuovinen S, Pesonen AK, Lahti J, Heinonen K, et al. Maternal Depressive Symptoms During and After Pregnancy and Psychiatric Problems in Children. J Am Acad Child Adolesc Psychiatry 2017;56(1):30–9 e7. [DOI] [PubMed] [Google Scholar]
- 16.Madigan S, Oatley H, Racine N, Fearon RMP, Schumacher L, Akbari E, et al. A Meta-Analysis of Maternal Prenatal Depression and Anxiety on Child Socioemotional Development. J Am Acad Child Adolesc Psychiatry 2018;57(9):645–57 e8. [DOI] [PubMed] [Google Scholar]
- 17.Huizink AC, de Medina PG, Mulder EJ, Visser GH, Buitelaar JK. Psychological measures of prenatal stress as predictors of infant temperament. J Am Acad Child Adolesc Psychiatry 2002;41(9):1078–85. [DOI] [PubMed] [Google Scholar]
- 18.Lobel M, Dunkel-Schetter C, Scrimshaw SC. Prenatal maternal stress and prematurity: a prospective study of socioeconomically disadvantaged women. Health Psychol 1992;11(1):32–40. [DOI] [PubMed] [Google Scholar]
- 19.Rice F, Harold GT, Boivin J, van den Bree M, Hay DF, Thapar A. The links between prenatal stress and offspring development and psychopathology: disentangling environmental and inherited influences. Psychol Med 2010;40(2):335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lautarescu A, Craig MC, Glover V. Chapter Two - Prenatal stress: Effects on fetal and child brain development. In: Clow A, Smyth N, editors. International Review of Neurobiology 150: Academic Press; 2020. p. 17–40. [DOI] [PubMed] [Google Scholar]
- 21.Kier EL, Kim JH, Fulbright RK, Bronen RA. Embryology of the human fetal hippocampus: MR imaging, anatomy, and histology. AJNR Am J Neuroradiol 1997;18(3):525–32. [PMC free article] [PubMed] [Google Scholar]
- 22.Humphrey T The development of the human amygdala during early embryonic life. J Comp Neurol 1968;132(1):135–65. [DOI] [PubMed] [Google Scholar]
- 23.Badihian N, Daniali SS, Kelishadi R. Transcriptional and epigenetic changes of brain derived neurotrophic factor following prenatal stress: A systematic review of animal studies. Neurosci Biobehav Rev 2020;117:211–31. [DOI] [PubMed] [Google Scholar]
- 24.McTeague LM, Rosenberg BM, Lopez JW, Carreon DM, Huemer J, Jiang Y, et al. Identification of Common Neural Circuit Disruptions in Emotional Processing Across Psychiatric Disorders. Am J Psychiatry 2020;177(5):411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Y, Lu YC, Jacobs M, Pradhan S, Kapse K, Zhao L, et al. Association of Prenatal Maternal Psychological Distress With Fetal Brain Growth, Metabolism, and Cortical Maturation. JAMA Netw Open 2020;3(1):e1919940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu A, Rifkin-Graboi A, Chen H, Chong YS, Kwek K, Gluckman PD, et al. Maternal anxiety and infants’ hippocampal development: timing matters. Transl Psychiatry 2013;3(9):e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehtola SJ, Tuulari JJ, Scheinin NM, Karlsson L, Parkkola R, Merisaari H, et al. Newborn amygdalar volumes are associated with maternal prenatal psychological distress in a sex-dependent way. NeuroImage Clinical 2020;28:102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rifkin-Graboi A, Bai J, Chen H, Hameed WB, Sim LW, Tint MT, et al. Prenatal maternal depression associates with microstructure of right amygdala in neonates at birth. Biol Psychiatry 2013;74(11):837–44. [DOI] [PubMed] [Google Scholar]
- 29.Moog NK, Nolvi S, Kleih TS, Styner M, Gilmore JH, Rasmussen JM, et al. Prospective association of maternal psychosocial stress in pregnancy with newborn hippocampal volume and implications for infant social-emotional development. Neurobiol Stress 2021;15:100368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Posner J, Cha J, Roy AK, Peterson BS, Bansal R, Gustafsson HC, et al. Alterations in amygdala-prefrontal circuits in infants exposed to prenatal maternal depression. Transl Psychiatry 2016;6(11):e935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu A, Anh TT, Li Y, Chen H, Rifkin-Graboi A, Broekman BF, et al. Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Transl Psychiatry 2015;5(2):e508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buss C, Entringer S, Wadhwa PD. Fetal programming of brain development: intrauterine stress and susceptibility to psychopathology. Sci Signal 2012;5(245):pt7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE. Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cereb Cortex 2016;26(1):288–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomason ME, Hect J, Waller R, Manning JH, Stacks AM, Beeghly M, et al. Prenatal neural origins of infant motor development: Associations between fetal brain and infant motor development. Dev Psychopathol 2018;30(3):763–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Shen D, Lin W. Resting-state functional MRI studies on infant brains: A decade of gap-filling efforts. Neuroimage 2019;185:664–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marek S, Tervo-Clemmens B, Calabro FJ, Montez DF, Kay BP, Hatoum AS, et al. Towards Reproducible Brain-Wide Association Studies Affiliations. Johnny Uriarte 2020;11:15–8. [Google Scholar]
- 37.Kharabian Masouleh S, Eickhoff SB, Hoffstaedter F, Genon S, Alzheimer’s Disease Neuroimaging I. Empirical examination of the replicability of associations between brain structure and psychological variables. Elife 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehl MR. Handbook of research methods for studying daily life Mehl MR, Conner TS, editors. New York, NY, US: The Guilford Press. xxvii, 676–xxvii, p. [Google Scholar]
- 39.Lazarides C, Ward EB, Buss C, Chen WP, Voelkle MC, Gillen DL, et al. Psychological stress and cortisol during pregnancy: An ecological momentary assessment (EMA)-Based within- and between-person analysis. Psychoneuroendocrinology 2020;121:104848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Entringer S, Buss C, Andersen J, Chicz-DeMet A, Wadhwa PD. Ecological momentary assessment of maternal cortisol profiles over a multiple-day period predicts the length of human gestation. Psychosom Med 2011;73(6):469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer U Neurodevelopmental Resilience and Susceptibility to Maternal Immune Activation. Trends Neurosci 2019;42(11):793–806. [DOI] [PubMed] [Google Scholar]
- 42.Instanes JT, Halmoy A, Engeland A, Haavik J, Furu K, Klungsoyr K. Attention-Deficit/Hyperactivity Disorder in Offspring of Mothers With Inflammatory and Immune System Diseases. Biol Psychiatry 2017;81(5):452–9. [DOI] [PubMed] [Google Scholar]
- 43.Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A 2012;109(16):5995–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zen M, Canova M, Campana C, Bettio S, Nalotto L, Rampudda M, et al. The kaleidoscope of glucorticoid effects on immune system. Autoimmun Rev 2011;10(6):305–10. [DOI] [PubMed] [Google Scholar]
- 45.Rohleder N Stimulation of systemic low-grade inflammation by psychosocial stress. Psychosom Med 2014;76(3):181–9. [DOI] [PubMed] [Google Scholar]
- 46.Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull 2004;130(4):601–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hantsoo L, Kornfield S, Anguera MC, Epperson CN. Inflammation: A Proposed Intermediary Between Maternal Stress and Offspring Neuropsychiatric Risk. Biol Psychiatry 2019;85(2):97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andersson NW, Li Q, Mills CW, Ly J, Nomura Y, Chen J. Influence of prenatal maternal stress on umbilical cord blood cytokine levels. Arch Womens Ment Health 2016;19(5):761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giurgescu C, Engeland CG, Templin TN, Zenk SN, Koenig MD, Garfield L. Racial discrimination predicts greater systemic inflammation in pregnant African American women. Appl Nurs Res 2016;32:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corwin EJ, Guo Y, Pajer K, Lowe N, McCarthy D, Schmiege S, et al. Immune dysregulation and glucocorticoid resistance in minority and low income pregnant women. Psychoneuroendocrinology 2013;38(9):1786–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blackmore ER, Moynihan JA, Rubinow DR, Pressman EK, Gilchrist M, O’Connor TG. Psychiatric symptoms and proinflammatory cytokines in pregnancy. Psychosom Med 2011;73(8):656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gayle DA, Beloosesky R, Desai M, Amidi F, Nunez SE, Ross MG. Maternal LPS induces cytokines in the amniotic fluid and corticotropin releasing hormone in the fetal rat brain. Am J Physiol Regul Integr Comp Physiol 2004;286(6):R1024–9. [DOI] [PubMed] [Google Scholar]
- 53.Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr Res 2001;47(1):27–36. [DOI] [PubMed] [Google Scholar]
