See rebuttal on page 1477. See counterpoint on page 1478.
The first bile acid, cholic acid, was discovered in 1848 from ox gallbladder, and the bile acid structure solved by Otto Wieland was recognized with the 1927 Nobel Prize in Chemistry.1 Primary bile acids are synthesized in the liver from cholesterol and conjugated to taurine or glycine for biliary secretion. Bile acid synthesis is catalyzed by approximately 17 enzymes via the classic or alternative pathways. The classic bile acid synthesis pathway is initiated by cholesterol 7α-hydroxylase (CYP7A1), followed by sterol 12α-hydroxylase (CYP8B1). On the other hand, the alternative pathway initiates with sterol 27-hydroxylase (CYP27A1), followed by oxysterol 7α-hydroxylase (CYP7B1). Upon secretion into the gut, microbiota deconjugate the taurine or glycine conjugated bile acids via the activity of 7α-dehydroxylase, thus generating secondary bile acids.1, 2, 3 The positioning of the hydroxyl groups on the 24-carbon steroid core renders the bile acid either hydrophilic or hydrophobic.1, 2, 3 Bile acids with a high hydrophobic index, such as lithocholic acid, deoxycholic acid, and chenodeoxycholic acid, are toxic and cause liver injury. In contrast, hydrophilic bile acid, such as ursodeoxycholic acid, stimulates biliary excretion of toxic bile acids and is protective against oxidative stress and cell death.1, 2, 3, 4
In addition to being essential for fat digestion and absorption, bile acids also are multifaceted signaling molecules that can activate the following: (1) calcium/calmodulin-dependent protein kinase (Ca2+/CaM-PK); (2) cyclic adenosine monophosphate/protein kinase A (PKA); (3) Protein Kinase C (PKC); (4) nuclear receptors, such as Farnesoid X receptor (FXR) and vitamin D receptor; or (5) G-protein–coupled receptors, including Takeda G-protein–coupled receptor 5, sphingosine-1-phosphate receptor 2, and muscarinic receptors to regulate many cellular processes.1, 2, 3, 4, 5, 6, 7, 8 In this article, we cite examples of the harmful effects triggered by pathogenic activation of these signaling pathways (Figure 1).
Figure 1.
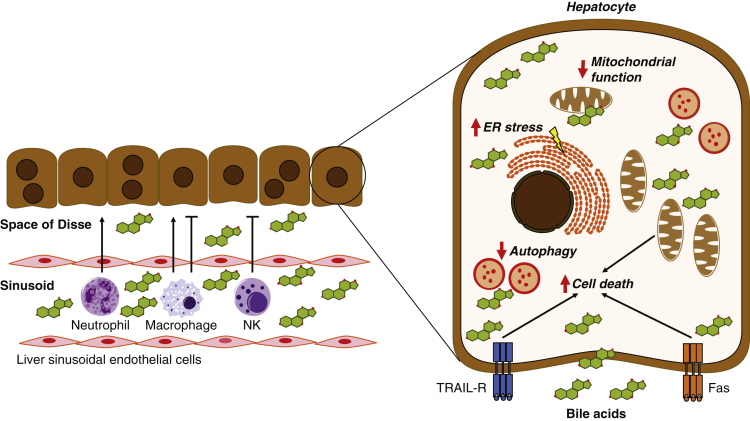
Different mechanisms by which bile acids can cause cellular injury and death. ER, endoplasmic reticulum; Fas, Fas cell surface death receptor; NK, natural killer; TRAIL-R, tumor necrosis factor–related apoptosis-inducing ligand receptor.
Many liver diseases result in cholestasis, which is defined as the accumulation of bile acids within the liver. The etiology of cholestasis ranges from genetic disorders, hormonal imbalances, immune-mediated destruction of bile ducts, drug-induced or virus-based injuries, and biliary obstruction, such as can be caused by gallstones or tumors.2,4 Biliary atresia destroys the biliary tree in neonates,9 while progressive familial intrahepatic cholestasis is the result of genetic defects in the bile acid transporter (bile salt export pump), the bile acid receptor FXR, phospholipid transporters (multidrug resistance protein 3), tight junction protein 2, or Myosin 5B.2 In contrast, autoimmune destruction of bile ducts occurs in primary biliary cholangitis, whereas inflammation and fibrosis in bile ducts result in primary sclerosing cholangitis. Finally, intrahepatic cholestasis of pregnancy involves hormonal, environmental, and genetic factors. Chronic cholestasis causes accumulation of bile acids in hepatocytes and the biliary system, with possible extracellular extravasation, which can lead to hepatic fibrosis, cirrhosis, hepatocellular carcinoma, and, ultimately, liver failure. Indeed, it is becoming increasingly evident that increased levels of cytotoxic hydrophobic bile acids are a driving factor for hepatocyte injury and cell death.
