Abstract
Setting
Congenital anomalies (CAs) can cause lifelong morbidity and accounted for 23.2% of infant deaths from 2003 to 2007. In British Columbia (BC), surveillance of CAs has been irregular since the early 2000s. To enhance CAs surveillance in BC, the Public Health Agency of Canada has provided funding for the implementation of the BC Congenital Anomalies Surveillance System (BCCASS).
Intervention
BCCASS is a population-based surveillance system. The system leverages existing administrative data sources that capture information regarding vital events, disease status, drug prescription, and healthcare utilization. The system uses a series of algorithms to capture specific CAs diagnoses, some of which are further validated with the support of the Provincial Advisory Committee. This Advisory Committee is a multi-stakeholder coalition that includes the BC Office of the Provincial Health Officer, subject matter experts, data partners, users, and academia, and acts to provide support, expertise, and strategic guidance to BCCASS.
Outcomes
Through BCCASS, prevalence and historical trends for 35 CAs in BC are available. Information pertaining to maternal place of residence, risk, and protective factors can be used for association studies such as links to environmental hazards and cluster analysis.
Implications
BCCASS is a cost-effective and sustainable system that leverages existing data sources necessary to understand the overall burden of CAs across the BC population. This is fundamental to support data-driven decisions around policy development, program planning, and evaluation of preventive measures. Strong coalitions with stakeholders are instrumental to ensure successful implementation and expansion in the future.
Keywords: Congenital anomalies, Surveillance, Administrative data, Birth defects, Canada
Résume
Contexte
Les anomalies congénitales (AC) peuvent causer une morbidité à vie et ont représenté 23,2 % des décès infantiles de 2003 à 2007. En Colombie-Britannique, la surveillance des AC a été irrégulière depuis le début des années 2000. Afin d’améliorer la surveillance de l’AC en Colombie-Britannique, l’Agence de la santé publique du Canada a financé la mise en œuvre du BC Congenital Anomalies Surveillance System (BCCASS).
Intervention
Le BCCASS est un système de surveillance basé sur la population. Le système exploite les sources de données administratives existantes qui capturent des informations concernant les événements vitaux, les diagnostics médicaux, la prescription de médicaments et l’utilisation des soins de santé. Le système utilise une série d’algorithmes pour saisir des diagnostics d’AC spécifiques, dont certains sont ensuite validés avec le soutien du Comité consultatif provincial. Ce comité consultatif est une coalition multipartite entre le bureau de l’Agence de santé provincial de la Colombie-Britannique, des experts en la matière, des partenaires de données, des utilisateurs et des universitaires, qui agit pour fournir un soutien, une expertise et des conseils stratégiques au BCCASS.
Résultats
Par le BCCASS, la prévalence et les tendances historiques pour 35 AC en Colombie-Britannique sont disponibles. Les informations relatives au lieu de résidence de la mère, aux facteurs de risque et de protection peuvent être utilisées pour des études d’association telles que les liens avec les facteurs environnementaux et l’analyse typologique.
Incidences
Le BCCASS est un système rentable et durable qui tire parti des sources de données existantes nécessaires pour comprendre le fardeau global des CA dans l’ensemble de la population de la Colombie-Britannique. Ceci est fondamental pour soutenir les décisions fondées sur les données concernant l’élaboration de politiques, la planification de programmes et l’évaluation des mesures préventives. Des coalitions solides avec les parties prenantes sont essentielles pour assurer une mise en œuvre et une expansion réussie dans l’avenir.
Mots-clés: Anomalies congénitales, surveillance, données administratives, malformations congénitales, Canada
Introduction
Congenital anomalies (CAs) have a major impact on public health as a leading cause of fetal and infant mortality (Public Health Agency of Canada 2013; Christianson et al. 2006; BC Coroners Service 2012, 2016, 2015). Many children with CAs experience significant lifelong physical and/or intellectual disabilities that result in social and financial burdens on affected families and the healthcare system (Glinianaia et al. 2017; Christianson et al. 2006).
