Abstract
Background
Insertable cardiac monitors (ICMs) require an invasive procedure and are used for purely diagnostic purposes. Therefore, simplicity of the insertion procedure, low complication rate, long-term patient acceptance, sensing quality, and reliable remote monitoring are of great importance.
Objective
To evaluate a novel ICM (BIOMONITOR III) regarding all these aspects.
Methods
BIOMONITOR III has a miniaturized profile, long sensing vector (≈70 mm), a fast insertion tool for pocket formation and ICM placement in 1 step, and daily automatic Home Monitoring (HM) function. We evaluated the insertion procedure, complication rate, patient acceptance, sensing quality, and HM performance in 653 patients with BIOMONITOR III inserted for any ICM indication within 2 ongoing studies involving 51 sites in 11 countries.
Results
The median time from skin incision to wound closure was 4.0 minutes (interquartile range, 2.3–6.2 minutes). Median follow-up period was 274 days (interquartile range, 175–342 days). Serious adverse device-related events occurred in 6 patients (0.9%). No deep infections were reported in 334 patients without antibiotic prophylaxis. The wearing comfort was good or excellent in ≈95%. The mean R-wave amplitude (0.73 mV) and HM transmission rate (≈94% of days) were stable over 1.5 years. R-wave amplitudes were larger (mean 0.80 vs 0.62 mV, P < .001) and noise burden was lower (median 3.7 vs 14.5 minutes/day, P < .001) for ICM insertions parallel to the heart’s long axis (54.2%) vs parasternal (41.3%). A gross visibility of P waves was 95.1%.
Conclusion
The study demonstrated fast insertion times, low complication rate, high patient acceptance, and favorable long-term sensing and HM performance of the ICM.
Keywords: Insertable cardiac monitor, Implantable loop recorder, Remote monitoring, Home Monitoring, Cardiac arrhythmia
Key Findings.
-
▪
The miniaturized implantable cardiac monitor (ICM) BIOMONITOR III (Biotronik, Berlin, Germany) has a uniquely long sensing vector, a fast insertion tool for pocket formation and ICM placement in 1 step, and daily automatic Home Monitoring (HM) function.
-
▪
This real-world study of 653 patients shows ease of device insertion (typically <5 minutes until wound closure) with low complication rate (0.9% of patients had serious adverse device-related events). The wearing comfort was good or excellent in ≈95% of patients.
-
▪
The R-wave amplitude (mean ≈0.73 mV) and HM performance (transmission on ≈94% of days) were stable over 1.5 years. Most electrocardiograms show discernible P waves.
-
▪
The insertion parallel to the heart’s long axis may be preferable owing to higher R-wave amplitudes and a lower noise burden.
Introduction
Insertable cardiac monitors (ICMs) provide continuous long-term heart rhythm monitoring to identify or exclude arrhythmias as cause of symptoms and to quantify burden of known arrhythmia.1, 2, 3, 4, 5 The expanding indications for the use of ICMs, the miniaturization of the devices, the simplification of the subcutaneous insertion procedure, and the improved arrhythmia detection algorithms and diagnostic possibilities, as well as the remote monitoring technology, lead to an increasing use of ICMs in clinical practice.3,5,6 With increased use, more robust data on the complexity and success of ICM insertion and device functionality are being collected in a real-world population that exceeds experience from individual clinical trials.
BIOMONITOR III (Biotronik, Berlin, Germany) is a new state-of-the-art ICM with a miniaturized shape and a simplified insertion procedure.7 We present data from a large patient cohort regarding the BIOMONITOR III insertion procedure and >1-year follow-up data for sensing quality (R-wave amplitudes and noise burden), P-wave visibility, remote monitoring performance, and complications.
Methods
Patient selection
The present analysis included patients with a BIOMONITOR III device inserted before September 1, 2021, as part of the BIO|MASTER.BIOMONITOR III study (NCT04025710) or BIO|STREAM.ICM registry (NCT04075084). These 2 ongoing studies enroll patients with any indication for ICM implantation in accordance with current clinical practice, including syncope or presyncope, detection of atrial fibrillation (AF) after cryptogenic stroke, AF burden monitoring, palpitations, and other conditions. Investigational sites accepted a central Ethics Committee’s vote for the respective study or obtained a separate local approval. The research is conducted according to the principles of the Declaration of Helsinki.
