Abstract
Objectives
The recent emergence of the COVID-19 pandemic (caused by SARS-CoV-2) and the experience of its unprecedented alarming toll on humanity have shone a fresh spotlight on the weakness of global preparedness for pandemics, significant health inequalities, and the fragility of healthcare systems in certain regions of the world. It is imperative to identify effective drug treatments for COVID-19. Therefore, the objective of this review is to present a unique and contextualised collection of antiviral natural plants or remedies from the West African sub-region as existing or potential treatments for viral infections, including COVID-19, with emphasis on their mechanisms of action.
Evidence acquisition
Evidence was synthesised from the literature using appropriate keywords as search terms within scientific databases such as Scopus, PubMed, Web of Science and Google Scholar.
Results
While some vaccines and small-molecule drugs are now available to combat COVID-19, access to these therapeutic entities in many countries is still quite limited. In addition, significant aspects of the symptomatology, pathophysiology and long-term prognosis of the infection yet remain unknown. The existing therapeutic armamentarium, therefore, requires significant expansion. There is evidence that natural products with antiviral effects have been used in successfully managing COVID-19 symptoms and could be developed as anti-COVID-19 agents which act through host- and virus-based molecular targets.
Conclusion
Natural products could be successfully exploited for treating viral infections/diseases, including COVID-19. Strengthening natural products research capacity in developing countries is, therefore, a key strategy for reducing health inequalities, improving global health, and enhancing preparedness for future pandemics.
Graphical abstract
Keywords: Antiviral, Medicinal Plants, Traditional Medicine, SARS-CoV-2, COVID-19, West Africa
Introduction: burden of SARS-CoV-2
The emergence in late 2019 of the novel SARS-CoV-2 virus (named COVID-19) and its consequent worldwide transmission has led to a significant burden on health care systems in almost every country on planet earth [1, 2]. COVID-19, the disease caused by the virus, exponentially expanded from the first reported case in Wuhan, China, on the 31st of December, 2019 to 364,191,494 confirmed cases and 5,631,457 deaths reported by the World Health Organisation (WHO) as of 28th January, 2022 [3]. So far in the course of the pandemic there has been a worrying trend of an abatement followed by a resurgence, especially in countries that were originally considered to have done very well in managing the pandemic, with the resurgence (termed “second or third or new waves”) being linked to several factors, including the emergence of new variants of the virus, differences in the extent and effectiveness of countries’ lockdown, quarantine and other preventive measures, overwhelming of healthcare capacity for treating the infected, and the fact that there are several aspects of the new infection that not much is known about to date.
SARS-CoV-2 is one of seven strains of coronaviruses (CoVs) recorded to date [4]. It belongs to Beta-coronaviruses-type Human Coronaviruses, the same group as the Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV). Data from the WHO indicate that SARS and MERS coronaviruses were the most destructive strains of CoVs until the current outbreak. MERS has a mortality rate of 36% and SARS 10% [5].
Even though reasonable progress has been made against COVID-19 in terms of finding effective preventive measures with the introduction of vaccines and treatment measures owing to the identification of some drugs or drug combinations, there is to date continuing profound burden of the infection on health care systems, as well as attendant disruptions to living and livelihoods occasioned by the varying quarantine, lockdown, and social distancing measures introduced by countries. Therefore, there is still significant pressure on the WHO, governments, academic institutions, pharmaceutical industries, charities, and related organisations to find curative treatments (drugs) to complement the current armamentarium. The growing emergence of SARS-CoV-2 variants of concern also suggests vaccines will have to keep being modified to retain acceptable levels of effectiveness. While some countries are currently doing well in their vaccine roll-out programmes, many countries still appear to be struggling in this regard and, unfortunately, their infection and death rates continue to soar.
While the current realities and future threats of COVID-19 are shared globally, the ominous potential for the developing countries to be disproportionately hard-hit in the short- and long-term presents a frightening spectre, although it currently appears the numbers of cases in those countries are generally lower than for other regions of the world. The higher vulnerability of developing countries is due to several factors. Chiefly, health systems in those countries are weak, fragile and lack the capacity [6] to contain full-blown infections within populations. These systems are already burdened by a slew of other infectious diseases such as malaria, tuberculosis, and Human Immunodeficiency Virus (HIV), amongst others [7]. Also, the poverty status and the culture in these countries [8] make extended lockdowns and social distancing measures near impossible. It is, therefore, important that solutions proposed for tackling SARS-CoV-2 and its effects in developing countries are sensitive to the dynamics of existing opportunities and challenges in those environments and how these might impact the effectiveness, affordability and accessibility of therapeutic options and strategies for tackling SARS-CoV-2 and COVID-19.
Notably, however, the current reality that COVID-19 infection rates and deaths in most African countries (and other countries considered underdeveloped), which were expected to buckle under the burden of COVID-19, are surprisingly much lower than predicted deserves to be investigated. Questions should be asked about what the people of those countries are doing to combat COVID-19 and whether or not, and to what extent, their massive use of traditional medicines plays a role in recording that relative success.
This review first explores natural products for use generally as antivirals, including their mechanisms of action. It then focusses on antiviral medicinal plants from the West African region, providing information about their identity, constituent compounds and their chemical structures, and the viral disease(s) they are used or reported to treat. It then discusses how these plants or herbal medicines containing them might be useful in the treatment of COVID-19 and similar coronavirus infections, based on their molecular mechanisms of action against other viruses, whether by direct antiviral effects or indirectly as anti-inflammatory and immunomodulatory agents. This work does provide detailed and contextualised understanding of the rationale and ramifications for the antiviral use of West African medicinal plants and how such existing knowledge repository and potential could be leveraged upon to investigate the plants for the treatment of COVID-19 or similar future infections, using an approach that integrates evidence-based herbal medicine into mainstream healthcare.
Natural products and antiviral therapy
Evidence indicates that up to 80% of the population in developing countries use herbal medicines as the primary form of healthcare [9–11] due to several reasons, including relatively lower cost and perceived safety of traditional therapies compared with conventional medicines, unavailability or inaccessibility of conventional medical facilities and healthcare practitioners, and cultural and religious practices. Consequently, as COVID-19 emerges in those countries, it is not inconceivable that citizens will turn to herbal remedies for the prophylaxis, treatment, and symptomatic management of COVID-19. There are reports of the use of natural products and traditional medicines for such purposes.
Consistent with the fact that nature has influenced human health and well-being since ancient times, medicinal plants and other natural products have become integral components of health systems in developing countries [12, 13]. Modern drug discovery has also benefitted significantly from natural products [14–16].
The search for nature-derived or nature-inspired chemical leads that could be developed for the treatment of diverse diseases has also accelerated in recent years [17]. Scientists are increasingly exploring diverse natural sources: microbes, marine organisms and animals. In fact, there are numerous examples of antiviral drugs or drug candidates sourced from nature: Bevirimat (PA-457), an HIV maturation inhibitor and a semi-synthetic derivative of the ubiquitous betulinic acid (a triterpenoid) that is found in several species, including Syzygium claviflorum [18]; calanolide A, a pyranocoumarin non-nucleoside reverse transcriptase inhibitor (anti-HIV-1) from Calophyllum lanigerum [19, 20, 21]; ceglosivir, an alpha-glucosidase 1 inhibitor (for treating Hepatitis C Virus, HCV) that is a semi-synthetic derivative of castanospermine, an alkaloid from Castanospermum australe [22]; alisporivir, a cyclophilin-inhibiting anti-HCV drug, which is a non-immunosuppressive derivative of ciclosporin isolated from the fungus Tolypocladium inflatum [23] and has been reported to inhibit SARS-CoV-2 RNA production [24]; acyclovir (for treating herpes simplex virus infections, chickenpox and shingles) and zidovudine (anti-HIV), synthetic derivatives of arabinosyl nucleosides (nucleoside analogues) from Tethya cripta [25, 26] and cyanovirin-N, a protein with virucidal activity against several viruses (including HIV), isolated from the cyanobacterium, Nostoc ellipsosporum [27].
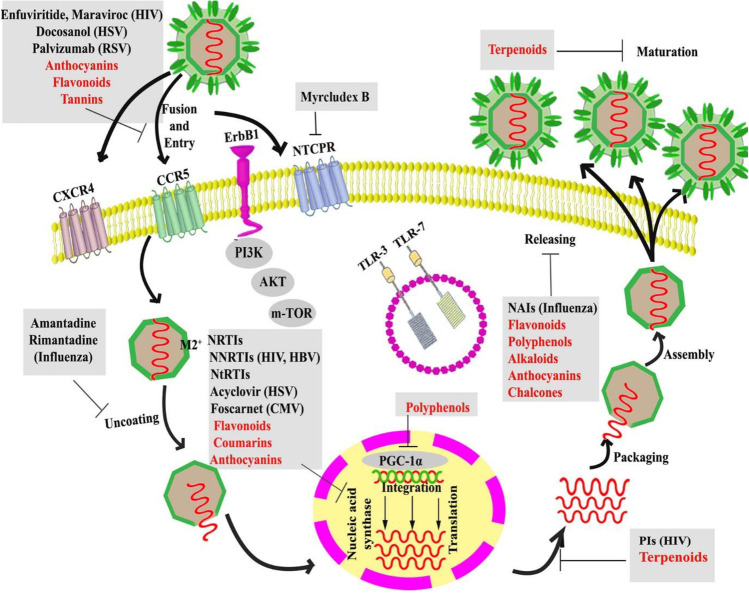
The majority of the antiviral herbs documented in literature have been found to contain active components such as flavones, alkaloids and polyphenols [28]. Flavonoids are said to constitute the largest source of antiviral agents in the entire plant kingdom [12]. For example, the flavone artogomezianone has been shown to possess anti-herpetic properties [29]; naringin has shown activity against HCV and HIV [30]; and quercetin reduced the infectivity and intracellular replication of Herpes Simplex Virus (HSV-1), Polio-virus type 1, Parainfluenza virus type 3 (Pf-3), and Respiratory Syncytial Virus (RSV) in cell culture monolayers [31]. Similarly, the alkaloid berberine, from Rhizoma Coptidis (RC), has been shown to prevent HSV penetration [32]; Farnsworth et al. [33] documented that nine of thirty-six alkaloids from Catharanthus roseus or C. lanceus were effective as antiviral agents, with pericalline being the most effective. Figure 1 shows the known or suggested mechanisms of antiviral action of flavonoids, polyphenols, terpenoids, coumarins, anthocyanins and chalcones, highlighting the various extracellular and intracellular drug targets, including host (entry) receptors and life cycle stages of the virus within the host. A recent review by Orhan and Senol Deniz [34] explored various articles from which they compiled the IC50/EC50 values for the anti-SARS-CoV activities of several flavonoids, some alkaloids, a few terpenes, diterpenes, saponins, diarylheptanoids and lectins, and a chalcone.
Fig. 1.
Mechanisms of antiviral action of various classes of natural compounds (indicated in red), with examples of some conventional antiviral therapeutics (for context and comparison), showing their extracellular and intracellular host- or virus-based drug targets. HIV, human immunodeficiency viruses; RSV, respiratory syncytial virus; HBV, hepatitis B virus; HSV-1/2, herpes simplex virus-1/2, NtRTIs, nucleotide reverse transcriptase inhibitors; NRTIs, nucleoside reverse transcriptase inhibitors; NNRTIs, non-nucleoside reverse transcriptase inhibitors; NAIs, neuraminidase inhibitors; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; CMV, cytomegalovirus; PI3K, Phosphatidylinositol-3-Kinase; TLR 3 or 7, toll-like receptor-3 or 7; AKT, Protein Kinase B; mTOR, mechanistic target of rapamycin; CCR5, C–C chemokine receptor type 5; CXCR4, C-X-C chemokine receptor type 4; ErbB1, epidermal growth factor receptor-1; NTCP Na + /taurocholate co-transporting polypeptide; PI, Protease Inhibitor. Figure reproduced with permission [35]
Despite the progress made in immunisation and antiviral drugs development, many viruses yet lack preventive vaccines and efficient and safe antiviral therapies. Thus, identifying novel antiviral drugs is of critical importance and natural products are an excellent source and may guide such discoveries [31, 36–38]. Interestingly, herbal remedies and natural products with antiviral activity have been mentioned in ethnobotanical surveys and reports of biological assays conducted in Africa. It should be emphasised that, beyond looking for chemical leads for the development of mono-component drugs, efforts, encouraged to be led by African nations, must also be directed towards deploying natural products known to have antiviral effects in developing standardised antiviral formulations, just as is now done with Traditional Chinese Medicine [39]. In this mini-review, we summarize available data on antiviral natural products, especially medicinal plants, focusing on those indigenous to, or found in, West Africa. We also highlight documented cases in the literature where these plants or constituents thereof have been shown to have positive effects specifically on coronaviruses.
