Abstract
The activity of three new, 8-methoxy-nonfluorinated quinolones (NFQs) against multiple-drug-resistant staphylococci was investigated. First, using Staphylococcus aureus strains containing point mutations in the serine 84–80 hot spots of the target genes (gyrA and grlA), cell growth inhibition potencies of the NFQs as a result of DNA gyrase and topoisomerase IV inhibition were estimated and compared with those of known fluoroquinolones. The NFQs and clinafloxacin showed higher affinities toward both the targets than ciprofloxacin, trovafloxacin and gatifloxacin. Furthermore, the ratio of the calculated affinity parameter for DNA gyrase to that for topoisomerase IV was lower in the case of the NFQs, clinafloxacin, and gatifloxacin than in the case of ciprofloxacin and trovafloxacin. These results suggest that the former group of quinolones is better able to exploit both the targets. Next, using clinical isolates of methicillin-resistant S. aureus (MRSA; n = 34) and coagulase-negative staphylococci (CoNS; n = 24), the NFQs and clinafloxacin were shown to be more potent (MIC at which 90% of the isolates are inhibited [MIC90] = 2 μg/ml for MRSA and 0.5 μg/ml for CoNS) than ciprofloxacin, trovafloxacin, and gatifloxacin (MIC90 = 16 to >64 μg/ml for MRSA and 4 to >32 μg/ml for CoNS). Bactericidal kinetics experiments, using two MRSA isolates, showed that exposure to the NFQs at four times the MIC reduced the bacterial counts (measured in CFU per milliliter) by ≥3 log units in 2 to 4 h. Overall, the NFQs and clinafloxacin were less susceptible than the other quinolones to existing mechanisms of quinolone resistance in staphylococci.
Quinolones exhibit potent antibacterial activity by targeting DNA gyrase (gyrase) and topoisomerase IV (topo IV), two enzymes that are essential for bacterial growth and survival (4, 6). Wide variations in the antibacterial potency and spectrum of quinolones are presumably attributable, in part, to their variable affinity toward these molecular targets. Although both gyrase and topo IV can be inhibited by a quinolone, bacterial growth inhibition and death can apparently be accomplished by inhibiting only one of these two targets. For example, a mutation in one of the target genes can reduce the potency of a quinolone like ciprofloxacin by severalfold, while a corresponding mutation in the other target gene may not affect its antibacterial potency (5, 13). Thus, antibacterial activity of certain quinolones is effectively dependent on one target, thereby increasing the likelihood of resistance development via mutation.
To better explain this phenomenon, it was previously proposed that in certain bacteria, such as Escherichia coli, gyrase is the primary target for quinolones while in others, such as Staphylococcus aureus, topo IV is the primary target (5, 10, 13, 19). However, in Streptococcus pneumoniae, gyrase and topo IV were shown to be dual targets of clinafloxacin (14). Thus, it seems appropriate to slightly modify the primary and/or secondary target theory by factoring in the variable affinity of quinolones toward their molecular targets to envision a target-ligand-specific phenomenon. The primary target for a given quinolone in a given bacterial species is the one for which it has a higher affinity. If the affinities of a quinolone for the two targets are relatively close, the extent of its exclusive dependence on one target to exhibit its antibacterial activity would be relatively low and the compound would be more likely to partially exploit both the targets. Consequently, the impact of mutations in either target alone would be relatively low in terms of lowering the antibacterial potency of a given quinolone. This appears to be true in the case of clinafloxacin in S. pneumoniae (14).
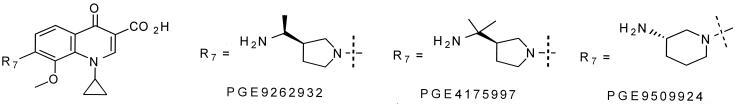
Recently, widespread quinolone resistance has been observed in clinical isolates of multidrug-resistant bacteria such as methicillin-resistant S. aureus (MRSA) and coagulase-negative staphylococci (CoNS) (9, 16). This is in part attributable to point mutations in the gyrA and grlA genes encoding the A subunits of gyrase and topo IV (7, 8, 15, 17). Thus, the effectiveness of newer quinolones against these pathogens will depend on their ability to (i) overcome existing resistance due to specific point mutations and (ii) inhibit both the targets simultaneously, thereby lowering the likelihood of stepwise, de novo resistance development. In this report, we describe the activity of three 8-methoxy-nonfluorinated quinolones (NFQs) (Fig. 1) against (i) their molecular targets, gyrase and topo IV, using whole-cell studies of specific S. aureus mutants and (ii) clinical isolates of MRSA and CoNS.