- 54.Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, et al. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci 2006;26(18):4752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deverman BE, Patterson PH. Cytokines and CNS development. Neuron 2009;64(1):61–78. [DOI] [PubMed] [Google Scholar]
- 56.Boulanger LM. Immune proteins in brain development and synaptic plasticity. Neuron 2009;64(1):93–109. [DOI] [PubMed] [Google Scholar]
- 57.Garbett KA, Hsiao EY, Kalman S, Patterson PH, Mirnics K. Effects of maternal immune activation on gene expression patterns in the fetal brain. Transl Psychiatry 2012;2(4):e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.da Silveira VT, Medeiros DC, Ropke J, Guidine PA, Rezende GH, Moraes MF, et al. Effects of early or late prenatal immune activation in mice on behavioral and neuroanatomical abnormalities relevant to schizophrenia in the adulthood. Int J Dev Neurosci 2017;58:1–8. [DOI] [PubMed] [Google Scholar]
- 59.Piontkewitz Y, Arad M, Weiner I. Tracing the development of psychosis and its prevention: what can be learned from animal models. Neuropharmacology 2012;62(3):1273–89. [DOI] [PubMed] [Google Scholar]
- 60.Crum WR, Sawiak SJ, Chege W, Cooper JD, Williams SCR, Vernon AC. Evolution of structural abnormalities in the rat brain following in utero exposure to maternal immune activation: A longitudinal in vivo MRI study. Brain Behav Immun 2017;63:50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J, Amaral DG. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun 2008;22(6):806–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hava G, Vered L, Yael M, Mordechai H, Mahoud H. Alterations in behavior in adult offspring mice following maternal inflammation during pregnancy. Dev Psychobiol 2006;48(2):162–8. [DOI] [PubMed] [Google Scholar]
- 63.Sullivan EL, Grayson B, Takahashi D, Robertson N, Maier A, Bethea CL, et al. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J Neurosci 2010;30(10):3826–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sasaki A, de Vega WC, St-Cyr S, Pan P, McGowan PO. Perinatal high fat diet alters glucocorticoid signaling and anxiety behavior in adulthood. Neuroscience 2013;240:1–12. [DOI] [PubMed] [Google Scholar]
- 65.Wu WL, Hsiao EY, Yan Z, Mazmanian SK, Patterson PH. The placental interleukin-6 signaling controls fetal brain development and behavior. Brain Behav Immun 2017;62:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci 2007;27(40):10695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meyer U, Feldon J. Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behav Brain Res 2009;204(2):322–34. [DOI] [PubMed] [Google Scholar]
- 68.Hsueh PT, Wang HH, Liu CL, Ni WF, Chen YL, Liu JK. Expression of cerebral serotonin related to anxiety-like behaviors in C57BL/6 offspring induced by repeated subcutaneous prenatal exposure to low-dose lipopolysaccharide. PLoS One 2017;12(6):e0179970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luchicchi A, Lecca S, Melis M, De Felice M, Cadeddu F, Frau R, et al. Maternal Immune Activation Disrupts Dopamine System in the Offspring. Int J Neuropsychopharmacol 2016;19(7):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bronson SL, Bale TL. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology 2014;155(7):2635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rahman T, Zavitsanou K, Purves-Tyson T, Harms LR, Meehan C, Schall U, et al. Effects of Immune Activation during Early or Late Gestation on N-Methyl-d-Aspartate Receptor Measures in Adult Rat Offspring. Front Psychiatry 2017;8(SEP):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Z, Bassam B, Thomas AG, Williams M, Liu J, Nance E, et al. Maternal inflammation leads to impaired glutamate homeostasis and up-regulation of glutamate carboxypeptidase II in activated microglia in the fetal/newborn rabbit brain. Neurobiol Dis 2016;94:116–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manjeese W, Mvubu NE, Steyn AJC, Mpofana T. Mycobacterium tuberculosis causes a leaky blood-brain barrier and neuroinflammation in the prefrontal cortex and cerebellum regions of infected mice offspring. Int J Dev Neurosci 2021;81(5):428–37. [DOI] [PubMed] [Google Scholar]