West African antiviral natural products
A review of medicinal plants in West Africa mentioned as part of ethnobotanical surveys for antiviral use within local populations and scientific investigations into possible antiviral properties showed that there are at least 124 species employed in West African traditional medicine (Table 1). These plants, whose leaves, roots, bark, flowers, latex and rhizomes form components of traditional antiviral remedies, are distributed across 50 plant families, exemplifying the recognised diversity of plants employed in traditional medicine systems [40–42]. The most prominent families were Amaryllidaceae, Anacardiaceae, Combretaceae, Compositae, Cucurbitaceae, Euphorbiaceae, Leguminosae, Malvaceae, Myrtaceae, Piperaceae, Rubiaceae, Rutaceae and Solanaceae. The Leguminosae and Compositae have been mentioned as part of the most species-rich medicinal plant families [43]. These plants (or the plant parts) are employed by the local population in the management of diseases, where viral infection is indicated, such as fevers, chickenpox, common cold, enteric conditions such as dysentery and diarrhoea, syphilis and other Sexually Transmitted Infections [44], measles, yellow fever, jaundice and hepatitis. From the literature review, one mushroom (Hypoxylon fuscum) and one lichen (Ramalina farinacea) were also reported to possess antiviral activities.
Table 1.
West African Traditional Medicines with Suggested Antiviral Activity
| S/N | Traditional Medicine | Family | Common name | Part used | Local Indication | Investigated Antiviral Activity | References |
|---|---|---|---|---|---|---|---|
| 1 | Adansonia digitata L. | Malvaceae | Monkey-bread tree | Bark, Root, Leaf | Intestinal and skin disorders, poliomyelitis asthma | NDV, HSV HCV, PV | [46–49] |
| 2. | Aframomum melegueta K.Schum. | Zingiberaceae | Alligator pepper | Seed | Cholera, smallpox and chickenpox, measles | MV, YFV | [50–53] |
| 3. | Ageratum conyzoides (L.) L | Compositae | Goat weed | Leaf, whole plant | Smallpox poliomyelitis, measles, yellow fever | EV 7, 19 HIV-1, HIV-2 | [52, 54–56] |
| 4. | Allanblackia floribunba Oliv. | Clusiaceae | Tallow tree | Leaf | Chickenpox, measles | [52] | |
| 5. | Allium ascalonicum L. | Amaryllidaceae | Shallot | Leaf, rhizome | Common cold Chickenpox | [52, 55] | |
| 6. | A. sativum L. | Amaryllidaceae | Garlic | Bulb | Poliomyelitis | [52] | |
| 7. | Alstonia boonei De Wild. | Apocynaceae | Cheese wood | Bark, Leaf | Yellow fever, jaundice | [52, 55] | |
| 8. | Amaranthus viridis L. | Amaranthaceae | Green amaranth | Leaf | Mumps | MV | [57, 58] |
| 9. | Anacardium occidentale L. | Anacardiaceae | Cashew | Bark | Enteric conditions, worms, jaundice, measles, chickenpox, shingles | PV, AV, HSV 1, Equine HSV, BPV, CPV | [52, 59, 60] |
| 10. | Annickia chlorantha (Oliv.) Setten & Maas | Annonaceae | African yellow wood | Bark | Fever, malaria | NDV | [61] |
| 11. | Anogeissus leiocarpa (DC.) Guill. & Perr. | Combretaceae | African birch | Leaf | Fever, diarrhoea, dressings | PV, AV, HSV 1, Equine HSV | [59] |
| 12. | Argyreia nervosa (Burm. f.) Bojer | Convolvulaceae | Elephant Creeper | Leaf | Chickenpox | [52] | |
| 13. | Azadirachta indica A. Juss. | Meliaceae | Neem tree | Leaf, bark | Fever, jaundice | DV, CV | [62, 63] |
| 14. | Bambusa vulgaris Schrad. | Poaceae | Tropical bamboo | Leaf | Measles | MV | [50–52] |
| 15. | Bauhinia thonningii Schum. | Leguminosae | Camel’s foot tree | Leaf | Diarrhoea, fever, influenza, cold, dysentery | PV, AV, HSV 1, Equine HSV, BPV and CPV | [59] |
| 16. | Boswellia dalzielii Hutch. | Burseraceae | Nigerian Frankincense | Bark | Diarrhoea, fever, gastrointestinal disorders | PV, AV, HSV 1, Equine HSV, BPV, CPV | [59, 61] |
| 17. | Brachiaria ciliaris Vanderyst | Poaceae | Buffalo grass | Leaf | Measles | [49] | |
| 18. | Bryophyllum pinnatum (Lam.) Oken | Crassulaceae | Life plant | Leaf | cold, pneumonia and respiratory tract infections, measles | EV 7, 19, HSV | [52, 54, 55] |
| 19. | Caesalpinia bonduc (L.) Roxb. | Leguminosae | Warri tree | Leaf | Measles | [51] | |
| 20. | Cajanus cajan (L.) Millsp. | Leguminosae | Pigeon pea | Whole plant | Measles | MV | [52, 64, 65] |
| 21. | Capsicum annuum L. | Solanaceae | Cayenne pepper | Seed | Measles | [51] | |
| 22. | Carica papaya L. | Caricaceae | Pawpaw | Leaf | Poliomyelitis, jaundice | [52, 64] | |
| 23. | Cassia fistula L. | Leguminosae | Golden shower | Seed | Common cold | [64, 66] | |
| 24. | Ceratotheca sesamoides Endl. | Pedaliaceae | False sesame | Leaf stem, root | Rhinitis, influenza, hepatitis, dysentery | MV | [57] |
| 25. | Chasmanthera dependens Hochst. | Menispermaceae | Climbing plant | Leaf | Poliomyelitis | [52] | |
| 26. | Citrullus colocynthis (L.) Schrad. | Cucurbitaceae | Bitter cucumber | Seed | Measles | [51] | |
| 27. | C. aurantiifolia (Christm.) Swingle | Rutaceae | Lime | Fruit, leaf | Hepatitis measles, jaundice | [52, 64, 67] | |
| 28. | C. paradisi Macfad. | Rutaceae | Grapefruit | Leaf | Hepatitis | [64, 67] | |
| 29. | Clausena anisata (Willd.) Hook.f. ex Benth. | Rutaceae | Horsewood | Whole Plant | Whooping cough, syphilis, sore throat | HIV-1, HIV-2 | [56] |
| 30. | Combretum indicum (L.) DeFilipps | Combretaceae | Rangoon creeper | Leaf | Fever, Diarrhoea | FPV, NDV | [68] |
| 31. | C. mucronatum Schumach. & Thonn. | Combretaceae | Leaf | Measles | [49] | ||
| 32. | Corchorus olitorius L. | Malvaceae | Jute plant | Whole plant | Measles | [51, 52] | |
| 33. | Crinum jagus (J.Thomps.) Dandy | Amaryllidaceae | St. Christopher’s Lily | Bulb | Tuberculosis, epilepsy, asthma, infections | EV 7, 19 | [54] |
| 34. | Cucumis metuliferus E.Mey. ex Naudin | Cucurbitaceae | Horned melon | Fruit | Hepatitis, HIV/AIDS | NDV | [69] |
| 35. | Cymbopogon citratus (DC.) Stapf | Poaceae | Lemongrass | Leaf | Jaundice, yellow fever | [52, 64] | |
| 36. | Deinbollia pinnata (Poir.) Schumach. & Thonn. | Sapindaceae | Indian beech | Seed | Measles | [51] | |
| 37. | Detarium microcarpum Guill. & Perr. | Leguminosae | Sweet detar | Bark | Dysentery, syphilis | HCV | [70] |
| 38. | D. senegalense J.F.Gmel. | Leguminosae | Tallow tree | Leaf | Fever, dysentery, Boils | PV, AV, HSV 1, Equine HSV, BPV and CPV | [59] |
| 39. | Dichrostachys cinerea (L.) Wight & Arn. | Leguminosae | Sickle bush | Leaf | Skin conditions, fever, diarrhoea measles, chickenpox, varicella | PV, AV, HSV 1, Equine HSV, BPV and CPV | [59] |
| 40. | Dioclea reflexa Hook. f. | Leguminosae | Brown hamburger bean | Seed | Measles | [52] | |
| 41. | Dioscorea cayennensis Lam | Dioscoreaceae | Yellow yam | Leaf | Poliomyelitis | [49] | |
| 42. | D. cayennensis subsp. rotundata (Poir.) J.Miège | Dioscoreaceae | West African yam | Leaf | Measles | [51] | |
| 43. | Diospyros barteri Hiern | Ebenaceae | Leaf | PV Type 2 | [71] | ||
| 44. | D. mespiliformis Hochst. ex A.DC. | Ebenaceae | Jackalberry | Leaf, fruit, roots | Herpes, mumps, hepatitis | FPV, NDV | [68, 72] |
| 45. | D. monbuttensis Gurke | Ebenaceae | Walking stick ebony | Seed | Herpes | PV Type 2 | [71] |
| 46. | Ehretia cymosa Thonn. | Boraginaceae | Leaf | Poliomyelitis, measles | [52] | ||
| 47. | Elaeis guineensis Jacq. | Arecaceae | African oil palm | Oil | Herpes simplex, Measles | [51, 64] | |
| 48. | Elytraria marginata Vahl | Acanthaceae | Leaf | Measles | [51] | ||
| 49. | Emilia coccinea (Sims) G.Don | Compositae | Tassel flower | Leaf | Mumps, herpes simplex, smallpox | [49] | |
| 50. | Erigeron aegyptiacus L. | Compositae | Leaf | Skin diseases, herpes, hepatitis | HSV, PV | [46, 47] | |
| 51. | Eucalyptus camaldulensis Dehnh. | Myrtaceae | Red river gum | Leaf | Fever, hepatitis, flu, rhinitis | PV type I, CV and EV 6 | [73] |
| 52. | E. globulus Labill. | Myrtaceae | Tasmanian blue gum | Leaf | Flu, fever, rhinitis | PV type I, CV and EV 6 | [73] |
| 53. | Euphorbia Lateriflora Schumach. | Euphorbiaceae | Crown of thorns | Leaf | MV | [57] | |
| 54. | Ficus laurifolia Lam. | Moraceae | Black fig | Root, bark | Tetanus convulsions | HSV | [46, 74] |
| 55. | Ficus polita Vahl | Moraceae | Heart-leaved fig | Whole Plant | Hepatitis, fever | HIV-1, HIV-2 | [56, 75] |
| 56. | Ficus thonningii Blume | Moraceae | Common wild fig | Leaf | Jaundice, measles | [52] | |
| 57. | Garcinia kola Heckel | Clusiaceae | Bitter kola | Seed, root | Hepatitis, smallpox | [52, 64] | |
| 58. | Gossypium arboreum L. | Malvaceae | Tree cotton | Leaf | Hepatitis | [52] | |
| 59. | G. barbadense L. | Malvaceae | Egyptian cotton | Seed | Common cold | [49] | |
| 60. | Guiera senegalensis J.F.Gmel. | Combretaceae | Leaf | Enteric problems, Worms | PV, AV, HSV 1, Equine HSV | [59] | |
| 61. | Hoslundia opposita Vahl | Lamiaceae | Leaf | Measles, chickenpox, varicella | [76] | ||
| 62. | Hymenostegia afzelii (Oliv.) Harms | Leguminosae | Fruit | Mumps | [64, 77] | ||
| 63. | H. fuscum Pers. Fr. | Xylariaceae | Hazel woodwart | Whole mushroom | EV 7, 19 | [78] | |
| 64. | Hyptis pectinata (L.) Poit. | Lamiaceae | Mint weed | Leaf | Poliomyelitis | [52] | |
| 65. | Ipomoea asarifolia (Desr.) Roem. & Schult. | Convolvulaceae | Ginger-leaf morning-glory | Leaf | Skin infections, abdominal cramps, diarrhoea | EV 7 | [54] |