FIG. 1.
Chemical structures of the NFQs.
(This study was reported in part at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, Calif. [S. Roychoudhury, K. M. Makin, E. J. McIntosh, H. D. McKeever, T. L. Twinem, P. M. Koenigs, and C. E. Catrenich, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 50.F-547, p. 304, 1999].)
MATERIALS AND METHODS
Materials and bacterial strains.
Ciprofloxacin was purchased from Miles, Inc. (West Haven, Conn.). All other benchmark and test quinolones were synthesized in-house. Laboratory-generated mutants of S. aureus used in this study were obtained from David C. Hooper (Massachusetts General Hospital [2, 13]). Strain ISP794 was the quinolone-sensitive parent strain used to construct genetically engineered mutants. Strain SS1 contained a point mutation (Ser84-Leu) in the gyrA gene coding for the A subunit of gyrase. Strain MT5224c4 contained a point mutation (Ser80-Phe) in the grlA gene coding for the A subunit of topo IV. Strain EN1252a was a double mutant containing both the gyrA and the grlA mutations mentioned above. Clinical isolates of MRSA and CoNS were obtained from major hospitals in the greater Cincinnati, Ohio, area (Health Alliance Laboratories). The isolates were collected from patients during the spring and fall of 1998.
Whole-cell target inhibition.
Colonies from overnight cultures were resuspended in brain heart infusion (BHI) broth to produce an optical density (OD) of 0.1 at a 600-nm wavelength with a theoretical yield of 108 CFU/ml of broth. These cultures were further diluted 200-fold with BHI broth to produce an expected cell density of ≈5 × 105 CFU/ml. The bacterial cultures along with antibacterial agents (in twofold serial dilutions) were transferred into microtiter plates. Each sample was tested in duplicate. To measure bacterial growth kinetics, the plates were incubated at 37°C in a THERMOmax microplate reader (Molecular Devices, Menlo Park, Calif.), which was programmed to read the OD of the cultures in the wells at 600 nm every 15 min (with shaking) for up to 24 h. The data were recorded using SOFTmax software (Molecular Devices) and then converted into an Excel spreadsheet to generate time course curves.
MICs.
Microtiter plates with bacterial culture and test compounds were incubated in BHI broth at 37°C overnight (18 to 24 h), and the MIC was recorded as the lowest concentration of the compound inhibiting visible growth of bacteria (12). In analyzing multiple isolates of MRSA and CoNS, the MIC50 and MIC90 were defined as the minimum compound concentration at which growth of ≥50 and ≥90%, respectively, of the strains was inhibited. To measure the bactericidal kinetics of the test compounds, log-phase cultures, starting with ≈5 × 105 CFU of MRSA isolates/ml, were incubated in Mueller-Hinton broth at 37°C with shaking in the presence of test compounds at one, two, four, and eight times the MIC. Bacterial colonies were counted over time by the colony count method using a Spiral Plater (Spiral Biotech, Inc., Bethesda, Md.).
RESULTS
Whole-cell target inhibition assay.
To monitor the effect of quinolones on cell growth at concentrations below and above the MIC, the MICs of these compounds for the S. aureus mutants were initially determined (Table 1). Next, time course curves of cell growth at different concentrations of the quinolones were generated. Based on these data, growth after 10 h of incubation, corresponding to the onset of the stationary phase, appeared to be an optimal indication of partial inhibition by ciprofloxacin. Using the 10-h time point, the OD at 600 nm was plotted against different concentrations of ciprofloxacin for each mutant in comparison to the parent strain (Fig. 2). Data presented in Fig. 2A show that there was no effect of the gyrA mutation (Ser84-Leu) on the potency of ciprofloxacin as judged by the inhibition of cell growth. These data are consistent with the notion that gyrA is not a primary target for ciprofloxacin in S. aureus (13) and suggest that the inhibitory concentration range for gyrase is higher than that at which ciprofloxacin inhibits cell growth via topo IV inhibition.
TABLE 1.