- 74.Singh G, Segura BJ, Georgieff MK, Gisslen T. Fetal inflammation induces acute immune tolerance in the neonatal rat hippocampus. J Neuroinflammation 2021;18(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, et al. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 2015;300:141–54. [DOI] [PubMed] [Google Scholar]
- 76.Hassan W, Noreen H, Castro-Gomes V, Mohammadzai I, da Rocha JB, Landeira-Fernandez J. Association of Oxidative Stress with Psychiatric Disorders. Curr Pharm Des 2016;22(20):2960–74. [DOI] [PubMed] [Google Scholar]
- 77.Gustafsson HC, Sullivan EL, Nousen EK, Sullivan CA, Huang E, Rincon M, et al. Maternal prenatal depression predicts infant negative affect via maternal inflammatory cytokine levels. Brain Behav Immun 2018;73:470–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Graham AM, Rasmussen JM, Rudolph MD, Heim CM, Gilmore JH, Styner M, et al. Maternal Systemic Interleukin-6 During Pregnancy Is Associated With Newborn Amygdala Phenotypes and Subsequent Behavior at 2 Years of Age. Biol Psychiatry 2018;83(2):109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rasmussen JM, Graham AM, Entringer S, Gilmore JH, Styner M, Fair DA, et al. Maternal Interleukin-6 concentration during pregnancy is associated with variation in frontolimbic white matter and cognitive development in early life. Neuroimage 2019;185:825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rudolph MD, Graham AM, Feczko E, Miranda-Dominguez O, Rasmussen JM, Nardos R, et al. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat Neurosci 2018;21(5):765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spann MN, Monk C, Scheinost D, Peterson BS. Maternal Immune Activation During the Third Trimester Is Associated with Neonatal Functional Connectivity of the Salience Network and Fetal to Toddler Behavior. J Neurosci 2018;38(11):2877–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci U S A 2012;109(20):E1312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pfaff AW, Mousli M, Senegas A, Marcellin L, Takikawa O, Klein JP, et al. Impact of foetus and mother on IFN-gamma-induced indoleamine 2,3-dioxygenase and inducible nitric oxide synthase expression in murine placenta following Toxoplasma gondii infection. Int J Parasitol 2008;38(2):249–58. [DOI] [PubMed] [Google Scholar]
- 84.Zavitsanou K, Lim CK, Purves-Tyson T, Karl T, Kassiou M, Banister SD, et al. Effect of maternal immune activation on the kynurenine pathway in preadolescent rat offspring and on MK801-induced hyperlocomotion in adulthood: amelioration by COX-2 inhibition. Brain Behav Immun 2014;41(1):173–81. [DOI] [PubMed] [Google Scholar]
- 85.Reisinger SN, Kong E, Khan D, Schulz S, Ronovsky M, Berger S, et al. Maternal immune activation epigenetically regulates hippocampal serotonin transporter levels. Neurobiol Stress 2016;4:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shields GS, Spahr CM, Slavich GM. Psychosocial Interventions and Immune System Function: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Psychiatry 2020;77(10):1031–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morgan N, Irwin MR, Chung M, Wang C. The effects of mind-body therapies on the immune system: meta-analysis. PLoS One 2014;9(7):e100903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.O’Toole MS, Bovbjerg DH, Renna ME, Lekander M, Mennin DS, Zachariae R. Effects of psychological interventions on systemic levels of inflammatory biomarkers in humans: A systematic review and meta-analysis. Brain Behav Immun 2018;74:68–78. [DOI] [PubMed] [Google Scholar]
- 89.Lopresti AL. Cognitive behaviour therapy and inflammation: A systematic review of its relationship and the potential implications for the treatment of depression. Aust N Z J Psychiatry 2017;51(6):565–82. [DOI] [PubMed] [Google Scholar]
- 90.Gazal M, Souza LD, Fucolo BA, Wiener CD, Silva RA, Pinheiro RT, et al. The impact of cognitive behavioral therapy on IL-6 levels in unmedicated women experiencing the first episode of depression: a pilot study. Psychiatry Res 2013;209(3):742–5. [DOI] [PubMed] [Google Scholar]
- 91.Segal ZV, Williams JMG, Teasdale JD. Mindfulness-based cognitive therapy for depression New York: Guilford Press; 2013. [Google Scholar]
- 92.Kabat-Zinn J, editor Full catastrophe living : using the wisdom of your body and mind to face stress, pain, and illness 1990.