| 66. | Jatropha tanjorensis J.L. Ellis & Saroja | Euphorbiaceae | Catholic vegetable | Leaf | Fever | HIV | [79] |
| 67. | Khaya ivorensis A.Chev. | Meliaceae | African Mahogany | Bark | Jaundice | [52] | |
| 68. | K. senegalensis (Desv.) A.Juss. | Meliaceae | Khaya wood | Bark | Helminths | PV, AV, HSV 1, Equine HSV | [59] |
| 69. | Kigelia africana(Lam.) Benth. | Bignoniaceae | Sausage tree | Bark | Poliomyelitis | [49] | |
| 70. | Lactuca taraxacifolia Schumach. & Thonn. | Compositae | African Lettuce | Leaf | Sores, measles, chickenpox, varicella | MV | [57, 80] |
| 71. | L. virosa Habl. | Compositae | Wild lettuce | Bark | Poliomyelitis | [52] | |
| 72. | Lagenaria breviflora (Benth.) Roberty | Cucurbitaceae | Wild colocynth | Fruit, whole plant | Measles | NDV | [52, 66, 81] |
| 73. | Lannea humilis (Oliv.) Engl. | Anacardiaceae | Bark | Diarrhoea, fever | PV, AV, HSV 1, Equine HSV | [59] | |
| 74. | Lawsonia inermis L. | Lythraceae | Henna tree | Leaf | Poliomyelitis, measles | [52] | |
| 75. | Lippia multiflora Moldenke | Verbenaceae | Bush tree | Leaf | Fever, ear and eye infections | EV 7, PV | [82] |
| 76. | Loranthus micranthus Hook. f. | Loranthaceae | Green mistletoe | Leaf | Diarrhoea, diabetes, and microbial invasions | RSV | [83] |
| 77. | Macaranga barteri Mull. Arg. | Euphorbiaceae | Macaranga plant | Leaf | Gonorrhoea, syphilis, skin infections | EV 7, 19 | [54, 84] |
| 78. | Mangifera indica L. | Anacardiaceae | Mango | Bark | Jaundice | [52] | |
| 79. | Mimosa pigra L. | Leguminosae | Giant sensitive plant | Leaf | Poliomyelitis | [52] | |
| 80. | Mitracarpus hirtus (L.) DC. | Rubiaceae | White eye | Leaf | Skin diseases | HSV, PV | [46, 47] |
| 81. | Momordica balsamina L. | Cucurbitaceae | Balsam apple | Fruit Leaf | Measles, Yellow fever, skin disease | NDV, HIV | [51, 52, 85] |
| 82. | Mondia whitei (Hook.f.) Skeels | Apocynaceae | White Ginger | Leaf | Malaria | EV 7, 19 | [54] |
| 83. | Morinda lucida Benth. | Rubiaceae | Brimstone tree | Roots | Yellow fever | [52] | |
| 84. | M. oleifera Lam. | Moringaceae | Moringa | Seed | Hepatitis | NDV | [85, 86] |
| 85. | Musa x paradisiaca L. | Musaceae | Plantain | Leaf | Smallpox | [64] | |
| 86. | Newbouldia laevis (P.Beauv.) Seem. | Bignoniaceae | Boundary tree | Measles | [51] | ||
| 87. | Nicotiana tabacum L. | Solanaceae | Tobacco | Leaf | Common cold, Poliomyelitis | [52, 64] | |
| 88. | Olax subscorpioides Oliv. | Olacaceae | Stink ant forest | Roots | Poliomyelitis | [52] | |
| 89. | Palisota hirsuta (Thunb.) K. Schum. | Commelinaceae | Leaf | Diarrhoea, skin disease | HSV, PV | [46, 47] | |
| 90. | Parkia biglobosa (Jacq.) G. Don | Leguminosae | African Locust Bean | Bark | Chickenpox, measles | [52] | |
| 91. | Paullinia pinnata L. | Sapindaceae | Supple jack | Whole Plant | Diarrhoea | HSV | [46, 47] |
| 92. | Peperomia pellucida (L.) Kunth | Piperaceae | Pepper elder | Leaf, whole plant | Mumps, herpes simplex, measles | [51, 64, 77] | |
| 93. | Persea americana Mill. | Lauraceae | Avocado | Leaf | Poliomyelitis, hepatitis | [49] | |
| 94. | Phyllanthus amarus Schumach. & Thonn. | Phyllanthaceae | Sleeping plant | Leaf | Hepatitis, shingles | NDV | [87, 88] |
| 95. | Piper guineense Schumach. & Thonn. | Piperaceae | West African Pepper | Seed | Measles,chickenpox | [52], [ [51, 53] | |
| 96. | Plumbago zeylanica L. | Plumbaginaceae | Ceylon leadwort | Seed | Smallpox | [49] | |
| 97. | Psidium guajava L. | Myrtaceae | Common guava | Leaf, bark | Gastrointestinal disorders, jaundice | NDV | [52, [89] |
| 98. | Pycnanthus angolensis (Welw.) Warb. | Myristicaceae | African nutmeg | Roots | Chickenpox | [52] | |
| 99. | R. farinacea (L.) Ach. | Ramalinaceae | Whole (Lichen) | HIV-1, Adenovirus, RSV | [90–92] | ||
| 100. | Raphia hookeri G. Mann & H. Wendl. | Arecaceae | Ivory Coast raffia palm | Latex | Measles | [51] | |
| 101. | Sarcocephalus latifolius (Sm.) E. A. Bruce | Rubiaceae | African peach | Root | Jaundice, fever, diarrhoea, dysentery | RSV, NDV | [52, 93] |
| 102. | Securidaca longipedunculata Fresen. | Polygalaceae | Violet tree | Seed | Smallpox | [49] | |
| 103. | Senna occidentalis (L.) Link | Leguminosae | Coffee weed | Leaf | Measles | [52, 51] | |
| 104. | S. siamea (Lam.) H. S. Irwin & Barneby | Leguminosae | Cassia tree | Bark | PV | [82] | |
| 105. | S. singueana (Delile) Lock | Leguminosae | Wild cassia | Leaf | Fever, worms | PV, AV, BPV, CPV | [59] |
| 106. | Sida acuta Burm. f. | Malvaceae | Broom weed | Leaf | Yellow fever | HSV | [46, 64] |
| 107. | Solanum torvum Sw. | Sapotaceae | Prickly solanum | Leaf | Yellow fever | [49] | |
| 108. | Sphenocentrum jollyanum Pierre | Menispermaceae | Morning seed | Leaf Root | Fever, hepatitis | PV Type 2 | [94, 95] |
| 109. | Spondias mombin L. | Anacardiaceae | Hog plum | Bark | Stomach ache, abdominal discomfort chickenpox, jaundice | EV 7 | [52, 54] |
| 110. | Sterculia setigera Delile | Malvaceae | Karaya gum tree | Bark | STIs, fever | PV, AV, HSV 1, Equine HSV, BPV and CPV | [59] |
| 111. | Symphonia globulifera L.f | Clusiaceae | Boarwood | Root | Poliomyelitis | [52] | |
| 112. | Terminalia ivorensis A. Chev. | Combretaceae | Ivory Coast almond | Bark | Syphilis, burns, bruises, arthritis and haemorrhoids | EV 7 | [54] |
| 113. | T. superba Engl. & Diels | Combretaceae | Shingle wood | Bark | Yellow fever | [52] | |
| 114. | Tetracera alnifolia Willd. | Dilleniaceae | Ware vine | Leaf | Leprosy, cough | EV 7 | [54] |
| 115. | T. potatoria Afzel. ex G.Don | Dilleniaceae | Water tree | Bark | Jaundice | [52] | |
| 116. | Uvaria chamae P. Beauv. | Annonaceae | Finger root | Leaf, Bark | Fever, hepatitis | MV | [65, 96] |
| 117. | Vernonia amygdalina Delile | Compositae | Bitter leaf | Leaf | Common cold, Measles, jaundice | VSV, PV, HSV | [51, 52, 77, 97] |
| 118. | Vitellaria paradoxa C. F. Gaertn. | Sapotaceae | Shea tree | Fruits, Bark | Measles, Fever, dressing, Boils | PV, AV | [51, 59] |
| 119. | Vitex grandifolia Gurke | Lamiaceae | Black plum | Leaf | Herpes simplex | [64, 77] | |
| 120. | Xylopia aethiopica (Dunal) A. Rich. | Annonaceae | Guinea pepper | Leaf, Bark, Fruit | Chickenpox, measles | MV | [52, 65] |
| 121. | Zea mays L. | Poaceae | Maize | Flower | Chickenpox | [52] | |
| 122. | Zephyranthes candida (Lindl.) Herb. | Amaryllidaceae | White windflower | PV | [82] | ||
| 123. | Zingiber officinale Roscoe | Zingiberaceae | Ginger | Rhizome | Yellow fever | [52] | |
| 124. | Ziziphus mucronata Willd. | Rhamnaceae | Buffalo thorn | Leaf | Enteric conditions | PV, AV | [59] |
Table is an alphabetical list of plants employed as antivirals in traditional West African medicine (numbers 63 and 99 are not plants but a fungus and a lichen, respectively, but they were added for some context). The plant names, families, common names, part(s) employed as medicines, traditional indications, and viruses they are investigated for efficacy against are described
AV is Astrovirus, BPV is Bovine Parvovirus, CPV is Canine Parvovirus, CV is Coxsackie Virus, DV is Dengue Virus, EV is Echovirus, FPV is Fowlpox Virus, HCV is Hepatitis C Virus, HIV is Human immunodeficiency Virus, HSV is Herpes Simplex Virus, MV is Measles Virus, NDV is Newcastle Disease Virus, PV is Polio Virus, RSV is Respiratory Syncytial Virus, VSV is Vesicular Stomatitis Virus, YFV is Yellow Fever Virus
Over the years, the study of the therapeutic potentials of medicinal plants has not been consistently adequate, with only a small fraction of all flowering plant species in the world exhaustively studied for their potential pharmacological activity [9, 14, 45]. Consistent with this, in our review, only sixty-five (65/124; 52%) of the documented natural antiviral remedies have been scientifically evaluated for acclaimed therapeutic efficacies. Researchers have investigated the possible antiviral effects of these plants against RSV, Echoviruses, Measles Virus (Measles morbillivirus), HSV, HIV, Coxsackievirus and Dengue Virus. Others have also investigated the use of these plants against animal viruses such as Newcastle Disease Virus, Bovine and Canine Parvovirus, as well as Equine Herpesvirus.
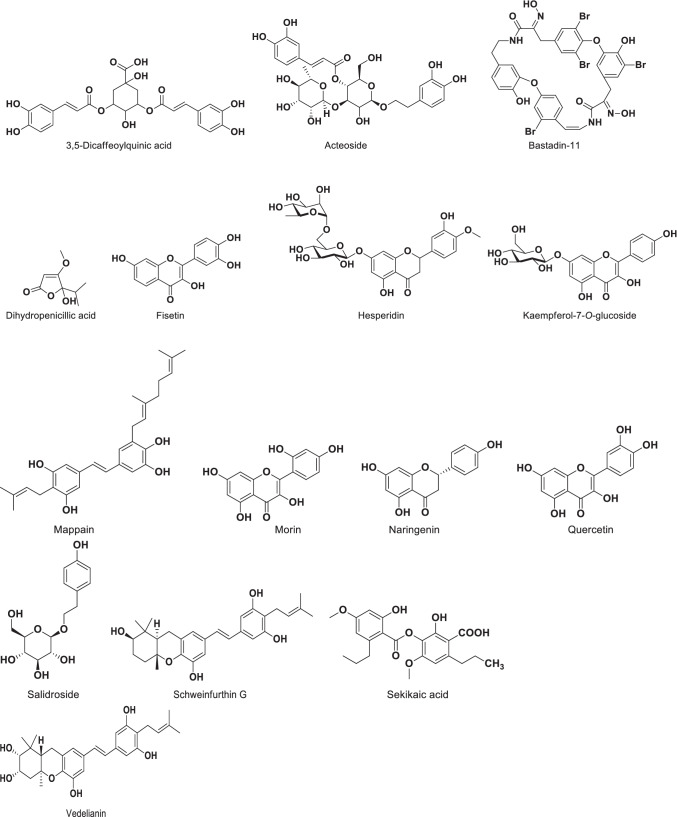
Of the 65 plants scientifically investigated, just four had their constituent phytochemicals potentially responsible for the observed activities isolated and identified, as shown in Table 2. The phytochemicals include the flavonoids quercetin, morin, fisetin, naringenin and hesperidin from Citrus aurantifolia and C. paradisi; alkaloids from Cucumis metuliferus; salidroside (2-(4-hydroxyphenyl)ethyl β-D-glucopyranoside) from Loranthus micranthus; flavonoids (3,5-dicaffeoylquinic acid, acteoside, kaempferol 7-O-glucoside, bastadin-11) and stilbenes (vedelianin, schweinfurthin G, mappain) from Macaranga barteri. In addition, dihydropenicillic acid was isolated as the active antiviral component of the mushroom H. fuscum, while sekikaic acid and other phenolic compounds were obtained from the lichen R. farinacea. The chemical structures of the compounds are shown in Fig. 2. We recommend that the drug targets mediating the antiviral activities of the remedies and isolated compounds should be investigated, using existing knowledge of the different potential antiviral drug targets as shown in Fig. 3.