MICs of the NFQs and other quinolones for S. aureus mutants
| Compound | MIC for strainsa
|
|||
|---|---|---|---|---|
| ISP794 | SS1 | MT5224c4 | EN1252a | |
| PGE9262932 | 0.016–0.031b | 0.063–0.125 | 0.063 | 0.25 |
| PGE4175997 | 0.031–0.063 | 0.063–0.125 | 0.125 | 0.5 |
| PGE9509924 | 0.063 | 0.125 | 0.125–0.5 | 0.5–1.0 |
| Clinafloxacin | 0.031 | 0.063 | 0.125 | 0.5 |
| Ciprofloxacin | 0.25 | 0.25–0.5 | 2.0 | 32 |
| Trovafloxacin | 0.063 | 0.063–0.125 | 0.25–0.5 | 4.0 |
| Gatifloxacin | 0.063–0.125 | 0.25 | 0.5 | 4.0 |
Relevant genotypes are as follows: ISP794, 8325 pig-131; SS1, gyrA (Ser84-Leu); MT5224c4, grlA (Ser80-Phe); EN1252a, gyrA (Ser84-Leu) grlA (Ser80-Phe).
Ranges are shown when traces of bacterial growth were observed with the lower concentration of the test compound. PGE9509924 was used as a racemic mixture in this experiment.
FIG. 2.
Effects of gyrA and grlA mutations on S. aureus cell growth in the presence of ciprofloxacin. With OD600 after a 10-h incubation (described under Materials and Methods), cell growth of strains SS1 (gyrA; Ser84-Leu) (A), MT5224c4 (grlA; Ser80-Phe) (B), and EN1252a (gyrA, Ser84-Leu; grlA, Ser80-Phe) (C) were compared with that of the parent strain ISP794. Error bars represent standard deviations due to well-to-well variations in results. These and similar data with all the quinolones included in this study were used to generate the ETI50 values.
Figure 2B shows the effect of the grlA mutation (Ser80-Phe) on the potency of ciprofloxacin. Unlike the mutation in gyrA, the grlA mutation had a major effect on reducing the potency of ciprofloxacin. With topo IV (grlA) mutated, ciprofloxacin could cause cell growth inhibition using either gyrase or the mutated form of topo IV as a target. Figure 2C shows the effect of mutation in both the gyrase and the topo IV genes. A comparison of Fig. 2B and C shows that when only topo IV is mutated, ciprofloxacin inhibits growth at ≥0.25 μg/ml, whereas when both gyrase and topo IV are mutated, there is no detectable inhibition at ciprofloxacin concentrations of ≤4 μg/ml. This suggests that when only topo IV is mutated, gyrase is the target via which ciprofloxacin mediates its inhibitory effect. Thus, the growth inhibition profile of the topo IV mutant (strain MT5224c4) reflects that of gyrase as the target, while the growth inhibition profile of the gyrase mutant (strain SS1) reflects that of topo IV as the target. Based on this analysis, the concentration of ciprofloxacin at which it inhibits 50% of cell growth is 0.5 μg/ml when gyrase is the target and 0.05 μg/ml when topo IV is the target (Fig. 2A and B and Table 2). This number is used in this study as a parameter to gauge the effective potency of quinolones toward the molecular targets as reflected in cell growth inhibition and is termed 50% effective target inhibition (ETI50). The assumptions underlying this analysis of quinolone potency against molecular targets are that (i) wild-type gyrase and topo IV are the only molecular targets of quinolones in S. aureus and (ii) serine hot spot mutations in either one of these targets leave the other target as the primary one via which cell growth inhibition occurs.
TABLE 2.
ETI50 values of NFQs and other quinolonesa
| Compound | ETI50 (gyrase) (μg/ml) | ETI50 (topo IV) (μg/ml) | ETI50 (gyrase)/ ETI50 (topo IV) |
|---|---|---|---|
| PGE9262932 | 0.014 | 0.004 | 3.5 |
| PGE4175997 | 0.024 | 0.008 | 3.0 |
| PGE9509924 | 0.05 | 0.012 | 4.2 |
| Clinafloxacin | 0.028 | 0.009 | 3.1 |
| Ciprofloxacin | 0.50 | 0.05 | 10 |
| Trovafloxacin | 0.10 | 0.006 | 16 |
| Gatifloxacin | 0.17 | 0.057 | 3.0 |
The ETI50 values were generated from growth inhibition curves of strains MT5224c4 [for ETI50 (gyrase)] and SS1 [for ETI50 (topo IV)], such as those shown in Fig. 2A and B for ciprofloxacin. Nonlinear regression analysis (SigmaPlot software) was used to estimate the ETI50 values. PGE9509924 was used as a racemic mixture in this experiment.