CAs surveillance systems vary within countries and globally. Many programs that undertake surveillance for CAs prefer active surveillance because it produces the most accurate and reliable information; however, it requires dedicated staff for case finding and data abstraction (Mburia-Mwalili and Yang 2014; World Health Organization 2020). Alternatively, passive surveillance captures cases from available information in hospitals or administrative databases and thus requires less staff hours, but their utility can be hindered by reporting errors, under-ascertainment, and issues regarding the validity of CAs diagnostic codes (Metcalfe et al. 2014; Salemi et al. 2018). That said, case definition algorithms can be used in passive systems to enhance case ascertainment under limited funding (Blais et al. 2010; Salemi et al. 2018). Hybrid case ascertainment can further address some limitations present in passive surveillance by applying active verification of defects to decrease the risk of false positive cases, thereby enhancing data quality and trustworthiness to better support data-driven decisions (World Health Organization 2020).
Historically, CAs in BC were monitored using the Health Status Registry (HSR). Developed in the 1950s, this registry uses passive case ascertainment to monitor CAs and developmental disabilities (Mott 1963). Until the early 2000s, the HSR was considered one of the best surveillance systems for CAs in the Americas (Lowry and Bedard 2013). However, the HSR is isolated from other surveillance initiatives and lacks a clear governance structure. In 2015, the BC Ministry of Health (MoH) conducted an environmental scan that concluded the HSR data were no longer actively monitored for completeness and accuracy, the number of data sources contributing to the registry had diminished, and the quality of data had declined such that it is no longer considered complete enough for surveillance purposes.
Setting
The Public Health Agency of Canada (PHAC) provided funding to the BC Office of the Provincial Health Officer (OPHO) to enhance CAs surveillance. The OPHO conducts surveillance on non-communicable diseases through the Chronic Disease Registry using case definition algorithms to monitor chronic disease incidence, prevalence, and mortality in BC. Aligning CAs surveillance efforts with existing surveillance initiatives allows for better allocation and utilization of resources. Moreover, it is within the Provincial Health Officer’s mandate to monitor and report on CAs as one aspect of the health of British Columbians. Finally, CAs surveillance supports several of the goals and objectives outlined within the BC Guiding Framework for Public Health, which establishes the long-term public health vision for BC (BC Ministry of Health 2017). Given this experience with various surveillance systems, legislated mandates, and the overall public health vision for BC, the OPHO is well positioned to develop and implement the BC Congenital Anomalies Surveillance System (BCCASS).
Intervention
The newly developed BCCASS is a province-wide, population-based, hybrid surveillance system that uses administrative data to capture CAs cases.
We will use BCCASS data to:
Provide accurate and timely data on the burden of CAs in BC
Provide data for jurisdictional comparisons
Track trends and identify temporal and geographic variation in the occurrence of CAs
Provide fundamental data required for program planning and policy development
Enable research on associated risk factors and evaluation of the impact of interventions
Data sources that feed BCCASS
The Perinatal Data Registry (PDR)
Implemented on April 1, 2000, the PDR contains information from obstetrical and neonatal charts for virtually all births in the province, including deliveries that occurred at home or in the hospital. The system undergoes rigorous quality checks at the hospital and provincial level (Frosst et al. 2015). We conducted a preliminary data assessment of the PDR and concluded that the completeness of key variables is high. For instance, maternal personal health number (PHN) required for data linkage is valid in 98% of the records. Information in the PDR is coded using the International Statistical Classification of Diseases and Related Health Problems (ICD) 10th revision.
Hospital Discharge Abstract Database (DAD)
The DAD contains detailed patient-level data for hospitalizations in BC as well as those that occur in other Canadian jurisdictions involving BC residents. Hospitals submit data to the Canadian Institute for Health Information, which in turn provides the validated data to the MoH data warehouse, Healthideas. Information in DAD is coded using ICD-10 Canadian revision.
Vital Events (VE) tables
The VE tables include births, stillbirths, and deaths that occurred in BC. Data are provided to Healthideas by the Vital Statistics Agency (VSA) and originate from the Notices of Births and Stillbirths, Medical Certificate of Death, Medical Certificate of Stillbirth, and autopsy reports with images when available. All information is captured in VISION, the VSA database (Vital Statistics Agency 2015). While data transferred from VISION to Healthideas do not contain a text description of CAs, the VSA database may contain images of the documentation used to derive diagnostic codes, including autopsy reports. Therefore, we can access text descriptions during the case validation for some records, provided related documents have been imaged. Information in the VSA database is coded using ICD 10th coding scheme.