BIOMONITOR III
The BIOMONITOR III ICM combines a large sensing vector with a miniaturized profile. It has a 47.5-mm-long rigid component and a 30.5-mm-long flexible “antenna” that can adapt to the curvature of the body while expanding the sensing vector to ≈70 mm for improved R-wave amplitudes (Figure 1). With a cross-section of 8.3 mm × 4.3 mm, total volume of 1.97 cm3, and weight of 5 g, BIOMONITOR III has a cross-sectional profile similar to other currently available ICM devices.4 A dedicated insertion tool set, consisting of the Incision Tool and the Fast Insertion Tool “FIT OneStep,” was developed to enable a simple, injection-like implantation procedure with pocket formation and ICM placement in 1 step. Further details on the ICM design and the insertion tool set can be found in the recently published first-in-human study with 47 patients.7
Figure 1.
The BIOMONITOR III (Biotronik, Berlin, Germany) implantable cardiac monitor (ICM) with a long sensing vector (≈70 mm, distance between the 2 electrocardiogram electrodes on the opposite ends of the device), the Fast Insertion Tool “FIT OneStep” with the ICM premounted for tunneling under the skin, and the Incision Tool with the stainless steel blade.
Based on the rate and stability of the R-wave sequence, BIOMONITOR III can detect 5 different types of heart rhythm disturbances: bradycardia, pause, AF, high ventricular rate, and sudden ventricular rate drop. A 1-minute electrocardiogram (ECG) strip is recorded upon detection of an arrhythmia or at a scheduled time of the day (“periodic ECG”).7 ECG recordings of 7.5 minutes can be triggered by the patient. To quantify the amount of noise and artefacts, the device records “noise burden” as the proportion of a 24-hour period during which very fast signals (<180 ms) prevent the evaluation of the rhythm.
The ICM uses the established Biotronik Home Monitoring® system (HM) to transmit up to 6 ECG strips, arrhythmia detection statistics, and sensing performance parameters to the HM Service Center once a day via wireless links without patient participation.7,8
ICM insertion procedure and follow-up schedule
BIOMONITOR III was inserted in accordance with institutional standards (regarding the place of procedure, incision closure, and wound protection) and the manufacturer’s instructions. The study protocols recommend 3 insertion positions: parallel to the heart’s long axis, parasternal, or inframammary, wherever large signal amplitudes and minimal device movement owing to positional changes and body and arm activities are expected. After selection of the insertion position and injection of a local anesthetic agent, an incision through the skin was made by the Incision Tool, the ICM was inserted using the FIT OneStep tool, the incision was closed, and the wound was protected.
In the BIO|MASTER.BIOMONITOR III study, follow-up visits are planned after 10–28 days, 3 months, and 12 months of ICM insertion. In the BIO|STREAM-ICM study, no follow-up visits were mandatory, but in-office and remote follow-ups were reported as conducted. Adverse events related to the insertion procedure or study device were reported during the study and are presented according to the categories and severity definition used in a large study of an alternative ICM model, to allow for comparisons.9
Data evaluation
During the insertion of ICM devices, investigators recorded procedure location; insertion position; wound closure method; duration from skin incision to tool removal, wound closure, and wound cleaned; antibiotic prophylaxis; and the need for ICM repositioning.
Based on the HM Service Center data, R-wave amplitudes, intraindividual stability of the R wave, noise burden, time to first HM message, and HM transmission success were assessed during follow-up. R waves and noise burden were summarized in 3 ways: per patient, by device position (long heart axis vs parasternally), and as temporal trend in pooled data for all patients. The intraindividual stability of the R wave was assessed by the ratio of the standard deviation (SD) and the mean value of all R-wave amplitudes. HM transmission success was defined as the percentage of days with HM message between the first transmission and study termination or data freeze, and was calculated per patient and as temporal trend.
Study investigators evaluated the visibility of P waves in periodic ECG strips transmitted by HM. The BIO|MASTER.BIOMONITOR III study protocols defined that at 3 follow-up visits, a total of 4 periodic ECG strips were to be evaluated if they showed regular 1:1 conducted rhythm. The numbers of heart cycles and discernible P waves in these ECG strips were counted.
The study investigators rated wound healing at in-office follow-ups and the patients rated their comfort with the device during in-office and remote follow-ups as very poor, poor, adequate, good, or excellent.