Table 2.
Compounds with antiviral activity which were isolated from antiviral West African Natural Products
| S/N | Natural Product | Constituent Antiviral Compounds | Antiviral Activity | References | |
|---|---|---|---|---|---|
| 1 | H. fuscum |
Dihydropenicillic acid |
Extract IC50 – EV7: 0.3811 µg/ml; EV19: 1.575 µg/ml | [78] | |
| 2 | M. barteri |
Flavonoids: 3,5-dicaffeoylquinic acid, acteoside, kaempferol-7- O-glucoside and bastadin-11 Stilbenes: vedelianin, schweinfurthin G and mappain |
Mappain IC50 – EV7: 1.23 µM; EV19: 0.24 µM Vedelianin IC50 – EV7: 0.025 nM; EV19: 0.0036 nM Schweinfurthin G IC50 – EV7: 0.043 nM; EV19: 0.018 nM |
[54, 84] | |
| 3 | C. aurantifolia | Flavonoids: quercetin, motin, fisetin, naringenin, hesperidin | [64, 67] | ||
| C. paradisi | Flavonoids: quercetin, motin, fisetin, naringenin, hesperidin | [64, 67] | |||
| 4 | L. micranthus | Salidroside (2-(4-hydroxyphenyl) ethyl-β-D-glucopyranoside) | Salidroside IC50—RSV: 10.3 ± 1.50 μg/ml | [83] | |
| 5 | R. farinacea | Sekikaic acid (and other phenolic compounds) |
Ethyl acetate-soluble fraction (ET4) IC50 – HSV-1: 6.09 μg/ml; RSV: 3.65 μg/ml; HIV-1: 0.33 μg/ml; HIV-1 RT 0.022 μg/ml Sekikaic acid IC50 Recombinant RSV: 5.69 µg/ml; RSV A2: 7.73 µg/ml |
[91, 92] |
Table lists antiviral compounds isolated from West African plants following investigations into their antiviral activity
EV7 is Echovirus 7, EV19 is Echovirus 19, HIV is Human Immunodeficiency Virus, HIV RT is Human Immunodeficiency Virus Reverse Transcriptase, HSV is Herpes Simplex Virus, RSV is Respiratory Syncytial Virus
Fig. 2.
Chemical structures of compounds isolated from West African plants and reported to have antiviral activity
Fig. 3.
Antiviral drug targets that could mediate the antiviral effects of natural products. Figure reproduced with permission [98]
Potential of West African plants with antiviral activity as sources of drugs or herbal formulations to combat coronaviruses, including the current COVID-19 pandemic
While to date there are no direct ethnobotanical or other scientific reports from West Africa on the use of the plants listed in Table 1 against MERS-CoV, SARS-CoV or indeed SARS-CoV-2, there are numerous reports from elsewhere that suggest that natural products and traditional medicines may play a role in the fight against the current pandemic [99]. This work, therefore, highlights the potential of these plants to aid current and future drug discovery efforts aimed at identifying chemical leads for the development of anti-COVID-19 therapeutics, as well as the potential for developing the plants in the most easily acceptable forms as phytomedicines for the developing nations from where the plants originate. In this regard, it is important to note that, while the development of effective vaccines for the prevention of SARS-CoV-2 infection is considered a top priority in current thinking, the development of effective, anti-COVID-19 small-molecule drugs and phytomedicines should also continue to be prioritised, as any effective vaccines will have their limitations and contraindications, such that the need will always be there not only to prevent SARS-CoV-2 infection but also to treat those already infected or those who, for some reasons, are unable to access or be administered the vaccines. In a similar vein, with traditional medicines using natural products such as medicinal plants being part of the health care systems in some countries, the process of encouraging all hands globally to be on deck in tackling SARS-CoV-2/COVID-19 should include a clear recognition of the potential for such natural products to be part of the anti-COVID-19 armamentarium.
In some countries such as China and India in Asia and Mozambique in Africa, traditional medical remedies are officially recognised and integrated into the response to COVID-19. China’s response includes Traditional Chinese Medicine regimens such as the Lung Cleansing and Detoxifying Decoction (LCCD), which is widely used and approved by local authorities [99]. The decoction, amongst other things, contains Dioscorea polystachya, Citrus aurantium and Citrus peel. Both Dioscorea and Citrus species are mentioned in Table 1. An extract prepared from Dioscorea spp. patented in the USA (patent no. 20090041803) in 2008 was mentioned as potent against a host of viruses, including HSV-1, MV, RSV and SARS-CoV [28]. In a recent study, it was reported that many patients infected with COVID-19 in several African countries recovered from the infection using therapies made from herbal remedies which usually included garlic, ginger, lemon, turmeric, honey and neem (A. indica) leaves [100]. These reported therapeutic effects of those remedies are consistent with current evidence; for example, garlic is known to have antiviral properties [101]. The World Health Organization has approved a protocol for African herbal medicines to undergo clinical trials as potential treatments for COVID-19 and other epidemics and has also endorsed a charter and terms of reference to establish a data and safety monitoring board for the trials [102]. There is a recognition now that "the onset of COVID-19, like the Ebola outbreak in West Africa, has highlighted the need for strengthened health systems and accelerated research and development programmes, including on traditional medicines” [103].
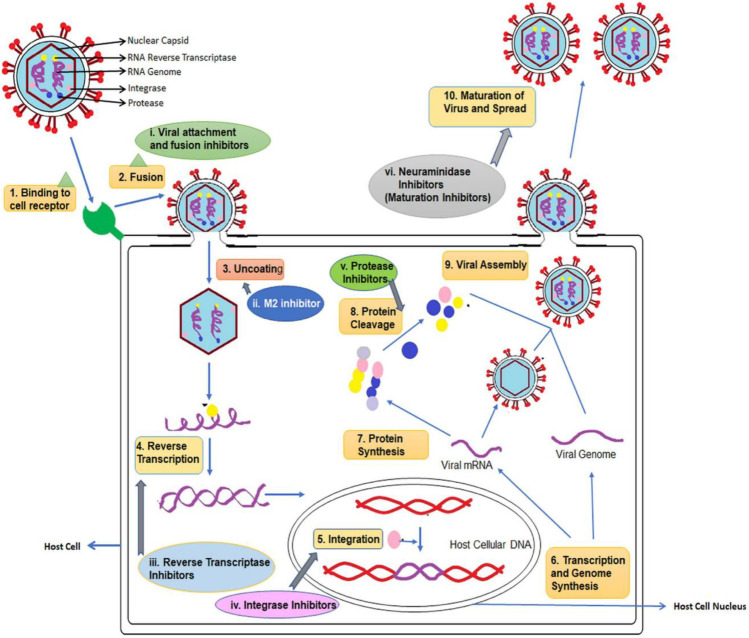
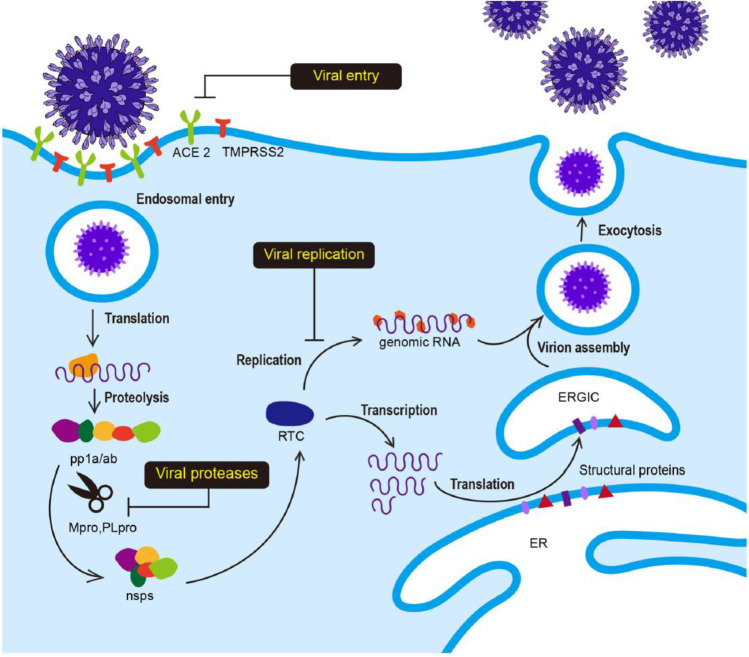
In the development of small-molecule therapeutics against SARS-CoV-2 (which causes COVID-19), many approaches have been identified, based on molecular targets linked to SARS-CoV-2 entry, replication and spike protein priming (see Fig. 4 for the life cycle of, and potential drug targets in, SARS-CoV-2). These approaches include binding to the viral 3-chymotrypsin-like cysteine protease 3CLpro (Mpro) enzyme that controls coronavirus replication and is essential for its life cycle [103]; inhibition of Angiotensin-Converting Enzyme 2 (ACE2), a host entry receptor for SARS-CoV-2; and inhibition of Transmembrane Protease, Serine 2 (TMPRSS2), a host serine protease that SARS-CoV-2 uses for its spike (S) protein priming [104] (Fig. 4). Interestingly, some natural compounds have been shown to possess efficacy against some of the targets [103]. Quercetin from Citrus fruits has been shown to have a high binding affinity for the SARS-CoV main proteinase (Mpro or 3CLpro) [105]. Hesperetin, an aglycone derivative of hesperidin and a naturally occurring flavanone-glycoside, the main flavonoid in lemons and sweet oranges, showed a concentration-dependent inhibitory effect on cleavage activity of 3CLpro in cell-free (IC50 60 µM) and cell-based (IC50 8.3 µM) assays [106]. Hesperetin also showed significant ACE2 inhibition activity [107]. Both SARS-CoV and SARS-CoV-2 engage the receptor ACE2 for cell entry [104], thus suggesting possible anti-SARS-CoV-2 activity of hesperetin. Also, hesperetin, when used with chloroquine, had shown positive antiviral activity in vitro [108]. Other citrus flavonoids in lemon and orange peel, such as nobiletin, tangeretin and naringenin, have shown good affinities for SARS-CoV 3CLpro and its receptors in molecular docking studies [105, 109, 110]. Naringenin was described in an earlier section as one of the compounds isolated from some West African Citrus plants and reported to have antiviral activity. Its mechanisms of anti-COVID-19 action, including directly targeting the virus as well as targeting the associated inflammation, are shown in Fig. 5.
Fig. 4.
The life cycle of SARS-CoV-2 infection. The Angiotensin-Converting Enzyme 2 (ACE2) is a host entry receptor for viral entry, while Transmembrane Protease, Serine 2 (TMPRSS2) is a host serine protease that the virus uses to prime its spike (S) protein. The viral 3-chymotrypsin-like cysteine protease 3CLpro (Mpro) controls coronavirus replication. ER is Endoplasmic Reticulum, RTC is Replicase-Transcriptase Complex and ERGIC ER-Golgi Intermediate Compartment. Figure reproduced with permission [111]
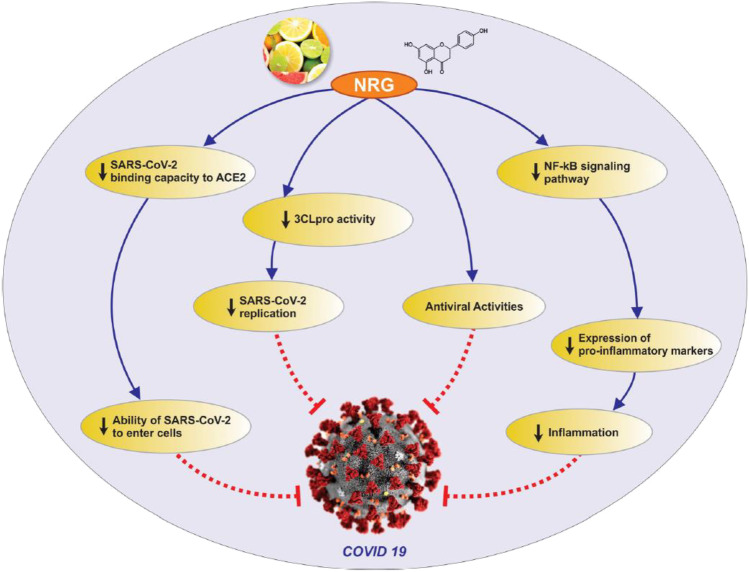
Fig. 5.