Medical Service Plan
The Medical Service Plan (MSP) is a data holding of Healthideas and is the BC public health insurance plan. Under the Medicare Protection Act, MSP enrollment is mandatory for all BC residents. MSP contains records on all medically required services from general practitioners, specialists, laboratory services, and diagnostic procedures. The diagnostic codes used the ICD 9th coding scheme and up to five diagnostic codes can be included per record. Reports suggest these codes are valid at the population level (Hu 1996). Linkage to other databases is done using the client PHN to avoid duplicated cases.
BCCASS produces rich information on 35 major CAs for surveillance. Inclusion criteria comprise (1) live births, stillbirths, and termination of pregnancies in pregnancies of at least 20 weeks of gestational age, diagnosed with one or more CAs, and born to a BC resident after April 1, 2000; and (2) deaths that occurred among infants under 1 year of age who were diagnosed with one or more congenital anomaly(ies) and were born to a BC mother.
We apply the same definition for stillbirths as VSA, “the complete expulsion or extraction from its mother after at least 20 weeks of pregnancy or after attaining a weight of at least 500 grams of a product of conception in which, after expulsion or extraction, there is no breathing, beating of the heart, pulsation of the umbilical cord or unmistakable movement of voluntary muscle” (Vital Statistics Agency 2017). While this definition includes termination of pregnancies, the data collection also allows for identification of stillbirths that are the result of termination of pregnancies. Information extracted from contributing data sources are linked together using a hierarchical, stepwise, deterministic record linkage approach that uses unique identifiers consistent across sources. Once all the data sources are linked, we use case definition algorithms to capture all cases with specific CAs diagnosed within the first year of life (Table 1). Some anomalies are further confirmed with external data sources such as the BC Cytogenetics Database and the VISION database (further described below) prior to inclusion into the system. The Medical Coding Unit at the BC VSA and the Genetic Screening Program at Perinatal Services BC are data partners that support case validation.
Table 1.
Congenital anomalies monitored by the BC Congenital Anomalies Surveillance System
| Anencephaly/craniorachischisis | |
| Anomalies of the corpus callosum | |
| Anorectal atresia | |
| Atresia of bile ducts | |
| Cataract | |
| Cleft lip | |
| Cleft lip with cleft palate | |
| Cleft palate | |
| Coarctation of the aorta | |
| Common truncus | |
| Congenital diaphragmatic hernia | |
| Cryptorchidism | |
| Cystic kidney | |
| Encephalocele | |
| Endocardial cushion defects | |
| Epispadias | |
| Esophageal atresia | |
| Exstrophy of urinary bladder | |
| Gastroschisis | |
| Hirschsprung disease | |
| Holoprosencephaly | |
| Hydrocephalus | |
| Hypoplastic left heart syndrome | |
| Hypospadias | |
| Indeterminate sex | |
| Lower urinary tract obstruction | |
| Omphalocele | |
| Renal agenesia | |
| Small intestine atresia | |
| Spina bifida | |
| Tetralogy of Fallot | |
| Transposition of great arteries | |
| Trisomy 13 | |
| Trisomy 18 | |
| Trisomy 21 (Down syndrome) |
We have one dedicated research officer and one epidemiologist who are tasked with the data extraction, preparation, linkage, analysis, and reporting. We use R-Studio and R Markdown for the entire data process, a widely used open-source data analytics platform that supports transparency and reproducibility and facilitates the integration of the code, analysis, results, and interpretation within the same document (Peng 2011). The scripts developed for data extraction, cleaning, and linkage can be rerun each year. The initial preparation process to create the full 20-year cohort took about 3 months of the two full-time staff. Subsequent annual refreshments should take about 1 to 2 weeks. Based on our experience, R Markdown expedites the process from data analysis to knowledge dissemination.