The present analysis considers data available by September 1, 2021 (the time of data freeze). Results are presented with standard summary statistics. Temporal trends were calculated with a linear regression, weighted with the number of data points. No study hypotheses or statistical endpoints were predefined.
Results
In total, 653 patients from 51 investigational sites in 11 countries were enrolled either in the BIO|MASTER.BIOMONITOR III study (n = 159) or BIO|STREAM-ICM registry (n = 494) before data freeze. The main contributing countries were Germany (28.8%), France (20.2%), Spain (13.6%), and Australia (12.3%). The patients were 63.3 ± 16.0 years old and 41.4% were women. The most frequent indications for ICM implantation were syncope or presyncope (57.2%), cryptogenic stroke or suspected transient ischemic attack (25.1%), and management of AF (7.1%) (Table 1).
Table 1.
Characteristics of included patients (n = 653) and contribution of countries
| N | Value | |
|---|---|---|
| Age, years | 649 | |
| Mean ± SD (range) | 63.3 ± 16.0 (18–90) | |
| Median (IQR) | 66 (54–76) | |
| Sex, male/female | 649 | 380 (58.6%) / 269 (41.4%) |
| Body mass index, kg/m2 | 535 | |
| Mean ± SD | 27.8 ± 5.6 | |
| Median (IQR) | 27.0 (24.0–30.6) | |
| Primary indication for ICM | 649 | |
| Syncope or presyncope | 371 (57.2%) | |
| Cryptogenic stroke | 163 (25.1%) | |
| Management of atrial fibrillation | 46 (7.1%) | |
| Other | 69 (10.6%) | |
| Coronary artery disease | 651 | 111 (17.1%) |
| Hypertension | 651 | 367 (56.4%) |
| History of heart failure | 651 | 76 (11.7%) |
| History of atrial fibrillation | 651 | 115 (17.7%) |
| History of ventricular arrhythmia | 651 | 49 (7.5%) |
| History of stroke / transient ischemic attack | 651 | 184 (28.3%) |
| Diabetes | 651 | 111 (17.1%) |
| Contributing country | 653 | |
| Germany | 188 (28.8%) | |
| France | 132 (20.2%) | |
| Spain | 89 (13.6%) | |
| Australia | 80 (12.3%) | |
| Switzerland | 53 (8.1%) | |
| Italy | 31 (4.7%) | |
| Portugal | 28 (4.3%) | |
| Austria | 22 (3.4%) | |
| Latvia | 19 (2.9%) | |
| Hungary | 8 (1.2%) | |
| Denmark | 3 (0.5%) |
Data are shown as n (%) if not stated otherwise.
ICM = insertable cardiac monitoring; IQR = interquartile range.
The locations of ICM insertions were catheterization lab (56.0%), operating theatre (25.4%), and a consultation room without specific equipment (18.5%). The most frequently used insertion positions were parallel to the heart’s long axis (54.2%) and parasternal (41.3%); the incisions were predominately made in the fourth (54.1%), third (32.0%), and fifth (8.6%) intercostal space. The wound was sutured in 79.2% of cases or, alternatively, closed with staples or adhesive strips (Table 2).
Table 2.
Insertion procedure
| N | Value | |
|---|---|---|
| Place of procedure | 653 | |
| Catheterization laboratory | 366 (56.0%) | |
| Operating theatre | 166 (25.4%) | |
| Other | 121 (18.5%) | |
| ICM insertion position | 653 | |
| Parallel to the heart's long axis | 354 (54.2%) | |
| Parasternal | 270 (41.3%) | |
| Other (mostly 2nd/3rd intercostal) | 29 (4.5%) | |
| Wound closure | 643 | |
| Sutures | 509 (79.2%) | |
| Staples | 75 (11.7%) | |
| Adhesive strips | 59 (9.2%) | |
| ICM repositioned | 653 | 2 (0.3%) |
| Antibiotic prophylaxis | 653 | |
| Systemic | 285 (43.6%) | |
| Local only | 34 (5.2%) | |
| None | 334 (51.2%) | |
| Procedure durations | ||
| Minutes from skin cut to tool removal | 650 | |
| Mean ± SD | 1.9 ± 3.2 | |
| Median (IQR) | 1.0 (0.8–2.0) | |
| From skin cut to wound closure | 651 | |
| Mean ± SD | 5.0 ± 4.5 | |
| Median (IQR) | 4.0 (2.3–6.2) | |
| From skin cut to wound cleaned | 649 | |
| Mean ± SD | 7.5 ± 6.0 | |
| Median (IQR) | 5.6 (4.0–9.0) |
Data are shown as n (%) if not stated otherwise.