Bile acid–mediated cellular injury is not limited to hepatocytes. Increased serum bile acids in pregnant patients with cholestasis has been linked to fetal cardiac dysfunctions.10 Similarly, altered levels of free nonmicellar bile acids resulting from altered biliary phospholipid secretion, such as can occur with ABCB4 mutation, has been linked to cholangiocyte injury in patients with progressive intrahepatic cholestasis-3 and animal models of progressive sclerosing cholangitis.11,12 Indeed, as a result of constant bile exposure, cholangiocytes are uniquely susceptible to bile acid–mediated injury and this has been proposed as a potential injury mechanism in primary biliary cholangitis and extrahepatic biliary atresia, in combination with exposure to environmental agents.13,14
Bile acids induce inflammation by cell-type–specific mechanisms within the liver.15 For example, bile acids induce the expression and release of proinflammatory cytokines via early growth response protein 1 or Toll-like receptor 9 in hepatocytes. In contrast, bile acids increase the expression and secretion of osteopontin from cholangiocytes, which indirectly recruits immune cells, thus playing a role in their activation. Bile acids also activate neutrophils to increase proinflammatory cytokine interleukin 17 through T helper 17 cells, and they can inhibit inflammation via effects on natural killer cells. Liver macrophages produce both proinflammatory and anti-inflammatory cytokines in response to bile acid signaling via Ca2+/CaM-PK16 and vitamin D receptor. For example, activation of vitamin D receptor in liver macrophages ameliorates liver inflammation. Thus, bile acids modulate inflammation within the liver through multiple mechanisms.
High concentrations of hydrophobic bile acids can increase plasma membrane fluidity and make them leaky by solubilizing membrane lipid components such as phospholipids and/or cholesterol.17 Previous studies with unilamellar vesicles have shown that, when present at low concentrations, bile acids and lipid aggregate in the outer membrane monolayer, whereas they cause the formation of transient membrane holes when present at high concentrations. Studies with synthetic liposomes and isolated plasma membranes have confirmed that bile acids can disrupt plasma membrane domains, and the hydrophobic index of the bile acid concentration determines the extent of membrane damage.
Increased extracellular levels of bile acid can induce hepatocyte apoptosis through the activation of cell surface death receptors, including Fas cell surface death receptor and tumor necrosis factor–related apoptosis-inducing ligand receptor. Bile acids have been shown to up-regulate tumor necrosis factor–related apoptosis-inducing ligand receptor expression and oligomerization, and initiate ligand-dependent and independent activation of Fas. Mechanistically, cyclic adenosine monophosphate/PKA, PKC, FXR, Takeda G-protein–coupled receptor 5, and sphingosine-1-phosphate receptor 2 signaling all have been implicated in bile acid–mediated apoptosis in hepatocytes.
Cytotoxic bile acids can be incorporated into the mitochondrial membrane, causing mitochondrial dysfunction. Bile acid signaling via Ca2+/CaM-PK also has been shown to mediate oxidative stress–induced mitochondrial defects in hepatocytes.6 Excess intracellular bile acids can stimulate the generation of reactive oxygen species (ROS) and trigger alterations in mitochondrial permeability. This can lead to the collapse of the mitochondrial membrane potential, reduced adenosine triphosphate synthesis, and further ROS generation, thus activating a feed-forward injury cycle that causes cytochrome c release and, eventually, cell death.
Excess hydrophobic bile acids within hepatocytes also can trigger the release of calcium ions from the endoplasmic reticulum (ER) and stimulate ER stress. In addition, bile acid–mediated release of ROS from mitochondria can promote ER stress in a positive feed-forward loop that is initiated by concomitant mitochondrial calcium release. Finally, FXR-dependent and independent mechanisms have been shown to inhibit autophagosomal–lysosomal fusion.18 Thus, bile acids can induce hepatocyte ER stress and regulate their clearance of cellular debris and recycling efforts. However, it is interesting to note that bile acid–mediated activation of the FXR nuclear hormone receptor also has been shown to alleviate ER stress–induced hepatocyte death,5 and bile acid–mediated activation of the vitamin D receptor in liver macrophages mitigates hepatic ER stress and subsequent liver injury.7
In summary, both the concentration and the composition of bile acids are central to their biology. In this discussion, we have presented evidence showing that bile acids must be tightly regulated to avoid detrimental cellular effects, ranging from activation of inflammation, to membrane disruption, to multi-organelle stress. Because of this, one may consider bile acids to be intrinsically harmful molecules. However, given their fundamental role in digestive physiology, when discussing bile acids it may be best to paraphrase Lanoree Brock (of Star Wars fame): their (bile acid) force is neither light nor dark, master nor slave, but a balance between extremes.
Footnotes
Conflicts of interest The authors disclose no conflicts.