Antiviral and anti-inflammatory targets of the anti-COVID-19 activity of the natural compound naringenin. Naringenin targets the virus (SARS-CoV-2), as well as the inflammation associated with the infection (naringenin was one of the compounds isolated from some West African Citrus plants and reported to have antiviral activity). Figure reproduced with permission [112]
In a separate assay to evaluate its inhibitory effect on ACE2, C. aurantium showed 100% activity, while A. sativum (Garlic) showed just above 70% activity [113]. A. sativum extract has been shown to possess activity against Infectious Bronchitis Virus (IBV), a coronavirus in poultry [114]. In a study that evaluated the activities of plant lectins against SARS-CoV using Vero and CrFK cells, A. sativum lectin was not active, but A. porrum (Leek) agglutinin was effective [115]. Alliin, a sulfoxide that is a natural constituent in fresh garlic, is a good inhibitor of SARS-CoV-2 Mpro as suggested by results of a molecular docking study [116].
Essential oil from Lemongrass (C. citratus) has been shown to exhibit anti-influenza activities [117]. Berberine, an alkaloid from B. vulgaris, has been found to significantly reduce RSV replication by reducing the synthesis of mRNA and viral proteins [118, 119]. Lactucopicrin-15-oxalate (from L. virosa, previously documented for antioxidant and antimalarial properties), biorobin (from Ficus spp.), and phyllaemblicin B (from Phyllantus spp.) were shown in in silico studies to have a high affinity for SARS-CoV-2 Mpro, RNA-dependent RNA polymerase (RdRP) and human ACE2 [120].
Other studies have also reported the possible anti-coronavirus Mpro activities of rutin from A. indica, T. chebula and O. basilicum; amentoflavone from M. indica and G. kola; agathisflavone (a biflavonoid) from A. occidentale; rubusic acid from S. nigrum; chlorogenin from S. torvum; lupeol from C. papaya and A. indica and cyanin from Z. officinale [37, 121–123]. Nallusamy and colleagues [121] also showed that agathisflavone, corilagin (from Terminalia spp.) and cyanin have high binding affinities for the RdRP responsible for the replication of SARS-CoV-2.
A. indica has been widely considered to be of value against COVID-19 in Indian Traditional Medicine (Ayurvedic Medicine), where it is used to treat fever, cough, asthma and diarrhoea, which are associated symptoms of COVID-19. In an in vivo assay, it showed significant inhibitory activity against viral entry in mouse hepatitis virus (MHV) – a β-coronavirus—without adverse effects to the mice [124]. Nimocin, phytosterol, β-amyrin, nimbolin A are examples of phytoconstituents from A. indica with significant binding affinity and interaction with M protease of SARS-CoV-2 [125]. Another study showed that meliacinanhydride and other compounds such as nimocinol, isomeldenin, nimbolide and nimbin may be potential treatment options against COVID-19 [126]. Maurya et al. [127] also reported significant binding affinity of nimbin, piperine (from P. guineense), mangiferin (from M. indica) and berberine (from Bambusa vulgaris) for the spike glycoprotein of SARS-CoV-2, suggesting them as therapeutic or prophylactic options due to their inhibiting viral attachment.
N-acetyl glucosamine-specific agglutinins in N. tabacum showed positive results against SARS-CoV, with an effective concentration (EC50) of 1.7 ± 0.3 µg/ml and a cytotoxic concentration (CC50) > 100 µg/ml [115]. SARS-CoV has 23 putative N-glycosylation sites [128], and SARS-CoV-2 has been shown to have extensively glycosylated Spike protein on its surface [129]. Other studies have also recommended the use of N. tabacum as an oral vaccine (viral S or N antigen) [130, 131]. Of the 22 triterpenoids isolated from E. neriifolia, the frieldelane derivatives 3β-friedelanol, 3β-acetoxyfriedelane, friedelin and epitaraxerol showed significant anti-CoV activity in silico [132].
It is useful to remark that, concerning the development of phytomedicines, especially from medicinal food plants that have been used safely for hundreds of years, compounds that have been isolated from such plants and which show antiviral activity could be used as markers for quality assurance of the phytomedicines developed from them. Such products might not need to undergo the entire range of rigorous toxicity studies as are usually undertaken for isolated compounds, which when tested as single entities have been known to elicit toxicity not observed in the extract or the plant (containing them) when taken as such.
Indirect anti-CoV activities of medicinal plants (anti-inflammatory and immunomodulatory effects)
Inflammation is now recognised as a critical mechanism in the pathophysiology of COVID-19. A sizeable number of COVID-19 patients develop cytokine storm, a severe hyper-immune response that leads to organ damage in some of those patients [133]. The use of some anti-inflammatory agents has recorded some degree of success in the management of the infection [134]. Some reports on the anti-CoV or anti-COVID-19 potentials of the plants detailed in Table 1 point to their significant immunomodulatory activities as a basis for such suggestions. Examples include:
The hemicellulose fraction of A. floribunda, due to its significant antioxidant and immunomodulatory activities, especially its effect on Interferon-gamma (IFN-γ) production and Peripheral blood mononuclear cells (PBMC) [135].
A garlic plus honey mixture may enhance the immune system due to the presence of sulphur-containing proteins and polyphenols [101, 136, 137].
M. indica bark has shown possible immunomodulatory properties [138].
P. guineense, C. papaya, Z. officinale and Citrus fruits all possess immunomodulatory properties [119].
Ginger (Z. officinale), banana (M. paradisiaca) and Solanum muricatum are all suggested to develop the immunity of individuals against COVID-19 [139].
A. indica possesses significant anti-inflammatory and potent immunostimulant activity [140].
C. fistula is recommended in Unani Medicine for the preservation of health during epidemics because of its immunomodulatory and antioxidant properties [141].
Naringin from citrus peel inhibits the expression of pro-inflammatory mediators COX-2, i-NOS, IL-1β and IL-6 in lipopolysaccharide (LPS)-induced RAW macrophages [142].
Documented evidence shows that naringenin, the aglycone of naringin, might exert therapeutic effects against coronaviruses through the inhibition of 3CLpro and reduction of ACE receptor activity. However, it might also exert a therapeutic effect against COVID-19 by attenuating inflammatory responses [143]. See Fig. 5.
There have been calls for accelerated production of hesperidin-rich citrus pectin from citrus peels, as they possess immunomodulatory activity in addition to activity against 3CLpro and ACE2 [144].
Dioscorea plants have also shown immunomodulatory properties. Dioscorin, a tuber protein, possesses systemic and mucosal immunomodulatory activities [145]. It induces macrophage activation via stimulation of signalling molecules (ERK, JNK, NF-κB) and induction of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) [146, 147].
Guava (P. guajava) leaf, mango (M. indica) stem bark and leaf, lemongrass (C. citratus) leaf, ginger (Z. officinale) rhizome, garlic (A. sativum) bulb and cinnamon (Cinnamomum zeylanicum) stem bark are immune-boosting herbs that are used in powdered form or as a decoction for oral administration [66].
Conclusions and recommendations
The discovery and development of anti-coronavirus drugs, or specifically anti-COVID-19 drugs, including those from natural resources such as medicinal plants, will play a vital role in combatting the scourge of the current and future pandemics. Anecdotal knowledge is emerging of the successful use of certain medicinal food plants to manage symptoms of COVID-19. These natural resources and the knowledge of their therapeutic usefulness and promise abound in developing countries where, in contrast, the prohibitive cost of research on the development of synthetic drugs is generally unaffordable and technological facilities are lacking [148]. Considering this reality, alongside the added challenges posed by fragile and under-resourced health care management systems in many of those countries, the use of more affordable and more accessible herbal or other naturally-derived medicines to manage disease conditions, not least of which is the currently ravaging COVID-19, is undoubtedly an attractive alternative [149]. In line with this claim, the WHO also actively encourages these countries to develop and integrate traditional and alternative medicines into their health systems [150], as means to cope with their significant health care burden [151]. It is quite reassuring to note that, in many African countries, some phytomedicines to address serious disease conditions have now been well researched, packaged and produced, and some other phytomedicines are currently undergoing clinical trials, with yet some others in the pipeline. However, these research and development (R & D) efforts need to be further supported and expanded, including through substantial funding, both at the pre-clinical research level (high-throughput screening (phenotypic and target-based), phytochemical analysis, standardisation and quality control of herbs, dosage forms design, etc.), and clinical research level (involving clinical trials) [148].
This review briefly chronicles evidence demonstrating the rich diversity and potentials of medicinal plants in traditional medicine practice in West Africa for the treatment of viral infections. There is now an imperative to investigate, through coordinated approaches, these plants and their constituents for antiviral efficacy and safety. Collaborative, interdisciplinary studies involving scientists and indigenous people with authentic herbal medicine knowledge should be facilitated to promote antiviral drug discovery and identify herbal remedies and/or natural compounds that could be efficacious in preventing, treating, and managing symptoms of COVID-19 or other existing, emerging or future coronavirus diseases. Such cohesive research efforts ranging from the bench to the bedside could even furnish additional insights into disease mechanisms and therapeutics development beyond the antiviral domains of research and which encompass solutions to other areas of unmet clinical need.
Acknowledgements
AAF is grateful to Liverpool John Moores University (LJMU) for the award of a competitive Global Challenges Research Fund (GCRF) Grant to undertake this work. LN gratefully acknowledges the financial support of the European Regional Development Fund–Project ENOCH (No. CZ.02.1.01/0.0/0.0/16_019/0000868).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.HS Kumar A. Molecular docking of natural compounds from Tulsi (Ocimum sanctum) and neem (Azadirachta indica) against SARS-CoV-2 protein targets. BEMS Reports. 2021;6(1):11–13.
- 2.Wang S-x, Wang Y, Lu Y-b, Li J-y, Song Y-j, Nyamgerelt M, Wang X-x. Diagnosis and treatment of novel coronavirus pneumonia based on the theory of traditional Chinese medicine. J Integr Med. 2020;18(4):275–83. [DOI] [PMC free article] [PubMed]
- 3.World Health Organisation. WHO Coronavirus Disease (COVID-19) Dashboard. 2020 09, August. Available from: https://covid19.who.int/.
- 4.Anandan R, Suseendran G, Zaman N, Brohi SN. Echinacea purpurea to treat Novel Coronavirus (2019-nCoV). 2020. 10.36227/techrxiv.12241223.v1
- 5.Elfiky AA. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020; 248:117477. [DOI] [PMC free article] [PubMed]
- 6.O'Hare B. Weak health systems and Ebola. Lancet Glob Health. 2015;3(2):e71–e72. doi: 10.1016/S2214-109X(14)70369-9. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. A heavy burden: the productivity cost of illness in Africa. 2019. Brazzaville: WHO Regional Office for Africa; 2019. Licence: CC BY-NC-SA 3.0 IGO. https://www.afro.who.int/publications/heavy-burden-productivity-cost-illness-africa
- 8.Bougrine H, Rochon L-P. Austerity, unemployment and poverty in developing countries. In. Aggregate Demand and Employment: Edward Elgar Publishing; 2020. [Google Scholar]
- 9.Okello J, Ssegawa P. Medicinal plants used by communities of Ngai Subcounty, Apac District, northern Uganda. Afr J Ecol. 2007;45:76–83. doi: 10.1111/j.1365-2028.2007.00742.x. [DOI] [Google Scholar]
- 10.Simoben CV, Ntie-Kang F, Lifongo LL, Babiaka SB, Sippl W, Mbaze LM. The uniqueness and therapeutic value of natural products from West African medicinal plants, part III: least abundant compound classes. RSC Adv. 2014;4(75):40095–40110. doi: 10.1039/C4RA05376A. [DOI] [Google Scholar]
- 11.Oreagba IA, Oshikoya KA, Amachree M. Herbal medicine use among urban residents in Lagos, Nigeria. BMC Complement Altern Med. 2011;11(1):117. doi: 10.1186/1472-6882-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naithani R, Mehta RG, Shukla D, Chandersekera SN, Moriarty RM. Antiviral activity of phytochemicals: a current perspective. In. Dietary Components and Immune Function: Springer; 2010. p. 421–468.