ICM = insertable cardiac monitor; IQR = interquartile range.
The insertions took a median of 1.0 minute until removal of the insertion tool (interquartile range [IQR], 0.8–2.0 minutes), 4.0 minutes until wound closure (IQR, 2.3–6.2 minutes), and 5.6 minutes until wound cleaning (IQR, 4.0–9.0 minutes) (Table 2). Less than half of all patients received antibiotic prophylaxis (48.8%). The ICM was repositioned in 2 cases (0.3%).
The median follow-up period until study termination or data freeze was 274 days (IQR, 175–342 days). Total follow-up duration was 469 patient-years.
Wound healing was rated as excellent or good in 91.1% (at month 1; n = 179), 97.6% (month 2–3; n = 166), and 99.5% (month 4–12; n = 192) of patients. At the same follow-up points, the wearing comfort was perceived as excellent or good by 93.4% (n = 197), 97.1% (n = 207), and 91.7% (n = 242) of patients, respectively.
HM service center data
The mean R-wave amplitude was 0.73 ± 0.40 mV. The R-waves were larger in devices inserted parallel to the heart’s long axis (0.80 ± 0.43 mV) than parasternally (0.62 ± 0.34 mV) (P < .001, t test). The daily R-wave amplitudes varied by 8.6% on average (Table 3).
Table 3.
R-wave amplitudes and noise burden (Home Monitoring Service Center data)
| R wave and noise by ICM position | N= | Mean ± SD | Median (IQR) |
|---|---|---|---|
| R-wave amplitude, mV | |||
| All insertion positions | 621 | 0.73 ± 0.40 | 0.60 (0.42–0.97) |
| Parallel to the heart's long axis† | 339 | 0.80 ± 0.43 | 0.71 (0.46–1.09) |
| Parasternal position† | 259 | 0.62 ± 0.34 | 0.53 (0.38–0.77) |
| Noise burden, minutes/day | |||
| All insertion positions | 621 | 43.3 ± 102.4 | 9.4 (0.3–36.4) |
| Parallel to the heart's long axis‡ | 339 | 36.3 ± 102.7 | 3.7 (0.0–26.8) |
| Parasternal position‡ | 259 | 53.3 ± 104.8 | 14.5 (3.5–49.1) |
| R-wave amplitude stability: SD/mean, % | 621 | 8.6 ± 6.5 | 7.0 (5.1–9.6) |
ICM = insertable cardiac monitor; IQR = interquartile range; SD, standard deviation.
P < .001 (t test) and
P < .001 (Mann–Whitney U test).
The distribution of noise burden across patients was highly oblique: many patients had no noise and few had high values. The median noise burden was 9.4 minutes (IQR, 0.3–36.4 minutes). Like R-wave amplitudes, also the noise burden had more favorable values in devices positioned along the heart axis than parasternally (median 3.7 vs 14.5 minutes/day, P < .001, Mann–Whitney U test) (Table 3).
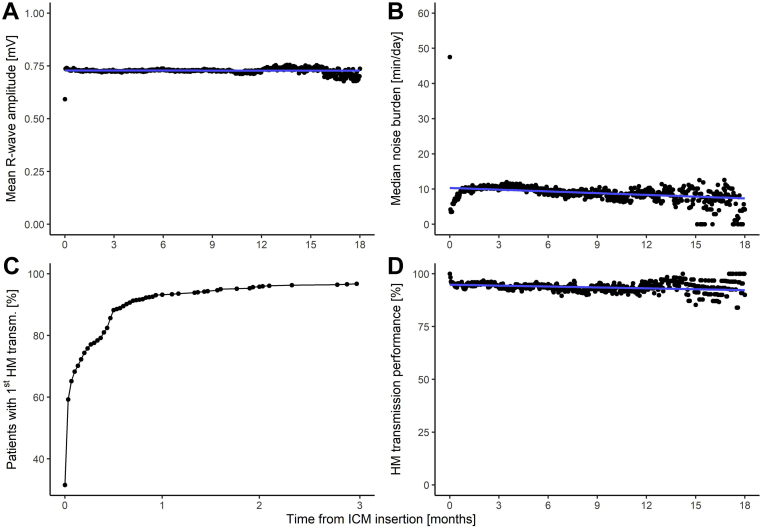
Over the first year of follow-up, the mean R-wave amplitude decreased by <0.1% (P < .001) and the median noise burden decreased by 2.0 minutes (P = .10) (Figure 2).