References
- 1.Hofmann A.F., Hagey L.R. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. J Lipid Res. 2014;55:1553–1595. doi: 10.1194/jlr.R049437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li T., Chiang J.Y. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev. 2014;66:948–983. doi: 10.1124/pr.113.008201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell D.W. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 4.Kuipers F., Bloks V.W., Groen A.K. Beyond intestinal soap--bile acids in metabolic control. Nat Rev Endocrinol. 2014;10:488–498. doi: 10.1038/nrendo.2014.60. [DOI] [PubMed] [Google Scholar]
- 5.Han C.Y., Rho H.S., Kim A., Kim T.H., Jang K., Jun D.W., Kim J.W., Kim B., Kim S.G. FXR inhibits endoplasmic reticulum stress-induced NLRP3 inflammasome in hepatocytes and ameliorates liver injury. Cell Rep. 2018;24:2985–2999. doi: 10.1016/j.celrep.2018.07.068. [DOI] [PubMed] [Google Scholar]
- 6.Toledo F.D., Perez L.M., Basiglio C.L., Ochoa J.E., Sanchez Pozzi E.J., Roma M.G. The Ca(2)(+)-calmodulin-Ca(2)(+)/calmodulin-dependent protein kinase II signaling pathway is involved in oxidative stress-induced mitochondrial permeability transition and apoptosis in isolated rat hepatocytes. Arch Toxicol. 2014;88:1695–1709. doi: 10.1007/s00204-014-1219-5. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Y., Dong B., Kim K.H., Choi S., Sun Z., Wu N., Wu Y., Scott J., Moore D.D. Vitamin D receptor activation in liver macrophages protects against hepatic endoplasmic reticulum stress in mice. Hepatology. 2020;71:1453–1466. doi: 10.1002/hep.30887. [DOI] [PubMed] [Google Scholar]
- 8.Sinal C.J., Tohkin M., Miyata M., Ward J.M., Lambert G., Gonzalez F.J. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 9.Bezerra J.A., Wells R.G., Mack C.L., Karpen S.J., Hoofnagle J.H., Doo E., Sokol R.J. Biliary atresia: clinical and research challenges for the twenty-first century. Hepatology. 2018;68:1163–1173. doi: 10.1002/hep.29905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasavan T., Deepak S., Jayawardane I.A., Lucchini M., Martin C., Geenes V., Yang J., Lövgren-Sandblom A., Seed P.T., Chambers J., Stone S., Kurlak L., Dixon P.H., Marschall H.U., Gorelik J., Chappell L., Loughna P., Thornton J., Pipkin F.B., Hayes-Gill B., Fifer W.P., Williamson C. Fetal cardiac dysfunction in intrahepatic cholestasis of pregnancy is associated with elevated serum bile acid concentrations. J Hepatol. 2021;74:1087–1096. doi: 10.1016/j.jhep.2020.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Vries E., Mazzetti M., Takkenberg B., Mostafavi N., Bikker H., Marzioni M., de Veer R., van der Meer A., Doukas M., Verheij J., Beuers U. Carriers of ABCB4 gene variants show a mild clinical course, but impaired quality of life and limited risk for cholangiocarcinoma. Liver Int. 2020;40:3042–3050. doi: 10.1111/liv.14662. [DOI] [PubMed] [Google Scholar]
- 12.Fickert P., Fuchsbichler A., Wagner M., Zollner G., Kaser A., Tilg H., Krause R., Lammert F., Langner C., Zatloukal K., Marschall H.U., Denk H., Trauner M. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Theise N.D., Crawford J.M., Nakanuma Y., Quaglia A. Canal of Hering loss is an initiating step for primary biliary cholangitis (PBC): a hypothesis. Med Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109680. [DOI] [PubMed] [Google Scholar]
- 14.Lorent K., Gong W., Koo K.A., Waisbourd-Zinman O., Karjoo S., Zhao X., Sealy I., Kettleborough R.N., Stemple D.L., Windsor P.A., Whittaker S.J., Porter J.R., Wells R.G., Pack M. Identification of a plant isoflavonoid that causes biliary atresia. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aaa1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li M., Cai S.Y., Boyer J.L. Mechanisms of bile acid mediated inflammation in the liver. Mol Aspects Med. 2017;56:45–53. doi: 10.1016/j.mam.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng D., Li Z., Wei X., Liu R., Shen A., He D., Tang C., Wu Z. Role of miR-148a in mitigating hepatic ischemia-reperfusion injury by repressing the TLR4 signaling pathway via targeting CaMKIIalpha in vivo and in vitro. Cell Physiol Biochem. 2018;49:2060–2072. doi: 10.1159/000493716. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y., Maxwell K.N., Sezgin E., Lu M., Liang H., Hancock J.F., Dial E.J., Lichtenberger L.M., Levental I. Bile acids modulate signaling by functional perturbation of plasma membrane domains. J Biol Chem. 2013;288:35660–35670. doi: 10.1074/jbc.M113.519116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panzitt K., Jungwirth E., Krones E., Lee J.M., Pollheimer M., Thallinger G.G., Kolb-Lenz D., Xiao R., Thorell A., Trauner M., Fickert P., Marschall H.U., Moore D.D., Wagner M. FXR-dependent Rubicon induction impairs autophagy in models of human cholestasis. J Hepatol. 2020;72:1122–1131. doi: 10.1016/j.jhep.2020.01.014. [DOI] [PubMed] [Google Scholar]