Using the methodology described above, ETI50 values for several quinolones were estimated and are summarized in Table 2. The ETI50 values for topo IV were compared with the published concentrations at which 50% of S. aureus topo IV activity was inhibited (IC50) by ciprofloxacin, gatifloxacin, and clinafloxacin (18), and a linear correlation was observed (Fig. 3), suggesting that the ETI50 values for topo IV could be used as a parameter to ascertain the affinity of a quinolone toward this target, in lieu of the IC50 values generated using the purified form of the enzyme.
FIG. 3.
Correlation between the S. aureus topo IV ETI50 values estimated during this study and previously reported IC50 values measured using purified topo IV by Takei et al. (18).
Activity of the NFQs for MRSA and CoNS.
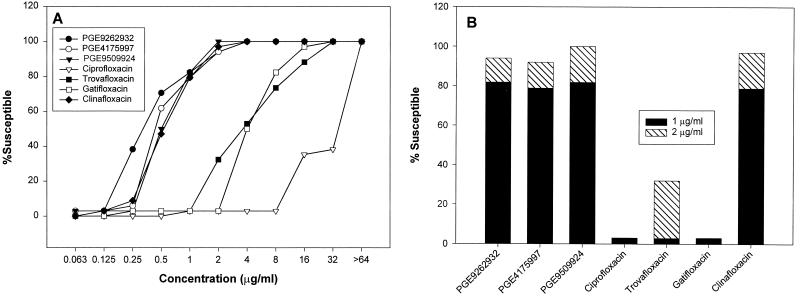
To study the in vitro potencies of the NFQs and several other antibacterials against MRSA isolates, MICs of these compounds were determined and summarized in Table 3. A high level of resistance (33 of 34 isolates) was observed not only to ciprofloxacin (MIC90 = >64 μg/ml) but also to newer quinolones such as trovafloxacin (MIC90 = 32 μg/ml) and gatifloxacin (MIC90 = 16 μg/ml). Figure 4A presents the cumulative susceptibility of the MRSA isolates to the NFQs and four other quinolones included in this study. Figure 4B shows the percentage of MRSA susceptibility at 1- and 2-μg/ml concentrations. These data suggest that the NFQs and clinafloxacin are more potent than ciprofloxacin, trovafloxacin, and gatifloxacin against MRSA. In vitro potency results, obtained with CoNS isolates from blood and surface wound infections, are summarized in Table 4. Based on the MIC90 values, the NFQs and clinafloxacin were >64-, 32-, and 8-fold more potent than ciprofloxacin, trovafloxacin, and gatifloxacin, respectively, against CoNS.
TABLE 3.
MICs of the NFQs and other compounds for MRSA (n = 34)
| Compound | MIC range (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) |
|---|---|---|---|
| PGE9262932 | 0.063–4.0 | 0.5 | 2.0 |
| PGE4175997 | 0.063–4.0 | 0.5 | 2.0 |
| PGE9509924 | 0.063–2.0 | 0.5 | 2.0 |
| Clinafloxacin | 0.125–4.0 | 1.0 | 2.0 |
| Ciprofloxacin | 1.0–>64 | 64 | >64 |
| Trovafloxacin | 0.25–32 | 4.0 | 32 |
| Gatifloxacin | 0.25–32 | 4.0 | 16 |
| Vancomycin | 1.0–2.0 | 1.0 | 2.0 |
| Ceftriaxone | 8.0–>16 | 16 | >16 |
| Clarithromycin | >200 | >200 | >200 |
| Azithromycin | >200 | >200 | >200 |
FIG. 4.
Cumulative susceptibility of the MRSA isolates to the NFQs and other quinolones. Percent susceptibility through the whole range of concentrations (A) and at 1- and 2-μg/ml concentrations (B) is shown.
TABLE 4.