Figure 2.
Temporal trends of Home Monitoring (HM) Service Center data. A: R-wave amplitudes. B: Noise burden. C: Proportion of patients connected to HM. D: Daily HM transmission success of all patients after the first transmission (patients are included on the day at which the transmission started). Blue lines indicate linear regression. ICM = insertable cardiac monitor.
Overall, 621 patients (95.1%) transmitted at least 1 HM message. Median delay from ICM insertion to the first HM message was 1 day (IQR, 0–7 days; Figure 2C). The HM transmission success until 18 months was fairly stable, with a value of 94.8% at the beginning and 93.1% at 1 year (Figure 2D). The median individual transmission success was 93.7% (IQR, 70.4%–98.6%).
P-wave visibility
The 159 BIO|MASTER.BIOMONITOR III study patients had 331 follow-up visits and 490 ECG strips scheduled. Of the 490 strips, 140 did not show sinus rhythm with a regular 1:1 conduction (owing to extrasystoles or other arrhythmias). The remaining 350 ECG strips in 135 patients were suitable for P-wave analysis. On average, ECG strips contained 65.1 heart cycles with 61.9 visible P waves, resulting in a gross visibility of 95.1% (21,670 P waves in 22,778 heart cycles). Of the 135 patients, 94 (69.6%) had all P waves visible and 123 (91.1%) had ≥80% of P waves visible in their ECGs. The visibility did not depend on the position of the inserted device.
Complications
Twelve adverse events related to the insertion procedure or study device occurred in 12 patients (1.8%). Of these, 6 events were serious (in 0.9% of patients), comprising device extrusion (n = 3), pocket erosion with device migration and impending extrusion (n = 1), and postimplant pain and/or discomfort (n = 2), all requiring ICM explantation. Two additional explantations were necessitated by postimplant pain after a device pocket had been formed by surgical equipment instead of the dedicated insertion tool (separately counted cases).
Six nonserious adverse device-related events included vasovagal syncope caused by painful insertion after insufficient anesthesia (n = 1), minor bleeding (n = 1) and wound healing disorder (n = 1) treated with an additional suture, superficial infection (n = 1), ICM migration to a new acceptable position (n = 1), and ICM migration solved by repositioning (n = 1).
No pocket infection requiring invasive intervention was reported. Eight patients died, 6 of them for documented nonarrhythmic causes. In 2 patients, the cause of death was unknown: in an 89-year-old multimorbid male patient and in a 66-year-old female patient with hypertension and heart failure. No evidence of bradyarrhythmias or tachyarrhythmias has been obtained by HM in these patients, but they had received the device for a presyncope/syncope and an arrhythmic death can therefore not be excluded.
Explantation after diagnosis and complication
There were 44 attempts of ICM explantation after arrhythmia diagnosis was made or owing to complications. The explantation was performed in an operating theatre (50.0%, n = 22), catheterization lab (45.5%, n = 20), or consultation room (4.5%, n = 2), in most cases with a forceps. The ICM was explanted successfully in all cases, taking a median of 7 minutes (IQR, 4–15 minutes). A 1- to 2-cm-long incision for explantation was predominantly made close to the scar. Explantation was rated as “very easy” in 20 of 42 ratings (47.6%), “rather easy” in 12 (28.6%), “adequate” in 7 (16.7%), and “rather difficult” in 3 (7.1%).
Discussion
An ICM is a device requiring an invasive procedure and is used for purely diagnostic purposes. Therefore, simplicity of the insertion procedure, low complication rate, long-term patient acceptance, sensing quality, and reliable remote monitoring are of great importance. We provide data from a wide range of patients, indications, and countries.