The BCCASS Advisory Committee
The BCCASS Advisory Committee is a multi-stakeholder coalition that includes the OPHO, subject matter experts, data partners, users, and academia. Members have expertise in medical genetics, epidemiology, maternal and infant health, public health, data science, data privacy, and CAs surveillance. The Committee was created to provide support, expertise, and strategic guidance towards implementation of the system. Committee members have been instrumental in identifying new data sources, advising on data source strengths and weaknesses, facilitating the development of data agreements, supporting networking activities, and validating conditions.
There are monthly meetings between the BCCASS lead and the Committee co-chair. The entire Committee meets 3–4 times per year, but more frequent meetings occur with specific committee members as necessary. A summary of action points, results, and outstanding points is presented at every meeting. This keeps members engaged and committed to advancing the system and promotes transparency and accountability, which we believe are vital to foster a trusting relationship between members; this is the foundation of respectful collaboration, communication, and success.
Case validation
Evidence suggests that the level of agreement between passive and active surveillance is dependent on the type of anomaly (Salemi et al. 2016). Agreement tends to be lower for conditions that are more likely to be terminated during pregnancy (such as Down syndrome) or not easily diagnosed at birth (Metcalfe et al. 2014; Salemi et al. 2018). Evidence also suggests that false positive cases are common for neural tube defects (NTDs) in passive systems (Metcalfe et al. 2014). Therefore, we validate cases of NTDs defects and Down syndrome prior to inclusion in the system.
We validate cases on a yearly basis with the support of members of the BCCASS Advisory Committee. The validation process involves cross-referencing cases with the VSA database, the Prenatal Genetic Screening Program, or reviewing documentation that feeds the DAD. If there is a discrepancy, we review individual cases with a BCCASS Advisory Committee medical expert, who helps determine the final decision for case inclusion or exclusion with the available information. Fortunately, the prevalence rates of both NTDs and Down syndrome are low in BC; thus, the time required to validate each case on an annual basis is minimal (e.g., it took about 12 h to validate all cases of Down syndrome that occurred in 2018).
Preliminary case validation for Down syndrome and NTD suggests that BCCASS algorithms are accurately capturing most of the cases in BC. For instance, we confirmed 97% (n=62) of Down syndrome cases (including live birth and stillbirths) in 2018 using the Prenatal Genetic Screening Program or the Medical Coding Unit, and only missed 4% (n=3) of the cases via the BCCASS.
Outcomes
The goal of BCCASS is to monitor CAs and produce high-quality data that can inform program planning and health policy. There are three main outcomes of our system: an established structure for data sharing, CAs algorithms, and surveillance data.
Established structure for data sharing
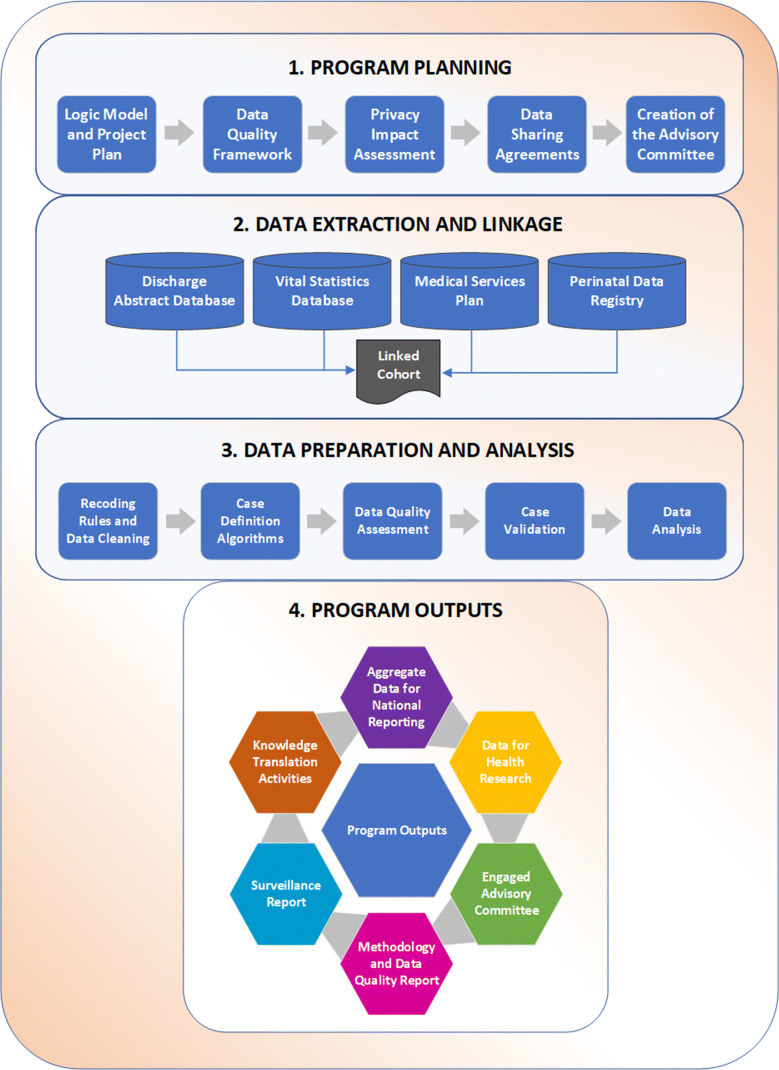