- 93.Goyal M, Singh S, Sibinga EM, Gould NF, Rowland-Seymour A, Sharma R, et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med 2014;174(3):357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.O’Reilly GA, Cook L, Spruijt-Metz D, Black DS. Mindfulness-based interventions for obesity-related eating behaviours: a literature review. Obes Rev 2014;15(6):453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits. A meta-analysis. J Psychosom Res 2004;57(1):35–43. [DOI] [PubMed] [Google Scholar]
- 96.Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. J Consult Clin Psychol 2010;78(2):169–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Khoury B, Lecomte T, Fortin G, Masse M, Therien P, Bouchard V, et al. Mindfulness-based therapy: a comprehensive meta-analysis. Clin Psychol Rev 2013;33(6):763–71. [DOI] [PubMed] [Google Scholar]
- 98.Creswell JD, Taren AA, Lindsay EK, Greco CM, Gianaros PJ, Fairgrieve A, et al. Alterations in Resting-State Functional Connectivity Link Mindfulness Meditation With Reduced Interleukin-6: A Randomized Controlled Trial. Biol Psychiatry 2016;80(1):53–61. [DOI] [PubMed] [Google Scholar]
- 99.Rosenkranz MA, Davidson RJ, Maccoon DG, Sheridan JF, Kalin NH, Lutz A. A comparison of mindfulness-based stress reduction and an active control in modulation of neurogenic inflammation. Brain Behav Immun 2013;27(1):174–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fang CY, Reibel DK, Longacre ML, Rosenzweig S, Campbell DE, Douglas SD. Enhanced psychosocial well-being following participation in a mindfulness-based stress reduction program is associated with increased natural killer cell activity. J Altern Complement Med 2010;16(5):531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Eisendrath S Mindfulness-Based Cognitive Therapy Associated with Decreases in C-Reactive Protein in Major Depressive Disorder: A Pilot Study. Alternative, Complementary & Integrative Medicine 2016;2(1):1–3. [Google Scholar]
- 102.Black DS, Slavich GM. Mindfulness meditation and the immune system: a systematic review of randomized controlled trials. Ann N Y Acad Sci 2016;1373(1):13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pascoe MC, Thompson DR, Jenkins ZM, Ski CF. Mindfulness mediates the physiological markers of stress: Systematic review and meta-analysis. J Psychiatr Res 2017;95:156–78. [DOI] [PubMed] [Google Scholar]
- 104.Radmark L, Sidorchuk A, Osika W, Niemi M. A Systematic Review and Meta-Analysis of the Impact of Mindfulness Based Interventions on Heart Rate Variability and Inflammatory Markers. J Clin Med 2019;8(10):1638-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol 2010;63(6):425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci 2009;16(2):206–15. [DOI] [PubMed] [Google Scholar]
- 107.Leff-Gelman P, Mancilla-Herrera I, Flores-Ramos M, Cruz-Fuentes C, Reyes-Grajeda JP, Garcia-Cuetara Mdel P, et al. The Immune System and the Role of Inflammation in Perinatal Depression. Neurosci Bull 2016;32(4):398–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Force USPST, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, et al. Interventions to Prevent Perinatal Depression: US Preventive Services Task Force Recommendation Statement. JAMA 2019;321(6):580–7. [DOI] [PubMed] [Google Scholar]
- 109.Yonkers KA, Smith MV, Forray A, Epperson CN, Costello D, Lin H, et al. Pregnant women with posttraumatic stress disorder and risk of preterm birth. JAMA Psychiatry 2014;71(8):897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bayrampour H, Kapoor A, Bunka M, Ryan D. The Risk of Relapse of Depression During Pregnancy After Discontinuation of Antidepressants: A Systematic Review and Meta-Analysis. J Clin Psychiatry 2020;81(4). [DOI] [PubMed] [Google Scholar]
- 111.Latendresse G, Ruiz RJ, Wong B. Psychological distress and SSRI use predict variation in inflammatory cytokines during pregnancy. Open J Obstet Gynecol 2013;3(1a):184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kohler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N, et al. Peripheral Alterations in Cytokine and Chemokine Levels After Antidepressant Drug Treatment for Major Depressive Disorder: Systematic Review and Meta-Analysis. Mol Neurobiol 2018;55(5):4195–206. [DOI] [PubMed] [Google Scholar]