MICs of the NFQs and other quinolones for CoNS (n = 24)
| Compound | MIC range (μg/ml) | MIC50 (μg/ml) | MIC90 (μg/ml) |
|---|---|---|---|
| PGE9262932 | ≤0.031–1.0 | 0.5 | 0.5 |
| PGE4175997 | ≤0.031–1.0 | 0.5 | 0.5 |
| PGE9509924 | 0.063–1.0 | 0.5 | 0.5 |
| Clinafloxacin | ≤0.031–0.5 | 0.5 | 0.5 |
| Ciprofloxacin | 0.125–>32 | 32 | >32 |
| Trovafloxacin | 0.063–32 | 8.0 | 16 |
| Gatifloxacin | 0.125–4.0 | 4.0 | 4.0 |
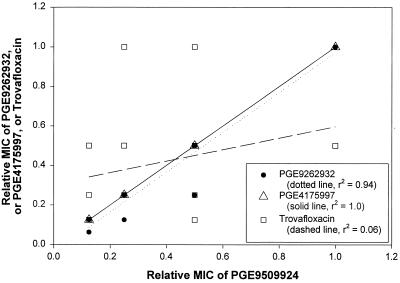
To further analyze the relative potencies of the NFQs and other quinolones against MRSA isolates with higher levels of quinolone resistance, isolates (n = 23) for which a trovafloxacin MIC was >2 μg/ml were studied. The relative MIC of a quinolone for each isolate was calculated by dividing the MIC by the highest MIC of that quinolone for this set of MRSA isolates. Relative MICs for these 23 isolates were either 1/16, 1/8, 1/4, 1/2, or 1/1. As shown in Fig. 5, the relative MICs of one of the NFQs, PGE9509924, were then plotted against those of two other NFQs, PGE9262932 and PGE4175997, and trovafloxacin. A linear correlation was observed between the relative MICs of PGE9509924 and PGE9262932 (linear regression coefficient [r2] = 0.94) and those of PGE9509924 and PGE4175997 (r2 = 1.0), while no such correlation was observed between the relative MICs of PGE9509924 and trovafloxacin (r2 = 0.06). Similar analyses using gatifloxacin and clinafloxacin (instead of trovafloxacin) yielded r2 values of 0.43 and 0.35, respectively.
FIG. 5.
Correlation of relative susceptibilities of MRSA isolates with high levels of quinolone resistance. These isolates were chosen based on the MIC of trovafloxacin (>2 μg/ml, i.e., in the 4- to 32-μg/ml range). Linear regression analyses were performed with data points correlating the relative MICs of PGE9509924 with those of PGE9262932, PGE4175997, and trovafloxacin.
Bactericidal kinetics.
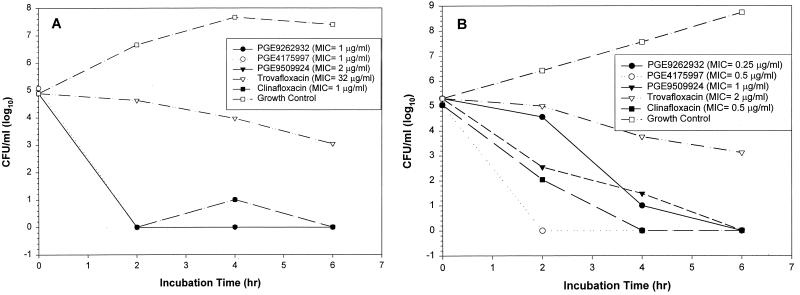
The NFQs were compared with trovafloxacin and clinafloxacin in bactericidal kinetics experiments using two MRSA isolates, Mi407 and Mi414. Like other quinolones (3), the NFQs demonstrated concentration-dependent killing kinetics. Results from experiments using four times the MIC of the test compounds are shown in Fig. 6. Against Mi407, all three NFQs and clinafloxacin showed a rapid bactericidal effect by reducing the bacterial count by >10,000-fold (to <10 CFU/ml) in ≤2 h of incubation, while trovafloxacin reduced the number of CFU per milliliter by approximately 100-fold in 6 h (Fig. 6A). Against Mi414, the NFQs and clinafloxacin reduced the bacterial count by >1,000-fold in ≤4 h, while trovafloxacin had a similar effect as in the case of Mi407 (Fig. 6B). The level of quinolone resistance was higher in Mi407 than in Mi414, as judged by the MICs (see the legend to Fig. 6). These data suggest that irrespective of the level of quinolone resistance, the NFQs and clinafloxacin have a potent bactericidal effect against the MRSA isolates used in this study.