Data sharing between organizations is a complex process that benefits from having clear strategies for secure data transfer, storage, and use. This includes having explicit objectives and deliverables, identifying what resources are needed, what activities will be performed, and what potential risks exist. We completed a Privacy Impact Assessment prior to implementing the program to ensure the protection of personal information. We created a logic model to depict the relationships between the main components of our program. Outputs from this logic model can be used to assess the system’s performance without engaging in a formal evaluation, as well as to identify areas for potential investment and disinvestment. Figure 1 displays the different processes involved in BCCASS. We developed several multiyear data sharing agreements to ensure continuous access to CA information. Amendments to existing agreements may be negotiated if data requirements evolve.
Fig. 1.
Program overview of the different processes involved in BCCASS
Case definition algorithms
Algorithms are an integral component of the BCCASS program. We have developed algorithms to ascertain 35 major congenital anomalies, which were prioritized based on the Canadian Congenital Anomalies Enhancement Initiative and the WHO Manual for Birth Defect Surveillance (World Health Organization 2020). We intend to expand the number of conditions we ascertain and monitor in 2023. An example of an algorithm is included in Appendix 1.
Case definition algorithms use a specific combination of health encounters, diagnostic and/or procedure codes, and data sources to ascertain CAs. Algorithms used in BCCASS are informed by the literature and subject matter experts and reviewed by the Advisory Committee. Before and after applying the algorithms, we use a series of rules to flag potential issues with diagnostic codes. For instance, cases that have concurrent diagnoses of spina bifida and hydrocephalus are flagged and reclassified as spina bifida with hydrocephalus to be a more specific diagnosis. This re-classification is necessary because diagnostic codes are pulled from multiple administrative data sources when the cohort is created, and these databases were not originally developed to collect information pertaining to CAs.
Given the value of using validated algorithms, we are undertaking a study to compare ascertainment of cases using BCCASS algorithms and the HSR for the years 2000 and 2002, the only years of overlap between the two systems when the HSR had a very high rate of case ascertainment.
Data features
BCCASS collects information of children born with specific CAs and their mothers. The dataset contains information regarding the pregnancy and delivery, including demographics, maternal risk factors, and information pertaining to the pregnancy outcome (see Appendix 2). Additionally, maternal residential street address or postal codes are known for different developmental time windows allowing for potential linkage to socioeconomic and environmental exposure data. Although BCCASS data are not currently available to external researchers, we plan to create the protocols and governance structures that would make it available for third parties in the future.
Data quality is assessed for any incoming data as well as the data produced for the final cohort using a modified version of the Data Quality Framework from the Manitoba Centre for Health Policy (Smith et al. 2018). Once the framework is applied, we generate two outputs. The first output is the Valid, Invalid, Missing, and Outlier (VIMO) table that contains a summary of variables to rapidly identify issues that could threaten the system’s internal validity. The second output is an R Markdown script rendered to produce the Data Quality Report. This is a detailed description of BCCASS data quality indicators that can be used to monitor data quality over time and identify areas for improvement.