- 113.Arch JJ. Cognitive behavioral therapy and pharmacotherapy for anxiety: treatment preferences and credibility among pregnant and non-pregnant women. Behav Res Ther 2014;52(1):53–60. [DOI] [PubMed] [Google Scholar]
- 114.Arch JJ, Dimidjian S, Chessick C. Are exposure-based cognitive behavioral therapies safe during pregnancy? Arch Womens Ment Health 2012;15(6):445–57. [DOI] [PubMed] [Google Scholar]
- 115.Brouwer ME, Williams AD, van Grinsven SE, Cuijpers P, Lambregtse-van den Berg MP, Burger H, et al. Offspring outcomes after prenatal interventions for common mental disorders: a meta-analysis. BMC Med 2018;16(1):208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lever Taylor B, Cavanagh K, Strauss C. The Effectiveness of Mindfulness-Based Interventions in the Perinatal Period: A Systematic Review and Meta-Analysis. PLoS One 2016;11(5):e0155720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van Ravesteyn LM, Lambregtse-van den Berg MP, Hoogendijk WJ, Kamperman AM. Interventions to treat mental disorders during pregnancy: A systematic review and multiple treatment meta-analysis. PLoS One 2017;12(3):e0173397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Goodman JH, Watson GR, Stubbs B. Anxiety disorders in postpartum women: A systematic review and meta-analysis. J Affect Disord 2016;203:292–331. [DOI] [PubMed] [Google Scholar]
- 119.Maguire PN, Clark GI, Wootton BM. The efficacy of cognitive behavior therapy for the treatment of perinatal anxiety symptoms: A preliminary meta-analysis. J Anxiety Disord 2018;60:26–34. [DOI] [PubMed] [Google Scholar]
- 120.Hall HG, Beattie J, Lau R, East C, Anne Biro M. Mindfulness and perinatal mental health: A systematic review. Women Birth 2016;29(1):62–71. [DOI] [PubMed] [Google Scholar]
- 121.Shi Z, MacBeth A. The Effectiveness of Mindfulness-Based Interventions on Maternal Perinatal Mental Health Outcomes: a Systematic Review. Mindfulness (N Y) 2017;8(4):823–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vieten C, Astin J. Effects of a mindfulness-based intervention during pregnancy on prenatal stress and mood: results of a pilot study. Arch Womens Ment Health 2008;11(1):67–74. [DOI] [PubMed] [Google Scholar]
- 123.Dimidjian S, Goodman SH, Felder JN, Gallop R, Brown AP, Beck A. Staying well during pregnancy and the postpartum: A pilot randomized trial of mindfulness-based cognitive therapy for the prevention of depressive relapse/recurrence. J Consult Clin Psychol 2016;84(2):134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Goodman SH, Cullum KA, Dimidjian S, River LM, Kim CY. Opening windows of opportunities: Evidence for interventions to prevent or treat depression in pregnant women being associated with changes in offspring’s developmental trajectories of psychopathology risk. Dev Psychopathol 2018;30(3):1179–96. [DOI] [PubMed] [Google Scholar]
- 125.Brown H, Krogh-Jespersen S, Tandon D, Graham A, Mackiewicz Seghete K, Wakschlag L. Looking Ahead: Pre- and Perinatal Interventions for Maternal Distress to Prevent Neurodevelopmental Vulnerability. In: Wazana A, Székely E, Oberlander TF, editors. Prenatal Stress and Child Development Cham: Springer International Publishing; 2021. p. 595–622. [Google Scholar]
- 126.Andersson S, Bathula DR, Iliadis SI, Walter M, Skalkidou A. Predicting women with depressive symptoms postpartum with machine learning methods. Sci Rep 2021;11(1):7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Missler M, Donker T, Beijers R, Ciharova M, Moyse C, de Vries R, et al. Universal prevention of distress aimed at pregnant women: a systematic review and meta-analysis of psychological interventions. BMC Pregnancy Childbirth 2021;21(1):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Buultjens M, Farouque A, Karimi L, Whitby L, Milgrom J, Erbas B. The contribution of group prenatal care to maternal psychological health outcomes: A systematic review. Women Birth 2020. [DOI] [PubMed]
- 129.Lomonaco-Haycraft KC, Hyer J, Tibbits B, Grote J, Stainback-Tracy K, Ulrickson C, et al. Integrated perinatal mental health care: a national model of perinatal primary care in vulnerable populations. Prim Health Care Res Dev 2018;20:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Miller ES, Jensen R, Hoffman MC, Osborne LM, McEvoy K, Grote N, et al. Implementation of perinatal collaborative care: a health services approach to perinatal depression care. Prim Health Care Res Dev 2020;21:e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gustafsson HC, Young AS, Doyle O, Nagel BJ, Mackiewicz Seghete K, Nigg JT, et al. Trajectories of perinatal depressive symptoms in the context of the COVID-19 pandemic. Child Dev 2021;n/a(n/a). [DOI] [PMC free article] [PubMed]