FIG. 6.
Bactericidal kinetics of the NFQs, trovafloxacin, and clinafloxacin at four times the MIC. Results using two isolates of MRSA, Mi407 (A) and Mi414 (B), are shown. PGE9509924 was used as a racemic mixture in this experiment.
DISCUSSION
Based on the MIC analysis, the NFQs and clinafloxacin are more potent than ciprofloxacin, trovafloxacin, and gatifloxacin against different mutants of S. aureus (Table 1). These data are consistent with the ETI50 data suggesting that the NFQs and clinafloxacin have higher potency against the two known molecular targets, gyrase and topo IV, than the other quinolones used in this study (Table 2). While similar conclusions could be reached from the MIC data presented in Table 1, the ETI50 analysis provides a more sensitive and accurate estimation of effective target inhibition, since these values are derived from regression analyses of data corresponding to a range of drug concentrations that cause partial inhibition of a target leading to partial inhibition of cell growth. Variations in the partial target inhibition profile, potentially detectable in the ETI50 analysis, may not necessarily lead to a variation in potency as judged by the MIC analysis, which reflects complete growth inhibition. In addition, the correlation between the ETI50 (topo IV) values and the previously reported IC50 (topo IV) values (18) (Fig. 3) suggests that ETI50 could be a useful whole-cell parameter to gauge quinolone potency toward topo IV. The ETI50 (gyrase)/ETI50 (topo IV) ratio could be an important factor in potential resistance development. The higher the ETI50 (gyrase)/ETI50 (topo IV) ratio, the higher is the likelihood that in a sensitive strain of S. aureus, the antibacterial potency of a given quinolone is dependent on only one target, i.e., topo IV. This phenomenon was evident in the case of ciprofloxacin, which showed no detectable effect in cell growth inhibition kinetics in the gyrA mutant (SS1) relative to the parent strain (ISP794) (Fig. 2A). A similar observation was made with respect to trovafloxacin (data not shown). Theoretically, a relatively high ETI50 (gyrase)/ETI50 (topo IV) ratio could also lead to a relatively high level of potency reduction via a first-step mutation in topo IV (grlA).
As shown in Table 2, the ETI50 (gyrase)/ETI50 (topo IV) ratios range from 3 to 4 for the NFQs, clinafloxacin, and gatifloxacin, and from 10 to 16 for ciprofloxacin and trovafloxacin. While the ETI50 ratios suggest that for both groups of quinolones the effective target potency is higher toward topo IV than toward gyrase, it is likely for the former group of quinolones that in a sensitive strain of S. aureus both gyrase and topo IV are inhibited simultaneously, albeit to varying degrees. Thus, the primary and/or secondary target concept appears less applicable in the case of these compounds in that mutations in either target gene could lead to relatively low levels of increase in the MIC, as shown in Table 1. However, whether the potential advantage of dual-target inhibition in S. aureus will translate into an advantage for the NFQs in the development of resistance in vivo will depend on favorable pharmacodynamic parameters, such as area under the concentration-time curve/MIC90 and maximum concentration of drug in serum/MIC90 ratios.
Results using the S. aureus mutant strain EN1252a (gyrA: Ser84-Leu; grlA: Ser80-Phe) suggest that, compared to other quinolones, the NFQs and clinafloxacin are more potent against strains with the serine hot spots mutated in both gyrA and grlA (Table 1). Relative to the parent strain (ISP794), MICs of ciprofloxacin, trovafloxacin, and gatifloxacin for EN1252a were elevated 64- to 128-fold, while those of the NFQs and clinafloxacin were elevated 8- to 16-fold. Mutation in the serine-aspartate hot spots in gyrA and grlA is a mechanism by which MRSA isolates are known to develop quinolone resistance (17). All but one of the 34 MRSA isolates tested in this study appeared resistant to currently marketed quinolones. Consistent with the ETI50 results, the NFQs and clinafloxacin were much more potent than other quinolones used in this study (Table 2).