We have analyzed BCCASS data to assess birth prevalence and annual trends, and are currently working on the surveillance report and validation of administrative algorithms. The first annual report will be focused on basic epidemiological analysis by time, person, and space. We envision to have annual reports available in a data dashboard within the OPHO website.
Due to the nature of our system, we have the capacity to rapidly produce surveillance data upon received external data. Our main timeliness limitation is that the PDR, our main data source, is transferred only once per year with data dating back one fiscal year. Therefore, our current report can only report on data up to December 2019.
Since 2019, we have contributed to national surveillance efforts by providing aggregated provincial CAs data to the Canadian Congenital Anomalies Surveillance System. We have prepared all processes of extraction, data linkage, algorithm creation, algorithm application, and data quality assessment as reproducible R programming scripts that can be modified or adapted as needed; therefore, it is possible to implement the same or similar methodology to other Canadian jurisdictions.
Implications
Since 2002, we have not been able to conduct consistent surveillance of CAs in BC, resulting in limited knowledge about the state of CAs in the province. The introduction and implementation of BCCASS is filling this gap. The possibility to standardize CAs surveillance across Canadian regions is one of the most relevant aspects of BCCASS. While jurisdictional comparison is complex because cases are not ascertained consistently, the uniform collection and availability of administrative data across the country creates an opportunity for standardization.
A challenge with this system is the proportion of missing data for some variables as administrative databases were not specifically designed for CA surveillance. Another issue is the inclusion of false positive cases for conditions that are not validated; therefore, validation of algorithms is an unqualified necessity. Restrictive algorithms (e.g., those that require diagnostic codes to appear in multiple databases) significantly improve accuracy. However, for routine monitoring of defects or healthcare planning, as previously suggested by Salemi et al. (2018), programs need to find the right tradeoffs between accuracy and completeness.
The BCCASS approach involves innovative elements that can serve as a guiding framework for the creation of resource-efficient and comprehensive surveillance systems for jurisdictions with an interest in surveillance but facing the challenge of limited resources or where case verification is not possible. The utilization of algorithms for case ascertainment, the continuous access and usage of existing administrative data, the validation of certain defects, the assessment of data quality, and the guidance of the Advisory Committee are all integral and complementary factors to the system. Due to the nature of our system, we rely heavily on the advice of the Advisory Committee. We continually learn what works best for the BCCASS system and will evolve based on feedback from stakeholders.
Implications for policy and practice
What are the innovations in this policy or program?
BCCASS hybrid case ascertainment is an effective way of conducting CAs surveillance as it maximizes available resources.
The use of existing administrative data sources saves resources for data collection.
Engagement with the Provincial Advisory Committee provides a practice-supported evidence-based system.
BCCASS will provide a comprehensive, timely overview of the burden of CAs in BC, which is necessary to inform data-driven decisions, healthcare planning, and health policy.
What are the burning research questions for this innovation?
To enhance BCCASS data quality, there is an ongoing process to conduct validation of algorithms and report on measures of algorithm performance.
Case ascertainment using case definition algorithms may standardize how conditions are ascertained across other regions and therefore may improve CA surveillance at the national level.
Acknowledgements
We would like to thank all members of the BCCASS Advisory Committee and the Alberta Congenital Anomalies Surveillance System for their support and guidance. We would also like to thank our stakeholders and partners for their invaluable support, in particular, Perinatal Services BC, the BC Vital Statistics Agency, the OPHO reporting team and the Health Sector Information, Analysis and Reporting division at the BC Ministry of Health.