Simplicity of the insertion procedure
The BIOMONITOR III ICM has a small cross-section and is supplied with a dedicated insertion tool for a fast and simple insertion procedure. Under real-world conditions, the time from skin incision to the insertion tool removal was ≤1 minute in one-half of patients and ≤2 minutes in three-quarters of patients. The median time from incision to closure was 4.0 minutes (IQR, 2.3–6.2 minutes) as compared to 4.0 minutes (IQR, 3.0–6.0 minutes) reported in another state-of-the-art ICM with a similar cross-sectional profile (Reveal LINQ; Medtronic Inc, Minneapolis, MN).10 In 18.5% of our patients, the ICM was inserted in a consultation room instead of a catheterization lab or operation theatre. Miniaturization of ICMs enables the procedure to be relocated to this new environment, giving hospitals flexibility in planning the use of their facilities without compromising patient safety or outcomes.9, 10, 11, 12 From the patients’ perspective in a large-cohort study described by Rogers and colleagues,11 far more patients perceived the procedure location to be “very convenient” when it was performed in office (85%) than when it was performed in hospital (29%).
Low complication rate, long-term patient acceptance
We observed no deep pocket infection requiring explantation, whereas a few infections were managed by local antibiotics. Since half of the insertions in our population were performed without antibiotic prophylaxis, prophylaxis may generally be unnecessary. Wound healing was good or excellent in 99.5% of patients. The overwhelming majority of patients felt comfortable with the implant (good or excellent wearing comfort in ≈95%).
The rate of serious adverse device-related events of 0.9% (6/653) is similar to the 0.6% (9/1420) and 0.8% (10/1222) rate of serious complications requiring ICM explantation reported from 2 large patient cohorts implanted with the Reveal LINQ ICM.9,13 It is noteworthy that all 3 cases of device dislocation or extrusion that required explantation in our study occurred in patients without sutures, who made up only 20.8% of our study cohort. We therefore strongly recommend sutures to prevent ICM explantations caused by dislocation or extrusion.
Sensing quality
The extended sensing vector of ≈70 mm vs <50 mm in other contemporary ICM models yields a larger mean R-wave amplitude in BIOMONITOR III (0.73 mV) than in other ICM modes (≤0.60 mV), which may improve sensing capabilities.7,14, 15, 16 Furthermore, R-wave amplitudes were significantly larger (mean 0.80 vs 0.62 mV) when the device was inserted along the heart axis (54.2% of patients) than when it was inserted parasternally (41.3% of patients). Therefore, the insertion parallel to the heart’s long axis can be recommended as a first-line solution in clinical practice. This is further supported by the finding of significantly lower noise burden in this position (median 3.7 vs 14.5 minutes/day).
The trend of mean R-wave amplitudes over the first 12 months was stable (<0.1% decrease in the linear fit) and no increase in noise burden was observed (median burden <10 minutes/day), demonstrating the longitudinal stability of the ICM’s sensing performance. The intraindividual stability of R-wave amplitudes during follow-up was excellent (SD/mean value <10%) in three-quarters of patients. Of note, ICM models other than BIOMONITOR 2 or III also suspend heart rhythm classification in the presence of noise, but to the best of our knowledge they do not quantify noise burden, which prevents comparisons.
P-wave visibility is an increasingly recognized ICM parameter of relevance for an appropriate medical decision based on a relatively short single ECG strip of an arrhythmia episode.7 Moreover, P-wave visibility is an indicator of high signal fidelity: the presence or absence of P waves not only helps with rhythm classification, but also indicates that the QRS-complex and T-wave morphologies can aid classification. In the present study, a gross visibility of P waves in ECG strips with sinus rhythm and regular 1:1 conduction was very satisfactory, 95.1%, with 69.6% of patients having all P waves visible. P-wave visibility did not depend on the insertion position. With alternative ICM models, P waves were reported to be well visible in 58%–72% of patients.15,17,18
Reliable remote monitoring
Patients with an ICM are an ideal group for pure remote follow-up, as they do not require adjustment of therapies delivered by the device. The remote monitoring capabilities with BIOMONITOR III are similar to what has been shown for other device types (pacemakers, defibrillators) and other patient groups using the studied HM technology. A median individual transmission success of ≈94% in the present study means that a daily message in one-half of the patients is missing only once in up to 15 days. The transmission success remains stable over 1.5 years, showing that remote follow-up is feasible and can therefore unburden both the investigator and the patient.