The DNA sequences of the quinolone resistance-determining regions of two MRSA isolates, Mi407 and Mi414, were found to have the known Ser84-Leu and Ser80-Phe mutations in gyrA and grlA, respectively. For Mi414, MICs of ciprofloxacin, trovafloxacin, and gatifloxacin are in the 4- to 16-μg/ml range, while MICs of the NFQs and clinafloxacin are in the 0.25- to 0.5-μg/ml range. Overall, these results are consistent with those obtained with strain EN1252a (gyrA, Ser84-Leu; grlA, Ser80-Phe) (Table 1) and suggest that the level of the observed quinolone resistance is due to mutations in the target genes. However, for Mi407, MICs of ciprofloxacin, trovafloxacin, and gatifloxacin are in the 16- to >64-μg/ml range, while MICs of the NFQs and clinafloxacin are in the 1-μg/ml range. Since Mi407 has the same mutations in the quinolone resistance-determining regions as Mi414, these data suggest that additional mechanisms of quinolone resistance are responsible for this higher level of quinolone resistance. However, unlike in the case of ciprofloxacin, trovafloxacin, and gatifloxacin, these additional mechanisms appear less effective in causing high-level resistance to the NFQs and clinafloxacin.
The efflux pump NorA has been implicated in fluoroquinolone resistance in S. aureus (11). The MIC data for SA1199B, a norA-overexpressing mutant of S. aureus, suggest that overexpression of NorA leads to a 2- to 4-fold increase (relative to the parent strain SA1199) in MICs of all the quinolones used in this study, except ciprofloxacin (16-fold increase) (data not shown). It is therefore possible that an efflux mechanism, along with point mutations in the target genes, is responsible for higher levels of quinolone resistance in MRSA. However, the relative MIC analysis using strains with higher levels of quinolone resistance (trovafloxacin MIC, >2 μg/ml) revealed an interesting possibility (Fig. 5). This analysis was done with the notion that if the variation in quinolone potency against these strains was due to variable expression of a resistance mechanism, such as an efflux pump, then the potency of one quinolone should have varied in proportion to that of another, assuming the substrate preference of the putative efflux pump did not change with its level of expression. Thus, a linear correlation between the relative MICs of the NFQs was not surprising. However, the lack of such a correlation between the relative MICs of PGE9509924 and that of trovafloxacin (as well as gatifloxacin and clinafloxacin) showed that the MICs of the NFQs could vary in a disproportionate manner relative to other quinolones for these MRSA strains. For example, the MIC of trovafloxacin was eightfold higher for one particular MRSA strain than another, while the corresponding MIC of PGE9509924 remained unchanged. This suggests that certain resistance mechanisms might be effective against one group of quinolones and not another. Further research is needed to shed light on the molecular basis for this observation.
In summary, the NFQs reported in this study have higher affinities for both the molecular targets, gyrase and topo IV, than ciprofloxacin, trovafloxacin, and gatifloxacin. In addition, the NFQs, clinafloxacin, and gatifloxacin appear to inhibit both the molecular targets more evenly than ciprofloxacin or trovafloxacin. The NFQs have higher potency against quinolone-resistant staphylococci than other quinolones such as ciprofloxacin, trovafloxacin, and gatifloxacin and are comparable to clinafloxacin. Bactericidal activity of the NFQs and clinafloxacin is unaffected by the level of quinolone resistance. Overall, the NFQs and clinafloxacin appear to be relatively unaffected by preexisting and characterized mechanisms of quinolone resistance in MRSA.
ACKNOWLEDGMENTS
We thank David C. Hooper and Glen W. Kaatz for the generous gifts of strains, Jan Pennington for the supply of clinical isolates, Laura J. V. Piddock for DNA sequence analysis, and R. D. Leunk, K. S. Howard-Nordan, W. L. Seibel, C. C. McOsker, J. J. Ares, and T. A. Inglin for managerial support.