- 13.Niharika A, Aquicio JM, Anand A. Antifungal properties of neem (Azadirachta indica) leaves extract to treat hair dandruff. E-ISRJ. 2010;2:244–252. [Google Scholar]
- 14.Farnsworth NR. Screening plants for new medicines. Biodiversity. 1988;15(3):81–99. [Google Scholar]
- 15.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 16.Popoola TD, Awodele O, Omisanya A, Obi N, Umezinwa C, Fatokun AA. Three indigenous plants used in anti-cancer remedies, Garcinia kola Heckel (stem bark), Uvaria chamae P. Beauv. (root) and Olax subscorpioidea Oliv. (root) show analgesic and anti-inflammatory activities in animal models. J Ethnopharmacol. 2016;194:440–449. doi: 10.1016/j.jep.2016.09.046. [DOI] [PubMed] [Google Scholar]
- 17.Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochim Biophy Acta (BBA) Gen Subj. 2013;1830(6):3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butler MS. The role of natural product chemistry in drug discovery. J Nat Prod. 2004;67(12):2141–2153. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- 19.Flavin MT, Rizzo JD, Khilevich A, Kucherenko A, Sheinkman AK, Vilaychack V, Lin L, Chen W, Greenwood EM, Pengsuparp T. Synthesis, chromatographic resolution, and anti-human immunodeficiency virus activity of (±)-calanolide A and its enantiomers. J Med Chem. 1996;39(6):1303–1313. doi: 10.1021/jm950797i. [DOI] [PubMed] [Google Scholar]
- 20.Wilson EO. What is nature worth? The Wilson Quarterly (1976-). 2002;26(1):20–39.
- 21.Nahar L, Talukdar AD, Nath D, Nath S, Mehan A, Ismail FMD, Sarker SD. Naturally Occurring Calanolides: Occurrence, Biosynthesis, and Pharmacological Properties Including Therapeutic Potential. Molecules. 2020;25(21):4983. doi: 10.3390/molecules25214983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitby K, Taylor D, Patel D, Ahmed P, Tyms AS. Action of celgosivir (6 O-butanoyl castanospermine) against the pestivirus BVDV: implications for the treatment of hepatitis C. Antiviral Chem Chemother. 2004;15(3):141–151. doi: 10.1177/095632020401500304. [DOI] [PubMed] [Google Scholar]
- 23.Butler MS, Robertson AA, Cooper MA. Natural product and natural product derived drugs in clinical trials. Nat Prod Rep. 2014;31(11):1612–1661. doi: 10.1039/C4NP00064A. [DOI] [PubMed] [Google Scholar]
- 24.Softic L, Brillet R, Berry F, Ahnou N, Nevers Q, Morin-Dewaele M, Hamadat S, Bruscella P, Fourati S, Pawlotsky J-M, Ahmed-Belkacem A. Inhibition of SARS-CoV-2 Infection by the Cyclophilin Inhibitor Alisporivir (Debio 025) Antimicrob Agents Chemother. 2020;64(7):e00876–e1820. doi: 10.1128/AAC.00876-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elion GB, Furman PA, Fyfe JA, De Miranda P, Beauchamp L, Schaeffer HJ. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci. 1977;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwitz JP, Chua J, Noel M, Nucleosides V. The Monomesylates of 1-(2'-Deoxy-β-D-lyxofuranosyl) thymine1, 2. J Org Chem. 1964;29(7):2076–2078. doi: 10.1021/jo01030a546. [DOI] [Google Scholar]
- 27.Boyd M, Gustafson K, McMahon J, Shoemaker R. Discovery of cyanovirin-N, a novel HIV-inactivating protein from Nostoc ellipsosporum that targets viral gp120. In: Int. Conf. AIDS; 1996. p. 71.
- 28.Ganjhu RK, Mudgal PP, Maity H, Dowarha D, Devadiga S, Nag S, Arunkumar G. Herbal plants and plant preparations as remedial approach for viral diseases. Virusdisease. 2015;26(4):225–236. doi: 10.1007/s13337-015-0276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Likhitwitayawuid K, Chaiwiriya S, Sritularak B, Lipipun V. Antiherpetic flavones from the heartwood of Artocarpus gomezianus. Chem Biodivers. 2006;3(10):1138–1143. doi: 10.1002/cbdv.200690115. [DOI] [PubMed] [Google Scholar]
- 30.Prendergast PT. Use of cirsiliol and derivatives to treat infections. In: Google Patents; 2003. https://patents.google.com/patent/US6555523B1/en
- 31.Naithani R, Huma LC, Holland LE, Shukla D, McCormick DL, Mehta RG, Moriarty RM. Antiviral activity of phytochemicals: a comprehensive review. Mini Rev Med Chem. 2008;8(11):1106–1133. doi: 10.2174/138955708785909943. [DOI] [PubMed] [Google Scholar]
- 32.Warowicka A, Nawrot R, Goździcka-Józefiak A. Antiviral activity of berberine. Arch Virol. 2020;165(9):1935–1945. doi: 10.1007/s00705-020-04706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farnsworth N, Svoboda G, Blomster R. Antiviral activity of selected Catharanthus alkaloids. J Pharm Sci. 1968;57(12):2174–2175. doi: 10.1002/jps.2600571235. [DOI] [PubMed] [Google Scholar]
- 34.Orhan IE, Senol Deniz FS. Natural Products as Potential Leads Against Coronaviruses: Could They be Encouraging Structural Models Against SARS-CoV-2?. Nat Prod Bioprospecting. 2020;10(4):171–186. doi: 10.1007/s13659-020-00250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohammadi Pour P, Fakhri S, Asgary S, Farzaei MH, Echeverría J. The Signaling Pathways, and Therapeutic Targets of Antiviral Agents: Focusing on the Antiviral Approaches and Clinical Perspectives of Anthocyanins in the Management of Viral Diseases. Front Pharmacol. 2019;10(1207). [DOI] [PMC free article] [PubMed]
- 36.Martin KW, Ernst E. Antiviral agents from plants and herbs: a systematic review. Antivir Ther. 2003;8(2):77–90. doi: 10.1177/135965350300800201. [DOI] [PubMed] [Google Scholar]
- 37.Lin L-T, Hsu W-C, Lin C-C. Antiviral Natural Products and Herbal Medicines. J Tradit Complement Med. 2014;4(1):24–35. doi: 10.4103/2225-4110.124335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasmin A, Chia S, Looi Q, Omar A, Noordin M, Ideris A. Herbal extracts as antiviral agents. In. Feed Additives: Elsevier; 2020. p. 115–32.
- 39.Xian Y, Zhang J, Bian Z, Zhou H, Zhang Z, Lin Z, Xu H. Bioactive natural compounds against human coronaviruses: a review and perspective. Acta Pharmaceutica Sinica B. 2020;10(7):1163–1174. doi: 10.1016/j.apsb.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cole N. Diversity of medicinal plants in West African habitats. In: The Biodiversity of African Plants. Springer; 1996. pp. 704–13.
- 41.Sawadogo WR, Schumacher M, Teiten M-H, Dicato M, Diederich M. Traditional West African pharmacopeia, plants and derived compounds for cancer therapy. Biochem Pharmacol. 2012;84(10):1225–1240. doi: 10.1016/j.bcp.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 42.Ekanem AP, Udoh FV. The diversity of medicinal plants in Nigeria: An Overview. In: African Natural Plant Products: New Discoveries and Challenges in Chemistry and Quality. ACS Symposium Series (Vol. 1021); 2009. pp. 135–47. https://doi.org/10.1021/bk-2009-1021.ch007
- 43.Addo-Fordjour P, Belford EJD, Akonnor D. Diversity and conservation of medicinal plants in the Bomaa community of the Brong Ahafo region. Ghana Journal of medicinal plants research. 2013;2(9):226–233. [Google Scholar]
- 44.Alavi M, Ho T, Stisher C, Richardson E, Kelly C, McCrory K, Snellings J, Zurek K, Boltz MW. Factors That Influence Student Choice in Family Medicine A National Focus Group. Fam Med. 2019;51(2):143–148. doi: 10.22454/FamMed.2019.927833. [DOI] [PubMed] [Google Scholar]
- 45.Balick MJ, Cox PA. Plants, people, and culture: the science of ethnobotany: Scientific American Library, New York; 1996.
- 46.Anani K, Hudson J, De Souza C, Akpagana K, Tower G, Arnason J, Gbeassor M. Investigation of medicinal plants of Togo for antiviral and antimicrobial activities. Pharm Biol. 2000;38(1):40–45. doi: 10.1076/1388-0209(200001)38:1;1-B;FT040. [DOI] [PubMed] [Google Scholar]
- 47.Hudson J, Anani K, Lee M, De Souza C, Arnason J, Gbeassor M. Further Iinvestigations on the Antiviral Activities of Medicinal Plants of Togo. Pharm Biol. 2000;38(1):46–50. doi: 10.1076/1388-0209(200001)3811-BFT046. [DOI] [PubMed] [Google Scholar]
- 48.Sulaiman LK, Oladele OA, Shittu IA, Emikpe BO, Oladokun AT. Meseko CAJAJoB. In-ovo evaluation of the antiviral activity of methanolic root-bark extract of the African Baobab (Adansonia digitata Lin) 2011;10(20):4256–4258. [Google Scholar]
- 49.Ajaiyeoba EO, Ogbole OO. A phytotherapeutic approach to Nigerian anti-HIV and immunomodulatory drug discovery. Afr J Med Med Sci. 2006;35:71–6. [PubMed]
- 50.Ojo O, Oluyege J, Famurewa OJAJPS. Antiviral properties of two Nigerian plants. 2009;3(7):157–159. [Google Scholar]
- 51.Sonibare MA, Moody JO, Adesanya EO. Use of medicinal plants for the treatment of measles in Nigeria. J Ethnopharmacol. 2009;122(2):268–72. [DOI] [PubMed]
- 52.Buochuama A, Amiofori F. The Utilization of Plant Species in the Treatment of some Identifiable Viral Diseases in Southwestern Nigeria. World Scientific News. 2018;95:111–123. [Google Scholar]
- 53.Esimone C, Omabuwajo O, Amadi C, Adikwa M, Edrada R, Proksch P, Nabi G. Antiviral potentials of Nigerians aframomum melagueta roscoe and piper guineese schum. and thonn. Niger J Nat Prod Med. 2006;10(51):54. [Google Scholar]
- 54.Ogbole OO, Akinleye TE, Segun PA, Faleye TC. Adeniji AJJVj. In vitro antiviral activity of twenty-seven medicinal plant extracts from Southwest Nigeria against three serotypes of echoviruses. 2018;15(1):110. doi: 10.1186/s12985-018-1022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ajaiyeoba E, Ogbole O. A phytotherapeutic approach to Nigerian anti-HIV and immunomodulatory drug discovery. Afr J Med Med Sci. 2006;35:71–76. [PubMed] [Google Scholar]
- 56.Ayisi NK. Antiviral and antibacterial activities of extracts from eight plants. In: Google Patents; 2007. https://patents.google.com/patent/US7220437
- 57.Nnoruka E, Okoye O. Topical steroid abuse: its use as a depigmenting agent. J Natl Med Assoc. 2006;98(6):934. [PMC free article] [PubMed] [Google Scholar]
- 58.Mathieu G, Meissa D. Traditional leafy vegetables in Senegal: diversity and medicinal uses. Afr J Tradit Complement Altern Med. 2007;4(4):469–475. doi: 10.4314/ajtcam.v4i4.31239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kudi AC, Myint SH. Antiviral activity of some Nigerian medicinal plant extracts. J Ethnopharmacol. 1999;68(1):289–294. doi: 10.1016/S0378-8741(99)00049-5. [DOI] [PubMed] [Google Scholar]
- 60.Adjanohoun E, Adjakidje V, Ahyi M, Akoegninou A, d'Almeida J, Apovo F, Boukef K, Chadare M, Gusset G, Dramane KDK. In: Contribution aux études ethnobotaniques et floristiques en République populaire du Bénin. Agence de coopération culturelle et technique,(ACCT), Paris, 895 p. Système. [ONLINE] Disponible à l'adresse; 1989.
- 61.Ohemu T, Agunu A, Chollom S, Okwori V, Dalen D, Olotu P. Preliminary phytochemical screening and antiviral potential of methanol stem bark extract of Enantia chlorantha Oliver (Annonaceae) and Boswellia dalzielii Hutch (Burseraceae) against Newcastle disease in Ovo. European Journal of Medicinal Plants. 2018:1–8.
- 62.Atawodi SE, Atawodi JC. Azadirachta indica (neem): a plant of multiple biological and pharmacological activities. Phytochem Rev. 2009;8(3):601–620.