Comparison with the first-in-human study
The BIOMONITOR III ICM was initially evaluated in a first-in-human study of 47 patients in Australia.7 Mariani and colleagues7 reported study results, recognizing the small number of patients and a short mean follow-up period of 35.2 ± 18.5 days as main study limitations. These limitations have now been overcome, with 653 patients followed for a median of 274 days in 11 countries. The cumulative follow-up period in the present study (469 patient-years) is ≈102 times greater than that in the first-in-human study (4.6 patient-years).
The 2 studies enrolled similar patient populations with respect to age and other baseline characteristics, and had similar findings.7 In addition to confirming the results of a small study conducted in a more controlled settings, the present study demonstrated long-term stability of the ICM’s sensing quality and HM performance, assessed complication rates in a large patient cohort in the real-world conditions, and indicated the superiority of the sensing performance of the ICMs inserted parallel to the longitudinal axis of the heart.
Study limitations
In the present interim analysis of the 2 ongoing studies, no summary of the ICM-detected arrhythmia episodes has been generated and the detection performance of the ICM has not yet been evaluated. These important aspects of ICM performance remain to be analyzed after a significant number of arrhythmia episodes have occurred and all related clinical data have been collected and consolidated. Because the study did not randomize the insertion positions, the corresponding comparative results may have been influenced by unknown confounders.
Conclusion
The BIOMONITOR III is a miniaturized ICM with a uniquely long sensing vector. This real-world study shows ease of device insertion with low complication rate. Long-term follow-up confirms high patient acceptance, favorable sensing parameters, and stable HM performance. The insertion parallel to the heart’s long axis may be preferable owing to higher R-wave amplitudes and a lower noise burden.
Acknowledgments
The authors are thankful to Anja Viehrig, Sabrina Hoche, and Jens Buttgereit for study management; Katharina Ingel and Nils Jenke for statistical evaluations; Juergen Schrader for scientific advice; and Dejan Danilovic for critical reading and editing of the manuscript.
Acknowledgments
Funding Sources
The study was supported by Biotronik SE & Co. KG, Berlin, Germany. The sponsor assisted in study design, data collection, data analysis, data interpretation, and preparation of this report. The corresponding author had full access to the data and had final responsibility for the decision to submit for publication.
Disclosures
TD reports speaker fee from Biotronik (educational grant); PC declares having no conflicts of interest; DH reports educational grants, consultant or speaker fees, and fellowship support from European Heart Rhythm Organization (EHRA), Abbott, Medtronic, Biotronik, Boston Scientific, Biosense Webster, Novartis, Bayer, Pfizer, and Spectranetics/Philipps; TG declares having no conflicts of interest; BP declares being consultant for Abbott, Biotronik, Boston Scientific, Microport; GB declares having no conflicts of interest; RKP declares having no conflicts of interest; VMS declares having no conflicts of interest; EM reports speaker fee/consulting fee from Deutsche Gesellschaft für Kardiologie, Medtronic, Astra Zeneca, Bristol-Myers Squibb, Philips/Spectranetics, and Biotronik; JM declares having no conflicts of interest; ABS declares having no conflicts of interest; MW declares having no conflicts of interest; AH declares having no conflicts of interest; TP declares having no conflicts of interest; BW is employee of Biotronik; DL declares support by Mid-Career Fellowships from The Hospital Research Foundation. Dr Lau reports that the University of Adelaide has received on his behalf lecture and/or consulting fees from Abbott Medical, Boehringer Ingelheim, Biotronik, Medtronic, MicroPort CRM, and Pfizer.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Written informed consent was obtained from all patients.
Ethics Statement
The research reported in this study was conducted according to the principles of the Declaration of Helsinki.
Disclaimer
Given his role as Associate Editor, Dennis Lau had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Editors Ulrika Birgersdotter-Green and Jeanne E. Poole.