REFERENCES
- 1.Anderson V E, Zaniewski R P, Kaczmarek F S, Gootz T D, Osheroff N. Quinolones inhibit DNA religation mediated by Staphylococcus aureus topoisomerase IV. Changes in drug mechanism across evolutionary boundaries. J Biol Chem. 1999;274:35927–35932. doi: 10.1074/jbc.274.50.35927. [DOI] [PubMed] [Google Scholar]
- 2.Bisognano C, Vaudaux P E, Lew D P, Ng E Y W, Hooper D C. Increased expression of fibronectin-binding proteins by fluoroquinolone-resistant Staphylococcus aureus exposed to subinhibitory levels of ciprofloxacin. Antimicrob Agents Chemother. 1997;41:906–913. doi: 10.1128/aac.41.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canton E, Ramon M S, Jimenez M T, Martinez J P. Killing kinetics of four quinolones against gram-positive cocci. Chemotherapy. 1993;39:394–399. doi: 10.1159/000238983. [DOI] [PubMed] [Google Scholar]
- 4.Chen A Y, Lie L F. DNA topoisomerase: essential enzymes and lethal targets. Annu Rev Pharmacol Toxicol. 1994;34:191–218. doi: 10.1146/annurev.pa.34.040194.001203. [DOI] [PubMed] [Google Scholar]
- 5.Cullen E M, Wyke A W, Kuroda A, Fisher L M. Cloning and characterization of a DNA gyrase gene from Escherichia coli that confers clinical resistance to 4-quinolones. Antimicrob Agents Chemother. 1989;33:886–894. doi: 10.1128/aac.33.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drlica K, Zao X. DNA gyrase, topoisomerase, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61:377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubin D T, Fitzgibbon J E, Nahvi M D, John J F. Topoisomerase sequences of coagulase-negative staphylococcal isolates resistant to ciprofloxacin or trovafloxacin. Antimicrob Agents Chemother. 1999;43:1631–1637. doi: 10.1128/aac.43.7.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fey P D, Climo M W, Archer G L. Determination of the chromosomal relationship between mecA and gyrA in methicillin-resistant coagulase-negative staphylococci. Antimicrob Agents Chemother. 1998;42:306–312. doi: 10.1128/aac.42.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein F W, Acar J F. Epidemiology of quinolone resistance: Europe and North and South America. Drugs. 1995;49(Suppl. 2):36–42. doi: 10.2165/00003495-199500492-00007. [DOI] [PubMed] [Google Scholar]
- 10.Hooper D C. Mode of action of fluoroquinolones. Drugs. 1999;58(Suppl. 2):6–10. doi: 10.2165/00003495-199958002-00002. [DOI] [PubMed] [Google Scholar]
- 11.Kaatz G W, Seo S M, Ruble C A. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1993;37:1086–1094. doi: 10.1128/aac.37.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. M7–A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 13.Ng E Y, Trucksis M, Hooper D C. Quinolone resistance mutations in topoisomerase IV: relationship with flqA locus and genetic evidence that topoisomerase IV is the primary target and DNA gyrase is the secondary target of fluoroquinolones in Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1881–1888. doi: 10.1128/aac.40.8.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan X-S, Fisher L M. DNA gyrase and topoisomerase IV are dual targets of clinafloxacin action in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1998;42:2810–2816. doi: 10.1128/aac.42.11.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson L R, Willard K E, Sinn L M, Fasching C E, Gerding D N. gyrA sequence analysis of Staphylococcus aureus and methicillin-resistant S. aureus strains selected, in vitro, for high-level ciprofloxacin resistance. Diagn Microbiol Infect Dis. 1993;17:97–101. doi: 10.1016/0732-8893(93)90019-4. [DOI] [PubMed] [Google Scholar]
- 16.Skurray R A, Firth N. Molecular evolution of multiply-antibiotic-resistant staphylococci. Ciba Found Symp. 1997;207:167–183. doi: 10.1002/9780470515358.ch11. [DOI] [PubMed] [Google Scholar]
- 17.Takahata M, Yonezawa M, Kurose S, Futakuchi N, Matsubara N, Watanabe Y, Narita H. Mutations in the gyrA and grlA genes of quinolone-resistant clinical isolates of methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1996;38:543–546. doi: 10.1093/jac/38.3.543. [DOI] [PubMed] [Google Scholar]
- 18.Takei M, Fukuda H, Yasue T, Hosaka M, Oomori Y. Inhibitory activities of gatifloxacin (AM-1155), a newly developed fluoroquinolone, against bacterial and mammalian type II topoisomerases. Antimicrob Agents Chemother. 1998;42:2678–2681. doi: 10.1128/aac.42.10.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka M, Onodera Y, Uchida Y, Sato K, Hayakawa I. Inhibitory activities of quinolones against DNA gyrase and topoisomerase IV purified from Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:2362–2366. doi: 10.1128/aac.41.11.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]