- 63.Parida MM, Upadhyay C, Pandya G, Jana AM. Inhibitory potential of neem (Azadirachta indica Juss) leaves on Dengue virus type-2 replication. J Ethnopharmacol. 2002;79(2):273–278. doi: 10.1016/S0378-8741(01)00395-6. [DOI] [PubMed] [Google Scholar]
- 64.Ajaiyeoba E, Ogbole O, Ogundipe O. Ethnobotanical survey of Plants used in the traditional management of viral infections in Ogun State of Nigeria. Editorial Advisory Board e. 2005;13(1):64–73. [Google Scholar]
- 65.Oluremi BB, Adeniji JA. Anti-viral Activity Evaluation of Selected Medicinal Plants of Nigeria against Measles Virus. Microbiol Res J Int. 2015:218–25.
- 66.Gbadamosi IT. Stay Safe: Helpful Herbal remedies in COVID-19 infection. Afr J Biomed Res. 2020;23(2):131–133. [Google Scholar]
- 67.Abonyi DO, Abonyi MU, Esimone CO, Ibezim EC. Plants as sources of antiviral agents. Afr J Biotechnol. 2009;8(17):3989–94.
- 68.Chukwuma OJT. Antiviral Activities of the Aqueous, Ethanolic and Methanolic Extracts of Diospyros Mespiliformis leaf on some pathogenic Avian viruses. IDOSR J Exp Sci. 2017;2(3):35–49.
- 69.Wannang NN, Kwanashie HO, Ede SO. Antiviral activity of the fruit extract of Cucumis metuliferus E. Meye (Curcubitaceae) in chicks. AJBAS 2010;2(3-4): 89–93.
- 70.Olugbuyiro J. Inhibitory activity of Detarium microcarpum extract against hepatitis C virus. Afr J Biomed Res. 2009;12(2):149–151. [Google Scholar]
- 71.Moody JO, Robert VA. Hughes Jd-A. Antiviral activities of selected medicinal plants II: Effect of extracts of Diospyros barteri, Diospyros monbutensis and Sphenocentrum jollyanum on Cowpea Mosaic viruses. Pharm Biol. 2002;40(5):342–5.
- 72.Arnold H-J, Gulumian M. Pharmacopoeia of traditional medicine in Venda. J Ethnopharmacol. 1984;12(1):35–74. doi: 10.1016/0378-8741(84)90086-2. [DOI] [PubMed] [Google Scholar]
- 73.Adeniyi BA, Ayepola OO, Adu FD. The antiviral activity of leaves of Eucalyptus camaldulensis (Dehn) and Eucalyptus torelliana (R. Muell) Pak J Pharm Sci. 2015;28(5):1773–1776. [PubMed] [Google Scholar]
- 74.Hudson J, Lee M, Rasoanaivo P. Antiviral activities in plants endemic to Madagascar. Pharm Biol. 2000;38(1):36–39. doi: 10.1076/1388-0209(200001)3811-BFT036. [DOI] [PubMed] [Google Scholar]
- 75.Malzy P. Quelques plantes du Nord Cameroun et leurs utilisations. Journal d'agriculture traditionnelle et de botanique appliquée. 1954;1(5):148–179. doi: 10.3406/jatba.1954.2147. [DOI] [Google Scholar]
- 76.Adjanohoun E, Adjakidje V, Ahyi M, Akpagana K, Chibon P, El-Hadji A, Eyme J, Garba M, Gassita J, Gbeassor M, Goudote E, Guinko S, Hodouto K-K, Houngnon, Keita PA, Keoula Y, Kluga -Ocloo WP, Lo I, Siamevi KM, Taffame, KK. In: Contribution aux études ethnobotaniques et floristiques au Togo Agence de coopération culturelle et technique (ACCT), Paris; 1986. p. 671.
- 77.Omilabu S. Antiviral Properties of African Medicinal Plants. In: Odugbemi TA, editor. Textbook of Medicinal Plants from Nigeria; 2008.
- 78.Ogbole O, Segun P, Akinleye T, Fasinu P. Antiprotozoal, antiviral and cytotoxic properties of the Nigerian Mushroom, Hypoxylon fuscum Pers. Fr.(Xylariaceae). ACTA Pharm Sci. 2018;56(4):43–56.
- 79.Esimone C, Omobowajo O, Sowemimo A, Proksch P. Single-cycle vector-based antiviral screening assays for high throughput evaluation of potential anti-HIV medicinal plants: a pilot study on some Nigerian herbs. Recent progress in medicinal plant research. 2007;19:49–60. [Google Scholar]
- 80.Adjanohoun E, Ahyi MRA, Ake-Assi L, Elewude JA, Dramane K, Fadoju SO, Gbile ZO, Goudole E, Johnson CLA, Keita A, Morakinyo O, Ojewole JAO, Olatunji AO, Sofowora EA. Traditional Medicine and Pharmacopoeia. Contribution to Ethnobotanical Floristic Studies in Western Nigeria. Lagos, Nigeria: Organization of African Unity, Scientific Technical and Research Commission; 1991. p. 420.
- 81.Oridupa O, Saba A, Sulaiman L. Preliminary report on the antiviral activity of the ethanolic fruit extract of Lagenaria breviflora Roberts on Newcastle disease virus. Trop Vet. 2011;29(1):22–33. [Google Scholar]
- 82.Ogbole OO, Adeniji AJ, Ajaiyeoba EO, Adu FD. Anti-poliovirus activity of medicinal plants selected from the Nigerian ethno-medicine. Afr J Biotechnol. 2013;12(24):3878–83.
- 83.Agbo MO, Odimegwu DC, Okoye FBC, Osadebe PO. Antiviral activity of Salidroside from the leaves of Nigerian mistletoe (Loranthus micranthus Linn) parasitic on Hevea brasiliensis against respiratory syncytial virus. Pak J Pharm Sci. 2017;30(4):1251–1256. [PubMed] [Google Scholar]
- 84.Segun PA, Ogbole OO, Akinleye TE, Faleye TO, Adeniji AJ. In vitro anti-enteroviral activity of stilbenoids isolated from the leaves of Macaranga barteri. Nat Prod Res. 2021;35(11): 1909–13. [DOI] [PubMed]
- 85.Chollom S, Agada G, Gotep J, Mwankon S, Dus P, Bot Y, Nyango D, Singnap C, Fyaktu E, Okwori A. Investigation of aqueous extract of Moringa oleifera lam seed for antiviral activity against newcastle disease virus in ovo. J Med Plants Res. 2012;6(22):3870–3875. doi: 10.5897/JMPR12.394. [DOI] [Google Scholar]
- 86.Adjanohoun E, Aké Assi L, Ali A. Contribution aux études ethnobotaniques et floristiques aux Comores. Rapport présenté à l’ACCT; 1982.
- 87.Faeji C, Oladunmoye M, Adebayo I, Adebolu T. In-ovo biological activities of Phyllanthus amarus leaf extracts against Newcastle disease virus. J Med Plants Res. 2017;11:419–425. doi: 10.5897/JMPR2017.6379. [DOI] [Google Scholar]
- 88.Akoegninou A, Adjanohoun E, Adjakidje M, Ahyi L, Ake Assi A, Akoegninou J, d'Almeida F, Apovo K, Boukef M, Chadare G, Gusset K. Contribution aux études ethnobotaniques et floristiques en République Populaire du Bénin. Médecine traditionnelle et pharmacopée Agence de coopération culturelle et technique, (ACCT), Paris; 1989. p. 895.
- 89.Chollom S, Agada G, Bot D, Okolo M, Dantong D, Choji T, Echeonwu B, Bigwan E, Lokason S, Banwat E. Phytochemical analysis and antiviral potential of aqueous leaf extract of Psidium guajava against newcastle disease virus in ovo. J Appl Pharm Sci. 2012;2(10):045–049.
- 90.Esimone C, Grunwald T, Wildner O, Nchinda G, Tippler B, Proksch P, Ueberla K. In vitro pharmacodynamic evaluation of antiviral medicinal plants using a vector-based assay technique. J Appl Microbiol. 2005;99(6):1346–1355. doi: 10.1111/j.1365-2672.2005.02732.x. [DOI] [PubMed] [Google Scholar]
- 91.Esimone C, Grunwald T, Nworu C, Kuate S, Proksch P, Überla K. Broad spectrum antiviral fractions from the lichen Ramalina farinacea (L.) Ach. Chemotherapy. 2009;55(2):119–126. doi: 10.1159/000194974. [DOI] [PubMed] [Google Scholar]
- 92.Lai D, Odimegwu DC, Esimone C, Grunwald T, Proksch P. Phenolic compounds with in vitro activity against respiratory syncytial virus from the Nigerian lichen Ramalina farinacea. Planta Med. 2013;79(15):1440–1446. doi: 10.1055/s-0033-1350711. [DOI] [PubMed] [Google Scholar]
- 93.Odimegwu DC, Esimone CO. In vitro Antiviral Activity of Nauclea latifolia Root Bark Extract Against the Respiratory Syncytial Virus. European J Med Plants. 2018;22(2):1–7.
- 94.Moody J, Roberts V. Antiviral effect of selected medicinal Plants 1: effect of Diospyros bateri, Diospyros monbutensis and Sphenocentrum jollyanum on Polio Viruses. Niger J Nat Prod Med. 2002;6(1):4–6. [Google Scholar]
- 95.Olowokudejo J, Kadiri A, Travih V. An ethnobotanical survey of herbal markets and medicinal plants in Lagos State of Nigeria. Ethnobotanical Leaflets 2008;12:851–65.
- 96.Carrière M. Plantes de Guineée à l'usage des éleveurs et des vétérinaires: CIRAD-EMVT; 1994. p. 235.
- 97.Vlietinck A, Van Hoof L, Totte J, Lasure A, Berghe DV, Rwangabo P, Mvukiyumwami J. Screening of hundred Rwandese medicinal plants for antimicrobial and antiviral properties. J Ethnopharmacol. 1995;46(1):31–47. doi: 10.1016/0378-8741(95)01226-4. [DOI] [PubMed] [Google Scholar]
- 98.Patel B, Sharma S, Nair N, Majeed J, Goyal RK, Dhobi M. Therapeutic opportunities of edible antiviral plants for COVID-19. Mol Cell Biochem. 2021;476(6):2345–64. [DOI] [PMC free article] [PubMed]
- 99.Weng J-K. Plant Solutions for the COVID-19 Pandemic and Beyond: Historical Reflections and Future Perspectives. Mol Plant. 2020;13(6):803–7. [DOI] [PMC free article] [PubMed]
- 100.Iwuoha VC, Ezeibe EN, Ezeibe CC. Glocalization of COVID-19 responses and management of the pandemic in Africa. Local Environ. 2020;25(8):641–7.
- 101.Rouf R, Uddin SJ, Sarker DK, Islam MT, Ali ES, Shilpi JA, Nahar L, Tiralongo E, Sarker SD. Anti-viral potential of garlic (Allium sativum) and it's organosulfur compounds: A systematic update of pre-clinical and clinical data. Trends Food Sci Technol. 2020;104:219–234. [DOI] [PMC free article] [PubMed]
- 102.World Health Organisation (WHO). Expert panel endorses protocol for COVID-19 herbal medicine clinical trials. 2020. https://www.afro.who.int/news/expert-panel-endorses-protocol-covid-19-herbal-medicine-clinical-trials
- 104.ul Qamar MT, Alqahtani SM, Alamri MA, Chen LL. Structural Basis of SARS-CoV-2 3CLpro and Anti-COVID-19 Drug Discovery from Medicinal Plants J Pharm Anal. 2020;10(4):313–9. [DOI] [PMC free article] [PubMed]
- 104.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–80. [DOI] [PMC free article] [PubMed]
- 105.Nguyen TTH, Woo H-J, Kang H-K, Kim Y-M, Kim D-W, Ahn S-A, Xia Y, Kim D. Flavonoid-mediated inhibition of SARS coronavirus 3C-like protease expressed in Pichia pastoris. Biotechnol Lett. 2012;34(5):831–838. doi: 10.1007/s10529-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li SY, Chen C, Zhang HQ, Guo HY, Wang H, Wang L, Zhang X, Hua SN, Yu J, Xiao PG, Li RS, Tan X. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res. 2005;67(1):18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen H, Du Q. Potential natural compounds for preventing SARS-CoV-2 (2019-nCoV) infection. Preprints 2020, 2020010358. 10.20944/preprints202001.0358.v3.