Appendix 1
Example of the case definition algorithm used for neural tube defects
| Congenital anomalies | Algorithm | ICD-10-CA | ICD-9 | Exclusion | Data source |
|---|---|---|---|---|---|
| Anencephaly and similar defects | 1 VE record or 1 perinatal record or ≥ 1 hospitalization | Q00.0 and Q00.1 | 740.0, 740.1 | Amniotic band syndromea and acalvariab | PDR, VSA, DAD |
| Encephalocele | 1 VE record or 1 perinatal record or ≥ 1 hospitalization | Q01.0–Q01.9 | 742.0 | Q00.0 and amniotic band syndromea | PDR, VSA, DAD |
| Spina bifida | 1 VE record or 1 perinatal record or ≥ 1 hospitalization | Q05.0–Q05.9 | 741, 741.0, 741.9 | Q76.0, tethered cord or spinal lipomas | PDR, VSA, DAD |
Abbreviations: VE, vital event including birth, stillbirth, or death record; ICD-10-CA, Canadian modification of the International Statistical Classification of Diseases and Related Health Problems 10th revision; ICD-9, International Statistical Classification of Diseases and Related Health Problems 9th revision; PDR, Perinatal Data Registry; VSA, Vital Statistics Agency; DAD, Discharge Abstract Database
a Confirmed by the autopsy report and identified with ICD-10 code Q79.8
b Confirmed by the autopsy report
c Confirmed by autopsy or notice of birth or stillbirth and identified with ICD-10-CA code Q06.8
Appendix 2
Variables in the British Columbia Congenital Anomalies Surveillance System
| Variable | Type of variable |
|---|---|
| PHN (infant) | Character |
| PHN (mother) | Character |
| DOB (infant) | Date |
| Maternal DOB | Date |
| Maternal age | Numeric |
| Biological sex | Character |
| Mode of delivery | Character |
| Birth weight | Numeric |
| Gestational age | Numeric |
| Gravida | Numeric |
| Outcome | Character |
| Head circumference | Numeric |
| Maternal body mass index | Numeric |
| Maternal body mass index | Character |
| Health authority | Character |
| Health service delivery area | Character |
| Local health area | Character |
| Postal code | Character |
| Death flag | Character |
| Date of death | Date |
| Survival at 7 days | Character |
| Survival at 28 days | Character |
| Post-mortem examination flag | Character |
| Prior anomaly flag | Character |
| IVF flag | Character |
| No risk flag | Character |
| Alcohol flag | Character |
| Smoking flag | Character |
| Type of smoker | Character |
| Cigarettes per day | Numeric |
| Marijuana flag | Character |
| Substance use flag | Character |
| Preexisting diabetes | Character |
| Gestational diabetes | Character |
| Preeclampsia | Character |
| Maternal country of birth | Character |
| Marital statusa | Character |
| Congenital anomalies flag | Character |
| Character |
a Based on the mother
Abbreviations: PHN, Personal Health Number; DOB, date of birth; IVF, in vitro fertilization
Funding
Partial financial support for this project was received from the Public Health Agency of Canada, Memorandum of Agreement # 6D023-172941.
Data availability
Not applicable
Code availability
Not applicable
Declarations
Ethics approval
Not applicable
Consent to participate
Not applicable
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- BC Coroners Service. (2012). Child mortality in British Columbia. Report prepared by the Child Death Review Unit of the British Columbia Coroners Service. https://www2.gov.bc.ca/assets/gov/birth-adoption-death-marriage-anddivorce/deaths/coroners-service/child-death-review-unit/reports-publications/child-mortality-2012.pdf
- BC Coroners Service. (2015). Child mortality in British Columbia. Report prepared by the Child Death Review Unit of the British Columbia Coroners Service. https://www2.gov.bc.ca/assets/gov/birth-adoption-death-marriage-anddivorce/deaths/coroners-service/child-death-review-unit/reports-publications/child-mortality-2015.pdf
- BC Coroners Service. (2016). Child mortality in British Columbia. Report prepared by the Child Death Review Unit of the British Columbia Coroners Service. https://www2.gov.bc.ca/assets/gov/birth-adoption-death-marriage-anddivorce/deaths/coroners-service/child-death-review-unit/reports-publications/child-mortality-2016.pdf