References
- 1.Galli A., Ambrosini F., Lombardi F. Holter monitoring and loop recorders: from research to clinical practice. Arrhythm Electrophysiol Rev. 2016;5:136–143. doi: 10.15420/AER.2016.17.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciconte G., Giacopelli D., Pappone C. The role of implantable cardiac monitors in atrial fibrillation management. J Atr Fibrillation. 2017;10:1590. doi: 10.4022/jafib.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giancaterino S., Lupercio F., Nishimura M., Hsu J.C. Current and future use of insertable cardiac monitors. JACC Clin Electrophysiol. 2018;4:1383–1396. doi: 10.1016/j.jacep.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Sakhi R., Theuns D.A.M.J., Szili-Torok T., Yap S.C. Insertable cardiac monitors: current indications and devices. Expert Rev Med Devices. 2019;16:45–55. doi: 10.1080/17434440.2018.1557046. [DOI] [PubMed] [Google Scholar]
- 5.Bisignani A., De Bonis S., Mancuso L., Ceravolo G., Bisignani G. Implantable loop recorder in clinical practice. J Arrhythm. 2019;35:25–32. doi: 10.1002/joa3.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wechselberger S., Piorkowski C., Pohl M. Current rare indications and future directions for implantable loop recorders. Herzschrittmacherther Elektrophysiol. 2016;27:366–370. doi: 10.1007/s00399-016-0475-x. [DOI] [PubMed] [Google Scholar]
- 7.Mariani J.A., Weerasooriya R., van den Brink O., et al. Miniaturized implantable cardiac monitor with a long sensing vector (BIOMONITOR III): insertion procedure assessment, sensing performance, and home monitoring transmission success. J Electrocardiol. 2020;60:118–125. doi: 10.1016/j.jelectrocard.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Sogaard P., Behrens S., Konyi A., et al. Transmission and loss of ECG snapshots: remote monitoring in implantable cardiac monitors. J Electrocardiol. 2019;56:24–28. doi: 10.1016/j.jelectrocard.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Beinart S.C., Natale A., Verma A., et al. Real-world comparison of in-hospital Reveal LINQ insertable cardiac monitor insertion inside and outside of the cardiac catheterization or electrophysiology laboratory. Am Heart J. 2019;207:76–82. doi: 10.1016/j.ahj.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Sanders P., Piorkowski C., Kragten J.A., et al. Safety of in-hospital insertable cardiac monitor procedures performed outside the traditional settings: results from the Reveal LINQ in-office 2 international study. BMC Cardiovasc Disord. 2019;19:132. doi: 10.1186/s12872-019-1106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers J.D., Sanders P., Piorkowski C., et al. In-office insertion of a miniaturized insertable cardiac monitor: results from the Reveal LINQ In-Office 2 randomized study. Heart Rhythm. 2017;14:218–224. doi: 10.1016/j.hrthm.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Kipp R., Young N., Barnett A., et al. Injectable loop recorder implantation in an ambulatory setting by advanced practice providers: analysis of outcomes. Pacing Clin Electrophysiol. 2017;40:982–985. doi: 10.1111/pace.13155. [DOI] [PubMed] [Google Scholar]
- 13.Diederichsen S.Z., Haugan K.J., Hojberg S., et al. Complications after implantation of a new-generation insertable cardiac monitor: results from the LOOP study. Int J Cardiol. 2017;241:229–234. doi: 10.1016/j.ijcard.2017.03.144. [DOI] [PubMed] [Google Scholar]
- 14.Purerfellner H., Sanders P., Pokushalov E., Di Bacco M., Bergemann T., Dekker L.R. Miniaturized Reveal LINQ insertable cardiac monitoring system: first-in-human experience. Heart Rhythm. 2015;12:1113–1119. doi: 10.1016/j.hrthm.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 15.Maines M., Zorzi A., Tomasi G., et al. Clinical impact, safety, and accuracy of the remotely monitored implantable loop recorder Medtronic Reveal LINQTM. Europace. 2018;20:1050–1057. doi: 10.1093/europace/eux187. [DOI] [PubMed] [Google Scholar]
- 16.Lim W.Y., Papageorgiou N., Sukumar S.M., et al. A nurse-led implantable loop recorder service is safe and cost effective. J Cardiovasc Electrophysiol. 2019;30:2900–2906. doi: 10.1111/jce.14206. [DOI] [PubMed] [Google Scholar]
- 17.Lacour P., Dang P.L., Huemer M., et al. Performance of the new BioMonitor 2-AF insertable cardiac monitoring system: can better be worse? Pacing Clin Electrophysiol. 2017;40:516–526. doi: 10.1111/pace.13059. [DOI] [PubMed] [Google Scholar]
- 18.Bisignani G., De Bonis S., Bisignani A., Mancuso L., Giacopelli D. Sensing performance, safety, and patient acceptability of long-dipole cardiac monitor: an innovative axillary insertion. Pacing Clin Electrophysiol. 2018;41:277–283. doi: 10.1111/pace.13281. [DOI] [PubMed] [Google Scholar]