- 108.De Clercq E. Potential antivirals and antiviral strategies against SARS coronavirus infections. Expert Rev Anti Infect Ther. 2006;4(2):291–302. doi: 10.1586/14787210.4.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jo S, Kim S, Shin DH, Kim M-S. Inhibition of SARS-CoV 3CL protease by flavonoids. J Enzyme Inhib Med Chem. 2020;35(1):145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Utomo RY, Meiyanto E. Revealing the potency of citrus and galangal constituents to halt SARS-CoV-2 infection. Preprints 2020, 2020030214. 10.20944/preprints202003.0214.v1.
- 111.Jeong GU, Song H, Yoon GY, Kim D, Kwon Y-C. Therapeutic Strategies Against COVID-19 and Structural Characterization of SARS-CoV-2: A Review. Front Microbiol. 2020;11:1723. [DOI] [PMC free article] [PubMed]
- 112.Tutunchi H, Naeini F, Ostadrahimi A, Hosseinzadeh-Attar MJ. Naringenin, a flavanone with antiviral and anti-inflammatory effects: A promising treatment strategy against COVID-19. Phytotherapy research : PTR. 2020;34(12):3137–3147. doi: 10.1002/ptr.6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ziai SA, Heidari MR, Amin Gh, Koochemeshki A, Heidari M. Inhibitory Effects of Germinal Angiotensin Converting Enzyme by Medicinal Plants Used in Iranian Traditional Medicine as Antihypertensive. J Kerman Univ Med Sci. 2009;16(2):134–43.
- 114.Shojai TM, Langeroudi AG, Karimi V, Barin A, Sadri N. The effect of Allium sativum (Garlic) extract on infectious bronchitis virus in specific pathogen free embryonic egg. Avicenna J Phytomed. 2016;6(4):458–67. [PMC free article] [PubMed]
- 115.Keyaerts E, Vijgen L, Pannecouque C, Van Damme E, Peumans W, Egberink H, Balzarini J, Van Ranst M. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res. 2007;75(3):179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cheng B, Li T. Discovery of alliin as a putative inhibitor of the main protease of SARS-CoV-2 by molecular docking. Biotechniques. 2020;69(2):108–12. [DOI] [PMC free article] [PubMed]
- 117.Vimalanathan S, Hudson J. Anti-influenza virus activity of essential oils and vapors. American Journal of Essential Oils and Natural Products. 2014;2(1):47–53. [Google Scholar]
- 118.Shin H-B, Choi M-S, Yi C-M, Lee J, Kim N-J, Inn K-S. Inhibition of respiratory syncytial virus replication and virus-induced p38 kinase activity by berberine. Int Immunopharmacol. 2015;27(1):65–68. doi: 10.1016/j.intimp.2015.04.045. [DOI] [PubMed] [Google Scholar]
- 119.Fan Y, Zhang Y, Tariq A, Jiang X, Ahamd Z, Zhihao Z, Idrees M, Azizullah A, Adnan M, Bussmann RW. Food as medicine: a possible preventive measure against coronavirus disease (COVID‐19). Phytother Res. 2020;34(12):3124–36. [DOI] [PMC free article] [PubMed]
- 120.Joshi RS, Jagdale SS, Bansode SB, Shankar SS, Tellis MB, Pandya VK, Chugh A, Giri AP, Kulkarni MJ. Discovery of potential multi-target-directed ligands by targeting host-specific SARS-CoV-2 structurally conserved main protease. J Biomol Struct Dyn. 2021;39(9):3099–114. [DOI] [PMC free article] [PubMed]
- 121.Nallusamy S, Mannu J, Ravikumar C, Angamuthu K, Nathan B, Nachimuthu K, Ramasamy G, Muthurajan R, Subbarayalu M, Neelakandan K. Exploring Phytochemicals of Traditional Medicinal Plants Exhibiting Inhibitory Activity Against Main Protease, Spike Glycoprotein, RNA-dependent RNA Polymerase and Non-Structural Proteins of SARS-CoV-2 Through Virtual Screening. Front Pharmacol. 2021;12:667704. [DOI] [PMC free article] [PubMed]
- 122.Olubiyi OO, Olagunju M, Keutmann M, Loschwitz J, Strodel B. High throughput virtual screening to discover inhibitors of the main protease of the coronavirus SARS-CoV-2. Molecules. 2020;25(14):3193. [DOI] [PMC free article] [PubMed]
- 123.Anand AV, Balamuralikrishnan B, Kaviya M, Bharathi K, Parithathvi A, Arun M, Senthilkumar N, Velayuthaprabhu S, Saradhadevi M, Al-Dhabi NA. Medicinal Plants, Phytochemicals, and Herbs to Combat Viral Pathogens Including SARS-CoV-2. Molecules. 2021;26(6):1775. doi: 10.3390/molecules26061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roy S, Bhattacharyya P. Possible role of traditional medicinal plant Neem (Azadirachta indica) for the management of COVID-19 infection. Int J Res Pharm Sci. 2020;11(SPL1):122–125. doi: 10.26452/ijrps.v11iSPL1.2256. [DOI] [Google Scholar]
- 125.Borkotoky S, Banerjee M. A computational prediction of SARS-CoV-2 structural protein inhibitors from Azadirachta indica (Neem). J Biomol Struct Dyn. 2021;39(11):4111–21. [DOI] [PMC free article] [PubMed]
- 126.Subramanian SS. Some Compounds from Neem leaves extract exhibit binding affinity as high as -14.3 kcal/mol against COVID-19 Main Protease (Mpro): A Molecular Docking Study. 2020. 10.21203/rs.3.rs-25649/v1.
- 127.Maurya VK, Kumar S, Bhatt ML, Saxena SK. Therapeutic Development and Drugs for the Treatment of COVID-19. In: Saxena S, editor. Coronavirus Disease 2019 (COVID-19). Singapore: Springer; 2020. pp. 109–126. https://doi.org/10.1007/978-981-15-4814-7_10
- 128.Krokhin O, Li Y, Andonov A, Feldmann H, Flick R, Jones S, Stroeher U, Bastien N, Dasuri KV, Cheng K. Mass spectrometric characterization of proteins from the SARS virus: a preliminary report. Mol Cell Proteomics. 2003;2(5):346–356. doi: 10.1074/mcp.M300048-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Watanabe Y, Allen JD, Wrapp D, McLellan JS, Crispin M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science. 2020;369(6501):330–3. [DOI] [PMC free article] [PubMed]
- 130.Zhong X, Qi G, Yang J, Xing G, Liu J, Yang X. High-efficiency expression of a receptor-binding domain of SARS-CoV spike protein in tobacco chloroplasts. Sheng Wu Gong Cheng Xue Bao= Chin J Biotechnol. 2014;30(6):920–30. [PubMed]
- 131.Zheng N, Xia R, Yang C, Yin B, Li Y, Duan C, Liang L, Guo H, Xie Q. Boosted expression of the SARS-CoV nucleocapsid protein in tobacco and its immunogenicity in mice. Vaccine. 2009;27(36):5001–5007. doi: 10.1016/j.vaccine.2009.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chang F-R, Yen C-T, Ei-Shazly M, Lin W-H, Yen M-H, Lin K-H, Wu Y-C. Anti-human coronavirus (anti-HCoV) triterpenoids from the leaves of Euphorbia neriifolia. Nat Prod Commun. 2012;7(11):1415–7. [PubMed]
- 133.Caricchio R, Gallucci M, Dass C, Zhang X, Gallucci S, Fleece D, Bromberg M, Criner GJ. Preliminary predictive criteria for COVID-19 cytokine storm. Ann Rheum Dis. 2021;80:88–95. [DOI] [PubMed]
- 134.Campbell CM, Guha A, Haque T, Neilan TG, Addison D. Repurposing Immunomodulatory Therapies against Coronavirus Disease 2019 (COVID-19) in the Era of Cardiac Vigilance: A Systematic Review. J Clin Med. 2020;9(9):2935. doi: 10.3390/jcm9092935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boudjeko T, Megnekou R, Woguia AL, Kegne FM, Ngomoyogoli JEK, Tchapoum CDN, Koum O. Antioxidant and immunomodulatory properties of polysaccharides from Allanblackia floribunda Oliv stem bark and Chromolaena odorata (L.) King and HE Robins leaves. BMC Res Notes. 2015;8(1):759. [DOI] [PMC free article] [PubMed]
- 136.Ishikawa H, Saeki T, Otani T, Suzuki T, Shimozuma K, Nishino H, Fukuda S, Morimoto K. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J Nutr. 2006;136(3):816S–20S. doi: 10.1093/jn/136.3.816S. [DOI] [PubMed] [Google Scholar]
- 137.Anywar G, Kakudidi E, Byamukama R, Mukonzo J, Schubert A, Oryem-Origa H. Medicinal plants used by traditional medicine practitioners to boost the immune system in people living with HIV/AIDS in Uganda. European Journal of Integrative Medicine. 2019:101011.
- 138.Makare N, Bodhankar S, Rangari V. Immunomodulatory activity of alcoholic extract of Mangifera indica L. in mice. J Ethnopharmacol. 2001;78(2–3):133–37. [DOI] [PubMed]
- 139.De L, De T. Protective Foods to Develop Immunity of Individuals against COVID 19. Biotica Research Today. 2020;2(5 Spl.):287–90.
- 140.Subhrajyoti C, Sciences IM. Immunomodulatory herbs of Ayurveda and Covid-19: A Review Article. Journal of Ayurveda. 2020;5(2):203–208. [Google Scholar]
- 141.Rahmani AH. Cassia fistula Linn: Potential candidate in the health management. Pharmacognosy Res. 2015;7(3):217. doi: 10.4103/0974-8490.157956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cheng L, Zheng W, Li M, Huang J, Bao S, Xu Q, Ma Z. Citrus fruits are rich in flavonoids for immunoregulation and potential targeting ACE2. Preprints. 2020;2020020313. [DOI] [PMC free article] [PubMed]
- 143.Tutunchi H, Naeini F, Ostadrahimi A, Hosseinzadeh‐Attar MJ. Naringenin, a flavanone with antiviral and anti‐inflammatory effects: A promising treatment strategy against COVID‐19. Phytother Res. 2020;34:3137–47. [DOI] [PMC free article] [PubMed]
- 144.Meneguzzo F, Ciriminna R, Zabini F, Pagliaro M. Accelerated production of hesperidin-rich citrus pectin from waste citrus peel for prevention and therapy of COVID-19. Preprints. 2020. 10.20944/preprints202003.0386.v1.
- 145.Liu Y-W, Liu J-C, Huang C-Y, Wang C-K, Shang H-F, Hou W-C. Effects of oral administration of yam tuber storage protein, dioscorin, to BALB/c mice for 21-days on immune responses. J Agric Food Chem. 2009;57(19):9274–9279. doi: 10.1021/jf902245k. [DOI] [PubMed] [Google Scholar]
- 146.Fu S-L, Hsu Y-H, Lee P-Y, Hou W-C, Hung L-C, Lin C-H, Chen C-M, Huang Y-J. Dioscorin isolated from Dioscorea alata activates TLR4-signaling pathways and induces cytokine expression in macrophages. Biochem Biophys Res Commun. 2006;339(1):137–144. doi: 10.1016/j.bbrc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 147.Wen C-C, Chen H-M, Yang N-S. Chapter 6 - Developing Phytocompounds from Medicinal Plants as Immunomodulators. In: Shyur L-F, Lau ASY, editors. Advances in Botanical Research: Academic Press; 2012. vol. 62, pp. 197–272. [DOI] [PMC free article] [PubMed]
- 148.Muhammad BY, Awaisu A. The need for enhancement of research, development, and commercialization of natural medicinal products in Nigeria: Lessons from the Malaysian experience. Afr J Tradit Complement Altern Med. 2008;5(2):120–30. [PMC free article] [PubMed]
- 149.Awodele O, Daniel A, Popoola T, Salami E. A study on pharmacovigilance of herbal medicines in Lagos West Senatorial District. Nigeria Int J Risk Saf Med. 2013;25(4):205–217. doi: 10.3233/JRS-130604. [DOI] [PubMed] [Google Scholar]
- 150.World Health Organization. Programme on Traditional Medicine. National policy on traditional medicine and regulation of herbal medicines : report of a WHO global survey. World Health Organization; 2005. https://apps.who.int/iris/handle/10665/43229
- 151.Awodele O, Popoola T, Amadi K, Coker H, Akintonwa A. Traditional medicinal plants in Nigeria—Remedies or risks. J Ethnopharmacol. 2013;150(2):614–618. doi: 10.1016/j.jep.2013.09.015. [DOI] [PubMed] [Google Scholar]