- 132.Gustafsson HC, Young AS, Stamos G, Wilken S, Brito NH, Thomason ME, et al. Innovative Methods for Remote Assessment of Neurobehavioral Development. Developmental Cognitive Neuroscience 2021:101015. [DOI] [PMC free article] [PubMed]
- 133.Wadhwa PD, Culhane JF, Rauh V, Barve SS, Hogan V, Sandman CA, et al. Stress, infection and preterm birth: a biobehavioural perspective. Paediatr Perinat Epidemiol 2001;15 Suppl 2:17–29. [DOI] [PubMed] [Google Scholar]
- 134.Christian LM, Glaser R, Porter K, Iams JD. Stress-induced inflammatory responses in women: effects of race and pregnancy. Psychosom Med 2013;75(7):658–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gyllenhammer LE, Entringer S, Buss C, Simhan HN, Grobman WA, Borders AE, et al. Racial differences across pregnancy in maternal pro-inflammatory immune responsivity and its regulation by glucocorticoids. Psychoneuroendocrinology 2021;131:105333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Goodman SH, Dimidjian S, Williams KG. Pregnant African American women’s attitudes toward perinatal depression prevention. Cultur Divers Ethnic Minor Psychol 2013;19(1):50–7. [DOI] [PubMed] [Google Scholar]
- 137.Woodruff TJ, Parker JD, Kyle AD, Schoendorf KC. Disparities in exposure to air pollution during pregnancy. Environ Health Perspect 2003;111(7):942–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lindsay KL, Buss C, Wadhwa PD, Entringer S. The Interplay Between Nutrition and Stress in Pregnancy: Implications for Fetal Programming of Brain Development. Biol Psychiatry 2019;85(2):135–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Edlow AG. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat Diagn 2017;37(1):95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Polańska K, Jurewicz J, Hanke W. Smoking and alcohol drinking during pregnancy as the risk factors for poor child neurodevelopment - A review of epidemiological studies. Int J Occup Med Environ Health 2015;28(3):419–43. [DOI] [PubMed] [Google Scholar]
- 141.Madan JC, Davis JM, Craig WY, Collins M, Allan W, Quinn R, et al. Maternal obesity and markers of inflammation in pregnancy. Cytokine 2009;47(1):61–4. [DOI] [PubMed] [Google Scholar]
- 142.Kiecolt-Glaser JK. Stress, food, and inflammation: psychoneuroimmunology and nutrition at the cutting edge. Psychosom Med 2010;72(4):365–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Latini G, Massaro M, De Felice C. Prenatal exposure to phthalates and intrauterine inflammation: a unifying hypothesis. Toxicol Sci 2005;85(1):743. [DOI] [PubMed] [Google Scholar]
- 144.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun 2010;34(3):J258–65. [DOI] [PubMed] [Google Scholar]
- 145.Hutchinson MR, Shavit Y, Grace PM, Rice KC, Maier SF, Watkins LR. Exploring the neuroimmunopharmacology of opioids: an integrative review of mechanisms of central immune signaling and their implications for opioid analgesia. Pharmacol Rev 2011;63(3):772–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hurley KM, Caulfield LE, Sacco LM, Costigan KA, Dipietro JA. Psychosocial influences in dietary patterns during pregnancy. J Am Diet Assoc 2005;105(6):963–6. [DOI] [PubMed] [Google Scholar]
- 147.Monk C, Georgieff MK, Osterholm EA. Research review: maternal prenatal distress and poor nutrition–mutually influencing risk factors affecting infant neurocognitive development. Journal of Child Psychology and Psychiatry 2013;54(2):115–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Goodwin RD, Cheslack-Postava K, Nelson DB, Smith PH, Hasin DS, Janevic T, et al. Serious Psychological Distress and Smoking During Pregnancy in the United States: 2008–2014. Nicotine Tob Res 2017;19(5):605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Havens JR, Simmons LA, Shannon LM, Hansen WF. Factors associated with substance use during pregnancy: results from a national sample. Drug Alcohol Depend 2009;99(1–3):89–95. [DOI] [PubMed] [Google Scholar]
- 150.Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet 2001;357(9258):763–7. [DOI] [PubMed] [Google Scholar]