- BC Ministry of Health. (2017). Promote, protect, prevent: Our Health Begins Here: BC’s guiding framework for public health. https://www.health.gov.bc.ca/library/publications/year/2017/BC-guiding-framework-for-public-health-2017-update.pdf
- Blais L, Kettani FZ, Elftouh N, Forget A. Effect of maternal asthma on the risk of specific congenital malformations: A population-based cohort study. Birth Defects Res A Clin Mol Teratol. 2010;88(4):216–222. doi: 10.1002/bdra.20651. [DOI] [PubMed] [Google Scholar]
- Christianson, A., Howson, C., & Modell, B. (2006). Global report on birth defects. March of Dimes Birth Defects Foundation. https://www.marchofdimes.org/global-report-on-birth-defects-the-hidden-toll-of-dying-and-disabledchildren-full-report.pdf
- Frosst, G., Hutcheon, J., Joseph, K., Kinniburgh, B., Johnson, C., & Lee, L. (2015). Validating the British Columbia Perinatal Data Registry: A chart re-abstraction study. BMC Pregnancy and Childbirth, 15(1). 10.1186/s12884-015-0563-7 [DOI] [PMC free article] [PubMed]
- Glinianaia, S. V., Tennant, P. W. G., & Rankin, J. (2017). Risk estimates of recurrent congenital anomalies in the UK: A population-based register study. BMC Medicine, 15(1). 10.1186/s12916-017-0789-5 [DOI] [PMC free article] [PubMed]
- Hu, W. (1996). Diagnostic codes in MSP Claim Data. Report for the Program Monitoring and Information Management Branch. BC Ministry of Health. https://www.popdata.bc.ca/sites/default/files/documents/data/MSP%20Diagnostic%20Codes%20paper.pdf
- Lowry B, Bedard T. Birth defect registries: the vagaries of management—The British Columbia and Alberta Case Histories. Journal of Registry Management. 2013;40(2):98–103. [PubMed] [Google Scholar]
- Mburia-Mwalili, A., & Yang, W. (2014). Birth defects surveillance in the United States: Challenges and implications of International Classification of Diseases, Tenth Revision, Clinical Modification Implementation. International Scholarly Research Notices. 10.1155/2014/212874 [DOI] [PMC free article] [PubMed]
- Metcalfe, A., Sibbald, B., Lowry, R., Tough, S., & Bernier, F. (2014). Validation of congenital anomaly coding in Canada’s administrative databases compared with a congenital anomaly registry. Birth Defects Research. Part A, Clinical and Molecular Teratology, 100(2). 10.1002/bdra.23206 [DOI] [PubMed]
- Mott, G. A. (1963). The Registry of Handicapped Children and Adults in British Columbia. Canadian Journal of Public Health, 54(6), 239–245. [PubMed]
- Peng RD. Reproducible research in computational science. Science. 2011;334(6060):1226–1227. doi: 10.1126/science.1213847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health Agency of Canada. (2013). Congenital anomalies in Canada: A perinatal health surveillance report. Public Health Agency of Canada.
- Salemi, J. L., Tanner, J., Sampat, D., Anjohrin, S., Correia, J., Watkins, S., et al. (2016). The accuracy of hospital discharge diagnosis codes for major birth defects: Evaluation of a statewide registry with passive case ascertainment. Journal of Public Health Management and Practice: JPHMP, 22(3). 10.1097/PHH.0000000000000291 [DOI] [PubMed]
- Salemi J, Rutkowski R, Tanner JP, Matas J, Kirby R. Identifying algorithms to improve the accuracy of unverified diagnosis codes for birth defects. Public Health Reports. 2018;133(3):303–310. doi: 10.1177/0033354918763168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M, Lix LM, Azimaee M, Enns JE, Orr J, Hong S, et al. Assessing the quality of administrative data for research: A framework from the Manitoba Centre for Health Policy. Journal of the American Medical Informatics Association. 2018;25(3):224–229. doi: 10.1093/jamia/ocx078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vital Statistics Agency. (2015). Annual Report: Selected vital statistics and health status indicators. Vital Statistics Agency.
- Vital Statistics Agency. (2017). Medical certification of death and stillbirth: A handbook for physicians, nurse practitioners and coroners. https://www2.gov.bc.ca/assets/gov/birth-adoption-death-marriage-anddivorce/deaths/vsa051.pdf
- World Health Organization. (2020). Birth defect surveillance: A manual for programme managers. https://www.who.int/publications/i/item/9789240015395
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable
Not applicable